Hydrogels Modulating the Microbiome: Therapies for Tissue Regeneration with Infection Control
Abstract
1. Introduction
2. Design of Hydrogels with Antimicrobial and Modulatory Capacity
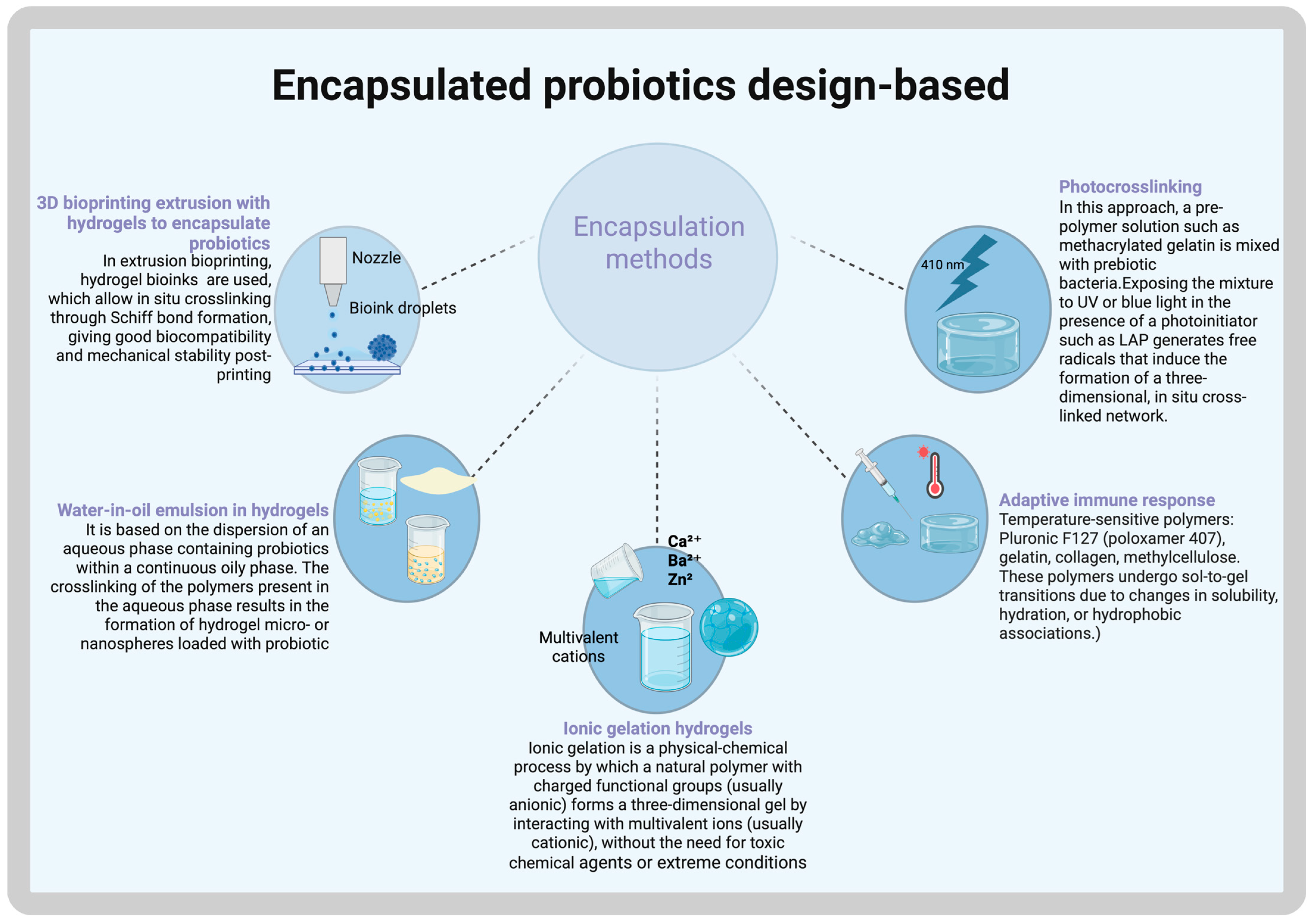
Encapsulated Probiotics
3. Customizing Hydrogel Methods
3D Bioprinting Techniques to Customize the Shape and Hydrogel Gelation via UV Irradiation or Thermal Self-Assembly
4. Mechanisms of Microbiome Modulation
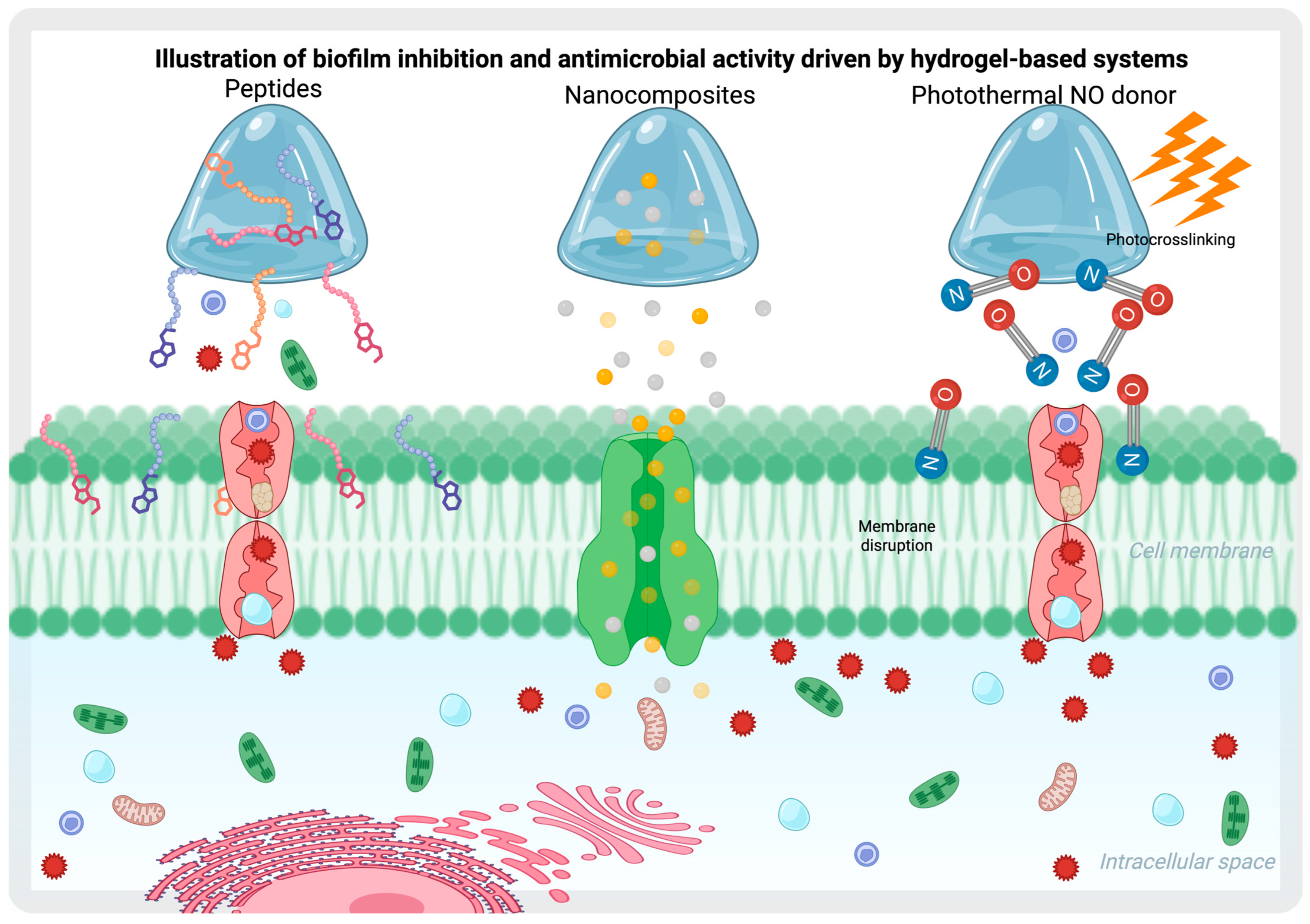
4.1. Biofilm Inhibition and Antimicrobial Effect Through Hydrogels
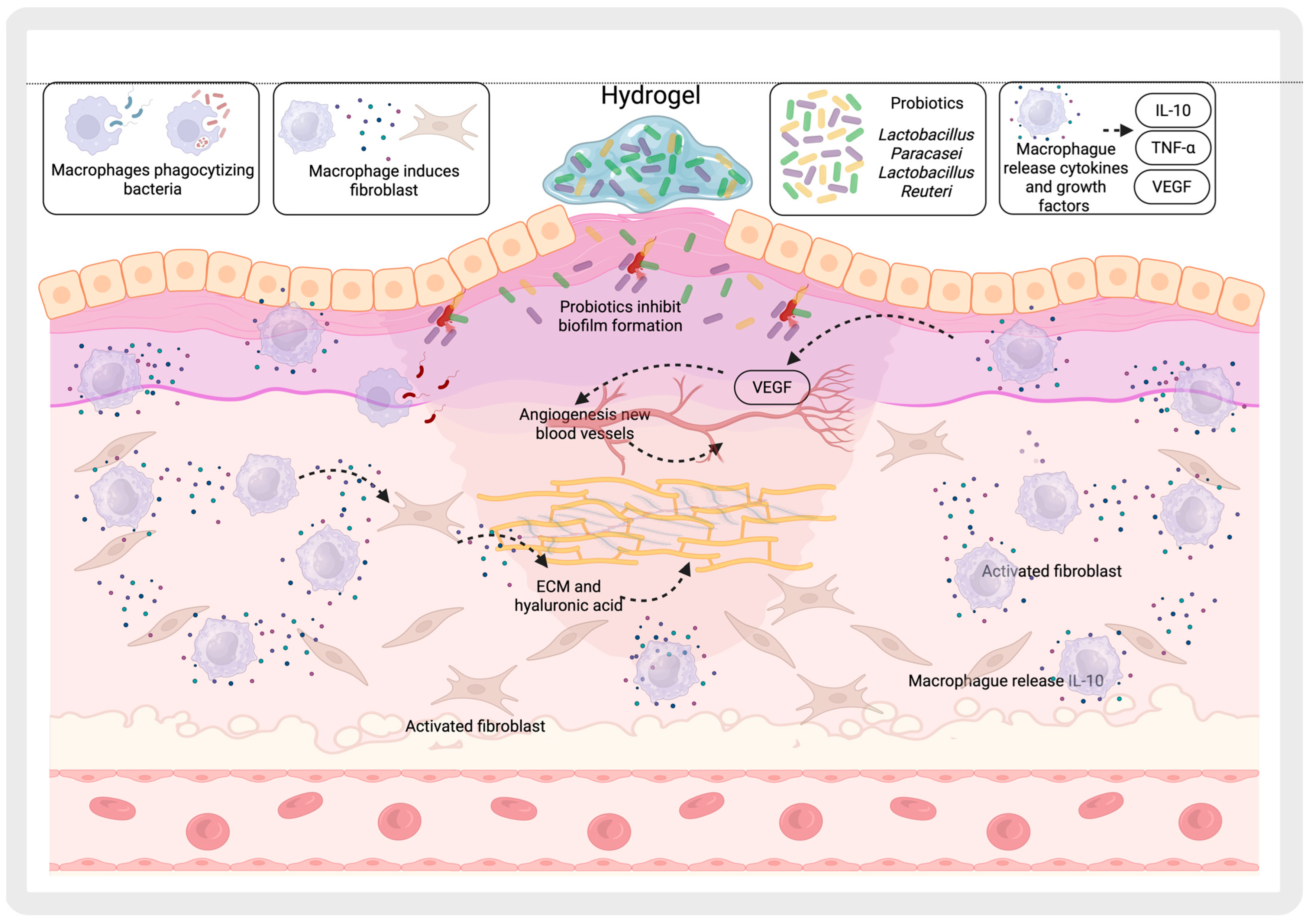
4.2. Promotion of Beneficial Microbiota
5. Interaction with the Immune System
Regulation of T Cells and Reduction in Immune Responses Through Hydrogels with Modified Bacteria
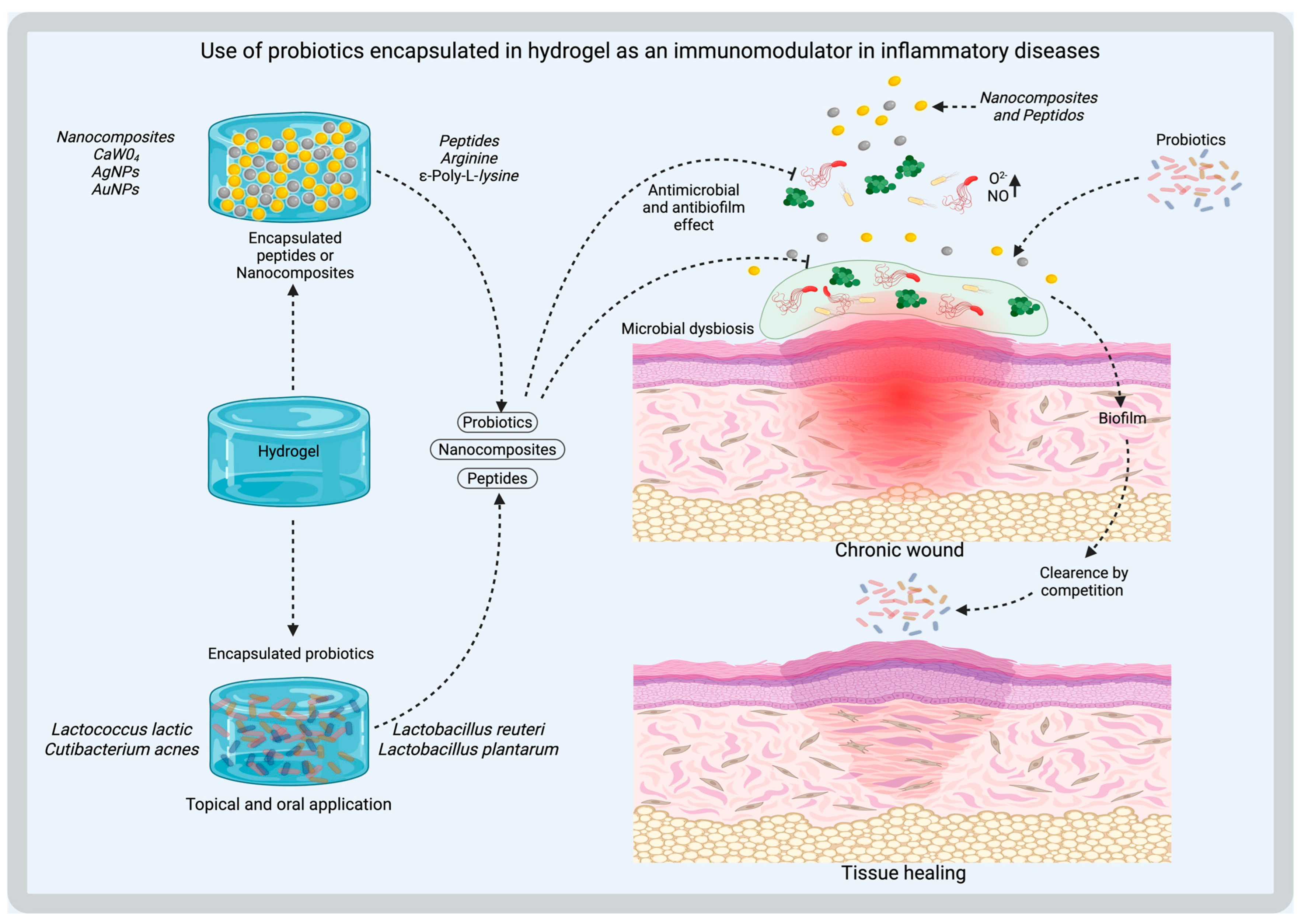
6. Applications in Tissue Regeneration and Infection Control
6.1. Chronic Wounds
6.2. Hydrogel Coatings with Hydroxyapatite to Prevent Infections in Bone Prostheses
7. Hydrogels Modulating the Microbiome: Suggested Uses in Preclinical and Clinical Studies
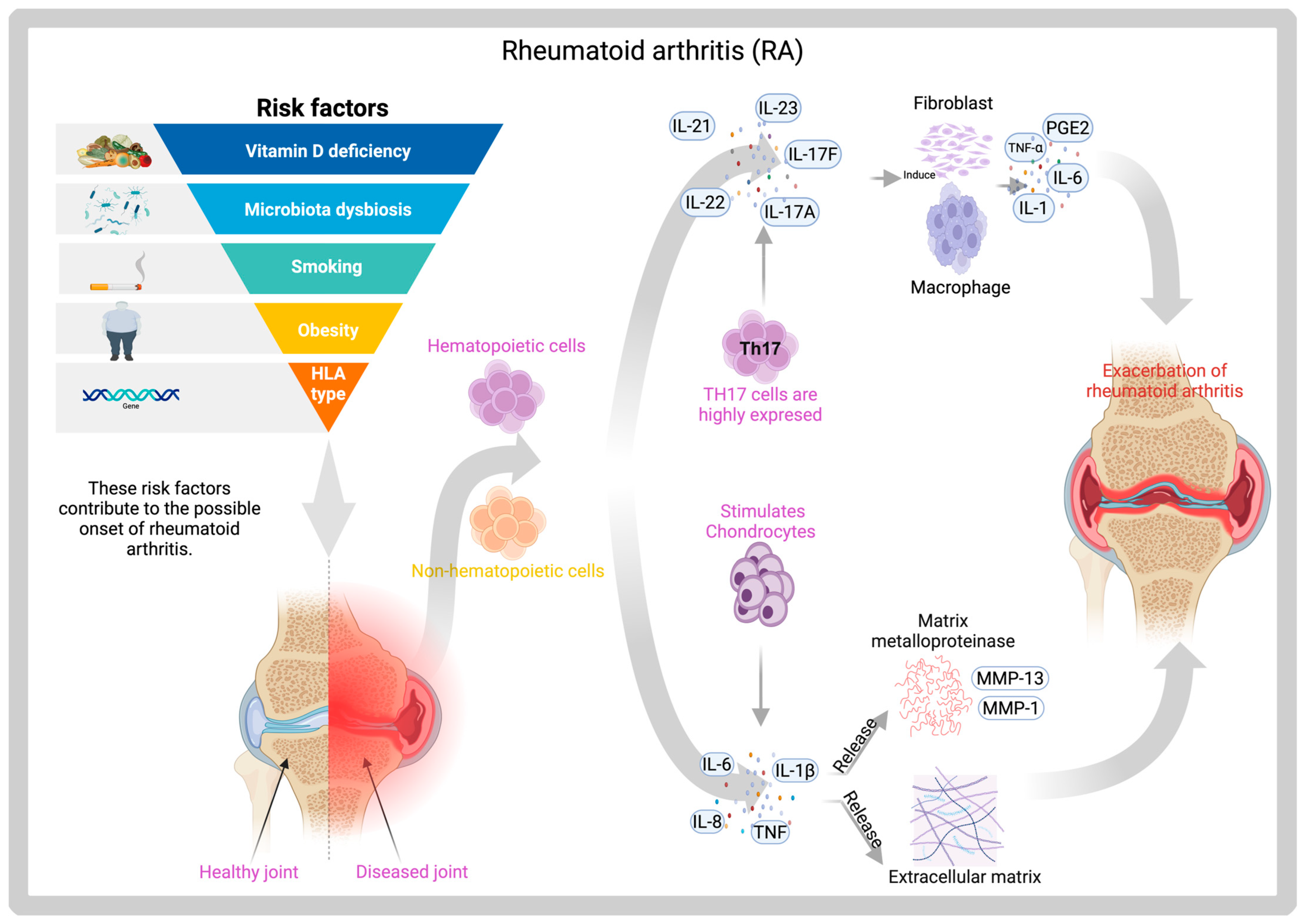
7.1. Hydrogels of Prevotella Histicola for Rheumatoid Arthritis
7.2. Hydrogel with Polylysine Against S. aureus in Infected Wound Models
8. Current Challenges
8.1. Poor Mechanical Properties
8.2. Scalability and Production Costs of Hydrogels
8.3. Interindividual Microbiome Variation
8.4. Reproducibility in the Fabrication of Multi-Functional Hydrogels
8.5. Risk of Immunogenicity in Hydrogels with Bacterial Components
9. Future Perspectives and Emerging Trends
9.1. Predictive Modeling to Design Hydrogels Adapted to the Patient’s Microbial Profile
9.2. CRISPR-Activated Hydrogels to Edit Pathogen or Commensal Bacterial Genes In Situ
9.3. Use of Marine-Derived Polypeptides or Recombinant Collagen to Reduce Costs and Risks
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cribier, B. Histología de la piel normal y lesiones histopatológicas elementales. EMC-Dermatol. 2021, 55, 1–14. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Scales, B.S.; Huffnagle, G.B. The microbiome in wound repair and tissue fibrosis. J. Pathol. 2013, 229, 323–331. [Google Scholar] [CrossRef]
- Serghiou, I.R.; Webber, M.A.; Hall, L.J. An update on the current understanding of the infant skin microbiome and research challenges. Curr. Opin. Microbiol. 2023, 75, 102364. [Google Scholar] [CrossRef]
- Williams, K.L.; Enslow, R.; Suresh, S.; Beaton, C.; Hodge, M.; Brooks, A.E. Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases. Biomedicines 2023, 11, 1582. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 7–15. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef]
- Ammons, M.C.; Morrissey, K.; Tripet, B.P.; Van Leuven, J.T.; Han, A.; Lazarus, G.S.; Zenilman, J.M.; Stewart, P.S.; James, G.A.; Copié, V.; et al. Biochemical association of metabolic profile and microbiome in chronic pressure ulcer wounds. PLoS ONE 2015, 10, e0126735. [Google Scholar] [CrossRef]
- March Rosselló, G.A.; Eiros Bouza, J.M. Quorum sensing en bacterias y levaduras. Med. Clínica 2013, 141, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Panchal, R.; Sakariya, B.; Gevariya, M.; Raiyani, R.; Soni, R.; Goswami, D. Combatting antibiotic resistance by exploring the promise of Quorum Quenching in targeting bacterial virulence. Microbe 2025, 6, 100224. [Google Scholar] [CrossRef]
- Uberoi, A.; Bartow-McKenney, C.; Zheng, Q.; Flowers, L.; Campbell, A.; Knight, S.A.B.; Chan, N.; Wei, M.; Lovins, V.; Bugayev, J.; et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe 2021, 29, 1235–1248.e1238. [Google Scholar] [CrossRef]
- Menendez, A.; Brett Finlay, B. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 2007, 19, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Zhang, L.; Zouboulis, C.C.; Gallo, R.L.; Huang, C.M. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J. Investig. Dermatol. 2010, 130, 985–994. [Google Scholar] [CrossRef]
- Mittal, R.; Kodiyan, J.; Gerring, R.; Mathee, K.; Li, J.D.; Grati, M.; Liu, X.Z. Role of innate immunity in the pathogenesis of otitis media. Int. J. Infect. Dis. 2014, 29, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hunt, R.L.; Villaruz, A.E.; Fisher, E.L.; Liu, R.; Liu, Q.; Cheung, G.Y.C.; Li, M.; Otto, M. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 2022, 30, 301–313.e309. [Google Scholar] [CrossRef]
