Polyampholytic Hydrogels from Chitosan Macromonomers with Aryl-Mono and Di-Sulfonated Groups: An Approach to the Removal of Copper Ions and Ciprofloxacin in Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
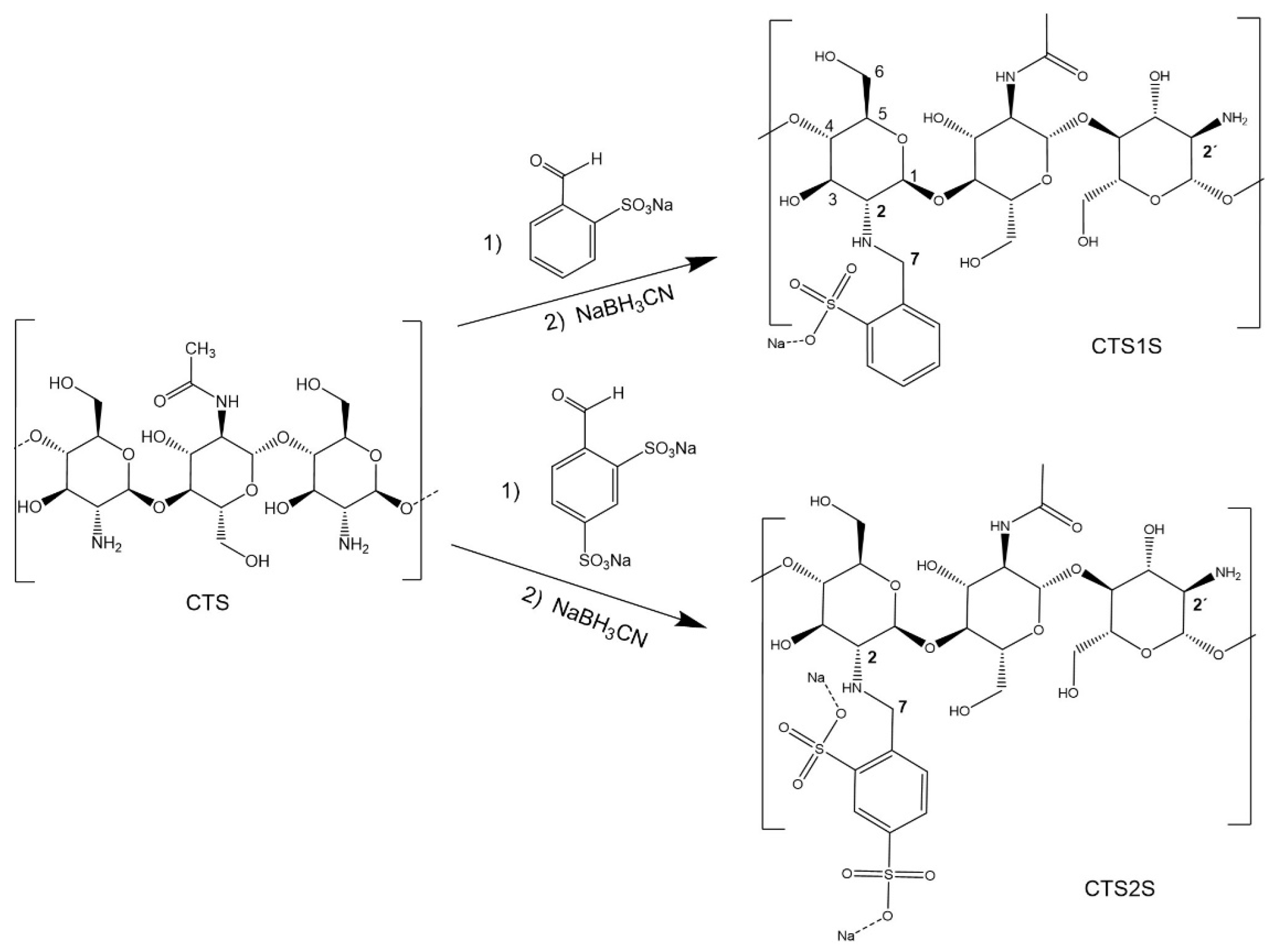
2.1. Functionalization of CTS with Sodium Sulfonates
2.1.1. Characterizations by FTIR, 1H-NMR, and TGA
2.1.2. Solubility
2.2. Preparation of Polyampholytic Hydrogels
3. Conclusions
4. Materials and Methods
4.1. General Materials and Procedures
4.2. CTS Characterization: Potentiometric Measurement of Deacetylation Degree (DDA) Determination, and 13C-NMR
- DDA = degree of deacetylation
- CNaOH = NaOH concentration (M)
- ∆VNaOH = volume difference between the two inflection points (L)
- mCTS = mass of the analyzed chitosan (g)
4.3. Functionalization of CTS with Sodium Sulfonates
4.4. Preparation of Polyampholytic Hydrogels
4.5. Characterization of Polyampholytic Hydrogels
- W = Degree of swelling
- W0 = Dry weight
- Wt = Weight over time
4.6. Application of the Hydrogels to Adsorption of Ciprofloxacin and Copper Ions in Aqueous Systems
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GMA | Glycidyl methacrylate |
| CTS | Chitosan |
| BIS | N,N-methylenebisacrylamide |
| APS | Ammonium persulfate |
| CPX | Ciprofloxacin |
| HG | Hydrogel |
| FB1S | 2-formylbenzylsulfonic acid sodium salt |
| FB2S | 4-formyl-1,3-benzenedisulfonic acid |
| TEMED | N,N,N’,N’-tetramethyl ethylenediamine |
| pHZCP | pH of zero charge point |
| DDA | Degree of deacetylation of the starting chitosan |
| DS | Degree of substitution |
| CTS1S | Chitosan modified with FB1S |
| CTS2S | Chitosan modified with FB2S |
| CTSV | Chitosan modified with GMA |
| CTSV1S | CTS1S modified with GMA |
| CTSV2S | CTS2S modified with GMA |
| HGCTS | Hydrogel obtained from CTS |
| HGCTS1S | Hydrogel obtained from CTSV1S |
| HGCTS2S | Hydrogel obtained from CTSV2S |
| 1H-NMR | Proton nuclear magnetic resonance |
| FTIR | Fourier transform infrared |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
References
- Zhao, J.; Xing, T.; Li, Q.; Chen, Y.; Yao, W.; Jin, S.; Chen, S. Preparation of chitosan and carboxymethylcellulose-based polyelectrolyte complex hydrogel via SD-A-SGT method and its adsorption of anionic and cationic dye. J. Appl. Polym. Sci. 2020, 137, 34. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.; Zhao, J.; Feng, H.; Li, P.; Tong, Z.; Yang, Z.; Li, S.; Yang, J.; Jin, S. Preparation of the chitosan/poly(glutamic acid)/alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohydr. Polym. 2018, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar] [CrossRef]
- Ceper, T.; Mohotti, S.W.; Lange, L.X.; Schacher, F.H. Polyampholyte Hydrogels with pH-Dependent Swelling for Controlled Catch & amp; Release of Model Dyes. Org. Mater. 2024, 6, 1–11. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Y.; Tang, S.; Yang, J.; Chen, Y.; Chen, S.; Li, P.; Yang, Z. Construction of chitosan/polyacrylate/graphene oxide composite physical hydrogel by semi-dissolution/acidification/sol-gel transition method and its simultaneous cationic and anionic dye adsorption properties. Carbohydr. Polym. 2020, 229, 115431. [Google Scholar] [CrossRef]
- Lipin, V.A.; Poshvina, T.A.; Petrova, Y.A. Interaction of polyampholytic hydrogels based on partially hydrolyzed polyacrylamide with divalent metals. Russ. Chem. Bull. 2023, 72, 1299–1306. [Google Scholar] [CrossRef]
