Redox-Active Anthraquinone-1-Sulfonic Acid Sodium Salt-Loaded Polyaniline for Dual-Functional Electrochromic Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
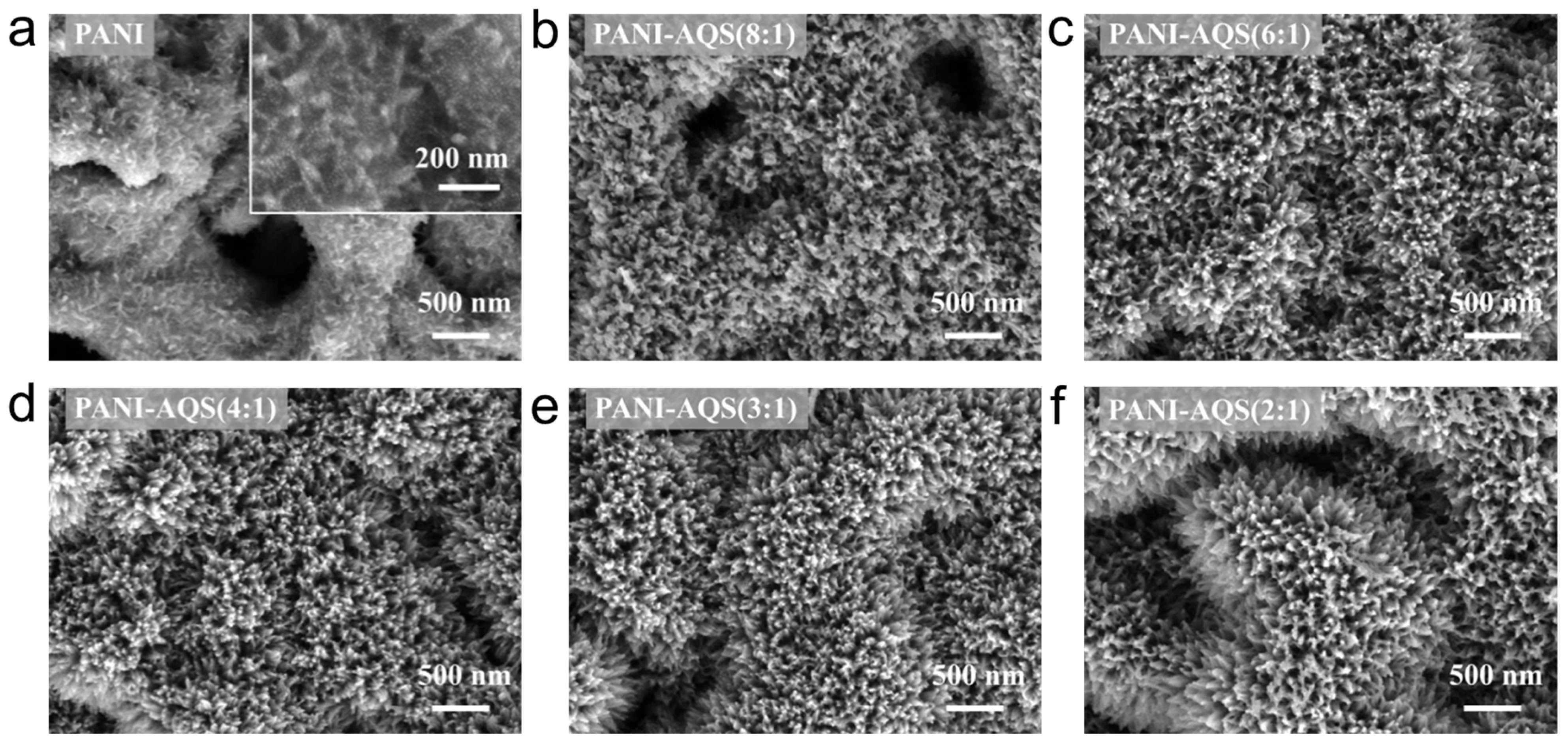
2.1. Optimization, Preparation, and Characterization of PANI-AQS Films
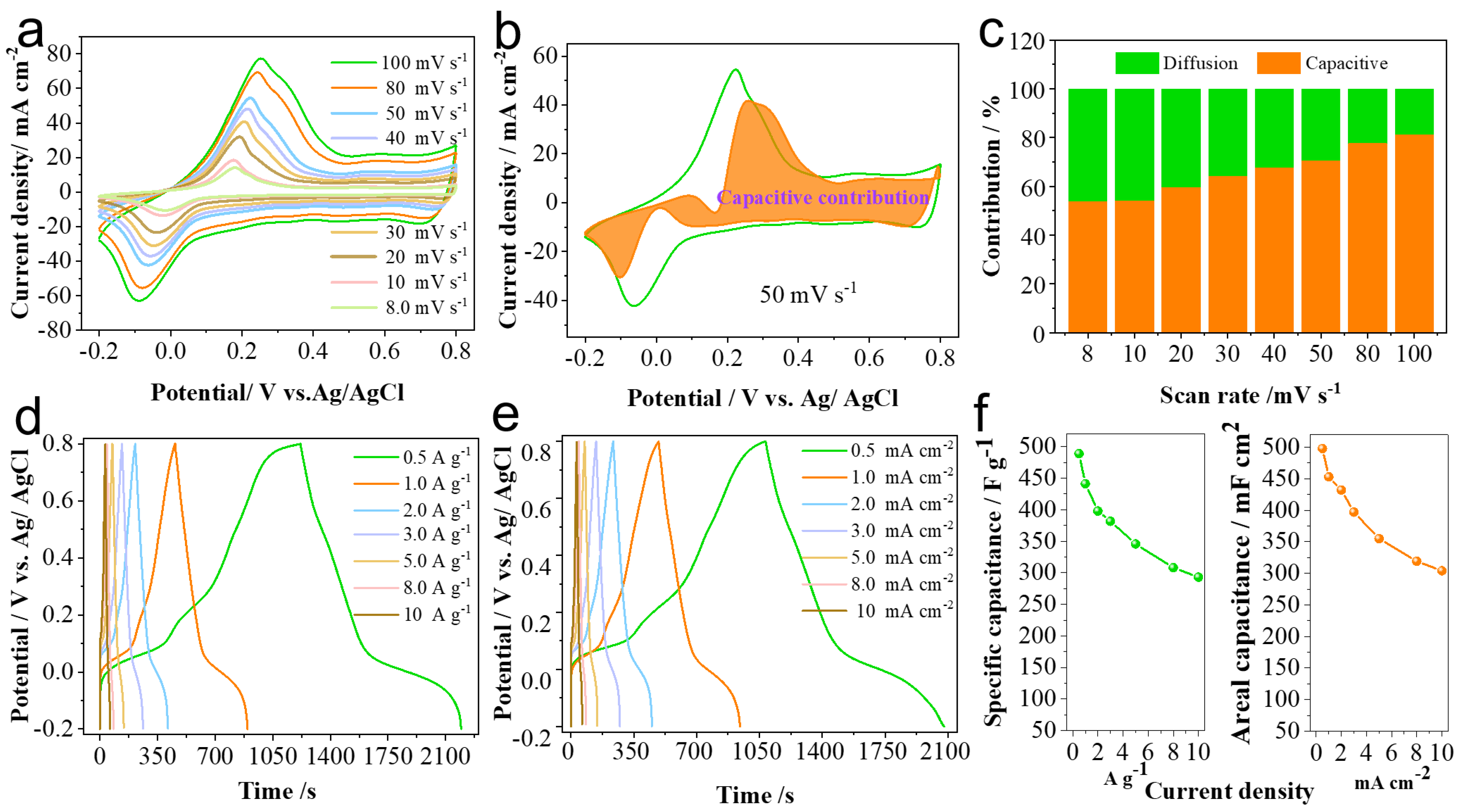
2.2. Electrochemical Performance Study of PANI-AQS Films
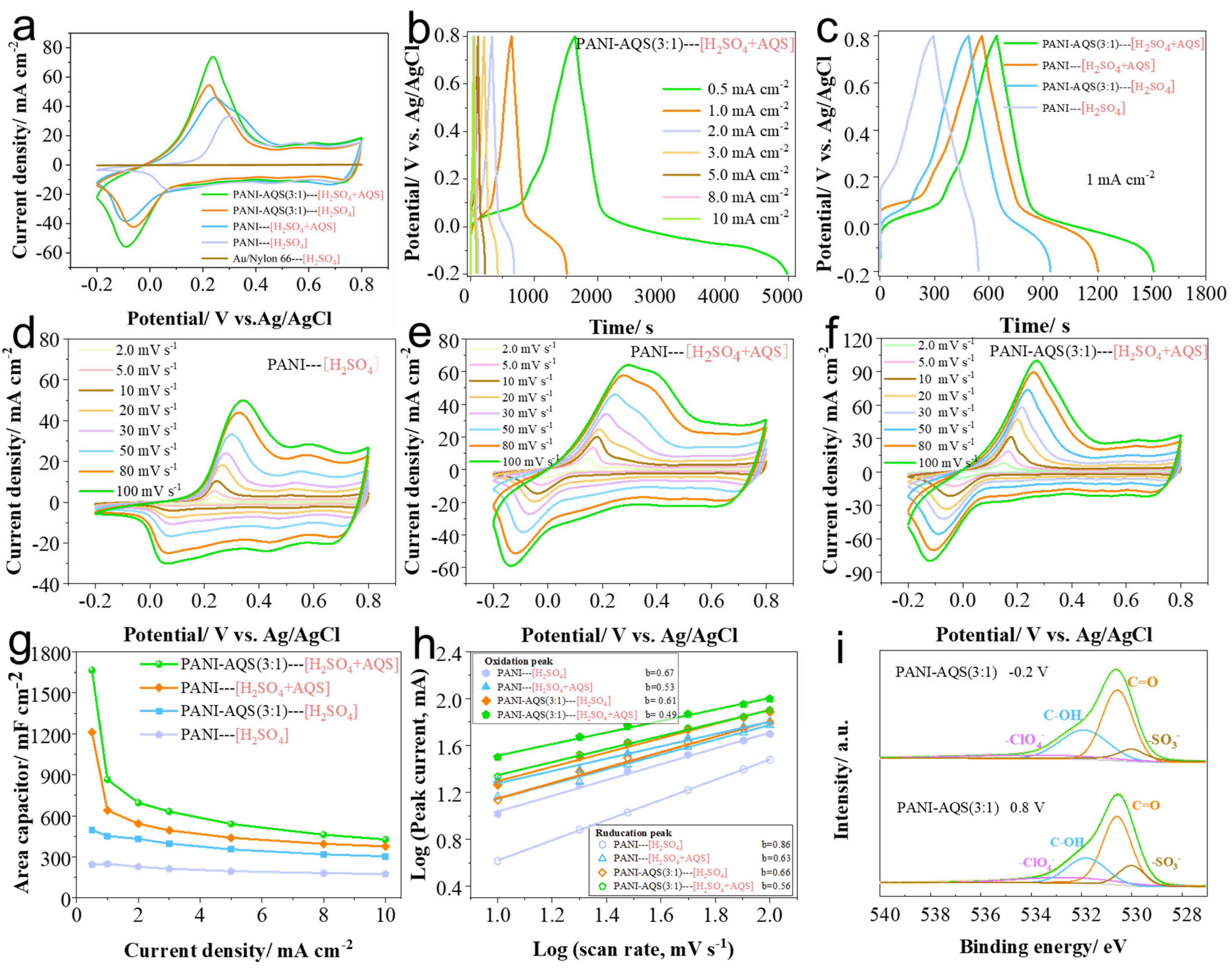
2.3. Effect of AQS Electrolyte Additive on the Electrochemical Performance of Films
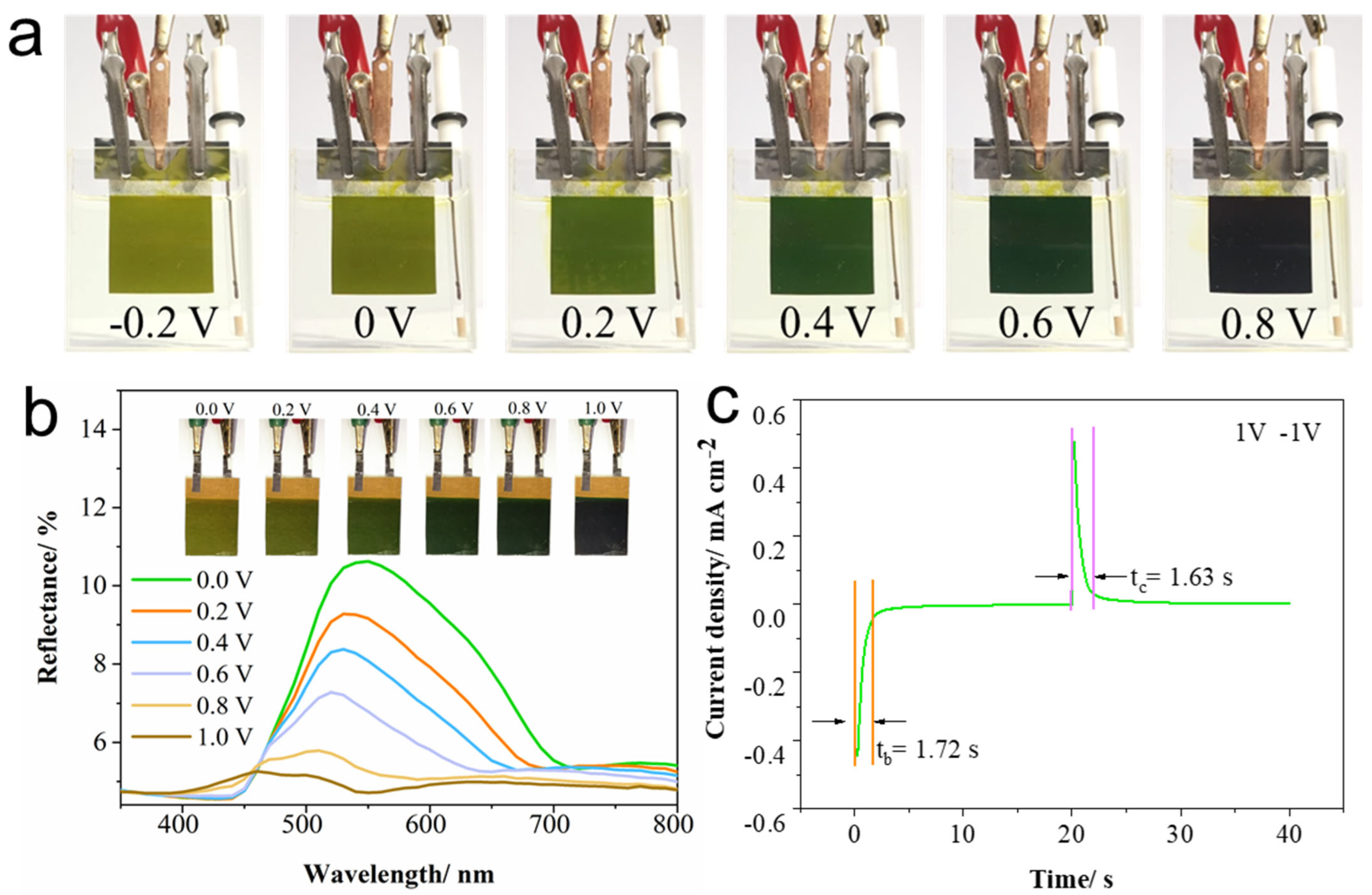
2.4. Electrochromic Performance of PANI-AQS Films
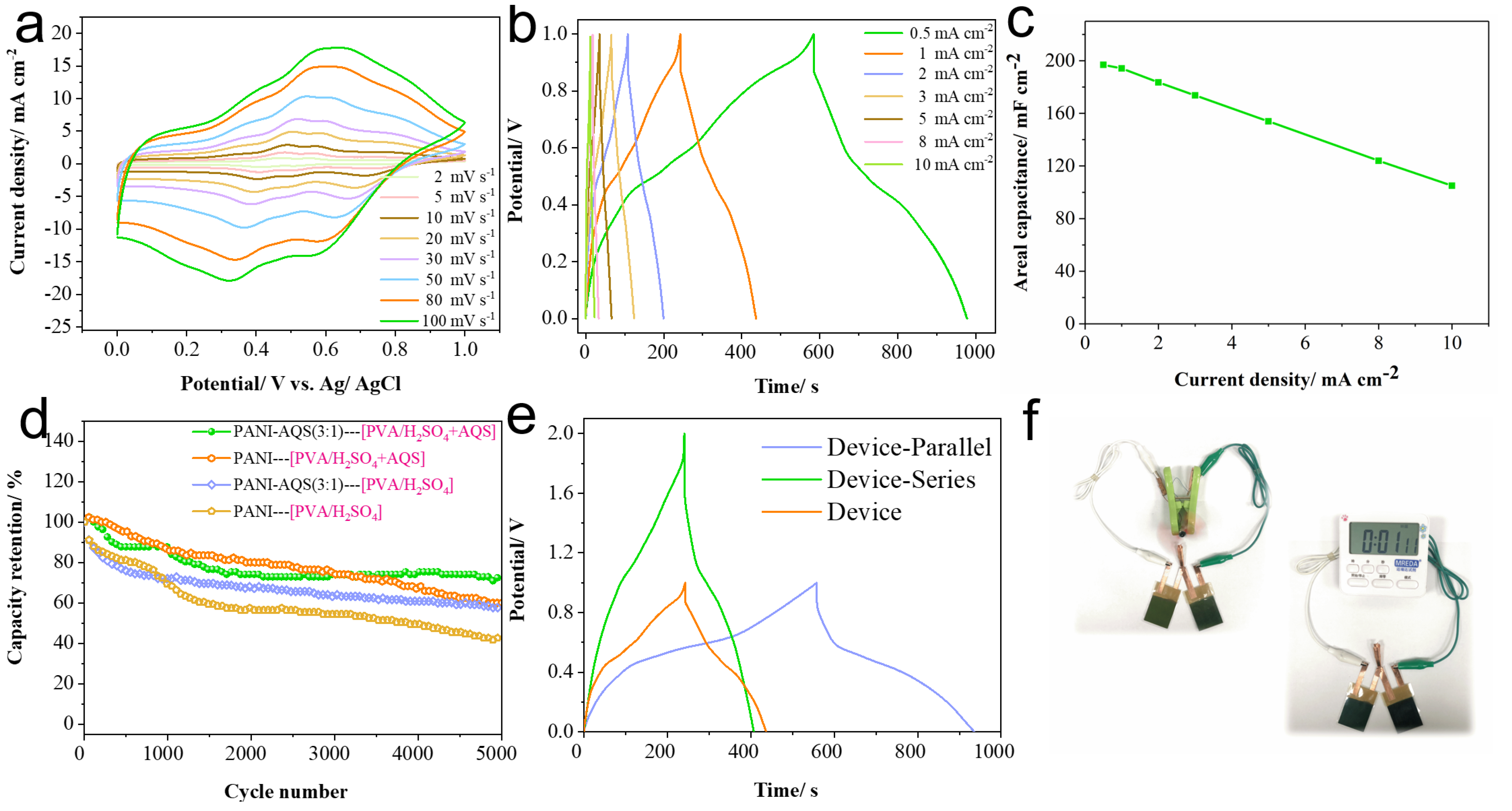
2.5. Performance Study of Dual-Functional Electrochromic-Supercapacitor Film Devices
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Electrochemical Deposition Solutions
4.3. Fabrication of PANI-AQS(x:y) Electrodes and Film Device
4.4. Preparation of Gel Electrolytes
4.5. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Wang, L.; Jin, S.; Xie, T.; Liu, G.; Meng, Z.; Liu, T.; Cui, Y.; Zhang, H.; Liu, W.; et al. Electrochromic harvester for all-day energy savings in buildings. ACS Energy Lett. 2025, 10, 3231–3240. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, Z.; Yan, Y.; Feng, T.; Xie, A. High-performance electrochromic energy storage devices based on hexagonal WO3 and SnO2/PB composite films. Materials 2025, 18, 2871. