Fabrication of Cellulose-Based Hydrogels Through Ionizing Radiation for Environmental and Agricultural Applications
Abstract
1. Introduction
2. Cellulose and Its Derivatives
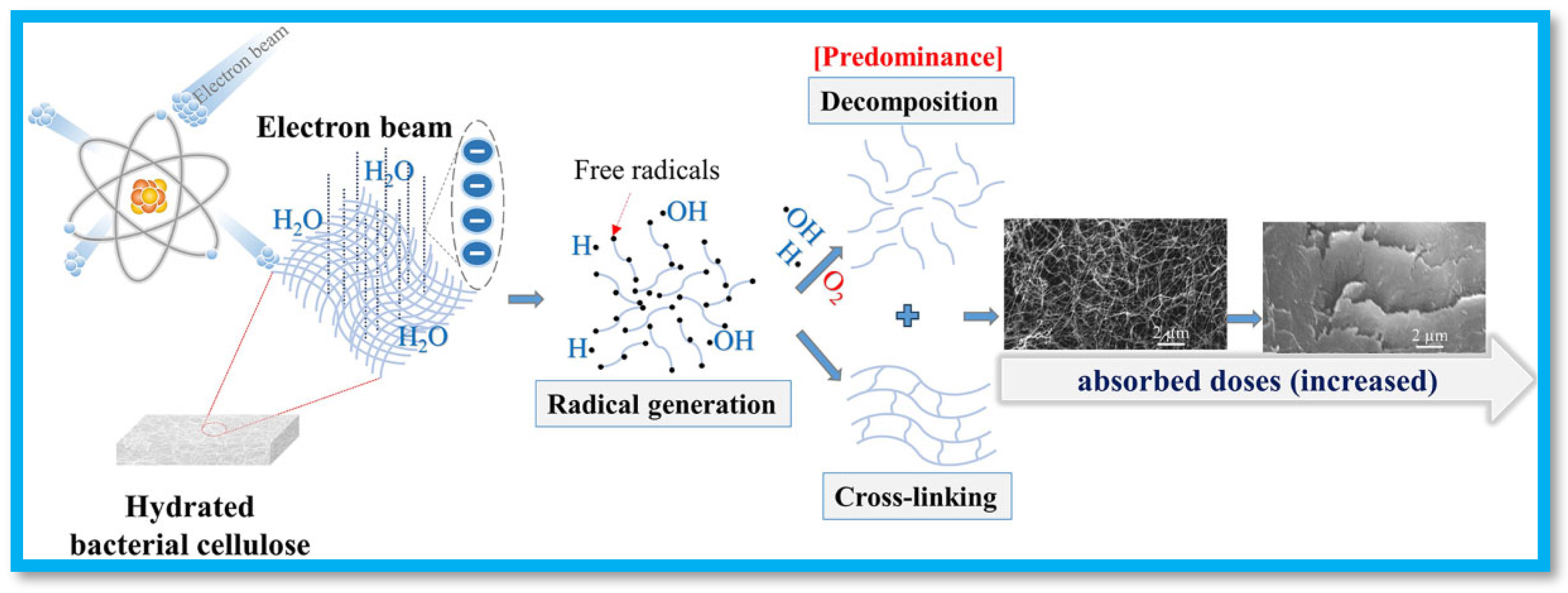
3. Ionizing Radiation
4. Fabrication of Cellulose-Based Hydrogels Using Ionizing Radiation
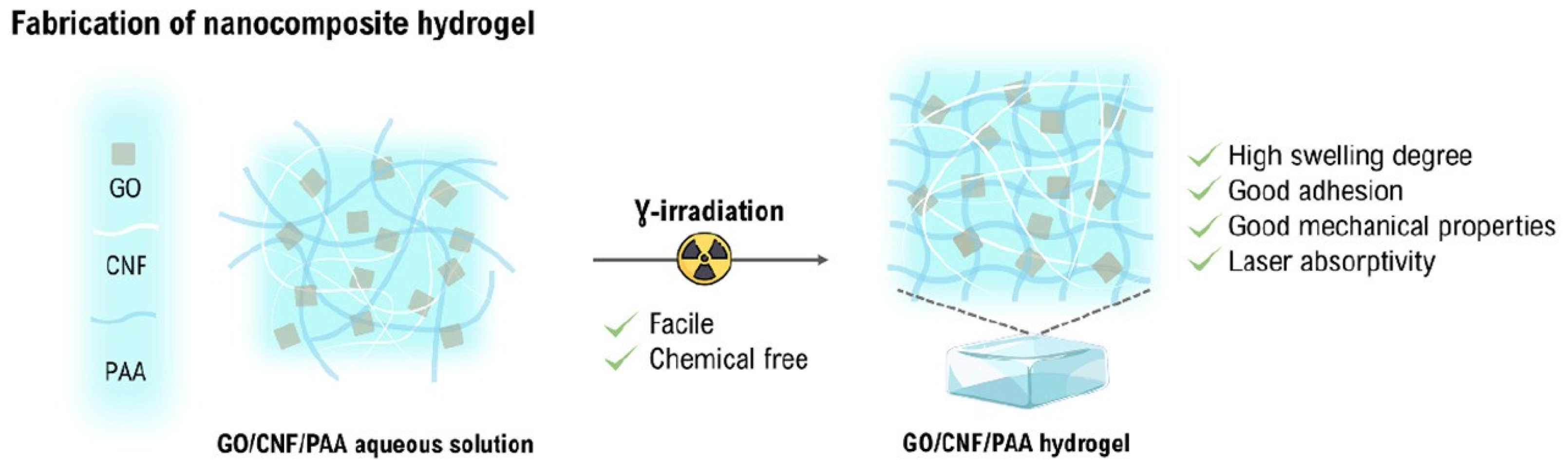
5. Environmental Applications
5.1. Water Purifications
5.1.1. Removal of Heavy Metals
5.1.2. Removal of Dyes
5.1.3. Removal of Organic Contaminants
| Adsorbent Constituents | Fabrication Technology (Ionizing Radiation) | Potential for Adsorbate Removal, Adsorption Kinetics, Isotherms, and Regeneration Efficiency | Applications | References |
|---|---|---|---|---|
| Cellulose/Urea/NaOH/AAM | γ (dose rate: 5 kGy/h) | 449.5 mg/g of U(VI), desorption rate 87.1%, PSO kinetic model, Langmuir model | Potential for wastewater treatment | [59] |
| Zr(IV) immobilized CMC/MW-CCNTs | EB absorbed dose: 5–30 kGy, (dose rate: 5 kGy/pass) | 36.657 mg/g of F−, PSO kinetic model, Freundlich and Langmuir models | Selectivity for F− removal from the hydrometallurgical system and water body | [60] |
| Removal of heavy metals | ||||
| HEC/AAC | γ absorbed dose: 10, 20, 30, 40, and 50 kGy, (dose rate: 0.7 kGy/h) | 26.65 mg/g of Pb(II), PSO kinetic model, Freundlich isotherm | Pb(II) removal | [62] |
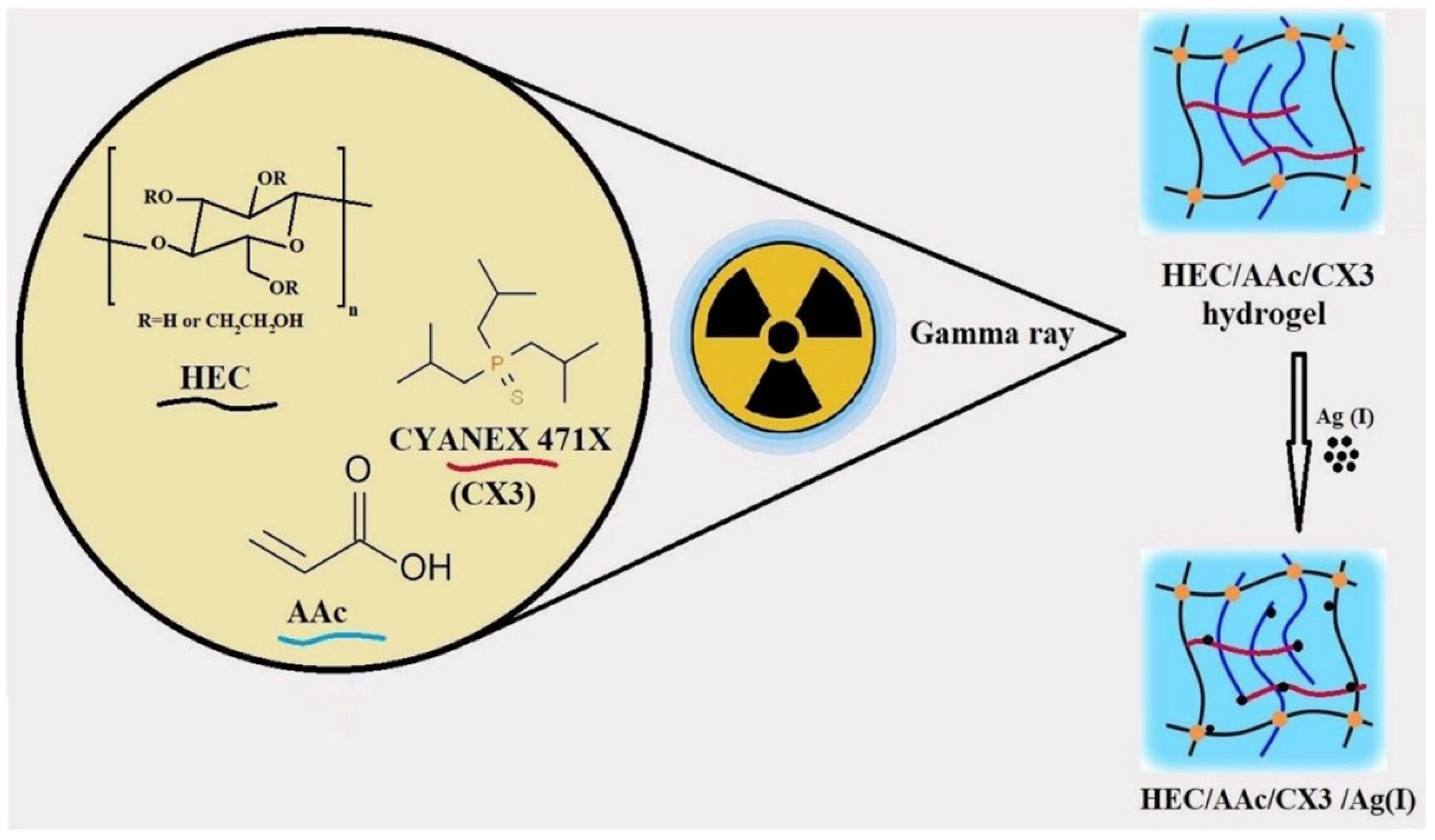
| CYANEX 471X/HEC/AAC | γ absorbed doses: 10, 20, 30, 40, and 50 kGy, (dose rate: 0.9 kGy/h) | 12 mg/g of Ag(I), maximum desorption 70%, non-Fickian diffusion, Five cycles | Ag(I) ions capture from acidic nitrate medium | [63] |
| CMC/Ca-MMT clay | γ absorbed doses: 5, 10, and 15 kGy, (dose rate: 0.33 Gy/s) | 54.6 mg/g of Cu(II), PSO kinetic model, Langmuir isotherm model | Removal of Cu(II) ions from wastewater | [65] |
| Cellulose/MAAC/AAM | γ absorbed dose: 20 kGy, (dose rate: 0.74 Gy/s) | Metal ions (Cu(II) and Co(II)), Dyes (acid blue dye and methyl green), Non-Fickian transport mechanism | Removal of pollutants from wastewater | [66] |
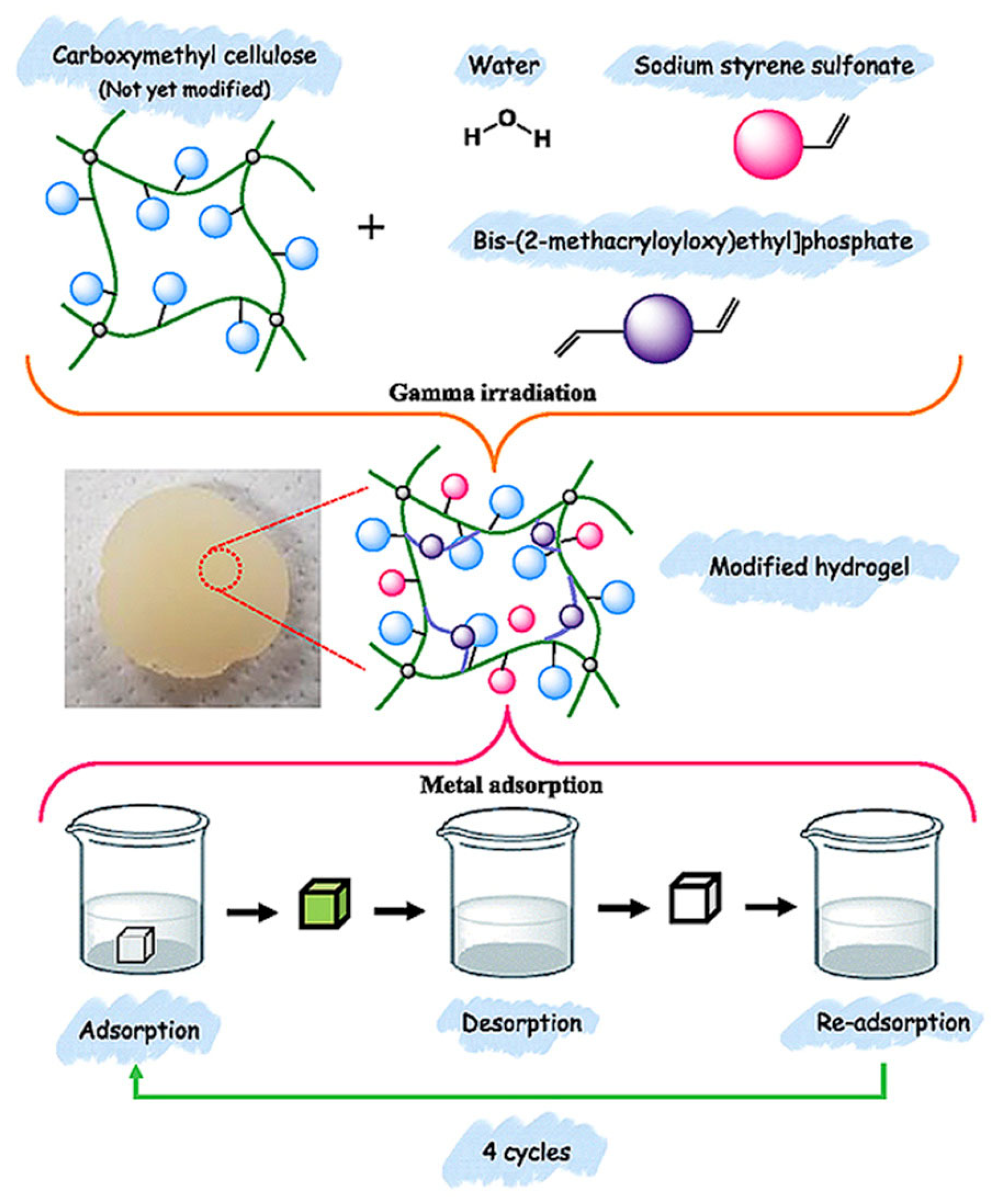
| CMC/SSS/BMEP | γ absorbed doses: 10–100 kGy | 70% for Ni(II), adsorption-desorption performance (~81%), Four cycles | Heavy metal ions adsorption | [68] |
| CMC/SSS | γ absorbed doses: 20 to 100 kGy | 79.78 μg/g of Fe(II), 3.60 µg/g of Pb(II), and 36.65 µg/g of Cr(III), PSO kinetic model, Langmuir isotherm model | Removal of metal ions from aqueous solutions | [67] |
| CMC/SA | γ absorbed dose: 20 kGy, (dose rate: 6.92 kGy/h) | Heavy metals (Ni(II) and Fe(III)) | Heavy metal ions from wastewater | [69] |
| CMC/AMPS | γ absorbed dose: 10–20 kGy, (dose rate: 10.28 kGy/h) | Heavy metals (Co(II), Mn(II), Fe(III), and Cu(II)), Langmuir isotherm model, Five cycles | Removal of heavy metals from model wastewater | [70] |
| CMC/HPMCP/CM-CS | γ absorbed dose: 40 and 80 kGy (dose rate: 20 Gy/min) | Sr(II) removal: 83.3 mg/g for HPMCP, 99.0 mg/g for CMCS, and 108.7 mg/g for CMC | Adsorption and desorption of Sr(II) ions | [64] |
| Removal of dyes | ||||
| CMC/AAC/ZnO/ZnO@Ag | γ absorbed doses: 10–30 kGy, (dose rate: 0.614 kGy/h) | Decolorization efficiency 93% of LABB dye, 60% performance after five cycles | Efficient photocatalytic remediation | [76] |
| NIPAAM/Substituted HPC/g-C3N4 | EB absorbed dose: 25 kGy, (dose rate: 5 kGy/pass) | Adsorption–photocatalytic removal of rhodamine B 71.4% | Promising new material with extensive applications in wastewater treatment | [84] |
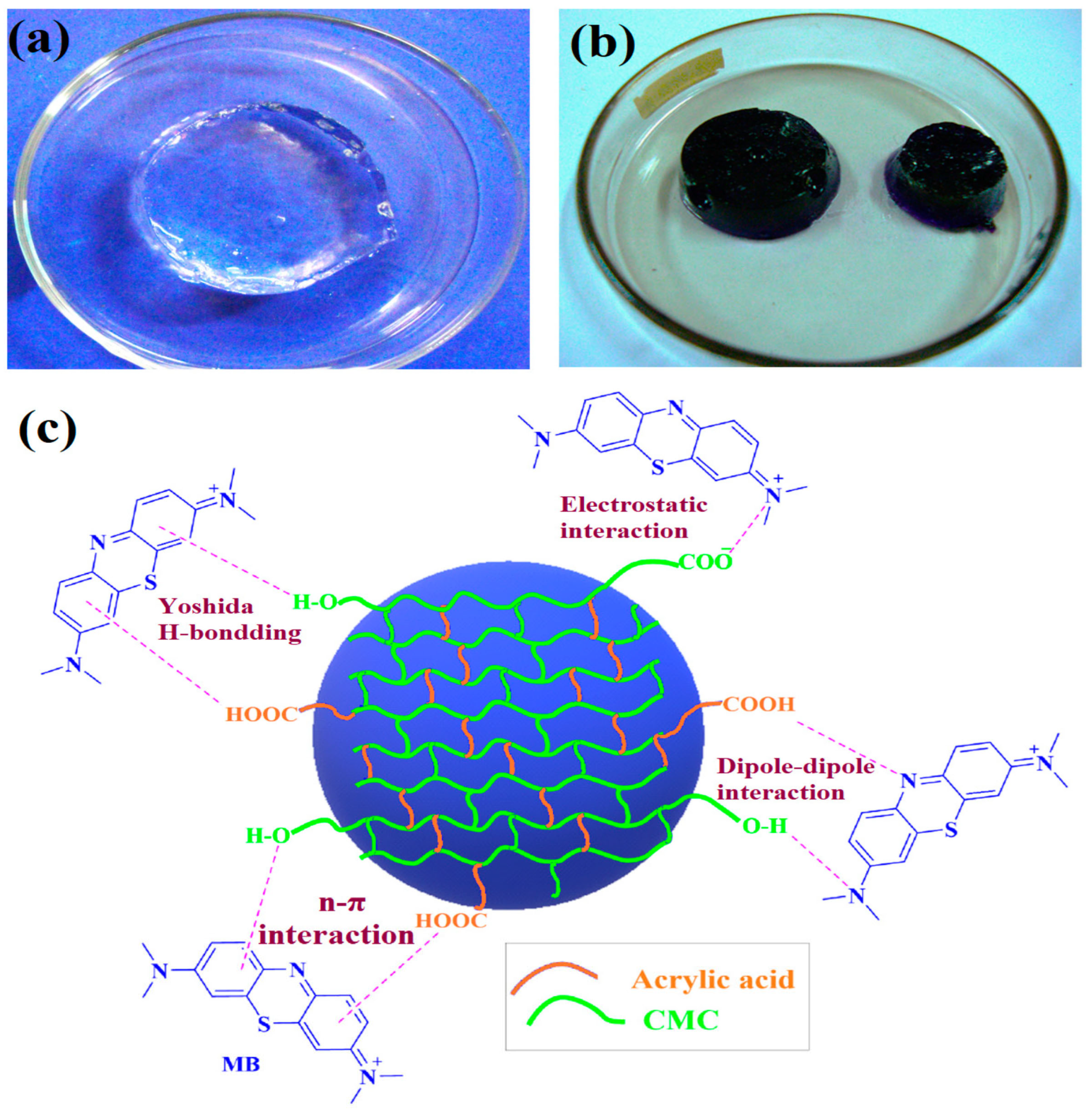
| CMC/AAC | γ absorbed doses: 1–15 kGy, (dose rate: 3 kGy/h) | 681 mg/g of MB, Desorption efficiency 95%, Schott’s PSO model | Dye removal | [77] |
| CNC/PEC/AAC | γ absorbed dose: 20 kGy | 576.62 mg/g of MB, Avrami kinetic model, Langmuir isotherm model | Remediation of basic dyes | [81] |
