Recent Progress in Flexible Wearable Sensors Utilizing Conductive Hydrogels for Sports Applications: Characteristics, Mechanisms, and Modification Strategies
Abstract
1. Introduction
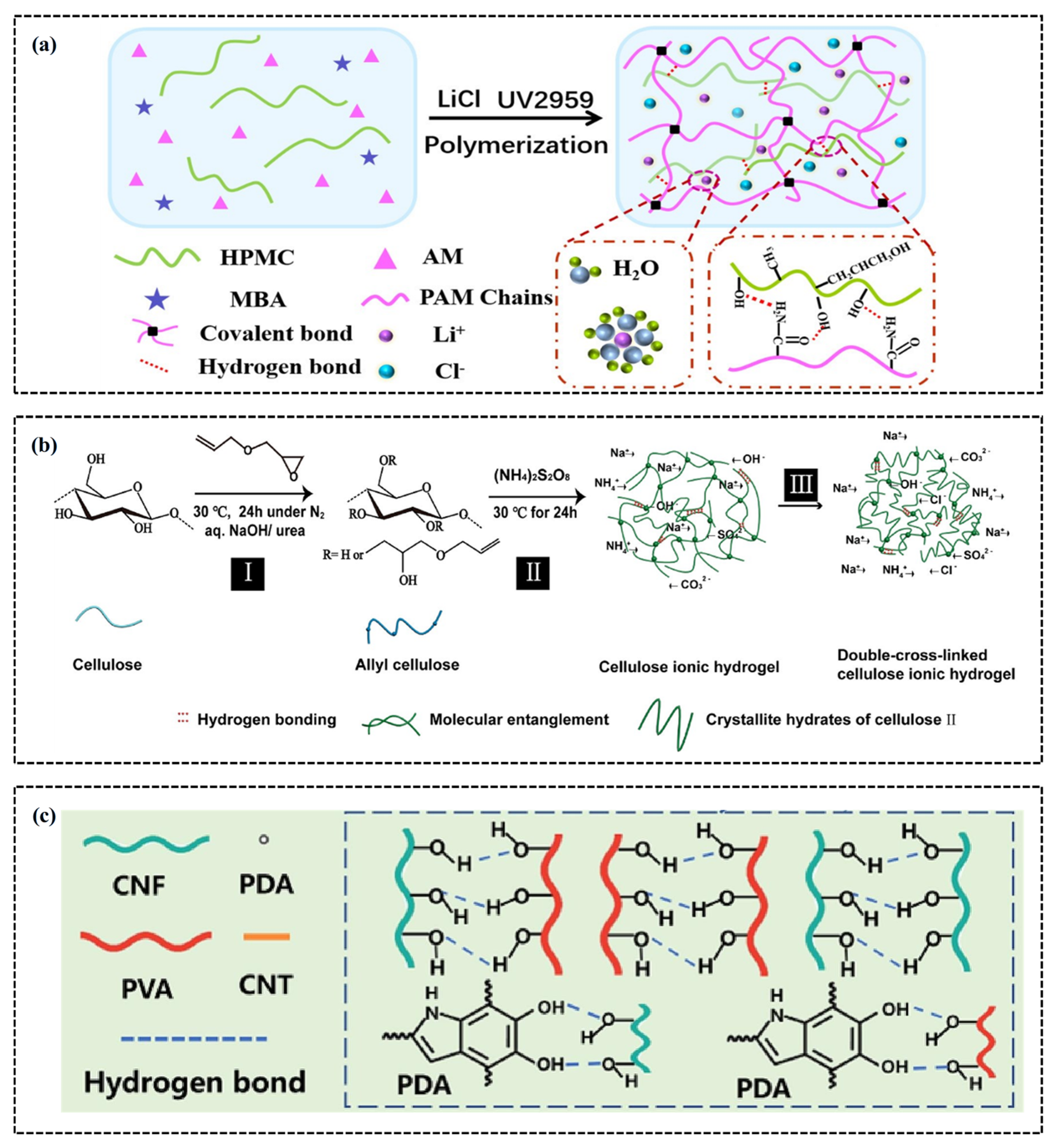
2. Characteristics of Conductive Hydrogels
| Sensing Mechanism | Materials | Mechanical Strength | Freeze Tolerance | Tensile Strain | Sensitivity (GF) | Conductivity | Performance Characteristics | Application Scenarios | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Resistance mode sensors | A, AM, KPS, LiCl | 0.206 MPa | −37 °C | 680% | 3.12 | R = 1.35 | Frost resistance, elastic recoverability, toughness, adhesion, self-healing properties | Monitoring human fingers, wrists, and elbows during exercise | [12] |
| PVA, CNTs, GO | - | - | 900% | 52.7 | - | Re-plasticity, self-healing properties, elastic strain (900%), mechanical pressure (10 kPa) | Wearable electronic devices, soft robots | [13] | |
| HPMC, AA, AM, TP, Al3+ | 0.032 MPa | - | 2225% | 5.13 | R = 3.03 | Stretchability (GF5.13), adhesion, self-healing performance (99.4%) | Monitoring joint and vocal cord vibrations during various movements | [14] | |
| CS, OHA, HPMC, PAA, TA, Al3+ | 0.0338 MPa | - | 3168% | <1 | R = 2.33 | Self-healing performance (95.5%), high transparency (98.5% transmittance), stretchability (GF4.12), adhesion | Monitoring joint bending movements, swallowing, and speaking during exercise | [15] | |
| PAM, CA, EtOH, DN | 0.581 MPa | - | 1545% | 1.53 | R = 1.53 | Biocompatibility, stretchability, high sensitivity (GF1.63), adhesion | Sports wearable devices and human–machine interface systems | [16] | |
| Starch, PHMB, glycerol, PVA | 0.209 MPa | −20 °C | 406% | 1.81 | - | Stretching resistance (GF 3.28), frost resistance (−128.9 °C), antibacterial properties, water retention properties | Monitoring various human movements, including speaking, finger bending, and knee bending | [17] | |
| NaSS, DMC, MBAA, NaSS/DMC | 0.06 MPa | - | 418% | 2.9 | - | Mechanical properties, self-healing properties, stretchability (GF 2.9), high cycling stability | Monitoring human movements, including bending of fingers, elbows, and knees | [18] | |
| MBAA, FeCl3·6H2O, APS, EDTA-2Na, MLA | 0.063 MPa | - | 556% | 2.6 | - | Stretchability (556%), high sensitivity, self-healing performance (99%). | Monitoring human movements, including finger, wrist, and elbow bending, throat swallowing, and pulse | [19] | |
| PVA, SA, NaCl, TENG | 0.58 MPa | - | - | - | R = 4.1 | Tensile strength (0.58 MPa), mechanical properties, conductivity | Human motion monitoring, pedometer, step frequency meter | [20] | |
| SA, PANI, GO, An, CaCl2 | 10.32 MPa | - | 176% | - | R = 0.5 | Cyclic stretchability, high sensitivity, repeatability, swelling, mechanical properties | Monitoring human movements, including palms, elbows, and knees | [21] | |
| PVA, O-CMCS, AgNPs, PDO, ZnSO4 | 2.2 MPa | −60 °C | 407% | 2.26 | R = 1.4 | High strength (2.2 MPa), frost resistance, ductility, high toughness, self-healing ability | Monitoring human motion at low temperatures, including body movement and bioelectric signals | [22] | |
| PVA, SA, CNF, MXene, GO | 0.21 MPa | - | 611.5% | 2.77 | - | Stretchability (GF 2.77), sensitivity, high toughness, temperature sensing | Wearable smart devices | [23] | |
| Capacitive mode sensors | CCNF, DES, NaOH, DA, PDA, AM | - | - | - | - | C = 0.99 | Adhesion, frost resistance, moisture retention, mechanical properties | Monitoring human electrocardiogram and bioelectrode adhesion | [24] |
| MoO2, a-CNTs, rGO, PVA | - | - | 246.7% | - | - | Cycle stability, stretchability, high mechanical properties | Wearable portable electronic devices, self-healing energy storage devices | [25] | |
| AA, AM, CMC, ZnSO4 | 1.22 MPa | - | 424% | 2.9 | C = 38 | Mechanical performance, flexibility, tensile strain, sensing performance, electrochemical performance | Flexible energy storage and application in wearable electronic devices | [26] | |
| PAM, SA, HCl, ZnSO4 | 0.1654 MPa | −20 °C | 408.2% | 5.08 | - | Mechanical properties (GF = 5.8), water retention, tensile sensing, adhesion, high sensitivity, and cycling stability | Detecting human movements, including fingers, arms, knees, ankles, and pulse | [27] | |
| PVA, AM, MBA, KPS, NMP | 0.1622 MPa | −17.3 °C | 826% | 3.0 | C = 21.7 | High compressibility, frost resistance (−85 °C), high stretchability, self-adhesive elasticity, fatigue resistance | Distinguishing various human movements | [28] | |
| Piezoelectric mode sensors | PVDF, AN, AAm, NaSS, APS | 3.15 MPa | −20 °C | 780% | - | - | High elongation, high toughness, frost resistance, self-powering performance, high sensitivity | Monitoring human movements, including finger and elbow bending, speaking, pulse, and gesture signal acquisition | [29] |
| PVA, PSS, MXene, CNFs, Glycerol | - | −20 °C | 15.3% | 3.37 | - | Frost resistance (−20 °C), tensile strength, piezoelectric mode activity, mechanical properties, moisturizing properties | Distinguishing between written letters and spoken words, and application in flexible wearable electronic products | [30] | |
| BNNT, PVDF, DMF, NMP | 18.10 MPa | - | 52.12% | - | - | Thermal conductivity, high waterproof and moisture-proof performance, mechanical strength, stretchability | Wearable sensing devices | [31] | |
| NaCl, CMC, BAPO-OH, Na-CMC | - | - | 600% | 2.27 | - | Biocompatibility, stretchability, high sensitivity, repeatability | Joint movement, heart rate, vocal cord vibration, and surface muscle contraction | [32] | |
| BaTiO3, AM, DMAA, CQ/DPI, PEGDA | 0.3 MPa | - | >100% | - | p > 1.5 | Tensile performance (>100% strain), mechanical properties, sensing properties, high sensitivity | Monitoring human motions, including finger, wrist, and knee movements | [33] | |
| CS, APTES, EG, Gel/OCS-ABTO | 0.01127 MPa | - | 225% | 2.18 | - | High sensitivity, stretchability, biocompatibility, mechanical response characteristics, self-powering performance | Human motion gesture recognition, elbow flexion, knee flexion, and plantar pressure distribution | [34] | |
| Battery-based sensing mode sensors | Ur, SA, ZIB, ZnCl2 | - | - | - | - | - | Mechanical properties, self-healing properties, biocompatibility | Monitoring human motion and physiological signal | [35] |
| HEA, AS, Gly, PTFE | 0.16 MPa | −42 °C | 3487% | 1.65 | B = 2.64 | Stretching performance (160 kPa), self-healing (95%) performance, fatigue resistance, frost resistance | Monitoring various human movements and medical tests | [36] | |
| AAm, BR, DMSO, MBAA, Zn | - | −36.9 °C | - | - | B = 24 | Fatigue resistance, frost resistance (−30 °C), stability, stretchability | Monitoring human movement at low temperatures | [37] | |
| NaCl, LiOH, ECH, K4Fe (CN)6·3H2O | - | - | - | - | B = 26.1 | High mechanical properties, sensing performance, stretchability | Monitoring body temperature and human movement | [38] | |
| PAAM, CMC, TA, ZnSO4, AAM, PVDF | 0.1063 MPa | −10 °C | 469.2% | 5.07 | B = 0.76 ± 0.04 | Stretchability (132 kPa), self-powered sensing performance, self-healing performance, self-adhesive performance | Monitoring various human movements | [39] |
2.1. Conductivity
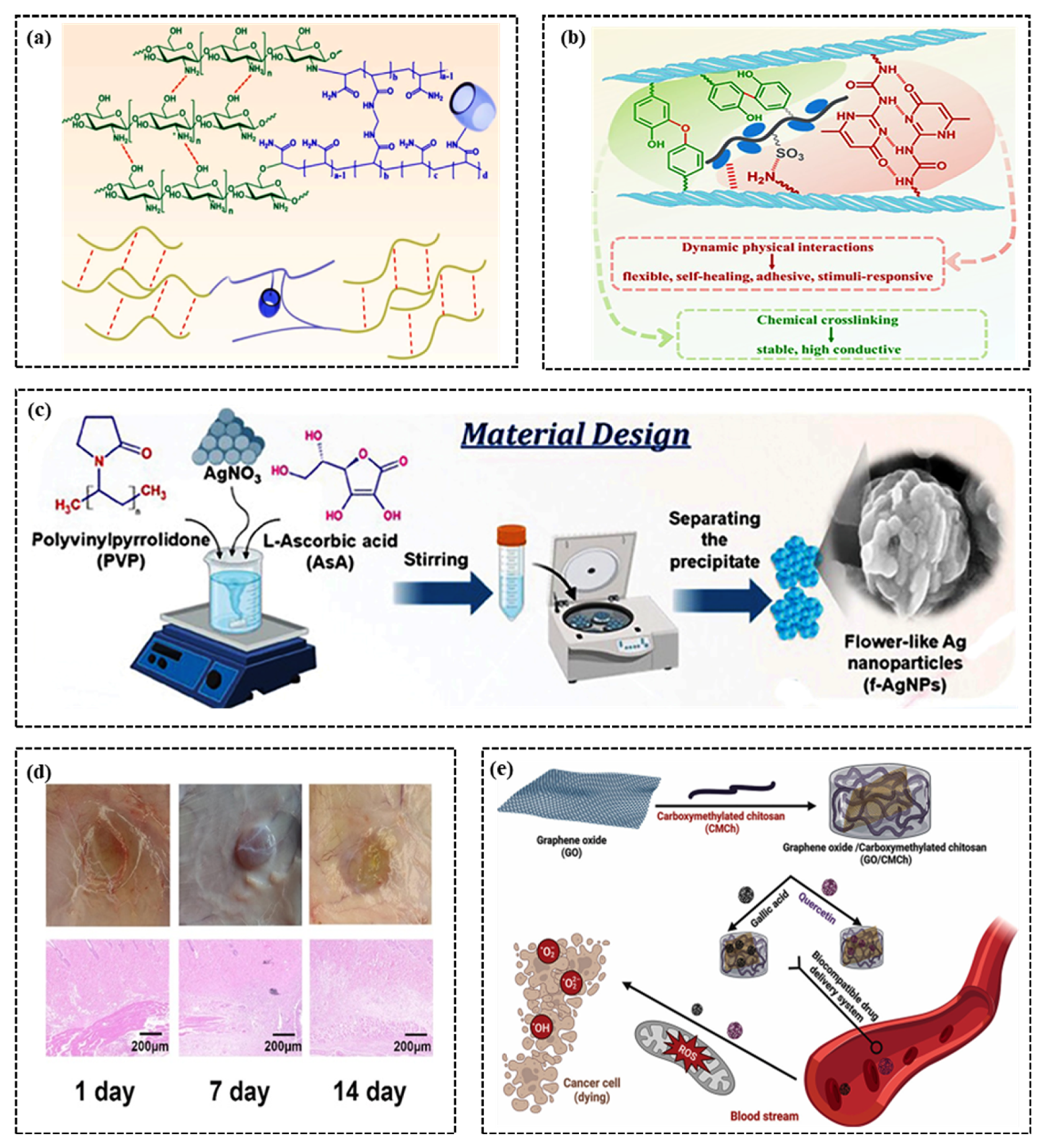
2.2. Adhesiveness

2.3. Self-Healing Ability

2.4. Biocompatibility
2.5. Antifreezing and Antiswelling Properties
2.6. Degradability and Sustainability
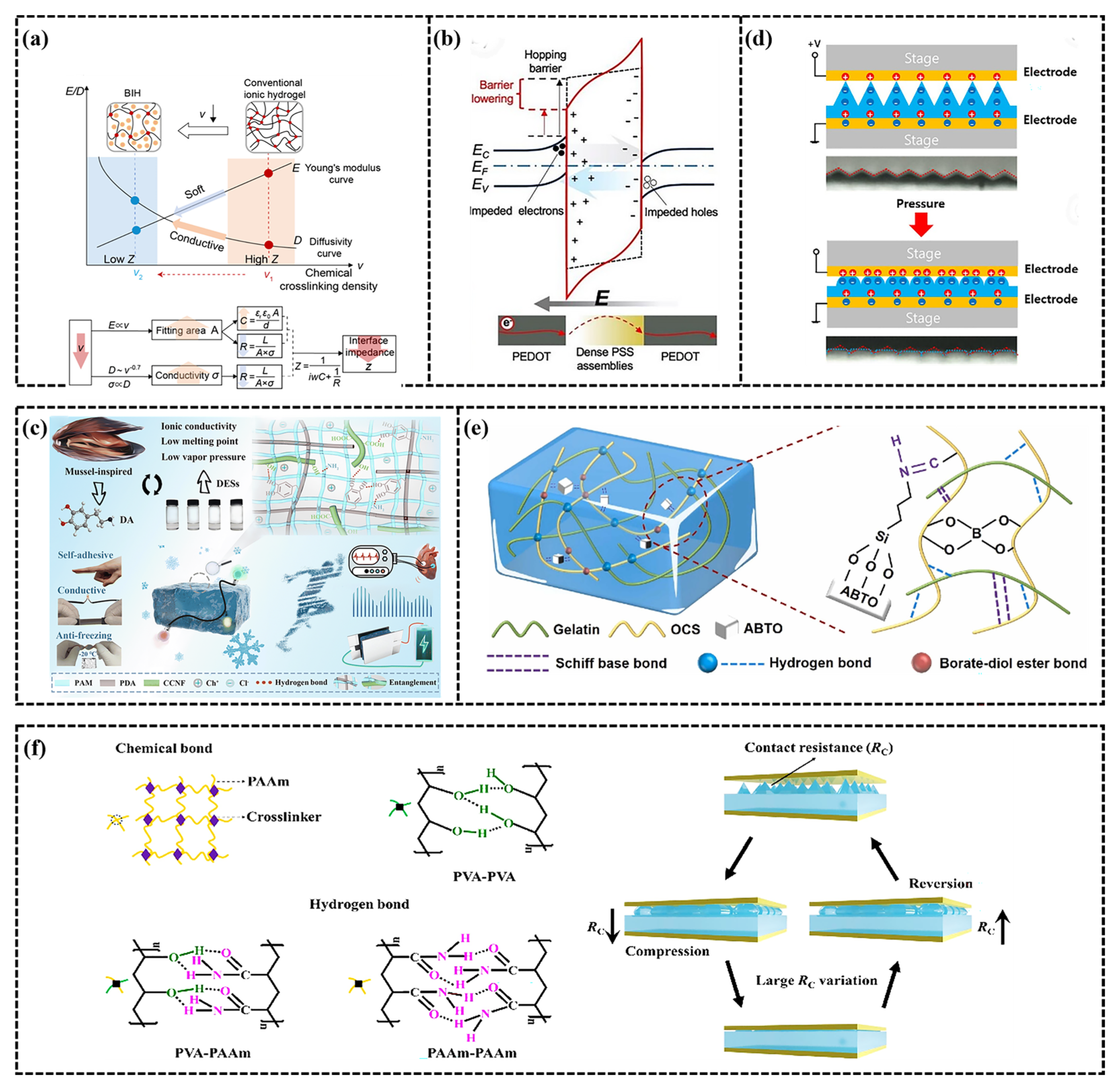
3. Conductivity Mechanism of CH-FWSs
3.1. Resistance Mode
3.2. Capacitive Mode
3.3. Piezoelectric Mode
3.4. Triboelectric Mode
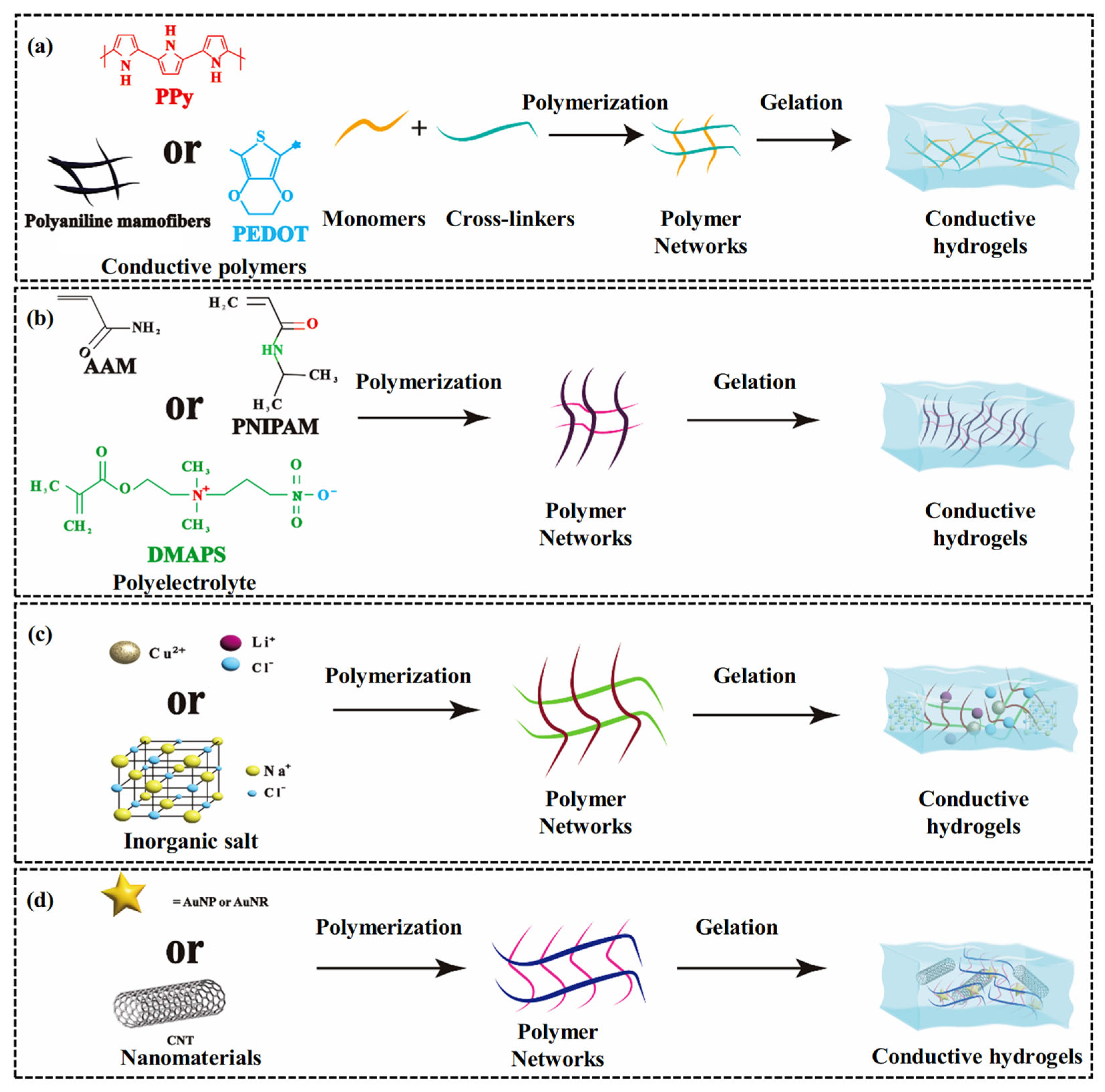
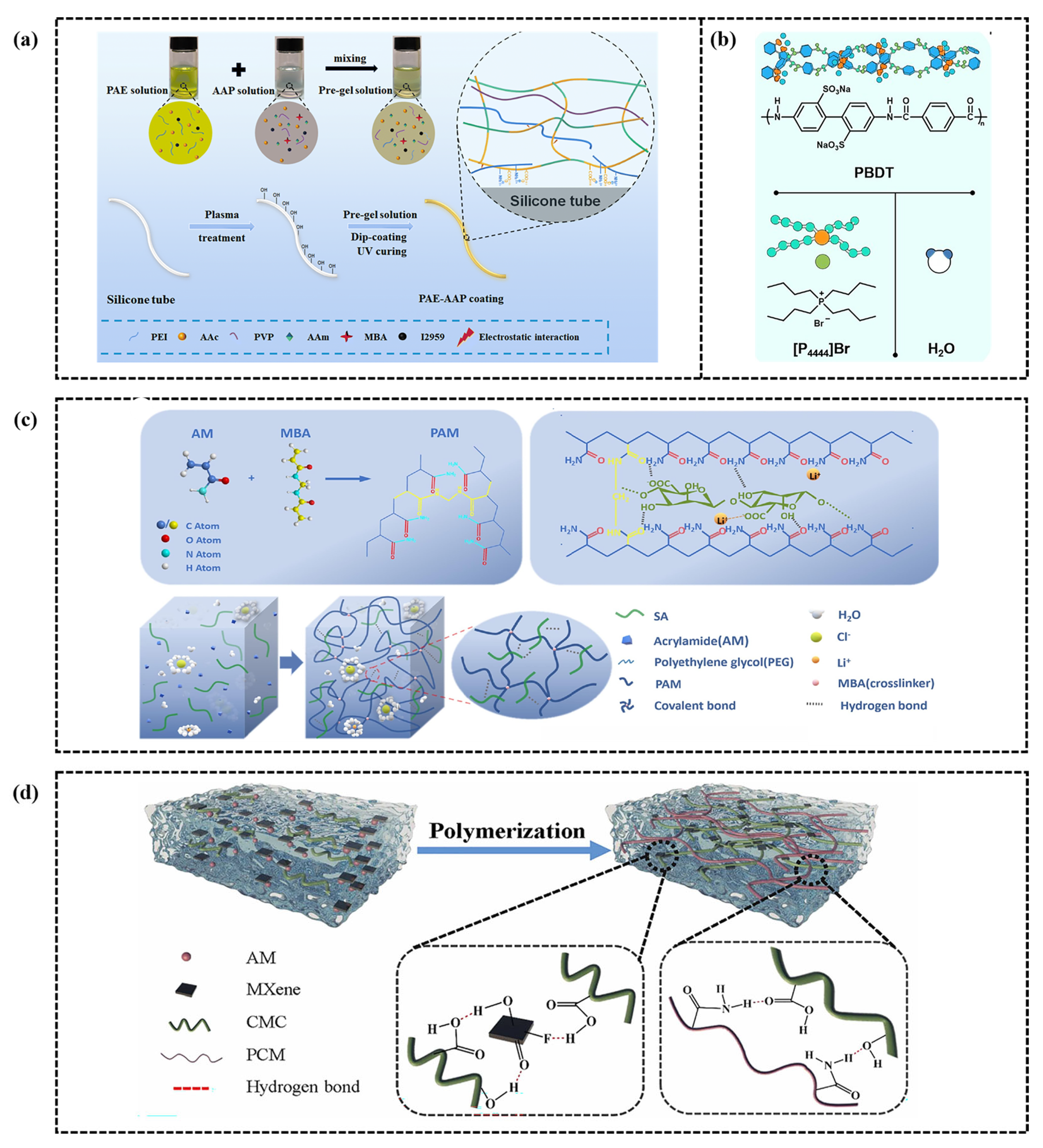
4. Modification Strategies of CH-FWSs
4.1. Bonding of Conductive Polymers
4.2. Combination of Polyelectrolytes
4.3. Inorganic Salt Doping
4.4. Integration of Nanomaterials
| Modification Strategy | Conductive Material | Hydrogel Network | Sensitivity (GF) | Stretchability | Conductivity (S/m) | Ref. |
