Formulation of Sustainable Materials from Agar/Glycerol/Water Gels: An Alternative to Polyurethane Foams in Single-Use Applications
Abstract
1. Introduction
2. Results and Discussion
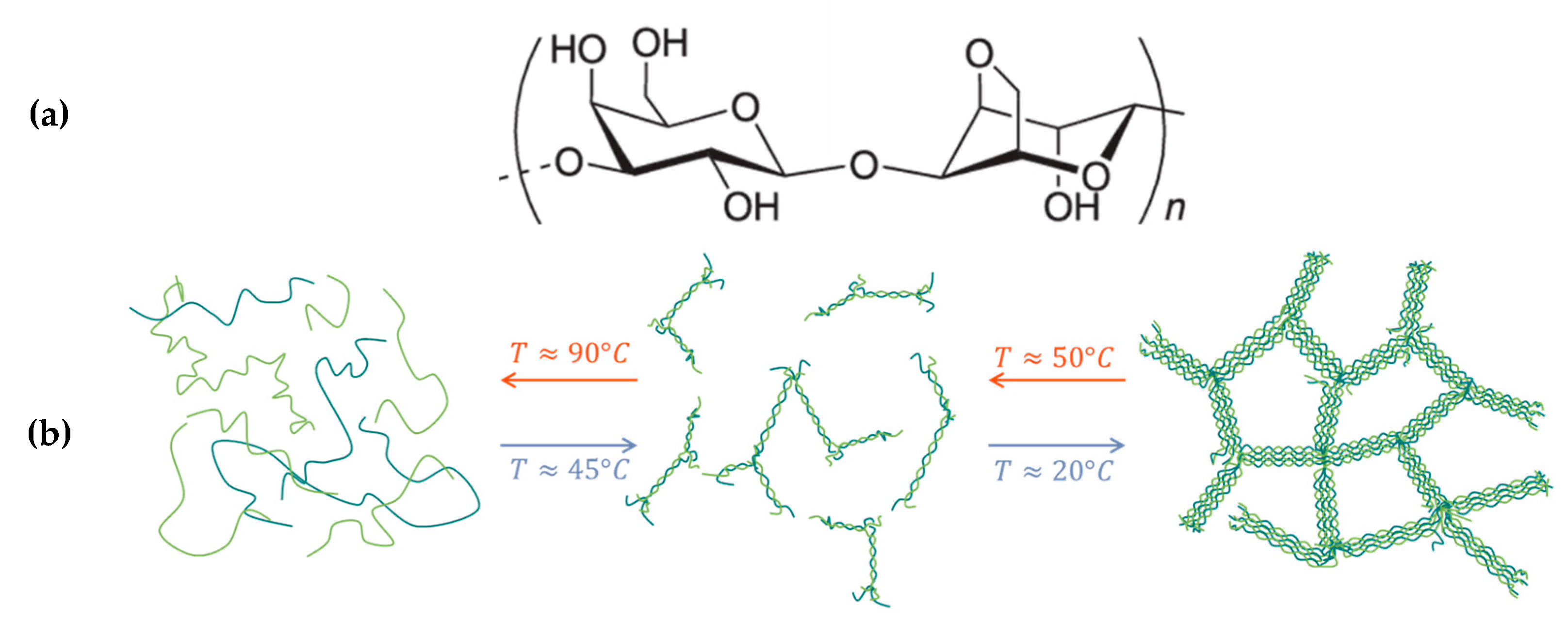
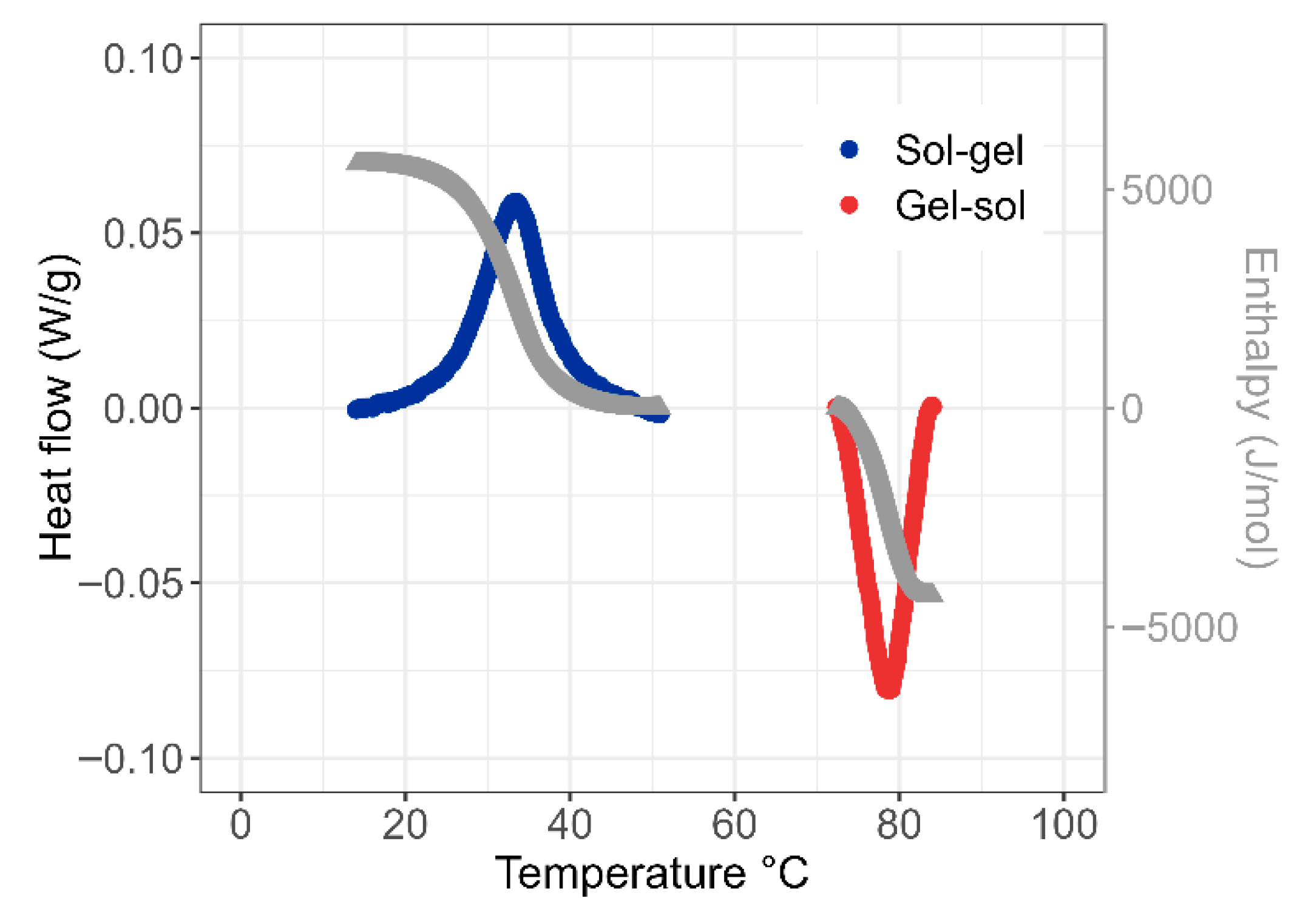
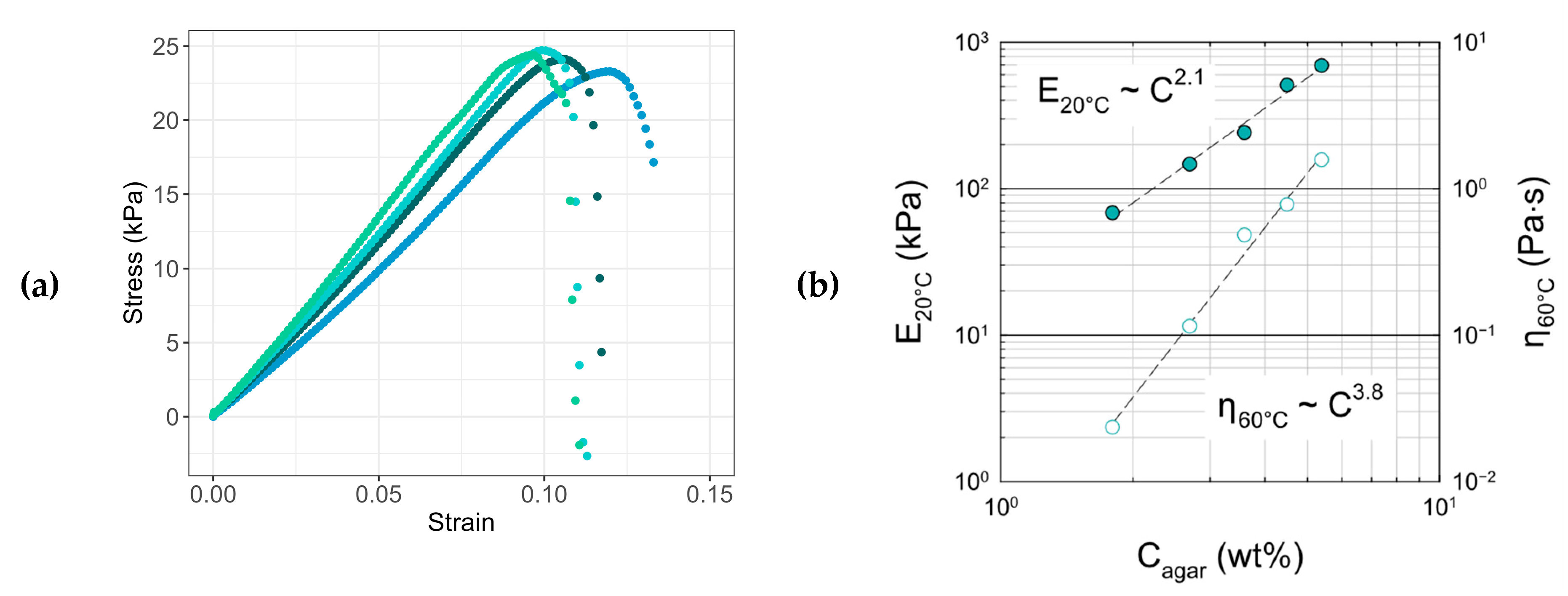
2.1. Agar Hydrogels
- -
- Between 60 and 45 °C, the agar solution behaves as a viscous liquid with a viscous modulus (G″) higher than the elastic modulus (G′). The complex viscosity increases gradually as the temperature decreases according to Andrade’s law with the activation energy for the viscous flow. The temperature at which the viscosity diverges from the Andrade relation marks the beginning of the transition: Tas ≅ 47 °C.
- -
- Between 45 and 30 °C, the moduli increase sharply with a crossover of G′ and G″ marking the sol/gel transition temperature (Tsol-gel ≅ 39 °C), below which the agar formulation exhibits a dominant elastic behavior.
- -
- Below 30 °C, the sample is in the gel state with G′ much higher than G″, and moduli remain practically independent of the frequency. At room temperature, the elastic modulus stabilizes around 80 kPa.
2.2. Water/Glycerol Agar Gels
2.3. Composite Agar Gels
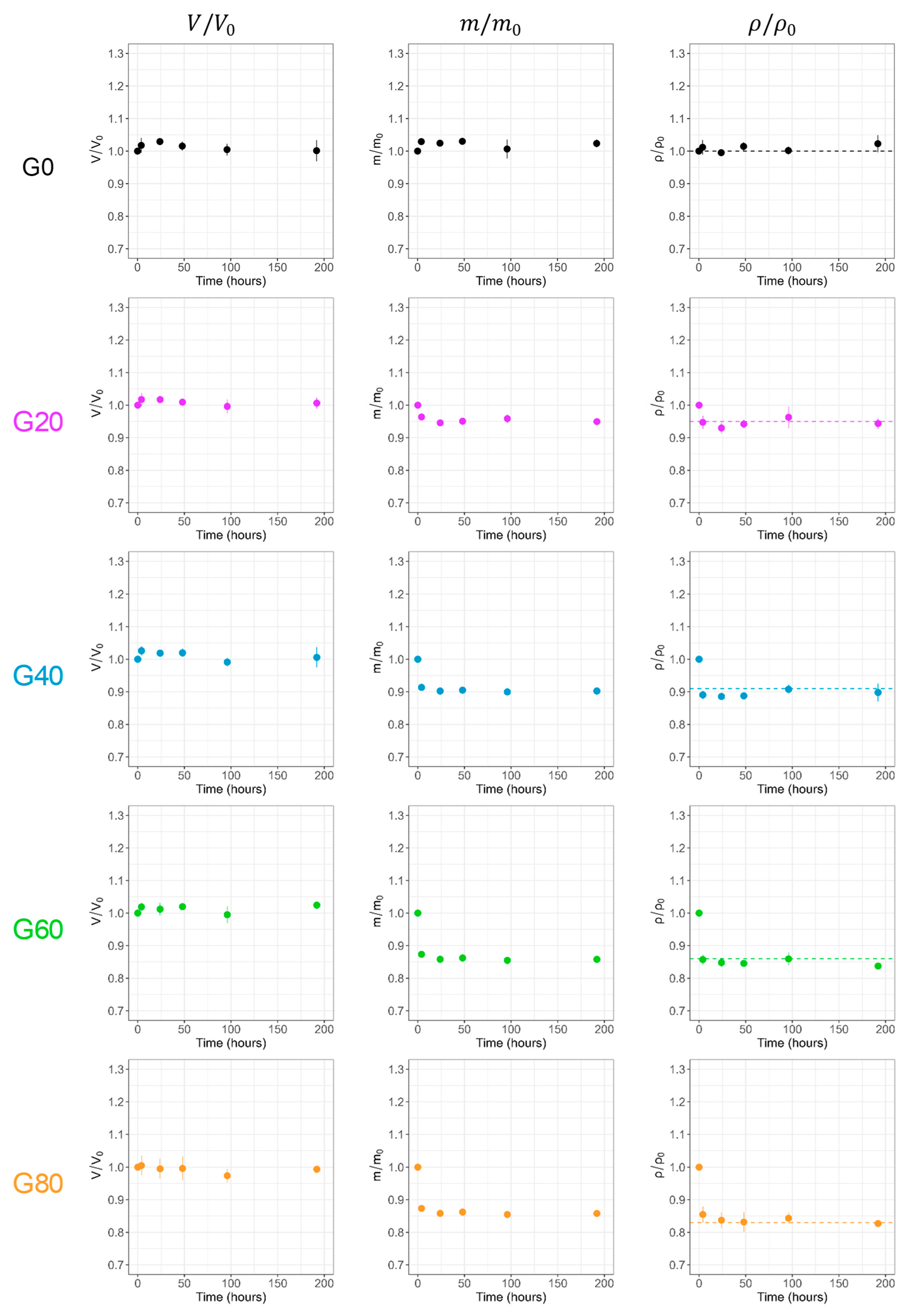
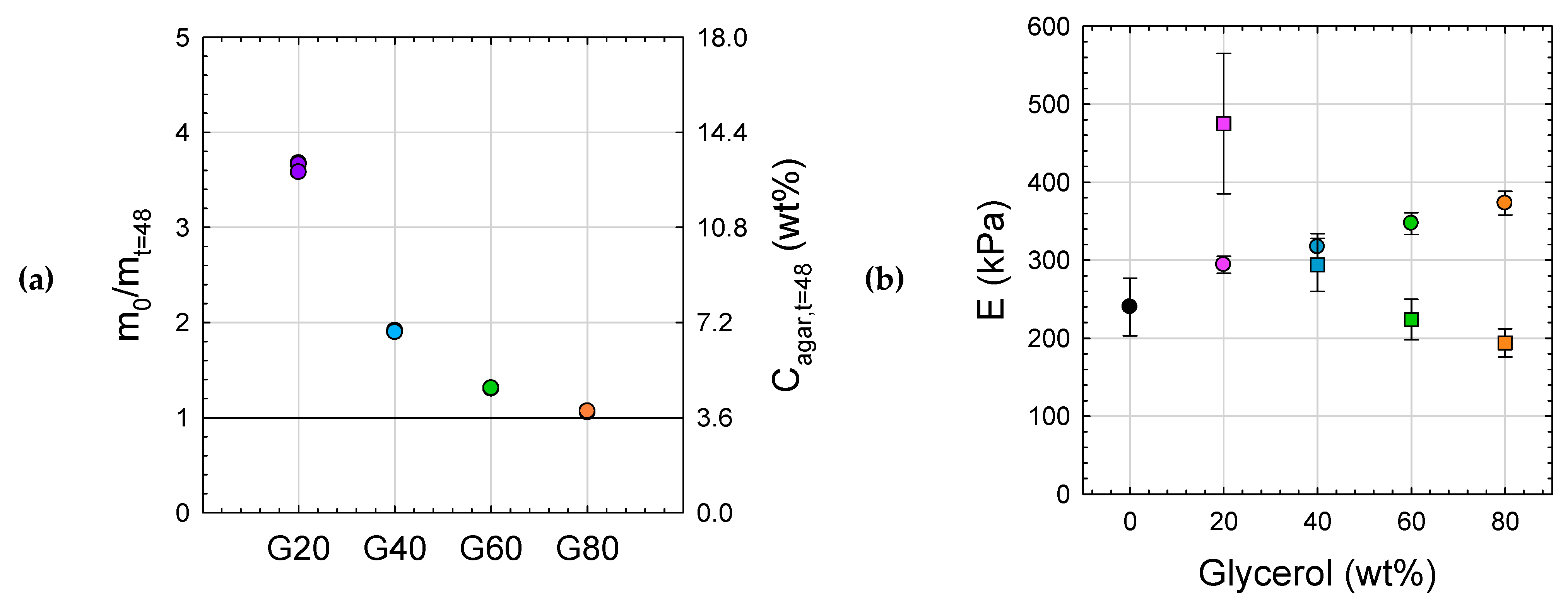
2.4. Stability of Agar Gels
2.4.1. Swelling Behavior of Agar Gels in Water
2.4.2. Swelling Behavior of Agar Gels in Glycerol
2.4.3. Aging of Agar Gels in Air
2.5. Towards Application
2.5.1. Aging in Open Air
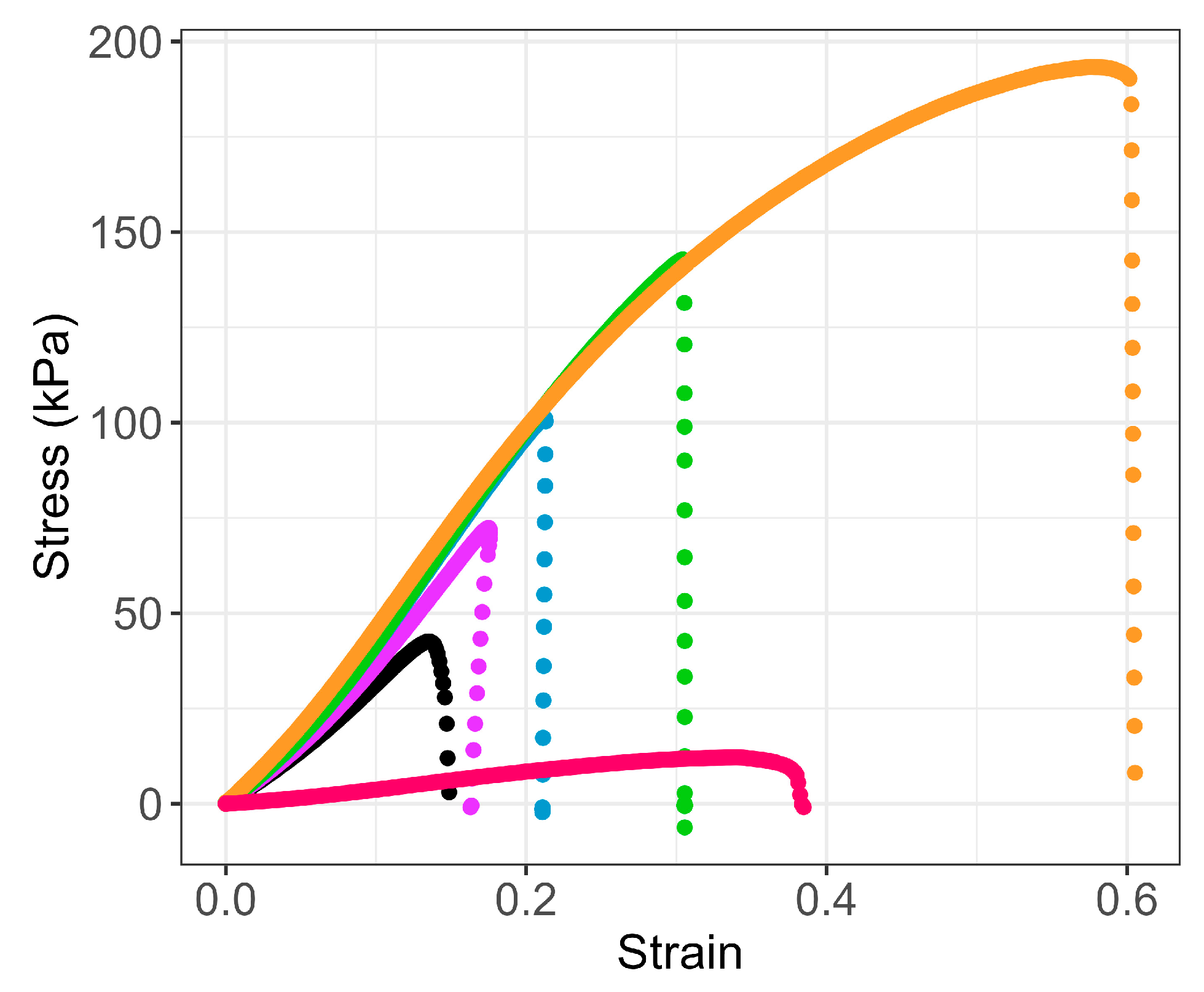
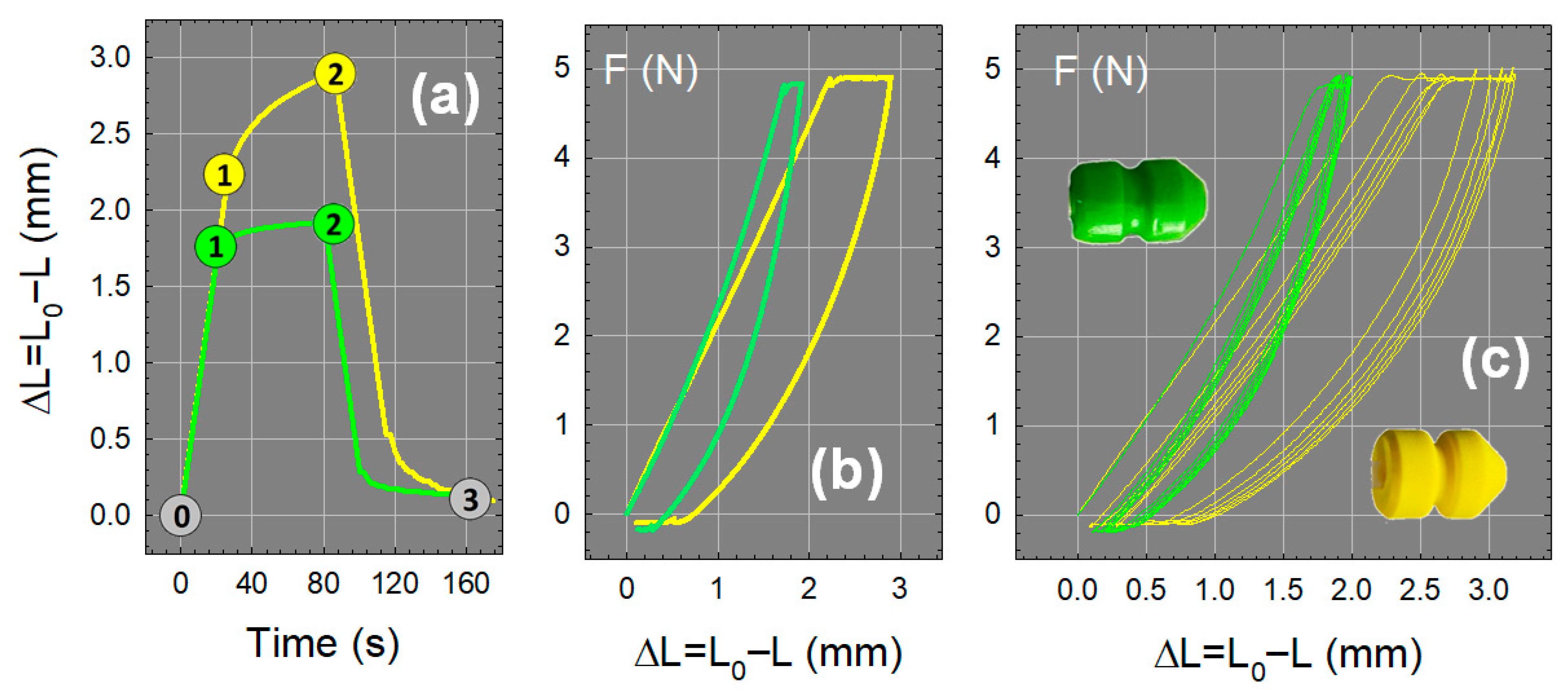
2.5.2. Mechanical Properties: G60T Versus PUR
Tensile Tests
Compression Tests
2.5.3. Compostability
2.5.4. In Vitro Toxicity
3. Conclusions
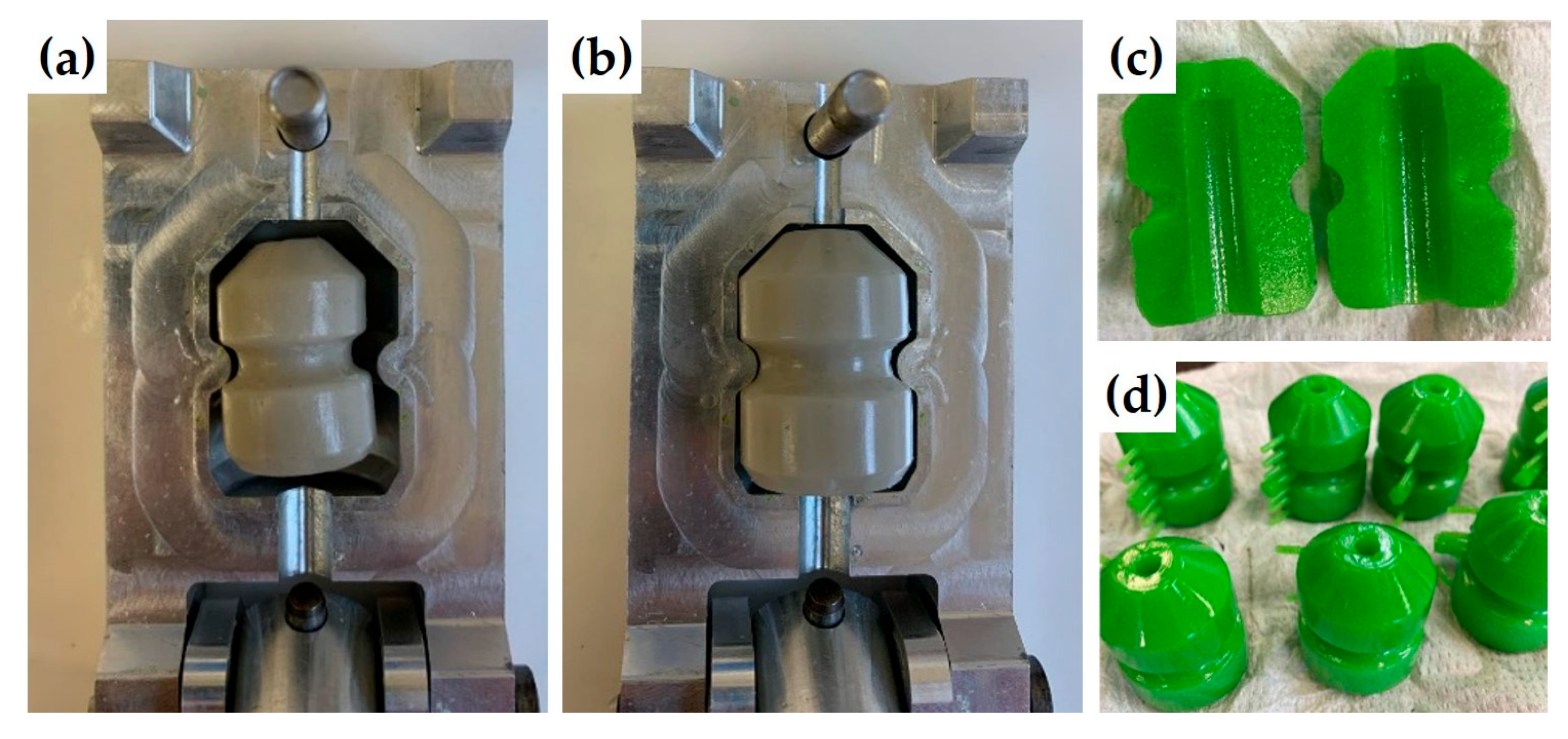
4. Materials and Methods
4.1. Formulations and Gel Formation
4.2. Linear Rheology
4.3. Mechanical Testing
4.3.1. Tensile Tests
4.3.2. Cyclic Compression Tests
4.4. Differential Scanning Calorimetry
4.5. Thermal Gravimetric Analysis (TGA)
4.6. Swelling and Drying Experiments
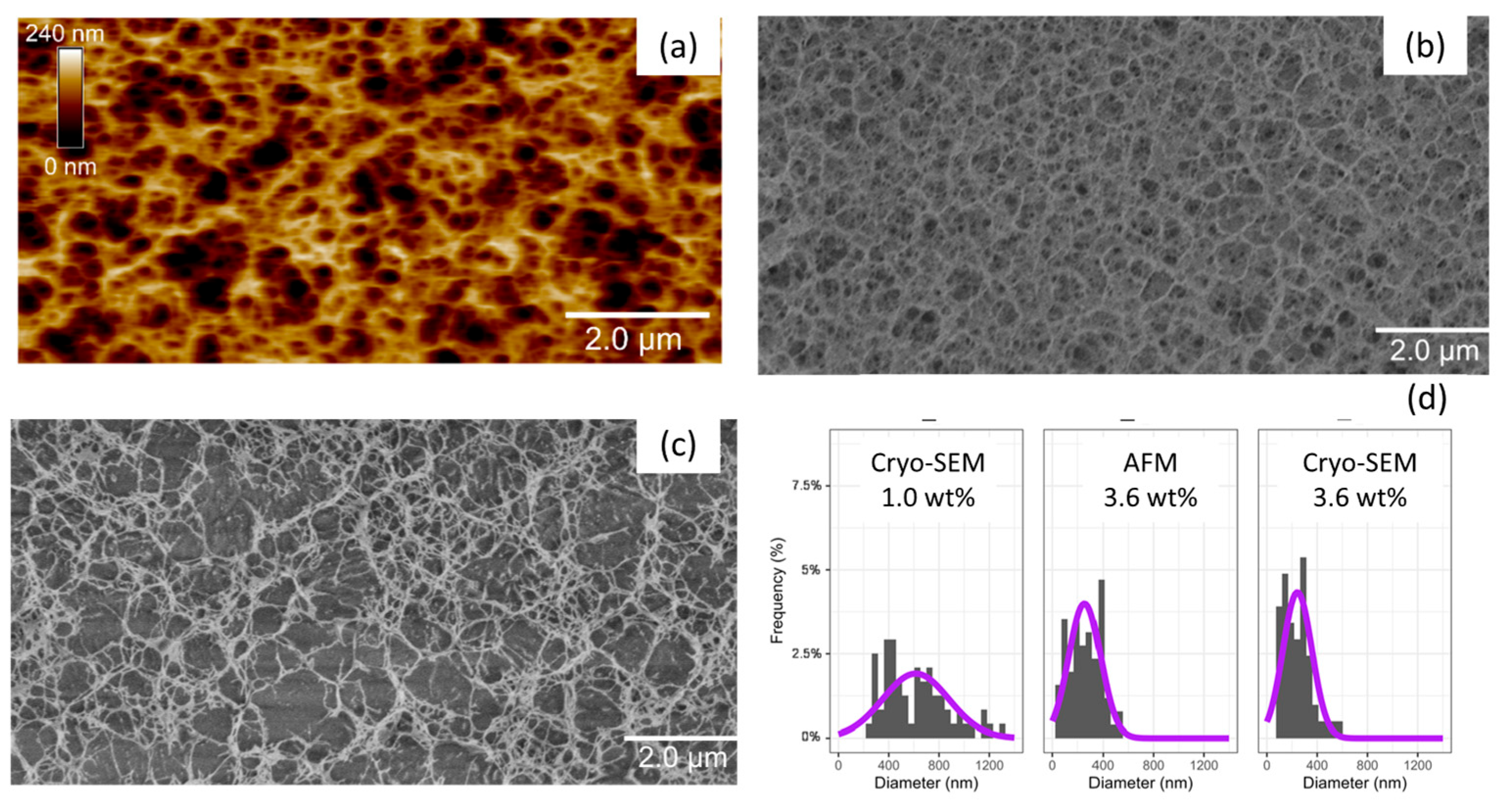
