The Potential of Functional Hydrogels in Burns Treatment
Abstract
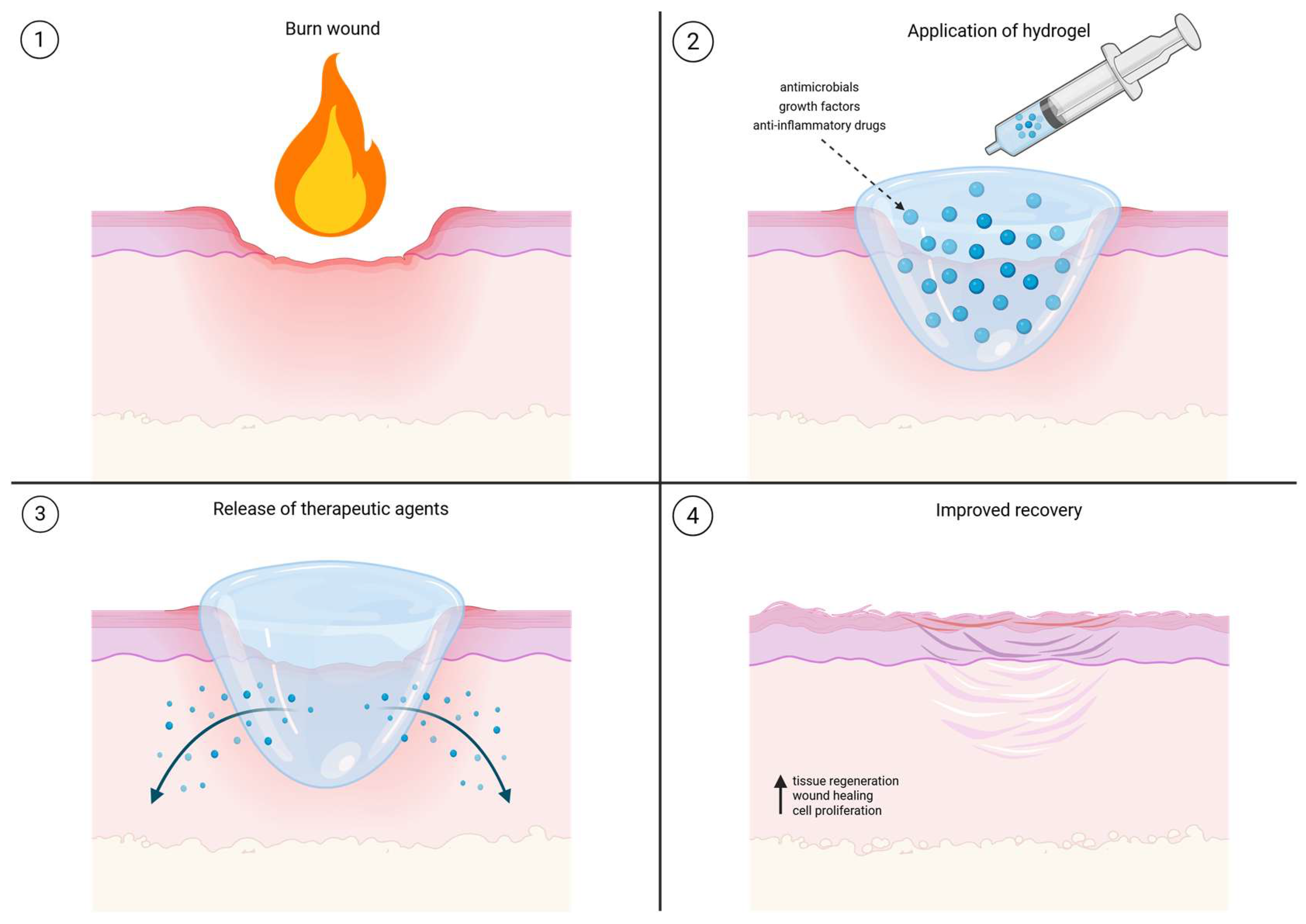
1. Introduction
2. Burn Wound Management: Characteristics and Therapeutic Interventions
2.1. Burn Wound Categorization
2.2. The Post-Burn Immune Response
2.3. Interventions and Treatments
2.3.1. Skin Grafting
Limitations and Challenges of Skin Grafts
2.3.2. Artificial Substitutes
Acellular Dermal Matrices
Criteria for Burn Dressings
3. Comprehensive Applications of Hydrogels in Burn Care
3.1. Hydrogel Structure, Properties and Advantages for Burn Treatment
Advantages of Hydrogels
3.2. Multifunctional Application of Hydrogels in Burn Care
3.2.1. Surgical Applications of Hydrogels and Role as a Skin Substitutes
3.2.2. Antimicrobial Hydrogels
3.2.3. Hydrogels in Nerve Damage Treatment
3.2.4. Hydrogel-Based Cooling and First-Aid Applications
3.2.5. Future Perspectives on Functional Hydrogels in Burn Care
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Schaefer, T.J.; Nunez Lopez, O. Burn Resuscitation and Management. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Anyanwu, J.A.; Cindass, R. Burn Debridement, Grafting, and Reconstruction. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y.; Park, K. 1—Hydrogel Swelling Behavior and Its Biomedical Applications. In Biomedical Hydrogels; Rimmer, S., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 3–24. [Google Scholar]
- Sudhakar, C.K.; Upadhyay, N.; Jain, A.; Verma, A.; Narayana Charyulu, R.; Jain, S. Chapter 5—Hydrogels—Promising Candidates for Tissue Engineering. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 77–94. [Google Scholar]
- Walker, A.; Baumber, R.; Robson, B. Pre-hospital management of burns by the UK fire service. Emerg. Med. J. 2005, 22, 205–208. [Google Scholar] [CrossRef]
- Bratland, B. Tekutinová Resuscitace Při Popáleninovém Šoku; Univerzita Karlova: Prague, Czechia, 2010. [Google Scholar]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Korkmaz, H.I.; Flokstra, G.; Waasdorp, M.; Pijpe, A.; Papendorp, S.G.; de Jong, E.; Rustemeyer, T.; Gibbs, S.; van Zuijlen, P.P.M. The Complexity of the Post-Burn Immune Response: An Overview of the Associated Local and Systemic Complications. Cells 2023, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Teot, L.; Otman, S.; Brancati, A.; Mittermayr, R. Burn Wound Healing: Pathophysiology. In Handbook of Burns: Reconstruction and Rehabilitation Volume 2; Kamolz, L.-P., Jeschke, M.G., Horch, R.E., Küntscher, M., Brychta, P., Eds.; Springer: Vienna, Austria, 2012; pp. 47–54. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef]
- LaPelusa, A.; Dave, H.D. Physiology, Hemostasis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lu, M.; Zhao, J.; Wang, X.; Zhang, J.; Shan, F.; Jiang, D. Research advances in prevention and treatment of burn wound deepening in early stage. Front. Surg. 2022, 9, 1015411. [Google Scholar] [CrossRef]
- Hicks, K.E.; Huynh, M.N.Q.; Jeschke, M.; Malic, C. Dermal regenerative matrix use in burn patients: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1741–1751. [Google Scholar] [CrossRef]
- Lund, T.; Onarheim, H.; Reed, R.K. Pathogenesis of edema formation in burn injuries. World J. Surg 1992, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Ünal, S.; Ersoz, G.; Demirkan, F.; Arslan, E.; Tütüncü, N.; Sari, A. Analysis of Skin-Graft Loss Due to Infection: Infection-Related Graft Loss. Ann. Plast. Surg. 2005, 55, 102–106. [Google Scholar]
