Engineering Marrow-Mimetic Hydrogel Platforms Enhance Erythropoiesis: A Mechanobiology-Driven Approach for Transfusion Red Blood Cell Production
Abstract
1. Introduction
2. Results
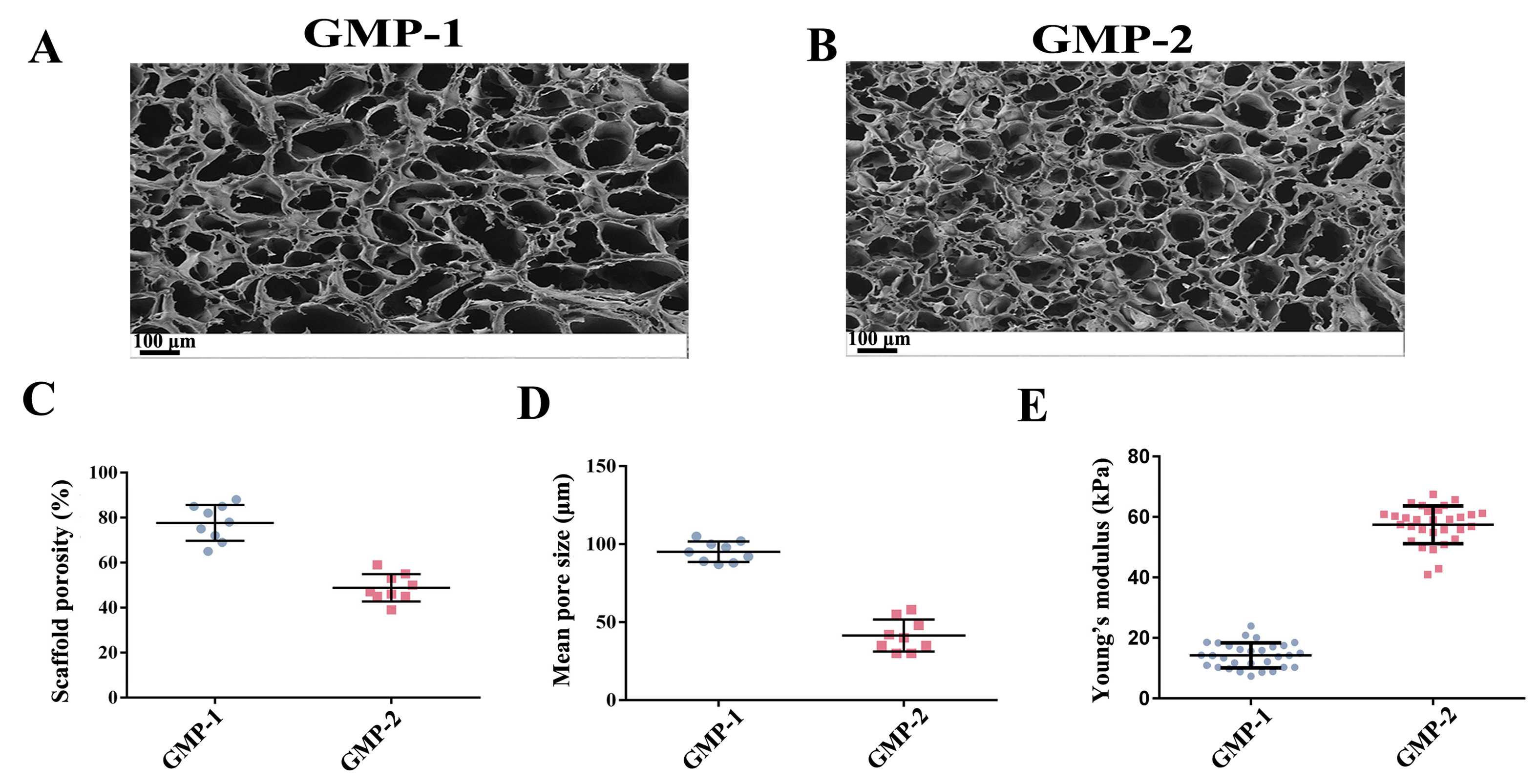
2.1. Preparation and Properties of Scaffolds
2.2. Preparation of Scaffolds with Varying Matrix Stiffness
2.3. Culture LSKs in Scaffolds with Varying Young’s Modulus
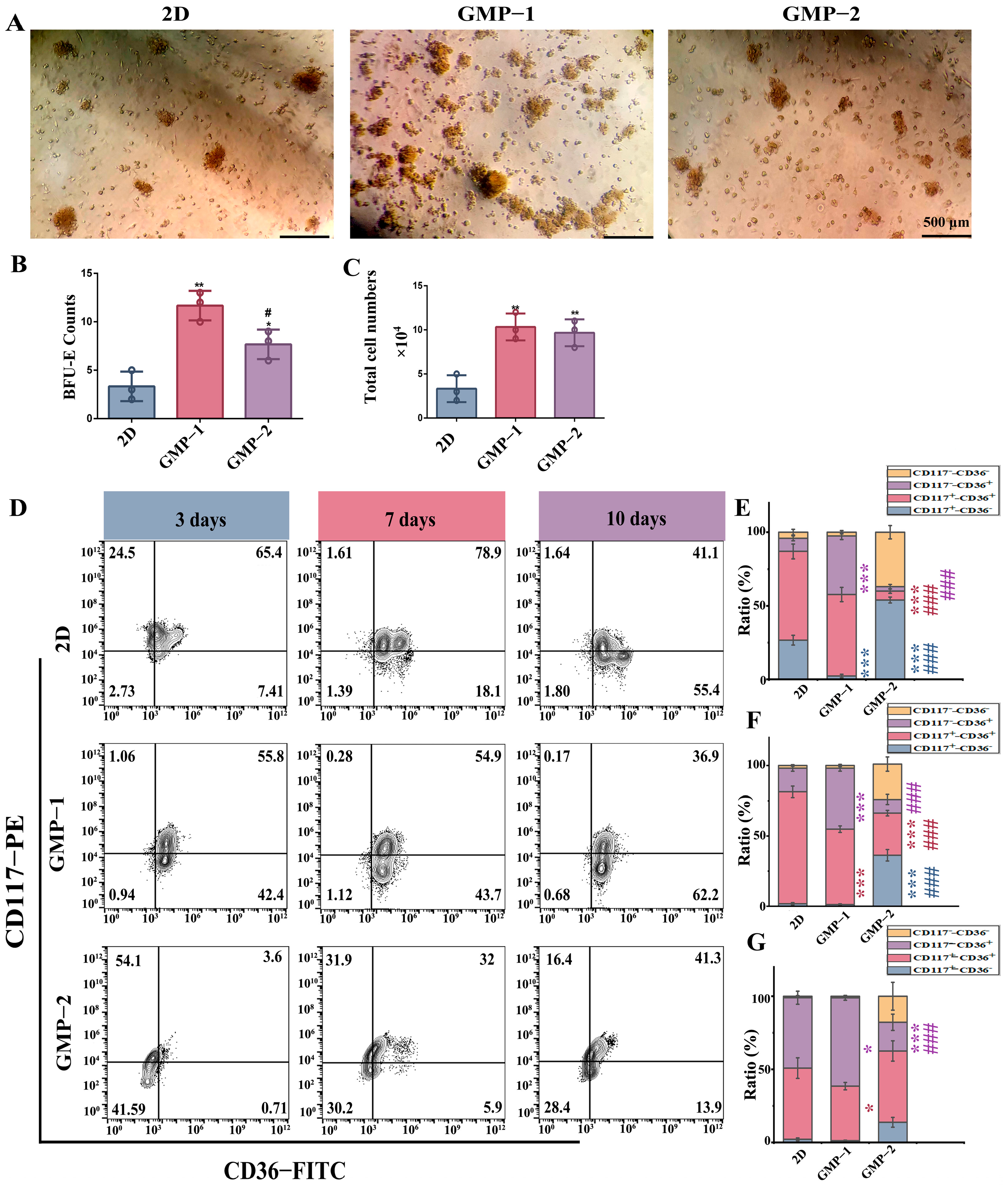
2.4. Matrix Stiffness Promotes Early Erythroid Progenitor Cell Differentiation and Proliferatio
2.5. The Influence of Mechanism Stiffness on the Differentiation Efficiency of Erythroid Cells and Enucleation of Terminal Cells
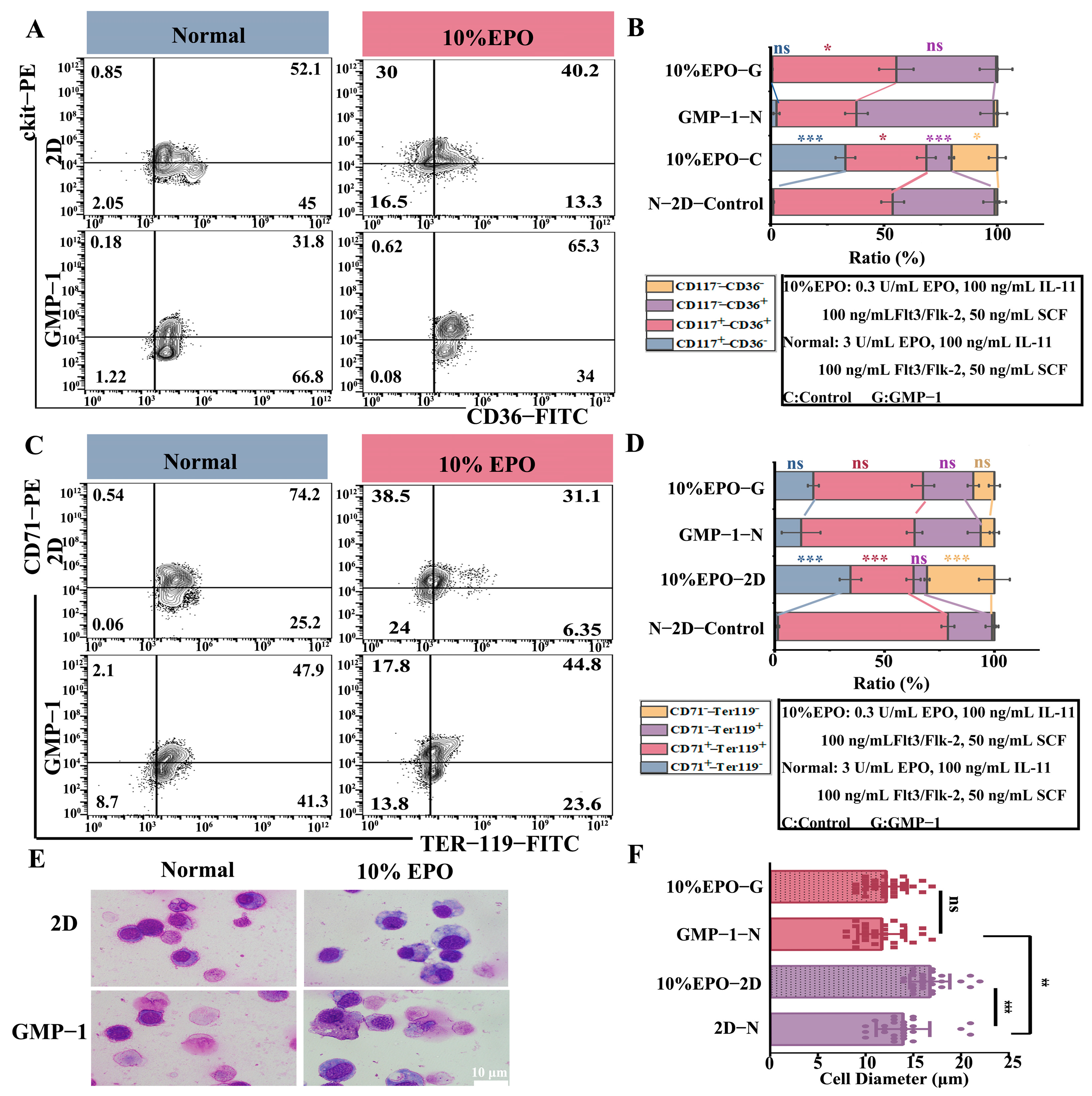
2.6. Matrix Stiffness Improves Erythroid Differentiation After Cytokine Restriction
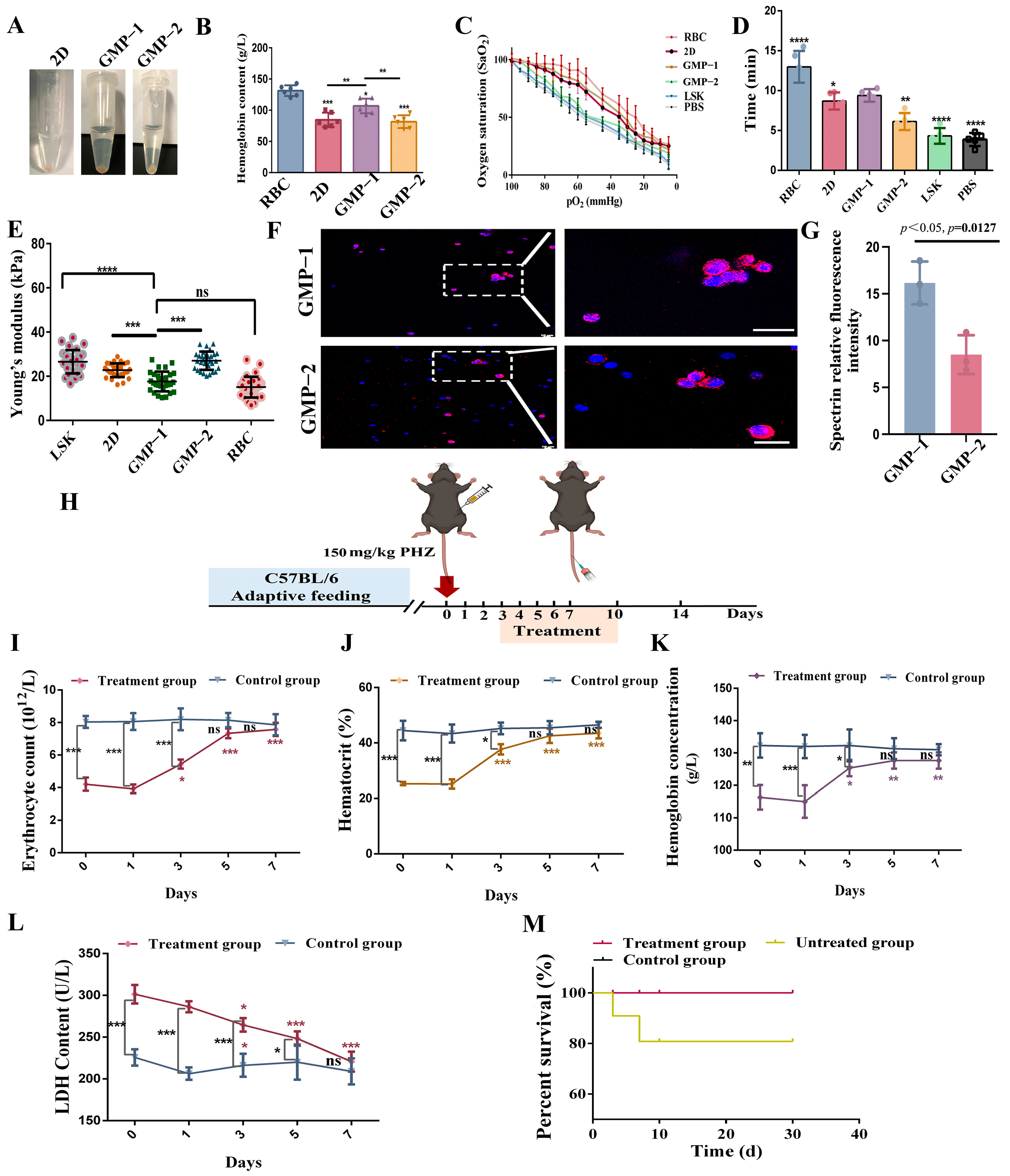
2.7. Transplantation Experiment of NOD/SCID Mice In Vivo
2.8. Cell Function Acquisition
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolation and Culture of Hematopoietic Stem Cells
5.2. Preparation of Marrow-Mimetic Hydrogel Platforms—GelMA-PEGDA
5.3. 3D Cell Culture in Scaffolds
5.4. Fourier-Transform Infrared (ATR-FTIR)
5.5. Material Hydrophilicity Test
5.6. Detection of Scaffold Degradation Rate
5.7. Young’s Modulus
5.8. Scanning Electron Microscopy (SEM)
5.9. Mercury Intrusion Porosimetry (MIP)
5.10. Cytocompatibility
5.11. Biocryo-Scanning Electron Microscopy (Cryo-SEM)
5.12. EDU Detection of Cell Proliferation
5.13. BFU-E Clone Formation Experiment
5.14. Immunofluorescence Staining
5.15. Flow Cytometry
5.16. Hemoglobin Detection
5.17. Oxygen Dissociation Curve
5.18. Wright-Giemsa Stain
5.19. Phenylhydrazine Hemolysis Recovery
5.20. Fate of Erythroid Cells in NOD/SCID Mice
5.21. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RBC | red blood cells |
| HSCs | hematopoietic stem cells |
| EPO | erythropoietin |
| SCF | stem cell factor |
| Il-11 | interleukin-11 |
| Flt3/Flk-2 | Fms-like tyrosine kinase 3/fetal liver kinase-2 |
| ECM | extracellular matrix |
| GelMA | gelatin methylallylamine |
| PEGDA | poly (ethylene glycol) diacrylate |
References
