Silk Fibroin–Alginate Aerogel Beads Produced by Supercritical CO2 Drying: A Dual-Function Conformable and Haemostatic Dressing
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Beads for Aerogel Processing
2.2. Evaluation of Physical and Morphological Properties of Aerogels
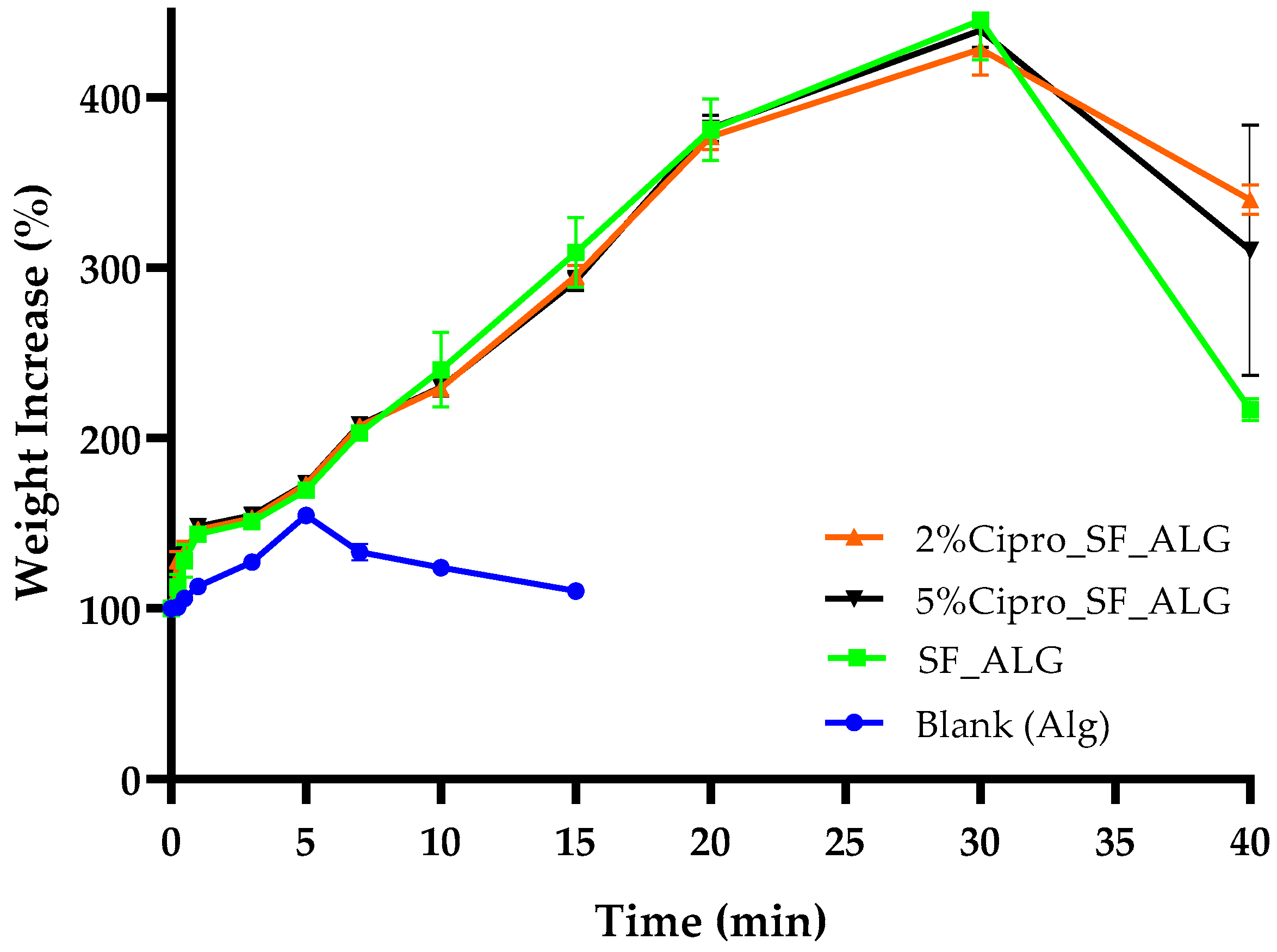
2.3. Aerogel Beads Fluid Uptake Ability
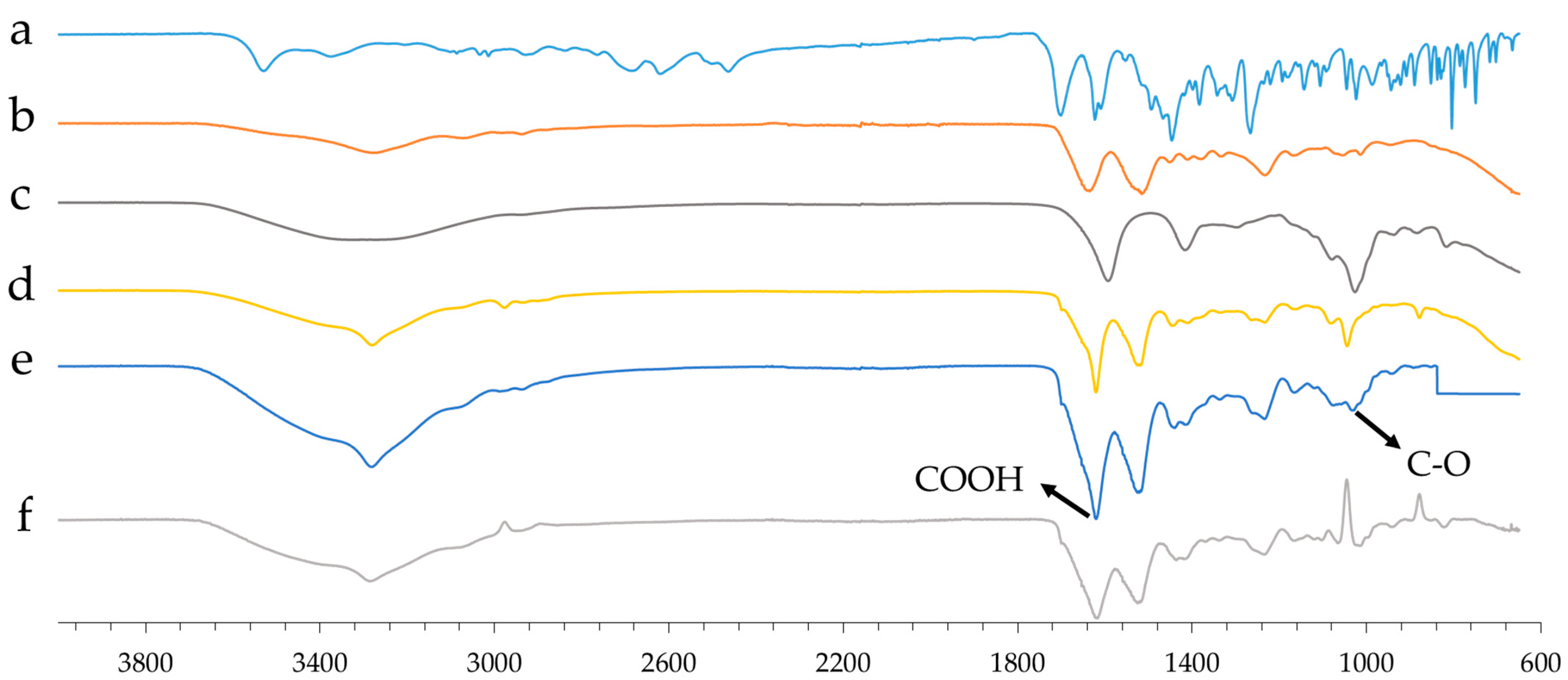
2.4. Aerogel Beads FT-IR Analysis
2.5. Aerogel Beads In Vitro Degradation
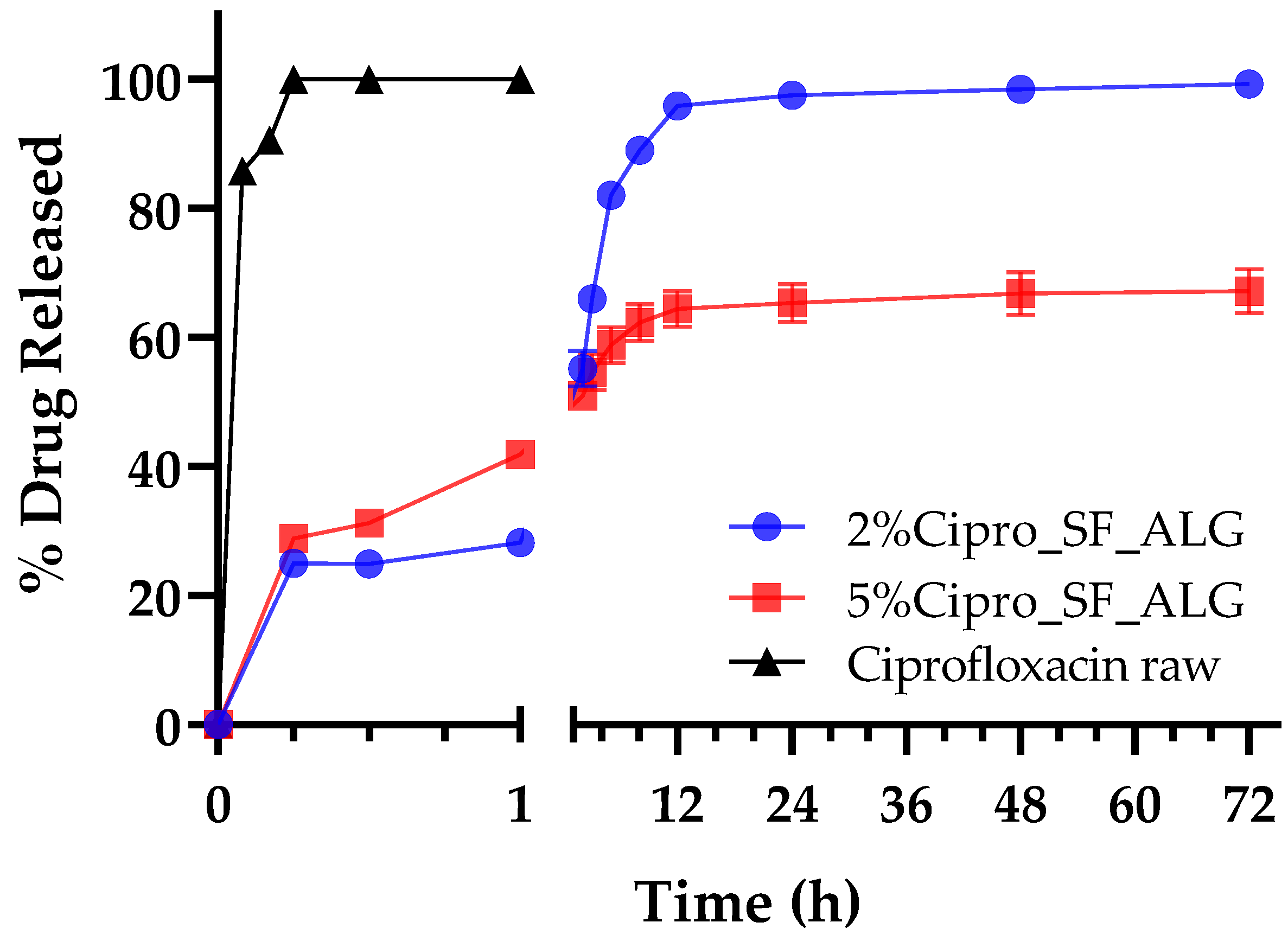
2.6. In Vitro Drug Release
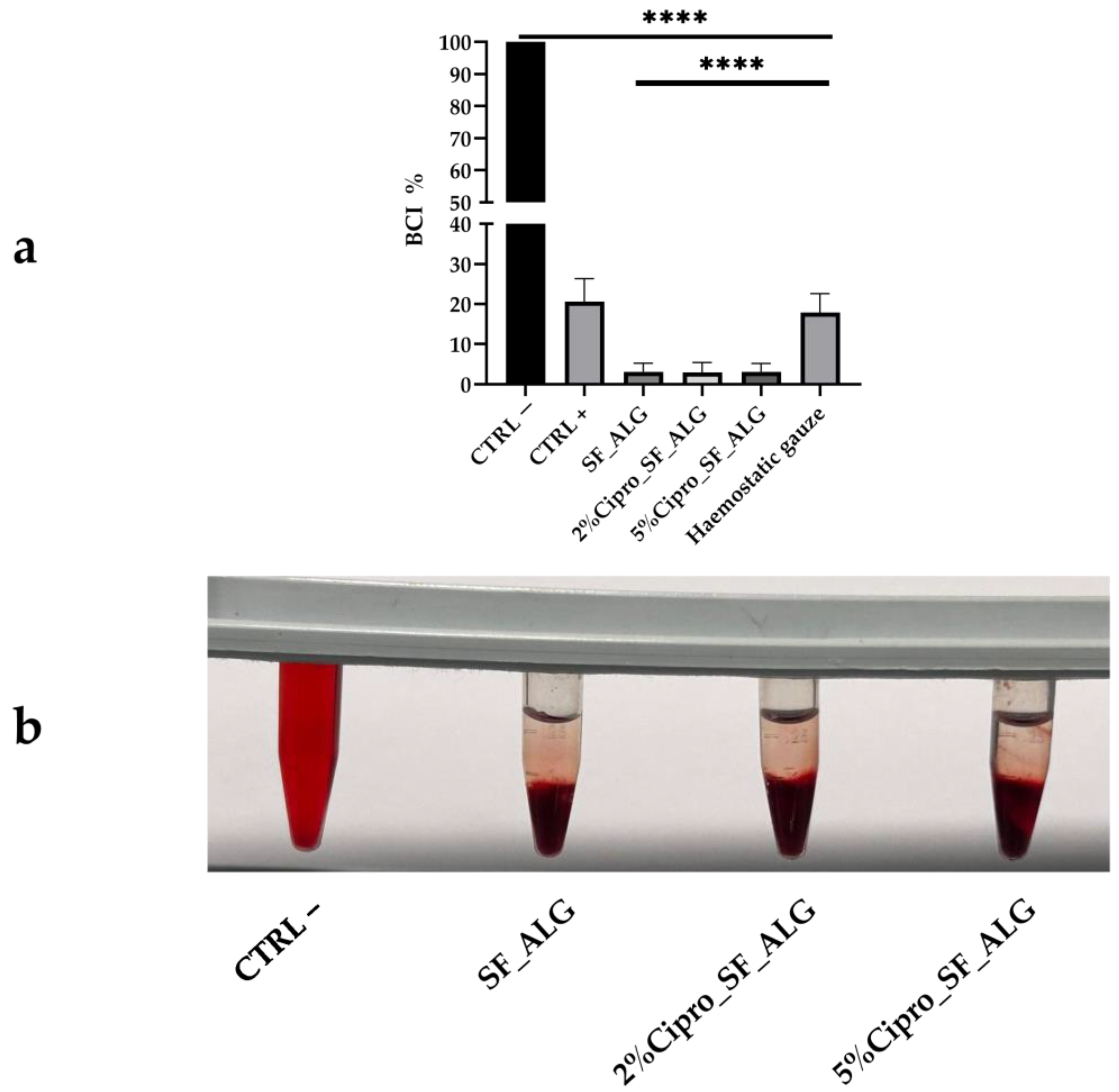
2.7. Aerogel Beads In Vitro Whole Blood Clotting Ability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. SF Extraction from Bombyx Mori Cocoons
4.2.2. Production of Drug-Loaded Gel Beads
4.2.3. Production of Drug-Loaded Aerogel Beads
4.2.4. Evaluation of Physical and Morphology Properties
4.2.5. Drug Content and Encapsulation Efficiency
4.2.6. Fluid Uptake Ability
4.2.7. In Vitro Drug Release
4.2.8. In Vitro Degradation Test
4.2.9. FT-IR Analysis
4.2.10. In Vitro Whole Blood Clotting
4.2.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACD | Acid–Citrate–Dextrose |
| BPR | Back Pressure Regulator |
| BCI | Blood Clotting Index |
| CO2 | Carbon Dioxide |
| EE% | Encapsulation Efficiency |
| FBS | Foetal Bovine Serum |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| LiBr | Lithium Bromide |
| MRD | Maximum Recovery Diluent |
| PBS | Phosphate Buffered Saline |
| PES | Polyethersulfone |
| RBCs | Red Blood Cells |
| SEM | Scanning Electron Microscopy |
| SF | Silk Fibroin |
| SS | Silk Sericin |
| SWF | Simulated Wound Fluid |
| ALG | Sodium Alginate |
| Sc-CO2 | Supercritical Carbon Dioxide |
References