|---|---|---|---|---|---|---|
| Conductive polymer | PPy | Silk/PPy | 0.54 | - | 26 | [157] |
| PPy | RCF-PPy | 2 | 13.9% | 0.8766 | [158] | |
| PANi/Fe3+ | P(AAm-co-AA) | 0.48 | 145–880% | 3.67 | [159] | |
| PANi/CNC | PAAm/HA | 3.7 | 2400% | 21.7 | [160] | |
| PPy | AG/SBMA | 4.58 | 510% | 0.514 | [161] | |
| PEDOT: PSS/MBA | PAA | 3.2 | 894% | 8.06 | [162] | |
| Polyelectrolyte | P(AM-AMPS) | PVA/DN | 1.57 | 1282% | 3.85 | [163] |
| AMPS | AAm/VIPS | 4.2 | 604% | 0.51 | [164] | |
| Alginate/AAc/Al2+ | AAV/APS | 1.2 | 1198% | 12.5 | [165] | |
| AAc/Fe | P(AAc-co-AAm) | 1.3 | 820% | 3.24 ± 0.12 | [166] | |
| DMAPS/MAA | SA/PMAA | - | 1353% | - | [167] | |
| SA/SBMA | AAm/MBAA | 4.56 | 1800% | 0.15 | [168] | |
| Inorganic salt doping | LiCl | PAM/SA | - | - | 21.7 | [169] |
| LiCl | PVA | - | 43–120% | 24.29 | [170] | |
| ZnCl2/CaCl2 | Cotton cellulose | 0.42 | 500% | 7.49 | [171] | |
| NaCl | PVA | 5.98 | 400% | 7.14 | [172] | |
| NaCl | PVA/Pullulan | 2.69 | 800% | 10.44 | [173] | |
| LiCl | SBMA/AA/HEMA | 2.08 | 505.54% | 2.25 | [174] | |
| Ag/Cu | Gel/Na2SO4 | 6.8 | 732.9% | 1.35 | [175] | |
| Nanomaterial | CNT/CaCl2 | AAm/SA | 2.83 | 1821% | 0.48 | [176] |
| AP/AgNPs | PVA/Borax | - | 480% | 0.39 | [177] | |
| rGO | NIPAM | 1.12 | 400% | 4.3 | [178] | |
| AgNWs | PVA | - | - | 17.39 | [179] | |
| MXene/Na/Li | PVA/TA | 5.42 | 900% | 8.1 | [180] | |
| CNT | PNIPAM | 2.15 | 1800% | 0.071 | [181] | |
| AgNW/AgF | PVP/PU | 4.8 | 2700% | 83.836 | [182] | |
| MXene | PVA/Aam | 1.2 | 1400% | 0.07 | [183] |
4.5. Material Advancement and Detection Limit Evaluation
5. Sports Application of CH-FWSs
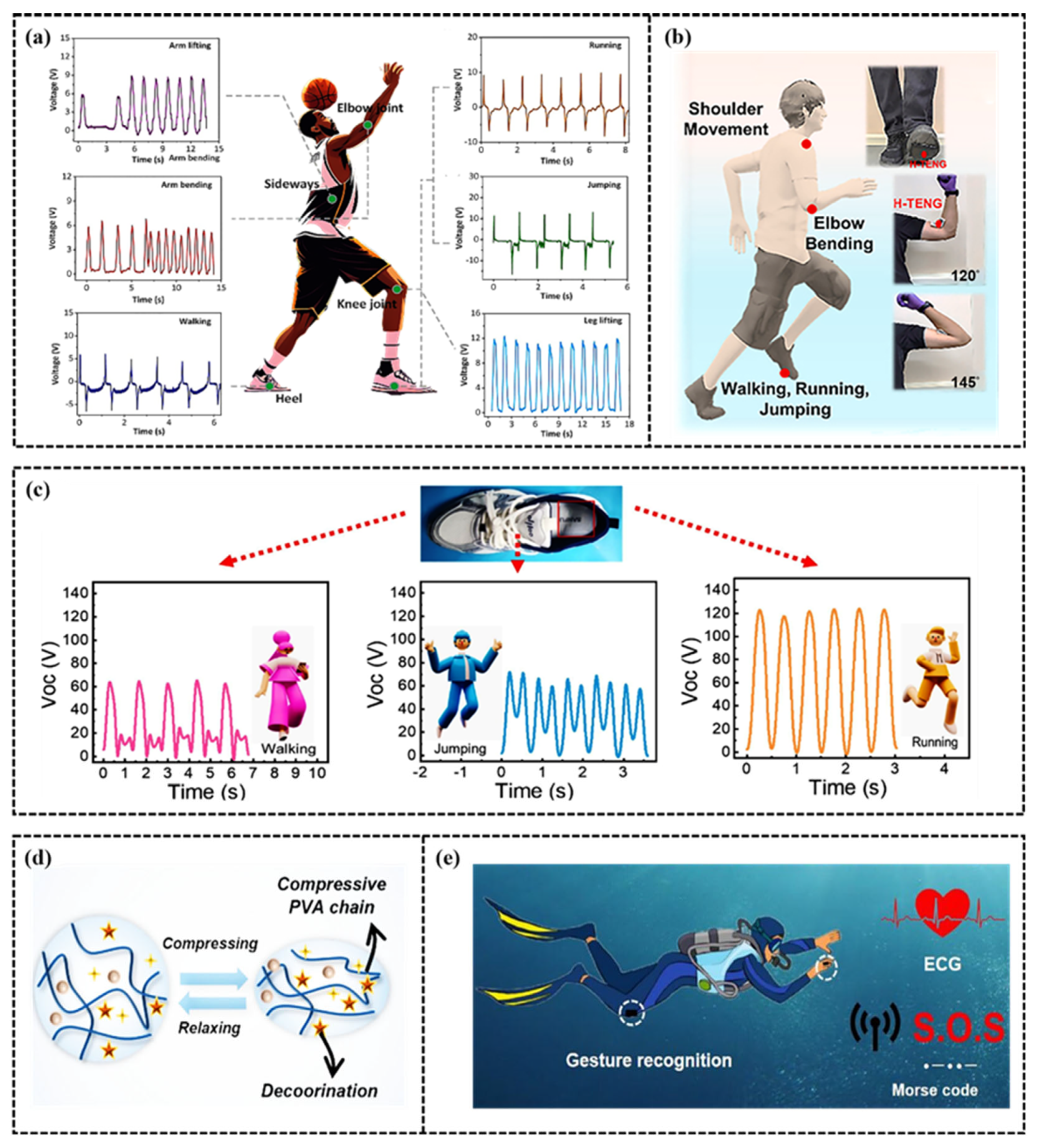
5.1. Human Motion Monitoring
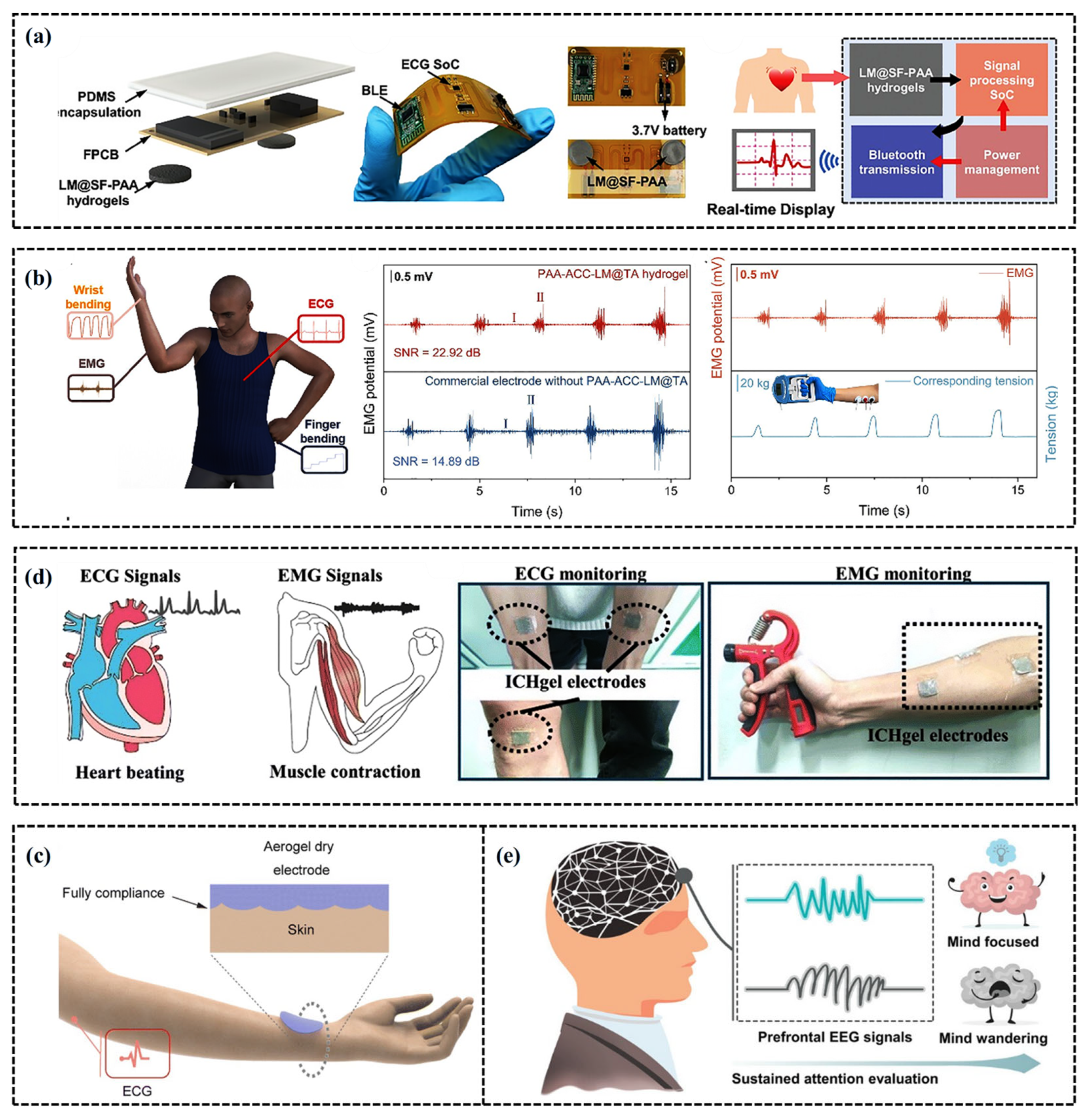
5.2. Exercise Electrophysiological Signals
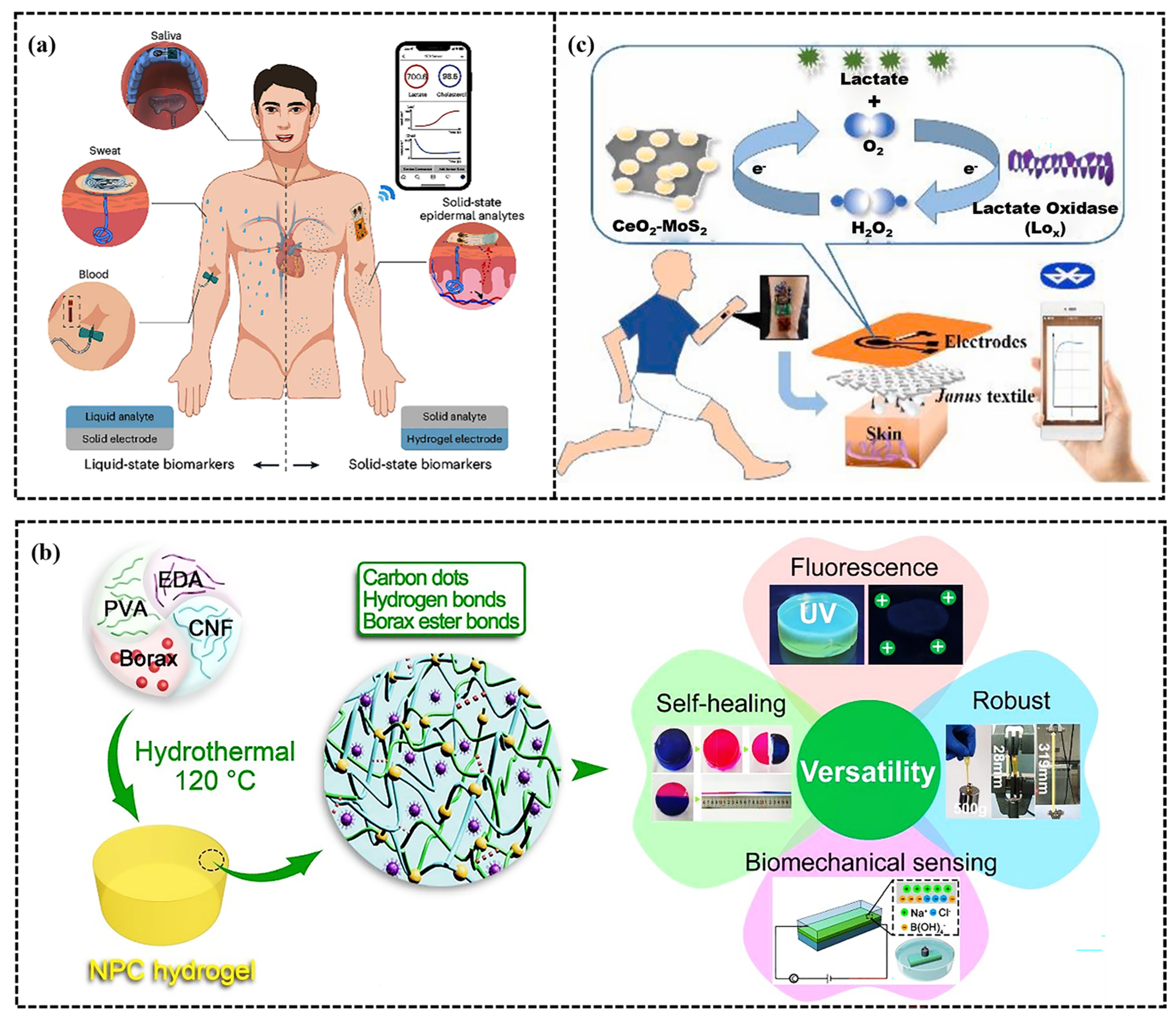
5.3. Sports Electrochemical Biological Monitoring
5.4. Commercial Viability
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.; Choi, Y.; Bae, Y.M.; Nam, S.; Shin, K. Stretchable and Shape-Transformable Organohydrogel with Gallium Mesh Frame. Gels 2024, 10, 769. [Google Scholar] [CrossRef]
- Lingling, M.; Liu, D.; Ding, S.; Li, W.; Liu, E. Preparation of Dual-Network Conductive Hydrogels As Wearable Flexible Strain Sensors Using One-Pot Method and Soaking Strategy. Polym. Sci. Ser. A 2024, 66, 76–85. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.-H.; et al. Soft and Elastic Hydrogel-Based Microelectronics for Localized Low-Voltage Neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–67. [Google Scholar] [CrossRef]
- Cao, X.; He, T.; Sui, J.; Yan, Y.; Liu, X.; Liu, L.; Lv, S. PVA/KGM dual network hydrogels doped with carbon nanotube-collagen corona as flexible sensors for human motion monitoring. J. Mater. Chem. C 2024, 12, 3333–3344. [Google Scholar] [CrossRef]
- Su, N. Advances and Prospects in the Study of Spherical Polyelectrolyte Brushes as a Dopant for Conducting Polymers. Molecules 2024, 29, 1315. [Google Scholar] [CrossRef]
- Singh Negi, S.; Rawat, S.; Singh, P.K.; Savilov, S.V.; Yadav, T.; Yahya, M.Z.A.; Chandra Singh, R. Conducting Carbon Black Nano-Filler Doped Polymer Electrolyte for Electrochemical Application. ChemistrySelect 2024, 9, e202400847. [Google Scholar] [CrossRef]
- Meng, L.; Li, W.; Ding, S.; Liu, E.; Liu, D. A Cellulose Reinforced Polyacrylamide/Polyvinyl Alcohol Dual Network Hydrogel for Flexible Sensors. ChemistrySelect 2024, 9, e202400710. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Hong, S.; Park, T.; Lee, J.; Ji, Y.; Dai, Y.; Yi, J.; Kim, J.J.; Kim, D.R.; Lee, C.H. Tough Conductive Organohydrogel for Wearable Sensing in Extreme Environmental Conditions. Adv. Mater. Technol. 2023, 9, 202301398. [Google Scholar] [CrossRef]
- Zhang, X.; Pang, J. A self-healing and wearable hydrogel sensor with a dynamic physical cross-linking structure can detect strain stimulus in a wide temperature range. J. Mater. Chem. C 2023, 11, 11988–11999. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, G.; Sun, Y.; Li, C.; Zhao, N.; Chen, Z.; Guo, R.; Zheng, Z.; Zhou, F.; Liu, W. Mussel-Inspired Wet-Adhesive Multifunctional Organohydrogel with Extreme Environmental Tolerance for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2023, 15, 44342–44353. [Google Scholar] [CrossRef]
- Di, X.; Li, J.; Yang, M.; Zhao, Q.; Wu, G.; Sun, P. Bioinspired, nucleobase-driven, highly resilient, and fast-responsive antifreeze ionic conductive hydrogels for durable pressure and strain sensors. J. Mater. Chem. A 2021, 9, 20703–20713. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, E.; Li, A.; Cui, C.; Guo, R.; Tang, H.; Xiao, H.; Zhou, M.; Qin, W.; Wang, X.; et al. A porous self-healing hydrogel with an island-bridge structure for strain and pressure sensors. J. Mater. Chem. B 2021, 9, 719–730. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, W.; Li, R.; Zhang, M.; Li, Y.; Yang, W. Ultra-stretchable, fast self-healing, adhesive, and strain-sensitive wearable sensors based on ionic conductive hydrogels. New J. Chem. 2024, 48, 11705–11716. [Google Scholar] [CrossRef]
- Li, R.; Ren, J.; Zhang, M.; Li, M.; Li, Y.; Yang, W. Highly Stretchable, Fast Self-Healing, Self-Adhesive, and Strain-Sensitive Wearable Sensor Based on Ionic Conductive Hydrogels. Biomacromolecules 2024, 25, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Wang, M.; Meng, C.; Guo, K.; Shariar Emon, N.; Li, J.; Qi, K.; Dai, Y.; Wang, B. Enhanced mechanical strength and stretchable ionic conductive hydrogel with double-network structure for wearable strain sensing and energy harvesting. Compos. Sci. Technol. 2024, 255, 110732. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Kong, X.; Zhou, X.; Gao, Y.; Wang, Y.; Gao, G.; Qu, W.; Shi, K. Ionic conductive soluble starch hydrogels for biocompatible and anti-freezing wearable sensors. Eur. Polym. J. 2024, 210, 112949. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, H.; Wang, Z.; Zhang, S.; Zhang, H. Ionically Conductive and Self-Healing Polyampholyte Hydrogels for Wearable Resistive Strain Sensors and Capacitive Pressure Sensors. ACS Appl. Polym. Mater. 2023, 5, 7581–7589. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, S.; Zhu, H.; Hu, H.; Yuan, N.; Ding, J. Self-healing hydrogel sensors with multiple shape memory properties for human motion monitoring. New J. Chem. 2021, 45, 314–320. [Google Scholar] [CrossRef]
- Long, K.; Zhang, Y.; Gao, X.; Li, J.; Luo, Y.; Huang, M.; Mao, Y.; Hu, C.; Guo, S. A Flexible, Conductive Hydrogel for Strain Sensor and Triboelectric Nanogenerator toward Human Motion Monitoring. ACS Appl. Electron. Mater. 2024, 6, 5496–5506. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Yi, J.; Wang, D.; Wu, W. Facile fabrication of flexible alginate/polyaniline/graphene hydrogel fibers for strain sensor. J. Eng. Fibers Fabr. 2022, 17, 15589250221114641. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Zhang, J.; Ren, Z.; Zhou, J.; Gu, H. Coordination/salting-out synergistic construction of multifunctional PVA/chitosan conductive organohydrogel as strain and bioelectrical sensors. Polymer 2024, 298, 126889. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, Z.; Wang, Y.; Yu, L.; Liu, K.; Wu, H.; Huang, L.; Chen, L.; Ni, Y. A tough organohydrogel-based multiresponsive sensor for a triboelectric nanogenerator and supercapacitor toward wearable intelligent devices. J. Mater. Chem. A 2022, 10, 12092–12103. [Google Scholar] [CrossRef]
- Linghu, C.; Liu, Y.; Yang, X.; Chen, Z.; Feng, J.; Zhang, Y.; Li, Y.; Zhao, Z.; Seo, Y.; Li, J.; et al. Versatile adhesive skin enhances robotic interactions with the environment. Sci. Adv. 2025, 11, eadi3347. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Zhao, T.; Jalil, A.; Jiang, T.; Shu, Y. MoO2/a-CNT/rGO as electrode material for all-in-one flexible supercapacitor with integrated structure and function. Diam. Relat. Mater. 2024, 142, 110858. [Google Scholar] [CrossRef]
