Orodispersible Hydrogel Film Technology for Optimized Galantamine Delivery in the Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Results and Discussion
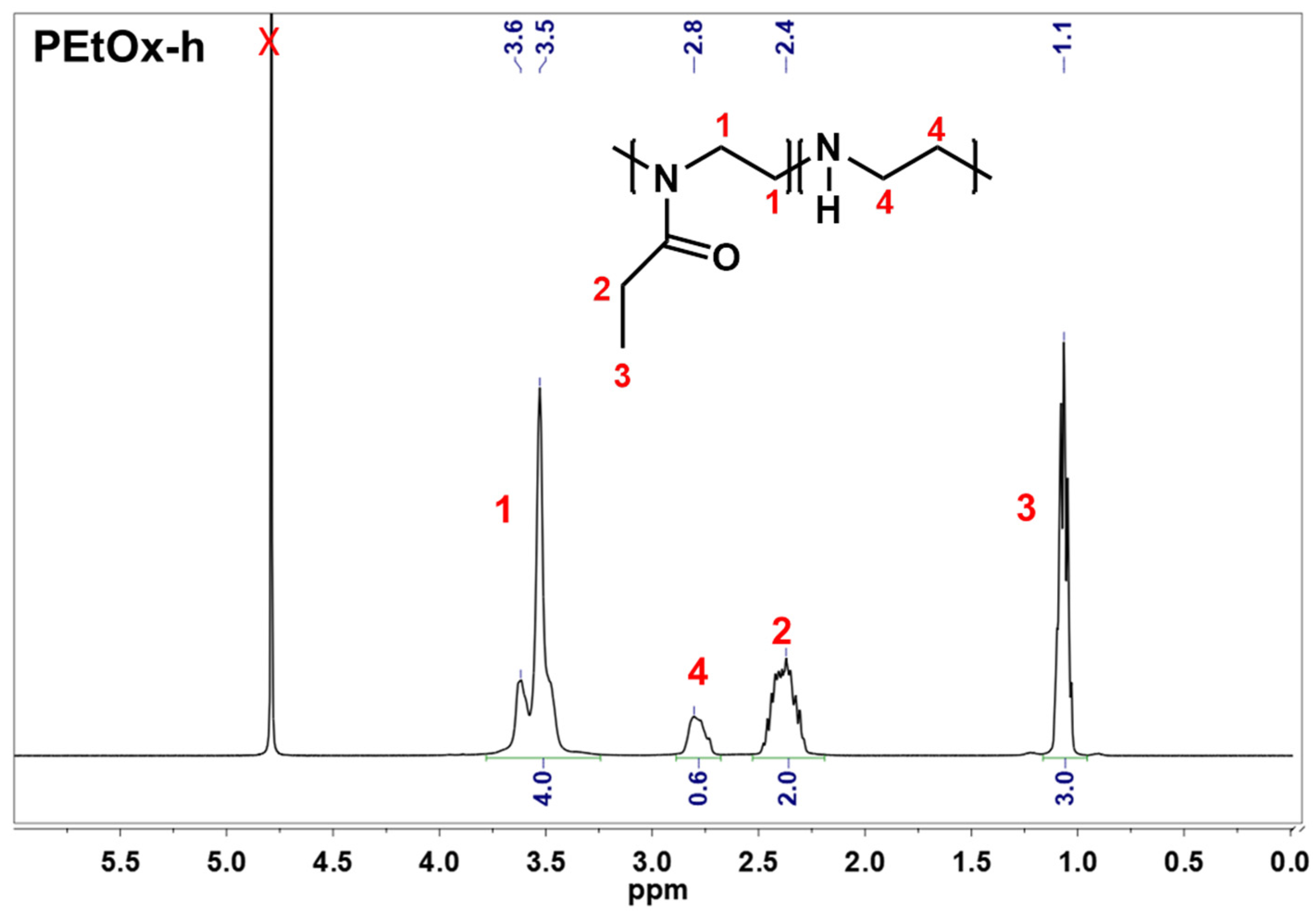
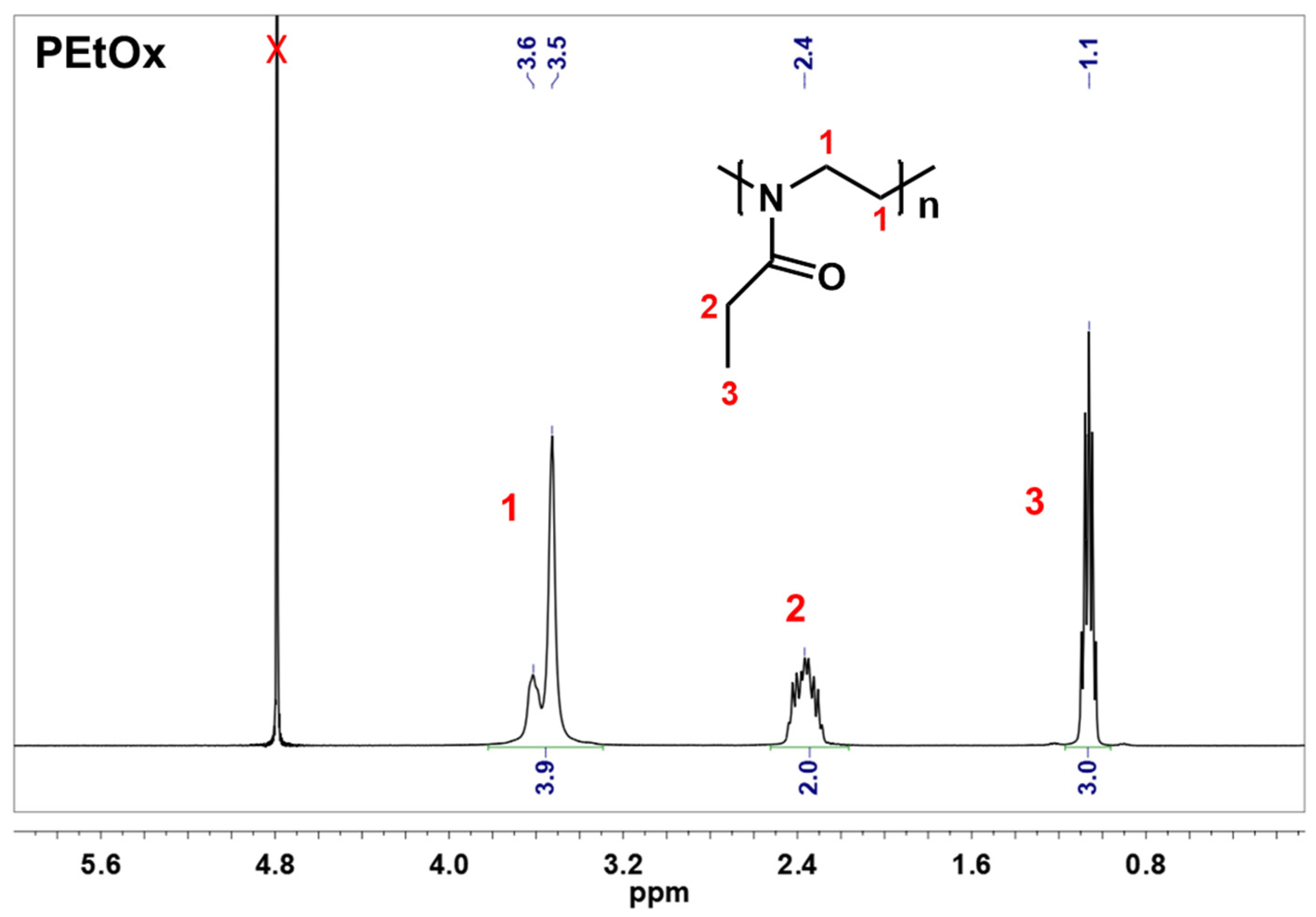
2.1. Preparation of Partially Hydrolyzed Poly(2-ethyl-2-oxazoline)
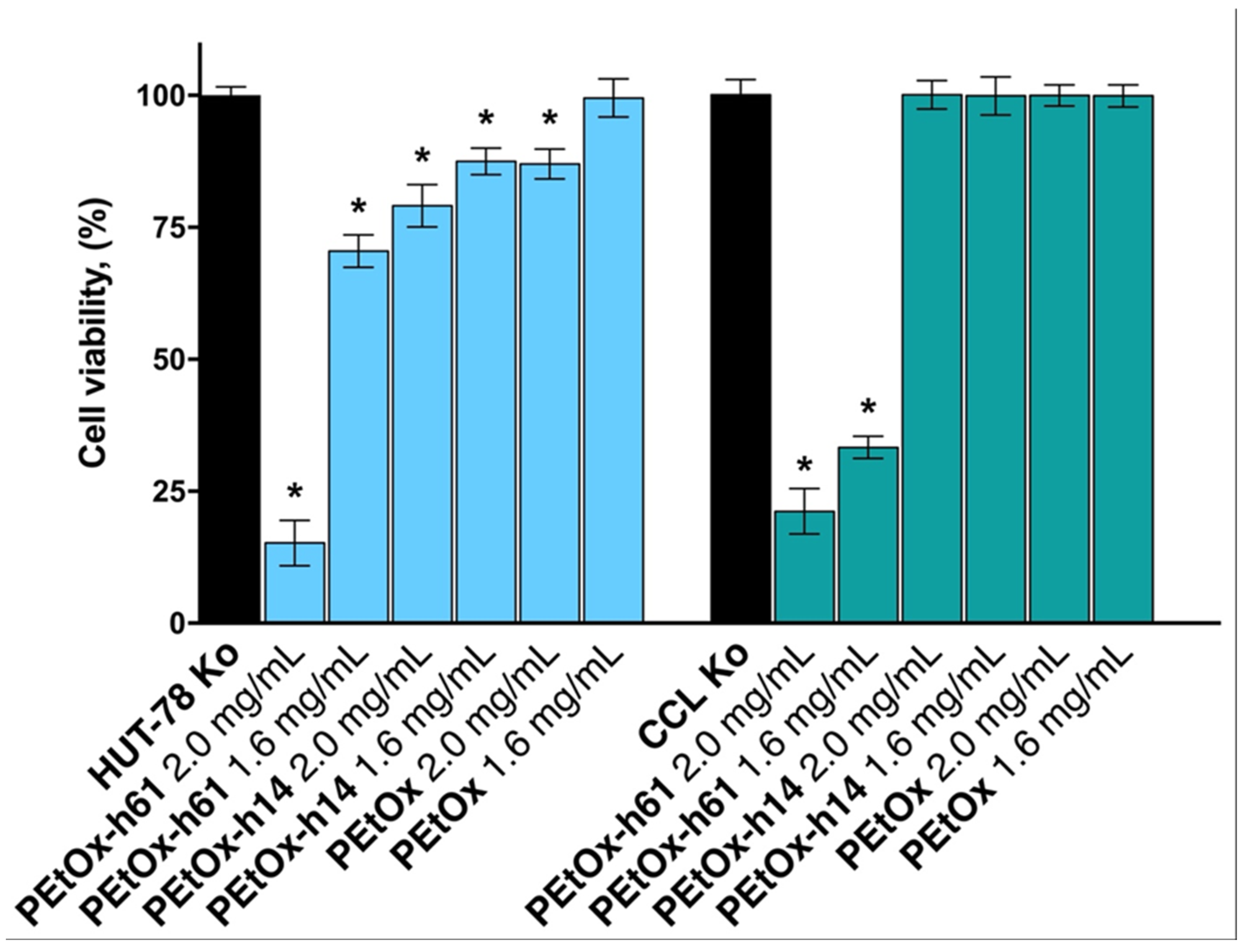
2.2. Citotoxicity Assay
2.3. Preparation of Galantamine-Loaded and Not-Loaded Orodispersible Films
2.4. Characterization of the Obtained Orodispersible Films
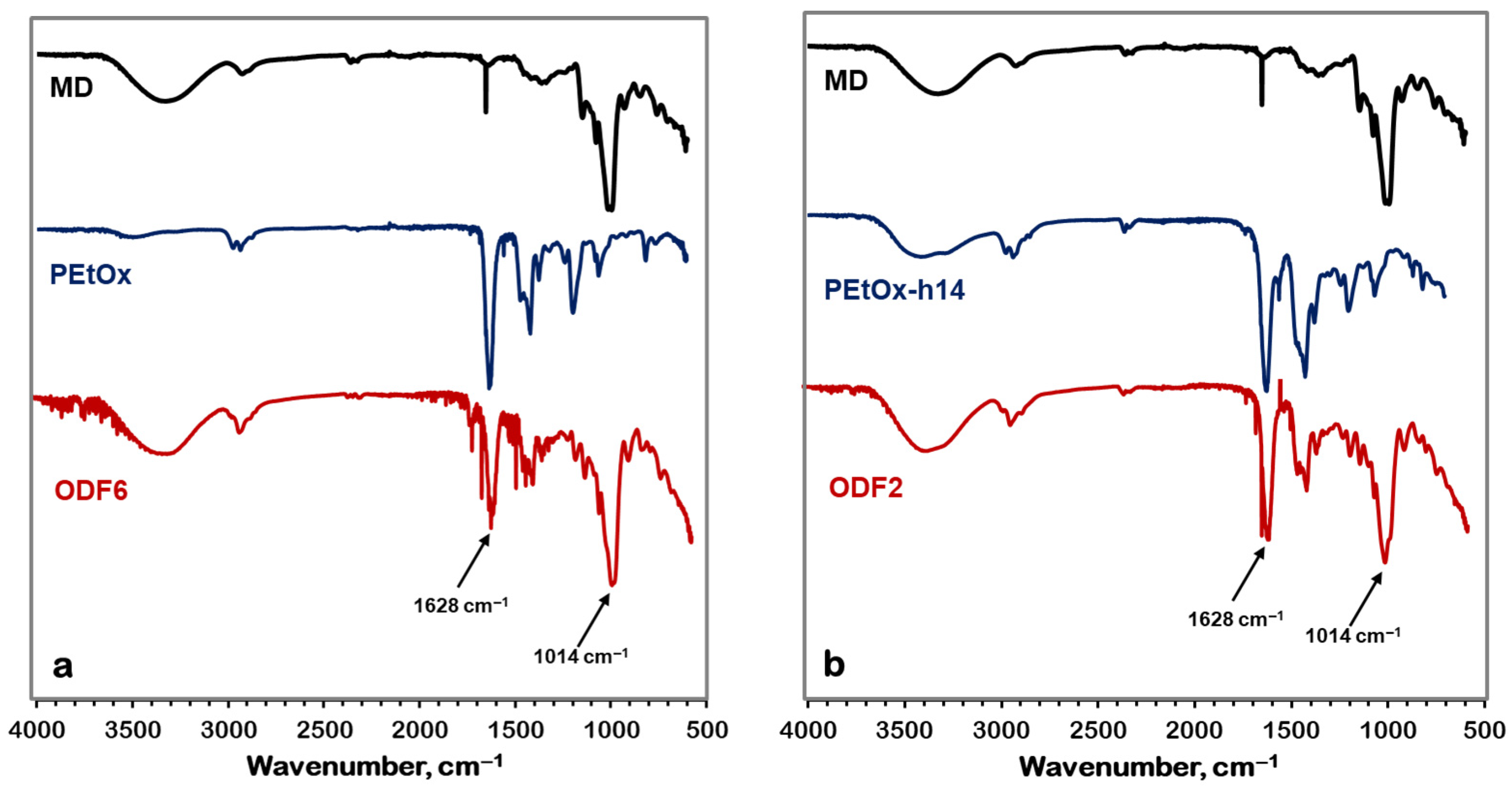
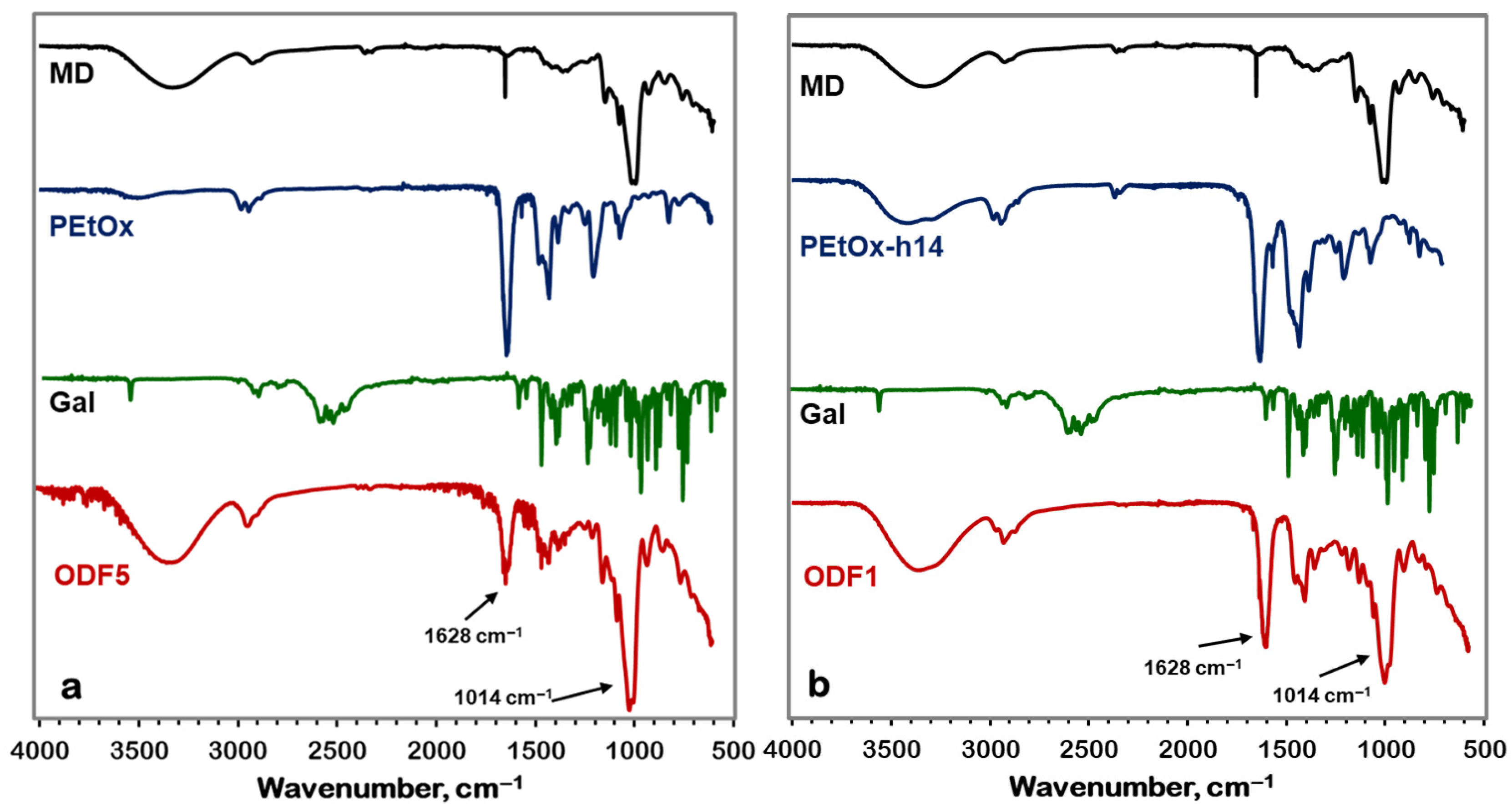
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
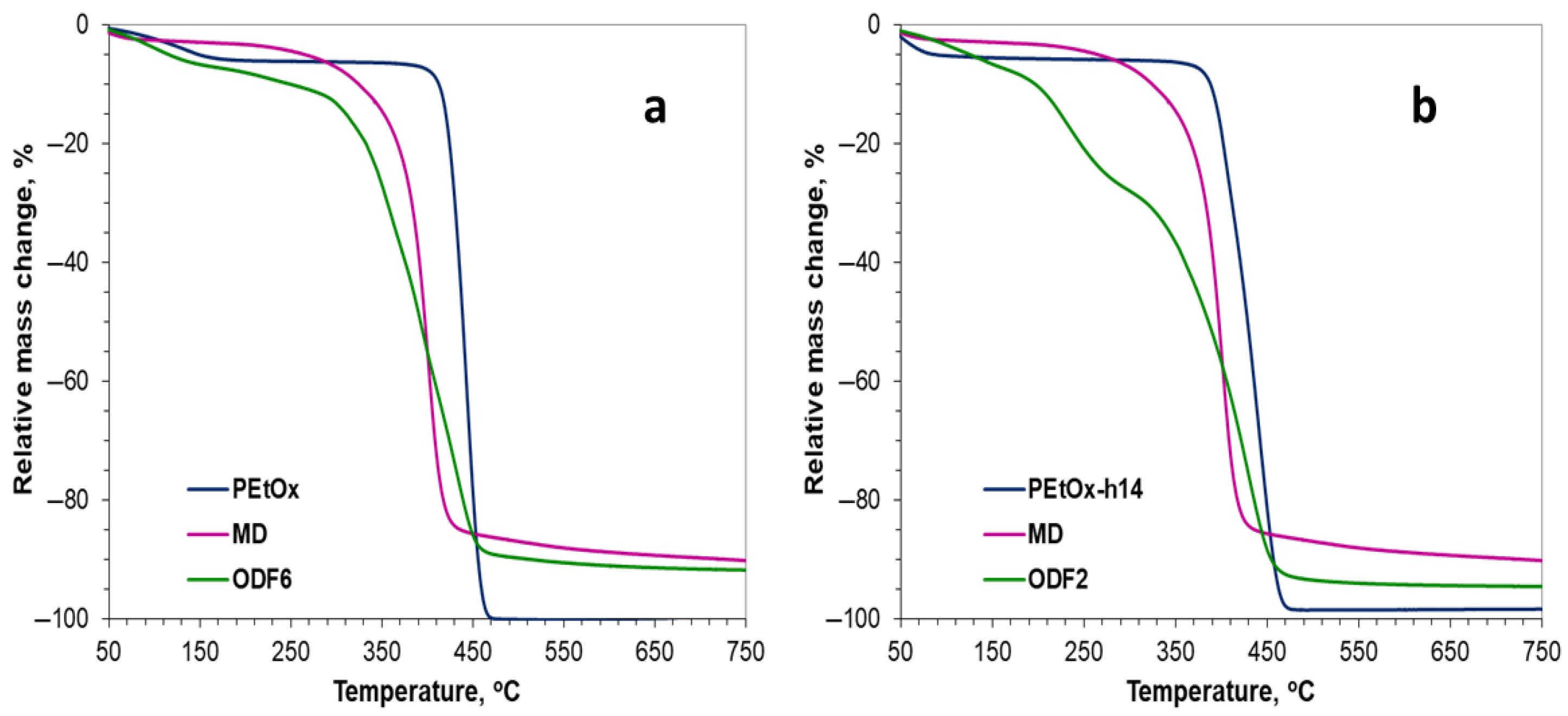
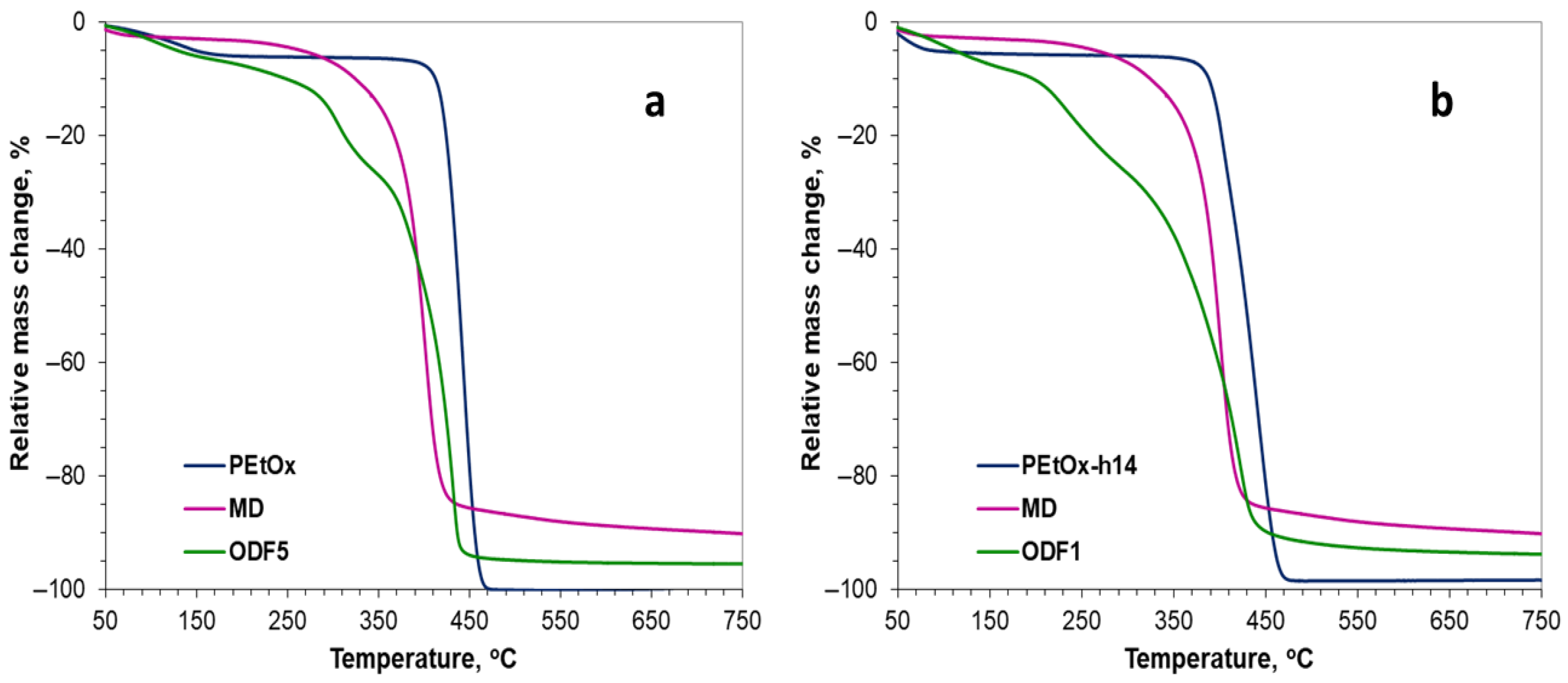
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Shore Hardness Assessment
2.4.4. Disintegration
2.4.5. In Vitro Drug Release Study
2.4.6. Drug Release Kinetics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis and Characterization of Partially Hydrolyzed Poly(2-ethyl-2-oxazoline)
- Synthesis of partially hydrolyzed poly(2-ethyl-2-oxazoline)
- Nuclear magnetic resonance (NMR)
- Citotoxicity assay
4.2.2. Preparation of Galantamine-Loaded and Non-Loaded Orodispersible Films
4.2.3. Characterization of the Obtained Orodispersible Films
- Fourier transform infrared spectrometry (FTIR)
- Thermogravimetric analysis (TGA)
- Shore (A) hardness assessment
- Disintegration
- In vitro drug release study