- Yang, F.; Bai, X.; Dai, X.; Li, Y. The biological processes during wound healing. Regen. Med. 2021, 16, 373–390. [Google Scholar] [CrossRef]
- Bennett, B.; Towler, H.M. Haemostatic response to trauma. Br. Med. Bull. 1985, 41, 274–280. [Google Scholar] [CrossRef]
- Strodtbeck, F. Physiology of wound healing. Newborn Infant Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef]
- Sellitto, M.R.; Amante, C.; Aquino, R.P.; Russo, P.; Rodríguez-Dorado, R.; Neagu, M.; García-González, C.A.; Adami, R.; Del Gaudio, P. Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds. Gels 2023, 9, 492. [Google Scholar] [CrossRef]
- Siviello, C.; Larobina, D. Bio-based aerogels by supercritical CO2. In Biofoams: Science and Applications of Bio-Based Cellular and Porous Materials; CRC Press: Boca Raton, FL, USA, 2015; p. 227. [Google Scholar]
- Eckelman, M.J.; Sherman, J. Environmental impacts of the U.S. health care system and effects on public health. PLoS ONE 2016, 11, e0157014. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Wang, M.; Du, Z.; Tong, A.; Zhang, Y.; Luo, Y.; Shi, Z.; Qiao, S.; Huang, Z.; He, A.; Chen, X. Silkworm Silk Can Become a High-Strength and Super-Toughness Spider-Silk-Like Fiber: Multipathway Engineering Strategies and Applications. Small Struct. 2025, 6, 2400639. [Google Scholar] [CrossRef]
- Zhu, J.; Du, Y.; Backman, L.J.; Chen, J.; Ouyang, H.; Zhang, W. Cellular Interactions and Biological Effects of Silk Fibroin: Implications for Tissue Engineering and Regenerative Medicine. Small 2025, 21, 2409739. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Bernardes, B.G.; Veiga, A.; Barros, J.; García-González, C.A.; Oliveira, A.L. Sustainable Silk-Based Particulate Systems for the Controlled Release of Pharmaceuticals and Bioactive Agents in Wound Healing and Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 3133. [Google Scholar] [CrossRef]