4.7. Microscopic Observations
4.7.1. Atomic Force Microscopy (AFM)
4.7.2. Scanning Electron Cryomicroscopy (Cryo-SEM)
4.8. Compostability
- * A mature compost has a uniform and homogeneous texture that resembles potting soil in which dry and green organic waste have completely decomposed.
4.9. In Vitro Toxicity
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- OCDE. Plastics Flows and Their Impacts on the Environment; OCDE: Paris, France, 2022. [Google Scholar]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Yates, J.; Deeney, M.; Rolker, H.B.; White, H.; Kalamatianou, S.; Kadiyala, S. A systematic scoping review of environmental, food security and health impacts of food system plastics. Nat. Food 2021, 2, 80–87. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Singh, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable materials alternative to petrochemical plastics pollution: A review analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Irie, M. Stimuli-Responsive Poly(N-Isopropylacrylamide)-Photoinduced and Chemical-Induced Phase-Transitions. Adv. Polym. Sci. 1993, 110, 49–65. [Google Scholar]
- Shibayama, M.; Ikkai, F.; Inamoto, S.; Nomura, S.; Han, C.C. pH and salt concentration dependence of the microstructure of poly(N-isopropylacrylamide-co-acrylic acid) gels. J. Chem. Phys. 1996, 105, 4358–4366. [Google Scholar] [CrossRef]
- Barbier, L.; Pipart, P.; Vahdati, M.; Lorthioir, C.; Tran, Y.; Hourdet, D. Injectable hydrogels based on alginates grafted with LCST side-chains of different chemistry. Carbohydr. Polym. 2024, 336, 122126. [Google Scholar] [CrossRef]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Gu, Z.P.; Huang, K.Q.; Luo, Y.; Zhang, L.B.; Kuang, T.R.; Chen, Z.; Liao, G.C. Double network hydrogel for tissue engineering. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for Soft Machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Haraguchi, K.; Farnworth, R.; Ohbayashi, A.; Takehisa, T. Compositional Effects on Mechanical Properties of Nanocomposite Hydrogels Composed of Poly(N,N-dimethylacrylamide) and Clay. Macromolecules 2003, 36, 5732–5741. [Google Scholar] [CrossRef]
