Design, Synthesis, and Morphological Behavior of Polymer Gel-Based Materials for Thermoelectric Devices: Recent Progress and Perspectives
Abstract
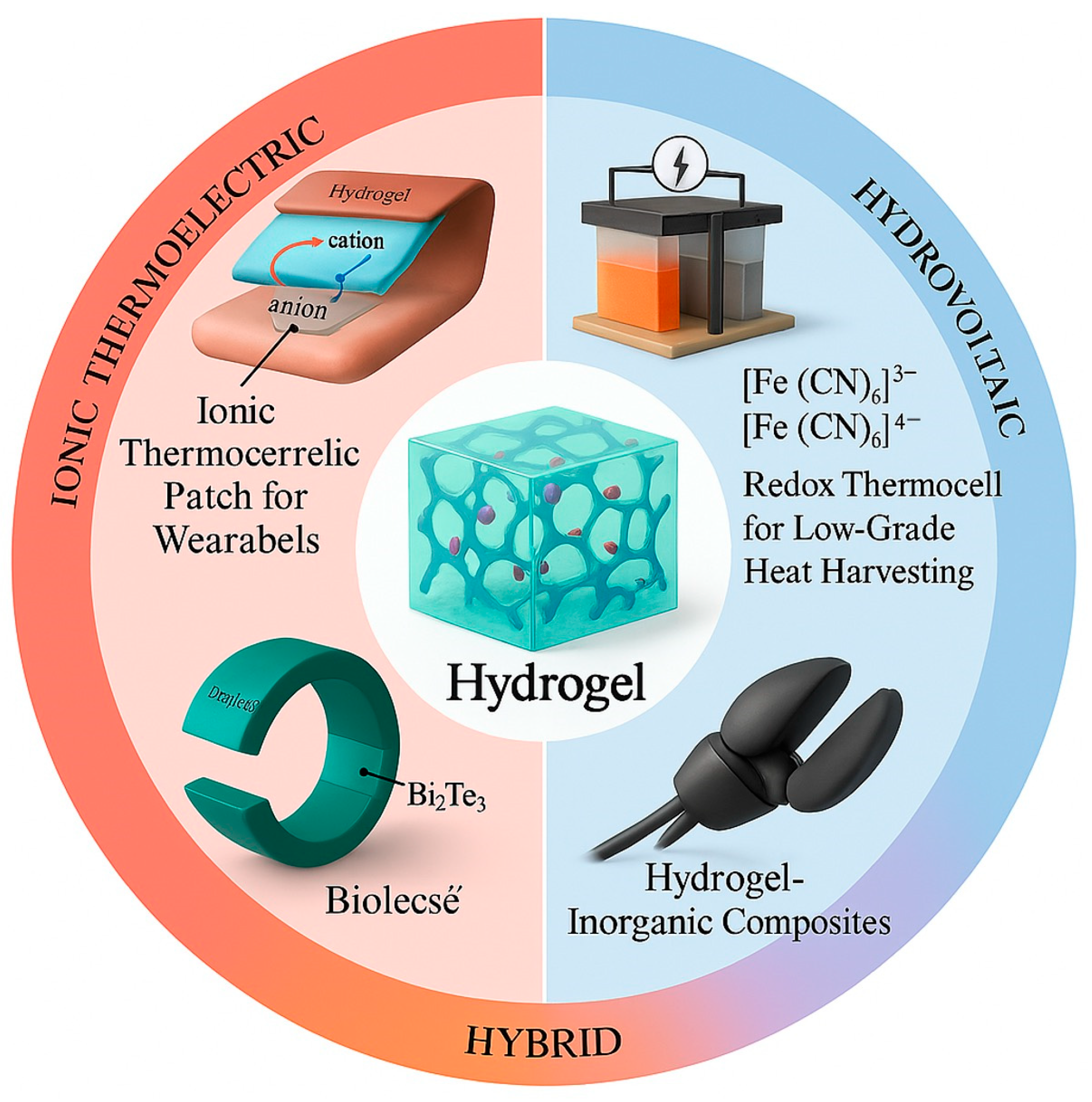
1. Introduction
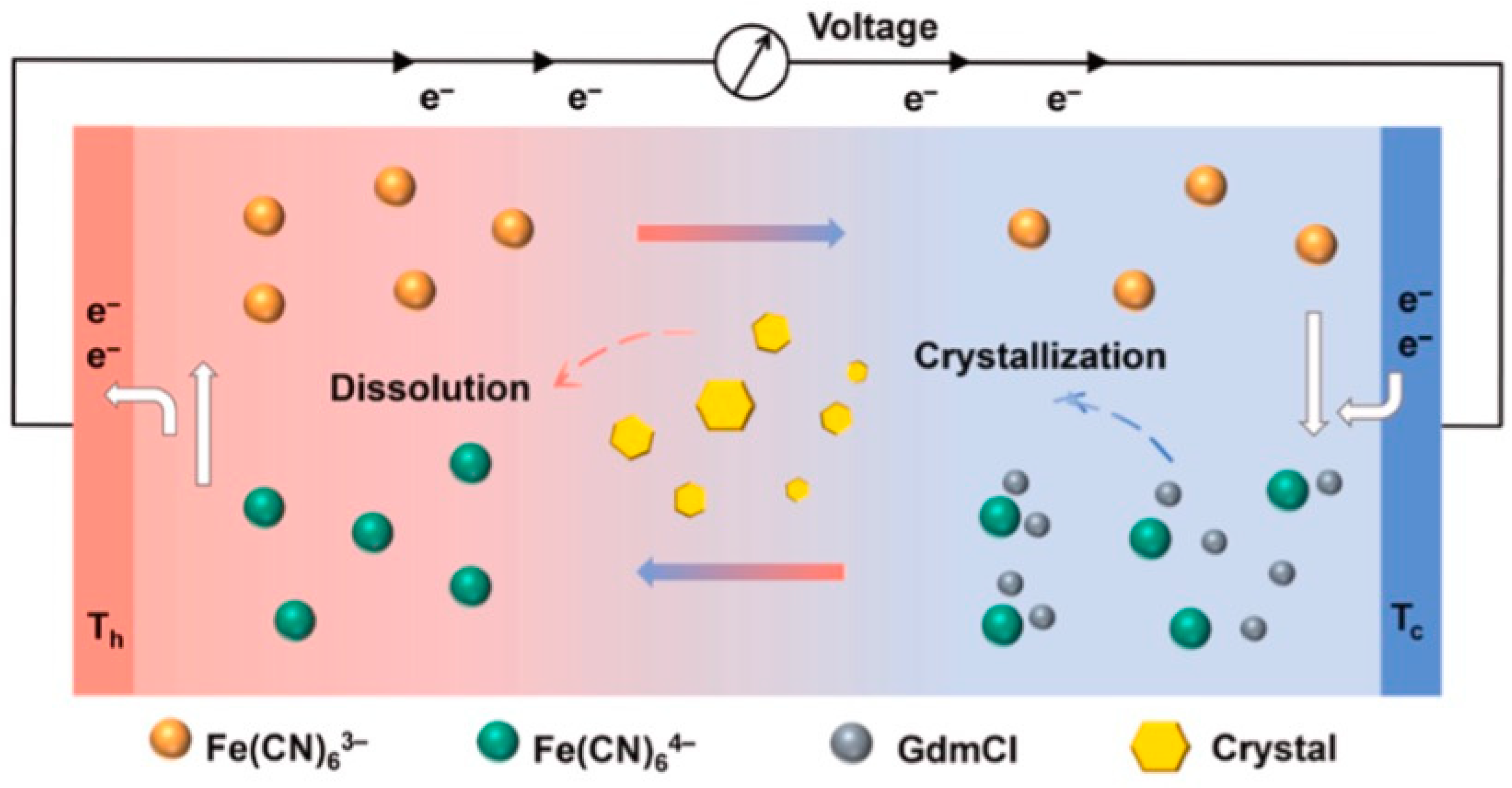
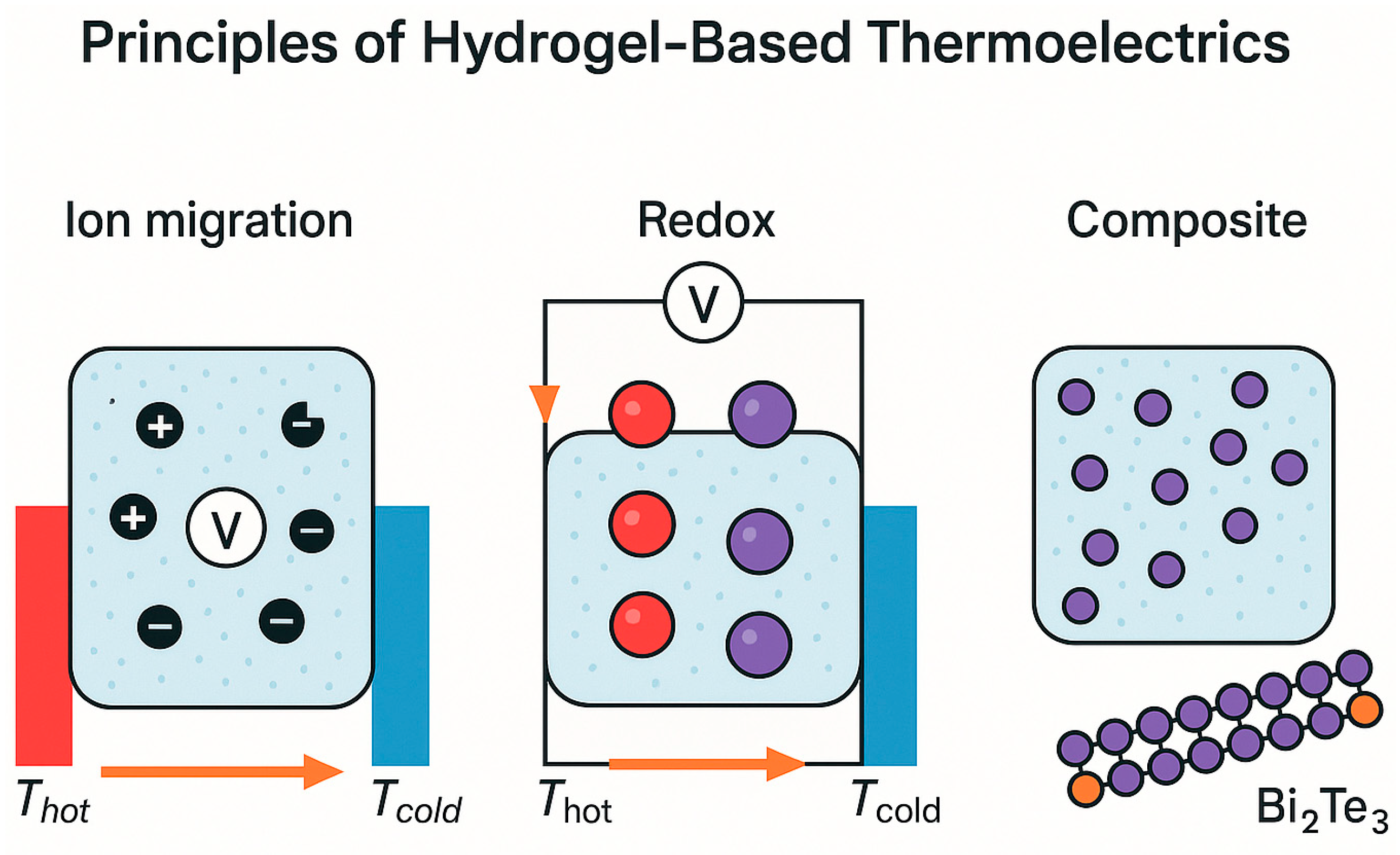
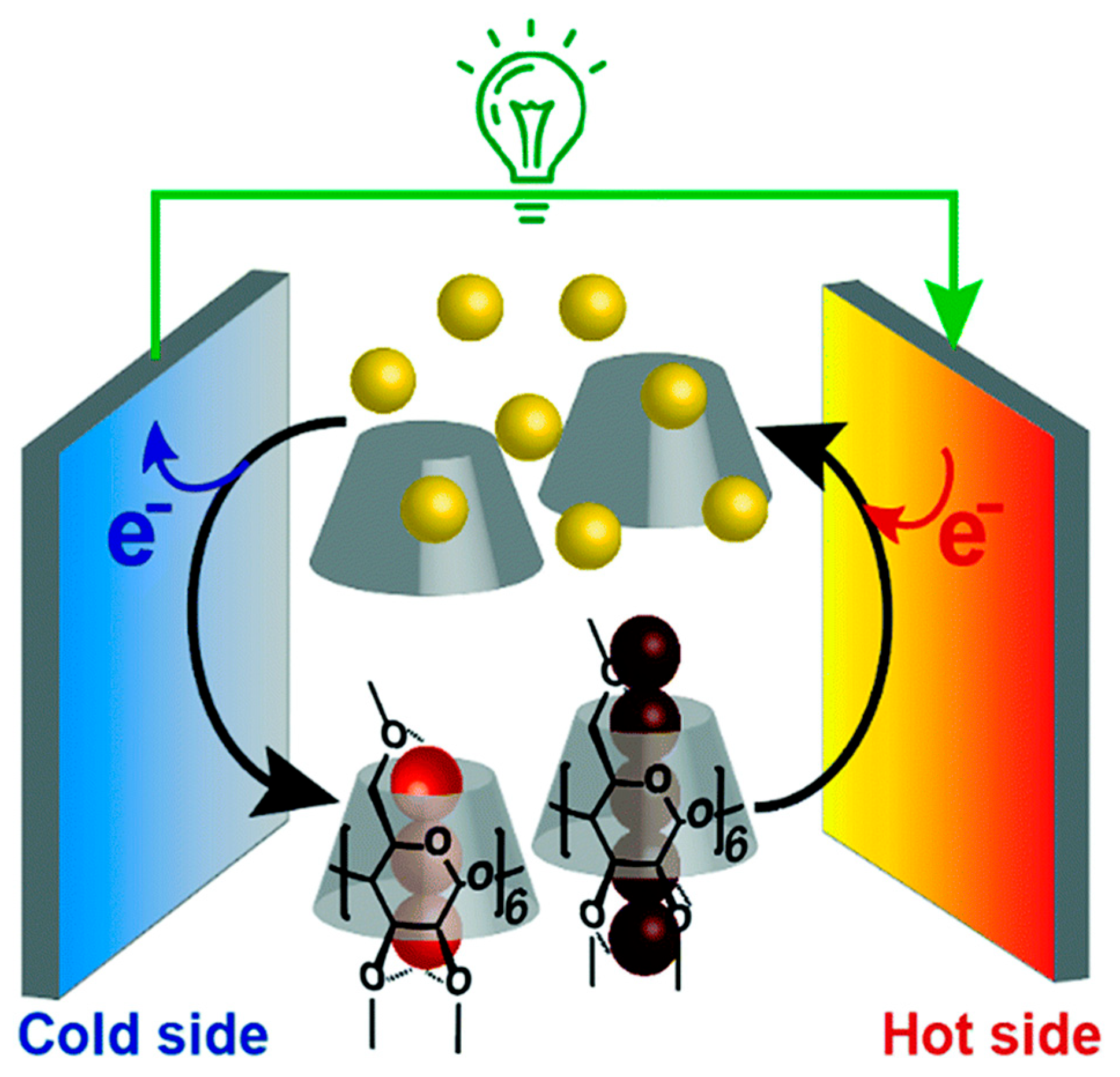
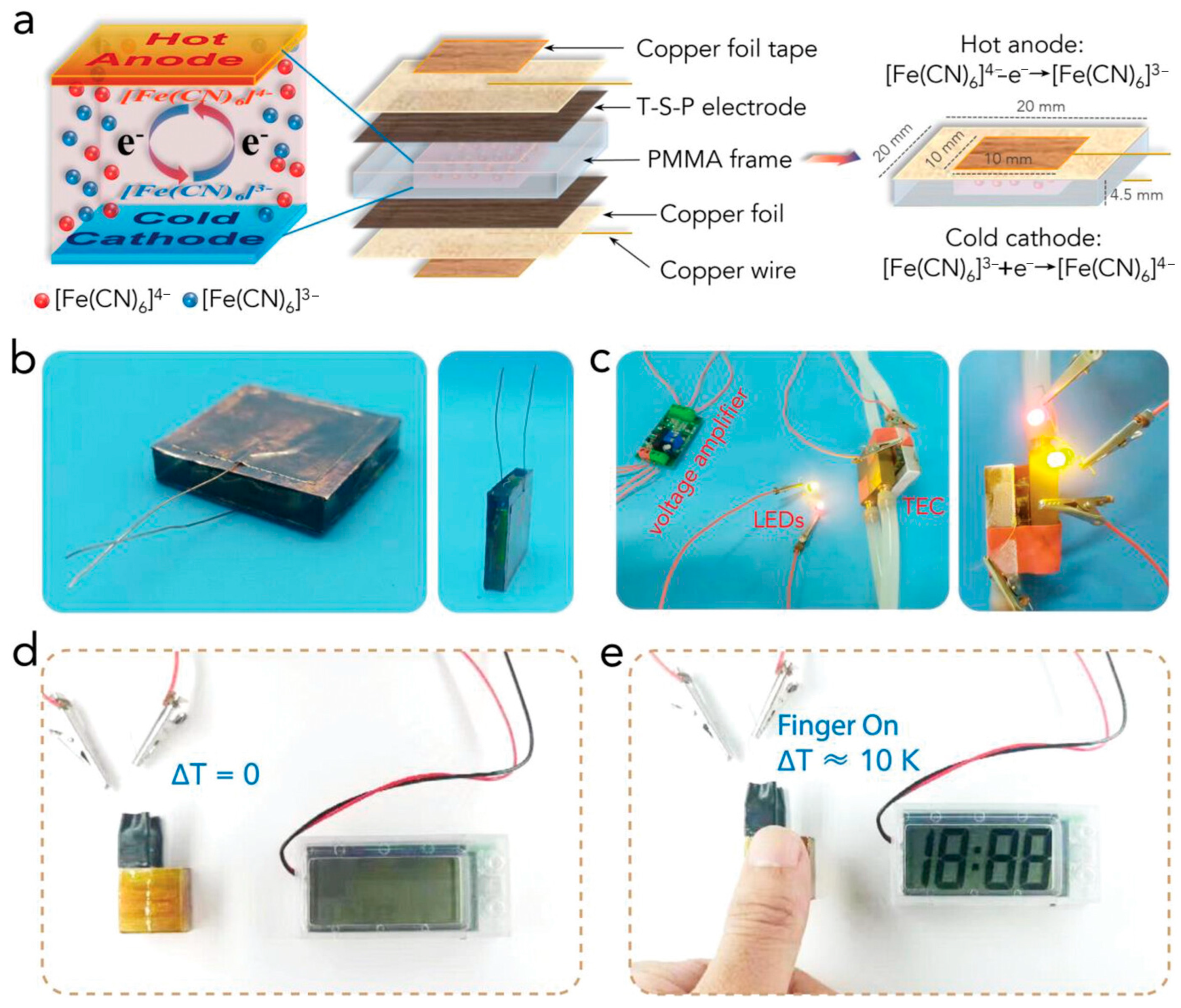
2. Principles of Hydrogel-Based Thermoelectric
3. Different Hydrogels for Thermoelectrics and Their Key Properties
3.1. Strategies for Improving Thermoelectric Properties in Hydrogel-Based Thermocells: Enhancing Thermopower
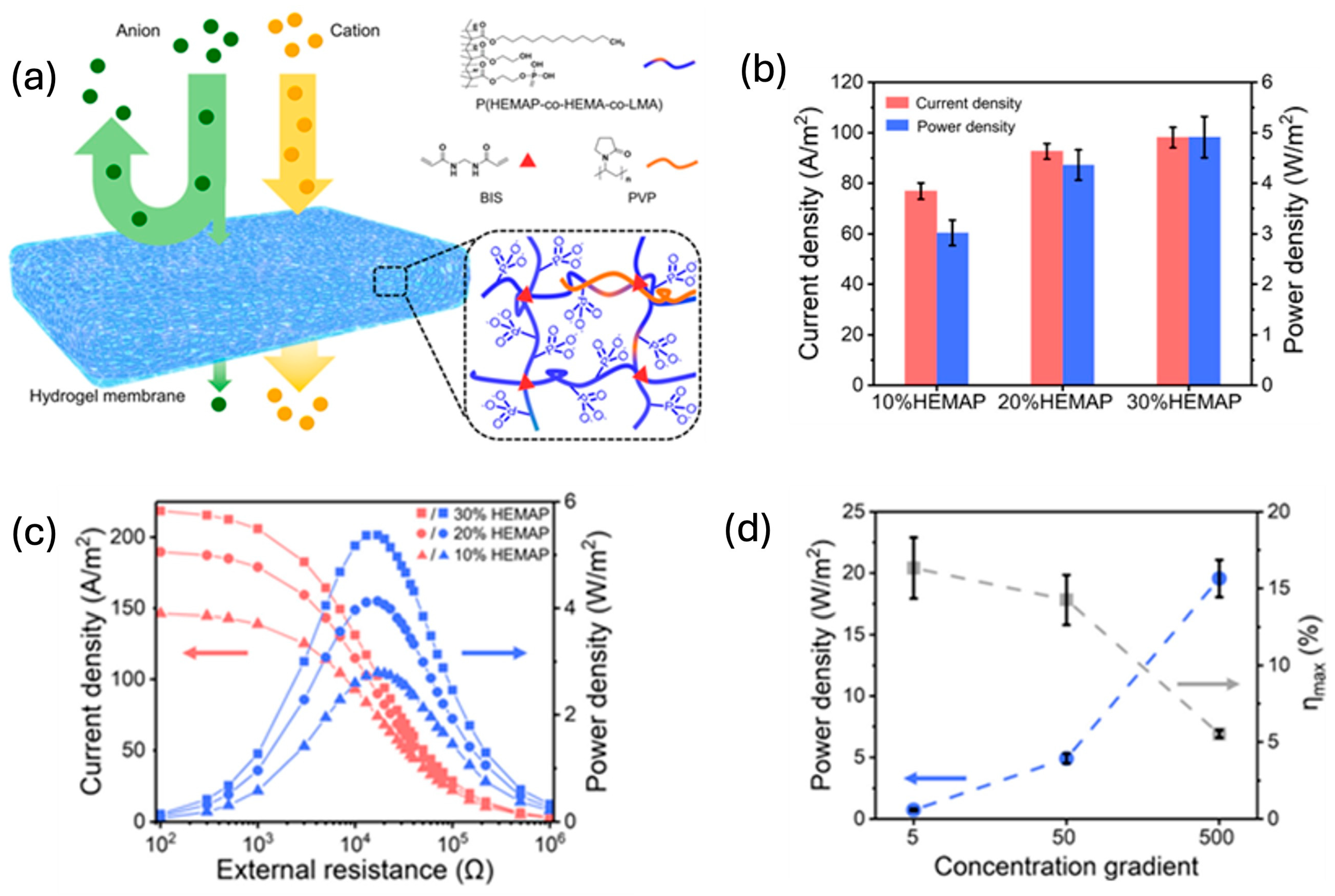
3.2. Improving Ionic Conductivity
4. Gel Matrix and Its Influence on Ionic Thermoelectric Conversion
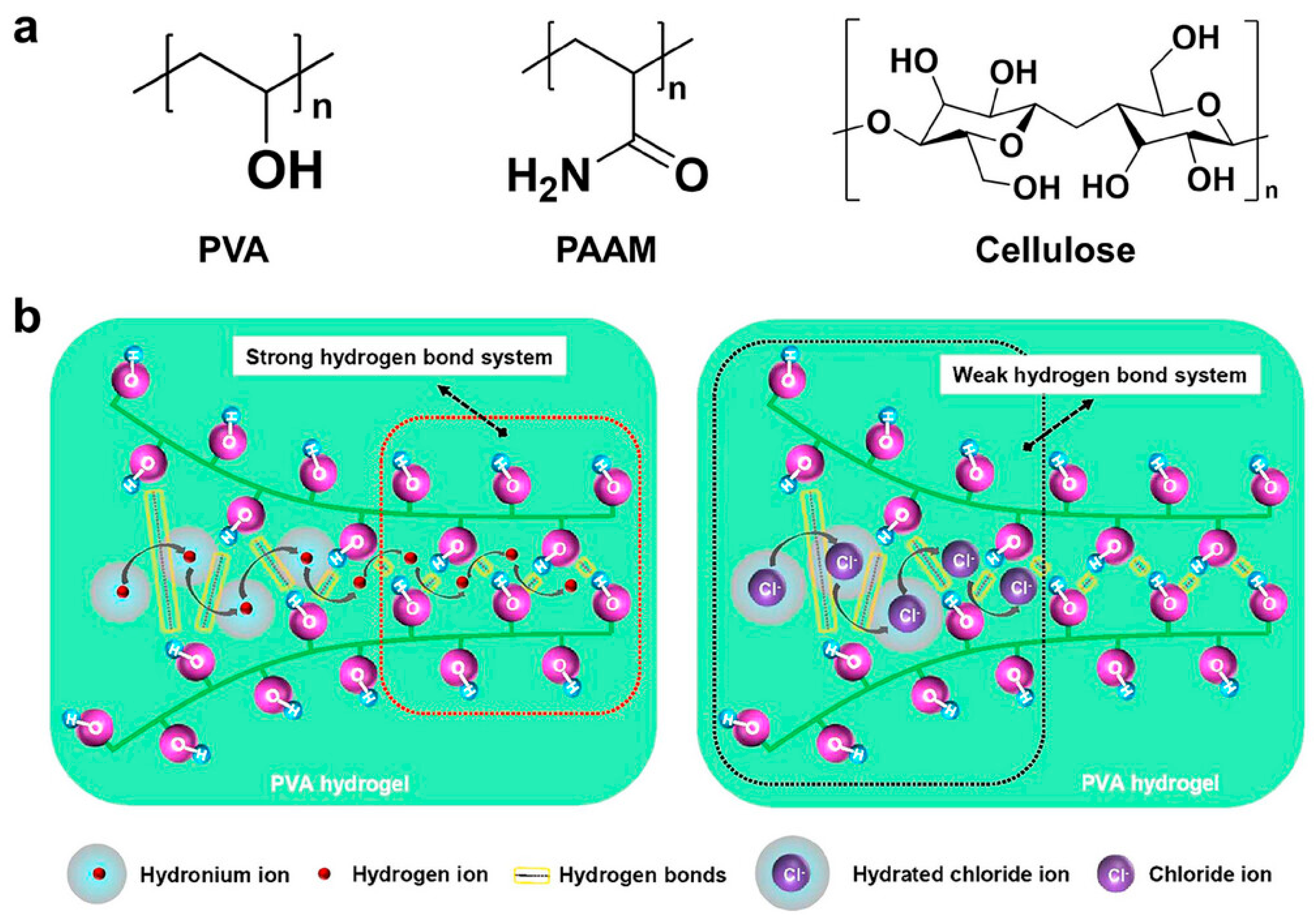
4.1. Polyvinyl Alcohol
4.2. Polyacrylamide
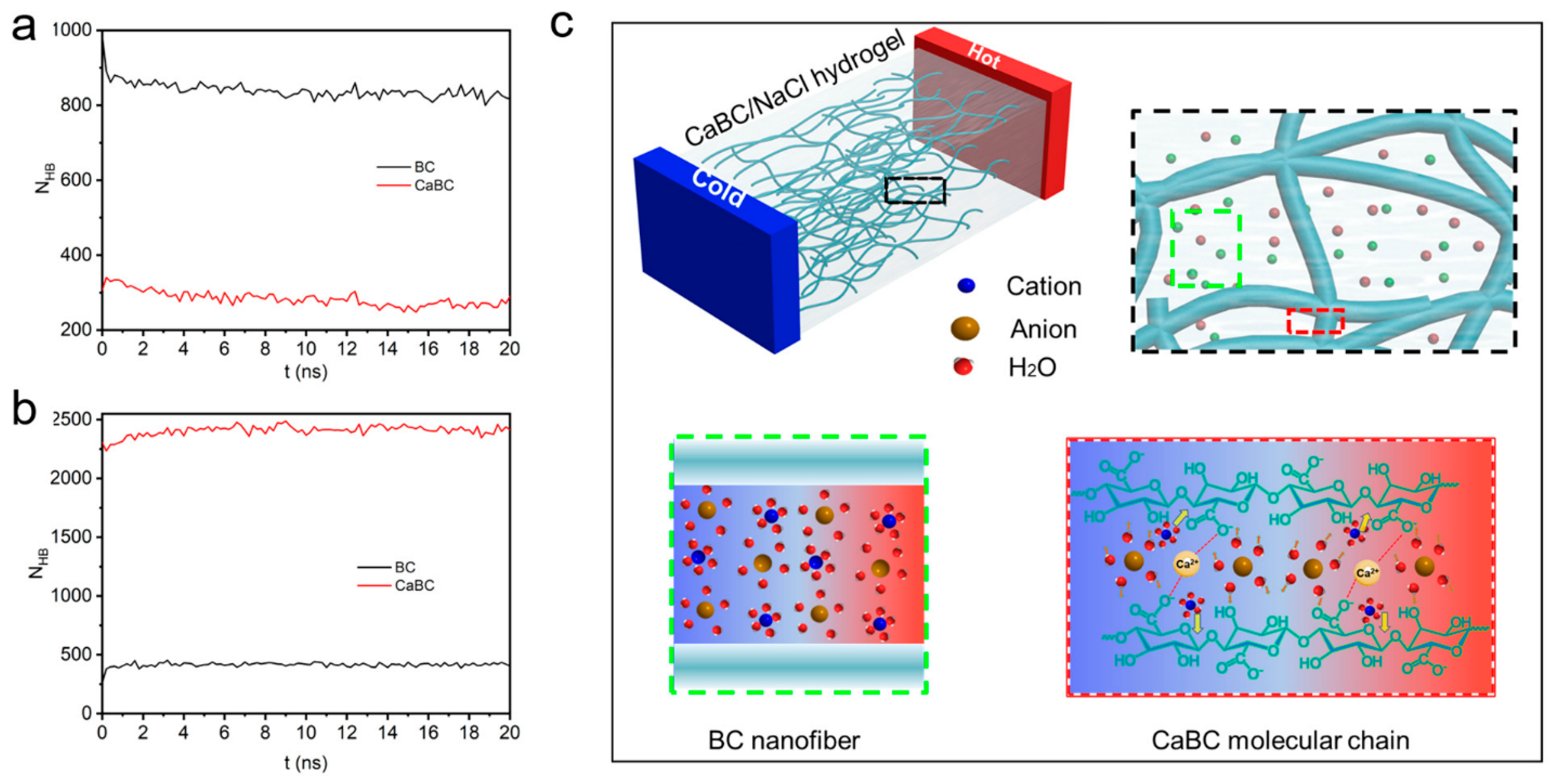
4.3. Cellulose
4.4. Other Polymer Matrices
5. Mechanistic Insights into Hydrogel Degradation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumon, M.M.H. Advances in cellulose-based hydrogels: Tunable swelling dynamics and their versatile real-time applications. RSC Adv. 2025, 15, 11688–11729. [Google Scholar] [CrossRef] [PubMed]
- Rumon, M.M.H.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-healing hydrogels: Development, biomedical applications, and challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Sarkar, S.D.; Uddin, M.M.; Alam, M.M.; Karobi, S.N.; Ayfar, A.; Azam, M.S.; Roy, C.K. Graphene oxide based crosslinker for simultaneous enhancement of mechanical toughness and self-healing capability of conventional hydrogels. RSC Adv. 2022, 12, 7453–7463. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Liu, L.; Guo, X.; Liu, W.; Lee, C. Recent Progress in the Energy Harvesting Technology—From Self-Powered Sensors to Self-Sustained IoT, and New Applications. Nanomaterials 2021, 11, 2975. [Google Scholar] [CrossRef] [PubMed]