- Shander, A.; Goobie, S.M.; Warner, M.A.; Aapro, M.; Bisbe, E.; Perez-Calatayud, A.A.; Callum, J.; Cushing, M.M.; Dyer, W.B.; Erhard, J.; et al. Essential Role of Patient Blood Management in a Pandemic: A Call for Action. Anesth. Analg. 2020, 131, 74–85. [Google Scholar] [CrossRef]
- Giarratana, M.-C.; Rouard, H.; Dumont, A.; Kiger, L.; Safeukui, I.; Le Pennec, P.-Y.; François, S.; Trugnan, G.; Peyrard, T.; Marie, T.; et al. Proof of Principle for Transfusion of in Vitro-Generated Red Blood Cells. Blood 2011, 118, 5071–5079. [Google Scholar] [CrossRef]
- Seita, J.; Weissman, I.L. Hematopoietic Stem Cell: Self-Renewal versus Differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone Marrow Niches in Haematological Malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The Bone Marrow Niche for Haematopoietic Stem Cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Choi, J.S.; Harley, B.A.C. Marrow-Inspired Matrix Cues Rapidly Affect Early Fate Decisions of Hematopoietic Stem and Progenitor Cells. Sci. Adv. 2017, 3, e1600455. [Google Scholar] [CrossRef]
- Choi, J.S.; Harley, B.A.C. The Combined Influence of Substrate Elasticity and Ligand Density on the Viability and Biophysical Properties of Hematopoietic Stem and Progenitor Cells. Biomaterials 2012, 33, 4460–4468. [Google Scholar] [CrossRef]
- Weissman, I.L.; Shizuru, J.A. The Origins of the Identification and Isolation of Hematopoietic Stem Cells, and Their Capability to Induce Donor-Specific Transplantation Tolerance and Treat Autoimmune Diseases. Blood 2008, 112, 3543–3553. [Google Scholar] [CrossRef]
- Laurenti, E.; Göttgens, B. From Haematopoietic Stem Cells to Complex Differentiation Landscapes. Nature 2018, 553, 418–426. [Google Scholar] [CrossRef]
- Scadden, D.T. The Stem-Cell Niche as an Entity of Action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef]
- Schofield, R. The Relationship between the Spleen Colony-Forming Cell and the Haemopoietic Stem Cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Lee-Thedieck, C.; Spatz, J.P. Biophysical Regulation of Hematopoietic Stem Cells. Biomater. Sci. 2014, 2, 1548–1561. [Google Scholar] [CrossRef]