- Lipin, V.A.; Evdokimov, A.N.; Poshvina, T.A.; Petrova, Y.A.; Garcia, D.D.H. Sorption Capacity of Polyampholytic Hydrogels with Respect to Dyes of Different Origins. Russ. J. Phys. Chem. A 2024, 98, 1285–1292. [Google Scholar] [CrossRef]
- Lipin, V.A.; Evdokimov, A.N.; Alekseev, V.G.; Sustavova, T.A.; Petrova, Y.A. Sorption of Anionic Dyes by Polyampholyte Hydrogels Based on Hydrolized Polyacrylamide Modified with Aliphatic Diamines. Russ. J. Phys. Chem. A 2022, 96, 387–390. [Google Scholar] [CrossRef]
- Yan, J.; Li, K. A magnetically recyclable polyampholyte hydrogel adsorbent functionalized with β-cyclodextrin and graphene oxide for cationic/anionic dyes and heavy metal ion wastewater remediation. Sep. Purif. Technol. 2021, 277, 119469. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Application of Chitin and Chitosan Derivatives in the Pharmaceutical Field. Curr. Pharm. Biotechno. 2003, 4, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Shahsavarifar, S. Chemical functionalization of chitosan biopolymer and chitosan-magnetite nanocomposite with sulfonic acid for acid-catalyzed reactions. Chin. J. Chem. Eng. 2021, 39, 154–161. [Google Scholar] [CrossRef]
- Shirdast, A.; Sharif, A.; Abdollahi, M. Effect of the incorporation of sulfonated chitosan/sulfonated graphene oxide on the proton conductivity of chitosan membranes. J. Power Sources 2016, 306, 541–551. [Google Scholar] [CrossRef]
- Balan, V.; Verestiuc, L. Strategies to improve chitosan hemocompatibility: A review. Eur. Polym. J. 2014, 53, 171–188. [Google Scholar] [CrossRef]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Oliveira, A.L.B.; Mota, G.F.; da Silva Souza, J.E.; de Aguiar Falcão, I.R.; et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.D.; Alí, F.; Zuluaga, F. Síntesis de quitosano modificado con poli (ácido acrílico) vía polimerización por transferencia atómica iniciada desde la superficie(SIP-ATRP). Iberoam. Polímeros 2010, 11, 505–519. [Google Scholar]
- Liu, P.; Yang, X.; Chen, W.; Hao, Y. Preparation of the modified chitosan flocculant introduced acryloyloxyethyl trimethyl ammonium chloride and 2-acrylamido-2-methyl propane sulfonic acid for the treatment of papermaking wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132934. [Google Scholar] [CrossRef]
- Montenegro, M.H.E.; Vega, M.D.; Hernández, M.A. Preparation and characterization of some derivatives of chitosan. Sci. Rev. Investig. Univ. Panamá 2019, 29, 10–33. [Google Scholar]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Dimassi, S.; Tabary, N.; Leclercq, L.; Degoutin, S.; Chai, F.; Pierlot, C.; Cazaux, F.; Ung, A.; Staelens, J.-N.; et al. Synthesis and characterization of polyampholytic aryl-sulfonated chitosans and their in vitro anticoagulant activity. Carbohydr. Polym. 2018, 196, 8–17. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Z.; Yu, C.; Deng, Y.; Ye, Q.; Niu, F.; Chen, Q.; Pan, W.; Wang, Y. Preparation, characterization and antibacterial activity of new ionized chitosan. Carbohydr. Polym. 2022, 290, 119490. [Google Scholar] [CrossRef]
- Institute of Public Health; Ministry of Health Chile ISP Informa Sobre la Resistencia a los Antimicrobianos y los Antibióticos más Vendidos en Chile. Ministerio de Salud Pública: Santiago, Chile, 2019. Available online: https://www.ispch.gob.cl/noticia/isp-informa-sobre-la-resistencia-a-los-antimicrobianos-y-los-antibioticos-mas-vendidos-en-chile/#:~:text=5 (accessed on 28 November 2023).