- Reddy, S.; El-Haddawi, F.; Fancourt, M.; Farrant, G.; Gilkison, W.; Henderson, N.; Kyle, S.; Mosquera, D. The incidence and risk factors for lower limb skin graft failure. Dermatol. Res. Pract. 2014, 2014, 582080. [Google Scholar] [CrossRef] [PubMed]
- Braza, M.E.; Marietta, M.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bayramiçli, M.; Jackson, I.T.; Herschman, B. Innervation of skin grafts over free muscle flaps. Br. J. Plast Surg. 2000, 53, 130–136. [Google Scholar] [CrossRef]
- Fatah, M.F.; Ward, C.M. The morbidity of split-skin graft donor sites in the elderly: The case for mesh-grafting the donor site. Br. J. Plast. Surg. 1984, 37, 184–190. [Google Scholar] [CrossRef]
- Adams, D.C.; Ramsey, M.L. Grafts in dermatologic surgery: Review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatol. Surg. 2005, 31, 1055–1067. [Google Scholar] [CrossRef]
- Younis, A.S.; Abdelmonem, I.M.; Mohamed, Y.R.; Alnaggar, H.E.; Villanueva, G.; Thompson, J.Y.; Areia, C.; El-Dessokey, H.A.; Nabhan, A.F. Hydrogel dressings for donor sites of split-thickness skin grafts. Cochrane Database Syst. Rev. 2020, 2020, CD013570. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Juma, A.; Oudit, D.; Ellabban, M. Patterns of Sensory and Autonomic Reinnervation of Long-Standing Myocutaneous Microvascular Flaps and Split-Skin Grafts Applied to Fascial Beds. Can. J. Plast. Surg. 2005, 13, 16–22. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Bee, Y.S.; Alonzo, B.; Ng, J.D. Review of AlloDerm Acellular Human Dermis Regenerative Tissue Matrix in Multiple Types of Oculofacial Plastic and Reconstructive Surgery. Ophthalmic. Plast Reconstr. Surg. 2015, 31, 348–351. [Google Scholar] [CrossRef]
- Pirayesh, A.; Hoeksema, H.; Richters, C.; Verbelen, J.; Monstrey, S. Glyaderm® dermal substitute: Clinical application and long-term results in 55 patients. Burns 2015, 41, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.; Lee, K.C.; Abdulsalam, A.; Amirize, E.; Kankam, H.K.N.; Ter Horst, B.; Gardiner, F.; Bamford, A.; Hejmadi, R.K.; Moiemen, N. A Histological and Clinical Study of MatriDerm® Use in Burn Reconstruction. J. Burn Care Res. 2023, 44, 1100–1109. [Google Scholar] [CrossRef]
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The Basics of Integra Dermal Regeneration Template and its Expanding Clinical Applications. Semin. Plast Surg. 2019, 33, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent developments in biopolymer-based hydrogels for tissue engineering applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.P.; Vermonden, T. Stimuli-Responsive Hydrogels: The Dynamic Smart Biomaterials of Tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Zhao, E.; Tang, X.; Li, X.; Zhao, J.; Wang, S.; Wei, G.; Yang, L.; Zhao, M. Bioactive multifunctional hydrogels accelerate burn wound healing via M2 macrophage-polarization, antioxidant and anti-inflammatory. Mater. Today Bio. 2025, 32, 101686. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef]
- Lapidot, S.A.; Kost, J. Hydrogels. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3878–3882. [Google Scholar]