- Drug release kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Ayyoubi, S.; Cerda, J.R.; Fernandez-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Agache, I.; Hellings, P.W. Chapter 1—The Audacious Goal of Precision Medicine. In Implementing Precision Medicine in Best Practices of Chronic Airway Diseases; Agache, I., Hellings, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–4. [Google Scholar] [CrossRef]
- Kasztura, M.; Richard, A.; Bempong, N.E.; Loncar, D.; Flahault, A. Cost-effectiveness of precision medicine: A scoping review. Int. J. Public Health 2019, 64, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, X.; Jiang, C.; Zhao, H. Difficulties and challenges in the development of precision medicine. Clin. Genet. 2019, 95, 569–574. [Google Scholar] [CrossRef]
- Duffy, D.J. Problems, challenges and promises: Perspectives on precision medicine. Brief. Bioinform. 2016, 17, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Englezos, K.; Wang, L.; Tan, E.C.K.; Kang, L. 3D printing for personalised medicines: Implications for policy and practice. Int. J. Pharm. 2023, 635, 122785. [Google Scholar] [CrossRef]
- Visser, J.V.; Woerdenbag, H.J.; Hanff, L.M.; Frijlink, H.W. Personalized Medicine in Pediatrics: The Clinical Potential of Orodispersible Films. AAPS PharmSciTech 2017, 18, 267–272. [Google Scholar] [CrossRef]
- Visser, J.C.; Wibier, L.; Mekhaeil, M.; Woerdenbag, H.J.; Taxis, K. Orodispersible flms as a personalized dosage form for nursing home residents, an exploratory study. Int. J. Clin. Pharm. 2020, 42, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2019, 576, 118963. [Google Scholar] [CrossRef]
