Structuring the Future of Cultured Meat: Hybrid Gel-Based Scaffolds for Edibility and Functionality
Abstract
1. Introduction
2. Hybrid Bio-Hydrogel-Based Composite Materials
2.1. Structurally Functional Natural Polymers
- (1)
- Gelatin and Collagen
- (2)
- Chitosan
- (3)
- Cellulose and its derivatives
- (4)
- Plant-derived proteins
- (5)
- Other food-compatible polysaccharides
2.2. Synergy Through Bio-Derived and Food-Grade Additives
- (1)
- Nanocellulose and Cellulose-derived reinforcements
- (2)
- Natural crosslinkers and functional binders
- (3)
- Dietary fibers, modified starches, and rheological modifiers
3. Summary and Outlook
- Quantitative structure–function mapping between scaffold composition, microarchitecture, and cellular behavior (ex., proliferation, alignment, and differentiation);
- Mechanistic insights into material–cell interactions to inform bioinspired scaffold design;
- Advanced hybridization strategies that optimize the trade-offs between printability, structural fidelity, and sensory performance;
- Integration with bioprinting process parameters to enable precision fabrication of edible, tissue-like constructs.
- Scale-up: Material formulations optimized at the lab scale may not be directly transferable to industrial bioreactors. Scaffold systems must be engineered to withstand long-term immersion in culture media while maintaining geometry, porosity, and bioactivity. Compatibility with high-throughput fabrication processes, including extrusion, molding, and 3D printing, will be essential for cost-effective production.
- Regulatory consideration: All scaffold components must comply with food safety standards, such as FDA GRAS or EFSA Novel Food requirements. Ingredients like chitosan, zein, or functionalized polysaccharides may require thorough evaluation of toxicity, allergenicity, metabolic impact, and residual presence in the final product. Therefore, the establishment of a standardized framework for evaluating scaffold safety, functionality, and nutritional impact is urgently needed.
- Commercial prospects: While scaffolds may contribute a relatively small portion of production costs, they exert a disproportionately large influence on product texture, structure, cookability, and consumer acceptability. The next generation of scaffolds must be designed not only to support cell growth but also to align with culinary expectations, enabling the fabrication of premium, structured meat analogs with palatable texture and sensory appeal.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef]
- Tuomisto, H.L. Importance of considering environmental sustainability in dietary guidelines. Lancet Planet. Health 2018, 2, e331–e332. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, e2102908. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choi, W.; Lee, J.M.; Lee, S.T.; Koh, W.-G.; Hong, J. Flavor-switchable scaffold for cultured meat with enhanced aromatic properties. Nat. Commun. 2024, 15, 5450. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, S.; Choi, B.; Choi, W.; Lee, H.; Lee, J.M.; Lee, S.T.; Yoo, K.H.; Han, D.; Bang, G.; et al. Cultured meat with enriched organoleptic properties by regulating cell differentiation. Nat. Commun. 