- Wanke, I.; Steffen, H.; Christ, C.; Krismer, B.; Götz, F.; Peschel, A.; Schaller, M.; Schittek, B. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Investig. Dermatol. 2011, 131, 382–390. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin. Immunother. 2014, 10, 3270–3285. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Yoo, T.H.; Kim, S.G.; Choi, S. Roles of toll-like receptors in cancer: A double-edged sword for defense and offense. Arch. Pharm. Res. 2012, 35, 1297–1316. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Díaz-Díaz, L.M.; Rodríguez-Villafañe, A.; García-Arrarás, J.E. The Role of the Microbiota in Regeneration-Associated Processes. Front. Cell Dev. Biol. 2021, 9, 768783. [Google Scholar] [CrossRef]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef]
- Yan, J.; Charles, J.F. Gut Microbiome and Bone: To Build, Destroy, or Both? Curr. Osteoporos. Rep. 2017, 15, 376–384. [Google Scholar] [CrossRef]
- Kunimitsu, M.; Nakagami, G.; Minematsu, T.; Koudounas, S.; Sanada, H. An in vivo critically colonised wound model with dysbiotic wound microbiota. Int. Wound J. 2023, 20, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Ji, S.-F.; Fu, X.-B.; Jiang, Y.-F.; Sun, X.-Y. Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration. Mil. Med. Res. 2024, 11, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Chen, X.; Huang, H.; Guo, C.; Zhu, X.; Chen, J.; Liang, J.; Yang, R.; Shao, D.; Chen, F.; Shi, B.; et al. Controlling Alveolar Bone Loss by Hydrogel-Based Mitigation of Oral Dysbiosis and Bacteria-Triggered Proinflammatory Immune Response. Adv. Funct. Mater. 2025, 35, 2409121. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Neresyan, T.; Gatina, Y.; Kolmanič Bučar, V.; Skrt, M.; Dogša, I.; Bogovič Matijašić, B.; Kulikova, I.; Lodygin, A.; Poklar Ulrih, N. Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions. Molecules 2021, 26, 7484. [Google Scholar] [CrossRef]
- Li, Z.; Behrens, A.M.; Ginat, N.; Tzeng, S.Y.; Lu, X.; Sivan, S.; Langer, R.; Jaklenec, A. Biofilm-Inspired Encapsulation of Probiotics for the Treatment of Complex Infections. Adv. Mater. 2018, 30, e1803925. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly active probiotic hydrogels matrixed on bacterial EPS accelerate wound healing via maintaining stable skin microbiota and reducing inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-encapsulation of probiotic Lactobacillus acidophilus and Reishi medicinal mushroom (Ganoderma lingzhi) extract in moist calcium alginate beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef]
- Rittisak, S.; Charoen, R.; Seamsin, S.; Buppha, J.; Ruangthong, T.; Muenkhling, S.; Riansa-Ngawong, W.; Eadmusik, S.; Phattayakorn, K.; Jantrasee, S.; et al. Effects of incorporated collagen/prebiotics and different coating substances on the survival rate of encapsulated probiotics. Food Sci. Biotechnol. 2025, 34, 1383–1399. [Google Scholar] [CrossRef]
- Serrano-Casas, V.; Pérez-Chabela, M.L.; Cortés-Barberena, E.; Totosaus, A. Improvement of lactic acid bacteria viability in acid conditions employing agroindustrial co-products as prebiotic on alginate ionotropic gel matrix co-encapsulation. J. Funct. Foods 2017, 38, 293–297. [Google Scholar] [CrossRef]
- Azam, M.; Saeed, M.; Pasha, I.; Shahid, M. A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Biosci. 2020, 37, 100679. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of homogenisation on bead size and survival of encapsulated probiotic bacteria. Food Res. Int. 2007, 40, 1261–1269. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán Mdel, C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Z.; Ma, S.; Chen, X.; Li, L.; Liu, W.; Ren, G.; Duan, X.; Cao, W.; Xu, Y.; et al. Effects of Transglutaminase Concentration and Drying Method on Encapsulation of Lactobacillus plantarum in Gelatin-Based Hydrogel. Molecules 2023, 28, 8070. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Luo, X.; Zhao, Z.; Yuan, J.; Song, Y.; Liu, C.; Huang, M.; Dong, L.; Xie, H.; Cai, L.; et al. Enhancing viability of Lactobacillus plantarum encapsulated by alginate-gelatin hydrogel beads during gastrointestinal digestion, storage and in the mimic beverage systems. Int. J. Biol. Macromol. 2023, 224, 94–104. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, J.; Zhang, J.; Yu, H.; Dai, W.; Yan, W.; Sun, M.; Ding, G.; Li, Q.; Meng, Q.; et al. Comparison of three different acidic solutions in tendon decellularized extracellular matrix bio-ink fabrication for 3D cell printing. Acta Biomater. 