| CMC/TiO2 | γ absorbed doses: 5, 10, and 15 kGy, (dose rate: 0.33 Gy/s) | 123.6 mg/g of violet 7 dye, PSO kinetic model, Langmuir model | Removal of basic dye from wastewater | [17] |
| BC/AAC | γ absorbed dose: 30 kGy | MB | Removal of MB dye in aqueous solution | [82] |
| PVA/CMC | EB absorbed doses: 5–20 kGy | Dye removal order: Direct pink 3B > acid green B > ismative violet 2R, Freundlich model | Removal of dyes | [85] |
| Removal of organic contaminants | ||||
| MAAC/CMC | γ absorbed doses: 20 kGy, (dose rate: 0.74 Gy/s) | 4-chlorophenol and 2,4-D | Removal of organic contaminants | [90] |
| CMC/AAM/MAAC | γ absorbed dose: 20 kGy, (dose rate: 0.74 Gy/s) | Organic contaminants (2,4-D and 4-chlorophenol), dyes (methyl green and acid blue dye), and heavy metals (Co(II) and Cu(II)), Non-Fickian transport mechanism | Removal of hazardous water pollutants | [91] |
6. Agricultural Applications
Release of Agrochemicals
| Superabsorbent Constituents | Fabrication Technology (Ionizing Radiation) | Applications | References |
|---|---|---|---|
| CMC/AAM | γ absorbed doses: 10, 20, 30, 40, and 50 kGy, (dose rate: 0.68 kGy/h) | For enhancing Beta vulgaris under drought stress | [93] |
| PVA/CMC/CS | γ absorbed doses: 2.5, 5, 10, and 20 kGy, (dose rate: 2.5 kGy/h) | Water-holding capacity and water-retention of sandy soils | [83] |
| HEC/PVA/Cu2O-rGO/BiVO4 | EB absorbed doses: 25, 35, and 45 kGy | Suitability for the irrigation of gardens and playgrounds | [95] |
| CSB/PAAC | EB absorbed doses: 5–100 kGy | SS amendment | [96] |
| HEC/AAM | γ absorbed doses: 10, 20, 30, 40, and 50 kGy, (dose rate: 0.83 kGy/h) | Maize planting in drought conditions | [100] |
| CMC/AAC/Ca-MMT clay | γ absorbed doses: 2.5,5, 7.5, and 10 kGy | Potential as water-managing material for agriculture and horticulture in desert and drought-prone areas | [97] |
| CMC/PVP | γ absorbed doses: 20, 25, and 30 kGy | Agriculture applications | [101] |
| CMC/PVP | γ absorbed doses: 5, 10, 20, and 30 kGy, (dose rate: 2.05 kGy/h) | Controlled release fertilizers | [99] |
| CMC/PAAM/Si | γ | Controlled release of some agrochemicals | [6] |
| HPC/CMC/PEGDA | EB absorbed doses: 5–100 kGy; (dose rate: 5 kGy/min ± 10%) | Water reservoirs for biodegradable microelements and nutrient delivery depots | [103] |
7. Conclusions, Limitations, and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; InTech: Houston, TX, USA, 2011. [Google Scholar]
- Behera, S.; Mahanwar, P.A. Superabsorbent Polymers in Agriculture and Other Applications: A Review. Polym. Plast. Technol. Mater. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Sun, M.-T.; Song, F.-P.; Zhang, G.-D.; Li, J.-Z.; Wang, F. Polymeric Superabsorbent Hydrogel-Based Kinetic Promotion for Gas Hydrate Formation. Fuel 2021, 288, 119676. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels 2021, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan Gum-Derived Materials for Applications in Environment and Eco-Friendly Materials: A Review. J. Environ. Chem. Eng. 2021, 9, 104702. [Google Scholar] [CrossRef]
- Maziad, N.A.; Abou El Fadl, F.I.; El-Kelesh, N.A.; El-Hamouly, S.H.; Zeid, I.F.; Gayed, H.M. Radiation Synthesis and Characterization of Super Absorbent Hydrogels for Controlled Release of Some Agrochemicals. J. Radioanal. Nucl. Chem. 2016, 307, 513–521. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Kumar, H.; Christopher, L.P. Recent Trends and Developments in Dissolving Pulp Production and Application. Cellulose 2017, 24, 2347–2365. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent Advances in Polysaccharide-Based Adsorbents for Wastewater Treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Tree Gum-Based Renewable Materials: Sustainable Applications in Nanotechnology, Biomedical and Environmental Fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Foroughi, F.; Rezvani Ghomi, E.; Morshedi Dehaghi, F.; Borayek, R.; Ramakrishna, S. A Review on the Life Cycle Assessment of Cellulose: From Properties to the Potential of Making It a Low Carbon Material. Materials 2021, 14, 714. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Abbas, A.; Raza, M.A. Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications. Gels 2025, 11, 47. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-Friendly Polyvinyl Alcohol/Carboxymethyl Cellulose Hydrogels Reinforced with Graphene Oxide and Bentonite for Enhanced Adsorption of Methylene Blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Rahman, S.; Hasan, S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl Cellulose-Based Materials for Infection Control and Wound Healing: A Review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Gad, Y.H.; Ali, H.E.; Hegazy, A.E.-S. Synergistic Effect of Titanium Dioxide (TiO2) and Ionizing Radiation on Thermal and Mechanical Properties of Carboxymethyl Cellulose (CMC) for Potential Application in Removal of Basic Dye from Wastewater. J. Polym. Environ. 2021, 29, 3887–3899. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective Removal of Anionic Textile Dyes Using Adsorbent Synthesized from Coffee Waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, E.I.; Agunwamba, J.C. A Review on the Applicability of Activated Carbon Derived from Plant Biomass in Adsorption of Chromium, Copper, and Zinc from Industrial Wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef] [PubMed]
- Fijul Kabir, S.M.; Ur Rashid, T.; Negulescu, I.I. Gelation of Textile Dye Solution Treated with Fish Scales. Gels 2019, 5, 37. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Cueto, R.; Balamurugan, S.; Romeo, L.D.; Kuttruff, J.T.; Marx, B.D.; Negulescu, I.I. Removal of Acid Dyes from Textile Wastewaters Using Fish Scales by Absorption Process. Clean Technol. 2019, 1, 311–324. [Google Scholar] [CrossRef]
- Ding, C.; Chen, J.; Zhu, F.; Chai, L.; Lin, Z.; Zhang, K.; Shi, Y. Biological Toxicity of Heavy Metal (Loid) s in Natural Environments: From Microbes to Humans. Front. Environ. Sci. 2022, 10, 920957. [Google Scholar] [CrossRef]
- Diaconu, L.I.; Covaliu-Mierlă, C.I.; Păunescu, O.; Covaliu, L.D.; Iovu, H.; Paraschiv, G. Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae. Biology 2023, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Manivasagan, R. Effect of Operating Parameters on Dye Wastewater Treatment Using Prosopis Cineraria and Kinetic Modeling. Environ. Eng. Res. 2020, 25, 788–793. [Google Scholar] [CrossRef]
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and Personal Care Products as Emerging Contaminants: Need for Combined Treatment Strategy. J. Hazard. Mater. Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Melvin, S.D.; Leusch, F.D.L. Removal of Trace Organic Contaminants from Domestic Wastewater: A Meta-Analysis Comparison of Sewage Treatment Technologies. Environ. Int. 2016, 92–93, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, E.R.V.; Snyder, S.A.; Sedlak, D.L.; Drewes, J.E. Indicator Compounds for Assessment of Wastewater Effluent Contributions to Flow and Water Quality. Water Res. 2011, 45, 1199–1212. [Google Scholar] [CrossRef]
- Rahman, F.B.A.; Akter, M. Removal of Dyes Form Textile Wastewater by Adsorption Using Shrimp Shell. Int. J. Waste Resour. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Schwartz, F.W.; Jeong, Y.; Song, H. Magnetic Chitosan Composite for Adsorption of Cationic and Anionic Dyes in Aqueous Solution. J. Ind. Eng. Chem. 2015, 28, 60–66. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-Based Materials in Wastewater Treatment of Petroleum Industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Dey, P.; Mandal, S.; Brahma, R.K. Cellulose-Based Hydrogels: Biocompatibility, Environmental Impact, and Sustainable Management. In Cellulose Based Hydrogels: Production, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 439–458. [Google Scholar] [CrossRef]