2024, 15, 77. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, A.; Wang, T.; Zhang, Y.; Zhu, G.; Ling, S.; Wu, Z.; Jin, Y.; Chen, H.; Lai, Y.; et al. Growing meat on autoclaved vegetables with biomimetic stiffness and micro-patterns. Nat. Commun. 2025, 16, 161. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Ding, X.; Ding, S.; Tang, C.; Zeng, X.; Wang, J.; Zhou, G. Programmable scaffolds with aligned porous structures for cell cultured meat. Food Chem. 2024, 430, 137098. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Park, S.-H.; Gil, E.S.; Xia, X.-X.; Weiss, A.S.; Kaplan, D.L. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials 2011, 32, 8979–8989. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lotz, E.M.; Schwartz, Z. Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties. Tissue Eng. Part. A 2017, 23, 1479–1489. [Google Scholar] [CrossRef]
- Li, C.H.; Yang, I.H.; Ke, C.J.; Chi, C.Y.; Matahum, J.; Kuan, C.Y.; Celikkin, N.; Swieszkowski, W.; Lin, F.H. The Production of Fat-Containing Cultured Meat by Stacking Aligned Muscle Layers and Adipose Layers Formed from Gelatin-Soymilk Scaffold. Front. Bioeng. Biotechnol. 2022, 10, 875069. [Google Scholar] [CrossRef]
- Andreassen, R.C.; Rønning, S.B.; Solberg, N.T.; Grønlien, K.G.; Kristoffersen, K.A.; Høst, V.; Kolset, S.O.; Pedersen, M.E. Production of food-grade microcarriers based on by-products from the food industry to facilitate the expansion of bovine skeletal muscle satellite cells for cultured meat production. Biomaterials 2022, 286, 121602. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Jing, L.; Zeng, X.; Chen, T.; Liu, H.; Kong, Y.; Wang, X.; Yang, X.; Fu, C.; Sun, J.; et al. 3D-Printed Prolamin Scaffolds for Cell-Based Meat Culture. Adv. Mater. 2023, 35, 2207397. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-m.; Han, W.-m.; Hou, L.-y.; Lin, D.-d.; Li, J.-y.; Lin, S.-t.; Yang, J.-p.; Liao, L.; Zeng, X.-a. Glutenin-chitosan 3D porous scaffolds with tunable stiffness and systematized microstructure for cultured meat model. Int. J. Biol. Macromol. 2024, 267, 131438. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, B.N.; Flanagan, C.C.; Griffin, D.R. Microporous Annealed Particle (MAP) Scaffold Pore Size Influences Mesenchymal Stem Cell Metabolism and Proliferation Without Changing CD73, CD90, and CD105 Expression over Two Weeks. Adv. Biol. 2024, 8, e2300482. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, L.; Shijie, D.; Wang, C.; Wang, J.; Ibeogu, I.H.; Li, C.; Zhou, G.H. An Overview of Recent Progress in Cultured Meat: Focusing on Technology, Quality Properties, Safety, Industrialization, and Public Acceptance. J. Nutr. 2025, 155, 745–755. [Google Scholar] [CrossRef]
- Xia, P.; Miyajima, H.; Fujita, S. Development of Biomimetic Edible Scaffolds for Cultured Meat Based on the Traditional Freeze-Drying Method for Ito-Kanten (Japanese Freeze-Dried Agar). Gels 2025, 11, 299. [Google Scholar] [CrossRef]