2021, 131, 262–275. [Google Scholar] [CrossRef]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-Printable Bioactivated Nanocellulose-Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Wei, Q.; Zhang, J.; Chen, X.; An, Y. A High-Stretching, Rapid-Self-Healing, and Printable Composite Hydrogel Based on Poly (Vinyl Alcohol), Nanocellulose, and Sodium Alginate. Gels 2024, 10, 258. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Li, X.; Liu, Z.; Zhang, Z. Application of photo-crosslinkable gelatin methacryloyl in wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1303709. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, L.; Liu, D.; Tang, D.; Liu, K.; Rao, L.; Yang, J.; Liu, Y.; Li, Y.; Chen, H.; et al. Controllable Preparation and Research Progress of Photosensitive Antibacterial Complex Hydrogels. Gels 2023, 9, 571. [Google Scholar] [CrossRef]
- Oguntoye, M.A.; Ezekiel, O.O.; Oridupa, O.A. Viability of Lactobacillus rhamnosus GG in provitamin A cassava hydrolysate during fermentation, storage, in vitro and in vivo gastrointestinal conditions. Food Biosci. 2021, 40, 100845. [Google Scholar] [CrossRef]
- Chen, B.; Pu, W.; Zhang, J. Functional Microgel Enables Effective Delivery and Colonization of Probiotics for Treating Colitis. ACS Cent. Sci. 2023, 9, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yu, X.; Gao, F.; Zang, M.; Huang, L.; Liu, W.; Xu, J.; Yu, S.; Wang, T.; Sun, H.; et al. Fighting bacteria with bacteria: A biocompatible living hydrogel patch for combating bacterial infections and promoting wound healing. Acta Biomater. 2024, 181, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, H.; Wang, J.; Yao, M.; Peng, Y.; Liu, T.; Li, Z.; Luo, G.; Deng, J. Engineering Bacteria-Activated Multifunctionalized Hydrogel for Promoting Diabetic Wound Healing. Adv. Funct. Mater. 2021, 31, 2105749. [Google Scholar] [CrossRef]
- Xu, Y.; Gan, Y.; Qi, F.; Lu, X.; Zhang, X.; Zhang, J.; Wang, H.; Li, Y.; Zhou, Z.; Wang, X.; et al. Innate lymphoid cell-based immunomodulatory hydrogel microspheres containing Cutibacterium acnes extracellular vesicles for the treatment of psoriasis. Acta Biomater. 2024, 184, 296–312. [Google Scholar] [CrossRef]
- Su, D.; Li, M.; Xie, Y.; Xu, Z.; Lv, G.; Jiu, Y.; Lin, J.; Chang, C.J.; Chen, H.; Cheng, F. Gut commensal bacteria Parabacteroides goldsteinii-derived outer membrane vesicles suppress skin inflammation in psoriasis. J. Control. Release 2025, 377, 127–145. [Google Scholar] [CrossRef]
- Corona-Escalera, A.F.; Tinajero-Díaz, E.; García-Reyes, R.A.; Luna-Bárcenas, G.; Seyfoddin, A.; Padilla-de la Rosa, J.D.; González-Ávila, M.; García-Carvajal, Z.Y. Enzymatic Crosslinked Hydrogels of Gelatin and Poly (Vinyl Alcohol) Loaded with Probiotic Bacteria as Oral Delivery System. Pharmaceutics 2022, 14, 2759. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Kong, L.; Wang, X.; Li, Q.; Zhang, L.; Shi, J.; Duan, J.; Mu, H. ROS-responsive hyaluronic acid hydrogel for targeted delivery of probiotics to relieve colitis. Int. J. Biol. Macromol. 2022, 222, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Deol, P.K.; Khare, P.; Singh, D.P.; Bishnoi, M.; Kondepudi, K.K.; Kaur, I.P. Pharmabiotic beads with improved encapsulation and gut survival for management of colonic inflammation associated gut derangements. J. Drug Target. 2020, 28, 1053–1062. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Du, T.; Xiao, Z.; Zhang, G.; Wei, L.; Cao, J.; Zhang, Z.; Li, X.; Song, Z.; Wang, W.; Liu, J.; et al. An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing. Acta Biomater. 2023, 161, 112–133. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, L.; Zhang, Y.; Su, H.; Jiang, X.; Liu, H.; Huang, X.; Zhou, L.; Shen, X.C.; Liu, C. Photothermal synergistic nitric oxide controlled release injectable self-healing adhesive hydrogel for biofilm eradication and wound healing. J. Mater. Chem. B 2023, 12, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bi, D.; Yu, B.; Chen, Q.; Du, S.; Xie, G.; Zhu, J.; Zhang, L. Photonic hydrogels combining the slow photon effect and NO gas therapy for synergetic enhanced photodynamic antibacterial therapy. J. Colloid. Interface Sci. 2025, 682, 1185–1194. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Lee, Y.; Le Thi, P.; Park, K.D. Nitric oxide-releasing injectable hydrogels with high antibacterial activity through in situ formation of peroxynitrite. Acta Biomater. 2018, 67, 66–78. [Google Scholar] [CrossRef]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; Medeiros, S.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Katas, H.; Mohd Akhmar, M.A.; Suleman Ismail Abdalla, S. Biosynthesized silver nanoparticles loaded in gelatine hydrogel for a natural antibacterial and anti-biofilm wound dressing. J. Bioact. Compat. Polym. 2021, 36, 111–123. [Google Scholar] [CrossRef]