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.-Z.; Wu, Z.-A.; Liu, J.; Guan, K.-L.; Zhang, Z.-L.; Wan, H.-Z.; Wang, H.; Sun, D.-Y.; Xie, A. Nanofullerene regulated electric field to achieve stable Sn metal anode for aqueous Sn batteries. Rare Met. 2025, 44, 3869–3880. [Google Scholar] [CrossRef]
- Li, R.; Ma, X.; Li, J.; Cao, J.; Gao, H.; Li, T.; Zhang, X.; Wang, L.; Zhang, Q.; Wang, G.; et al. Flexible and high-performance electrochromic devices enabled by self-assembled 2D TiO2/MXene heterostructures. Nat. Commun. 2021, 12, 1587. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, Y.; Jia, C.; Wan, Z.; Luo, J.; Malik, H.A.; Weng, X.; Xie, J.; Deng, L. Intelligent biomimetic chameleon skin with excellent self-healing and electrochromic properties. ACS Appl. Energy Mater. 2018, 10, 35533–35538. [Google Scholar] [CrossRef]
- Yun, T.G.; Park, M.; Kim, D.H.; Kim, D.; Cheong, J.Y.; Bae, J.G.; Han, S.M.; Kim, I.D. All-transparent stretchable electrochromic supercapacitor wearable patch device. ACS Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, Z.; Yang, X.; Yang, Y.; Li, X.; Gao, Y.; Wang, L.; Lü, W. Pendulum-style integrated dual-function electrochromic energy storage device. Chem. Eng. J. 2025, 512, 162491. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, Z.; Zuo, Y.; Zhou, H.; Sun, D.; Li, Y.; Yan, Y.; Feng, T.; Xie, A. Self-seeded growth of hexagonal-phase WO3 film by a one-step hydrothermal method for high-performance electrochromic energy storage devices. J. Power Sources 2025, 633, 236350. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Tang, S.; Weng, X.; Xiang, Y.; Jia, C. Multi-spectrum compatible electrochromic device combining microwave transmission with ultra-wide emissivity modulation. Chem. Eng. J. 2024, 498, 155419. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.; Jiu, J.; Liu, J.; Yan, H.; Suganuma, K. Polyaniline films with modified nanostructure for bifunctional flexible multicolor electrochromic and supercapacitor applications. Chem. Eng. J. 2018, 345, 290–299. [Google Scholar] [CrossRef]
- Tian, M.; Liu, X.; Diao, X.; Zhong, X. High performance PANI/MnO2 coral-like nanocomposite anode for flexible and robust electrochromic energy storage device. Sol. Energy Mater. Sol. Cells 2023, 253, 112239. [Google Scholar] [CrossRef]
- Sui, Y.; Ma, Y.; Gao, Y.; Song, J.; Ye, Y.; Niu, H.; Ma, W.; Zhang, P.; Qin, C. PANI/MoO3−x shell–core composites with enhanced rate and cycling performance for flexible solid-state supercapacitors and electrochromic applications. New J. Chem. 2021, 45, 10654–10663. [Google Scholar] [CrossRef]