- Dagès, B.A.S.; Fabian, J.A.; Polakova, D.; Rysova, M.; Topham, P.D.; Souppez, J.-B.R.G.; Hanga, M.P.; Theodosiou, E. Edible electrospun materials for scalable cultivated beef production. Food Bioprod. Process. 2025, 149, 118–129. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Munawar, N.; Zan, L.; Zhu, J. 3D edible scaffolds with yeast protein: A novel alternative protein scaffold for the production of high-quality cell-cultured meat. Int. J. Biol. Macromol. 2024, 259, 129134. [Google Scholar] [CrossRef]
- Nurul Alam, A.M.M.; Kim, C.-J.; Kim, S.-H.; Kumari, S.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Scaffolding fundamentals and recent advances in sustainable scaffolding techniques for cultured meat development. Food Res. Int. 2024, 189, 114549. [Google Scholar] [CrossRef]
- Jeong, D.; Jang, G.; Jung, W.K.; Park, Y.H.; Bae, H. Stretchable zein-coated alginate fiber for aligning muscle cells to artificially produce cultivated meat. npj Sci. Food 2024, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, D.H.; Ryoo, J.H.; Park, S.M.; Kwon, H.C.; Keum, D.H.; Shin, D.M.; Han, S.G. Influence of Gelatin on Adhesion, Proliferation, and Adipogenic Differentiation of Adipose Tissue-Derived Stem Cells Cultured on Soy Protein-Agarose Scaffolds. Foods 2024, 13, 2247. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
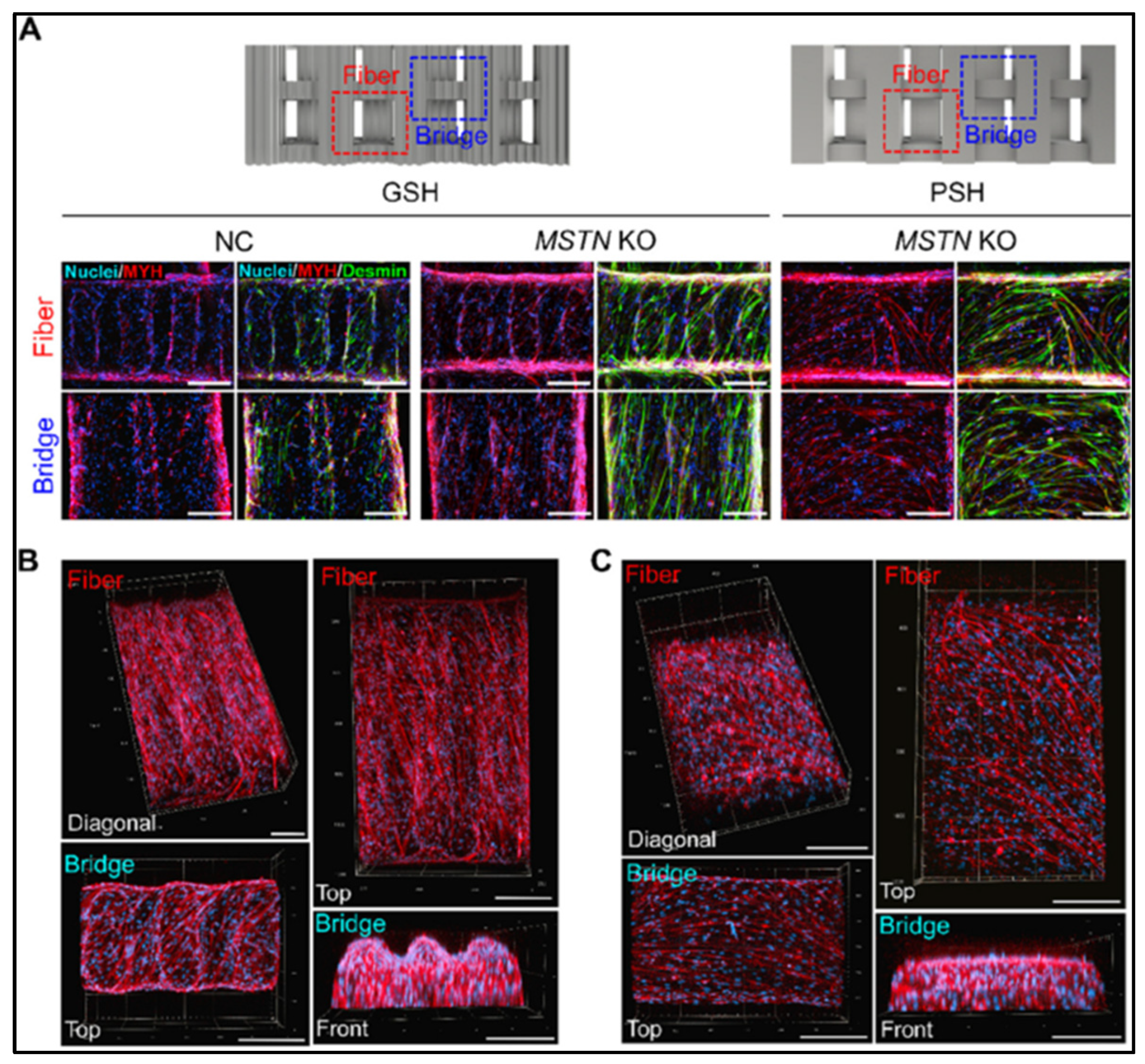
- Eom, K.-H.; Jeong, D.; Choi, J.-Y.; Gim, G.-M.; Yum, S.-Y.; Jin, S.; Bae, H.; Jang, G. MSTN knockout enhances the production of MYOD1-mediated steak-type cultivated meat. J. Anim. Sci. Biotechnol. 2025, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Xu, Y.; Yin, J.; Hu, J. A 3D-printable gelatin/alginate/ε-poly-l-lysine hydrogel scaffold to enable porcine muscle stem cells expansion and differentiation for cultured meat development. Int. J. Biol. Macromol. 2024, 271, 131980. [Google Scholar] [CrossRef]
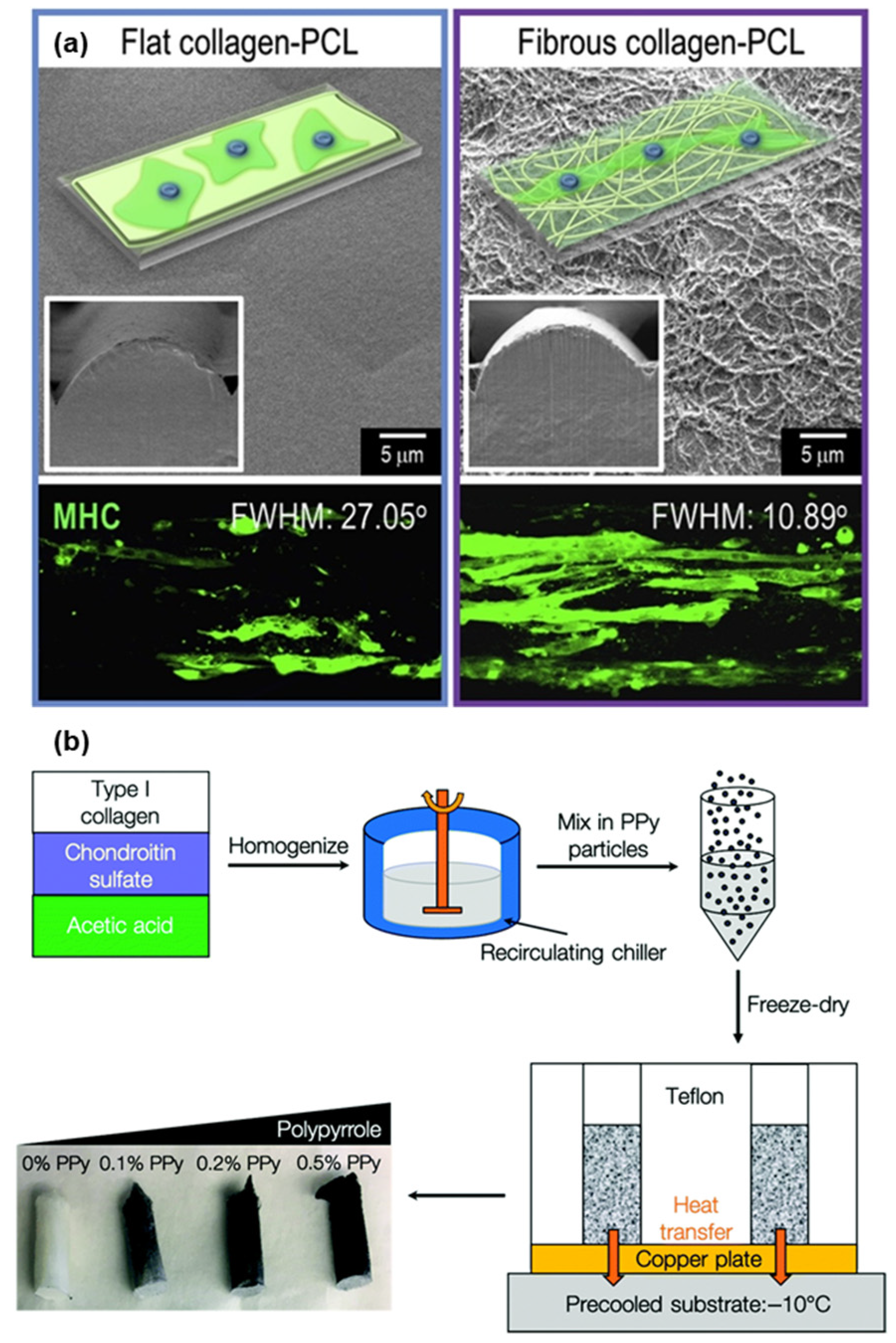
- Chae, S.; Lee, J.; Kim, G. Skeletal myotube formation enhanced through fibrillated collagen nanofibers coated on a 3D-printed polycaprolactone surface. Colloids Surf. B Biointerfaces 2019, 181, 408–415. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Long, X.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ogura, T.; Wang, D.O.; Ikejima, T. Type I collagen promotes the migration and myogenic differentiation of C2C12 myoblasts via the release of interleukin-6 mediated by FAK/NF-κB p65 activation. Food Funct. 2020, 11, 328–338. [Google Scholar] [CrossRef]
- Basurto, I.M.; Mora, M.T.; Gardner, G.M.; Christ, G.J.; Caliari, S.R. Aligned and electrically conductive 3D collagen scaffolds for skeletal muscle tissue engineering. Biomater. Sci. 2021, 9, 4040–4053. [Google Scholar] [CrossRef]
- Yun, Y.R.; Lee, S.; Jeon, E.; Kang, W.; Kim, K.H.; Kim, H.W.; Jang, J.H. Fibroblast growth factor 2-functionalized collagen matrices for skeletal muscle tissue engineering. Biotechnol. Lett. 2012, 34, 771–778. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef] [PubMed]