- Wanyan, H.; Li, Q.; Huang, H.; Li, J.; Huang, L.; Chen, L.; Wei, J.; Zhou, X.; Tang, Z.; Wu, H. Flexible high electrochemical active hydrogel for wearable sensors and supercapacitor electrolytes. Int. J. Biol. Macromol. 2024, 277, 134356. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Xing, Z.; Ge, X.; Zhang, X.; Zhang, Q.; Wang, Z.-X. Acid-mediated strategies designed for stretchable and durable polyacrylamide/sodium alginate dual-network hydrogels toward flexible capacitors and wearable sensors. Int. J. Biol. Macromol. 2024, 276, 134065. [Google Scholar] [CrossRef]
- Dong, X.; Chen, W.; Ge, X.; Li, S.; Xing, Z.; Zhang, Q.; Wang, Z.-X. Stretchable, self-adhesion and durable polyacrylamide/polyvinylalcohol dual-network hydrogel for flexible supercapacitor and wearable sensor. J. Energy Storage 2024, 89, 111793. [Google Scholar] [CrossRef]
- Shi, Y.; Guan, Y.; Liu, M.; Kang, X.; Tian, Y.; Deng, W.; Yu, P.; Ning, C.; Zhou, L.; Fu, R.; et al. Tough, Antifreezing, and Piezoelectric Organohydrogel as a Flexible Wearable Sensor for Human–Machine Interaction. ACS Nano 2024, 18, 3720–3732. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Z.; Zheng, S.; Liu, A.; Wang, Y.; Chen, L.; Miao, Q. High Ion-Conducting PVA Nanocomposite Hydrogel-Based Wearable Piezoelectric and Triboelectric Sensors for Harsh Environments. Biomacromolecules 2024, 25, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Yanar, N.; Kim, T.Y.-S.; Jung, J.; Dinh, D.K.; Choi, K.-i.; Pornea, A.G.; Yadav, D.; Hanif, Z.; Park, E.; Kim, J. Boron Nitride Nanotube-Aligned Electrospun PVDF Nanofiber-Based Composite Films Applicable to Wearable Piezoelectric Sensors. ACS Appl. Nano Mater. 2024, 7, 11715–11726. [Google Scholar] [CrossRef]
- Mogli, G.; Chiappone, A.; Sacco, A.; Pirri, C.F.; Stassi, S. Ultrasensitive Piezoresistive and Piezocapacitive Cellulose-Based Ionic Hydrogels for Wearable Multifunctional Sensing. ACS Appl. Electron. Mater. 2022, 5, 205–215. [Google Scholar] [CrossRef]
- Wu, M.; Wang, G.; Zhang, M.; Li, J.; Wang, C.; Sun, G.; Zheng, J. A tough and piezoelectric poly(acrylamide/N, N-dimethylacrylamide) hydrogel-based flexible wearable sensor. Soft Matter 2024, 20, 6800–6807. [Google Scholar] [CrossRef]
- Fu, R.; Zhong, X.; Xiao, C.; Lin, J.; Guan, Y.; Tian, Y.; Zhou, Z.; Tan, G.; Hu, H.; Zhou, L.; et al. A stretchable, biocompatible, and self-powered hydrogel multichannel wireless sensor system based on piezoelectric barium titanate nanoparticles for health monitoring. Nano Energy 2023, 114, 108617. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, X.; Li, J.; Zhang, H.; Li, D.; Ge, H.; Liang, S.; Lu, B.; Zhao, J.; Zhou, J. Biocompatible and stable quasi-solid-state zinc-ion batteries for real-time responsive wireless wearable electronics. Energy Environ. Sci. 2024, 17, 3878–3887. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Tian, X.; Liu, S.; Xu, Z.; Sun, G.; Qin, G.; Chen, Q. Self-powered wearable sensing devices based on a flexible ammonium-ion battery with fatigue resistance and frost resistance based on a strong and tough hydrogel. J. Mater. Chem. C 2022, 10, 17675–17683. [Google Scholar] [CrossRef]
- Li, B.; Ullah Khan, M.; Qv, C.; Chen, L.; Xiong, Y.; Zhang, L.; Wu, M. A wearable zinc-air battery based on an Agar hydrogel electrolyte for stable operation at −30 °C. Chem. Phys. Lett. 2023, 832, 140883. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Wu, Z.; Han, Z.; Qu, X.; Jin, M.; Jia, Y.; Zhou, Z.; Wang, H. Bacterial cellulose hydrogel-based wearable thermo-electrochemical cells for continuous body heat harvest. Nano Energy 2023, 112, 108482. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, Y.; Yang, H.Y.; Fu, Y.; Xi, M.; Jiang, Y.; Li, Y. Cellulose nanocrystals-strengthened, anti-drying, and anti-freezing hydrogels for human motion sensing and 3D printing. Polymer 2024, 296, 126837. [Google Scholar] [CrossRef]
- Li, Y.; Miao, R.; Yang, Y.; Han, L.; Han, Q. A zinc-ion battery-type self-powered strain sensing system by using a high-performance ionic hydrogel. Soft Matter 2023, 19, 8022–8032. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Huang, Y.-X.; Yu, J.-B.; Yang, X.; Luo, M.-D.; Chen, X.-P. A synergistic enhancement strategy for mechanical and conductive properties of hydrogels with dual ionically cross-linked κ-carrageenan/poly(sodium acrylate-co-acrylamide) network. Carbohydr. Polym. 2024, 346, 122638. [Google Scholar] [CrossRef]
- Reynolds, M.; Stoy, L.M.; Sun, J.; Opoku Amponsah, P.E.; Li, L.; Soto, M.; Song, S. Fabrication of Sodium Trimetaphosphate-Based PEDOT:PSS Conductive Hydrogels. Gels 2024, 10, 115. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, S.; Bai, J.; Yin, J.; Li, N.; Jiao, T. Ionic conductive hydroxypropyl methyl cellulose reinforced hydrogels with extreme stretchability, self-adhesion and anti-freezing ability for highly sensitive skin-like sensors. Int. J. Biol. Macromol. 2022, 220, 90–96. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Pan, D.; Tian, J.; Qi, H.; Li, R.a.; Lu, F.; He, M. Ultrastretchable and Antifreezing Double-Cross-Linked Cellulose Ionic Hydrogels with High Strain Sensitivity under a Broad Range of Temperature. ACS Sustain. Chem. Eng. 2019, 7, 14256–14265. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Zhang, D.; Yang, M.; Huang, X.; Han, L.; Chen, K.; Li, X.; Pang, R.; Shang, Y.; et al. Conductive hydrogels incorporating carbon nanoparticles: A review of synthesis, performance and applications. Particuology 2023, 83, 212–231. [Google Scholar] [CrossRef]
- Liao, G.; Hu, J.; Chen, Z.; Zhang, R.; Wang, G.; Kuang, T. Preparation, Properties, and Applications of Graphene-Based Hydrogels. Front. Chem. 2018, 6, 450. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, T.; Wang, X.; Liang, L.; Lu, H.; Xie, Z.; Yuan, T.; Fang, G. Intrinsically conductive polymer reinforced hydrogel with synergistic strength, toughness, and sensitivity for flexible motion-monitoring sensors. Cell Rep. Phys. Sci. 2024, 5, 102178. [Google Scholar] [CrossRef]
- Peng, Z.; Zhong, W. Facile Preparation of an Excellent Mechanical Property Electroactive Biopolymer-Based Conductive Composite Film and Self-Enhancing Cellulose Hydrogel to Construct a High-Performance Wearable Supercapacitor. ACS Sustain. Chem. Eng. 2020, 8, 7879–7891. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, A.; Yang, Y.; Zhu, Y.; Song, Y.; Li, Y.; Li, J. Mussel-inspired cellulose nanofiber/poly(vinyl alcohol) hydrogels with robustness, self-adhesion and antimicrobial activity for strain sensors. Int. J. Biol. Macromol. 2023, 245, 125469. [Google Scholar] [CrossRef]
- Santoro, L.; Vaiani, L.; Boccaccio, A.; Lamberti, L.; Lo Muzio, L.; Ballini, A.; Cantore, S. Finite Element Modeling of Cells Adhering to a Substrate: An Overview. Appl. Sci. 2024, 14, 2596. [Google Scholar] [CrossRef]
- Pardo-Rendón, A.G.; Mejía-Méndez, J.L.; López-Mena, E.R.; Bernal-Chávez, S.A. Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery. Gels 2024, 10, 574. [Google Scholar] [CrossRef]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef]