- Singh, N.S.; Mukherjee, I.; Das, S.K.; Varghese, E. Leaching of Clothianidin in Two Different Indian Soils: Effect of Organic Amendment. Bull. Environ. Contam. Toxicol. 2018, 100, 553–559. [Google Scholar] [CrossRef]
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable Intensification of Agriculture for Human Prosperity and Global Sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hernandez, S.; Salazar, N. Biopolymer-Based Hydrogels for Harvesting Water from Humid Air: A Review. Sustainability 2023, 15, 848. [Google Scholar] [CrossRef]
- Ranganathan, N.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Cellulose-Based Hydrogels for Agricultures. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Hou, X.; Pan, Y.; Xiao, H.; Liu, J. Controlled Release of Agrochemicals Using PH and Redox Dual-Responsive Cellulose Nanogels. J. Agric. Food Chem. 2019, 67, 6700–6707. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel Ethanol Production from Lignocellulosic Biomass: An Overview on Feedstocks and Technological Approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Sozcu, S.; Frajova, J.; Wiener, J.; Venkataraman, M.; Tomkova, B.; Militky, J. Synthesis of Acetobacter Xylinum Bacterial Cellulose Aerogels and Their Effect on the Selected Properties. Gels 2025, 11, 272. [Google Scholar] [CrossRef]
- Oshima, T.; Taguchi, S.; Ohe, K.; Baba, Y. Phosphorylated Bacterial Cellulose for Adsorption of Proteins. Carbohydr. Polym. 2011, 83, 953–958. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl Cellulose Hydrogel for Wound Dressing: Fabrication, Characterization and in Vitro Evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef]
- Naomi, R.; Idrus, R.B.H.; Fauzi, M.B. Plant-vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Skorik, Y.A. Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review. Polymers 2023, 15, 440. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, M.H.; Ferreira, L.M.; Leal, J.P.; Pereira, C.C.L.; Monteiro, B. Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications. Membranes 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Güven, O. Ionizing radiation: A versatile tool for nanostructuring of polymers. Pure Appl. Chem. 2016, 88, 1049–1061. [Google Scholar] [CrossRef]
- Rimdusit, S.; Somsaeng, K.; Kewsuwan, P.; Jubsilp, C.; Tiptipakorn, S. Comparison of Gamma Radiation Crosslinking and Chemical Crosslinking on Properties of Methylcellulose Hydrogel. Eng. J. 2012, 16, 15–28. [Google Scholar] [CrossRef]
- Ahmed, S.; Islam, M.; Hasan, K.; Nam, K.-W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Raza, M.A.; Lim, Y.M.; Lee, S.W.; Seralathan, K.K.; Park, S.H. Synthesis and Characterization of Hydrogels Based on Carboxymethyl Chitosan and Poly(Vinylpyrrolidone) Blends Prepared by Electron Beam Irradiation Having Anticancer Efficacy, and Applications as Drug Carrier for Controlled Release of Drug. Carbohydr. Polym. 2021, 258, 117718. [Google Scholar] [CrossRef]
- More, A.P.; Chapekar, S. Irradiation Assisted Synthesis of Hydrogel: A Review. Polym. Bull. 2024, 81, 5839–5908. [Google Scholar] [CrossRef]
- Le, V.T.; Joo, S.W.; Berkani, M.; Mashifana, T.; Kamyab, H.; Wang, C.; Vasseghian, Y. Sustainable Cellulose-Based Hydrogels for Water Treatment and Purification. Ind. Crops Prod. 2023, 205, 117525. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Wach, R.A.; Rokita, B.; Bartoszek, N.; Katsumura, Y.; Ulanski, P.; Rosiak, J.M. Hydroxyl Radical-Induced Crosslinking and Radiation-Initiated Hydrogel Formation in Dilute Aqueous Solutions of Carboxymethylcellulose. Carbohydr. Polym. 2014, 112, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Passornraprasit, N.; Siripongpreda, T.; Ninlapruk, S.; Rodthongkum, N.; Potiyaraj, P. γ-Irradiation Crosslinking of Graphene Oxide/Cellulose Nanofiber/Poly (Acrylic Acid) Hydrogel as a Urea Sensing Patch. Int. J. Biol. Macromol. 2022, 213, 1037–1046. [Google Scholar] [CrossRef]