- Serna-Galvis, E.A.; Vélez-Peña, E.; Osorio-Vargas, P.; Jiménez, J.N.; Salazar-Ospina, L.; Guaca-González, Y.M.; Torres-Palma, R.A. Inactivation of carbapenem-resistant Klebsiella pneumoniae by photo-Fenton: Residual effect, gene evolution and modifications with citric acid and persulfate. Water Res. 2019, 161, 354–363. [Google Scholar] [CrossRef]
- Xiao, R.; He, Z.; Diaz-Rivera, D.; Pee, G.Y.; Weavers, L.K. Sonochemical degradation of ciprofloxacin and ibuprofen in the presence of matrix organic compounds. Ultrason. Sonochem. 2014, 21, 428–435. [Google Scholar] [CrossRef]
- World Health Organization. Vigilancia de la Resistencia a Los Antimicrobianos; WHO: Geneva, Switzerland, 2018; Volume 4, pp. 20–33. [Google Scholar]
- Nasri, A.; Allouche, M.; Hannachi, A.; Harrath, A.H.; Aldahmash, W.; Alwasel, S.; Mahmoudi, E.; Beyrem, H.; Boufahja, F. Restructuring of a meiobenthic assemblage after sediment contamination with an antibacterial compound: Case study of ciprofloxacin. Ecotoxicol. Environ. Saf. 2020, 205, 111084. [Google Scholar] [CrossRef]
- Gomes, M.C.; Mano, J.F. Chemical modification strategies to prepare advanced protein-based biomaterials. Biomater. Biosyst. 2021, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Punia, A. Role of temperature, wind, and precipitation in heavy metal contamination at copper mines: A review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zou, B.; Li, N.Y.; Li, Z.A. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health. Environ. Geochem. Health 2009, 31, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Shan, S.; Sun, X.-F.; Xie, Y.; Li, W.; Ji, T. High-Performance Hydrogel Adsorbent Based on Cellulose, Hemicellulose, and Lignin for Copper(II) Ion Removal. Polymers 2021, 13, 3063. [Google Scholar] [CrossRef]
- Palacio, D.A.; Becerra, Y.; Urbano, B.F.; Rivas, B.L. Antibiotics removal using a chitosan-based polyelectrolyte in conjunction with ultrafiltration membranes. Chemosphere 2020, 258, 127416. [Google Scholar] [CrossRef]
