Anatase-Free Nanosized Hierarchical Titanosilicate TS-1 Synthesis via Nitric Acid-Catalyzed Gel Preparation
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Titanosilicate Catalysts
2.1.1. Structural Properties of Titanosilicates
2.1.2. Morphology of Titanosilicates
2.1.3. Coordination of Ti Atoms
2.1.4. Textural Properties
2.1.5. FTIR Study of Concentration of Brønsted and Lewis Acid Sites and Hydroxyl Group Bands
2.1.6. Catalytic Performance in Epoxidation of Cyclohexene
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Titanosilicates TS-1 via Gels Formation
4.2. Catalyst Characterization
4.3. Catalytic Performance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPPO | Hydrogen Peroxide to Propylene Oxide |
| Cy | Cyclohexene |
| epoxy | epoxycyclohexane |
| diol | cyclohexane-1,2-diol |
| enon | 2-cyclohexen-1-one |
| enol | 2-cyclohexen-1-ol |
| LAS | Lewis acid sites |
| BAS | Brønsted acid sites |
| FTIR | Fourier transform infrared spectroscopy |
| DR UV–vis spectroscopy | Ultra-violet–visible diffuse reflectance spectroscopy |
| BJH method | Barrett–Joyner–Halenda method |
References
- Millini, R.; Previde Massara, E.; Perego, G.; Bellussi, G. Framework Composition of Titanium Silicalite-1. J. Catal. 1992, 137, 497–503. [Google Scholar] [CrossRef]
- Schmidt, F.; Bernhard, M.; Morell, H.; Pascaly, M. HPPO Process Technology A Novel Route to Propylene Oxide without Coproducts. Chim. Oggi/Chem. Today 2014, 32, 31–35. [Google Scholar]
- Our Proprietary Catalyst Technology. Available online: https://versalis.eni.com/en-IT/portfolio/technologies-licensing/catalyst-technology.html (accessed on 16 July 2025).
- Perego, C.; Carati, A.; Ingallina, P.; Mantegazza, M.A.; Bellussi, G. Production of Titanium Containing Molecular Sieves and Their Application in Catalysis. Appl. Catal. A Gen. 2001, 221, 63–72. [Google Scholar] [CrossRef]