- Gomes, V.; Salgueiro, S.P. From small to large-scale: A review of recombinant spider silk and collagen bioproduction. Discov. Mater. 2022, 2, 3. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Álvarez-López, C.; Restrepo-Osorio, A. Structural and thermal properties of silk fibroin films obtained from cocoon and waste silk fibers as raw materials. Procedia Eng. 2017, 200, 384–388. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Barakat, N.A.; Pichiah, P.T.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.-S.; Jung, C.-H.; El-Newehy, M.; Kim, H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Mao, Y.; Zhao, Z.; Zhang, J.; Zhu, L.; Chen, L.; Cao, L. Novel fabrication of antibiotic containing multifunctional silk fibroin injectable hydrogel dressing to enhance bactericidal action and wound healing efficiency on burn wound: In vitro and in vivo evaluations. Int. Wound J. 2022, 19, 679–691. [Google Scholar] [CrossRef]
- Bhat, U.M.; Khan, N.A.; Raza, S.N.; Ali, M.; Mehdi, S.; Mohiuddin, I.; Shakeel, F.; Bhat, Z.A.; Bader, G.N.; Chashoo, I.A.; et al. Ciprofloxacin hydrochloride-loaded ocular silk fibroin liposomes: Formulation, characterisation, in vitro cytotoxicity, and antimicrobial activity. Heliyon 2024, 10, e38777. [Google Scholar] [CrossRef]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Blandino, A.; Macias, M.; Cantero, D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef] [PubMed]