- Rose, S.; Dizeux, A.; Narita, T.; Hourdet, D.; Marcellan, A. Time Dependence of Dissipative and Recovery Processes in Nanohybrid Hydrogels. Macromolecules 2013, 46, 4095–4104. [Google Scholar] [CrossRef]
- Guo, H.; Sanson, N.; Hourdet, D.; Marcellan, A. Thermoresponsive Toughening with Crack Bifurcation in Phase-Separated Hydrogels under Isochoric Conditions. Adv. Mater. 2016, 28, 7043. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Q.; Hu, R.; Wang, H.; Newby, B.-m.Z.; Chang, Y.; Zheng, J. Mechanically strong hybrid double network hydrogels with antifouling properties. J. Mater. Chem. B 2015, 3, 5426–5435. [Google Scholar] [CrossRef]
- Zhang, B.; da Silva, M.; Johnston, I.; Aspinall, S.; Cook, M. Poly(vinyl alcohol)-Agar Double Network Hydrogels: Linking Formulation to Mechanical and Rheological Properties. Macromol. Chem. Phys. 2025, e00257. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Le, H.H.; Cui, J.; Jeon, I. Double-Hydrophobic-Coating through Quenching for Hydrogels with Strong Resistance to Both Drying and Swelling. Adv. Sci. 2020, 7, 1903145. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Dou, S.; Sun, J.; Cong, Z.; Jiang, C.; Du, C.; Pu, X.; Hu, W.; Wang, Z.L. Triboelectric-Nanogenerator-Based Soft Energy-Harvesting Skin Enabled by Toughly Bonded Elastomer/Hydrogel Hybrids. ACS Nano 2018, 12, 2818–2826. [Google Scholar] [CrossRef]
- Niu, C.; An, L.; Zhang, H. Mechanically Robust, Antifatigue, and Temperature-Tolerant Nanocomposite Ionogels Enabled by Hydrogen Bonding as Wearable Sensors. ACS Appl. Polym. Mater. 2022, 4, 4189–4198. [Google Scholar] [CrossRef]
- Yu, K.; Gao, Y.; Wang, R.; Wu, L.; Ma, X.; Fang, Y.; Fang, X.; Dou, Q. Ultra-Tough and Highly Stretchable Dual-Crosslinked Eutectogel Based on Coordinated and Non-Coordinated Two Types Deep Eutectic Solvent Mixture. Macromol. Rapid Commun. 2024, 45, 2300557. [Google Scholar] [CrossRef]