- Luan, H.; Xu, C.; Wu, Q.; Xiao, F.-S. Recent Advances in the Synthesis of TS-1 Zeolite. Front. Chem. 2022, 10, 1080554. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized Zeolites: Quo Vadis? Comptes Rendus Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef]
- Rodionova, L.I.; Knyazeva, E.E.; Konnov, S.V.; Ivanova, I.I. Application of Nanosized Zeolites in Petroleum Chemistry: Synthesis and Catalytic Properties (Review). Pet. Chem. 2019, 59, 455–470. [Google Scholar] [CrossRef]
- Smeets, V.; Gaigneaux, E.M.; Debecker, D.P. Titanosilicate Epoxidation Catalysts: A Review of Challenges and Opportunities. ChemCatChem 2022, 14, e202101132. [Google Scholar] [CrossRef]
- Fu, G.; Dib, E.; Lang, Q.; Zhao, H.; Wang, S.; Ding, R.; Yang, X.; Valtchev, V. Acidic Medium Synthesis of Zeolites—An Avenue to Control the Structure-Directing Power of Organic Templates. Dalton Trans. 2022, 51, 11499–11506. [Google Scholar] [CrossRef]
- Pope, E.J.A.; Mackenzie, J.D. Sol-Gel Processing of Silica. J. Non-Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Tsikoza, L.T.; Shikina, N.V.; Zarytova, V.F.; Zinoviev, V.V.; Zagrebelnyi, S.N. Synthesis and Stabilization of Nano-Sized Titanium Dioxide. Russ. Chem. Rev. 2009, 78, 873–885. [Google Scholar] [CrossRef]
- Antonenko, M.V.; Ogurtsova, Y.N.; Strokova, V.V.; Gubareva, E.N. The Effect of Titanium Dioxide Sol Stabilizer on the Properties of Photocatalytic Composite Material. In Innovations and Technologies in Construction; Lecture Notes in Civil Engineering ; Klyuev, S.V., Lesovik, V.S., Vatin, N.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 95, pp. 16–22. ISBN 978-3-030-54651-9. [Google Scholar]