- Tian, Y.; Hou, L.X.; Zhang, X.N.; Du, M.; Zheng, Q.; Wu, Z.L. Engineering Tough Supramolecular Hydrogels with Structured Micropillars for Tunable Wetting and Adhesion Properties. Small 2024, 20, 2308570. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.; Shi, C.; Liu, Y.; Zhu, Y.; Song, Y. Self-healing hydrogel with multiple adhesion as sensors for winter sports. J. Colloid Interface Sci. 2023, 629, 1021–1031. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Xu, X.; Ren, L.; Zhou, X.; Xu, H.; Zhao, L.; Xin, J.; Wang, S.; Wang, Z. Chitosan-based multifunctional hydrogel with bio-adhesion and antioxidant properties for efficient wound hemostasis. Colloids Surf. B Biointerfaces 2024, 234, 113697. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Stevanović, M.; Filipović, N. A Review of Recent Developments in Biopolymer Nano-Based Drug Delivery Systems with Antioxidative Properties: Insights into the Last Five Years. Pharmaceutics 2024, 16, 670. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, Z.h.; Tang, X.-G.; Liang, Z.; Feng, J.; Ye, L.; Tan, Y.; Jiang, Y.p.; Lan, M.; Zhu, D. Wireless Wearable System Based on Multifunctional Conductive Peg-Hema Hydrogel with Anti-Freeze, Anti-Uv, Self-Healing, and Self-Adhesive Performance for Health Monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134196. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Q.; Yao, X.; Bai, R.; Hong, W.; Yang, C. Fatigue of amorphous hydrogels with dynamic covalent bonds. Extrem. Mech. Lett. 2022, 53, 101679. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Fang, X.; Zhang, Z.; Li, S.; Sun, J. Healable and Recyclable Polymeric Materials with High Mechanical Robustness. ACS Mater. Lett. 2022, 4, 554–571. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, J.; Ren, A.; Wei, Z.; Xiang, T.; Zhou, S. Ultra-fast cryogenic self-healing ionic hydrogel for flexible wearable bioelectronics. Chem. Eng. J. 2024, 495, 153734. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Ling, Q.; Fan, X.; Gu, H. Ultra-fast preparation of multifunctional conductive hydrogels with high mechanical strength, self-healing and self-adhesive properties based on Tara Tannin-Fe3+ dynamic redox system for strain sensors applications. Polymer 2022, 240, 124513. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, X.; Liu, A.; Wang, S.; Chen, B.; Chen, Z.; Lyu, M.; Guo, W.; Cao, X.; Ye, M. Tough Hydro-Aerogels with Cation Specificity Enabled Ultra-High Stability for Multifunctional Sensing and Quasi-Solid-State Electrolyte Applications. Adv. Mater. 2024, 36, 202313088. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Bian, T.; Yang, P.; Yang, Y.; Zhang, L. Fabrication and Characterization of π–π Stacking Peptide-Contained Double Network Hydrogels. ACS Biomater. Sci. Eng. 2023, 9, 4761–4769. [Google Scholar] [CrossRef]
- Morariu, S. Advances in the Design of Phenylboronic Acid-Based Glucose-Sensitive Hydrogels. Polymer 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, S.; Guo, Z.; Hu, Y. Double dynamic bonds tough hydrogel with high self-healing properties based on acylhydrazone bonds and borate bonds. Polym. Adv. Technol. 2022, 33, 2528–2541. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- Wang, K.; Dong, R.; Tang, J.; Li, H.; Dang, J.; Zhang, Z.; Yu, Z.; Guo, B.; Yi, C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 2022, 430, 132664. [Google Scholar] [CrossRef]
- Yang, X.; Guo, M.; Wu, Y.; Xue, S.; Xia, Y.; Zhang, R.; Wang, H.; Guo, Q. A facile approach for polymer hydrogels with enhanced strength, self–healing and multi–responsive shape memory properties. Mater. Res. Express 2019, 6, 125340. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically Tunable Conductive Interpenetrating Network Hydrogels That Mimic the Elastic Moduli of Biological Tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Xu, H.; Huang, C.; Lu, Y.; Cui, H.; Tan, Y. Chitosan-driven biocompatible hydrogel based on water-soluble polypyrrole for stable human-machine interfaces. Carbohydr. Polym. 2022, 295, 119890. [Google Scholar] [CrossRef]
- Sun, J.; Wu, X.; Xiao, J.; Zhang, Y.; Ding, J.; Jiang, J.; Chen, Z.; Liu, X.; Wei, D.; Zhou, L.; et al. Hydrogel-Integrated Multimodal Response as a Wearable and Implantable Bidirectional Interface for Biosensor and Therapeutic Electrostimulation. ACS Appl. Mater. Interfaces 2023, 15, 5897–5909. [Google Scholar] [CrossRef]
- Cheng, H.; Keerthika Devi, R.; Huang, K.Y.; Ganesan, M.; Ravi, S.K.; Lin, C.C. Highly Biocompatible Antibacterial Hydrogel for Wearable Sensing of Macro and Microscale Human Body Motions. Small 2024, 20, 202401201. [Google Scholar] [CrossRef]
- Dai, R.; Gao, Y.; Sun, Y.; Shi, K.; Gao, G.; Zhang, H. Ionic conductive amylopectin hydrogels for biocompatible and anti-freezing wearable sensors. Eur. Polym. J. 2023, 200, 112496. [Google Scholar] [CrossRef]
- Salama, A.M.; Alqarni, Z.; Hamed, Y.S.; Yang, K.; Nour, H.F.; Lu, J. Biocompatible graphene oxide/carboxymethylated chitosan nanocomposite hydrogel for controlled release of polyphenolic antioxidants. J. Drug Deliv. Sci. Technol. 2024, 95, 105630. [Google Scholar] [CrossRef]
- Niu, W.; Tian, Q.; Liu, Z.; Liu, X. Solvent-Free and Skin-Like Supramolecular Ion-Conductive Elastomers with Versatile Processability for Multifunctional Ionic Tattoos and On-Skin Bioelectronics. Adv. Mater. 2023, 35, 202304157. [Google Scholar] [CrossRef]
- Zhao, Y.; Ohm, Y.; Liao, J.; Luo, Y.; Cheng, H.-Y.; Won, P.; Roberts, P.; Reis Carneiro, M.; Islam, M.F.; Ahn, J.H.; et al. A Self-Healing Electrically Conductive Organogel Composite. Nat. Electron. 2023, 6, 206–215. [Google Scholar] [CrossRef]
- Yang, J.; Chang, L.; Deng, H.; Cao, Z. Zwitterionic Eutectogels with High Ionic Conductivity for Environmentally Tolerant and Self-Healing Triboelectric Nanogenerators. ACS Nano 2024, 18, 18980–18991. [Google Scholar] [CrossRef]
- Li, P.; Sun, W.; Li, J.; Chen, J.-P.; Wang, X.; Mei, Z.; Jin, G.; Lei, Y.; Xin, R.; Lei, T.; et al. N-type Semiconducting Hydrogel. Science 2024, 384, adj4397. [Google Scholar] [CrossRef]
- Gao, X.; Wu, J.; Wang, Y.; Wang, Y.; Zhang, Y.; Nguyen, T.T.; Guo, M. Anti-Freezing Hydrogel Regulated by Ice-Structuring Proteins/Cellulose Nanofibers System as Flexible Sensor for Winter Sports. Int. J. Biol. Macromol. 2024, 265, 131118. [Google Scholar] [CrossRef]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(ether urethane)s and α-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Wu, J.; Xue, Z.; Yan, C.; Zhang, H.; Wang, Y.; Zhang, Y.; Jiang, M.; Zhao, Y. Lignin-Ethylene Glycol Improved Hydrogel with Antifreezing and Antiswelling Properties As a Flexible Sensor for Underwater Motion Monitoring. ACS Sustain. Chem. Eng. 2024, 12, 15159–15172. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Wang, S.; Feng, W. Design of High-Temperature-Tolerant, Self-Healing, and Antiswelling Ion-Conductive Hydrogels for Reliable Flexible Electronics. Macromolecules 2025, 58, 5c01230. [Google Scholar] [CrossRef]
- Aljarid, A.A.K.; Doty, K.L.; Wei, C.; Salvage, J.P.; Boland, C.S. Food-Inspired, High-Sensitivity Piezoresistive Graphene Hydrogels. ACS Sustain. Chem. Eng. 2023, 11, 2c06101. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef]
- Zhao, D.; Pang, B.; Zhu, Y.; Cheng, W.; Cao, K.; Ye, D.; Si, C.; Xu, G.; Chen, C.; Yu, H. A Stiffness-Switchable, Biomimetic Smart Material Enabled by Supramolecular Reconfiguration. Adv. Mater. 2022, 34, 2107857. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Ding, M.; Xia, T.; Gong, Q.; Zeng, X.; Cai, Z.; Hu, Y. From network to channel: Crack-based strain sensors with high sensitivity, stretchability, and linearity via strain engineering. Nano Energy 2023, 116, 108832. [Google Scholar] [CrossRef]
- Pan, X.; Xu, Z.; Bao, R.; Pan, C. Research Progress in Stretchable Circuits: Materials, Methods, and Applications. Adv. Sens. Res. 2023, 2, 2300065. [Google Scholar] [CrossRef]
- Saeed, A.; Zaidi, S.F.A.; Park, C.G.; Lee, J.H. Enhancing Multifunctionality and Performance Indicators of Resistive-Type Strain Sensors with Advanced Conductive Hydrogels. Adv. Mater. Technol. 2024, 9, 2400355. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, X.; Wang, Y.; Wang, P.; Jiang, X.; Song, Z.; Ding, J.; Liu, G.; Li, X.; Sun, W.; et al. Gel-based strain/pressure sensors for underwater sensing: Sensing mechanisms, design strategies and applications. Compos. Part B Eng. 2023, 255, 110631. [Google Scholar] [CrossRef]