- Cebin, A.V.; Bunic, M.; Jaric, A.M.; Seremet, D.; Komes, D. Physicochemical and Sensory Stability Evaluation of Gummy Candies Fortified with Mountain Germander Extract and Prebiotics. Polymers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Bulca, E.N.; Akdeniz, E.; Mutlu, Z.; Tireki, S.; Karimidastjerd, A.; Toker, O.S. Influence of various corn syrup types on the quality and sensory properties of gelatin-based jelly confectionery. J. Food Meas. Charact. 2024, 18, 8408–8422. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Stabilization of gelatin gels by sugars and polyols. Food Hydrocoll. 1986, 1, 163–175. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.; Wang, J.H.; Li, T.Z.; Zhou, X.H.; Gan, T.S.; Handschuh-Wang, S.; Zhou, X.C. Rational Fabrication of Anti-Freezing, Non-Drying Tough Organohydrogels by One-Pot Solvent Displacement. Angew. Chem.-Int. Ed. 2018, 57, 6568–6571. [Google Scholar] [CrossRef]
- Chen, H.J.; Lee, P.Y.; Chen, C.Y.; Huang, S.L.; Huang, B.W.; Dai, F.J.; Chau, C.F.; Chen, C.S.; Lin, Y.S. Moisture retention of glycerin solutions with various concentrations: A comparative study. Sci. Rep. 2022, 12, 10232. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Lee, Y.S.; Varma, A.V.R.; Gupta, T.; Wadu, R.R.M.; Jeon, I. Tardigrade-inspired extremotolerant glycerogels. NPG Asia Mater. 2023, 15, 22. [Google Scholar] [CrossRef]
- Sher, M.; Shah, L.A.; Fu, J.; Yoo, H.-M.; Ullah, R.; Ibrahim, M.A. Facile fabrication of stretchable, anti-freezing, and stable organohydrogels for strain sensing at subzero temperatures. Mater. Adv. 2024, 5, 8164–8176. [Google Scholar] [CrossRef]
- Richard, F.; Hermans, J.J.; Angelova, L. Rigid Solvent-Gels in Paper Conservation: A New Approach to Sticky Problems. J. Pap. Conserv. 2024, 25, 86–106. [Google Scholar] [CrossRef]
- Wei, C.Y.; Li, K.; Lin, F.; Mi, S.L.; Dong, L.A.; Song, Y.; Zhang, T. High stability PAM/glycerol hydrogels and its applications in lenses and actuators. J. Appl. Polym. Sci. 2023, 140, e54322. [Google Scholar] [CrossRef]
- Efe, N.; Dawson, P. A review: Sugar-based confectionery and the importance of ingredients. Eur. J. Agric. Food Sci. 2022, 4, 1–8. [Google Scholar] [CrossRef]