- Watson, S.S.; Beydoun, D.; Scott, J.A.; Amal, R. The Effect of Preparation Method on the Photoactivity of Crystalline Titanium Dioxide Particles. Chem. Eng. J. 2003, 95, 213–220. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Kalashnikova, G.O.; Shefer, K.I.; Mel’gunova, E.A.; Panchenko, V.N.; Nikolaev, A.I.; Gil, A. Effect of the Acid Activation on a Layered Titanosilicate AM-4: The Fine-Tuning of Structural and Physicochemical Properties. Appl. Clay Sci. 2020, 186, 105445. [Google Scholar] [CrossRef]
- Llabrés i Xamena, F.X.; Calza, P.; Lamberti, C.; Prestipino, C.; Damin, A.; Bordiga, S.; Pelizzetti, E.; Zecchina, A. Enhancement of the ETS-10 Titanosilicate Activity in the Shape-Selective Photocatalytic Degradation of Large Aromatic Molecules by Controlled Defect Production. J. Am. Chem. Soc. 2003, 125, 2264–2271. [Google Scholar] [CrossRef]
- Lee, S.L.; Nur, H.; Wei, S.C. Effect of Acid Treatment on Silica-Titania Aerogel as Oxidative-Acidic Bifunctional Catalyst. Appl. Mech. Mater. 2012, 110–116, 457–464. [Google Scholar] [CrossRef]
- Xiong, G.; Jia, Q.; Cao, Y.; Liu, L.; Guo, Z. The Effect of Acid Treatment on the Active Sites and Reaction Intermediates of the Low-Cost TS-1 in Propylene Epoxidation. RSC Adv. 2017, 7, 24046–24054. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Wu, H.; Liu, Y.; Li, X.; Jiang, J.; He, M.; Wu, P. Postsynthesis of Mesoporous MOR-Type Titanosilicate and Its Unique Catalytic Properties in Liquid-Phase Oxidations. J. Catal. 2011, 281, 263–272. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Q.; Xu, B.; Lin, K.; Deng, M.; Lu, X.; Ma, R.; Fu, Y.; Zhu, W. Ti-MWW Synthesis via Acid-Modulated Isomorphous Substitution of ERB-1 for Efficient Ethylene Oxidative Hydration to Ethylene Glycol. J. Catal. 