- Li, N.; Liu, W.; Zheng, X.; Wang, Q.; Shen, L.; Hui, J.; Fan, D. Antimicrobial hydrogel with multiple pH-responsiveness for infected burn wound healing. Nano Res. 2023, 16, 11139–11148. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.; Akelma, H. Hydrogel burn dressing effectiveness in burn pain. Burns 2024, 50, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.L.; Tzeng, Y.S.; Chiao, H.Y.; Chiu, H.T.; Chen, C.Y.; Chu, T.S.; Huang, D.W.; Hsu, K.F.; Chang, C.K.; Wang, C.H.; et al. Clinical Performance of Hydrogel-Based Dressing in Facial Burn Wounds: A Retrospective Observational Study. Ann. Plast. Surg. 2021, 86, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Dhaliwal, K.; Lopez, N. Hydrogel dressings and their application in burn wound care. Br. J. Community Nurs. 2018, 23, S24–S27. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. N. J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Muhammad, N.; Wang, W.; Zhang, J. Latest advance anti-inflammatory hydrogel wound dressings and traditional Lignosus rhinoceros used for wound healing agents. Front. Bioeng. Biotechnol. 2024, 12, 1488748. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef]
- Keen, E.F., 3rd; Robinson, B.J.; Hospenthal, D.R.; Aldous, W.K.; Wolf, S.E.; Chung, K.K.; Murray, C.K. Incidence and bacteriology of burn infections at a military burn center. Burns 2010, 36, 461–468. [Google Scholar] [CrossRef]
- Tronci, G.; Yin, J.; Holmes, R.A.; Liang, H.; Russell, S.J.; Wood, D.J. Protease-sensitive atelocollagen hydrogels promote healing in a diabetic wound model. J. Mater. Chem. B 2016, 4, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio. 2022, 16, 100429. [Google Scholar] [CrossRef]
- Krishnan, P.; Frew, Q.; Green, A.; Martin, R.; Dziewulski, P. Cause of death and correlation with autopsy findings in burns patients. Burns 2013, 39, 583–588. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Harish, D.; Singh, V.P.; Bangar, S. Septicemia as a cause of death in burns: An autopsy study. Burns 2006, 32, 545–549. [Google Scholar] [CrossRef]
- Uppuluri, V.; Shanmugarajan, T.S. Icariin-Loaded Polyvinyl Alcohol/Agar Hydrogel: Development, Characterization, and In Vivo Evaluation in a Full-Thickness Burn Model. Int. J. Low Extrem. Wounds 2019, 18, 323–335. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burns Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef]
- Weller, C. 4—Interactive Dressings and Their Role in Moist Wound Management. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 97–113. [Google Scholar]
- Yasin, S.N.N.; Said, Z.; Halib, N.; Rahman, Z.A.; Mokhzani, N.I. Polymer-Based Hydrogel Loaded with Honey in Drug Delivery System for Wound Healing Applications. Polymers 2023, 15, 3085. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Seetharaman, S.; Wrice, N.L.; Christy, R.J.; Natesan, S. Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration. PLoS ONE 2019, 14, e0217965. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.-Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef]
- Nuutila, K.; Grolman, J.; Yang, L.; Broomhead, M.; Lipsitz, S.; Onderdonk, A.; Mooney, D.; Eriksson, E. Immediate Treatment of Burn Wounds with High Concentrations of Topical Antibiotics in an Alginate Hydrogel Using a Platform Wound Device. Adv. Wound Care 2020, 9, 48–60. [Google Scholar] [CrossRef]