- Baumgartner, M.; Hartmann, F.; Drack, M.; Preninger, D.; Wirthl, D.; Gerstmayr, R.; Lehner, L.; Mao, G.Y.; Pruckner, R.; Demchyshyn, S.; et al. Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat. Mater. 2020, 19, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.; Ibarra, D.; Triana, J.F.; Martínez-Soto, B.; Faúndez, M.; Vasco, D.A.; Gordillo, L.; Herrera, F.; García-Herrera, C.; Garmulewicz, A. Agar Biopolymer Films for Biodegradable Packaging: A Reference Dataset for Exploring the Limits of Mechanical Performance. Materials 2022, 15, 3954. [Google Scholar] [CrossRef] [PubMed]
- Armisén, R.; Gaiatas, F. 4—Agar. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: London, UK, 2009; pp. 82–107. [Google Scholar]
- Nussinovitch, A. Agar. In Hydrocolloid Applications: Gum Technology in the Food and Other Industries; Nussinovitch, A., Ed.; Springer: Boston, MA, USA, 1997; pp. 1–18. [Google Scholar]
- Djabourov, M.; Nishinari, K.; Ross-Murphy, S.B. Physical Gels from Biological and Synthetic Polymers; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Feke, G.T.; Prins, W. Spinodal Phase Separation in a Macromolecular Sol → Gel Transition. Macromolecules 1974, 7, 527–530. [Google Scholar] [CrossRef]
- Xiong, J.-Y.; Narayanan, J.; Liu, X.-Y.; Chong, T.K.; Chen, S.B.; Chung, T.-S. Topology Evolution and Gelation Mechanism of Agarose Gel. J. Phys. Chem. B 2005, 109, 5638–5643. [Google Scholar] [CrossRef]
- Guenet, J.-M.; Brûlet, A.; Rochas, C. Agarose chain conformation in the sol state by neutron scattering. Int. J. Biol. Macromol. 1993, 15, 131–132. [Google Scholar] [CrossRef]
- Arnott, S.; Fulmer, A.; Scott, W.E.; Dea, I.C.M.; Moorhouse, R.; Rees, D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef]
- Hayashi, A.; Kanzaki, T. Swelling of agarose gel and its related changes. Food Hydrocoll. 1987, 1, 317–325. [Google Scholar] [CrossRef]
- Nishinari, K.; Watase, M. Effects of Polyhydric Alcohols on Thermal and Rheological Properties of Polysaccharide Gels. Agric. Biol. Chem. 1987, 51, 3231–3238. [Google Scholar] [CrossRef]