- Sung, Y.; Kim, T.H.; Lee, B. Syntheses of Carboxymethylcellulose/Graphene Nanocomposite Superabsorbent Hydrogels with Improved Gel Properties Using Electron Beam Radiation. Macromol. Res. 2016, 24, 143–151. [Google Scholar] [CrossRef]
- Amoatey, P.; Bani, R. Wastewater Management. In Waste Water—Evaluation and Management; InTech: Houston, TX, USA, 2011. [Google Scholar]
- Tchobanoglous, G.; Burton, F.; Stensel, D.H. Wastewater Engineering: Treatment and Reuse; Metcalf & Eddy; McGraw Hill Companies, Inc.: New York, NY, USA, 2014; p. 421. [Google Scholar]
- Chakraborty, S.K.; Sanyal, P.; Ray, R. Pollution, Environmental Perturbation and Consequent Loss of Wetlands. In Wetlands Ecology; Springer International Publishing: Cham, Switzerland, 2023; pp. 521–582. [Google Scholar]
- Zhang, N.; Li, W.; Li, J.; Wang, Q.; Li, B.; Zhang, J.; Zhang, T.; Li, Z.; Li, Y.; Tian, B.; et al. Chemically and Physically Dual Cross-Linked Phosphorylated Cellulose Hydrogel for Uranium Removal from Aqueous Solution. Sep. Purif. Technol. 2025, 368, 132907. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, K.; Zhang, F.; Yang, X.; Du, J.; Dong, Z.; Bao, Q.; Zhao, L. Facile Preparation of Zirconium(IV) Immobilized Carboxymethyl Cellulose/Multiwalled Carbon Nanotubes Gels by Radiation Technique and Its Selective Fluoride Removal. Int. J. Biol. Macromol. 2024, 282, 137447. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of Novel Magnetic Chitosan Grafted with Graphene Oxide to Enhance Adsorption Properties for Methyl Blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.S.; Abdel-Salam, F.H.; Maziad, N.A.; Mahmoud, F.Z.; Gayed, H.M. Radiation-Assisted Synthesis of Hydroxyethyl Cellulose and Acrylic Acid Hydrogel Modified with Novel Surfactants for Pb (II) Removal. Int. J. Environ. Anal. Chem. 2025, 1–24. [Google Scholar] [CrossRef]
- Masry, B.A.; Gayed, H.M.; Daoud, J.A. Gamma Radiation Synthesis of Hydroxyethyl Cellulose/Acrylic Acid/CYANEX 471X Hydrogel for Silver Ions Capture from Acidic Nitrate Medium. Cellulose 2024, 31, 4329–4346. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Peng, J.; Zhai, M.; Li, J.; Wei, G. Adsorption and Desorption of Sr(II) Ions in the Gels Based on Polysaccharide Derivates. J. Hazard. Mater. 2009, 171, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Gad, Y.H.; Ali, H.E.; Hegazy, E.S.A. Radiation-Induced Improving Mechanical and Thermal Properties of Carboxymethyl Cellulose/Clay Composite for Application in Removal of Copper(II) Ions from Wastewater. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2083–2094. [Google Scholar] [CrossRef]
- El-Arnaouty, M.B.; Abdel Ghaffar, A.M.; Abdel Baky, A.A.K.; Shama, S.A. Radiation Synthesis of Hydrogels Based on Carboxymethyl Cellulose and Its Application in Removal of Pollutants from Wastewater. J. Vinyl Addit. Technol. 2019, 25, E35–E43. [Google Scholar] [CrossRef]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Removal of Metal Ions from Aqueous Solutions Using Carboxymethyl Cellulose/Sodium Styrene Sulfonate Gels Prepared by Radiation Grafting. Carbohydr. Polym. 2017, 157, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.T.; Okabe, H.; Hidaka, Y.; Omoldi, B.A.; Hara, K. Radiation Induced Modified CMC-Based Hydrogel with Enhanced Reusability for Heavy Metal Ions Adsorption. Polymer 2019, 181, 121772. [Google Scholar] [CrossRef]