- Ferlak, J.; Guzenda, W.; Osmałek, T. Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics 2023, 15, 361. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, J.; Jing, H.; Wang, Q.; Wu, Z.; Duan, Y. Recent progress in orodispersible films-mediated therapeutic applications: A review. MedComm—Biomat. Appl. 2023, 2, e34. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible Thin Film: A new patient-centered innovation. J. Drug Deliv. Sci. Tech. 2020, 59, 101843. [Google Scholar] [CrossRef]
- Salawi, A. An Insight into Preparatory Methods and Characterization of Orodispersible Film—A Review. Pharmaceuticals 2022, 15, 844. [Google Scholar] [CrossRef]
- Gupta, M.S.; Gowda, D.V.; Kumar, T.P.; Rosenholm, J.M. A Comprehensive Review of Patented Technologies to Fabricate Orodispersible Films: Proof of Patent Analysis (2000–2020). Pharmaceutics 2022, 14, 820. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Crediet, S.; Gerrits, E.; Lesschen, M.A.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W. Orodispersible films in individualized pharmacotherapy: The development of a formulation for pharmacy preparations. Int. J. Pharm. 2015, 478, 155–163. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Davidson, R.; Kuppu, G.R.; Pathak, K.; Gowda, D.V. Printing Methods in the Production of Orodispersible Films. AAPS PharmSciTech 2021, 22, 129. [Google Scholar] [CrossRef]