- Ouyang, M.; Hu, X.; Shao, X.; Chen, L.; Li, W.; Bai, R.; Zhang, L.; Lv, X.; Tameev, A.; Zhang, C. In situ preparation and determination of electrochemical and electrochromic properties of copper phthalocyanine-polyaniline nanocomposite films. RSC Adv. 2019, 9, 34382–34388. [Google Scholar] [CrossRef]
- Xinming, W.; Qiguan, W.; Wenzhi, Z.; Yan, W.; Weixing, C. Enhanced electrochemical performance of hydrogen-bonded graphene/polyaniline for electrochromo-supercapacitor. J. Mater. Sci. 2016, 51, 7731–7741. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Z.; Yan, Y.; Xie, A.; Feng, T.; Jia, C. Construction of symmetric flexible electrochromic and rechargeable supercapacitors based on a 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt-loaded polyaniline nanostructured film. Materials 2025, 18, 2836. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Han, C.; Duan, H.; Xu, L.; Zhou, D.; Li, H.; Li, J.; Kang, F.; Li, B.; Wang, G. Redox-active organic sodium anthraquinone-2-sulfonate (AQS) anchored on reduced graphene oxide for high-performance supercapacitors. Adv. Energy Mater. 2018, 8, 1802088. [Google Scholar] [CrossRef]
- Sheng, L.; Fang, D.; Wang, X.; Tang, J.; Han, Q.; Zhou, J.; Tang, W. Boosting PEDOT energy storage with redox dopant and electrolyte additive. Chem. Eng. J. 2020, 401, 126123. [Google Scholar] [CrossRef]
