Hydrogel-Based Nitric Oxide Delivery Systems for Enhanced Wound Healing
Abstract
1. Introduction
2. Pathology of Chronic Wounds
2.1. Hypoxia in Chronic Wounds
2.2. ROS in Chronic Wounds
3. The Role of NO in Wound Healing
3.1. NO in Oxygen Homeostasis and Hypoxia Adaptation
3.2. NO in Redox Balance and Inflammation Regulation
3.3. NO in Cellular Regeneration and Tissue Remodeling
4. NO-Releasing Hydrogels for Wound Healing
4.1. NO Donors and Their Integration into Hydrogels
4.2. NO-Releasing Hydrogels
4.2.1. Enzymatic NO-Releasing Hydrogels
4.2.2. Non-Enzymatic NO-Releasing Hydrogels
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Pugliese, E.; Coentro, J.Q.; Raghunath, M.; Zeugolis, D.I. Wound healing and scar wars. Adv. Drug Deliv. Rev. 2018, 129, 1–3. [Google Scholar] [CrossRef]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-healing studies in cornea and skin: Parallels, differences and opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Heo, T.-H.; Gu, B.K.; Ohk, K.; Yoon, J.-K.; Son, Y.H.; Chun, H.J.; Yang, D.-H.; Jeong, G.-J. Polynucleotide and hyaluronic acid mixture for skin wound dressing for accelerated wound healing. Tissue Eng. Regen. Med. 2025, 22, 515–526. [Google Scholar] [CrossRef]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Lee, D.-H.; Kim, Y.C.; Bhang, S.H. Enhancing Skin Regeneration Efficacy of Human Dermal Fibroblasts Using Carboxymethyl Cellulose-Coated Biodegradable Polymer. Tissue Eng. Regen. Med. 2024, 22, 505–513. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, Q.; Newland, B.; Foley, R.; Lara-Sáez, I.; Curtin, J.F.; Wang, W. Reactive oxygen species (ROS): Utilizing injectable antioxidative hydrogels and ROS-producing therapies to manage the double-edged sword. J. Mater. Chem. B 2021, 9, 6326–6346. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Eggert, A.; Ikegaki, N.; Kwiatkowski, J.; Zhao, H.; Brodeur, G.M.; Himelstein, B.P. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin. Cancer Res. 2000, 6, 1900–1908. [Google Scholar]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Cirino, G.; Vellecco, V.; Bucci, M. Nitric oxide and hydrogen sulfide: The gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017, 174, 4021–4031. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Lillo, M.A.; Gaete, P.S.; Riquelme, M.A.; Sáez, J.C. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology 2013, 75, 471–478. [Google Scholar] [CrossRef]
- Bloodsworth, A.; O’Donnell, V.B.; Freeman, B.A. Nitric oxide regulation of free radical–and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yoon, B.; Dey, A.; Park, J.H. Recent progress in nitric oxide-generating nanomedicine for cancer therapy. J. Control. Release 2022, 352, 179–198. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.S.; Cordon, B.; Ma, B.; Xie, J. Nitric oxide: Physiological functions, delivery, and Biomedical Applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, P.I. Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol. Pharm. 2010, 7, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Min, S.; Tian, Y. Injectable and cell-laden hydrogel in the contained bone defect animal model: A systematic review. Tissue Eng. Regen. Med. 2023, 20, 829–837. [Google Scholar] [CrossRef]