- Hoogenboom, R. The future of poly(2-oxazoline)s. Eur. Polym. J. 2022, 179, 111521. [Google Scholar] [CrossRef]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Harris, J.M.; Bentley, M.D.; Moreadith, R.W.; Viegas, T.X.; Fang, Z.; Yoon, K.; Weimer, R.; Dizman, B.; Nordstierna, L. Tuning drug release from polyoxazoline-drug conjugates. Eur. Polym. J. 2019, 120, 109241. [Google Scholar] [CrossRef]
- Mahand, S.N.; Aliakbarzadeh, S.; Moghaddam, A.; Moghaddam, A.S.; Kruppke, B.; Nasrollahzadeh, M.; Khonakdar, H.A. Polyoxazoline: A review article from polymerization to smart behaviors and biomedical applications. Eur. Polym. J. 2022, 178, 111484. [Google Scholar] [CrossRef]
- Christova, D.; Velichkova, R.; Loos, W.; Goethals, E.J.; Du Prez, F. New thermo-responsive polymer materials based on poly (2-ethyl-2-oxazoline) segments. Polymer 2003, 44, 2255–2261. [Google Scholar] [CrossRef]
- Kostova, B.; Ivanova-Mileva, K.; Rachev, D.; Christova, D. Study of the potential of amphiphilic conetworks based on poly(2-ethyl-2-oxazoline) as new platforms for delivery of drugs with limited solubility. AAPS PharmSciTech 2013, 14, 352–359. [Google Scholar] [CrossRef]
- Kostova, B.; Ivanova, S.; Balashev, K.; Rachev, D.; Christova, D. Evaluation of poly(2-ethyl-2-oxazoline) containing copolymer networks of varied composition as sustained metoprolol tartrate delivery systems. AAPS PharmSciTech 2014, 15, 939–946. [Google Scholar] [CrossRef][Green Version]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as Polymer Therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef]