2023, 425, 105–115. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Bai, R.; Gao, S.; Feng, Z.; Zhang, Q.; Yu, J. Amino Acid-Assisted Synthesis of TS-1 Zeolites Containing Highly Catalytically Active TiO6 Species. Chin. J. Catal. 2021, 42, 2189–2196. [Google Scholar] [CrossRef]
- Li, M.; Shen, X.; Liu, M.; Lu, J. Synthesis TS-1 Nanozelites via L-Lysine Assisted Route for Hydroxylation of Benzene. Mol. Catal. 2021, 513, 111779. [Google Scholar] [CrossRef]
- Tyablikov, I.A. Synthesis and Physicochemical Properties of Titanosilicate with MFI Structure as a Catalyst for Epoxidation of Alkenes. Ph.D. Thesis, Lomonosov Moscow State University, Moscow, Russia, 2019. [Google Scholar]
- Grieneisen, J.L.; Kessler, H.; Fache, E.; Le Govic, A.M. Synthesis of TS-1 in Fluoride Medium. A New Way to a Cheap and Efficient Catalyst for Phenol Hydroxylation. Microporous Mesoporous Mater. 2000, 37, 379–386. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Song, Y.; Xu, W.; Meng, X.; Li, J. High-Efficiency Synthesis of Enhanced-Titanium and Anatase-Free TS-1 Zeolite by Using a Crystallization Modifier. Inorg. Chem. Front. 2021, 8, 3077–3084. [Google Scholar] [CrossRef]
- Perera, A.S.; Trogadas, P.; Nigra, M.M.; Yu, H.; Coppens, M.-O. Optimization of Mesoporous Titanosilicate Catalysts for Cyclohexene Epoxidation via Statistically Guided Synthesis. J. Mater. Sci. 2018, 53, 7279–7293. [Google Scholar] [CrossRef]
- Geobaldo, F.; Bordiga, S.; Zecchina, A.; Giamello, E.; Leofanti, G.; Petrini, G. DRS UV-Vis and EPR Spectroscopy of Hydroperoxo and Superoxo Complexes in Titanium Silicalite. Catal. Lett. 1992, 16, 109–115. [Google Scholar] [CrossRef]