- Lee, G.; Ko, Y.-G.; Bae, K.H.; Kurisawa, M.; Kwon, O.K.; Kwon, O.H. Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications. Biomater. Res. 2022, 26, 62. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Rafe, A.; Vahedi, E.; Hasan-Sarei, A.G. Rheology and microstructure of binary mixed gel of rice bran protein–whey: Effect of heating rate and whey addition. J. Sci. Food Agric. 2016, 96, 3890–3896. [Google Scholar] [CrossRef]
- Omer, A.M.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Tamer, T.M.; Eldin, M.S.M.; Ouyang, X.-k.; Heydari, A. Advances in stimuli-responsive polymeric hydrogels for anticancer drug delivery: A review. J. Drug Deliv. Sci. Technol. 2024, 102, 106394. [Google Scholar] [CrossRef]
- Zhang, L.; Furst, E.M.; Kiick, K.L. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide–polysaccharide interactions. J. Control. Release 2006, 114, 130–142. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Q.; Zhou, J.; Li, Y.; Zheng, Y.; Chen, L. A reactive oxygen species-responsive hydrogel loaded with Apelin-13 promotes the repair of spinal cord injury by regulating macrophage M1/M2 polarization and neuroinflammation. J. Nanobiotechnology 2025, 23, 12. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative stress-induced angiogenesis is mediated by miR-205-5p. J. Cell. Mol. Med. 2020, 24, 1428–1436. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, Y.; Zhang, D.; Zhang, H.; Yu, A.; Li, Z. Roles of oxidative stress and raftlin in wound healing under negative-pressure wound therapy. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1745–1753. [Google Scholar] [CrossRef]
- Brem, H.; Jacobs, T.; Vileikyte, L.; Weinberger, S.; Gibber, M.; Gill, K.; Tarnovskaya, A.; Entero, H.; Boulton, A. Wound-healing protocols for diabetic foot and pressure ulcers. Surg. Technol. Int. 2003, 11, 85–92. [Google Scholar] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Honnegowda, T.M.; Kumar, P.; Udupa, E.G.P.; Kumar, S.; Kumar, U.; Rao, P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthetic Res. 2015, 2, 243–249. [Google Scholar] [CrossRef]
- Bosco, M.C.; Puppo, M.; Blengio, F.; Fraone, T.; Cappello, P.; Giovarelli, M.; Varesio, L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology 2008, 213, 733–749. [Google Scholar] [CrossRef]
- Lokmic, Z.; Musyoka, J.; Hewitson, T.D.; Darby, I.A. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int. Rev. Cell Mol. Biol. 2012, 296, 139–185. [Google Scholar]
- Bevensee, M.O.; Boron, W.F. Effects of acute hypoxia on intracellular-pH regulation in astrocytes cultured from rat hippocampus. Brain Res. 2008, 1193, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Erra Díaz, F.; Dantas, E.; Geffner, J. Unravelling the interplay between extracellular acidosis and immune cells. Mediat. Inflamm. 2018, 2018, 1218297. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Lucena, A.; Núñez de Castro, I.; Lozano, R.M.; Muñoz-Willery, I.; Zazo, M.; Giménez-Gallego, G. Effect of low pH and heparin on the structure of acidic fibroblast growth factor. Eur. J. Biochem. 1994, 222, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. -Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef]
- Pichiule, P.; Chavez, J.C.; LaManna, J.C. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 2004, 279, 12171–12180. [Google Scholar] [CrossRef]
- Hitchon, C.; Wong, K.; Ma, G.; Reed, J.; Lyttle, D.; El-Gabalawy, H. Hypoxia-induced production of stromal cell–derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002, 46, 2587–2597. [Google Scholar] [CrossRef]