- De Soricellis, C.; Amante, C.; Russo, P.; Aquino, R.P.; Del Gaudio, P. Prilling as an Effective Tool for Manufacturing Submicrometric and Nanometric PLGA Particles for Controlled Drug Delivery to Wounds: Stability and Curcumin Release. Pharmaceutics 2025, 17, 129. [Google Scholar] [CrossRef]
- Abdollahi, H.; Amiri, S.; Amiri, F.; Moradi, S.; Zarrintaj, P. Antibacterial biocomposite based on chitosan/pluronic/agarose noncovalent hydrogel: Controlled drug delivery by alginate/tetracycline beads system. J. Funct. Biomater. 2024, 15, 286. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Hilonga, A.; Kim, H.T. Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci. 2010, 12, 911–918. [Google Scholar] [CrossRef]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Mukhtar, M.; Csóka, I.; Martinović, J.; Šelo, G.; Bucić-Kojić, A.; Orosz, L.; Paróczai, D.; Burian, K.; Ambrus, R. Fabrication of Ciprofloxacin-Loaded Sodium Alginate Nanobeads Coated with Thiol-Anchored Chitosan Using B-390 Encapsulator Following Optimization by DoE. Pharmaceutics 2024, 16, 691. [Google Scholar] [CrossRef]
- Sadeghi-Aghbash, M.; Rahimnejad, M.; Adeli, H.; Feizi, F. Catecholamines polymerization crosslinking for alginate-based burn wound dressings developed with ciprofloxacin and zinc oxide interactions. Int. J. Biol. Macromol. 2024, 260, 129400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.-l.; Dai, F.-Y.; Zhang, H.-H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.-Z. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef]
- Gil, E.S.; Frankowski, D.J.; Spontak, R.J.; Hudson, S.M. Swelling behavior and morphological evolution of mixed gelatin/silk fibroin hydrogels. Biomacromolecules 2005, 6, 3079–3087. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Fogh, K. Clinical utility of foam dressings in wound management: A review. Chronic Wound Care Manag. Res. 2015, 2, 31–38. [Google Scholar] [CrossRef]