- Serrano, D.P.; Sanz, R.; Pizarro, P.; Moreno, I. Tailoring the Properties of Hierarchical TS-1 Zeolite Synthesized from Silanized Protozeolitic Units. Appl. Catal. A Gen. 2012, 435–436, 32–42. [Google Scholar] [CrossRef]
- Guo, T.; Wang, B.; Xue, C.; Liu, X.; Lin, C.; Xie, X.; Luo, Y.; Liao, W.; Shu, X. In Situ Tailoring the Crystalline Defects of Titanium Silicalite-1 (TS-1) to Improve the 1-Butene Epoxidation Performance. Ind. Eng. Chem. Res. 2024, 63, 11872–11880. [Google Scholar] [CrossRef]
- Li, N.; Chen, R.; Miao, J.; Zhou, P.; Yu, H.-B.; Chen, T.-H. Synthesis of Single Crystal-like Hierarchically Mesoporous Titanosilicate Ti-SBA-1. Chin. Chem. Lett. 2015, 26, 1269–1272. [Google Scholar] [CrossRef]
- Carati, A.; Flego, C.; Previde Massara, E.; Millini, R.; Carluccio, L.; Parker, W.O., Jr.; Bellussi, G. Stability of Ti in MFI and Beta structures: A comparative study. Microporous Mesoporous Mater. 1999, 30, 137–144. [Google Scholar] [CrossRef]
- Hou, W.; Lin, K.; Zhang, X.; Xu, B.; Wang, Y.; Lu, X.; Gao, Y.; Ma, R.; Fu, Y.; Zhu, W. Highly Stable and Selective Pt/TS-1 Catalysts for the Efficient Nonoxidative Dehydrogenation of Propane. Chem. Eng. J. 2023, 474, 145648. [Google Scholar] [CrossRef]
- Martínez, C.; Corma, A. Inorganic Molecular Sieves: Preparation, Modification and Industrial Application in Catalytic Processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-Modified Zeolites: Preparation, Characterization, and Applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef]
- Tekla, J.; Tarach, K.A.; Olejniczak, Z.; Girman, V.; Góra-Marek, K. Effective Hierarchization of TS-1 and Its Catalytic Performance in Cyclohexene Epoxidation. Microporous Mesoporous Mater. 2016, 233, 16–25. [Google Scholar] [CrossRef]