- Ma, J.; Xiong, Y.; Dai, X.; Yu, F. Coadsorption behavior and mechanism of ciprofloxacin and Cu(II) on graphene hydrogel wetted surface. Chem. Eng. J. 2020, 380, 122387. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Sun, X.-F.; Liu, J.; Song, C.; Wang, S.-G.; Javed, A. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total Environ. 2018, 639, 560–569. [Google Scholar] [CrossRef]
- Yu, F.; Sun, Y.; Yang, M.; Ma, J. Adsorption mechanism and effect of moisture contents on ciprofloxacin removal by three-dimensional porous graphene hydrogel. J. Hazard. Mater. 2019, 374, 195–202. [Google Scholar] [CrossRef]
- Ding, J.; Li, Q.; Xu, X.; Zhang, X.; Su, Y.; Yue, Q.; Gao, B. A wheat straw cellulose-based hydrogel for Cu (II) removal and preparation copper nanocomposite for reductive degradation of chloramphenicol. Carbohydr. Polym. 2018, 190, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Bieritz, L.; Mönnig, A.; Großmann, A.M. Development of Sustainable Mining Strategies in Chile with a Regionalized National Model: Project Introduction and Overview Standard-Nutzungsbedingungen; Gesellschaft für Wirtschaftliche Strukturforschung (GWS): Osnabrück, Germany, 2017. [Google Scholar]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mira, H.; Wei, Y.; Aboelenin, S.M.; Guibal, E.; Salem, W.M. Sulfonation of chitosan for enhanced sorption of Li(I) from acidic solutions—Application to metal recovery from waste Li-ion mobile battery. Chem. Eng. J. 2022, 441, 135941. [Google Scholar] [CrossRef]
- Palacio, D.A.; Urbano, B.F.; Rivas, B.L. Hydrogels based on alkylated chitosan and polyelectrolyte copolymers. J. Appl. Polym. Sci. 2018, 135, 46556. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Xie, W.; Chen, L.; Zheng, H.; Fan, L. Preparation and anticoagulant activity of N-succinyl chitosan sulfates. Int. J. Biol. Macromol. 2012, 51, 808–814. [Google Scholar] [CrossRef]