- Huang, F.; Gao, T.; Feng, Y.; Xie, Y.; Tai, C.; Huang, Y.; Ling, L.; Wang, B. Bioinspired Collagen Scaffold Loaded with bFGF-Overexpressing Human Mesenchymal Stromal Cells Accelerating Diabetic Skin Wound Healing via HIF-1 Signal Pathway Regulated Neovascularization. ACS Appl. Mater. Interfaces 2024, 16, 45989–46004. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. -Cell Physiol. 2011, 300, C385–C393. [Google Scholar] [CrossRef]
- Cooke, J.; Dryden, M.; Patton, T.; Brennan, J.; Barrett, J. The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Res. Notes 2015, 8, 20. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Huda, M.N.; Shin, Y.; Han, S.; Akter, S.; Kang, I.; Ha, J.; Choe, W.; Choi, T.G.; Kim, S.S. Correlation between oxidative stress and transforming growth factor-beta in cancers. Int. J. Mol. Sci. 2021, 22, 13181. [Google Scholar] [CrossRef] [PubMed]
- Gouzos, M.; Ramezanpour, M.; Bassiouni, A.; Psaltis, A.J.; Wormald, P.; Vreugde, S. Antibiotics affect ROS production and fibroblast migration in an in-vitro model of sinonasal wound healing. Front. Cell. Infect. Microbiol. 2020, 10, 110. [Google Scholar] [CrossRef]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell. Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar]
- Huo, Y.; Qiu, W.-Y.; Pan, Q.; Yao, Y.-F.; Xing, K.; Lou, M.F. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp. Eye Res. 2009, 89, 876–886. [Google Scholar] [CrossRef]
- Wu, L.; Pan, Y. Reactive oxygen species mediate TNF-α-induced inflammatory response in bone marrow mesenchymal cells. Iran. J. Basic Med. Sci. 2019, 22, 1296. [Google Scholar]
- Kaltalioglu, K.; Coskun-Cevher, S.; Tugcu-Demiroz, F.; Celebi, N. PDGF supplementation alters oxidative events in wound healing process: A time course study. Arch. Dermatol. Res. 2013, 305, 415–422. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef]
- Tan, J.L.; Lash, B.; Karami, R.; Nayer, B.; Lu, Y.-Z.; Piotto, C.; Julier, Z.; Martino, M.M. Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun. Biol. 2021, 4, 422. [Google Scholar] [CrossRef] [PubMed]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- An, Z.; Zhang, L.; Liu, Y.; Zhao, H.; Zhang, Y.; Cao, Y.; Zhang, Y.; Pei, R. Injectable thioketal-containing hydrogel dressing accelerates skin wound healing with the incorporation of reactive oxygen species scavenging and growth factor release. Biomater. Sci. 2022, 10, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zheng, Z.; Zhang, H.; Xie, E.; Chen, L.; Jiang, Z.; Gao, Y.; Ma, J.; Yang, L. Effects of reactive oxygen species and antioxidant strategies on wound healing in diabetes. Interdiscip. Med. 2025, 3, e20240062. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal cell responses and role of TNF-in impaired diabetic wound healing. BioMed Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef]
- Corredor, J.; Yan, F.; Shen, C.C.; Tong, W.; John, S.K.; Wilson, G.; Whitehead, R.; Polk, D.B. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am. J. Physiol. -Cell Physiol. 2003, 284, C953–C961. [Google Scholar] [CrossRef]
- McGettrick, A.; O’Neill, L. NLRP3 and IL-1β in macrophages as critical regulators of metabolic diseases. Diabetes Obes. Metab. 2013, 15, 19–25. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X.; Wang, Y.; Chen, H.; Chai, Y. ROS-activated NLRP3 inflammasome initiates inflammation in delayed wound healing in diabetic rats. Int. J. Clin. Exp. Pathol. 2017, 10, 9902. [Google Scholar]