- Hannum, C.; Culpepper, J.; Campbell, D.; McClanahan, T.; Zurawski, S.; Bazan, J.F.; Kastelein, R.; Hudak, S.; Wagner, J.; Mattson, J. Ligand for FLT3/FLK2 Receptor Tyrosine Kinase Regulates Growth of Haematopoietic Stem Cells and Is Encoded by Variant RNAs. Nature 1994, 368, 643–648. [Google Scholar] [CrossRef]
- Poulos, M.G.; Crowley, M.J.P.; Gutkin, M.C.; Ramalingam, P.; Schachterle, W.; Thomas, J.-L.; Elemento, O.; Butler, J.M. Vascular Platform to Define Hematopoietic Stem Cell Factors and Enhance Regenerative Hematopoiesis. Stem Cell Rep. 2015, 5, 881–894. [Google Scholar] [CrossRef][Green Version]
- Miller, C.L.; Eaves, C.J. Expansion in Vitro of Adult Murine Hematopoietic Stem Cells with Transplantable Lympho-Myeloid Reconstituting Ability. Proc. Natl. Acad. Sci. USA 1997, 94, 13648–13653. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell–Extracellular Matrix Mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Di Buduo, C.A.; Careddu, F.; Metti, S.; Lunghi, M.; Diprima, S.; Camilotto, V.; Bruni, G.; Gianelli, U.; Tosi, D.; Perotti, C.; et al. In Vitro Studies of Human Erythropoiesis Using a 3D Silk-Based Bone Marrow Model That Generates Erythroblastic Islands. Blood Adv. 2025, 9, 2192–2206. [Google Scholar] [CrossRef]
- Malara, A.; Abbonante, V.; Di Buduo, C.A.; Tozzi, L.; Currao, M.; Balduini, A. The Secret Life of a Megakaryocyte: Emerging Roles in Bone Marrow Homeostasis Control. Cell Mol. Life Sci. 2015, 72, 1517–1536. [Google Scholar] [CrossRef]
- Torisawa, Y.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone Marrow-on-a-Chip Replicates Hematopoietic Niche Physiology In Vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef]
- Griffiths, R.E.; Kupzig, S.; Cogan, N.; Mankelow, T.J.; Betin, V.M.S.; Trakarnsanga, K.; Massey, E.J.; Lane, J.D.; Parsons, S.F.; Anstee, D.J. Maturing Reticulocytes Internalize Plasma Membrane in Glycophorin A-Containing Vesicles That Fuse with Autophagosomes before Exocytosis. Blood 2012, 119, 6296–6306. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer Matrices for Intestinal Stem Cell and Organoid Culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Mahadik, B.P.; Bharadwaj, N.A.K.; Ewoldt, R.H.; Harley, B.A.C. Regulating Dynamic Signaling between Hematopoietic Stem Cells and Niche Cells via a Hydrogel Matrix. Biomaterials 2017, 125, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.H.; Abroun, S.; Soleimani, M.; Mowla, S.J. 3-Dimensional Nano-Fibre Scaffold for Ex Vivo Expansion of Cord Blood Haematopoietic Stem Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 740–748. [Google Scholar] [CrossRef]
- Uriel, S.; Huang, J.-J.; Moya, M.L.; Francis, M.E.; Wang, R.; Chang, S.-Y.; Cheng, M.-H.; Brey, E.M. The Role of Adipose Protein Derived Hydrogels in Adipogenesis. Biomaterials 2008, 29, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Lo Celso, C.; Scadden, D.T. The Haematopoietic Stem Cell Niche at a Glance. J. Cell Sci. 2011, 124, 3529–3535. [Google Scholar] [CrossRef]
- Szade, K.; Gulati, G.S.; Chan, C.K.F.; Kao, K.S.; Miyanishi, M.; Marjon, K.D.; Sinha, R.; George, B.M.; Chen, J.Y.; Weissman, I.L. Where Hematopoietic Stem Cells Live: The Bone Marrow Niche. Antioxid. Redox Signal. 2018, 29, 191–204. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, C.; Cui, X.; Liang, Y. Interactions of Hematopoietic Stem Cells with Bone Marrow Niche. Methods Mol. Biol. 2021, 2346, 21–34. [Google Scholar] [CrossRef]
- Eliasson, P.M.; Jönsson, J.-I. A Hypoxic Niche in the Mouse Bone Marrow Diminishes Proliferation and Differentiation of Hematopoietic Stem Cells. Blood 2008, 112, 4777. [Google Scholar] [CrossRef]
- Gomariz, A.; Helbling, P.M.; Isringhausen, S.; Suessbier, U.; Becker, A.; Boss, A.; Nagasawa, T.; Paul, G.; Goksel, O.; Székely, G.; et al. Quantitative Spatial Analysis of Haematopoiesis-Regulating Stromal Cells in the Bone Marrow Microenvironment by 3D Microscopy. Nat. Commun. 2018, 9, 2532. [Google Scholar] [CrossRef]
- Nilsson, S.K.; Johnston, H.M.; Whitty, G.A.; Williams, B.; Webb, R.J.; Denhardt, D.T.; Bertoncello, I.; Bendall, L.J.; Simmons, P.J.; Haylock, D.N. Osteopontin, a Key Component of the Hematopoietic Stem Cell Niche and Regulator of Primitive Hematopoietic Progenitor Cells. Blood 2005, 106, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.K.; Debatis, M.E.; Dooner, M.S.; Madri, J.A.; Quesenberry, P.J.; Becker, P.S. Immunofluorescence Characterization of Key Extracellular Matrix Proteins in Murine Bone Marrow in Situ. J. Histochem. Cytochem. 1998, 46, 371–377. [Google Scholar] [CrossRef]