- Pires Figueiredo, M.; Rodríguez-Fernández, S.; Copes, F.; Mantovani, D. Review of collagen type I-based hydrogels: Focus on composition-structure-properties relationships. npj Biomed. Innov. 2025, 2, 16. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2021, 10, 1097–1123. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Taborda, M.I.; Catalan, K.N.; Orellana, N.; Bezjak, D.; Enrione, J.; Acevedo, C.A.; Corrales, T.P. Micropatterned Nanofiber Scaffolds of Salmon Gelatin, Chitosan, and Poly(vinyl alcohol) for Muscle Tissue Engineering. ACS Omega 2023, 8, 47883–47896. [Google Scholar] [CrossRef]
- Smoak, M.M.; Hogan, K.J.; Grande-Allen, K.J.; Mikos, A.G. Bioinspired electrospun dECM scaffolds guide cell growth and control the formation of myotubes. Sci. Adv. 2021, 7, eabg4123. [Google Scholar] [CrossRef]
- Kang, Y.M.; Lee, B.N.; Ko, J.H.; Kim, G.H.; Kang, K.N.; Kim, D.Y.; Kim, J.H.; Park, Y.H.; Chun, H.J.; Kim, C.H.; et al. In vivo biocompatibility study of electrospun chitosan microfiber for tissue engineering. Int. J. Mol. Sci. 2010, 11, 4140–4148. [Google Scholar] [CrossRef]
- Ma, X.; Ge, J.; Li, Y.; Guo, B.; Ma, P.X. Nanofibrous electroactive scaffolds from a chitosan-grafted-aniline tetramer by electrospinning for tissue engineering. RSC Adv. 2014, 4, 13652–13661. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial Activities of Chitosan Derivatives. Pharmaceutics 2021, 13, 1639. [Google Scholar] [CrossRef] [PubMed]
- Waibel, K.H.; Haney, B.; Moore, M.; Whisman, B.; Gomez, R. Safety of chitosan bandages in shellfish allergic patients. Mil. Med. 2011, 176, 1153–1156. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Mikhailidi, A.; Samuil, C.; Ungureanu, O.C.; Fortună, M.E.; Ungureanu, E. Advances in Cellulose-Based Hydrogels: Current Trends and Challenges. Gels 2024, 10, 842. [Google Scholar] [CrossRef] [PubMed]
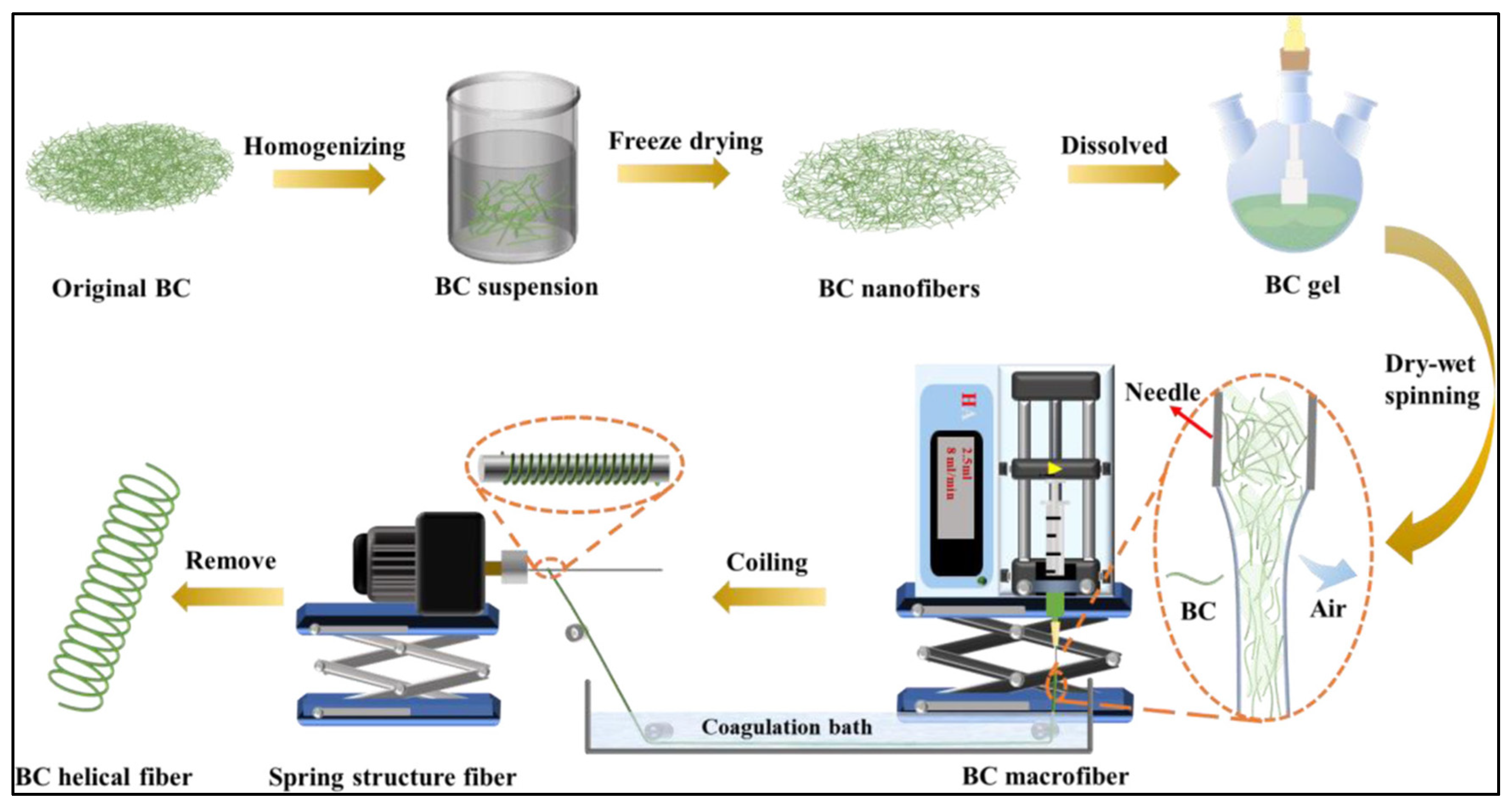
- Liang, Q.; Zhang, D.; Ji, P.; Sheng, N.; Zhang, M.; Wu, Z.; Chen, S.; Wang, H. High-Strength Superstretchable Helical Bacterial Cellulose Fibers with a “Self-Fiber-Reinforced Structure”. ACS Appl. Mater. Interfaces 2021, 13, 1545–1554. [Google Scholar] [CrossRef]
- Skogberg, A.; Mäki, A.J.; Mettänen, M.; Lahtinen, P.; Kallio, P. Cellulose Nanofiber Alignment Using Evaporation-Induced Droplet-Casting, and Cell Alignment on Aligned Nanocellulose Surfaces. Biomacromolecules 2017, 18, 3936–3953. [Google Scholar] [CrossRef]
- Murugarren, N.; Roig-Sanchez, S.; Antón-Sales, I.; Malandain, N.; Xu, K.; Solano, E.; Reparaz, J.S.; Laromaine, A. Highly Aligned Bacterial Nanocellulose Films Obtained During Static Biosynthesis in a Reproducible and Straightforward Approach. Adv. Sci. 2022, 9, 2201947. [Google Scholar] [CrossRef]
- Santos, A.E.A.d.; Cotta, T.; Santos, J.P.F.; Camargos, J.S.F.; Carmo, A.C.C.d.; Alcântara, E.G.A.; Fleck, C.; Copola, A.G.L.; Nogueira, J.M.; Silva, G.A.B.; et al. Bioactive cellulose acetate nanofiber loaded with annatto support skeletal muscle cell attachment and proliferation. Front. Bioeng. Biotechnol. 2023, 11, 1116917. [Google Scholar] [CrossRef]