- Ding, Y.; Ding, X.; Zhang, H.; Li, S.; Yang, P.; Tan, Q. Relevance of NLRP3 inflammasome-related pathways in the pathology of diabetic wound healing and possible therapeutic targets. Oxidative Med. Cell. Longev. 2022, 2022, 9687925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Fojtík, P.; Beckerová, D.; Holomková, K.; Šenfluk, M.; Rotrekl, V. Both hypoxia-inducible factor 1 and MAPK signaling pathway attenuate PI3K/AKT via suppression of reactive oxygen species in human pluripotent stem cells. Front. Cell Dev. Biol. 2021, 8, 607444. [Google Scholar] [CrossRef] [PubMed]
- Ripoli, M.; D’Aprile, A.; Quarato, G.; Sarasin-Filipowicz, M.; Gouttenoire, J.; Scrima, R.; Cela, O.; Boffoli, D.; Heim, M.H.; Moradpour, D. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1α-mediated glycolytic adaptation. J. Virol. 2010, 84, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Addabbo, F.; Montagnani, M.; Goligorsky, M.S. Mitochondria and reactive oxygen species. Hypertension 2009, 53, 885–892. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial nitric oxide synthase (eNOS) and the cardiovascular system: In physiology and in disease states. Am. J. Biomed. Sci. Res. 2022, 15, 153. [Google Scholar] [CrossRef]
- Sivaraj, D.; Noishiki, C.; Kosaric, N.; Kiwanuka, H.; Kussie, H.C.; Henn, D.; Fischer, K.S.; Trotsyuk, A.A.; Greco, A.H.; Kuehlmann, B.A. Nitric oxide-releasing gel accelerates healing in a diabetic murine splinted excisional wound model. Front. Med. 2023, 10, 1060758. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zuo, A.; Zhang, J.; Wen, W.; Jiang, W.; Chen, H.; Liang, D.; Sun, J.; Wang, M. HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury. Cell. Mol. Biol. Lett. 2021, 26, 40. [Google Scholar] [CrossRef]
- Campos, K.L.; Giovanelli, J.; Kaufman, S. Characteristics of the Nitric Oxide Synthase-catalyzed Conversion of Arginine to N-Hydroxyarginine, the First Oxygenation Step in the Enzymic Synthesis of Nitric Oxide (∗). J. Biol. Chem. 1995, 270, 1721–1728. [Google Scholar] [CrossRef]
- Leiper, J.; Nandi, M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat. Rev. Drug Discov. 2011, 10, 277–291. [Google Scholar] [CrossRef]
- Hildebrand, S.; Ibrahim, M.; Schlitzer, A.; Maegdefessel, L.; Röll, W.; Pfeifer, A. PDGF regulates guanylate cyclase expression and cGMP signaling in vascular smooth muscle. Commun. Biol. 2022, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Braverman, J.; Stanley, S.A. Nitric oxide modulates macrophage responses to Mycobacterium tuberculosis infection through activation of HIF-1α and repression of NF-κB. J. Immunol. 2017, 199, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dai, X.; He, J.; Yan, X.; Yang, C.; Fan, X.; Sun, S.; Chen, J.; Xu, J.; Deng, Z. Endothelial overexpression of metallothionein prevents diabetes-induced impairment in ischemia angiogenesis through preservation of HIF-1α/SDF-1/VEGF signaling in endothelial progenitor cells. Diabetes 2020, 69, 1779–1792. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Long, Y.; de Castro Barbosa, T.; Karlsson, H.; Glund, S.; Zavadoski, W.; Gibbs, E.; Koistinen, H.; Wallberg-Henriksson, H.; Zierath, J.R. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK) α1-specific activity and glucose transport in human skeletal muscle. Diabetologia 2010, 53, 1142–1150. [Google Scholar] [CrossRef]