- Van Poucke, C.; Verdegem, E.; Mangelinckx, S.; Stevens, C.V. Synthesis and unambiguous NMR characterization of linear and branched N-alkyl chitosan derivatives. Carbohydr. Polym. 2024, 337, 122131. [Google Scholar] [CrossRef]
- Elomaa, M. Determination of the degree of substitution of acetylated starch by hydrolysis, 1H NMR and TGA/IR. Carbohydr. Polym. 2004, 57, 261–267. [Google Scholar] [CrossRef]
- Echols, K.R.; Meadows, J.C.; Orazio, C.E. Pollution of Aquatic Ecosystems II: Hydrocarbons, Synthetic Organics, Radionuclides, Heavy Metals, Acids, and Thermal Pollution. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; pp. 120–128. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Gallardo, M.G.C.; Barbosa, R.C.; Fook, M.V.L.; Sabino, M.A. Synthesis and characterization of a novel biomaterial based on chitosan modified with amino acids. Matéria 2019, 24, e12397. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Dodi, G.; Hritcu, D.; Lisa, G.; Popa, M.I. Core–Shell magnetic chitosan particles functionalized by grafting: Synthesis and characterization. Chem. Eng. J. 2012, 203, 130–141. [Google Scholar] [CrossRef]
- Palacio, D.A.; Urbano, B.F.; Palencia, M.; Rivas, B.L. Preparation of alkylated chitosan-based polyelectrolyte hydrogels: The effect of monomer charge on polymerization. Eur. Polym. J. 2019, 118, 551–560. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Elella, M.H.A.; Sabaa, M.W. Cytotoxicity and metal ions removal using antibacterial biodegradable hydrogels based on N-quaternized chitosan/poly(acrylic acid). Int. J. Biol. Macromol. 2017, 98, 302–313. [Google Scholar] [CrossRef]
- Elzamly, R.A.; Mohamed, H.M.; Mohamed, M.I.; Zaky, H.T.; Harding, D.R.K.; Kandile, N.G. New sustainable chemically modified chitosan derivatives for different applications: Synthesis and characterization. Arab. J. Chem. 2021, 14, 103255. [Google Scholar] [CrossRef]