- MA, M.F.; Faris Taufeq, F.Y.; Suleman Ismail Abdalla, S.; Katas, H. Roles of chitosan in synthesis, antibacterial and anti-biofilm properties of bionano silver and gold. RSC Adv. 2022, 12, 19297–19312. [Google Scholar] [CrossRef]
- Suleman Ismail Abdalla, S.; Katas, H.; Chan, J.Y.; Ganasan, P.; Azmi, F.; Fauzi, M.B. Gelatin Hydrogels Loaded with Lactoferrin-Functionalized Bio-Nanosilver as a Potential Antibacterial and Anti-Biofilm Dressing for Infected Wounds: Synthesis, Characterization, and Deciphering of Cytotoxicity. Mol. Pharm. 2021, 18, 1956–1969. [Google Scholar] [CrossRef]
- Fathil, M.A.M.; Katas, H. Antibacterial, Anti-Biofilm and Pro-Migratory Effects of Double Layered Hydrogels Packaged with Lactoferrin-DsiRNA-Silver Nanoparticles for Chronic Wound Therapy. Pharmaceutics 2023, 15, 991. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Zou, Z.; Qi, D.; Geng, Y.; Wang, Z.; Zhang, Z.; He, C.; Yu, J. Tissue-Adhesive and Antibacterial Hydrogel Promotes MDR Bacteria-Infected Diabetic Wound Healing via Disrupting Bacterial Biofilm, Scavenging ROS and Promoting Angiogenesis. Adv. Healthc. Mater. 2025, 14, e2404889. [Google Scholar] [CrossRef]
- Srichaiyapol, O.; Maddocks, S.E.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Patramanon, R. TA-AgNPs/Alginate Hydrogel and Its Potential Application as a Promising Antibiofilm Material against Polymicrobial Wound Biofilms Using a Unique Biofilm Flow Model. Microorganisms 2022, 10, 2279. [Google Scholar] [CrossRef]
- Dragland, I.S.; Rukke, H.V.; Stenhagen, I.S.; Lönn-Stensrud, J.; Kopperud, H.M. Antibacterial and Antibiofilm Effect of Low Viscosity Chitosan against Staphylococcus epidermidis. Int. J. Microbiol. 2016, 2016, 9159761. [Google Scholar] [CrossRef] [PubMed]
- Orgaz, B.; Lobete, M.M.; Puga, C.H.; San Jose, C. Effectiveness of chitosan against mature biofilms formed by food related bacteria. Int. J. Mol. Sci. 2011, 12, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Cobrado, L.; Azevedo, M.M.; Silva-Dias, A.; Ramos, J.P.; Pina-Vaz, C.; Rodrigues, A.G. Cerium, chitosan and hamamelitannin as novel biofilm inhibitors? J. Antimicrob. Chemother. 2012, 67, 1159–1162. [Google Scholar] [CrossRef]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Pina-Vaz, C.; Rodrigues, A.G. In vivo antibiofilm effect of cerium, chitosan and hamamelitannin against usual agents of catheter-related bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 126–130. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Ma, S.; Moser, D.; Schneider-Stickler, B. Efficacy of carboxymethyl chitosan against Candida tropicalis and Staphylococcus epidermidis monomicrobial and polymicrobial biofilms. Int. J. Biol. Macromol. 2018, 110, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Pati, B.A.; Kurata, W.E.; Horseman, T.S.; Pierce, L.M. Antibiofilm activity of chitosan/epsilon-poly-L-lysine hydrogels in a porcine ex vivo skin wound polymicrobial biofilm model. Wound Repair Regen. 2021, 29, 316–326. [Google Scholar] [CrossRef]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Khan, F.; Lee, J.W.; Pham, D.T.N.; Kim, Y.M. Chitooligosaccharides as Antibacterial, Antibiofilm, Antihemolytic and Anti-Virulence Agent against Staphylococcus aureus. Curr. Pharm. Biotechnol. 2019, 20, 1223–1233. [Google Scholar] [CrossRef]
- Moon, A.Y.; Bailey, E.J.; Polanco, J.A.; Kurata, W.E.; Pierce, L.M. Antibacterial Efficacy of a Chitosan-Based Hydrogel Modified with Epsilon-Poly-l-Lysine Against Pseudomonas aeruginosa in a Murine-Infected Burn Wound Model. Mil. Med. 2023, 188, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Inda, M.E.; Lai, Y.; Lu, T.K.; Zhao, X. Engineered Living Hydrogels. Adv. Mater. 2022, 34, e2201326. [Google Scholar] [CrossRef]
- Quan, Y.; Shao, H.; Wang, N.; Gao, Z.; Jin, M. Microenvironment-sensitive hydrogels as promising drug delivery systems for co-encapsulating microbial homeostasis probiotics and anti-inflammatory drugs to treat periodontitis. Mater. Today Bio 2025, 32, 101711. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kong, W.; Lu, T.; Irudayaraj, J. Soft hydrogel-shell confinement systems as bacteria-based bioactuators and biosensors. Biosens. Bioelectron. 2023, 219, 114809. [Google Scholar] [CrossRef]