- Siler, U.; Seiffert, M.; Puch, S.; Richards, A.; Torok-Storb, B.; Müller, C.A.; Sorokin, L.; Klein, G. Characterization and Functional Analysis of Laminin Isoforms in Human Bone Marrow. Blood 2000, 96, 4194–4203. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Buxboim, A.; Spinler, K.R.; Swift, J.; Christian, D.A.; Hunter, C.A.; Léon, C.; Gachet, C.; Dingal, P.C.D.P.; Ivanovska, I.L.; et al. Contractile Forces Sustain and Polarize Hematopoiesis from Stem and Progenitor Cells. Cell Stem Cell 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Engler, A.J.; Richert, L.; Wong, J.Y.; Picart, C.; Discher, D.E. Surface Probe Measurements of the Elasticity of Sectioned Tissue, Thin Gels and Polyelectrolyte Multilayer Films: Correlations between Substrate Stiffness and Cell Adhesion. Surf. Sci. 2004, 570, 142–154. [Google Scholar] [CrossRef]
- Engler, A.J.; Rehfeldt, F.; Sen, S.; Discher, D.E. Microtissue Elasticity: Measurements by Atomic Force Microscopy and Its Influence on Cell Differentiation. Methods Cell Biol. 2007, 83, 521–545. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.; Watson, S.; Lord, M.S.; Eamegdool, S.S.; Bax, D.V.; Nivison-Smith, L.B.; Kondyurin, A.; Ma, L.; Oberhauser, A.F.; Weiss, A.S.; et al. Substrate Elasticity Provides Mechanical Signals for the Expansion of Hemopoietic Stem and Progenitor Cells. Nat. Biotechnol. 2010, 28, 1123–1128. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD Peptide in Cancer Targeting: Benefits, Challenges, Solutions, and Possible Integrin-RGD Interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Ulyanova, T.; Georgolopoulos, G.; Papayannopoulou, T. Reappraising the Role of A5 Integrin and the Microenvironmental Support in Stress Erythropoiesis. Exp. Hematol. 2020, 81, 16–31.e4. [Google Scholar] [CrossRef]