- Erusalimsky, J.D.; Moncada, S. Nitric oxide and mitochondrial signaling: From physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2524–2531. [Google Scholar] [CrossRef]
- Stoimenova, M.; Igamberdiev, A.U.; Gupta, K.J.; Hill, R.D. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007, 226, 465–474. [Google Scholar] [CrossRef]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 2000, 403, 103–108. [Google Scholar] [CrossRef]
- Geethika, M.; Singh, N.; Kumar, S.; Kumar, S.K.N.; Mugesh, G. A redox modulatory SOD mimetic nanozyme prevents the formation of cytotoxic peroxynitrite and improves nitric oxide bioavailability in human endothelial cells. Adv. Healthc. Mater. 2023, 12, 2300621. [Google Scholar] [CrossRef]
- Kiraz, S.; Ertenli, I.; Calguneri, M.; Ozturk, M.; Haznedaroglu, I.; Altun, B.; Erman, M.; Celik, I. Interactions of nitric oxide and superoxide dismutase in Behçet’s disease. Clin. Exp. Rheumatol. 2001, 19 (Suppl. S24), S25–S29. [Google Scholar]
- Xia, Y.; Zweier, J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA 1997, 94, 6954–6958. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Carreira, B.P.; Morte, M.I.; Inácio, Â.; Costa, G.; Rosmaninho-Salgado, J.; Agasse, F.; Carmo, A.; Couceiro, P.; Brundin, P.; Ambrósio, A.F. Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells 2010, 28, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Kumar, P.; Palmer, J.; Rophael, J.; Dolderer, J.; Thomas, G.; Morrison, W.; Penington, A.; Stewart, A.; Mitchell, G. The influence of nitric oxide synthase 2 on cutaneous wound angiogenesis. Br. J. Dermatol. 2011, 165, 1223–1235. [Google Scholar] [CrossRef]
- Cooke, J.P.; Losordo, D.W. Nitric oxide and angiogenesis. Circulation 2002, 105, 2133–2135. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, D.; Oh, Y.; Heo, J.; Hong, J. A Nanocoating Co-Localizing Nitric Oxide and Growth Factor onto Individual Endothelial Cells Reveals Synergistic Effects on Angiogenesis. Adv. Healthc. Mater. 2022, 11, 2102095. [Google Scholar] [CrossRef]
- Maloney, S.E.; Broberg, C.A.; Grayton, Q.E.; Picciotti, S.L.; Hall, H.R.; Wallet, S.M.; Maile, R.; Schoenfisch, M.H. Role of nitric oxide-releasing glycosaminoglycans in wound healing. ACS Biomater. Sci. Eng. 2022, 8, 2537–2552. [Google Scholar] [CrossRef]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of nitric oxide on cell proliferation: Novel insights. J. Am. Coll. Cardiol. 2013, 62, 89–95. [Google Scholar] [CrossRef]
- Thuraisingam, T.; Xu, Y.Z.; Eadie, K.; Heravi, M.; Guiot, M.-C.; Greemberg, R.; Gaestel, M.; Radzioch, D. MAPKAPK-2 signaling is critical for cutaneous wound healing. J. Investig. Dermatol. 2010, 130, 278–286. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/AKT pathway: Emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, L.; Redman, T.; Qi, S.; Zhao, F. Nitric oxide regulates cell behavior on an interactive cell-derived extracellular matrix scaffold. J. Biomed. Mater. Res. Part A 2015, 103, 3807–3814. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Witte, M.B.; Thornton, F.J.; Efron, D.T.; Barbul, A. Enhancement of fibroblast collagen synthesis by nitric oxide. Nitric Oxide 2000, 4, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, W.; Beeg, T.; Beck, K.-F.; Walpen, S.; Gauer, S.; Böhles, H.; Pfeilschifter, J. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000, 57, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Windhausen, A.N.; Isenberg, J.S.; Yeung, N.; Thomas, D.D.; Vitek, M.P.; Roberts, D.D.; Wink, D.A. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and-independent pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 16898–16903. [Google Scholar] [CrossRef]