- Tang, Z.; Yu, Y.; Liu, W.; Chen, Z.; Wang, R.; Liu, H.; Wu, H.; Liu, Y.; He, M. Deboronation-Assisted Construction of Defective Ti(OSi)3 OH Species in MWW-Type Titanosilicate and Their Enhanced Catalytic Performance. Catal. Sci. Technol. 2020, 10, 2905–2915. [Google Scholar] [CrossRef]
- Přech, J. Catalytic Performance of Advanced Titanosilicate Selective Oxidation Catalysts—A Review. Catal. Rev. 2018, 60, 71–131. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Ahn, W.-S.; Ryoo, R. MFI Titanosilicate Nanosheets with Single-Unit-Cell Thickness as an Oxidation Catalyst Using Peroxides. ACS Catal. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Tatsumi, T. Metal-Substituted Zeolites as Heterogeneous Oxidation Catalysts. In Modern Heterogeneous Oxidation Catalysis: Design, Reactions and Characterization; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; Volume 4. [Google Scholar]
- Shamzhy, M.; Přech, J.; Zhang, J.; Ruaux, V.; El-Siblani, H.; Mintova, S. Quantification of Lewis Acid Sites in 3D and 2D TS-1 Zeolites: FTIR Spectroscopic Study. Catal. Today 2020, 345, 80–87. [Google Scholar] [CrossRef]

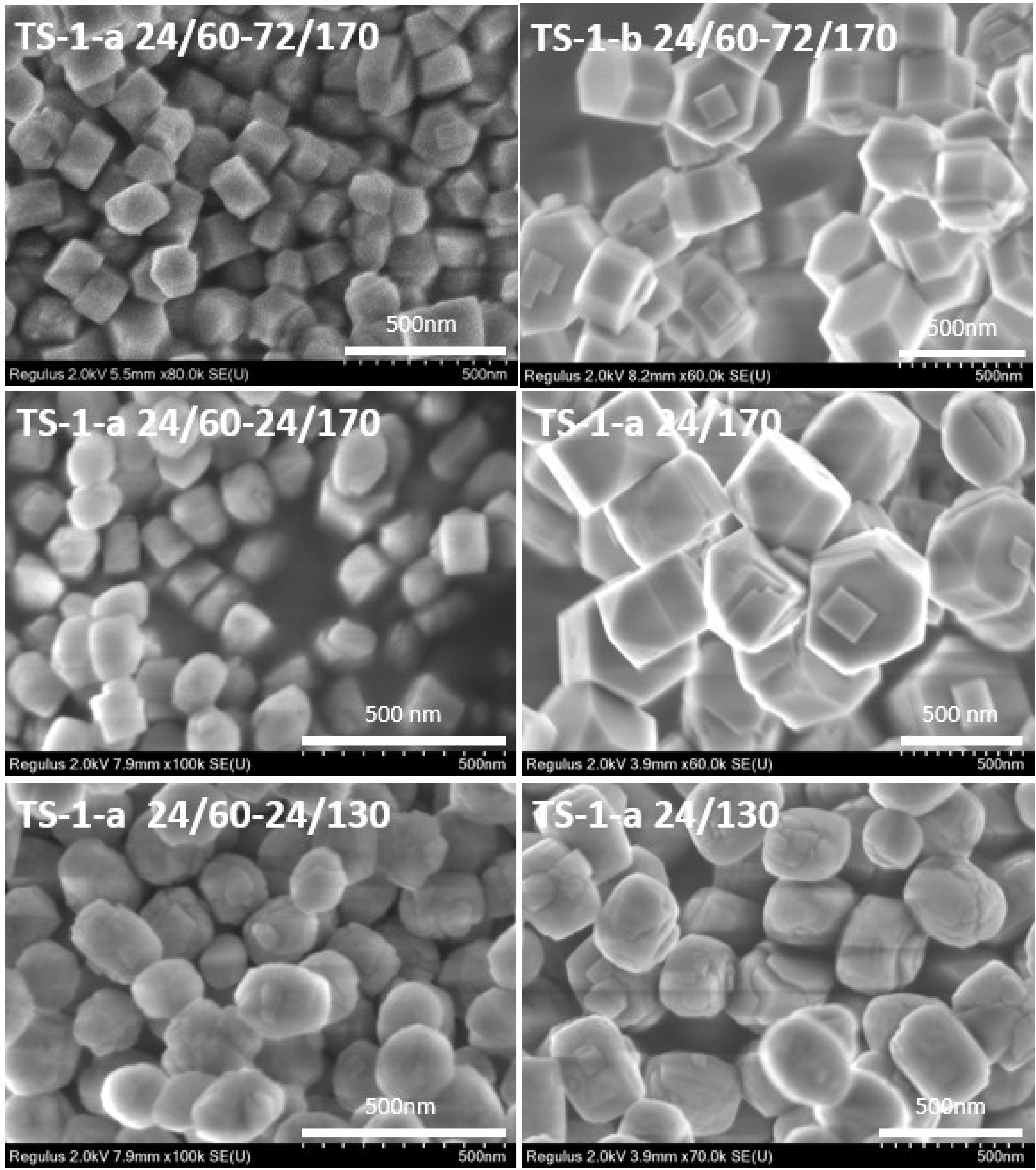

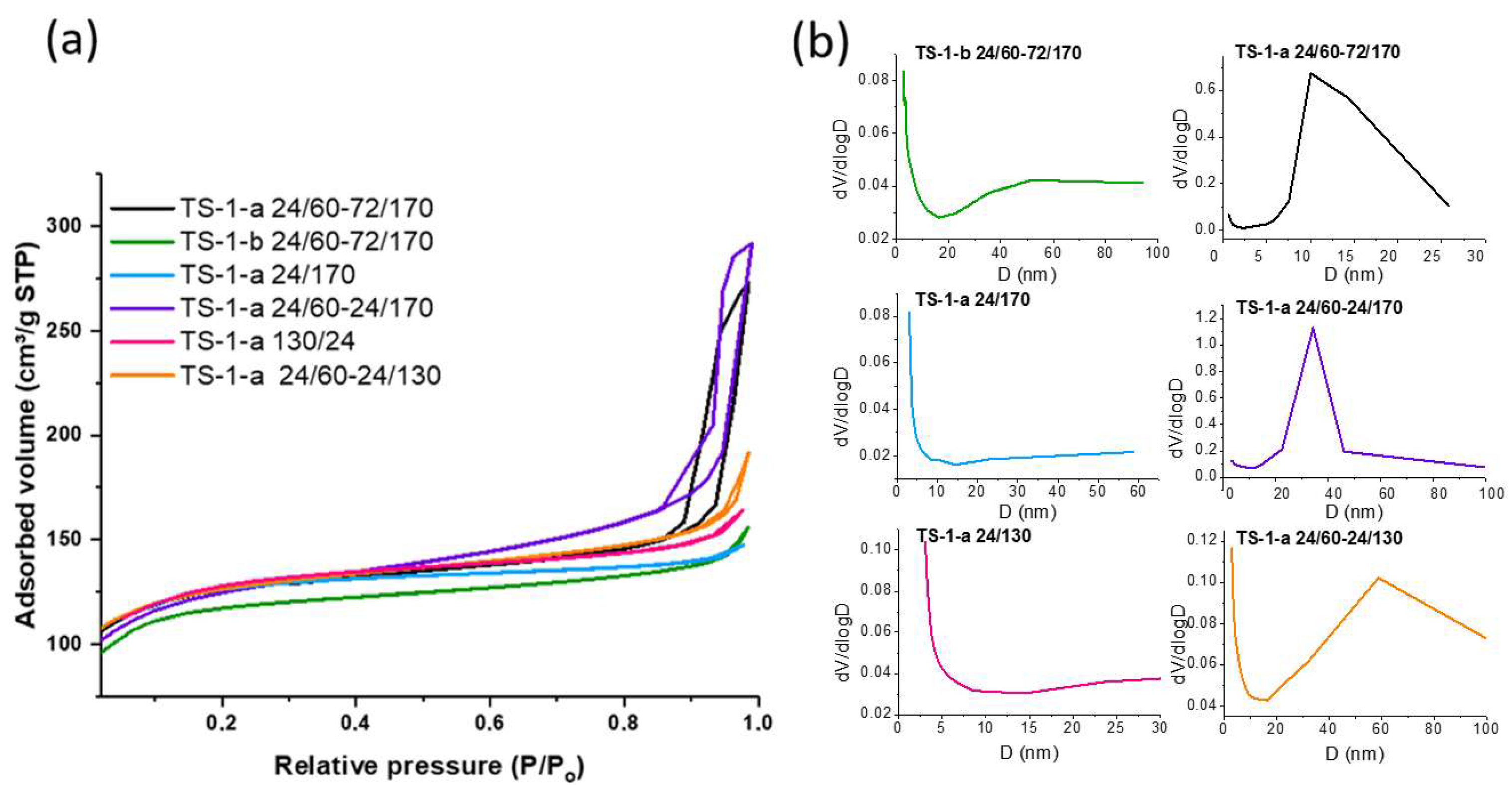
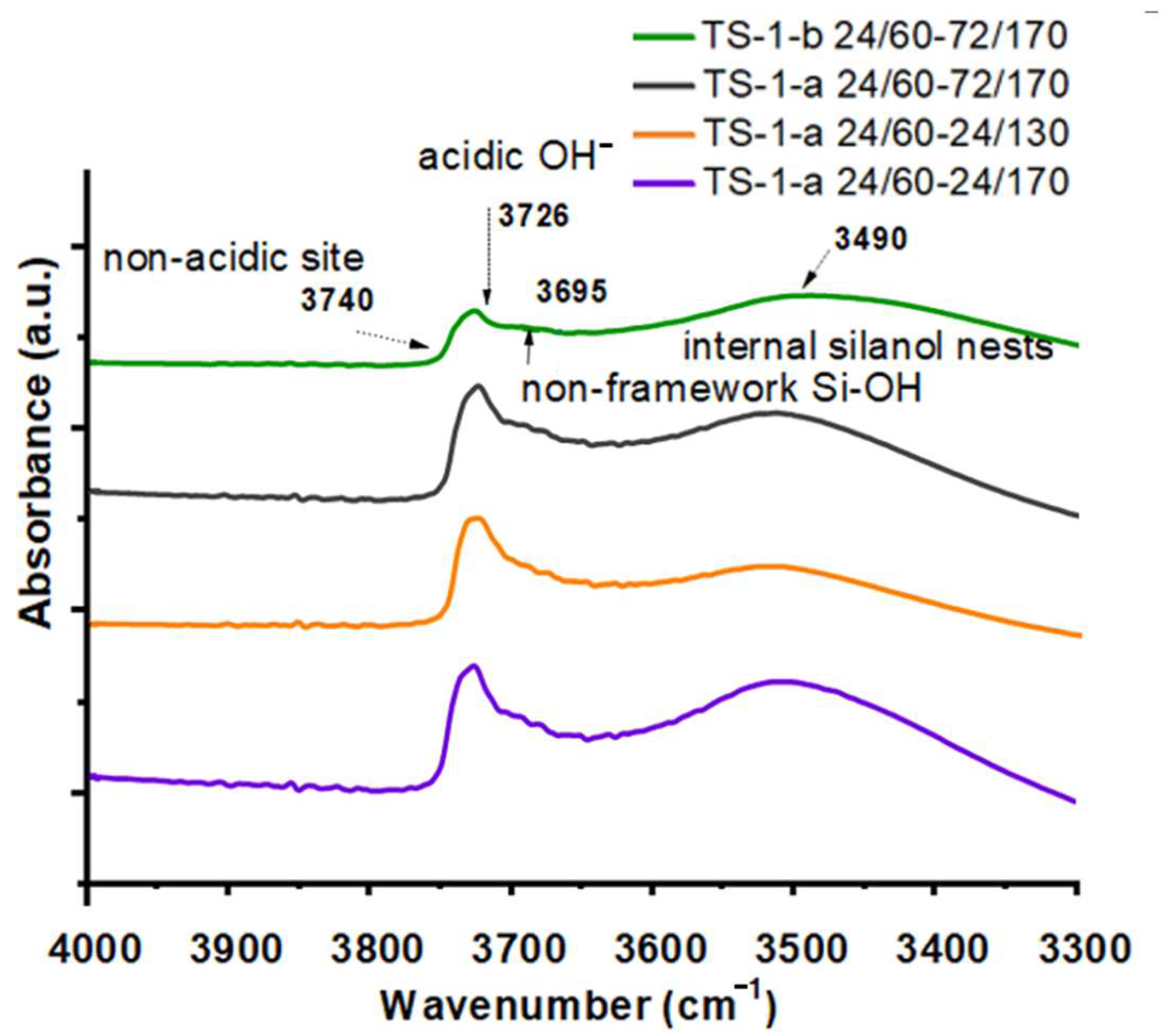


| Sample | SBET (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vtotal (cm3/g) |
|---|---|---|---|---|
| TS-1-b 24/60-72/170 | 350 | 0.15 | 0.04 | 0.19 |
| TS-1-a 24/60-72/170 | 406 | 0.12 | 0.30 | 0.42 |
| TS-1-a 24/60-24/170 | 461 | 0.15 | 0.30 | 0.45 |
| TS-1-a 24/60-24/130 | 464 | 0.16 | 0.14 | 0.3 |
| TS-1-a 24/130 | 394 | 0.17 | 0.08 | 0.25 |
| TS-1-a 24/170 | 380 | 0.18 | 0.05 | 0.23 |
| Sample | Concentration of Acid Sites, µmol Py/g | ||
|---|---|---|---|
| LAS | |||
| 150 °C | 200 °C | 250 °C | |
| TS-1-b 24/60-72/170 | 145 | 33 | 9 |
| TS-1-a 24/60-72/170 | 172 | 31 | 8 |
| TS-1-a 24/60-24/130 | 137 | 23 | 4 |