- Hasan, S.; Kouzani, A.Z.; Adams, S.; Long, J.; Mahmud, M.A.P. Recent progress in hydrogel-based sensors and energy harvesters. Sens. Actuators A Phys. 2022, 335, 113382. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Zhou, G.; Peng, M. Superhydrophobic, Anti-Freezing and Multi-Cross-Linked Wearable Hydrogel Strain Sensor for Underwater Gesture Recognition. ACS Sens. 2024, 9, 4617–4625. [Google Scholar] [CrossRef]
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Xiang, X.; Chen, K.; Yang, X.; Guan, L.; Jiang, X.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Wang, B.; Dong, F.; Sun, X.; Bu, Y.; Wang, H.; Tang, D.; Li, L. Biphase Ionic Hydrogels with Ultrasoftness and High Conductivity for Bio-Ionotronics. ACS Nano 2025, 19, 16488–16499. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Liu, X.; Xia, S.; Gao, Y.; Gao, G. Flexible and wearable strain sensors based on conductive hydrogels. J. Polym. Sci. 2022, 60, 2663–2678. [Google Scholar] [CrossRef]
- He, Q.; Cheng, Y.; Deng, Y.; Wen, F.; Lai, Y.; Li, H. Conductive Hydrogel for Flexible Bioelectronic Device: Current Progress and Future Perspective. Adv. Funct. Mater. 2023, 34, 2308974. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Abdiryim, T.; Liu, X. An overview of conductive composite hydrogels for flexible electronic devices. Adv. Compos. Hybrid Mater. 2024, 7, 35. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Pan, F.; Fu, Z. Polyvinyl Alcohol/Graphene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting Out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef]
- Li, Z.; Yun, H.; Yan, Y.; Zhao, Y.; Zhao, F. Boosting Electronic Charge Transport in Conductive Hydrogels via Rapid Ion-Electron Transduction. Angew. Chem. 2025, 2025, e202506560. [Google Scholar] [CrossRef]
- Qiu, J.; Nguyen, Q.N.; Lyu, Z.; Wang, Q.; Xia, Y. Bimetallic Janus Nanocrystals: Syntheses and Applications. Adv. Mater. 2021, 34, 2102591. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Sun, Q.; Ma, F.; Sun, Y.; Zhang, Y.; Xie, L.; Huang, Y.; Yang, X.; Hao, X. High-Sensitivity Capacitive Flexible Tactile Sensor Based on Hemispherical Shell Microstructure and ZnO/RTV Composite Dielectric Layer. ACS Appl. Electron. Mater. 2024, 6, 3440–3453. [Google Scholar] [CrossRef]
- Hou, X.-Y.; Guo, C.-F. Sensing mechanisms and applications of flexible pressure sensors. AcPSn 2020, 69, 178102. [Google Scholar] [CrossRef]
- Li, Q.; Tian, B.; Tang, G.; Zhan, H.; Liang, J.; Guo, P.; Liu, Q.; Wu, W. Multifunctional conductive hydrogels for wearable sensors and supercapacitors. J. Mater. Chem. A 2024, 12, 3589–3600. [Google Scholar] [CrossRef]
- Zhang, N.; Zong, X.; Ma, X.; Wang, J.; Zhang, C. A double-layer flexible pressure sensor with high performances based on etched hierarchical microstructures and serpentine electrodes. Surf. Interfaces 2024, 51, 104798. [Google Scholar] [CrossRef]
- Kang, B.S.; Moon, S.E.; Kim, J.H.; Kang, S.M. Broad-Range Supersensitive and Transparent Patterned Hydrogel-Based Pressure Sensor with Long-Term Stability. ACS Appl. Polym. Mater. 2025, 7, 1012–1019. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Zhang, Z.; Cui, X.; Zhang, H. Hydrogel-Based Energy Harvesters and Self-Powered Sensors for Wearable Applications. Nanoenergy Adv. 2023, 3, 315–342. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, Z.; Wang, B.; Guo, Y. Self-powered flexible piezoelectric sensors based on self-assembled 10 nm BaTiO3 nanocubes on glass fiber fabric. Nano Energy 2022, 99, 107400. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Tran, T.L.; Nguyen, H.; Chen, G.; Lai, P.K.; Song, P.A.; Tran, T.T.; Tran, C.D.; Bell, J.; Dinh, T. The concept of pressure-induced conduction band mismatch in soft–hard semiconductors for self-powered phototronic pressure sensing. ACS Appl. Mater. Interfaces 2025, 17, 32827–32837. [Google Scholar] [CrossRef]
- Yuan, X.; Yan, A.; Lai, Z.; Liu, Z.; Yu, Z.; Li, Z.; Cao, Y.; Dong, S. A poling-free PVDF nanocomposite via mechanically directional stress field for self-powered pressure sensor application. Nano Energy 2022, 98, 107340. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Liang, Y.; Tang, J.; Yang, Y.; Song, H.; Zrínyi, M.; Chen, Y. Sensitive piezoresistive pressure sensor based on micropyramid patterned tough hydrogel. Appl. Surf. Sci. 2023, 615, 156328. [Google Scholar] [CrossRef]
- Peng, S.; Feng, Y.; Liu, Y.; Feng, M.; Wu, Z.; Cheng, J.; Zhang, Z.; Liu, Y.; Shen, R.; Wang, D. New Blind Navigation Sensor Based on Triboelectrification and Electrostatic Induction. Nano Energy 2022, 104, 107899. [Google Scholar] [CrossRef]
- Zheng, C.; Gao, D.; Lyu, B.; Zhang, C.; Li, H.; Zhou, Y.; Li, N.; Ma, J. A Triboelectric Sensor with Highly Sensitive and Durable: Dual Regulation Strategy Based on Surface Morphology and Functional Groups on Negative/Positive Tribolayers. Chem. Eng. J. 2023, 477, 147071. [Google Scholar] [CrossRef]
- Yang, S.; Goncharenko, D.V.; Ji, P.; Grozova, N.A.; Semencha, A.V.; Larionova, T.V.; Tolochko, O.V. A Carbon Nanotube-Doped Polyurethane Nanocomposite-Based Triboelectric Nanogenerator: A Platform for Efficient Mechanical Energy Harvesting and Self-Powered Motion Sensing. ACS Appl. Mater. Interfaces 2025, 17, 5c05754. [Google Scholar] [CrossRef]
- Manchi, P.; Graham, S.A.; Paranjape, M.V.; Kurakula, A.; Kavarthapu, V.S.; Yu, J.S. Calcium Copper Titanate Incorporated Polydimethylsiloxane Flexible Composite Film-Based Triboelectric Nanogenerator for Energy Harvesting and Self-Powered Sensing Applications. J. Mater. Sci. Technol. 2024, 190, 56–66. [Google Scholar] [CrossRef]
- Motalebizadeh, A.; Fardindoost, S.; Jungwirth, J.; Tasnim, N.; Hoorfar, M. Microplastic In Situ Detection Based on a Portable Triboelectric Microfluidic Sensor. Anal. Methods 2023, 15, 4718–4727. [Google Scholar] [CrossRef]
- Xie, T.; Ou, F.; Ning, C.; Tuo, L.; Zhang, Z.; Gao, Y.; Pan, W.; Li, Z.; Gao, W. Dual-Network Carboxymethyl Chitosan Conductive Hydrogels for Multifunctional Sensors and High-Performance Triboelectric Nanogenerators. Carbohydr. Polym. 2024, 333, 121960. [Google Scholar] [CrossRef]
- He, W.; Lee, B.; Luo, Y. A Flexible SP/PVA Conductive Hydrogel-Based Triboelectric Nanogenerator for Energy Harvesting and Motion Monitoring in Basketball. Microsyst. Technol. 2025, 31, 1287–1295. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Y.; Tian, M.; Zhang, H.; Wang, R.; Yan, H.; Tan, H.; Esmaeely Neisiany, R.; Sun, W.; You, Z. Readily Recyclable, Degradable, Stretchable, Highly Conductive, Anti-Freezing and Anti-Drying Glycerohydrogel for Triboelectric Nanogenerator. Chem. Eng. J. 2024, 504, 158881. [Google Scholar] [CrossRef]
- Yu, M.; Luo, Y.; Yang, Q.; Duan, T.; Tang, Z.; Xu, L.; Li, N.; Xu, J. Tough and Adhesive Conductive Hydrogels with Fast Gelation from a Polyphenol–Aluminium Ion Dual Self-Catalysis System for Wearable Strain Sensors and Triboelectric Nanogenerators. J. Mater. Chem. C 2024, 41, 16872–16880. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, T.; Tang, W.; Li, Y.; Li, Y.; Lang, X.; Jiang, Q.; Tan, H. Rapidly Synthesized Single-Ion Conductive Hydrogel Electrolyte for High-Performance Quasi-Solid-State Zinc-ion Batteries. Angew. Chem. 2023, 135, e202312020. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Yang, J.; Kang, F.; Wang, X.; Zhang, Q. Design strategies for improving the crystallinity of covalent organic frameworks and conjugated polymers: A review. Mater. Horiz. 2022, 9, 121–146. [Google Scholar] [CrossRef]
- Kim, W.; Lee, D.; Wu, G.; Cha, Y.L.; Moazzem, M.S.; Cho, S.; Kim, D.-J. Molecularly Imprinted Chemiresistive Sensor for Specific Recognition of Furaneol as a Biomarker of Strawberry Flavor Conditions. ACS Sens. 2023, 8, 1542–1549. [Google Scholar] [CrossRef]
- Lett, J.A.; Sagadevan, S.; Alshahateet, S.F.; Murugan, B.; Jasni, A.H.; Fatimah, I.; Hossain, M.A.M.; Mohammad, F.; Oh, W.C. Synthesis and characterization of polypyrrole-coated iron oxide nanoparticles. Mater. Res. Express 2021, 8, 025007. [Google Scholar] [CrossRef]