- Kandile, N.G.; Elzamly, R.A.; Mohamed, M.I.; Zaky, H.T.; Harding, D.R.K.; Mohamed, H.M. New sustainable antimicrobial chitosan hydrogels based on sulfonamides and its nanocomposites: Fabrication and characterization. Int. J. Biol. Macromol. 2023, 239, 124280. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, T.H.; Nasr, A.S.; Bassioni, G.; Harding, D.R.; Kandile, N.G. Fabrication of sustainable hydrogels-based chitosan Schiff base and their potential applications. Arab. J. Chem. 2022, 15, 103511. [Google Scholar] [CrossRef]
- Jang, J.; Seol, Y.-J.; Kim, H.J.; Kundu, J.; Kim, S.W.; Cho, D.-W. Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering. J. Mech. Behav. Biomed. Mater. 2014, 37, 69–77. [Google Scholar] [CrossRef]
- Hajikarimi, P.; Nejad, F.M. Introduction to viscoelasticity. In Applications of Viscoelasticity; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Ortega, F.R.; Rodríguez, G.; Aguilar, M.R.; García-Sanmartín, J.; Martínez, A.; Román, J.S. Comportamiento reológico de geles biodegradables para aplicacions en medicina regenerativa. Biomecánica 2012, 20, 7–19. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Removal of ciprofloxacin antibiotic pollutants from wastewater using nano-composite adsorptive membranes. Environ. Res. 2022, 215, 114182. [Google Scholar] [CrossRef]
- Chaurasiya, A.; Pande, P.P.; Shankar, R.; Chaurasia, S.K.; Kumar, A.; Kumar, P.; Kashaudhan, K. Synthesis and characterization of allyl mannitol cross-linker based novel hydrogel for capturing of toxic metal ions from aqueous solution. Sep. Sci. Technol. 2024, 60, 1–20. [Google Scholar] [CrossRef]
- Akpor, O.B.; Otohinoyi, D.A.; Olaolu, T.D.; Aderiye, B.I. Pollutants in wastewater effluents: Impacts and remediation processes. Int. J. Environ. Res. Earth Sci. 2014, 3, 50–59. [Google Scholar]
- Shi, H.; Ni, J.; Zheng, T.; Wang, X.; Wu, C.; Wang, Q. Remediation of wastewater contaminated by antibiotics. A review. Environ. Chem. Lett. 2020, 18, 345–360. [Google Scholar] [CrossRef]