- El-Naggar, A.A. Radiation Synthesis of Superabsorbent Hydrogels Based on Carboxymethyl Cellulose/Sodium Alginate for Absorbent of Heavy Metal Ions from Waste Water. J. Thermoplast. Compos. Mater. 2016, 29, 16–27. [Google Scholar] [CrossRef]
- El-Hag Ali, A. Removal of Heavy Metals from Model Wastewater by Using Carboxymehyl Cellulose/2-Acrylamido-2-Methyl Propane Sulfonic Acid Hydrogels. J. Appl. Polym. Sci. 2012, 123, 763–769. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated Lignin-Chitosan Extruded Blends for Efficient Adsorption of Methylene Blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Bulut, E.; Özacar, M.; Şengil, İ.A. Adsorption of Malachite Green onto Bentonite: Equilibrium and Kinetic Studies and Process Design. Microporous Mesoporous Mater. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Hao, Y.; Yan, L.; Yu, H.; Yang, K.; Yu, S.; Shan, R.; Du, B. Comparative Study on Adsorption of Basic and Acid Dyes by Hydroxy-Aluminum Pillared Bentonite. J. Mol. Liq. 2014, 199, 202–207. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Photo-Catalyzed Degradation of Hazardous Dye Methyl Orange by Use of a Composite Catalyst Consisting of Multi-Walled Carbon Nanotubes and Titanium Dioxide. J. Colloid Interface Sci. 2012, 371, 101–106. [Google Scholar] [CrossRef]
- Ali, H.E.; Nasef, S.M.; Gad, Y.H. Remediation of Astrazon Blue and Lerui Acid Brilliant Blue Dyes from Waste Solutions Using Amphoteric Superparamagnetic Nanocomposite Hydrogels Based on Chitosan Prepared by Gamma Rays. Carbohydr. Polym. 2022, 283, 119149. [Google Scholar] [CrossRef]
- Elshahawy, M.F.; Ahmed, N.A.; Gad, Y.H.; Ali, A.E.H. Efficient Photocatalytic Remediation of Lerui Acid Brilliant Blue Dye Using Radiation- Prepared Carboxymethyl Cellulose/Acrylic Acid Hydrogel Supported by ZnO@Ag. Int. J. Biol. Macromol. 2024, 262, 129946. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Banik, N.; Islam, M.; Rahman Khan, M.M.; Jeong, J.-H. Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal. Gels 2024, 10, 785. [Google Scholar] [CrossRef]
- Salunkhe, B.; Schuman, T.P. Super-Adsorbent Hydrogels for Removal of Methylene Blue from Aqueous Solution: Dye Adsorption Isotherms, Kinetics, and Thermodynamic Properties. Macromol 2021, 1, 256–275. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Amar, A.; Loulidi, I.; Jabri, M.; Hadey, C.; Lgaz, H.; Alrashdi, A.A.; Boukhlifi, F. Characterization and Adsorption Capacity of Four Low-Cost Adsorbents Based on Coconut, Almond, Walnut, and Peanut Shells for Copper Removal. Biomass Convers. Biorefin. 2024, 14, 3655–3666. [Google Scholar] [CrossRef]
- Al-Gorair, A.S.; Sayed, A.; Mahmoud, G.A. Engineered Superabsorbent Nanocomposite Reinforced with Cellulose Nanocrystals for Remediation of Basic Dyes: Isotherm, Kinetic, and Thermodynamic Studies. Polymers 2022, 14, 567. [Google Scholar] [CrossRef]
- Hakam, A.; Rahman, I.A.; Jamil, M.S.M.; Othaman, R.; Amin, M.C.I.M.; Lazim, A.M. Removal of Methylene Blue Dye in Aqueous Solution by Sorption on a Bacterial-g-Poly-(Acrylic Acid) Polymer Network Hydrogel. Sains Malays. 2015, 44, 827–834. [Google Scholar] [CrossRef]
- Helal, I.M.; Ahmed, S.B.; Abdulrahim, M.R. Synthesis and Characterization of Sodium Carboxymethyl Cellulose/Chitosan/Polyvinyl Alcohol Hydrogels to Removal Ionic Dyes and Their Biodegaration. Polym. Adv. Technol. 2025, 36, e70061. [Google Scholar] [CrossRef]
- Liu, G.; Li, T.T.; Song, X.F.; Yang, J.Y.; Qin, J.T.; Zhang, F.F.; Wang, Z.X.; Chen, H.G.; Wu, M.H.; Li, Y.S. Thermally Driven Characteristic and Highly Photocatalytic Activity Based on N-Isopropyl Acrylamide/High-Substituted Hydroxypropyl Cellulose/g-C3N4 Hydrogel by Electron Beam Pre-Radiation Method. J. Thermoplast. Compos. Mater. 2022, 35, 2009–2031. [Google Scholar] [CrossRef]