- de la Rosa, V.R.; Bauwens, E.; Monnery, B.D.; De Geest, B.G.; Hoogenboom, R. Fast and accurate partial hydrolysis of poly(2-ethyl2-oxazoline) into tailored linear polyethylenimine copolymers. Polym. Chem. 2014, 5, 4957–4964. [Google Scholar] [CrossRef]
- Van Kuringen, H.P.C.; Lenoir, J.; Adriaens, E.; Bender, J.; De Geest, B.G.; Hoogenboom, R. Partial Hydrolysis of Poly(2-ethyl-2-oxazoline) and Potential Implications for Biomedical Applications? Macromol. Biosci. 2012, 12, 1114–1123. [Google Scholar] [CrossRef]
- Kroneková, Z.; Jankovič, L.; Mošková, Z.; Minarčíková, A.; Zhukouskaya, H.; Kučka, J.; Vetrík, M.; Hrubý, M.; Kronek, J. Partially hydrolyzed poly(2-oxazoline)s as a new class of biocompatible modifiers of montmorillonites for the adsorption and decontamination of the organophosphate paraoxon. Colloids Surf. A Physicochem. Eng. Asp. 2025, 713, 136534. [Google Scholar] [CrossRef]
- National Institutes of Health. A Study of Weekly Subcutaneous Injections of SER-214 in Subjects with Parkinson’s Disease (PD), to Determine the Safety, Tolerability and Pharmacokinetic (PK) Profile of SER-214. 2016. Available online: http://clinicaltrials.gov/show/NCT02579473 (accessed on 30 March 2025).
- Moreadith, R.W.; Viegas, T.X.; Bentley, M.D.; Harris, J.M.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Rae, B.P.; Li, X.; et al. Clinical development of a poly(2-oxazoline) (POZ) polymer therapeutic for the treatment of Parkinson’s disease—Proof of concept of POZ as a versatile polymer platform for drug development in multiple therapeutic indications. Eur. Polym. J. 2017, 88, 524–552. [Google Scholar] [CrossRef]