- Yang, J.; Peng, M.; Tan, S.; Ge, S.; Xie, L.; Zhou, T.; Liu, W.; Zhang, K.; Zhang, Z.; Liu, J.; et al. Calcium Tungstate Microgel Enhances the Delivery and Colonization of Probiotics during Colitis via Intestinal Ecological Niche Occupancy. ACS Cent. Sci. 2023, 9, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Peng, M.; Tan, S.; Ge, S.; Yang, X.; Liang, Y.; Wen, Z.; Xie, L.; Zhou, T.; et al. Bionic Regulators Break the Ecological Niche of Pathogenic Bacteria for Modulating Dysregulated Microbiome in Colitis. Adv. Mater. 2022, 34, e2204650. [Google Scholar] [CrossRef]
- Wang, J.; Hu, D.; Chen, Q.; Liu, T.; Zhou, X.; Xu, Y.; Zhou, H.; Gu, D.; Gao, C. Intracellular hydrogelation of macrophage conjugated probiotics for hitchhiking delivery and combined treatment of colitis. Mater. Today Bio 2023, 20, 100679. [Google Scholar] [CrossRef]
- Yanamandra, A.K.; Bhusari, S.; Del Campo, A.; Sankaran, S.; Qu, B. In vitro evaluation of immune responses to bacterial hydrogels for the development of living therapeutic materials. Biomater. Adv. 2023, 153, 213554. [Google Scholar] [CrossRef]
- Alam, A.; Neish, A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef]
- Smits, H.H.; Engering, A.; van der Kleij, D.; de Jong, E.C.; Schipper, K.; van Capel, T.M.; Zaat, B.A.; Yazdanbakhsh, M.; Wierenga, E.A.; van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef]
- Peng, P.; Feng, T.; Yang, X.; Nie, C.; Yu, L.; Ding, R.; Zhou, Q.; Jiang, X.; Li, P. Gastrointestinal Microenvironment Responsive Nanoencapsulation of Probiotics and Drugs for Synergistic Therapy of Intestinal Diseases. ACS Nano 2023, 17, 14718–14730. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Ni, W.; He, Y.; Li, M.; Wang, C.; Bai, Y.; Zhang, H.; Yao, M. An Immune-Regulating Polysaccharide Hybrid Hydrogel with Mild Photothermal Effect and Anti-Inflammatory for Accelerating Infected Wound Healing. Adv. Healthc. Mater. 2024, 13, e2400003. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Giardino Torchia, M.L.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Luo, X.Y.; Ma, K.; Liu, Z.Z.; Gao, Y.; Zhang, J.B.; Chen, W.; Yang, Y.J. Hyaluronic Acid/Gelatin-Based Multifunctional Bioadhesive Hydrogel Loaded with a Broad-Spectrum Bacteriocin for Enhancing Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 47226–47241. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Wang, S.; Gao, M.; Du, K.; Chen, X.; Lu, Y.; Hu, Q.; Du, A.; Du, S.; et al. A dynamically phase-adaptive regulating hydrogel promotes ultrafast anti-fibrotic wound healing. Nat. Commun. 2025, 16, 3738. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, Y.; Li, W.; Sun, J. Promotion of chronic wound healing by plant-derived active ingredients and research progress and potential of plant polysaccharide hydrogels. Chin. Herb. Med. 2025, 17, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, D.; Gao, B.; Huang, S.; Tang, Y.; Wa, Q.; Dong, Y.; Yu, S.; Huang, J.; Huang, S. A DNA-inspired injectable adhesive hydrogel with dual nitric oxide donors to promote angiogenesis for enhanced wound healing. Acta Biomater. 2024, 176, 128–143. [Google Scholar] [CrossRef]
- Zanchetta, F.C.; De Wever, P.; Morari, J.; Gaspar, R.C.; Prado, T.P.D.; De Maeseneer, T.; Cardinaels, R.; Araújo, E.P.; Lima, M.H.M.; Fardim, P. In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications. Bioengineering 2024, 11, 168. [Google Scholar] [CrossRef]
- Yao, W.D.; Zhou, J.N.; Tang, C.; Zhang, J.L.; Chen, Z.Y.; Li, Y.; Gong, X.J.; Qu, M.Y.; Zeng, Q.; Jia, Y.L.; et al. Hydrogel Microneedle Patches Loaded with Stem Cell Mitochondria-Enriched Microvesicles Boost the Chronic Wound Healing. ACS Nano 2024, 18, 26733–26750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Höglund, L.; Samanta, A.; Procter, P.; Persson, C. Hydroxyapatite particle shape affects screw attachment in cancellous bone when augmented with hydroxyapatite-containing hydrogels. J. Mech. Behav. Biomed. Mater. 2024, 150, 106241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, S.; Xu, Z.; Li, L.; Liu, Y.; Gao, X.; Diao, Y.; Chen, L.; Sun, J. Preparation and Characterization of Carboxymethyl Chitosan/Sodium Alginate Composite Hydrogel Scaffolds Carrying Chlorhexidine and Strontium-Doped Hydroxyapatite. ACS Omega 2024, 9, 22230–22239. [Google Scholar] [CrossRef]
- Yin, C.; Deng, M.; Yu, J.; Chen, Y.; Zheng, K.; Huang, Y.; Deng, X.; Tian, Y.; Ma, Y.; Zeng, B.; et al. An Andrias davidianus derived composite hydrogel with enhanced antibacterial and bone repair properties for osteomyelitis treatment. Sci. Rep. 2024, 14, 24626. [Google Scholar] [CrossRef]