- Huang, X.; Yang, J.; Zhang, R.; Ye, L.; Li, M.; Chen, W. Phloroglucinol Derivative Carbomer Hydrogel Accelerates MRSA-Infected Wounds’ Healing. Int. J. Mol. Sci. 2022, 23, 8682. [Google Scholar] [CrossRef]
- Chhibber, T.; Gondil, V.S.; Sinha, V.R. Development of Chitosan-Based Hydrogel Containing Antibiofilm Agents for the Treatment of Staphylococcus aureus-Infected Burn Wound in Mice. AAPS PharmSciTech 2020, 21, 43. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, M.; Liu, Q.; Yan, S.; Feng, L.; Lan, Y.; Shan, G.; Xue, W.; Guo, R. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater. Sci. 2018, 6, 2472–2486. [Google Scholar] [CrossRef]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-Containing Agarose Hydrogel for Wound and Burn Care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The concurrent use of probiotic microorganism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef]
- Rana, M.M.; Rahman, M.S.; Ullah, M.A.; Siddika, A.; Hossain, M.L.; Akhter, M.S.; Hasan, M.Z.; Asaduzzaman, S.M. Amnion and collagen-based blended hydrogel improves burn healing efficacy on a rat skin wound model in the presence of wound dressing biomembrane. Biomed. Mater. Eng. 2020, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tamam, Y.; Tamam, C.; Tamam, B.; Ustundag, M.; Orak, M.; Tasdemir, N. Peripheral neuropathy after burn injury. Eur. Rev. Med. Pharmacol. Sci. 2013, 17 (Suppl. S1), 107–111. [Google Scholar] [PubMed]
- Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, O.; Oladipo, O.; Mahmoud, R.H.; Yosipovitch, G. Itch: from the skin to the brain–peripheral and central neural sensitization in chronic itch. Front. Mol. Neurosci. 2023, 16, 1272230. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.S.; Saffle, J.R.; Schnebly, W.A.; Hayes-Lundy, C.; Reddy, R. Sensory loss over grafted areas in patients with burns. J. Burn Care Rehabil. 1989, 10, 536–538. [Google Scholar] [CrossRef]
- Coert, J.H. Pathophysiology of nerve regeneration and nerve reconstruction in burned patients. Burns 2010, 36, 593–598. [Google Scholar] [CrossRef]
- Dong, M.; Shi, B.; Liu, D.; Liu, J.-H.; Zhao, D.; Yu, Z.-H.; Shen, X.-Q.; Gan, J.-M.; Shi, B.-l.; Qiu, Y.; et al. Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve. ACS Nano 2020, 14, 16565–16575. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xiao, C.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater. Today Bio 2023, 20, 100668. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- Lan, D.; Wu, B.; Zhang, H.; Chen, X.; Li, Z.; Dai, F. Novel Bioinspired Nerve Scaffold with High Synchrony between Biodegradation and Nerve Regeneration for Repair of Peripheral Nerve Injury. Biomacromolecules 2023, 24, 5451–5466. [Google Scholar] [CrossRef]
- Su, W.; Xu, J.; Pei, D.; Li, X.; Yang, J.; Geng, Z.; Liu, Q.; Yang, L.; Yu, S. Hybrid Electrically Conductive Hydrogels with Local Nerve Growth Factor Release Facilitate Peripheral Nerve Regeneration. ACS Appl. Bio Mater. 2023, 6, 5854–5863. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, J.; Tang, Y.; Deng, X.; Liang, C.; Du, J.; Ge, Z.; Wang, D.; Shen, Y.; Lin, W.; et al. Dynamic adaptive hydrogel facilitates neuroregeneration in segmental nerve deficits via immunomodulation and mitochondrial homeostasis. Chem. Eng. J. 2024, 494, 152890. [Google Scholar] [CrossRef]