- Vernet, D.; Ferrini, M.G.; Valente, E.G.; Magee, T.R.; Bou-Gharios, G.; Rajfer, J.; Gonzalez-Cadavid, N.F. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric Oxide 2002, 7, 262–276. [Google Scholar] [CrossRef]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer hydrogels for wound dressing and tissue engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and synthetic polymers for biomedical and environmental applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Tavares, G.; Alves, P.; Simões, P. Recent advances in hydrogel-mediated nitric oxide delivery systems targeted for wound healing applications. Pharmaceutics 2022, 14, 1377. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef]
- Kang, M.-L.; Kim, H.-S.; You, J.; Choi, Y.S.; Kwon, B.-J.; Park, C.H.; Baek, W.; Kim, M.S.; Lee, Y.J.; Im, G.-I. Hydrogel cross-linking–programmed release of nitric oxide regulates source-dependent angiogenic behaviors of human mesenchymal stem cell. Sci. Adv. 2020, 6, eaay5413. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Li, L.-L. Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 2021, 13, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Lee, J.; Ahn, H.-J.; Hwang, W.R.; Bahar, M.A.; Habibie, H.; Amir, M.N.; Lallo, S.; Son, H.-J.; Yoo, J.-W. Nitric oxide-releasing bacterial cellulose/chitosan crosslinked hydrogels for the treatment of polymicrobial wound infections. Pharmaceutics 2021, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Riccio, D.A.; Schoenfisch, M.H. Nitric oxide release: Part I. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, H.; Zhang, J.; Li, X.; Wu, Y.; Wei, Y.; Ji, S.; Kong, D.; Zhao, Q. Functional poly (ε-caprolactone)/chitosan dressings with nitric oxide-releasing property improve wound healing. Acta Biomater. 2017, 54, 128–137. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Adler, B.L.; Friedman, A.J. Nitric oxide therapy for dermatologic disease. Future Sci. OA 2015, 1, FSO37. [Google Scholar] [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and promise of nitric oxide-releasing platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef] [PubMed]
- Suschek, C.V.; Schewe, T.; Sies, H.; Kröncke, K.-D. Nitrite, a naturally occurring precursor of nitric oxide that acts like a ‘prodrug’. Biol. Chem. 2006, 387, 449–506. [Google Scholar] [CrossRef] [PubMed]
- Nossaman, V.E.; Nossaman, B.D.; Kadowitz, P.J. Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol. Rev. 2010, 18, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, X.; Xian, M.; Wang, P.G. Glycosylated diazeniumdiolates: A novel class of enzyme-activated nitric oxide donors. Tetrahedron Lett. 2001, 42, 3779–3782. [Google Scholar] [CrossRef]
- Gutierrez Cisneros, C.; Bloemen, V.; Mignon, A. Synthetic, natural, and semisynthetic polymer carriers for controlled nitric oxide release in dermal applications: A review. Polymers 2021, 13, 760. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Xu, S.; Liu, X.; Lin, J.; Chen, H.; Yuan, Z. Light-triggered fluorescence self-reporting nitric oxide release from coumarin analogues for accelerating wound healing and synergistic antimicrobial applications. J. Med. Chem. 2021, 65, 424–435. [Google Scholar] [CrossRef]