- Szpak, D.; Turpin, C.; Goreke, U.; Bialkowska, K.; Bledzka, K.M.; Verbovetskiy, D.; Mohandas, N.; Gurkan, U.A.; Qin, J.; Plow, E.F.; et al. Kindlin-3 Deficiency Leads to Impaired Erythropoiesis and Erythrocyte Cytoskeleton. Blood Adv. 2023, 7, 1739–1753. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Alketbi, A.S.; Shi, Y.; Li, H.; Raza, A.; Zhang, T. Impact of PEGDA Photopolymerization in Micro-Stereolithography on 3D Printed Hydrogel Structure and Swelling. Soft Matter 2021, 17, 7188–7195. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Schertl, P.; Klein, G. The Extracellular Matrix of Hematopoietic Stem Cell Niches. Adv. Drug Deliv. Rev. 2022, 181, 114069. [Google Scholar] [CrossRef]
- Akhmanova, M.; Osidak, E.; Domogatsky, S.; Rodin, S.; Domogatskaya, A. Physical, Spatial, and Molecular Aspects of Extracellular Matrix of In Vivo Niches and Artificial Scaffolds Relevant to Stem Cells Research. Stem Cells Int. 2015, 2015, 167025. [Google Scholar] [CrossRef]
- Chen, X.; Hughes, R.; Mullin, N.; Hawkins, R.J.; Holen, I.; Brown, N.J.; Hobbs, J.K. Mechanical Heterogeneity in the Bone Microenvironment as Characterized by Atomic Force Microscopy. Biophys. J. 2020, 119, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic Stem Cell Activity and Interactions with the Niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef]
- Acar, M.; Kocherlakota, K.S.; Murphy, M.M.; Peyer, J.G.; Oguro, H.; Inra, C.N.; Jaiyeola, C.; Zhao, Z.; Luby-Phelps, K.; Morrison, S.J. Deep Imaging of Bone Marrow Shows Non-Dividing Stem Cells Are Mainly Perisinusoidal. Nature 2015, 526, 126–130. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The Bone Marrow Microenvironment at Single-Cell Resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Xu, L.; Xu, Y.; Gao, Z.; Pan, Y.; Jiang, M.; Wei, Y.; Wang, L.; Liao, Y.; et al. Harnessing Matrix Stiffness to Engineer a Bone Marrow Niche for Hematopoietic Stem Cell Rejuvenation. Cell Stem Cell 2023, 30, 378–395.e8. [Google Scholar] [CrossRef]
- Chitteti, B.R.; Kacena, M.A.; Voytik-Harbin, S.L.; Srour, E.F. Modulation of Hematopoietic Progenitor Cell Fate In Vitro by Varying Collagen Oligomer Matrix Stiffness in the Presence or Absence of Osteoblasts. J. Immunol. Methods 2015, 425, 108–113. [Google Scholar] [CrossRef]