- Akib, A.A.; Rumon, M.M.H.; Moniruzzaman, M.; Kumar Roy, C.; Chowdury, A.-N. PLA-PEG Diblock Copolymer Micelle as Nanocarrier for Anti-Obesity Drug Delivery System. ECS Trans. 2022, 107, 19031. [Google Scholar] [CrossRef]
- Akib, A.A.; Shakil, R.; Rumon, M.M.H.; Roy, C.K.; Chowdhury, E.H.; Chowdhury, A.-N. Natural and Synthetic Micelles for the Delivery of Small Molecule Drugs, Imaging Agents and Nucleic Acids. Curr. Pharm. Des. 2022, 28, 1389–1405. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Sayem, M.; Halder, S.K.; Brishti, R.S.; Das, A.; Hasan, M.A.; Shakil, M.S. The Promise of Functionalized Chitosan-Based Self-Healing Hydrogels. Adv. Polym. Technol. 2025, 2025, 4913728. [Google Scholar] [CrossRef]
- Hossain, M.A.; Roy, C.K.; Sarkar, S.D.; Roy, H.; Howlader, A.H.; Firoz, S.H. Improvement of the strength of poly(acrylic acid) hydrogels by the incorporation of functionally modified nanocrystalline Cellulose. Mater. Adv. 2020, 1, 2107–2116. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Uddin, M.M.; Roy, C.K.; Hossen, M.J.; Sujan, M.I.; Azam, M.S. Mechanically tough and highly stretchable poly(acrylic acid) hydrogel cross-linked by 2D graphene oxide. RSC Adv. 2020, 10, 10949–10958. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Qian, W.; Jia, S.; Yu, P.; Li, K.; Li, M.; Lan, J.; Lin, Y.-H.; Yang, X. Highly stretchable, low-hysteresis, and antifreeze hydrogel for low-grade thermal energy harvesting in ionic thermoelectric Supercapacitors. Mater. Today Phys. 2024, 49, 101589. [Google Scholar] [CrossRef]
- Zhou, Z.; Wan, Y.; Zi, J.; Ye, G.; Jin, T.; Geng, X.; Zhuang, W.; Yang, P. Flexible hydrogel with a coupling enhanced thermoelectric effect for low-grade heat harvest. Mater. Today Sustain. 2023, 21, 100293. [Google Scholar] [CrossRef]