- Mastrodimos, M.; Jain, S.; Badv, M.; Shen, J.; Montazerian, H.; Meyer, C.E.; Annabi, N.; Weiss, P.S. Human Skeletal Muscle Myoblast Culture in Aligned Bacterial Nanocellulose and Commercial Matrices. ACS Appl. Mater. Interfaces 2024, 16, 47150–47162. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Somerville, C. Cellulose synthase interacting protein: A new factor in cellulose synthesis. Plant Signal Behav. 2010, 5, 1571–1574. [Google Scholar] [CrossRef]
- Chen, J.; Ma, A.; Zhang, Y.; Sun, L.; Yang, K.; Vanegas Sáenz, J.R.; Hong, G. Mechanical and biological properties of cellulose nanofibers as a dental biomaterial. J. Dent. Sci. 2025, 20, 1436–1446. [Google Scholar] [CrossRef]
- Kummala, R.; Soto Véliz, D.; Fang, Z.; Xu, W.; Abitbol, T.; Xu, C.; Toivakka, M. Human Dermal Fibroblast Viability and Adhesion on Cellulose Nanomaterial Coatings: Influence of Surface Characteristics. Biomacromolecules 2020, 21, 1560–1567. [Google Scholar] [CrossRef]
- Cândido, A.; Fregonezi, N.; Carvalho, A.; Trovatti, E.; Resende, F. TEMPO-Oxidized Cellulose Nanofibers In Vitro Cyto-genotoxicity Studies. BioNanoScience 2020, 10, 766–772. [Google Scholar] [CrossRef]
- Hu, Y.J.; Wang, Y.; Huang, Y.H.; Bian, J.; Li, M.F.; Peng, F.; Sun, R.C. Benzoxazine enhanced amino cellulose-based composite films: Preparation, proposed mechanism, and improved performance. Carbohydr. Polym. 2019, 222, 115008. [Google Scholar] [CrossRef] [PubMed]
- Claro, A.; Amaral, N.; Colturato, V.; Andrade Aleixo, N.; Paiva, R.; Cruz, S.; Monteiro, G.; Carvalho, G.; Nogueira, F.; Deffune, E.; et al. Siloxane-modified bacterial cellulose as a promising platform for cell culture. Cellulose 2022, 29, 1–12. [Google Scholar] [CrossRef]
- Ahangari, H.; Ebrahimi, A.; Ehsani, A.; Amjadi, S. Multipurpose packaging system based on intelligent carboxymethyl cellulose film and activated cellulose acetate electrospun nanofibers for seafoods. Int. J. Biol. Macromol. 2025, 298, 140115. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jung, S.; Heo, J.; Koh, W.G.; Lee, S.; Hong, J. Chitosan/Cellulose-Based Porous Nanofilm Delivering C-Phycocyanin: A Novel Platform for the Production of Cost-Effective Cultured Meat. ACS Appl. Mater. Interfaces 2021, 13, 32193–32204. [Google Scholar] [CrossRef]
- Melzener, L.; Spaans, S.; Hauck, N.; Pötgens, A.J.G.; Flack, J.E.; Post, M.J.; Doğan, A. Short-Stranded Zein Fibers for Muscle Tissue Engineering in Alginate-Based Composite Hydrogels. Gels 2023, 9, 914. [Google Scholar] [CrossRef]
- Kawecki, N.S.; Norris, S.C.P.; Xu, Y.; Wu, Y.; Davis, A.R.; Fridman, E.; Chen, K.K.; Crosbie, R.H.; Garmyn, A.J.; Li, S.; et al. Engineering multicomponent tissue by spontaneous adhesion of myogenic and adipogenic microtissues cultured with customized scaffolds. Food Res. Int. 2023, 172, 113080. [Google Scholar] [CrossRef]
- Dey, S.; Hettiarachchy, N.; Bisly, A.A.; Luthra, K.; Atungulu, G.G.; Ubeyitogullari, A.; Mozzoni, L.A. Physical and textural properties of functional edible protein films from soybean using an innovative 3D printing technology. J. Food Sci. 2022, 87, 4808–4819. [Google Scholar] [CrossRef]
- Kim, W.-J.; Lu, Y.; Ovissipour, R.; Nitin, N. Evaluation of plant-based composite materials as 3D printed scaffolds for cell growth and proliferation in cultivated meat applications. Food Hydrocoll. 2024, 160, 110823. [Google Scholar] [CrossRef]