- Fan, J.; He, N.; He, Q.; Liu, Y.; Ma, Y.; Fu, X.; Liu, Y.; Huang, P.; Chen, X. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale 2015, 7, 20055–20062. [Google Scholar] [CrossRef]
- Liu, R.; Peng, Y.; Lu, L.; Peng, S.; Chen, T.; Zhan, M. Near-infrared light-triggered nano-prodrug for cancer gas therapy. J. Nanobiotechnol. 2021, 19, 443. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, B.; Wang, L.; Yang, L.; Chen, H.; Chen, W.; Qiao, H.; Qian, H. A glucose-responsive nitric oxide release hydrogel for infected diabetic wounds treatment. J. Ophthalmol. Clin. Res. 2023, 359, 147–160. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Wang, Y.; Qin, X.; Lu, Y.; Wu, Z.; Zhang, C.; Xu, L.; Han, J.; Yang, S. l-Arginine carboxymethyl cellulose hydrogel releasing nitric oxide to improve wound healing. Eur. Polym. J. 2023, 189, 111940. [Google Scholar] [CrossRef]
- Le Thi, P.; Tran, D.L.; Park, K.M.; Lee, S.; Oh, D.H.; Park, K.D. Biocatalytic nitric oxide generating hydrogels with enhanced anti-inflammatory, cell migration, and angiogenic capabilities for wound healing applications. J. Mater. Chem. B 2024, 12, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, D.; Gao, B.; Huang, S.; Tang, Y.; Wa, Q.; Dong, Y.; Yu, S.; Huang, J.; Huang, S. A DNA-inspired injectable adhesive hydrogel with dual nitric oxide donors to promote angiogenesis for enhanced wound healing. Acta Biomater. 2024, 176, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, L.; Huang, L.; Zhang, Y.; Tong, M.; Pan, H.; Shangguan, J.; Yao, Q.; Xu, S.; Xu, H. In situ hydrogel capturing nitric oxide microbubbles accelerates the healing of diabetic foot. J. Control. Release 2022, 350, 93–106. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, X.; Guo, H.; Huang, H.; Huang, S.; Huang, S.; Xue, W.; Zhu, P.; Guo, R. Nitric oxide released injectable hydrogel combined with synergistic photothermal therapy for antibacterial and accelerated wound healing. Appl. Mater. Today 2020, 20, 100781. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; He, Y.; Wang, L.; Deng, Z.; Liu, J.; Peng, D.; Ding, T.; Lu, L.; Ding, Y. Polydopamine nanosheets doped injectable hydrogel with nitric oxide release and photothermal effects for bacterial ablation and wound healing. Adv. Healthc. Mater. 2021, 10, 2101476. [Google Scholar] [CrossRef]
- He, C.; Bi, S.; Zhang, R.; Chen, C.; Liu, R.; Zhao, X.; Gu, J.; Yan, B. A hyaluronic acid hydrogel as a mild photothermal antibacterial, antioxidant, and nitric oxide release platform for diabetic wound healing. J. Control. Release 2024, 370, 543–555. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Lee, Y.; Le Thi, P.; Park, K.D. Nitric oxide-releasing injectable hydrogels with high antibacterial activity through in situ formation of peroxynitrite. Acta Biomater. 2018, 67, 66–78. [Google Scholar] [CrossRef]
- Zahid, A.A.; Ahmed, R.; ur Rehman, S.R.; Augustine, R.; Tariq, M.; Hasan, A. Nitric oxide releasing chitosan-poly (vinyl alcohol) hydrogel promotes angiogenesis in chick embryo model. Int. J. Biol. Macromol. 2019, 136, 901–910. [Google Scholar] [CrossRef]
- Ahmed, R.; Afreen, A.; Tariq, M.; Zahid, A.A.; Masoud, M.S.; Ahmed, M.; Ali, I.; Akram, Z.; Hasan, A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed. Mater. 2021, 16, 035014. [Google Scholar] [CrossRef] [PubMed]
- Durão, J.; Vale, N.; Gomes, S.; Gomes, P.; Barrias, C.C.; Gales, L. Nitric oxide release from antimicrobial peptide hydrogels for wound healing. Biomolecules 2018, 9, 4. [Google Scholar] [CrossRef]