- Khan, W.U.; Shen, Z.; Mugo, S.M.; Wang, H.; Zhang, Q. Implantable hydrogels as pioneering materials for next-generation brain–computer interfaces. Chem. Soc. Rev. 2025, 54, 2832–2880. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, M.Y.; Kim, D.-H.; Koo, J.H. Recent Advances in Hydrogel-Based Soft Bioelectronics and its Convergence with Machine Learning. Adv. Eng. Mater. 2024, 26, 2401432. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Zhang, Q. Constructing phase separation in polymer gels: Strategies, functions and applications. Prog. Polym. Sci. 2024, 154, 101847. [Google Scholar] [CrossRef]
- Boutry, C.M.; Negre, M.; Jorda, M.; Vardoulis, O.; Chortos, A.; Khatib, O.; Bao, Z. A hierarchically patterned, bioinspired e-skin able to detect the direction of applied pressure for robotics. Sci. Robot. 2018, 3, eaau6914. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Recent Progress on the synthesis, morphological topography, and battery applications of polypyrrole-based nanocomposites. Polymers 2024, 16, 3277. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H.; Islam, M. Synthesis, Rheology, Morphology, and Mechanical Properties of Biodegradable PVA-Based Composite Films: A Review on Recent Progress. Processes 2024, 12, 2880. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Sarkar, S.D.; Alam, M.M.; Roy, C.K. Nanomaterials for self-healing hydrogels. Emerg. Appl. Nanomater. 2023, 141, 270–293. [Google Scholar]
- Rani, R.; Sehrawat, M.; Verma, A.K.; Rani, M.; Gahtori, B.; Kumar, P.; Dhakate, S.R.; Singh, B.P. Surfactant-Mediated Control of Polyethylenimine Dopant for Enhanced Thermoelectric Performance of Carbon Nanotube Buckypaper for Varied Device Configurations. ACS Appl. Energy Mater. 2024, 7, 6996–7005. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, Y.; Chen, P.; Qi, H.; Lu, A. Regulation of thermal migration channel in cellulose hydrogel to enhance thermopower. Chem. Eng. J. 2024, 498, 155161. [Google Scholar] [CrossRef]
- Dechiraju, H.; Jia, M.; Luo, L.; Rolandi, M. Ion-conducting hydrogels and their applications in bioelectronics. Adv. Sustain. Syst. 2022, 6, 2100173. [Google Scholar] [CrossRef]