- Hu, M.; Qiu, L.; Huang, Y.; Wang, D.; Li, J.; Liang, C.; Wu, G.; Peng, F. An adhesive, low swelling and conductive tri-network hydrogel for wearable electronic devices. J. Mater. Chem. C 2024, 12, 8534–8544. [Google Scholar] [CrossRef]
- Xu, K.; Tan, L.; Sun, H.; Chong, C.; Li, L.; Sun, B.; Yao, Z.; Zhuang, Y.; Wang, L. Manipulating gelatinization, retrogradation, and hydrogel properties of potato starch through calcium chloride-controlled crosslinking and crystallization behavior. Carbohydr. Polym. 2025, 357, 123371. [Google Scholar] [CrossRef]
- Wei, W.; Wu, M.; Chen, Y. A Flexible and Wearable Strain Sensor from Polypyrrole-Doped Elastomers with Dual Functions in Motion Monitoring and Thermotherapy. Chin. J. Chem. 2023, 41, 1545–1551. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Yan, J.; Sun, J. Roles of Ionic Liquids in Adjusting Nature of Ionogels: A Mini Review. Adv. Funct. Mater. 2022, 32, 2203988. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Benetti, D.; Ni, Y.; Rosei, F. Polyelectrolyte hydrogel: A versatile platform for mechanical-electric conversion and self-powered sensing. Nano Energy 2022, 103, 107718. [Google Scholar] [CrossRef]
- Duan, M.-Y.; Chen, J.-D.; Liu, Y.-M.; Peng, Z.-F.; Chen, G. Swelling of Spherical Polyelectrolyte Gels. Chin. J. Polym. Sci. 2024, 42, 1386–1392. [Google Scholar] [CrossRef]
- Su, J.; Zhang, L.; Wan, C.; Deng, Z.; Wei, S.; Yong, K.-T.; Wu, Y. Dual-network self-healing hydrogels composed of graphene oxide@nanocellulose and poly(AAm-co-AAc). Carbohydr. Polym. 2022, 296, 119905. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Yan, C.; Wu, J.; Wang, Y.; Jiang, M.; Wang, Y. Yunnan Baiyao-enhanced cellulose nanofiber composite hydrogel wearable patch for transdermal drug delivery and anti-freezing applications. Int. J. Biol. Macromol. 2025, 315, 144684. [Google Scholar] [CrossRef] [PubMed]
- Oral, N.; Basal, G. The effects of crosslinker ratio and photoinitiator type on the properties of pnipam hydrogel. J. Polym. Res. 2022, 30, 34. [Google Scholar] [CrossRef]
- Daengmankhong, J.; Ross, S.; Pinthong, T.; Mahasaranon, S.; Viyoch, J.; Tighe, B.J.; Derry, M.J.; Topham, P.D.; Ross, G. Water-soluble macromers based on 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt (Na-AMPS) for rapid in situ hydrogel film formation. Polym. Chem. 2024, 15, 1620–1634. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, M.-J.; Chung, T.-S. Zwitterionic poly(sulfobetaine methacrylate-co-acrylic acid) assisted simultaneous anti-wetting and anti-fouling membranes for membrane distillation. Desalination 2023, 555, 116527. [Google Scholar] [CrossRef]
- Paschke, S.; Lienkamp, K. Polyzwitterions: From Surface Properties and Bioactivity Profiles to Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 129–151. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Y.; Fu, H.; Liang, Z.; Huang, B.; Jiang, R.; Wu, J.; Zhao, Y. Recent advances of multifunctional zwitterionic polymers for biomedical application. Acta Biomater. 2024, 181, 19–45. [Google Scholar] [CrossRef]
- Li, S.; Yu, P.; Dong, F.; Zhang, J.; Wang, T.; Zhang, P.; Gao, G.; Wang, Y.; Gao, Y. A lubricated and antibacterial hydrogel coating based on polyelectrolyte adhesion for medical catheters. J. Mater. Chem. A 2025, 13, 6687–6696. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, K.; Cui, Y.; Li, L.; Liu, Q.; Men, Y. Synthetic Reversible Fibrous Network Hydrogels Based on a Double-Helical Polyelectrolyte. Angew. Chem. 2025, 137, e202503030. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, R.; Zhang, H.; Zhang, Y.; Tang, X.; Wang, H.; Shao, A.; Ma, Y. A Hydrogel Electrolyte toward a Flexible Zinc-Ion Battery and Multifunctional Health Monitoring Electronics. ACS Nano 2024, 18, 7596–7609. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Wu, Z.; Gao, F.-L.; Gao, X.-Z.; Zhao, H.-Y.; Li, X.; Yu, Z.-Z. Self-adhesive, self-healing, biocompatible and conductive polyacrylamide nanocomposite hydrogels for reliable strain and pressure sensors. Nano Energy 2023, 109, 108324. [Google Scholar] [CrossRef]
- Xu, H.; Shen, Z.; Gu, G. Performance characterization of ionic-hydrogel based strain sensors. Sci. China Technol. Sci. 2020, 63, 923–930. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Li, S.; Zou, X.; Yin, H.; Huang, Y.; Dong, F.; Li, P.; Song, Y. Construction and characterization of highly stretchable ionic conductive hydrogels for flexible sensors with good anti-freezing performance. Eur. Polym. J. 2023, 186, 111827. [Google Scholar] [CrossRef]
- Huang, F.; Lv, J.; Li, H.; Xu, S. Regulation rule of cellulose nanocrystals on thixotropy of hydrogel for water shutoff in horizontal wells. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128735. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Yu, J.; Liao, H.; Yang, L.; Ren, E.; Lin, S.; Lan, J. Ionic Conductive Cellulose-Based Hydrogels with Superior Long-Lasting Moisture and Antifreezing Features for Flexible Strain Sensor Applications. Biomacromolecules 2024, 25, 838–852. [Google Scholar] [CrossRef]
- Wang, C.; Chai, Y.; Wen, X.; Ai, Y.; Zhao, H.; Hu, W.; Yang, X.; Ding, M.-Y.; Shi, X.; Liu, Q.; et al. Stretchable and Anisotropic Conductive Composite Hydrogel as Therapeutic Cardiac Patches. ACS Mater. Lett. 2021, 3, 1238–1248. [Google Scholar] [CrossRef]
- Li, R.; Zhang, C.; Wang, C.; Cheng, Y.; Hu, D. Study on the Mechanism of the Reversible Color Change of Polyacrylic Acid Modified Gold Nanoparticles Responding to pH. Materials 2021, 14, 3679. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Han, Y.; Liu, P.; Xu, H.; Yu, G.; Wang, Y.; Wen, T.; Ju, W.; Gu, J. Hierarchical construction of CNT networks in aramid papers for high-efficiency microwave absorption. Nano Res. 2023, 16, 7801–7809. [Google Scholar] [CrossRef]
- Jang, J.; Park, I.; Chee, S.-S.; Song, J.-H.; Kang, Y.; Lee, C.; Lee, W.; Ham, M.-H.; Kim, I.S. Graphene oxide nanocomposite membrane cooperatively cross-linked by monomer and polymer overcoming the trade-off between flux and rejection in forward osmosis. J. Membr. Sci. 2020, 598, 117684. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Jouyban, A.; Ostadi, A.; Gharakhani, A.; Rahimpour, E. A sensitive determination of morphine in plasma using AuNPs@UiO-66/PVA hydrogel as an advanced optical scaffold. Anal. Chim. Acta 2022, 1227, 340252. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Peng, H.; Zhang, B.; Chen, L.; Zhang, P.; Peng, Z.; Fu, X. Advances in carbon nanotube-based gas sensors: Exploring the path to the future. Coord. Chem. Rev. 2024, 518, 216049. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, Z.; Yang, L.; Luo, M.; Fu, L.; Lin, B.; Xu, C. A High-Performance, Sensitive, Wearable Multifunctional Sensor Based on Rubber/CNT for Human Motion and Skin Temperature Detection. Adv. Mater. 2021, 34, 202107309. [Google Scholar] [CrossRef]
- Wu, W.; Feng, W.; Yu, Y.; Li, L.; Lu, M.; Qian, G.; Chen, C.; Min, D. Highly sensitive MXene-enhanced polyacrylamide/ carboxymethyl cellulose double-network hydrogels with wide operation range for wearable electronics. Ind. Crops Prod. 2024, 214, 118573. [Google Scholar] [CrossRef]
- Xu, M.; Ge, R.; Wang, W.; Peng, Y.; Li, Z.; Liu, W.; Wang, L. Xanthan Gum-Toughened Nanocomposite Conductive Hydrogel for Wearable Sensors and Smart Livestock Monitoring. Int. J. Biol. Macromol. 2025, 318, 144983. [Google Scholar] [CrossRef]