- Palacio, D.A.; Rivas, B.L.; Urbano, B.F. Ultrafiltration membranes with three water-soluble polyelectrolyte copolymers to remove ciprofloxacin from aqueous systems. Chem. Eng. J. 2018, 351, 85–93. [Google Scholar] [CrossRef]
- Moes, S.; van Gestel, K.; van Beek, G. Metal Speciation. In Environmental Toxicology; Libretexts: Davis, CA, USA, 2023; Chapter 3.5, p. 2. [Google Scholar]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Dinculescu, D.D.; Apetroaei, M.R.; Gîjiu, C.L.; Anton, M.; Enache, L.; Schröder, V.; Isopescu, R.; Rău, I. Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste. Polymers 2024, 16, 170. [Google Scholar] [CrossRef]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.-Z.; Chen, W.; Bai, Z.-W. Data of 1H/13C NMR spectra and degree of substitution for chitosan alkyl urea. Data Brief 2016, 7, 1228–1236. [Google Scholar] [CrossRef]
- Palencia, M.S.; Mora, M.A.; Palencia, S.L. Biodegradable Polymer Hydrogels Based in Sorbitol and Citric Acid for Controlled Release of Bioactive Substances from Plants (Polyphenols). Curr. Chem. Biol. 2017, 11, 36–43. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; He, C.; Kang, Y.; Zhou, J. Ionically crosslinked chitosan/poly(acrylic acid) hydrogels with high strength, toughness and antifreezing capability. Carbohydr. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef]
- Saghandali, F.; Salehi, M.B.; Taghikhani, V. Hydrogel nanocomposite network elasticity parameters as a function of swelling ratio: From micro to macro flooding. J. Ind. Eng. Chem. 2023, 125, 163–177. [Google Scholar] [CrossRef]
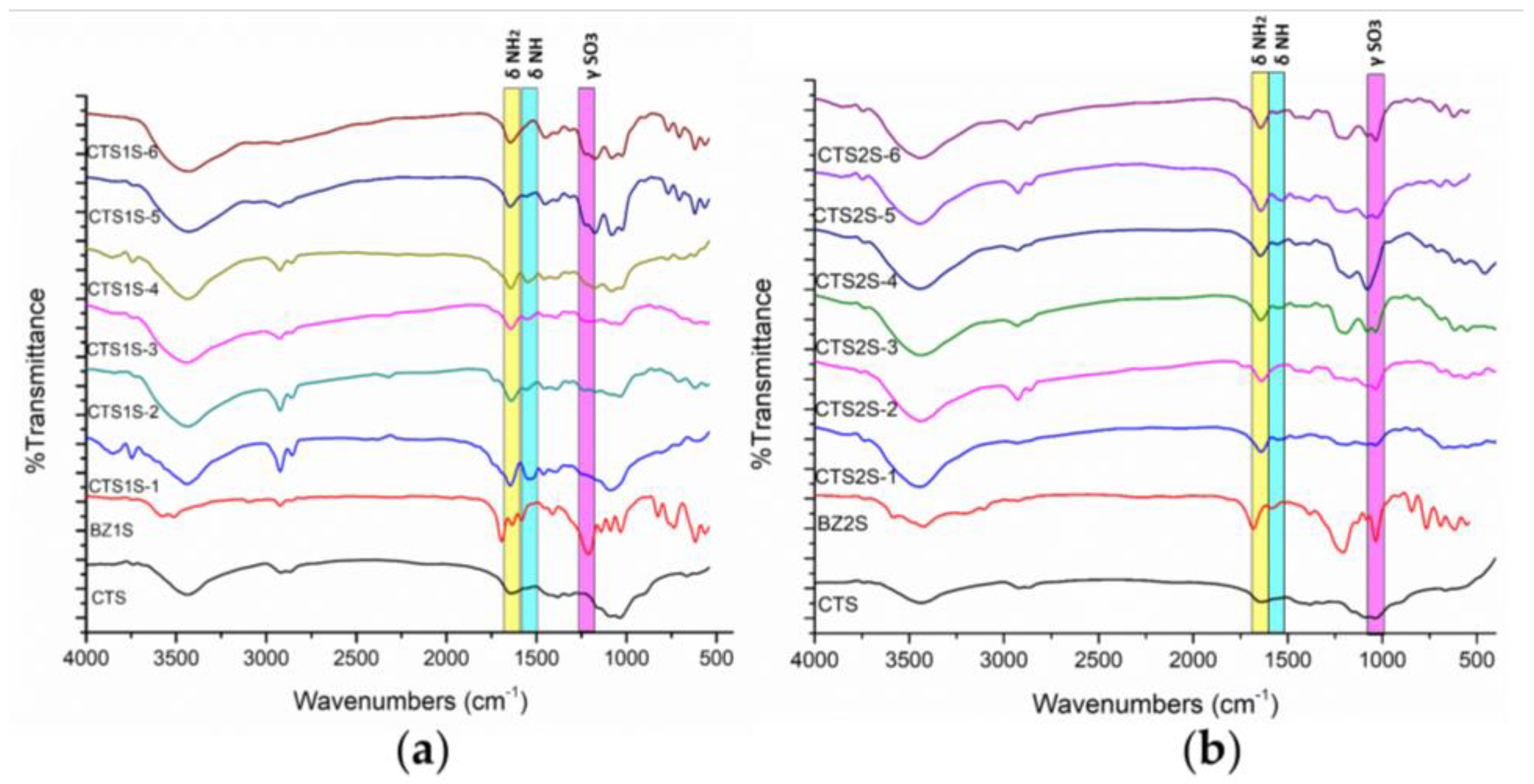

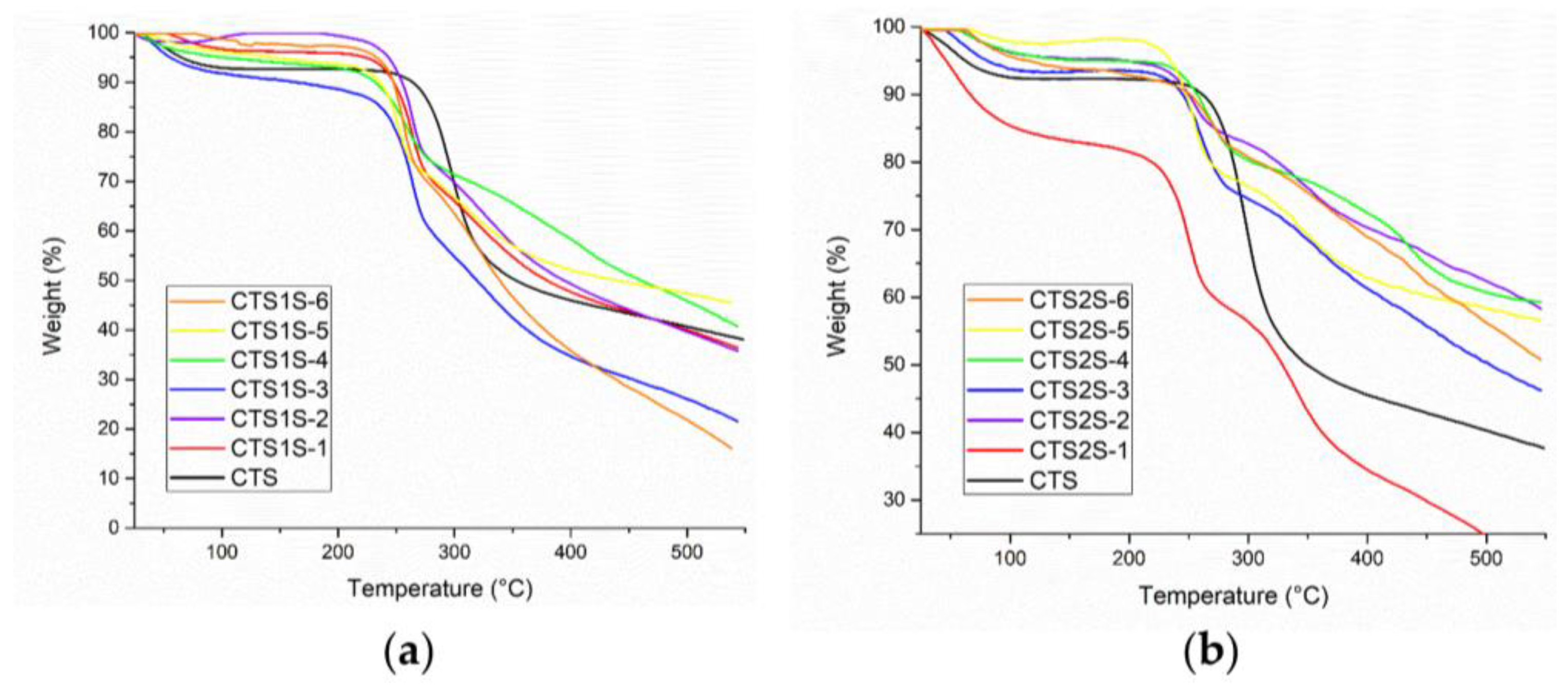
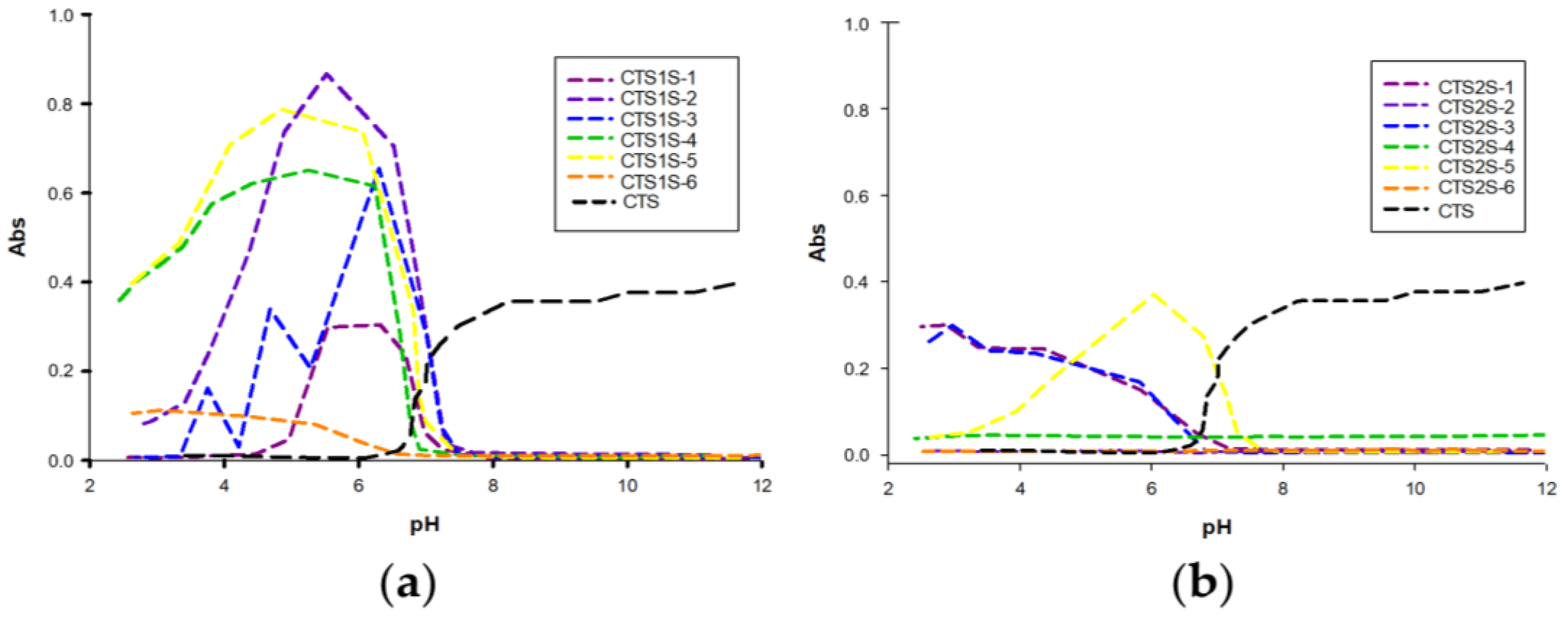
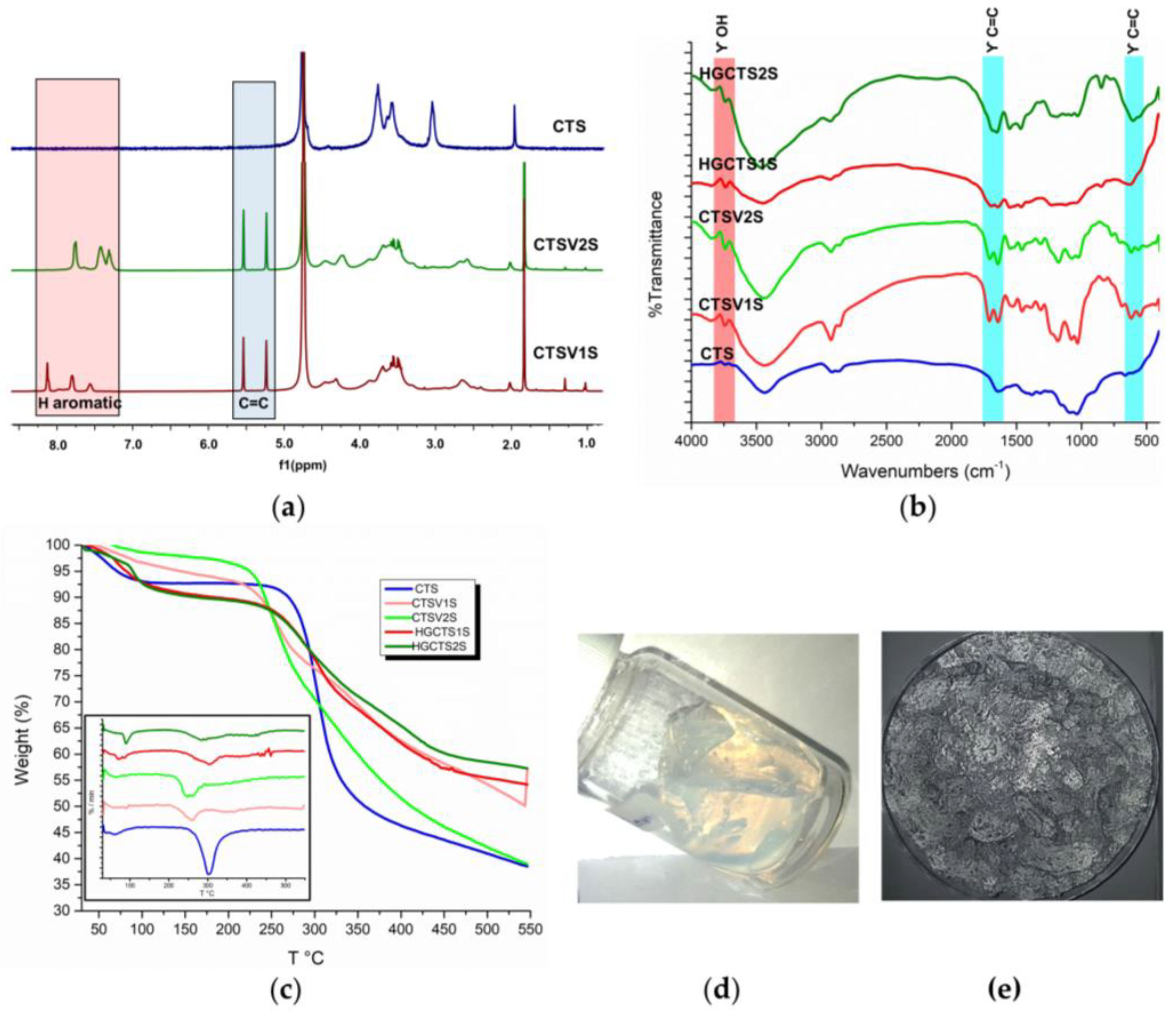

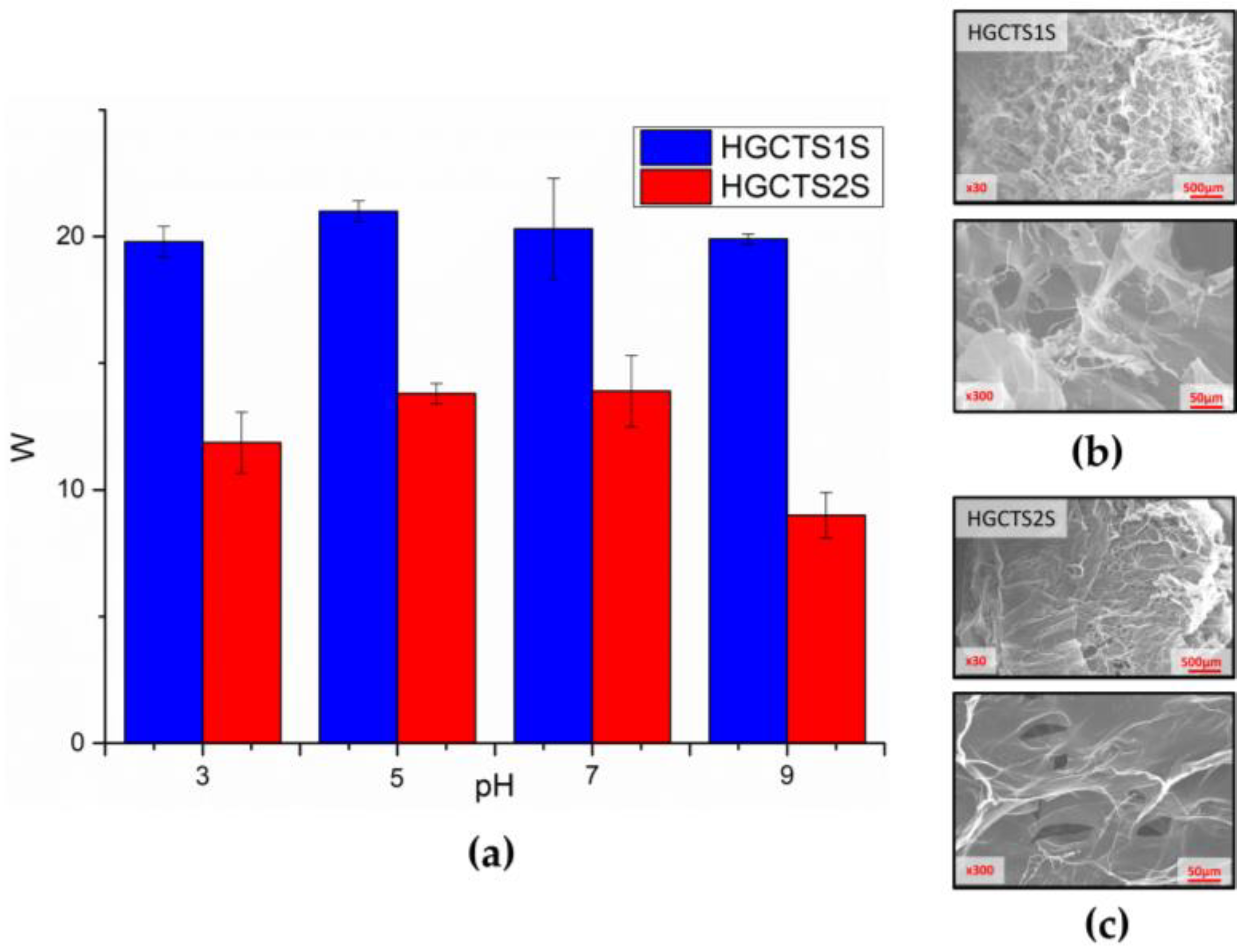

| Samples | 1 h—25 °C | 1 h—60 °C | 8 h—25 °C | 8 h—60 °C | 24 h—25 °C | 24 h—60 °C | |
|---|---|---|---|---|---|---|---|