- Heller, A. Electron-conducting redox hydrogels: Design, characteristics and synthesis. Curr. Opin. Chem. Biol. 2006, 10, 664–672. [Google Scholar] [CrossRef]
- Jiang, K.; Jia, J.; Chen, Y.; Li, L.; Wu, C.; Zhao, P.; Zhu, D.Y.; Zeng, W. An ionic thermoelectric generator with a giant output power density and high energy density enabled by synergy of thermodiffusion effect and redox reaction on electrodes. Adv. Energy Mater. 2023, 13, 2204357. [Google Scholar] [CrossRef]
- Katz, E. Modified electrodes and electrochemical systems switchable by temperature changes. Electroanalysis 2016, 28, 1916–1929. [Google Scholar] [CrossRef]
- Jia, S.; Ma, H.; Gao, S.; Yang, L.; Sun, Q. Thermoelectric Materials and Devices for Advanced Biomedical Applications. Small 2024, 20, 2405019. [Google Scholar] [CrossRef]
- Brishti, R.S.; Habib, M.A.; Ara, M.H.; Karim, K.M.R.; Islam, M.K.; Naime, J.; Rumon, M.M.H.; Khan, M.A.R. Green synthesis of ZnO NPs using aqueous extract of Epipremnum aureum leave: Photocatalytic degradation of Congo red. Results Chem. 2024, 7, 101441. [Google Scholar] [CrossRef]
- Shi, X.-L.; Wang, L.; Lyu, W.; Cao, T.; Chen, W.; Hu, B.; Chen, Z.-G. Advancing flexible thermoelectrics for integrated electronics. Chem. Soc. Rev. 2024, 53, 9254–9305. [Google Scholar] [CrossRef]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, X.L.; Liu, Q.; Chen, Z.G. Hydrogel-Based Functional Materials for Thermoelectric Applications: Progress and Perspectives. Adv. Funct. Mater. 2024, 34, 2410127. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Jiang, K.; Shen, G. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev. 2017, 46, 6764–6815. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable Thermocells Based on Gel Electrolytes for the Utilization of Body Heat. Angew. Chem. Int. Ed. 2016, 55, 12050–12053. [Google Scholar] [CrossRef]