- O′Meara, S.; Martyn-St James, M. Foam dressings for venous leg ulcers. Cochrane Database Syst. Rev. 2013, 2013, CD009907. [Google Scholar] [CrossRef]
- Alvarez, O.M.; Mertz, P.M.; Eaglstein, W.H. The effect of occlusive dressings on collagen synthesis and re-epithelialization in superficial wounds. J. Surg. Res. 1983, 35, 142–148. [Google Scholar] [CrossRef]
- Feng, F.; Zhao, Z.; Li, J.; Huang, Y.; Chen, W. Multifunctional dressings for wound exudate management. Prog. Mater. Sci. 2024, 146, 101328. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef]
- Lujerdean, C.; Baci, G.-M.; Cucu, A.-A.; Dezmirean, D.S. The contribution of silk fibroin in biomedical engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Li, G.; Wang, P.; Yang, Y. Tailoring degradation rates of silk fibroin scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 104–113. [Google Scholar] [CrossRef]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising nanostructured materials in fluid management, healing and regeneration of wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- Lai, C. Preparation chitosan lactate-hyaluronate sponges with unidirectional porous structure and their potential use as wound dressings. Int. J. Biosci. Biochem. Bioinform. 2014, 4, 71–77. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Dorado, R.; Landín, M.; Altai, A.; Russo, P.; Aquino, R.P.; Del Gaudio, P. A novel method for the production of core-shell microparticles by inverse gelation optimized with artificial intelligent tools. Int. J. Pharm. 2018, 538, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; McSpadden, S. Diffusional release of a solute from a polymer matrix. J. Membr. Sci. 1976, 1, 33–48. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Peterchristoper, G. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Papadokostaki, K.; Petrou, J. Combined experimental and computer simulation study of the kinetics of solute release from a relaxing swellable polymer matrix. I. Characterization of non-Fickian solvent uptake. J. Appl. Polym. Sci. 2004, 92, 2458–2467. [Google Scholar] [CrossRef]
- Paul, D. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Kolev, A.; Vassileva, V.; Radev, L. Antibacterial fibroin/alginate blended biomaterials containing Silver and Ciprofloxacin. Imp. J. Interdiscip. Res. 2016, 2. [Google Scholar]
- Pourseif, T.; Ghafelehbashi, R.; Abdihaji, M.; Radan, N.; Kaffash, E.; Heydari, M.; Naseroleslami, M.; Mousavi-Niri, N.; Akbarzadeh, I.; Ren, Q. Chitosan-based nanoniosome for potential wound healing applications: Synergy of controlled drug release and antibacterial activity. Int. J. Biol. Macromol. 2023, 230, 123185. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; D′Esposito, V.; Formisano, P.; Boateng, J.S.; Quaglia, F. Composite alginate-hyaluronan sponges for the delivery of tranexamic acid in postextractive alveolar wounds. J. Pharm. Sci. 2018, 107, 654–661. [Google Scholar] [CrossRef]
- Sultan, M.T.; Hong, H.; Lee, O.J.; Ajiteru, O.; Lee, Y.J.; Lee, J.S.; Lee, H.; Kim, S.H.; Park, C.H. Silk fibroin-based biomaterials for hemostatic applications. Biomolecules 2022, 12, 660. [Google Scholar] [CrossRef]
- Siddiqua, A.; Clutter, E.; Garklavs, O.; Kanniyappan, H.; Wang, R.R. Electrospun Silk-ICG Composite Fibers and the Application toward Hemorrhage Control. J. Funct. Biomater. 2024, 15, 272. [Google Scholar] [CrossRef]