- Xhauflaire-Uhoda, E.; Paquet, P.; Piérard, G.E. Dew point effect of cooled hydrogel pads on human stratum corneum biosurface. Dermatology 2008, 216, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Klifto, K.M.; Dellon, A.L.; Hultman, C.S. Prevalence and associated predictors for patients developing chronic neuropathic pain following burns. Burns Trauma 2020, 8, tkaa011. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Fu, X.; Han, N. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. Int. J. Mol. Sci. 2024, 25, 7882. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Liu, H.; Li, S.; An, Z.; Feng, Z. Construction of bupivacaine-loaded gelatin-based hydrogel delivery system for sciatic nerve block in mice. J. Biomed. Mater. Res. A 2024, 112, 1975–1984. [Google Scholar] [CrossRef]
- Zhang, Z.; Rouabhia, M.; Wang, Z.; Roberge, C.; Shi, G.; Roche, P.; Li, J.; Dao, L.H. Electrically conductive biodegradable polymer composite for nerve regeneration: Electricity-stimulated neurite outgrowth and axon regeneration. Artif. Organs 2007, 31, 13–22. [Google Scholar] [CrossRef]
- Liu, B.; Alimi, O.A.; Wang, Y.; Kong, Y.; Kuss, M.; Krishnan, M.A.; Hu, G.; Xiao, Y.; Dong, J.; DiMaio, D.J.; et al. Differentiated mesenchymal stem cells-derived exosomes immobilized in decellularized sciatic nerve hydrogels for peripheral nerve repair. J. Control Release 2024, 368, 24–41. [Google Scholar] [CrossRef]
- Allahham, A.; Rowe, G.; Stevenson, A.; Fear, M.W.; Vallence, A.-M.; Wood, F.M. The impact of burn injury on the central nervous system. Burns Trauma 2024, 12, tkad037. [Google Scholar] [CrossRef] [PubMed]
- Demling, R.H.; Mazess, R.B.; Wolberg, W. The effect of immediate and delayed cold immersion on burn edema formation and resorption. J. Trauma 1979, 19, 56–60. [Google Scholar] [CrossRef]
- Wright, E.H.; Tyler, M.; Vojnovic, B.; Pleat, J.; Harris, A.; Furniss, D. Human model of burn injury that quantifies the benefit of cooling as a first aid measure. Br. J. Surg. 2019, 106, 1472–1479. [Google Scholar] [CrossRef]
- Cuttle, L.; Kravchuk, O.; Wallis, B.; Kimble, R.M. An audit of first-aid treatment of pediatric burns patients and their clinical outcome. J. Burn Care Res. 2009, 30, 1028–1034. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-Demand Dissolution of a Dendritic Hydrogel-Based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 9984–9987. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringrose, N.S.; Balk, R.W.J.; Gibbs, S.; van Zuijlen, P.P.M.; Korkmaz, H.I. The Potential of Functional Hydrogels in Burns Treatment. Gels 2025, 11, 595. https://doi.org/10.3390/gels11080595
Ringrose NS, Balk RWJ, Gibbs S, van Zuijlen PPM, Korkmaz HI. The Potential of Functional Hydrogels in Burns Treatment. Gels. 2025; 11(8):595. https://doi.org/10.3390/gels11080595
Chicago/Turabian StyleRingrose, Nathalie S., Ricardo W. J. Balk, Susan Gibbs, Paul P. M. van Zuijlen, and H. Ibrahim Korkmaz. 2025. "The Potential of Functional Hydrogels in Burns Treatment" Gels 11, no. 8: 595. https://doi.org/10.3390/gels11080595
APA StyleRingrose, N. S., Balk, R. W. J., Gibbs, S., van Zuijlen, P. P. M., & Korkmaz, H. I. (2025). The Potential of Functional Hydrogels in Burns Treatment. Gels, 11(8), 595. https://doi.org/10.3390/gels11080595