- Ramzi, M.; Rochas, C.; Guenet, J.-M. Phase behavior of agarose in binary solvents. Macromolecules 1996, 29, 4668–4674. [Google Scholar] [CrossRef]
- Holland, S.; Tuck, C.; Foster, T. Fluid Gels: A New Feedstock for High Viscosity Jetting. Food Biophys. 2018, 13, 175–185. [Google Scholar] [CrossRef]
- Rochas, C. Calorimetric study of galactans. Food Hydrocoll. 1987, 1, 215–225. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K.; Clark, A.; Ross-Murphy, S. Differential scanning calorimetry, rheology, X-ray, and NMR of very concentrated agarose gels. Macromolecules 1989, 22, 1196–1201. [Google Scholar] [CrossRef]
- Clark, A.H.; Ross-Murphy, S.B. The concentration dependence of biopolymer gel modulus. Br. Polym. J. 1985, 17, 164–168. [Google Scholar] [CrossRef]
- Mohammed, Z.H.; Hember, M.; Richardson, R.; Morris, E. Kinetic and equilibrium processes in the formation and melting of agarose gels. Carbohydr. Polym. 1998, 36, 15–26. [Google Scholar] [CrossRef]
- Lahrech, K.; Safouane, A.; Peyrellasse, J. Sol state formation and melting of agar gels rheological study. Phys. A Stat. Mech. Its Appl. 2005, 358, 205–211. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New insight into agarose gel mechanical properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Rahbani, J.; Behzad, A.R.; Khashab, N.M.; Al-Ghoul, M. Characterization of internal structure of hydrated agar and gelatin matrices by cryo-SEM. Electrophoresis 2013, 34, 405–408. [Google Scholar] [CrossRef]
- Roux, D.C.; Caton, F.; Jeacomine, I.; Maîtrejean, G.; Rinaudo, M. Reversibility in the Physical Properties of Agarose Gels following an Exchange in Solvent and Non-Solvent. Polymers 2024, 16, 811. [Google Scholar] [CrossRef]
- Shimizu, S.; Matubayasi, N. Gelation: The role of sugars and polyols on gelatin and agarose. J. Phys. Chem. B 2014, 118, 13210–13216. [Google Scholar] [CrossRef]
- Normand, V.; Aymard, P.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Frith, W.J. Effect of sucrose on agarose gels mechanical behaviour. Carbohydr. Polym. 2003, 54, 83–95. [Google Scholar] [CrossRef]