- Miyaguchi, Y.; Hu, J. Physicochemical properties of silk fibroin after solubilization using calcium chloride with or without ethanol. Food Sci. Technol. Res. 2005, 11, 37–42. [Google Scholar] [CrossRef]
- Cerciello, A.; Auriemma, G.; Morello, S.; Pinto, A.; Del Gaudio, P.; Russo, P.; Aquino, R.P. Design and in vivo anti-inflammatory effect of ketoprofen delayed delivery systems. J. Pharm. Sci. 2015, 104, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Juhász, Á.; Ungor, D.; Berta, K.; Seres, L.; Csapó, E. Spreadsheet-based nonlinear analysis of in vitro release properties of a model drug from colloidal carriers. J. Mol. Liq. 2021, 328, 115405. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
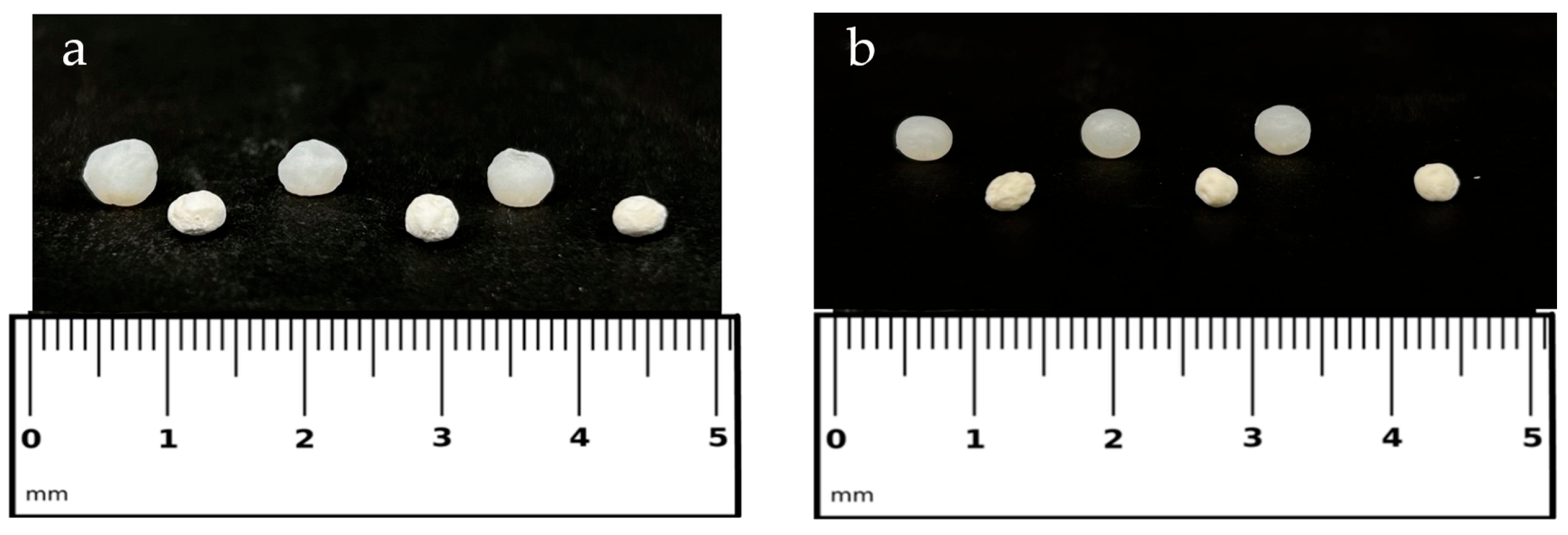
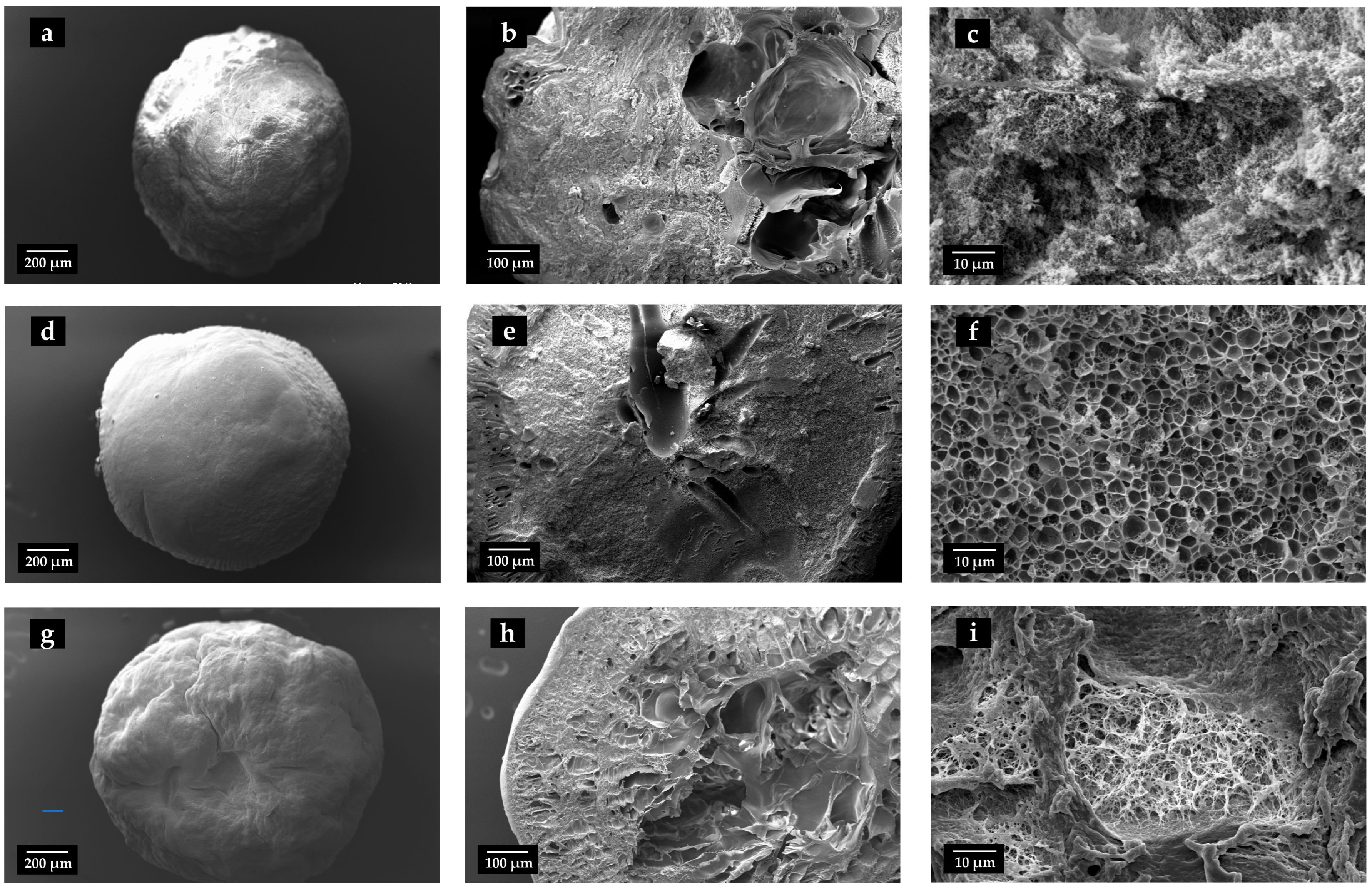

| Samples | Diameter ± sd ALCOGEL (mm) | Diameter ± sd AEROGEL (mm) | Apparent Density ± sd (g/cm3) | Sphericity Coefficient (SC) | EE ± sd (%) | Drug Content ± sd (%) |
|---|---|---|---|---|---|---|
| SF_ALG | 4.22 ± 0.08 | 3.48 ± 0.34 | 0.19 ± 0.03 | 0.93 | - | - |
| 2%Cipro_SF_ALG | 4.78 ± 0.04 *** | 3.42 ± 0.13 | 0.26 ± 0.05 | 0.90 | 42.75 ± 3.22 | 0.80 ± 0.08 |
| 5%Cipro_SF_ALG | 5.37 ± 0.09 **** | 4.34 ± 0.11 * | 0.31 ± 0.02 * | 0.93 | 49.05 ± 4.91 | 2.27 ± 0.23 |
| Model | 2%Cipro_SF_ALG (R2) | 5%Cipro_SF_ALG (R2) |
|---|---|---|
| First order | 0.9843 | 0.4253 |
| Second order | 0.9843 | 0.4253 |
| Hixson–Crowell | 0.4193 | 0.4193 |
| Higuchi | 0.9184 | 0.9729 |
| Weibull | 0.9870 (β = 0.84) | 0.8717 (β = 0.19) |
| Korsmeyer–Peppas | 0.9498 (n = 0.65) | 0.9673 (n = 0.62) |
| Peppas–Sahlin | 0.8671 (m = 0.08; k2 = 0.49) | 0.8671 (m; k2 = 0.08) |
| Hopfenberg | 0.7129 | 0.4195 |