- Kim, S.L.; Lin, H.T.; Yu, C. Thermally Chargeable Solid-State Supercapacitor. Adv. Energy Mater. 2016, 6, 1600546. [Google Scholar] [CrossRef]
- Zhao, D.; Martinelli, A.; Willfahrt, A.; Fischer, T.; Bernin, D.; Khan, Z.U.; Shahi, M.; Brill, J.; Jonsson, M.P.; Fabiano, S.; et al. Polymer gels with tunable ionic Seebeck coefficient for ultra-sensitive printed thermopiles. Nat. Commun. 2019, 10, 1093. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and limitations of two step metal oxide thermochemical redox cycles; a review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H.; Barai, H.R. Conductive Gels for Energy Storage, Conversion, and Generation: Materials Design Strategies, Properties, and Applications. Materials 2024, 17, 2268. [Google Scholar] [CrossRef]
- Yin, J.; Jia, P.; Ren, Z.; Zhang, Q.; Lu, W.; Yao, Q.; Deng, M.; Zhou, X.; Gao, Y.; Liu, N. Recent Advances in Self-Powered Sensors Based on Ionic Hydrogels. Research 2025, 8, 0571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, C.; Wang, Z.; Liu, X.; Li, X.; Cui, X. Low-grade thermal energy harvesting and self-powered sensing based on thermogalvanic hydrogels. Micromachines 2023, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hong, J.; Huang, C.; Zhang, X.; Shen, S.; Du, Z.; Zhou, P.; Miao, Y.-B.; Lin, Z.-H.; Lyu, X.; et al. A strong, tough, and high-efficiency hydrogel thermocell for thermal energy harvesting. Nano Energy 2025, 138, 110878. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Collins, H.; Dsouza, K.; Polash, M.H.; Hosseini, M.; Hyland, M.; Liu, J.; Malhotra, A.; Ortiz, F.M.; Mohaddes, F. Review of wearable thermoelectric energy harvesting: From body temperature to electronic systems. Appl. Energy 2020, 258, 114069. [Google Scholar] [CrossRef]
- Sun, T.; Wang, L.; Jiang, W. Pushing thermoelectric generators toward energy harvesting from the human body: Challenges and strategies. Mater. Today 2022, 57, 121–145. [Google Scholar] [CrossRef]