- Georgieva, D.; Kostova, B. HPMC-based fast-dissolving oral films with galantamine-loaded chitosan nanoparticles. Pharmacia 2024, 71, 1–6. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, M.; Verma, H. Optimization and evaluation of desloratadine oral strip: An innovation in paediatric medication. Sci. World J. 2013, 2013, 395681. [Google Scholar] [CrossRef]
- Bruschi, M.L. 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 9780081000922. [Google Scholar] [CrossRef]
- Özakar, E.; Sevinç-Özakar, R.; Yılmaz, B. Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films Containing Pregabalin and Methylcobalamin. Gels 2023, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Rençber, S.; Karavana, S.Y.; Yilmaz, F.F.; Eraç, B.; Nenni, M.; Gurer-Orhan, H.; Limoncu, M.H.; Güneri, P.; Ertan, G. Formulation and evaluation of fluconazole loaded oral strips for local treatment of oral candidiasis. J. Drug. Deliv. Sci. Technol. 2019, 49, 615–621. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics 2021, 13, 1613. [Google Scholar] [CrossRef] [PubMed]
- Lambermont-Thijs, H.M.; van der Woerdt, F.S.; Baumgaertel, A.; Bonami, L.; Du Prez, F.E.; Schubert, U.S.; Hoogenboom, R. Linear poly (ethylene imine)s by acidic hydrolysis of poly (2-oxazoline)s: Kinetic screening, thermal properties, and temperature-induced solubility transitions. Macromolecules 2010, 43, 927–933. [Google Scholar] [CrossRef]