| Model | Equation |
|---|---|
| First order | Mt = M∞ (1 − ekt) |
| Second order | dMt/dt = k (M∞ − Mt) |
| Hixson–Crowell | Mt1/3 = M∞1/3 − kt |
| Higuchi | Mt = kt1/2 |
| Weibull | Mt = M∞(1 − e−(t/T)β) |
| Korsmeyer–Peppas | Mt/M∞ = ktn |
| Peppas–Sahlin | Mt/M∞ = k1tm + k2t2m |
| Hopfenberg | Mt/M∞ = 1 − (1 − kt)n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellitto, M.R.; Larobina, D.; De Soricellis, C.; Amante, C.; Falcone, G.; Russo, P.; Bernardes, B.G.; Oliveira, A.L.; Del Gaudio, P. Silk Fibroin–Alginate Aerogel Beads Produced by Supercritical CO2 Drying: A Dual-Function Conformable and Haemostatic Dressing. Gels 2025, 11, 603. https://doi.org/10.3390/gels11080603
Sellitto MR, Larobina D, De Soricellis C, Amante C, Falcone G, Russo P, Bernardes BG, Oliveira AL, Del Gaudio P. Silk Fibroin–Alginate Aerogel Beads Produced by Supercritical CO2 Drying: A Dual-Function Conformable and Haemostatic Dressing. Gels. 2025; 11(8):603. https://doi.org/10.3390/gels11080603
Chicago/Turabian StyleSellitto, Maria Rosaria, Domenico Larobina, Chiara De Soricellis, Chiara Amante, Giovanni Falcone, Paola Russo, Beatriz G. Bernardes, Ana Leite Oliveira, and Pasquale Del Gaudio. 2025. "Silk Fibroin–Alginate Aerogel Beads Produced by Supercritical CO2 Drying: A Dual-Function Conformable and Haemostatic Dressing" Gels 11, no. 8: 603. https://doi.org/10.3390/gels11080603
APA StyleSellitto, M. R., Larobina, D., De Soricellis, C., Amante, C., Falcone, G., Russo, P., Bernardes, B. G., Oliveira, A. L., & Del Gaudio, P. (2025). Silk Fibroin–Alginate Aerogel Beads Produced by Supercritical CO2 Drying: A Dual-Function Conformable and Haemostatic Dressing. Gels, 11(8), 603. https://doi.org/10.3390/gels11080603