- Taleb, M.F.A.; El-Mohdy, H.L.A.; El-Rehim, H.A.A. Radiation Preparation of PVA/CMC Copolymers and Their Application in Removal of Dyes. J. Hazard. Mater. 2009, 168, 68–75. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging Pollutants of Severe Environmental Concern in Water and Wastewater: A Comprehensive Review on Current Developments and Future Research. Water Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- de Zwart, D.; Adams, W.; Galay Burgos, M.; Hollender, J.; Junghans, M.; Merrington, G.; Muir, D.; Parkerton, T.; De Schamphelaere, K.A.C.; Whale, G.; et al. Aquatic Exposures of Chemical Mixtures in Urban Environments: Approaches to Impact Assessment. Environ. Toxicol. Chem. 2018, 37, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. Regeneration and Reuse of Polymeric Nanocomposites in Wastewater Remediation: The Future of Economic Water Management. Polym. Bull. 2018, 76, 647–681. [Google Scholar] [CrossRef]
- Uva, M.; Tambasco, M.; Grassi, G.; Corsi, I.; Protano, G.; Atrei, A. Carboxymethylcellulose Hydrogels Cross-Linked with Magnetite Nanoparticles for the Removal of Organic and Inorganic Pollutants from Water. J. Environ. Chem. Eng. 2017, 5, 3632–3639. [Google Scholar] [CrossRef]
- Abdel Ghaffar, A.M.; El-Arnaouty, M.B.; Abdel Baky, A.A.; Shama, S.A. Radiation Synthesis of Carboxymethyl Cellulose Hydrogels for Removal of Organic Contaminants from Its Aqueous Solution. J. Vinyl Addit. Technol. 2020, 26, 362–369. [Google Scholar] [CrossRef]
- Abdel Ghaffar, A.M.; El-Arnaouty, M.B.; Abdel Baky, A.A.; Shama, S.A. Radiation-Induced Grafting of Acrylamide and Methacrylic Acid Individually onto Carboxymethyl Cellulose for Removal of Hazardous Water Pollutants. Des. Monomers Polym. 2016, 19, 706–718. [Google Scholar] [CrossRef]
- Kimura, A.; Nagasawa, N.; Taguchi, M. Cellulose Gels Produced in Room Temperature Ionic Liquids by Ionizing Radiation. Radiat. Phys. Chem. 2014, 103, 216–221. [Google Scholar] [CrossRef]
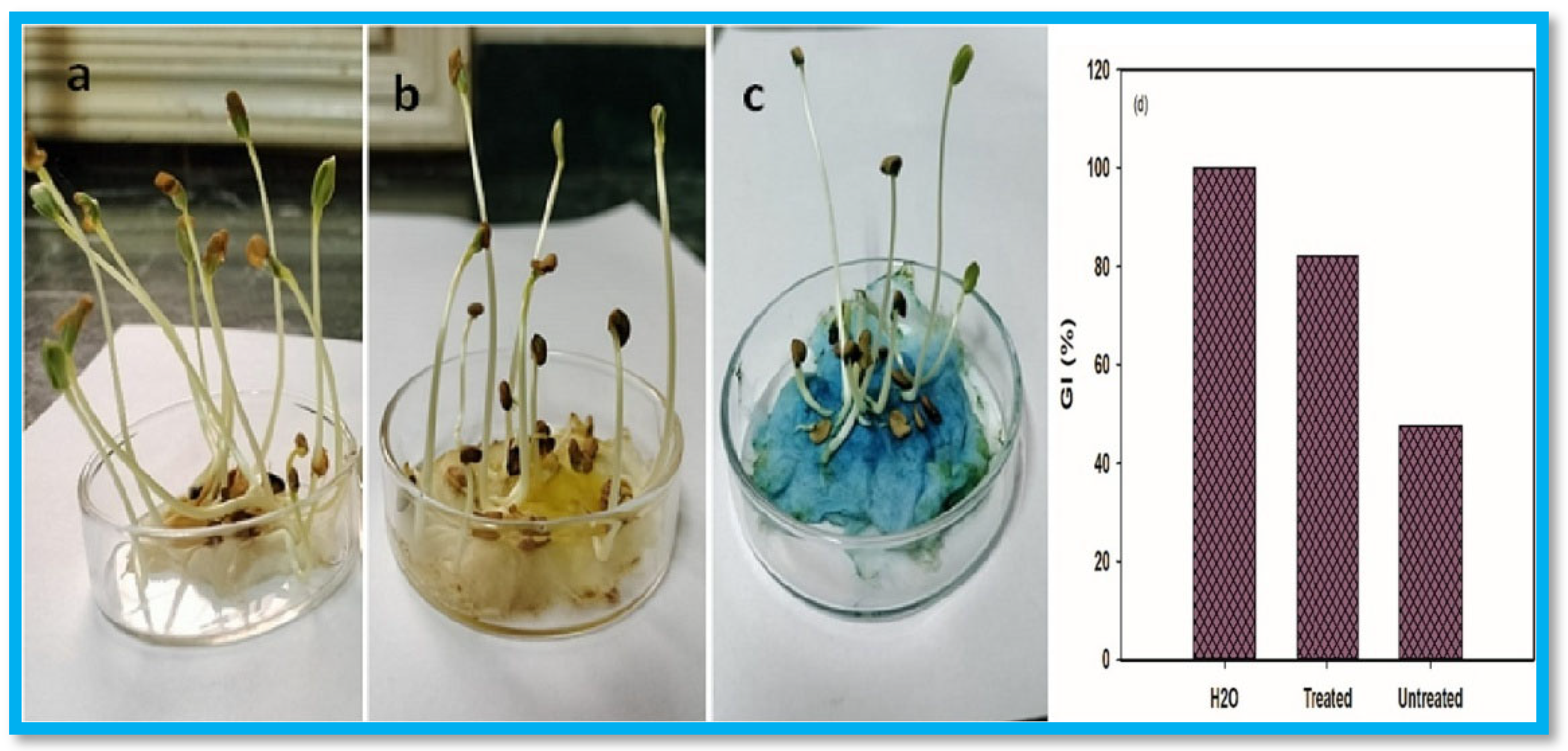
- El-diehy, M.A.; Farghal, I.I.; Amin, M.A.; Ghobashy, M.M.; Nowwar, A.I.; Gayed, H.M. Radiation-Assisted Tailoring of Swelling Behavior and Water Retention of Na-CMC/PAAm Hydrogels for Enhancing Beta Vulgaris under Drought Stress. Sci. Rep. 2025, 15, 1661. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H.; Yosh, F. Synthesis of PH-Sensitive and Biodegradable CM-Cellulose/Chitosan Polyampholytic Hydrogels with Electron Beam Irradiation. J. Bioact. Compat. Polym. 2008, 23, 319–333. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Abdelwahab, S.M.; Elsawy, N.M.; Ahmed, N.A.; Raafat, A.I. E-Beam Irradiation-Induced Synthesis of Hydroxyethyl Cellulose/(Cu2O-RGO)/BiVO4-Based Nanocomposite for Photocatalytic Remediation of Wastewater under Visible Light. Int. J. Biol. Macromol. 2024, 258, 128681. [Google Scholar] [CrossRef]
- Piroonpan, T.; Haema, K.; Hiangrat, K.; Sriroth, K.; Pasanphan, W. Sugarcane Bagasse Cellulose-PAA Micro-Composite Super-Water Absorbent for Sandy Soil Amendment: Exploring a Complete Set of Studies from One-Pot Electron Beam Processing to Pot-Testing. Ind. Crops Prod. 2024, 221, 119219. [Google Scholar] [CrossRef]
- Salmawi, K.M.E.; El-Naggar, A.A.; Ibrahim, S.M. Gamma Irradiation Synthesis of Carboxymethyl Cellulose/Acrylic Acid/Clay Superabsorbent Hydrogel. Adv. Polym. Technol. 2018, 37, 515–521. [Google Scholar] [CrossRef]
- Ghobashy, M.M. The Application of Natural Polymer-Based Hydrogels for Agriculture. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–356. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Controlled Release Fertilizers Using Superabsorbent Hydrogel Prepared by Gamma Radiation. Radiochim. Acta 2017, 105, 865–876. [Google Scholar] [CrossRef]
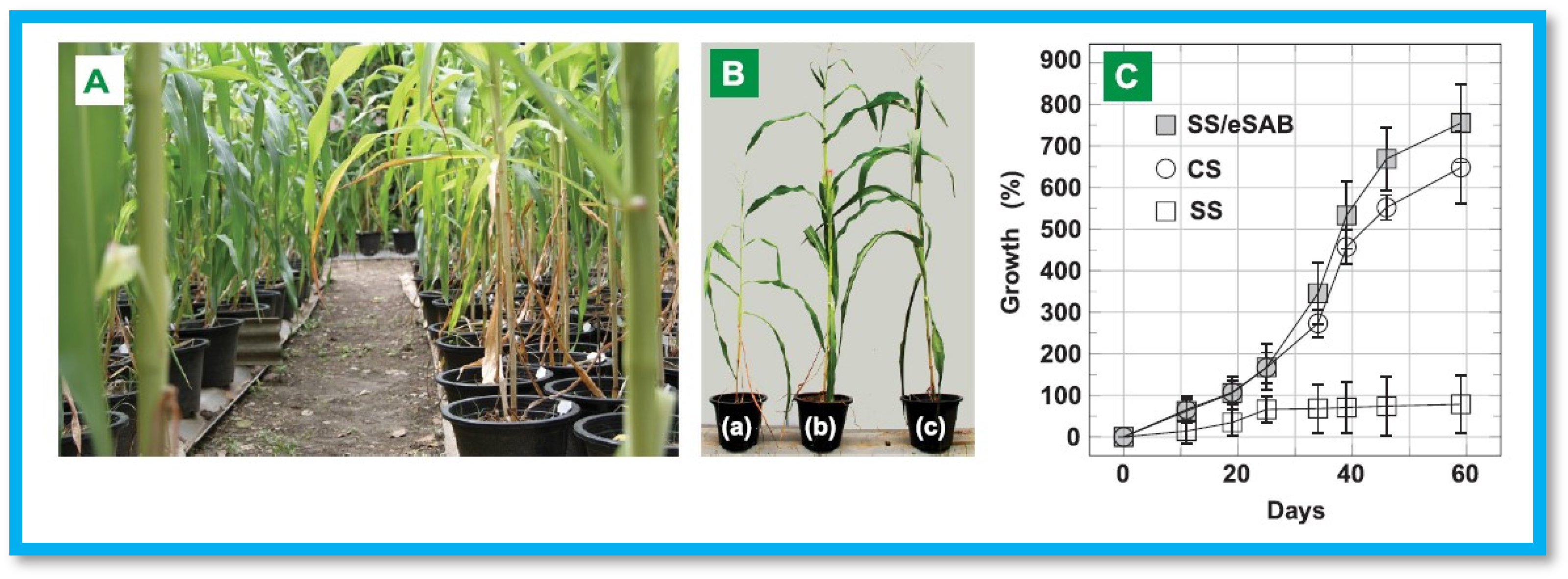