- Tabaie, Z.; Omidvar, A. Human body heat-driven thermoelectric generators as a sustainable power supply for wearable electronic devices: Recent advances, challenges, and future perspectives. Heliyon 2023, 9, e14707. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Rahman Khan, M.M. Progress in hydrogel toughening: Addressing structural and crosslinking challenges for biomedical applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Song, H.-S.; Rumon, M.M.H.; Rahman Khan, M.M.; Jeong, J.-H. Toward Intelligent Materials with the Promise of Self-Healing Hydrogels in Flexible Devices. Polymers 2025, 17, 542. [Google Scholar] [CrossRef]
- Shakil, A.R.; Begum, M.L.; Shaikh, M.A.A.; Sultana, S.; Rahman, M.S.; Rumon, M.M.H.; Roy, C.K.; Haque, M.A. Jute fiber reinforced hydrogel composite for removal of methylene blue dye from water. Dhaka Univ. J. Sci. 2022, 70, 59–64. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, H.; Qi, K.; Ma, X.; Wang, M.; Ma, Z.; Wang, Z.; Yang, Y.; Ramakrishna, S.; Ou, K. Electrode-dependent thermoelectric effect in ionic hydrogel fiber for self-powered sensing and low-grade heat harvesting. Chem. Eng. J. 2024, 497, 154970. [Google Scholar] [CrossRef]
- Sun, S.; Li, M.; Shi, X.L.; Chen, Z.G. Advances in ionic thermoelectrics: From materials to devices. Adv. Energy Mater. 2023, 13, 2203692. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, K.; Shao, C.; Hao, S.; Yang, J. Recent progress in bioinspired design strategies for freeze resistant hydrogel platforms toward flexible electronics. Chem. Mater. 2023, 35, 10316–10347. [Google Scholar] [CrossRef]
- Chenani, H.; Saeidi, M.; Rastkhiz, M.A.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and advances of hydrogel-based wearable electrochemical biosensors for real-time monitoring of biofluids: From lab to market. A review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lu, Y.; Yin, J.; Levin, A.; Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Lyu, Q. A review on thermal properties of hydrogels for electronic devices applications. Gels 2022, 9, 7. [Google Scholar] [CrossRef]