- Neildez-Nguyen, T.M.A.; Wajcman, H.; Marden, M.C.; Bensidhoum, M.; Moncollin, V.; Giarratana, M.-C.; Kobari, L.; Thierry, D.; Douay, L. Human Erythroid Cells Produced Ex Vivo at Large Scale Differentiate into Red Blood Cells in Vivo. Nat. Biotechnol. 2002, 20, 467–472. [Google Scholar] [CrossRef]
- Santos, E.J.F.; Dias, R.S.C.; Lima, J.F.d.B.; Filho, N.S.; dos Santos, A.M. Erythropoietin Resistance in Patients with Chronic Kidney Disease: Current Perspectives. Int. J. Nephrol. Renovasc. Dis. 2020, 13, 231–237. [Google Scholar] [CrossRef]
- Hu, Z.; Van Rooijen, N.; Yang, Y.-G. Macrophages Prevent Human Red Blood Cell Reconstitution in Immunodeficient Mice. Blood 2011, 118, 5938–5946. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Nocka, K.; Beier, D.R.; Chu, T.Y.; Buck, J.; Lahm, H.W.; Wellner, D.; Leder, P.; Besmer, P. The Hematopoietic Growth Factor KL Is Encoded by the Sl Locus and Is the Ligand of the C-Kit Receptor, the Gene Product of the W Locus. Cell 1990, 63, 225–233. [Google Scholar] [CrossRef]
- Audet, J.; Miller, C.L.; Rose-John, S.; Piret, J.M.; Eaves, C.J. Distinct Role of Gp130 Activation in Promoting Self-Renewal Divisions by Mitogenically Stimulated Murine Hematopoietic Stem Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.; Copley, M.; Benz, C.; Dykstra, B.; Bowie, M.; Eaves, C. Regulation of Hematopoietic Stem Cells by the Steel Factor/KIT Signaling Pathway. Clin. Cancer Res. 2008, 14, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Kumar, H.; He, X.; Lu, Q.; Bai, H.; Kim, K.; Hu, J. The Effect of Crosslinking Degree of Hydrogels on Hydrogel Adhesion. Gels 2022, 8, 682. [Google Scholar] [CrossRef]
- Raic, A.; Rödling, L.; Kalbacher, H.; Lee-Thedieck, C. Biomimetic Macroporous PEG Hydrogels as 3D Scaffolds for the Multiplication of Human Hematopoietic Stem and Progenitor Cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.E.; Cortiella, J.; Lee, J.; Niles, J.A.; Cuddihy, M.; Wang, S.; Bielitzki, J.; Cantu, A.; Mlcak, R.; Valdivia, E.; et al. In Vitro Analog of Human Bone Marrow from 3D Scaffolds with Biomimetic Inverted Colloidal Crystal Geometry. Biomaterials 2009, 30, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, J.