- D’Errico, G.; Ortona, O.; Capuano, F.; Vitagliano, V. Diffusion coefficients for the binary system glycerol+ water at 25 C. A velocity correlation study. J. Chem. Eng. Data 2004, 49, 1665–1670. [Google Scholar] [CrossRef]
- Nakagawa, H.; Oyama, T. Molecular basis of water activity in glycerol–water mixtures. Front. Chem. 2019, 7, 731. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P.; Soede, N.; Kemp, B. Uterine activity, sperm transport, and the role of boar stimuli around insemination in sows. Theriogenology 2005, 63, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Willenburg, K.; Miller, G.; Rodriguez-Zas, S.; Knox, R. Influence of hormone supplementation to extended semen on artificial insemination, uterine contractions, establishment of a sperm reservoir, and fertility in swine. J. Anim. Sci. 2003, 81, 821–829. [Google Scholar] [CrossRef][Green Version]
- EN 13432:2000/AC:2005; Packaging—Requirements for Packaging Recoverable Through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Committee for standardization: Brussels, Belgium, 2005.[Green Version]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. [Google Scholar] [CrossRef]



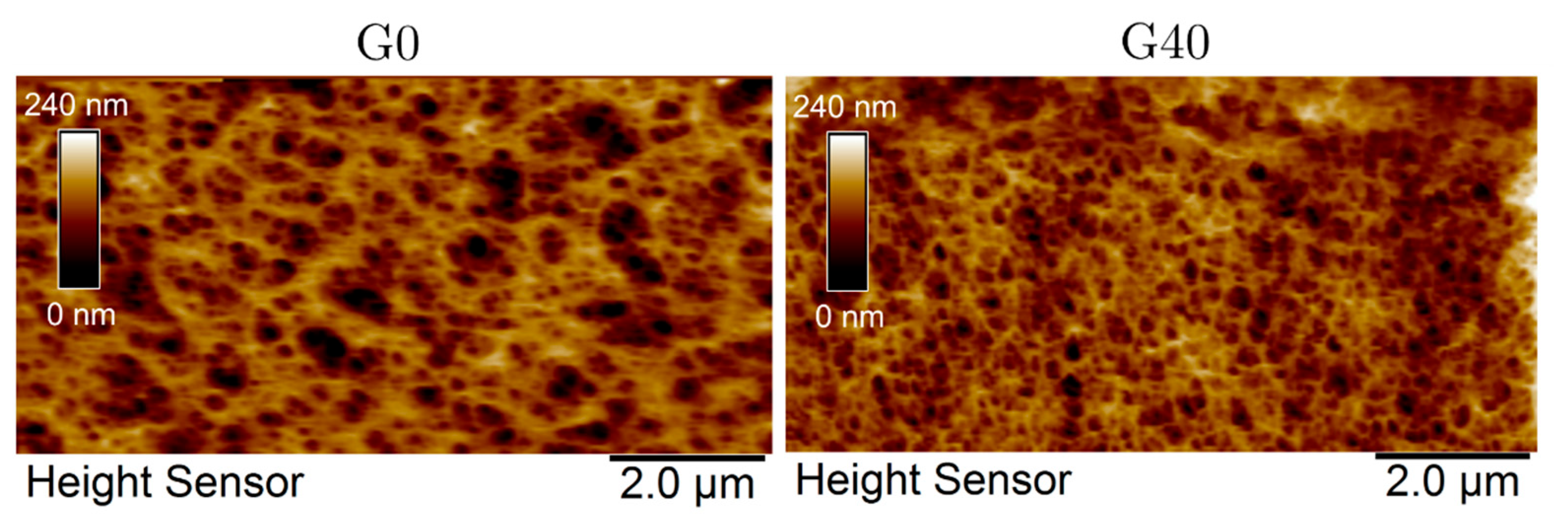
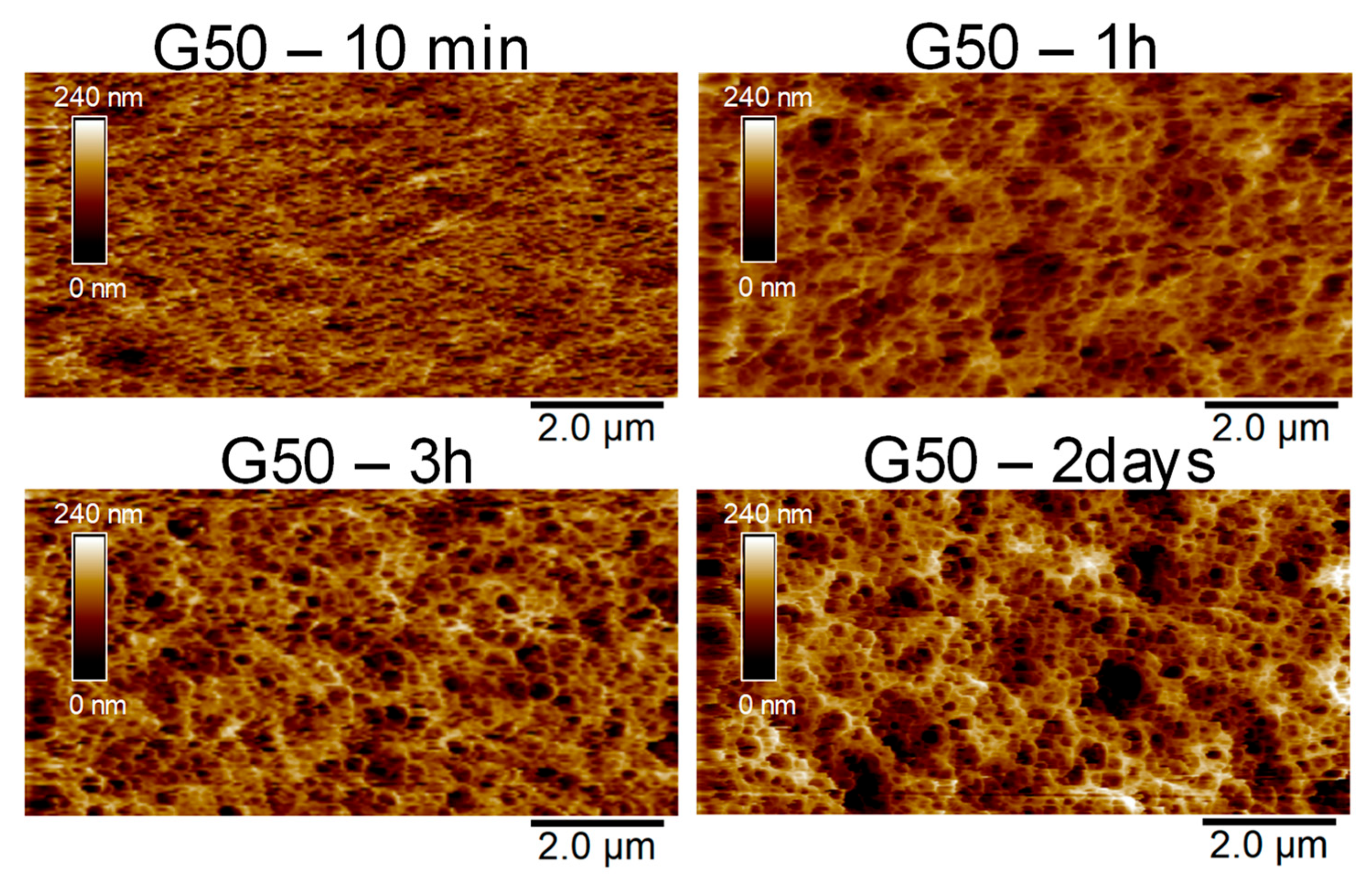

| Sample | G0 | G20 | G40 | G60 | G80 | G100 |
|---|---|---|---|---|---|---|
| ϕagar | 0.035 | 0.037 | 0.039 | 0.041 | 0.043 | 0.045 |
| ηsp | 930 ± 360 | 690 ± 145 | 685 ± 125 | 740 ± 140 | 590 ± 120 | 290 ± 6 |
| Gel | Without Talc | With Talc | |||||
|---|---|---|---|---|---|---|---|
| ϕagar | G’ (kPa) | E (kPa) | εR | ϕagar | E (kPa) | εR | |
| G0 | 0.035 | 81 | 240 ± 37 | 0.10 ± 0.02 | 0.0380 | 283 ±12 | 0.10 ± 0.02 |
| G20 | 0.037 | 93 | 294 ± 11 | 0.13 ± 0.04 | 0.040 | 399 ± 81 | 0.13 ± 0.02 |
| G40 | 0.039 | 103 | 317 ± 17 | 0.18 ± 0.03 | 0.0415 | 368 ± 93 | 0.13 ± 0.01 |
| G60 | 0.041 | 80 | 347 ± 14 | 0.27 ± 0.03 | 0.0434 | 443 ± 36 | 0.22 ± 0.06 |
| G80 | 0.043 | 46 | 373 ± 15 | 0.44 ± 0.08 | 0.0453 | 392 ± 85 | 0.31 ± 0.10 |
| G100 | 0.045 | 10 | 27 ± 5 | 0.29 ± 0.06 | |||