- Seo, J.W.; Jung, W.K.; Park, Y.H.; Bae, H. Development of cultivable alginate fibers for an ideal cell-cultivated meat scaffold and production of hybrid cultured meat. Carbohydr. Polym. 2023, 321, 121287. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Chen, X.; Chen, Y.; Ding, S.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; et al. Chitosan-sodium alginate-collagen/gelatin three-dimensional edible scaffolds for building a structured model for cell cultured meat. Int. J. Biol. Macromol. 2022, 209, 668–679. [Google Scholar] [CrossRef]
- Ahmad Hariza, A.M.; Mohd Yunus, M.H.; Fauzi, M.B.; Murthy, J.K.; Tabata, Y.; Hiraoka, Y. The Fabrication of Gelatin-Elastin-Nanocellulose Composite Bioscaffold as a Potential Acellular Skin Substitute. Polymers 2023, 15, 779. [Google Scholar] [CrossRef]
- LakshmiBalasubramaniam, S.; Tajvidi, M.; Skonberg, D. Hydrophobic corn zein-modified cellulose nanofibril (CNF) films with antioxidant properties. Food Chem. 2024, 458, 140220. [Google Scholar] [CrossRef]
- Zuber, J.; Lopes Cascabulho, P.; Gemini Piperni, S.; Farias Corrêa do Amaral, R.J.; Vogt, C.; Carre, V.; Hertzog, J.; Kontturi, E.; Trubetskaya, A. Fast, Easy, and Reproducible Fingerprint Methods for Endotoxin Characterization in Nanocellulose and Alginate-Based Hydrogel Scaffolds. Biomacromolecules 2024, 25, 6762–6772. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Wang, Z.; Hou, J.; Liu, S.; Yang, Q.; Yu, L.; Guo, W.; Wang, Y.; Guo, B.; et al. Injectable remote magnetic nanofiber/hydrogel multiscale scaffold for functional anisotropic skeletal muscle regeneration. Biomaterials 2022, 285, 121537. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, L.; Ma, L.; Sun, Y.; Yang, X. Controlled Hydrophobic Biosurface of Bacterial Cellulose Nanofibers through Self-Assembly of Natural Zein Protein. ACS Biomater. Sci. Eng. 2017, 3, 1595–1604. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.; Christensen, H.; Dusemund, B.; Durjava, M.; Kouba, M.; Lopez-Alonso, M.; López, S.; Marcon, F.; et al. Safety and efficacy of microcrystalline cellulose for all animal species. EFSA J. Eur. Food Saf. Auth. 2020, 18, e06209. [Google Scholar] [CrossRef]
- Park, S.-M.; Ryoo, J.-H.; Kwon, H.C.; Han, S.G. Scaffold Biomaterials in the Development of Cultured Meat: A Review. Food Sci. Anim. Resour. 2025, 45, 688–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Chen, L.; Shao, W.; Chen, X.; Fan, X.; Liu, Y.; Ding, S.; Xu, X.; Zhou, G.; et al. Gellan gum-gelatin scaffolds with Ca2+ crosslinking for constructing a structured cell cultured meat model. Biomaterials 2023, 299, 122176. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Transglutaminase in Foods and Biotechnology. Int. J. Mol. Sci. 2023, 24, 12402. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved thermal-stability and mechanical properties of type I collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef]
- Broderick, E.P.; O’Halloran, D.M.; Rochev, Y.A.; Griffin, M.; Collighan, R.J.; Pandit, A.S. Enzymatic stabilization of gelatin-based scaffolds. J. Biomed. Mater. Res. Part B 2004, 72B, 37–42. [Google Scholar] [CrossRef]