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; O’Kelly, M.B.; Nourigat, C.; Jain, P.; Delrow, J.J.; et al. Expansion of Primitive Human Hematopoietic Stem Cells by Culture in a Zwitterionic Hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7334-08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface Modification of Polycaprolactone Nanofibers through Hydrolysis and Aminolysis: A Comparative Study on Structural Characteristics, Mechanical Properties, and Cellular Performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef]
- ASTM F1635-16; Standard Test Method for In Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- Sneddon, I.N. The Relation between Load and Penetration in the Axisymmetric Boussinesq Problem for a Punch of Arbitrary Profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Li, W.; Liang, H.; Ao, Y.; Tang, B.; Li, J.; Li, N.; Wang, J.; Du, Y. Biophysical Cues of Bone Marrow-Inspired Scaffolds Regulate Hematopoiesis of Hematopoietic Stem and Progenitor Cells. Biomaterials 2023, 298, 122111. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Liu, R.; Wang, X. Engineering Marrow-Mimetic Hydrogel Platforms Enhance Erythropoiesis: A Mechanobiology-Driven Approach for Transfusion Red Blood Cell Production. Gels 2025, 11, 594. https://doi.org/10.3390/gels11080594
Yang Q, Liu R, Wang X. Engineering Marrow-Mimetic Hydrogel Platforms Enhance Erythropoiesis: A Mechanobiology-Driven Approach for Transfusion Red Blood Cell Production. Gels. 2025; 11(8):594. https://doi.org/10.3390/gels11080594
Chicago/Turabian StyleYang, Qinqin, Runjin Liu, and Xiang Wang. 2025. "Engineering Marrow-Mimetic Hydrogel Platforms Enhance Erythropoiesis: A Mechanobiology-Driven Approach for Transfusion Red Blood Cell Production" Gels 11, no. 8: 594. https://doi.org/10.3390/gels11080594
APA StyleYang, Q., Liu, R., & Wang, X. (2025). Engineering Marrow-Mimetic Hydrogel Platforms Enhance Erythropoiesis: A Mechanobiology-Driven Approach for Transfusion Red Blood Cell Production. Gels, 11(8), 594. https://doi.org/10.3390/gels11080594