- Ghobashy, M.M.; Amin, M.A.; Ismail, M.A.; Nowwar, A.I.; El-diehy, M.A.; Gayed, H.M. Radiation Cross-Linked Ultra-Absorbent Hydrogel to Rationalize Irrigation Water and Fertilizer for Maize Planting in Drought Conditions. Int. J. Biol. Macromol. 2023, 252, 126467. [Google Scholar] [CrossRef] [PubMed]
- Raafat, A.I.; Eid, M.; El-Arnaouty, M.B. Radiation Synthesis of Superabsorbent CMC Based Hydrogels for Agriculture Applications. Nucl. Instrum. Methods Phys. Res. B 2012, 283, 71–76. [Google Scholar] [CrossRef]
- El-Naggar, A.A.; El-Salmawi, K.; Ibrahim, S.M. Characterization of Gamma Irradiated Carboxymethyl Cellulose (CMC) Films Loaded with Metal Salts and Fertilizers. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 161–167. [Google Scholar] [CrossRef]
- Wach, R.A.; Palmeri, G.; Adamus-Wlodarczyk, A.; Rokita, B.; Olejnik, A.K.; Dispenza, C.; Ulanski, P. Dual Stimuli-Responsive Polysaccharide Hydrogels Manufactured by Radiation Technique. Appl. Sci. 2022, 12, 11764. [Google Scholar] [CrossRef]
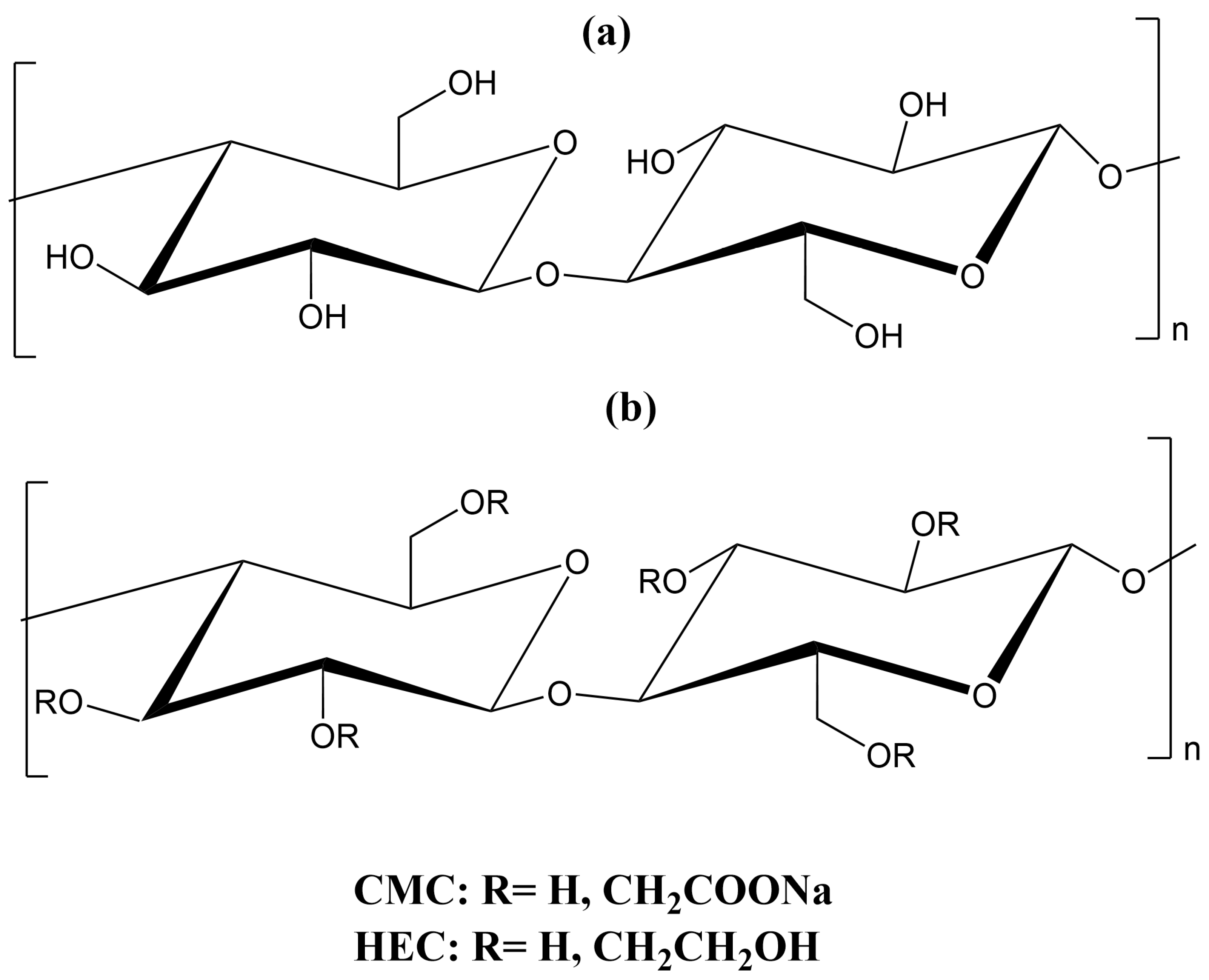

| Characteristics | Cellulose | BC | CMC |
|---|---|---|---|
| Sources | Cell wall of oomycetes, algae, and plants | Gluconacetobacter, Genera Agrobacterium, and Sarcina | CMC is a derivative of cellulose; however, the synthesis of CMC has been reported from wood residue, paper sludge, mixed office waste, textile waste, and terry towel waste |
| Synthesis route | − | − | Sodium hydroxides and sodium monochloroacetic acid |
| Solubility in water | Soluble | Insoluble | Insoluble |
| Availability | Abundance | Less abundant | Abundance |
| Mechanical properties | Moderate strength | Superior mechanical strength | Moderate strength |
| Toxicity | Non-toxic | Non-toxic | Non-toxic |
| Applications | Biomedical, textiles, electronics, and industrial | Medicines, food, industrial and commercial products | Absorbent, hydrogel, targeted delivery |
| Parameter | γ-Irradiation | EB |
|---|---|---|
| Origin | Radioactive source (Co-60) | Beams of electrons (generator/accelerator) |
| Processing time | Slow (hours) | Fast (seconds to minutes) |
| Dose delivery | Slow | Quick |
| Penetration depth | Deeper (excellent penetration) | Less deep (typically <5 cm; depends on energy and density) |
| Dose rate | Low | High |
| Direction | Non-directional (omnidirectional) | More directional (controllable) |
| Speed of irradiation | Slow pace | High speed |
| Capital investment | High (facility + source management) | High (accelerator + shielding) |
| Materials Preference | Bulky and high-density materials | Low-to medium-density, thin products |
| Environmental impact | Radioactive source management is required | No radioactive waste, only electricity is required |
| Dose uniformity | Very good (especially for complex shapes) | Good (best for simple or thin products) |
| Safety | Strict due to radioactive material, challenging waste disposal | Easier operation, no radioactive source, simpler regulation |
| Flexibility | Continuous emission, cannot be turned off instantly | Can be turned on/off instantly, adjustable dose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, M.A. Fabrication of Cellulose-Based Hydrogels Through Ionizing Radiation for Environmental and Agricultural Applications. Gels 2025, 11, 604. https://doi.org/10.3390/gels11080604
Raza MA. Fabrication of Cellulose-Based Hydrogels Through Ionizing Radiation for Environmental and Agricultural Applications. Gels. 2025; 11(8):604. https://doi.org/10.3390/gels11080604
Chicago/Turabian StyleRaza, Muhammad Asim. 2025. "Fabrication of Cellulose-Based Hydrogels Through Ionizing Radiation for Environmental and Agricultural Applications" Gels 11, no. 8: 604. https://doi.org/10.3390/gels11080604
APA StyleRaza, M. A. (2025). Fabrication of Cellulose-Based Hydrogels Through Ionizing Radiation for Environmental and Agricultural Applications. Gels, 11(8), 604. https://doi.org/10.3390/gels11080604