| Gel | Preparation State | After 13 Days in H2O | After 13 Days in Glycerol | |||
|---|---|---|---|---|---|---|
| E0 (kPa) | εR | E (kPa) | εR | E (kPa) | εR | |
| G0 | 240 ± 37 | 0.10 ± 0.02 | 148 ± 7 | 0.08 ± 0.05 | 51 ± 16 | 0.82 ± 0.04 |
| G40 | 317 ± 17 | 0.18 ± 0.03 | 205 ± 48 | 0.11 ± 0.03 | 56 ± 18 | 0.64 ± 0.29 |
| G60 | 347 ± 14 | 0.27 ± 0.03 | 221 ± 21 | 0.08 ± 0.02 | 44 ± 8 | 0.66 ± 0.16 |
| G80 | 373 ± 15 | 0.44 ± 0.08 | 226 ± 35 | 0.06 ± 0.02 | 35 ± 4 | 0.61 ± 0.17 |
| Gx | Without Talc | GxT | With Talc | ||
|---|---|---|---|---|---|
| E (kPa) | εR | E (kPa) | ɛR | ||
| G20 | 475 ± 90 | 0.58 | G20T | 1862 ± 126 | 0.81 |
| G40 | 294 ± 34 | 0.45 | G40T | 477 ± 66 | 0.63 |
| G60 | 224 ± 26 | 0.30 | G60 T | 334 ± 9 | 0.62 |
| G80 | 194 ± 18 | 0.30 | G80T | 301 ± 35 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipart, P.; Bresson, B.; Marcellan, A.; Merland, T.; Tran, Y.; Gorges, J.-C.; Carion, O.; Hourdet, D. Formulation of Sustainable Materials from Agar/Glycerol/Water Gels: An Alternative to Polyurethane Foams in Single-Use Applications. Gels 2025, 11, 842. https://doi.org/10.3390/gels11100842
Pipart P, Bresson B, Marcellan A, Merland T, Tran Y, Gorges J-C, Carion O, Hourdet D. Formulation of Sustainable Materials from Agar/Glycerol/Water Gels: An Alternative to Polyurethane Foams in Single-Use Applications. Gels. 2025; 11(10):842. https://doi.org/10.3390/gels11100842
Chicago/Turabian StylePipart, Perrine, Bruno Bresson, Alba Marcellan, Théo Merland, Yvette Tran, Jean-Charles Gorges, Olivier Carion, and Dominique Hourdet. 2025. "Formulation of Sustainable Materials from Agar/Glycerol/Water Gels: An Alternative to Polyurethane Foams in Single-Use Applications" Gels 11, no. 10: 842. https://doi.org/10.3390/gels11100842
APA StylePipart, P., Bresson, B., Marcellan, A., Merland, T., Tran, Y., Gorges, J.-C., Carion, O., & Hourdet, D. (2025). Formulation of Sustainable Materials from Agar/Glycerol/Water Gels: An Alternative to Polyurethane Foams in Single-Use Applications. Gels, 11(10), 842. https://doi.org/10.3390/gels11100842