| TS-1-a 24/60-24/170 | 214 | 48 | 15 |
| Sample | Conversion Cyclohexene, % | Selectivity, % | |||
|---|---|---|---|---|---|
| Epoxy 1 | Enol + Enon 3 + 4 | Dio l2 | Others | ||
| TS-1b 24/60-72/170 | 7 | 26 | 60 | 3 | 11 |
| TS-1a 24/130 | 6 | 41 | 42 | 8 | 9 |
| TS-1a 24/170 | 8 | 37 | 50 | 4 | 9 |
| TS-1a 24/60-72/170 | 15 | 26 | 57 | 7 | 10 |
| TS-1a 24/60-24/170 | 9 | 48 | 40 | - | 12 |
| TS-1a 24/60-24/130 | 10 | 67 | 23 | 3 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikbaeva, V.R.; Bubennov, S.V.; Serebrennikov, D.V.; Ogurechnikova, D.A.; Vakulin, E.V.; Kutepov, B.I.; Grigoreva, N.G.; Maximov, A.L. Anatase-Free Nanosized Hierarchical Titanosilicate TS-1 Synthesis via Nitric Acid-Catalyzed Gel Preparation. Gels 2025, 11, 605. https://doi.org/10.3390/gels11080605
Bikbaeva VR, Bubennov SV, Serebrennikov DV, Ogurechnikova DA, Vakulin EV, Kutepov BI, Grigoreva NG, Maximov AL. Anatase-Free Nanosized Hierarchical Titanosilicate TS-1 Synthesis via Nitric Acid-Catalyzed Gel Preparation. Gels. 2025; 11(8):605. https://doi.org/10.3390/gels11080605
Chicago/Turabian StyleBikbaeva, Vera R., Sergey V. Bubennov, Dmitry V. Serebrennikov, Daria A. Ogurechnikova, Evgenii V. Vakulin, Boris I. Kutepov, Nellia G. Grigoreva, and Anton L. Maximov. 2025. "Anatase-Free Nanosized Hierarchical Titanosilicate TS-1 Synthesis via Nitric Acid-Catalyzed Gel Preparation" Gels 11, no. 8: 605. https://doi.org/10.3390/gels11080605
APA StyleBikbaeva, V. R., Bubennov, S. V., Serebrennikov, D. V., Ogurechnikova, D. A., Vakulin, E. V., Kutepov, B. I., Grigoreva, N. G., & Maximov, A. L. (2025). Anatase-Free Nanosized Hierarchical Titanosilicate TS-1 Synthesis via Nitric Acid-Catalyzed Gel Preparation. Gels, 11(8), 605. https://doi.org/10.3390/gels11080605