- Ziyadullaev, S.K.; Khudaiberdiev, S.S.; Aripova, T.U.; Chirumbolo, S.; Kamalov, Z.S.; Bjørklund, G.; Rizaev, J.A.; Tashkenbaeva, E.N.; Khamidov, O.A.; Gaffarov, U.B. Synovial Fluid as a Crucial Component of the Joint Microenvironment in Rheumatoid Arthritis. Immune Netw. 2025, 25, e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Ren, F.; Zhang, J.; Song, W.; Ren, L. New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research. Gels 2025, 11, 136. [Google Scholar] [CrossRef]
- Xue, Z.; Li, N.; Du, K.; Shu, J.; Huang, Z.; Gao, Z.; Xie, X.; Li, Q.; Lu, Y. Inhibiting synovial inflammation and promoting cartilage repair in rheumatoid arthritis using a matrix metalloproteinase-binding hydrogel. Mater. Today Bio 2025, 32, 101792. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Johnson, S.; Luckey, D.; Marietta, E.; Murray, J.; Taneja, V. Small intestinal derived Prevotella histicola simulates biologic as a therapeutic agent. Sci. Rep. 2024, 14, 29217. [Google Scholar] [CrossRef]
- Mangalam, A.K.; Murray, J. Microbial monotherapy with Prevotella histicola for patients with multiple sclerosis. Expert. Rev. Neurother. 2019, 19, 45–53. [Google Scholar] [CrossRef]
- Marietta, E.V.; Murray, J.A.; Luckey, D.H.; Jeraldo, P.R.; Lamba, A.; Patel, R.; Luthra, H.S.; Mangalam, A.; Taneja, V. Suppression of Inflammatory Arthritis by Human Gut-Derived Prevotella histicola in Humanized Mice. Arthritis Rheumatol. 2016, 68, 2878–2888. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Luckey, D.; Bodhke, R.; Chen, J.; Marietta, E.; Jeraldo, P.; Murray, J.; Taneja, V. Prevotella histicola Protects from Arthritis by Expansion of Allobaculum and Augmenting Butyrate Production in Humanized Mice. Front. Immunol. 2021, 12, 609644. [Google Scholar] [CrossRef]
- Maeda, Y.; Takeda, K. Role of Gut Microbiota in Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 60. [Google Scholar] [CrossRef]
- Wang, H.; Ong, E.; Kao, J.Y.; Sun, D.; He, Y. Reverse Microbiomics: A New Reverse Dysbiosis Analysis Strategy and Its Usage in Prediction of Autoantigens and Virulent Factors in Dysbiotic Gut Microbiomes from Rheumatoid Arthritis Patients. Front. Microbiol. 2021, 12, 633732. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ghorbani, S.; Barani, M.; Singh Chauhan, N.P.; Khodadadi Yazdi, M.; Saeb, M.R.; Ramsey, J.D.; Hamblin, M.R.; Mozafari, M.; Mostafavi, E. Polylysine for skin regeneration: A review of recent advances and future perspectives. Bioeng. Transl. Med. 2022, 7, e10261. [Google Scholar] [CrossRef]
- Pi, Z.; Ye, M.; Huang, J.; Li, B.; Yan, C.; Wang, Q.; Ji, B.; Yu, X.; Tan, Z.; Li, D.; et al. Injectable polyethylene glycol/methacrylated polylysine double cross-linked hydrogel releases neuropeptides for infected wound healing. Int. J. Biol. Macromol. 2025, 284, 137972. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Z.; Li, J.; Liu, Y.; Li, R.; Zhao, Y.; Song, S.; Huang, H.; Guo, Q.; Wu, C.; et al. A multi-functional oxidative konjac glucomannan/ε-poly-l-lysine hydrogel with antibacterial, haemostatic, and chronic diabetic wound-treating properties. Int. J. Biol. Macromol. 2025, 290, 138859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, W.; Chen, Y.; Zhu, Z.; Chen, Y.; Ni, Y.; Liu, Z.; Jia, F.; Lu, Z.; Ye, Y.; et al. Poly (Glutamic Acid-Lysine) Hydrogels with Alternating Sequence Resist the Foreign Body Response in Rodents and Non-Human Primates. Adv. Sci. 2024, 11, e2308077. [Google Scholar] [CrossRef]
- Xie, H.; Tian, S.; Cui, C.; Sun, C.; Hu, Y.; Tang, C.; Gao, D.; Lu, L.; Jin, L.; Xu, F.; et al. A glycopeptide-based pH-responsive hydrogel promotes diabetic wound healing via antimicrobial and remodeling microenvironment. Colloids Surf. B Biointerfaces 2025, 251, 114614. [Google Scholar] [CrossRef]
- Xiao, F.; Yan, B.; Yuan, T.; He, Y.; Zhang, X.; He, X.; Peng, W.; Xu, Y.; Cao, J. Novel Nanozyme-Based Multicomponent in situ Hydrogels with Antibacterial, Hypoxia-Relieving and Proliferative Properties for Promoting Gastrostomy Tube Tract Maturation. Int. J. Nanomed. 2025, 20, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Syaifie, P.H.; Rochman, N.T.; Jaya Syaifullah, S.; Jauhar, M.M.; Mardliyati, E. A recent study of natural hydrogels: Improving mechanical properties for biomedical applications. Biomed. Mater. 2025, 20, 022010. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Q. Innovative modification strategies and emerging applications of natural hydrogel scaffolds for osteoporotic bone defect regeneration. Front. Bioeng. Biotechnol. 2025, 13, 1591896. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- HT, A.A.; Ismaeel, G.L.; Jalil, A.T.; Hadi, W.H.; Jasim, I.K.; Almulla, A.F.; Radhea, Z.A. Advanced injectable hydrogels for bone tissue regeneration. Biophys. Rev. 2023, 15, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Sunagawa, S.; Mende, D.R.; Bork, P. Inter-individual differences in the gene content of human gut bacterial species. Genome Biol. 2015, 16, 82. [Google Scholar] [CrossRef]