- Fan, Z.; Du, D.; Guan, X.; Ouyang, J. Polymer films with ultrahigh thermoelectric properties arising from significant seebeck coefficient enhancement by ion accumulation on surface. Nano Energy 2018, 51, 481–488. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, M.; Qin, C.; Zhang, J.; Lu, A. Cellulose ionic conductor with tunable Seebeck coefficient for low-grade heat harvesting. Carbohydr. Polym. 2022, 292, 119650. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Q.; Xiao, S.; Feng, J.; Zhang, X.; Wang, T. Giant negative thermopower of ionic hydrogel by synergistic coordination and hydration interactions. Sci. Adv. 2021, 7, eabi7233. [Google Scholar] [CrossRef]
- Cho, C.; Kim, B.; Park, S.; Kim, E. Bisulfate transport in hydrogels for self-healable and transparent thermoelectric harvesting films. Energy Environ. Sci. 2022, 15, 2049–2060. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, B.; Xiao, S.; Feng, J.; Yang, J.; Yue, Q.; Zhang, X.; Wang, T. Giant Thermopower of Hydrogen Ion Enhanced by a Strong Hydrogen Bond System. ACS Appl. Mater. Interfaces 2022, 14, 19304–19314. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Hsu, C.-C.; Hong, S.-H.; Lee, L.-C.; Jeng, U.S.; Chen, H.-L.; Tung, S.-H.; Liu, C.-L. Highly conductive triple network hydrogel thermoelectrochemical cells with low-grade heat harvesting. J. Power Sources 2024, 609, 234647. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, Y.; Liu, L.; Zhou, Y.; Bai, P.; Li, Q.; Guo, J.; Ma, R. Boosting Thermoelectric Performance of Thermogalvanic Hydrogels by Structure Engineering Induced by Liquid Nitrogen Quenching. Adv. Energy Mater. 2024, 14, 2303358. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Bai, P.; Mao, Y.; Li, Q.; Guo, J.; Fang, Y.; Ma, R. Strong Tough Thermogalvanic Hydrogel Thermocell With Extraordinarily High Thermoelectric Performance. Adv. Mater. 2023, 35, 2300696. [Google Scholar] [CrossRef]