- Chien, K.B.; Shah, R.N. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012, 8, 694–703. [Google Scholar] [CrossRef]
- Sengor, M. Transglutaminase crosslinked sodium caseinate/starch/tri Calcium Phosphate based flexible sponge grafts. Mater. Lett. 2022, 326, 132943. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Processed Food Additive Microbial Transglutaminase and Its Cross-Linked Gliadin Complexes Are Potential Public Health Concerns in Celiac Disease. Int. J. Mol. Sci. 2020, 21, 1127. [Google Scholar] [CrossRef]
- Choi, H.; Lee, K. Crosslinking Mechanisms of Phenol, Catechol, and Gallol for Synthetic Polyphenols: A Comparative Review. Appl. Sci. 2022, 12, 11626. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Shokrani, H.; Shokrani, A.; Seidi, F.; Mashayekhi, M.; Kar, S.; Nedeljkovic, D.; Kuang, T.; Saeb, M.R.; Mozafari, M. Polysaccharide-based biomaterials in a journey from 3D to 4D printing. Bioeng. Transl. Med. 2023, 8, e10503. [Google Scholar] [CrossRef] [PubMed]
- Amr, M.; Dykes, I.; Counts, M.; Kernan, J.; Mallah, A.; Mendenhall, J.; Van Wie, B.; Abu-Lail, N.; Gozen, B.A. 3D printed, mechanically tunable, composite sodium alginate, gelatin and Gum Arabic (SA-GEL-GA) scaffolds. Bioprinting 2021, 22, e00133. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Cao, G.; Li, Z.; Du, M. Effect of Heat Treatment on Gelatin Properties and the Construction of High Internal Phase Emulsions for 3D Printing. Foods 2024, 13, 4009. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, M.-M.; Manoliu, D.-R.; Ciobotaru, M.C.; Flocea, E.-I.; Boișteanu, P.-C. Dietary Fibres in Processed Meat: A Review on Nutritional Enhancement, Technological Effects, Sensory Implications and Consumer Perception. Foods 2025, 14, 1459. [Google Scholar] [CrossRef]
- Fraeye, I.; Kratka, M.; Vandenburgh, H.; Thorrez, L. Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred. Front. Nutr. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kamel, J.; Yadav, C.-J.; Yadav, U.; Afrin, S.; Son, Y.-M.; Won, S.-Y.; Han, S.-S.; Park, K.-M. Production of Plant-Based, Film-Type Scaffolds Using Alginate and Corn Starch for the Culture of Bovine Myoblasts. Foods 2024, 13, 1358. [Google Scholar] [CrossRef]
- Liu, P.; Dang, X.; Woo, M.W.; Chattha, S.A.; An, J.; Shan, Z. Feasibility Study of Starch-Based Biomass Incorporated 3D Printed Beef. Starch-Stärke 2022, 74, 2200030. [Google Scholar] [CrossRef]
- Piantino, M.; Muller, Q.; Nakadozono, C.; Yamada, A.; Matsusaki, M. Towards more realistic cultivated meat by rethinking bioengineering approaches. Trends Biotechnol. 2025, 43, 364–382. [Google Scholar] [CrossRef]
- Hubbe, M.; Sjostrand, B.; Lestelius, M.; Håkansson, H.; Swerin, A.; Henriksson, G. Swelling of cellulosic fibers in aqueous systems: A Review of chemical and mechanistic factors. BioResources 2024, 19, 6859–6945. [Google Scholar] [CrossRef]