- Liang, C.; Dudko, V.; Khoruzhenko, O.; Hong, X.; Lv, Z.-P.; Tunn, I.; Umer, M.; Timonen, J.V.I.; Linder, M.B.; Breu, J.; et al. Stiff and Self-Healing Hydrogels by Polymer Entanglements in Co-Planar Nanoconfinement. Nat. Mater. 2025, 24, 599–606. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Xu, L.; Yan, M.; Bi, H.; Wang, Q. Transparent Multifunctional Cellulose-Based Conductive Hydrogel for Wearable Strain Sensors and Arrays. Carbohydr. Polym. 2024, 329, 121784. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, L.; Wen, C.; Wang, Z.; Dai, J.; Shi, L. Flexible conductive silk-PPy hydrogel toward wearable electronic strain sensors. Biomed. Mater. 2022, 17, 024107. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, C.; Dong, Y.; Yao, J.; Mi, Q.; Ge, D.; Yang, L.; Yu, H.-Y. Freeze polymerization to modulate transverse-longitudinal polypyrrole growth on robust cellulose composite fibers for multi-scenario signal monitoring. Chem. Eng. J. 2024, 485, 149785. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Ding, Y.; Yao, Y.; Liu, Y.; Tang, J. Fe3+-Coordination mediated synergistic dual-network conductive hydrogel as a sensitive and highly-stretchable strain sensor with adjustable mechanical properties. J. Mater. Chem. B 2022, 10, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, P.; Shojaei, A.; Dickey, M.D. A highly conductive and ultra-stretchable polyaniline/cellulose nanocrystal/polyacrylamide hydrogel with hydrophobic associations for wearable strain sensors. J. Mater. Chem. A 2024, 12, 9552–9562. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhu, J.; Gao, C.; Wang, M.; Gao, Q. Anti-freezing and moisturizing PAA/PEDOT: PSS ionogels with multiple stimulus responses for flexible wearable electronics. Eur. Polym. J. 2024, 210, 112934. [Google Scholar] [CrossRef]
- Chen, G.; Hu, O.; Lu, J.; Gu, J.; Chen, K.; Huang, J.; Hou, L.; Jiang, X. Highly flexible and adhesive poly(vinyl alcohol)/poly(acrylic amide-co-2-acrylamido-2-methylpropane sulfonic acid)/glycerin hydrogel electrolyte for stretchable and resumable supercapacitor. Chem. Eng. J. 2021, 425, 131505. [Google Scholar] [CrossRef]
- Lee, G.; Seo, H.; Kim, D.; Shin, S.; Kwon, K. All polymeric conductive strain sensors with excellent skin adhesion, recovery, and long-term stability prepared from an anion-zwitterion based hydrogel. RSC Adv 2023, 13, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, H.; Zhang, Z.; Yao, Y.; Bao, X.; Hu, Q. Ultrastretchable, self-adhesive, strain-sensitive and self-healing GO@DA/Alginate/P(AAc-co-AAm) multifunctional hydrogels via mussel-inspired chemistry. Carbohydr. Polym. 2021, 254, 117316. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Hu, G.; Dong, F.; Xiong, Y.; Yuan, C. Tri-network, physical cross-linked sodium alginate hydrogel with strongly self-healing capacity and highly tough, for wearable flexible devices. Eur. Polym. J. 2024, 209, 112823. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, H.; Qiao, F.; Huang, W.; Bao, X.; Wang, Z.; Hu, Q. Fabrication of alginate-P(SBMA-co-AAm) hydrogels with ultrastretchability, strain sensitivity, self-adhesiveness, biocompatibility, and self-cleaning function for strain sensors. J. Appl. Polym. Sci. 2020, 138, 49697. [Google Scholar] [CrossRef]
- Li, T.; Wei, H.; Zhang, Y.; Wan, T.; Cui, D.; Zhao, S.; Zhang, T.; Ji, Y.; Algadi, H.; Guo, Z.; et al. Sodium alginate reinforced polyacrylamide/xanthan gum double network ionic hydrogels for stress sensing and self-powered wearable device applications. Carbohydr. Polym. 2023, 309, 120678. [Google Scholar] [CrossRef]
- Bai, Z.; Chinnappan, A.; Zhang, Y.; Kang, F.; Yi, X.; Deng, J.; Ramakrishna, S. Performance evaluation of PVA/PEO/LiCl composite as coated heat exchangers desiccants. Int. J. Refrig. 2023, 154, 258–267. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic Salts Induce Thermally Reversible and Anti-Freezing Cellulose Hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Qing, X.; Liu, Z.; Katsaounis, A.; Bouropoulos, N.; Taurino, I.; Fardim, P. Poly(vinyl alcohol)/Pullulan/NaCl Conductive Hydrogels with High Strength and Sensitivity for Wearable Strain Sensors. ACS Appl. Polym. Mater. 2024, 6, 8105–8115. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Wang, P.; Yu, Y.; Zhou, M.; Xu, B.; Cui, L.; Wang, Q. Stretchable, transparent, self-adhesive, anti-freezing and ionic conductive nanocomposite hydrogels for flexible strain sensors. Eur. Polym. J. 2023, 186, 111824. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Y.; Liu, M.; Wang, F.; Liu, P.; Yu, Y.; Feng, Q.; Xiao, X. Highly Stretchable, Tough, and Conductive Ag@Cu Nanocomposite Hydrogels for Flexible Wearable Sensors and Bionic Electronic Skins. Macromol. Mater. Eng. 2021, 306, 2100341. [Google Scholar] [CrossRef]
- Xiao, S.; Lao, Y.; Liu, H.; Li, D.; Wei, Q.; Ye, L.; Lu, S. A nanocomposite hydrogel loaded with Ag nanoparticles reduced by aloe vera polysaccharides as an antimicrobial multifunctional sensor. Int. J. Biol. Macromol. 2024, 267, 131541. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, C.; Huang, W.-Y.; Liu, C.-L.; Zhang, Y. Wearable Sensor Based on a Tough Conductive Gel for Real-Time and Remote Human Motion Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 11957–11972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, X.; Tan, R.; Zhang, S.; Zhang, K.; Hu, J. Hierarchically Structured Hydrogel Composites with Ultra-High Conductivity for Soft Electronics. Adv. Funct. Mater. 2023, 34, 2312667. [Google Scholar] [CrossRef]
- Yuan, S.; Bai, J.; Li, S.; Ma, N.; Deng, S.; Zhu, H.; Li, T.; Zhang, T. A Multifunctional and Selective Ionic Flexible Sensor with High Environmental Suitability for Tactile Perception. Adv. Funct. Mater. 2023, 34, 202309626. [Google Scholar] [CrossRef]
- Gregg, A.; De Volder, M.; Baumberg, J.J. Kinetics of Light-Responsive CNT/PNIPAM Hydrogel Microactuators. Small 2023, 20, 202305034. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liao, W.; Zhang, D.; Dai, Y.; Wu, C.; Wen, J.; Zeng, W. Stretchable conductive hydrogels integrated with microelectronic devices for strain sensing. J. Mater. Chem. C 2023, 11, 15873–15880. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cui, Y.; Liu, Y.; Wang, W.; Guo, Y.; Wang, Q.; Dong, X. Ionic Liquid/Water Binary Solvent Anti-Freezing Hydrogel for Strain and Temperature Sensors. ACS Appl. Mater. Interfaces 2024, 16, 5208–5216. [Google Scholar] [CrossRef]
- Qin, H.; Sun, M.; Li, P.; Li, J.; Zhang, Z.; Dai, S.; Huang, M.; Lu, B.; Pan, X.; Wu, L. Soft, Stretchable, and Conductive Hydrogel Based on Liquid Metal for Accurately Facial Expression Monitoring. Adv. Mater. Technol. 2023, 8, 202300406. [Google Scholar] [CrossRef]
- Gong, T.; Li, Z.n.; Liang, H.; Li, Y.; Tang, X.; Chen, F.; Hu, Q.; Wang, H. High-Sensitivity Wearable Sensor Based On a MXene Nanochannel Self-Adhesive Hydrogel. ACS Appl. Mater. Interfaces 2023, 15, 19349–19361. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Lu, J.; Xue, Y.; Wang, J.; Jia, B.; Han, G.; Zhao, Y.; Qureshi, M.A.K.; Yu, L.; et al. Highly stretchable, self-healable, and conductive gelatin methacryloyl hydrogel for long-lasting wearable tactile sensors. Adv. Sci. 2025, e02678. [Google Scholar] [CrossRef]
- Song, L.; Wang, Z.; Chen, S.; Shen, Y.; Yin, J.; Wang, R. Phytic acid-induced gradient hydrogels for highly sensitive and broad range pressure sensing. Adv. Mater. 2025, 37, 2417978. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H.; Wang, Y.; Fan, X.; Li, Z.; Zhang, X.; Liu, T. Highly stretchable, ultra-soft, and fast self-healable conductive hydrogels based on polyaniline nanoparticles for sensitive flexible sensors. Adv. Funct. Mater. 2022, 32, 2204366. [Google Scholar] [CrossRef]
- Li, J.; Ge, S.; Niu, Y.; Liu, S.; Geng, J.; Tian, L.; Xu, M.; Shi, Y.; Cui, X.; Jia, R.; et al. Intrinsically adhesive, conductive organohydrogel with high stretchable, moisture retention, anti-freezing and healable properties for monitoring of human motions and electrocardiogram. Sens. Actuators B Chem. 2023, 377, 133098. [Google Scholar] [CrossRef]
- Chen, T.; Liang, X. A flexible triboelectric nanogenerator based on PDA/MXene/NIPAM hydrogel for mechanical energy harvesting and basketball posture monitoring. AIP Adv. 2024, 14, 045105. [Google Scholar] [CrossRef]