- Yang, S.; Sun, L. Rational intramolecular and interface design of cellulosic paper electrode via PEDOT with AQS as dopant and electrolyte additives. Int. J. Biol. Macromol. 2024, 278, 134931. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chang, C.; Yau, S.; Fan, L.; Yang, Y.; Yang, L.O.; Itaya, K. Conformations of polyaniline molecules adsorbed on Au(111) probed by in situ STM and ex situ XPS and NEXAFS. J. Am. Chem. Soc. 2009, 131, 6468–6474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, Z.; Zhong, Q.; Qin, Y.; Xu, A.; Li, W.; Shi, J. In situ synthesis of trifluoroacetic acid-doped polyaniline/reduced graphene oxide composites for high-performance all-solid-state supercapacitors. ACS Appl. Energy Mater. 2020, 3, 8774–8785. [Google Scholar] [CrossRef]
- Habib, H.; Wani, I.S.; Husain, S. High performance nanostructured symmetric reduced graphene oxide/polyaniline supercapacitor electrode: Effect of polyaniline morphology. J. Energy Storage 2022, 55, 105732. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Li, G.; Li, C.; Qin, X. Ultra-simple and green two-step synthesis of sodium anthraquinone-2-sulfonate composite graphene (AQS/rGO) hydrogels for supercapacitor electrode materials. J. Alloys Compd. 2021, 862, 158472. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Li, B.; Lü, H.; Shen, Y. AQDS-guided growth of nanocilia-like polyaniline on graphene nanofiber as cathode material for high-performance asymmetric supercapacitors. Synth. Met. 2021, 272, 116660. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2019, 5, 5–19. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, J.-M.; Kim, S.J.; Kim, K.H.; Lee, S.H.; Bae, N.H.; Lee, K.G.; Choi, B.G. Large-area and 3D polyaniline nanoweb film for flexible supercapacitors with high rate capability and long cycle life. ACS Appl. Energy Mater. 2020, 3, 7746–7755. [Google Scholar] [CrossRef]
- Zhang, L.; Han, D.; Tao, Y.; Cui, C.; Deng, Y.; Dong, X.; Lv, W.; Lin, Z.; Wu, S.; Weng, Z.; et al. Dense organic molecules/graphene network anodes with superior volumetric and areal performance for asymmetric supercapacitors. J. Mater. Chem. A 2020, 8, 461–469. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; He, Z.; Yi, Y.; Wang, M.; Luo, Z.; Liu, Q.; Huang, J.; Zhong, X.; Du, K.; et al. All-solid-state electrochromic Li-ion hybrid supercapacitors for intelligent and wide-temperature energy storage. Chem. Eng. J. 2021, 414, 128892. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Zheng, R.; Pan, J.; Niu, J.; Zou, X.; Jia, C. A flexible, electrochromic, rechargeable Zn-ion battery based on actiniae-like self-doped polyaniline cathode. J. Mater. Chem. A 2020, 8, 12799–12809. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, J.; Wang, Y.; Hu, L.; Tang, S.; Wan, Z.; Jia, C.; Weng, X.; Deng, L. A light-weight, thin-thickness, flexible multifunctional electrochromic device integrated with variable optical, thermal management and energy storage. Electrochim. Acta 2022, 435, 141274. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, E.; Wang, Z.; Feng, T.; Xie, A. Redox-Active Anthraquinone-1-Sulfonic Acid Sodium Salt-Loaded Polyaniline for Dual-Functional Electrochromic Supercapacitors. Gels 2025, 11, 568. https://doi.org/10.3390/gels11080568
Wang Y, Lin E, Wang Z, Feng T, Xie A. Redox-Active Anthraquinone-1-Sulfonic Acid Sodium Salt-Loaded Polyaniline for Dual-Functional Electrochromic Supercapacitors. Gels. 2025; 11(8):568. https://doi.org/10.3390/gels11080568
Chicago/Turabian StyleWang, Yi, Enkai Lin, Ze Wang, Tong Feng, and An Xie. 2025. "Redox-Active Anthraquinone-1-Sulfonic Acid Sodium Salt-Loaded Polyaniline for Dual-Functional Electrochromic Supercapacitors" Gels 11, no. 8: 568. https://doi.org/10.3390/gels11080568
APA StyleWang, Y., Lin, E., Wang, Z., Feng, T., & Xie, A. (2025). Redox-Active Anthraquinone-1-Sulfonic Acid Sodium Salt-Loaded Polyaniline for Dual-Functional Electrochromic Supercapacitors. Gels, 11(8), 568. https://doi.org/10.3390/gels11080568