- Bai, J.; Ren, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; Rao, Z. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Xian, D.; Wu, L.; Lin, K.; Liu, P.; Wu, S.; Yuan, Y.; Xie, F. Augmenting corn starch gel printability for architectural 3D modeling for customized food. Food Hydrocoll. 2024, 156, 110294. [Google Scholar] [CrossRef]
- Colussi, R.; Halal, S.; Zanella Pinto, V.; Bartz, J.; Gutkoski, L.; Zavareze, E.; Dias, A. Acetylation of rice starch in an aqueous medium for use in food. LWT 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- Xie, X.; Li, X.; Lei, J.; Zhao, X.; Lyu, Y.; Mu, C.; Li, D.; Ge, L.; Xu, Y. Oxidized starch cross-linked porous collagen-based hydrogel for spontaneous agglomeration growth of adipose-derived stem cells. Mater. Sci. Eng. C 2020, 116, 111165. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Khoda, B. Rheological Analysis of Bio-ink for 3D Bio-printing Processes. J. Manuf. Process 2022, 76, 708–718. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, C.; Hu, Z.; Shen, W.; Ji, Z.; Li, F.; Guo, C. Effect of zein-pectin composite particles on the stability and rheological properties of gelatin/hydroxypropyl methylcellulose water-water systems. Int. J. Biol. Macromol. 2024, 269, 131846. [Google Scholar] [CrossRef]
- Mo, Q.; Huang, L.; Sheng, Y.; Wei, Z.; Zhang, S.; Li, Y.; Wang, X.; Wang, Y.; Lu, X.; Huang, C.; et al. Crosslinking strategy and promotion role of cellulose as a composite hydrogel component for three-dimensional printing—A review. Food Hydrocoll. 2024, 154, 110079. [Google Scholar] [CrossRef]
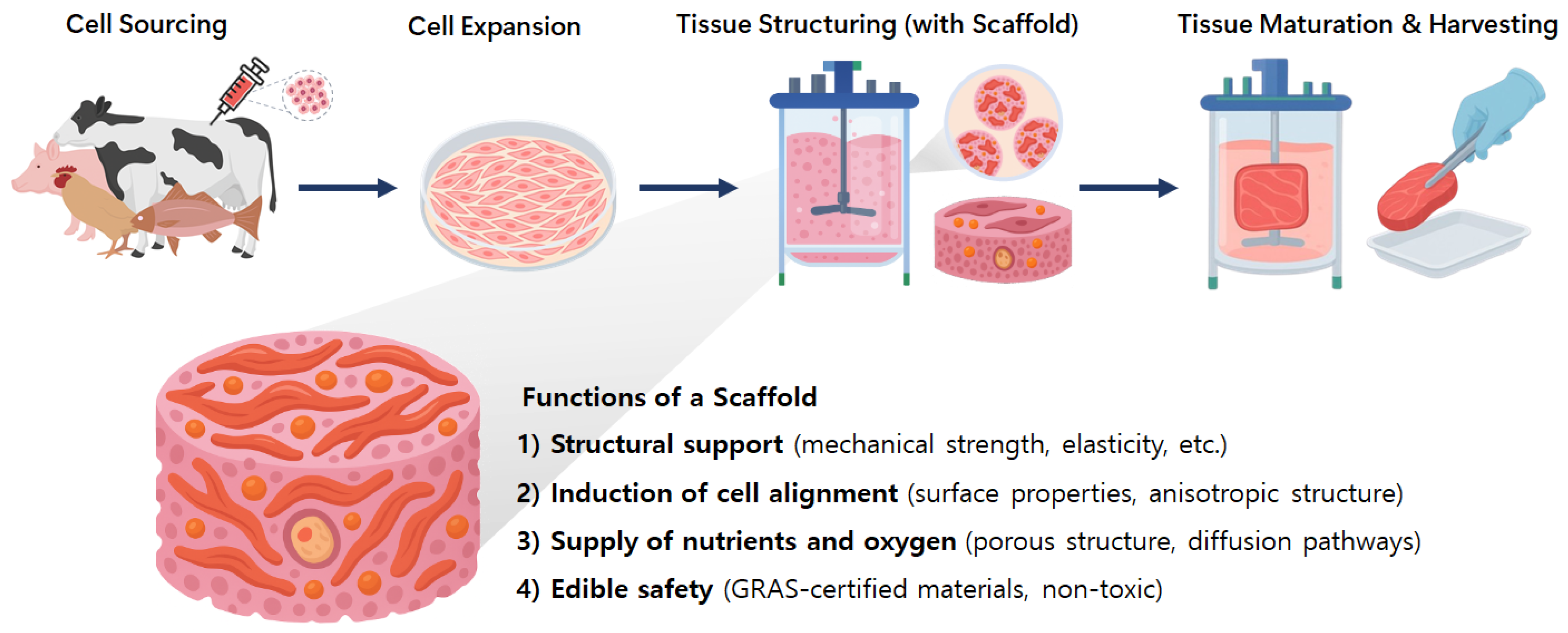


| Category | Description | Ref. |
|---|---|---|
| Biocompatibility | Requires a biofriendly surface that supports cell adhesion, growth, and differentiation | [7,8,9,10] |
| Edibility and Safety | Made from food-grade materials that are GRAS-certified, non-toxic, and digestible | [4,11,12] |
| Mechanical Properties | Elasticity similar to muscle tissue (10–100 kPa); durability to withstand cutting and cooking | [6,13,14] |
| Porosity and Structure | Pore size (50–200 μm) to enable nutrient/oxygen transport; structure that guides cell alignment | [4,15] |
| Scalability | Must allow for low-cost, large-scale production without the use of toxic chemicals | [3,5,16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zo, S.M.; Sood, A.; Won, S.Y.; Choi, S.M.; Han, S.S. Structuring the Future of Cultured Meat: Hybrid Gel-Based Scaffolds for Edibility and Functionality. Gels 2025, 11, 610. https://doi.org/10.3390/gels11080610
Zo SM, Sood A, Won SY, Choi SM, Han SS. Structuring the Future of Cultured Meat: Hybrid Gel-Based Scaffolds for Edibility and Functionality. Gels. 2025; 11(8):610. https://doi.org/10.3390/gels11080610
Chicago/Turabian StyleZo, Sun Mi, Ankur Sood, So Yeon Won, Soon Mo Choi, and Sung Soo Han. 2025. "Structuring the Future of Cultured Meat: Hybrid Gel-Based Scaffolds for Edibility and Functionality" Gels 11, no. 8: 610. https://doi.org/10.3390/gels11080610
APA StyleZo, S. M., Sood, A., Won, S. Y., Choi, S. M., & Han, S. S. (2025). Structuring the Future of Cultured Meat: Hybrid Gel-Based Scaffolds for Edibility and Functionality. Gels, 11(8), 610. https://doi.org/10.3390/gels11080610