- Gupta, B.; Bano, S.; Singh, R. High-performance silver nanoparticles embedded conductive PVA hydrogel for stretchable wearable triboelectric nanogenerators. J. Power Sources 2025, 632, 236271. [Google Scholar] [CrossRef]
- Ma, J.; Yang, W.; Chen, J.; Zhou, Y.; Ye, M.; Xu, X.; Xiao, H.; Han, J. Lignosulfonate-enhanced dispersion and compatibility of liquid metal nanodroplets in PVA hydrogel for improved self-recovery and fatigue resistance in wearable sensors. Int. J. Biol. Macromol. 2025, 306, 141653. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, L.; Ji, D.; Luo, M.; Zhang, Z.; Zhao, G.; Chang, X.; Zhu, Y. Highly stretchable, conductive, and self-adhesive starch-based hydrogel for high-performance flexible electronic devices. Carbohydr. Polym. 2025, 352, 123220. [Google Scholar] [CrossRef]
- Wang, H.; Lin, X.; Li, X.; Lv, D.; Zhang, J.; Wei, L.; Tang, J.; Lin, Y.; Wu, X.; Xu, X. Antiswelling, Ultrastretchable, and Ultrastable Hydrogel Sensors for Long-Term Underwater Monitoring. Small 2025, 2025, 2503067. [Google Scholar] [CrossRef]
- Wang, X.; Ji, H.; Gao, L.; Hao, R.; Shi, Y.; Yang, J.; Hao, Y.; Chen, J. Wearable hydrogel-based health monitoring systems: A new paradigm for health monitoring? Chem. Eng. J. 2024, 495, 153382. [Google Scholar] [CrossRef]
- Wu, J.; Xian, J.; He, C.; Lin, H.; Li, J.; Li, F. Asymmetric Wettability Hydrogel Surfaces for Enduring Electromyographic Monitoring. Adv. Mater. 2024, 36, 202405372. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Y.; Zhang, Y.; Zhu, P.; Mao, Y. Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials 2024, 14, 1398. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ji, S.; Chaturvedi, I.; Xia, H.; Wang, T.; Chen, G.; Pan, L.; Wan, C.; Qi, D.; Ong, Y.-S.; et al. Adhesive Biocomposite Electrodes on Sweaty Skin for Long-Term Continuous Electrophysiological Monitoring. ACS Mater. Lett. 2020, 2, 478–484. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Fu, Y.; Chen, X.; Gan, S.; Yang, W.; Chen, S.; Liu, L. Liquid Metal@Silk Fibroin Peptide Particles Initiated Hydrogels with High Toughness, Adhesion, and Conductivity for Portable and Continuous Electrophysiological Monitoring. Adv. Funct. Mater. 2025, 35, 202420240. [Google Scholar] [CrossRef]
- Wei, J.; Chen, H.; Pan, F.; Zhang, H.; Yang, K.; Yuan, T.; Fang, Y.; Ping, H.; Wang, Q.; Fu, Z. Reusable liquid metal-based hierarchical hydrogels with multifunctional sensing capability for electrophysiology electrode substitution. ACS Nano 2025, 19, 15554–15564. [Google Scholar] [CrossRef]
- Gao, X.; Su, T.; Bao, Y.; Lu, J.; Zhang, L.; He, C.; Ouyang, J. A moldable PEDOT:PSS dry electrode with excellent epidermal compliance for wearable electrocardiogram monitoring. J. Mater. Chem. C 2023, 11, 13387–13394. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, A.; Xu, Y.; Han, S.; Zhang, J.; Chen, Y.; Hang, J.; Yang, X.; Guan, L. Multiple physical crosslinked highly adhesive and conductive hydrogels for human motion and electrophysiological signal monitoring. Soft Matter 2024, 20, 3666–3675. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, C.; Guo, T.; Tian, Y.; Song, W.; Lei, J.; Li, Q.; Wang, A.; Zhang, M.; Bai, S.; et al. Hydrogel Nanoarchitectonics of a Flexible and Self-Adhesive Electrode for Long-Term Wireless Electroencephalogram Recording and High-Accuracy Sustained Attention Evaluation. Adv. Mater. 2023, 35, 202209606. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Liang, S.; Bai, Y. Sweat-enhanced adhesive hydrogel enables interfacial exchange coupling for wearable strain sensor. Chem. Eng. J. 2024, 495, 153385. [Google Scholar] [CrossRef]
- Tian, Z.; Zhu, Z.; Yue, S.; Liu, Y.; Li, Y.; Yu, Z.-Z.; Yang, D. Self-powered, self-healing, and anti-freezing triboelectric sensors for violation detection in sport events. Nano Energy 2024, 122, 109276. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Li, P.; Ling, Z.; Liu, X.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Yang, L.; Wei, D. Nanocomposite hydrogels flexible sensors with functional cellulose nanocrystals for monitoring human motion and lactate in sweat. Chem. Eng. J. 2023, 466, 143306. [Google Scholar] [CrossRef]
- Chen, T.; Pang, J.; Liu, X.; Chen, N.; Wu, C.; Duan, Y.; You, X.; Dou, Q.; Yuan, C.; Wang, Y.; et al. Anti-biofilm super-hydrophilic gel sensor for saliva glucose monitoring. Nano Today 2024, 55, 102141. [Google Scholar] [CrossRef]
- Arwani, R.T.; Tan, S.C.L.; Sundarapandi, A.; Goh, W.P.; Liu, Y.; Leong, F.Y.; Yang, W.; Zheng, X.T.; Yu, Y.; Jiang, C.; et al. Stretchable ionic–electronic bilayer hydrogel electronics enable in situ detection of solid-state epidermal biomarkers. Nat. Mater. 2024, 23, 1115–1122. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, F.; Cai, H.; Li, X.; Sun, J.; Wu, Y.; Wang, N.; Zhu, Y. Robust versatile nanocellulose/polyvinyl alcohol/carbon dot hydrogels for biomechanical sensing. Carbohydr. Polym. 2021, 259, 117753. [Google Scholar] [CrossRef]
- Weng, X.; Li, M.; Chen, L.; Peng, B.; Jiang, H. A wearable nanozyme–enzyme electrochemical biosensor for sweat lactate monitoring. Talanta 2024, 279, 126675. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, Y.; Chen, R.; Liang, S.; Tian, S.; Cao, Y.; Cui, N.; Yang, H. A multifunctional flexible sensor with dual-conductive networks for monitoring human motion signals and sweat pH/Lactic acid. Compos. Sci. Technol. 2025, 265, 111130. [Google Scholar] [CrossRef]
- Ning, Y.; Lu, F.; Liu, Y.; Yang, S.; Wang, F.; Ji, X.; He, Z. Glow-type chemiluminescent hydrogels for point-of-care testing (POCT) of cholesterol. Ana 2021, 146, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, X.; Yang, A.; Wu, M.; Yu, L.; Zeng, H.; Han, L. Nanomaterials-Enhanced, Stretchable, Self-Healing, Temperature-Tolerant and Adhesive Tough Organohydrogels with Long-Term Durability as Flexible Sensors for Intelligent Motion-Speech Recognition. Chem. Eng. J. 2023, 461, 141905. [Google Scholar] [CrossRef]
- Lu, N.; Kang, H.; Lu, Y.; Li, Y.; Li, J.; Xue, Y.; Qiu, H. High-Strength, Conductive Dual-Network Nanocomposite Hydrogel for Multi-Substrate Adhesion and Enhanced Wearable Sensor Performance. Polymer 2025, 334, 128743. [Google Scholar] [CrossRef]
- Chenani, H.; Saeidi, M.; Adel Rastkhiz, M.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and Advances of Hydrogel-Based Wearable Electrochemical Biosensors for Real-Time Monitoring of Biofluids: From Lab to Market. A Review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Q.; Wang, H.; Wu, Z.; Gui, X.; Li, C.; Hu, N.; Tao, K.; Wu, J. Engineering Smart Composite Hydrogels for Wearable Disease Monitoring. Nano-Micro Lett. 2023, 15, 105. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Hong, J.; Gao, X.; Wang, Y.; Wang, W.; Zhang, H.; Park, J.; Shi, W.; Guo, W. Recent Progress in Flexible Wearable Sensors Utilizing Conductive Hydrogels for Sports Applications: Characteristics, Mechanisms, and Modification Strategies. Gels 2025, 11, 589. https://doi.org/10.3390/gels11080589
Wu J, Hong J, Gao X, Wang Y, Wang W, Zhang H, Park J, Shi W, Guo W. Recent Progress in Flexible Wearable Sensors Utilizing Conductive Hydrogels for Sports Applications: Characteristics, Mechanisms, and Modification Strategies. Gels. 2025; 11(8):589. https://doi.org/10.3390/gels11080589
Chicago/Turabian StyleWu, Jie, Jingya Hong, Xing Gao, Yutong Wang, Wenyan Wang, Hongchao Zhang, Jaeyoung Park, Weiquan Shi, and Wei Guo. 2025. "Recent Progress in Flexible Wearable Sensors Utilizing Conductive Hydrogels for Sports Applications: Characteristics, Mechanisms, and Modification Strategies" Gels 11, no. 8: 589. https://doi.org/10.3390/gels11080589
APA StyleWu, J., Hong, J., Gao, X., Wang, Y., Wang, W., Zhang, H., Park, J., Shi, W., & Guo, W. (2025). Recent Progress in Flexible Wearable Sensors Utilizing Conductive Hydrogels for Sports Applications: Characteristics, Mechanisms, and Modification Strategies. Gels, 11(8), 589. https://doi.org/10.3390/gels11080589