- Barnes, C.J.; Clausen, M.L.; Asplund, M.; Rasmussen, L.; Olesen, C.M.; Yüsel, Y.T.; Andersen, P.S.; Litman, T.; Hansen, A.J.; Agner, T.; et al. Temporal and Spatial Variation of the Skin-Associated Bacteria from Healthy Participants and Atopic Dermatitis Patients. mSphere 2022, 7, e0091721. [Google Scholar] [CrossRef] [PubMed]
- Gabrilska, R.A.; Omeir, K.; Ancira, J.; Miller, C.; Tipton, C.D.; Rumbaugh, K.P.; Wolcott, J.; Noe, A.; Subasinghe, K.; Rowe, M.; et al. Functionally enriched human polymorphisms associate to species in the chronic wound microbiome. medRxiv 2025. [Google Scholar] [CrossRef]
- Govender, P.; Ghai, M. Population-specific differences in the human microbiome: Factors defining the diversity. Gene 2025, 933, 148923. [Google Scholar] [CrossRef] [PubMed]
- Acciaretti, F.; Vesentini, S.; Cipolla, L. Fabrication Strategies Towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights. Chem. Asian J. 2022, 17, e202200797. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A Novel Effective Formulation of Bioactive Compounds for Wound Healing: Preparation, In Vivo Characterization, and Comparison of Various Postbiotics Cold Creams in a Rat Model. Evid. Based Complement. Alternat Med. 2021, 2021, 8577116. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.; Song, W.; Yu, B.; Liu, H. Rational design in functional hydrogels towards biotherapeutics. Mater. Des. 2022, 223, 111086. [Google Scholar] [CrossRef]
- Zivari-Ghader, T.; Rashidi, M.R.; Mehrali, M. Biological macromolecule-based hydrogels with antibacterial and antioxidant activities for wound dressing: A review. Int. J. Biol. Macromol. 2024, 279, 134578. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, J.; Tan, W. CRISPR propels a smart hydrogel. Science 2019, 365, 754–755. [Google Scholar] [CrossRef]
- Kahn, J.S.; Trifonov, A.; Cecconello, A.; Guo, W.; Fan, C.; Willner, I. Integration of Switchable DNA-Based Hydrogels with Surfaces by the Hybridization Chain Reaction. Nano Lett. 2015, 15, 7773–7778. [Google Scholar] [CrossRef] [PubMed]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gel, C.-c. CRISPR turns gels into biological watchdogs Geologist’s sacking prompts outcry. Nature 2019, 572, 574. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Pallela, R.; Venkatesan, J.; Bhatnagar, I.; Shim, Y.; Kim, S. Applications of marine collagen-based scaffolds in bone tissue engineering. In Marine Biomaterials: Isolation, Characterization and Applications; CRC-Taylor & Francis: Boca Raton, FL, USA, 2013; pp. 519–528. [Google Scholar]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]


| Biomaterial/Crosslinking | Probiotics/Prebiotics | Reference |
|---|---|---|
| Alginate microbeads/Ionic crosslinking | Lactobacillus plantarum or Lactobacillus rhamnosus | [38] |
| Hyaluronic acid hydrogel/Photo-crosslinking | Lactobacillus paracasei/EPS-M76 | [40] |
| Alginate microbeads/Ionic crosslinking | Lactobacillus acidophilus/Reishi mushroom extract | [41] |
| Alginate Microbeads/Ionic crosslinking | Enterococcus. faecium or Aerococcus viridans/Inulin or apple marc flour | [43] |
| Fructooligosaccharides-Alginate microbeads/Ionic crosslinking | Lactobacillus rhamnosus/Fructooligosaccharides | [44] |
| Alginate microbeads/Ionic crosslinking | Lactobacillus rhamnosus/provitamin A | [55] |
| Chitosan-coated alginate microbeads/Ionic crosslinking | Lactobacillus gasseri or Bifidobacterium bifidum/Quercetin | [46] |
| Gelatin hydrogel/Enzymatic crosslinking | L. plantarum | [47] |
| Alginate-gelatin microbeads/Ionic crosslinking | Lactobacillus plantarum | [48] |
| Alginate microbeads/Ionic crosslinking | Bacillus coagulans | [56] |
| Bovine serum albumin hydrogel/Chemical crosslinking | E. coli | [57] |
| Heparin-poloxamer hydrogel/Thermosensitive crosslinking | L. lactis | [58] |
| Gelatin microbeads/Photo-crosslinking | Cutibacterium acnes-derived extracellular vesicles | [59] |
| PF-127 hydrogel/Thermosensitive crosslinking | Parabacteroides goldsteinii-derived outer membrane vesicles | [60] |
| Poly (vinyl alcohol)-Gelatin/Ionic crosslinking | Lactiplantibacillus plantarum | [61] |
| Methacrylate-Hyaluronic acid/Ionic crosslinking | Lactobacillus reuteri | [62] |