| Code | Component | ||||||
|---|---|---|---|---|---|---|---|
| PEtOx-h14 (%) | PEtOx (%) | Maltodextrin (%) | Glycerol (%) | PEG 400 (%) | GH (%) | Water (%) | |
| ODF1 | 3.3 | 0 | 2.7 | 0.9 | 0 | 0.2 | 92.9 |
| ODF 2 | 3.3 | 0 | 2.7 | 0.9 | 0 | 0 | 93.1 |
| ODF 3 | 3.3 | 0 | 2.7 | 0 | 1.5 | 0.2 | 92.3 |
| ODF 4 | 3.3 | 0 | 2.7 | 0 | 1.5 | 0 | 92.5 |
| ODF 5 | 0 | 3.3 | 2.7 | 0.9 | 0 | 0.2 | 92.9 |
| ODF 6 | 0 | 3.3 | 2.7 | 0.9 | 0 | 0 | 93.1 |
| ODF 7 | 0 | 3.3 | 2.7 | 0 | 1.5 | 0.2 | 92.3 |
| ODF 8 | 0 | 3.3 | 2.7 | 0 | 1.5 | 0 | 92.5 |
| Sample | Shore (A) Hardness |
|---|---|
| ODF1 | 92 ± 2 |
| ODF2 | 88 ± 2 |
| ODF3 | 80 ± 3 |
| ODF4 | 83 ± 2 |
| ODF5 | 90 ± 4 |
| ODF6 | 86 ± 2 |
| ODF7 | 85 ± 3 |
| ODF8 | 85 ± 3 |
| Sample | Disintegration Time at pH 1.2 (min) | Disintegration Time at pH 6.8 (min) |
|---|---|---|
| ODF1 | 0.57 ± 0.05 | 0.58 ± 0.16 |
| ODF3 | 0.52 ± 0.15 | 0.55 ± 0.29 |
| ODF5 | 1.40 ± 0.39 | 1.58 ± 0.67 |
| ODF7 | 1.36 ± 0.25 | 1.52 ± 0.48 |
| Sample | Zero Order | First Order | Higuchi | Korsmeyer-Peppas |
|---|---|---|---|---|
| ODF1 | k0 = 0.0778 R2 = 0.9907 | k1 = 0.0179 R2 = 0.8985 | kH = 8.4879 R2 = 0.9613 | n = 0.9593 R2 = 0.9977 |
| ODF3 | k0 = 0.0765 R2 = 0.9935 | k1 = 0.0179 R2 = 0.911 | kH = 8.3062 R2 = 0.9549 | n = 0.9531 R2 = 0.9985 |
| ODF5 | k0 = 0.0545 R2 = 0.9964 | k1 = 0.0157 R2 = 0.9622 | kH = 7.838 R2 = 0.946 | n = 0.9386 R2 = 0.9995 |