| 1H-NMR | %DS a CTS1S | 15 | 11 | 13 | 11 | 11 | 8 |
| %DS a CTS2S | 32 | 21 | 26 | 22 | 39 | 16 | |
| SEM-EDS | %S b CTS1S | 12.29 | 9.76 | 2.77 | 8.73 | 4.74 | 11.11 |
| %S b CTS2S | 11.15 | 13.13 | 7.86 | 10.98 | 12.21 | 14.83 | |
| TGA | %Wt c CTS1S | 21 | 20 | 18 | 13 | 16 | 17 |
| %Wt c CTS2S | 22 | 11 | 13 | 19 | 14 | 16 |
| 1 h 25 °C | 1 h 60 °C | 8 h 25 °C | 8 h 60 °C | 24 h 25 °C | 24 h 60 °C | |
|---|---|---|---|---|---|---|
| FB1S | CTS1S-1 | CTS1S-2 | CTS1S-3 | CTS1S-4 | CTS1S-5 | CTS1S-6 |
| FB2S | CTS2S-1 | CTS2S-2 | CTS2S-3 | CTS2S-4 | CTS2S-5 | CTS2S-6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Rodríguez, D.; Salas, A.; Meléndrez, M.F.; Gillies, E.R.; Palacio, D.A. Polyampholytic Hydrogels from Chitosan Macromonomers with Aryl-Mono and Di-Sulfonated Groups: An Approach to the Removal of Copper Ions and Ciprofloxacin in Aqueous Solutions. Gels 2025, 11, 622. https://doi.org/10.3390/gels11080622
Montoya-Rodríguez D, Salas A, Meléndrez MF, Gillies ER, Palacio DA. Polyampholytic Hydrogels from Chitosan Macromonomers with Aryl-Mono and Di-Sulfonated Groups: An Approach to the Removal of Copper Ions and Ciprofloxacin in Aqueous Solutions. Gels. 2025; 11(8):622. https://doi.org/10.3390/gels11080622
Chicago/Turabian StyleMontoya-Rodríguez, Diana, Alexis Salas, Manuel F. Meléndrez, Elizabeth R. Gillies, and Daniel A. Palacio. 2025. "Polyampholytic Hydrogels from Chitosan Macromonomers with Aryl-Mono and Di-Sulfonated Groups: An Approach to the Removal of Copper Ions and Ciprofloxacin in Aqueous Solutions" Gels 11, no. 8: 622. https://doi.org/10.3390/gels11080622
APA StyleMontoya-Rodríguez, D., Salas, A., Meléndrez, M. F., Gillies, E. R., & Palacio, D. A. (2025). Polyampholytic Hydrogels from Chitosan Macromonomers with Aryl-Mono and Di-Sulfonated Groups: An Approach to the Removal of Copper Ions and Ciprofloxacin in Aqueous Solutions. Gels, 11(8), 622. https://doi.org/10.3390/gels11080622