| Polyethylene glycol-alginate/Ionic crosslinking | Lactobacillus acidophilus | [63] |
| Biomaterial/Hydrogel System | Microorganism(s) Affected: Antibacterial and Anti-Biofilm Formation | Reference |
|---|---|---|
| PSPG hydrogel with Pt-decorated AuNPs + SNP-loaded PCN | General spectrum | [65] |
| Quaternized chitosan hydrogel + BNN6 in MPDA + NIR activation | Staphylococcus aureus | [66] |
| Chitosan hydrogel + PG@Arg/IR820 | S. aureus | [67] |
| S-nitrosothiolated gelatin hydrogel | Escherichia coli, Staphylococcus aureus | [68] |
| Chitosan-stabilized AgNPs gelatin hydrogel | S. aureus, B. subtilis, P. aeruginosa, E. coli | [70] |
| AgNPs and AuNPs in chitosan matrix | S. aureus, P. aeruginosa | [71] |
| LTF-functionalized AgNPs in gelatin hydrogel | S. aureus, P. aeruginosa | [72] |
| AgNP + LTF + DsiRNA in gelatin hydrogel | S. aureus, P. aeruginosa | [73] |
| Poly (ethylene glycol) DFO-loaded hydrogel + MXene + NIR | Methicillin-resistant S. aureus (MRSA) | [74] |
| Alginate hydrogel + TA-AgNPs | S. pyogenes, S. aureus, P. aeruginosa | [75] |
| Low viscosity chitosan | S. epidermidis | [76] |
| Medium molecular weight native chitosan | Listeria monocytogenes, B. cereus, S. enterica, P. fluorescens | [77] |
| Chitosan-coated catheters | S. epidermidis, C. albicans | [78,79] |
| Carboxymethyl-chitosan hydrogel | S. epidermidis, C. tropicalis | [80] |
| Chitosan hydrogel with epsilon-poly-L-lysine | P. aeruginosa, S. aureus, C. albicans | [81] |
| Chitosan-coated surfaces | K. pneumoniae, P. aeruginosa, C. albicans | [82] |
| Chitooligosaccharides (COS) | S. aureus | [83] |
| Biomaterial/Hydrogel System | Application In Vivo Study/Target Microorganism | Key Molecules Involved | Cytokines Regulated | Reference |
|---|---|---|---|---|
| Calcium alginate hydrogel + 5-aminosalicylic acid | Colitis model/Gut microbiota | 5-aminosalicylic acid | ↓ IL-6, IL-1β, TNF-α; ↑ IL-10 | [94] |
| Heparin–poloxamer hydrogel + Lactococcus lactis | Diabetic wound model inflammation/skin microbiota modulation | Lactic acid and VEGF | ↓ TNF-α, iNOS, NO; ↑ IL-10, CD206, ARG1 | [58] |
| Oxidized konjac glucomannan-chitosan-arginine hydrogel + protocatechualdehyde, Fe3+ | Full-thickness MRSA-infected wounds/skin microbiota modulation | Protocatechualdehyde, Fe3+ | ↓ TNF-α; ↑ IL-10, CD206 | [95] |
| Gelatin methacrylate hydrogel + Cutibacterium acnes-derived extracellular vesicles | Psoriasis model/skin microbiota modulation | Microbial extracellular vesicles | ↓ IL-17, IL-22; ↑ IL-10 | [59] |
| PF-127 hydrogel + Parabacteroides goldsteinii-derived outer membrane vesicles | Psoriasis model/skin microbiota modulation | Outer membrane vesicles (pentadecanoic acid) | ↓ IL-23, IL-17; ↑ IL-10 | [60] |
| Hyaluronic acid-Bacillus velezensis extracellular polysaccharides + Lactobacillus paracasei | Wound healing model/skin microbiota modulation | Lactic acid | ↓ TNF-α; ↑ IL-10, VEGF-α | [40] |
| Hyaluronic acid-gelatin hydrogel + jileicin | MRSA-infected diabetic wounds model/ skin microbiota modulation | Jileicin (bacteriocin) | ↑ IL-10; ↓ TNF-α | [97] |
| Poly (ethylene glycol) diacrylate gelated peritoneal macrophages + E. coli Nissle | Intestinal inflammation/Gut microbiota | TNFR2, IL1R2, TLR4, E. coli Nissle 1917 | Neutralization of TNF-α, IL-1β | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Gastelum, G.R.; Villegas-Mercado, C.E.; Cota-Quintero, J.L.; Arzola-Rodríguez, S.I.; Ramos-Payán, R.; Bermúdez, M. Hydrogels Modulating the Microbiome: Therapies for Tissue Regeneration with Infection Control. Gels 2025, 11, 584. https://doi.org/10.3390/gels11080584
Jiménez-Gastelum GR, Villegas-Mercado CE, Cota-Quintero JL, Arzola-Rodríguez SI, Ramos-Payán R, Bermúdez M. Hydrogels Modulating the Microbiome: Therapies for Tissue Regeneration with Infection Control. Gels. 2025; 11(8):584. https://doi.org/10.3390/gels11080584
Chicago/Turabian StyleJiménez-Gastelum, Germán Reynaldo, Carlos Esteban Villegas-Mercado, Juan Luis Cota-Quintero, Silvia Ivonne Arzola-Rodríguez, Rosalío Ramos-Payán, and Mercedes Bermúdez. 2025. "Hydrogels Modulating the Microbiome: Therapies for Tissue Regeneration with Infection Control" Gels 11, no. 8: 584. https://doi.org/10.3390/gels11080584
APA StyleJiménez-Gastelum, G. R., Villegas-Mercado, C. E., Cota-Quintero, J. L., Arzola-Rodríguez, S. I., Ramos-Payán, R., & Bermúdez, M. (2025). Hydrogels Modulating the Microbiome: Therapies for Tissue Regeneration with Infection Control. Gels, 11(8), 584. https://doi.org/10.3390/gels11080584