| ODF7 | k0 = 0.0532 R2 = 0.9979 | k1 = 0.0157 R2 = 0.97 | kH = 7.6282 R2 = 0.9395 | n = 0.9313 R2 = 0.9998 |
| Kinetic Models | Equation |
|---|---|
| Zero order | |
| First order | |
| Higuchi Diffusion Kinetic Model | |
| Korsmeyer-Peppas model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, D.; Bogdanova, I.; Mihaylova, R.; Alexandrova, M.; Bozhilova, S.; Christova, D.; Kostova, B. Orodispersible Hydrogel Film Technology for Optimized Galantamine Delivery in the Treatment of Alzheimer’s Disease. Gels 2025, 11, 629. https://doi.org/10.3390/gels11080629
Georgieva D, Bogdanova I, Mihaylova R, Alexandrova M, Bozhilova S, Christova D, Kostova B. Orodispersible Hydrogel Film Technology for Optimized Galantamine Delivery in the Treatment of Alzheimer’s Disease. Gels. 2025; 11(8):629. https://doi.org/10.3390/gels11080629
Chicago/Turabian StyleGeorgieva, Dilyana, Ivana Bogdanova, Rositsa Mihaylova, Mariela Alexandrova, Silvia Bozhilova, Darinka Christova, and Bistra Kostova. 2025. "Orodispersible Hydrogel Film Technology for Optimized Galantamine Delivery in the Treatment of Alzheimer’s Disease" Gels 11, no. 8: 629. https://doi.org/10.3390/gels11080629
APA StyleGeorgieva, D., Bogdanova, I., Mihaylova, R., Alexandrova, M., Bozhilova, S., Christova, D., & Kostova, B. (2025). Orodispersible Hydrogel Film Technology for Optimized Galantamine Delivery in the Treatment of Alzheimer’s Disease. Gels, 11(8), 629. https://doi.org/10.3390/gels11080629