- Ruan, L.; Pan, C.; Ran, X.; Wen, Y.; Lang, R.; Peng, M.; Cao, J.; Yang, J. Dual-Delivery Temperature-Sensitive Hydrogel with Antimicrobial and Anti-Inflammatory Brevilin A and Nitric Oxide for Wound Healing in Bacterial Infection. Gels 2024, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.T.; de Araujo Lima, B.; Do Nascimento, M.H.; Lombello, C.B.; Brocchi, M.; Seabra, A.B. Biocompatible and antibacterial nitric oxide-releasing pluronic F-127/chitosan hydrogel for topical applications. Polymers 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Ahn, H.-J.; Song, K.-W.; Yoo, J.-W. In situ hydrogel-forming/nitric oxide-releasing wound dressing for enhanced antibacterial activity and healing in mice with infected wounds. Pharmaceutics 2019, 11, 496. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, P.; Huang, L.; Tan, X.; Zhou, N.; Yang, T.; Qiu, H.; Dai, X.; Michael, S.; Tu, Q. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nat. Commun. 2021, 12, 7079. [Google Scholar] [CrossRef]
- Minehan, R.L.; Del Borgo, M.P. Controlled release of therapeutics from enzyme-responsive biomaterials. Front. Biomater. Sci. 2022, 1, 916985. [Google Scholar] [CrossRef]

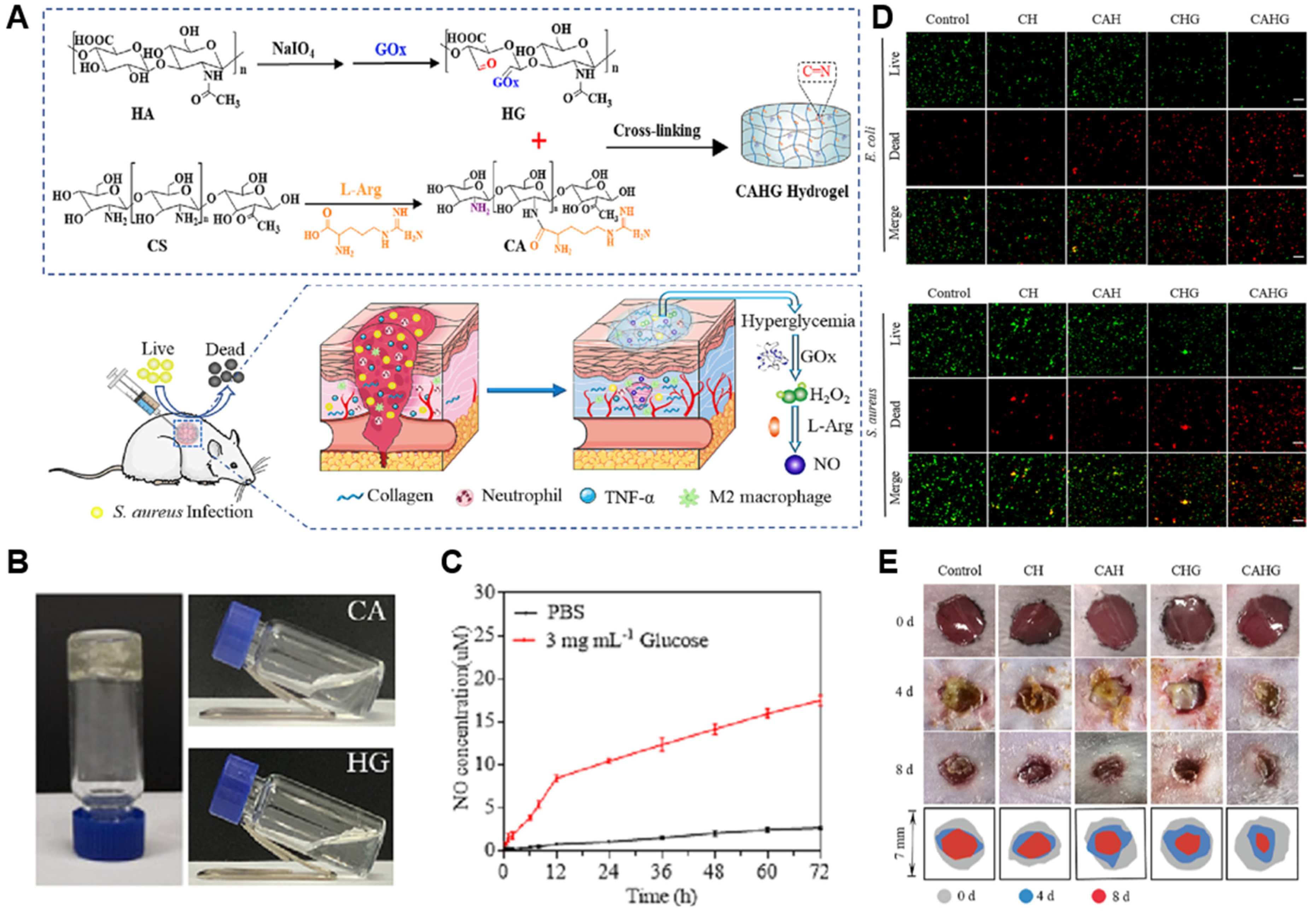

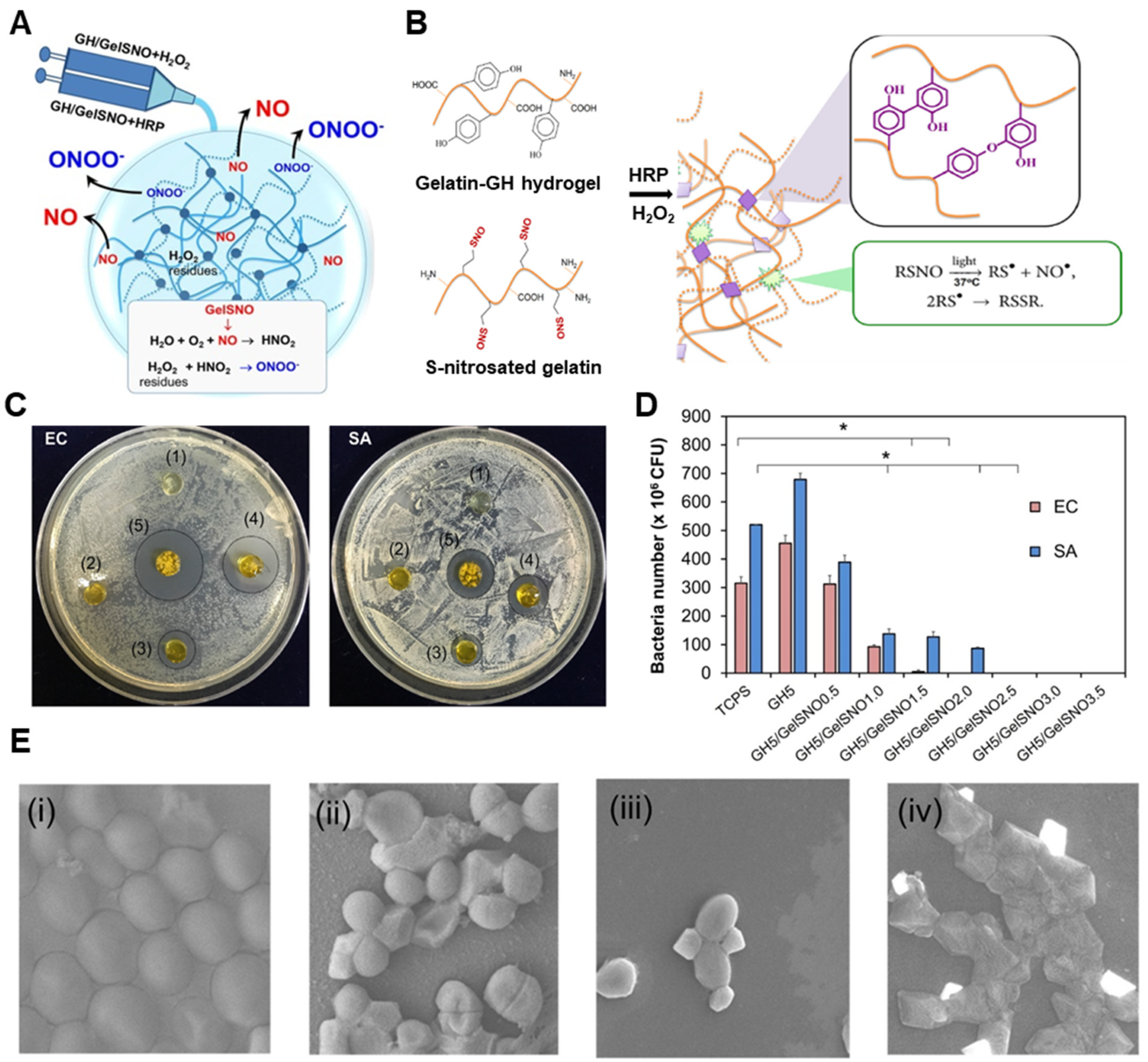
| Type | NO Donors | Polymers | Animal Model | Ref |
|---|---|---|---|---|
| Enzymatic | L-arg | Chitosan with GOx-modified hyaluronic acid | Diabetic mouse | [120] |
| Carboxymethyl cellulose/chitosan hydrogel | Rat | [121] | ||
| RSNO | Gelatin-tyramine hydrogel | Mouse | [122] | |
| Ammonia | Gelatin hydrogel | Mouse, Rat | [104] | |
| GSNO + L-Arg | DNA-inspired injectable adhesive hydrogel | Rat | [123] | |
| Non-Enzymatic | NO gas (microbubble) | Poloxamer 407 hydrogel | Diabetic Mouse | [124] |
| BNN6 | PDA | Mouse | [125] | |
| PDA nanosheet-embedded hydrogel | Mouse | [126] | ||
| Allomelanin-loaded hydrogel | Mouse | [127] | ||
| GO-BNN6 complex hydrogel | Mouse | [128] | ||
| RSNO | Gelatin-hydroxyphenylpropionic acid | - | [129] | |
| SNAP | Chitosan/PVA hydrogel | Chick embryo | [130] | |
| NONOate | Micelle-embedded PEI or PAA hydrogel | Rabbit | [131] | |
| Antimicrobial peptide-based hydrogel | - | [132] | ||
| GSNO | Temperature-sensitive SA/Pluronic F-127 hydrogel | Mouse | [133] | |
| Pluronic F-127/Chitosan hydrogel | [134] | |||
| Alginate/Pectin/PEG-based in situ hydrogel-forming powder | Mouse | [135] | ||
| Pluronic F127-based hydrogel | Rat | [136] | ||
| GSNO + L-Arg | DNA-inspired injectable adhesive hydrogel | Rat | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, T.-H.; Jang, H.-J.; Jeong, G.-J.; Yoon, J.-K. Hydrogel-Based Nitric Oxide Delivery Systems for Enhanced Wound Healing. Gels 2025, 11, 621. https://doi.org/10.3390/gels11080621
Heo T-H, Jang H-J, Jeong G-J, Yoon J-K. Hydrogel-Based Nitric Oxide Delivery Systems for Enhanced Wound Healing. Gels. 2025; 11(8):621. https://doi.org/10.3390/gels11080621
Chicago/Turabian StyleHeo, Tae-Hyun, Hye-Jeong Jang, Gun-Jae Jeong, and Jeong-Kee Yoon. 2025. "Hydrogel-Based Nitric Oxide Delivery Systems for Enhanced Wound Healing" Gels 11, no. 8: 621. https://doi.org/10.3390/gels11080621
APA StyleHeo, T.-H., Jang, H.-J., Jeong, G.-J., & Yoon, J.-K. (2025). Hydrogel-Based Nitric Oxide Delivery Systems for Enhanced Wound Healing. Gels, 11(8), 621. https://doi.org/10.3390/gels11080621