- Kong, S.; Huang, Z.; Hu, Y.; Jiang, Y.; Lu, Y.; Zhao, W.; Shi, Q.; Yuan, M.; Dai, B.; Li, J.; et al. Tellurium-nanowire-doped thermoelectric hydrogel with high stretchability and seebeck coefficient for low-grade heat energy harvesting. Nano Energy 2023, 115, 108708. [Google Scholar] [CrossRef]
- Chen, J.; Shi, C.; Wu, L.; Deng, Y.; Wang, Y.; Zhang, L.; Zhang, Q.; Peng, F.; Tao, X.-M.; Zhang, M.; et al. Environmentally Tolerant Ionic Hydrogel with High Power Density for Low-Grade Heat Harvesting. ACS Appl. Mater. Interfaces 2022, 14, 34714–34721. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, C.-G.; Chen, J.; Yang, L.; Ma, Y.; Guan, H.; Han, D.; Niu, L. Ultra-high performance of ionic thermoelectric-electrochemical gel cells for harvesting low grade heat. Energy Environ. Sci. 2024, 17, 4104–4114. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, Y.; Huang, A.; Jiang, M.; Wang, L.; Zhang, Q.; Jiang, W. Metal-Halogen Interactions Inducing Phase Separation for Self-Healing and Tough Ionogels with Tunable Thermoelectric Performance. Adv. Mater. 2024, 36, 2402386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, Y.; Xia, F.; Zhang, X. Gelatin/polyacrylamide ionic conductive hydrogel with skin temperature-triggered adhesion for human motion sensing and body heat harvesting. Nano Energy 2022, 104, 107977. [Google Scholar] [CrossRef]
- Guo, B.; Miura, Y.; Hoshino, Y. Rational Design of Thermocells Driven by the Volume Phase Transition of Hydrogel Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 32184–32192. [Google Scholar] [CrossRef]
- Li, J.; Xu, T.; Ma, Z.; Li, W.; Qian, Y.; Tao, Y.; Wei, Y.; Jiang, Q.; Luo, Y.; Yang, J. Self-Healable and Stretchable PAAc/XG/Bi2Se0.3Te2.7 Hybrid Hydrogel Thermoelectric Materials. Energy Environ. Mater. 2024, 7, e12547. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, Y.-T.; Hong, S.-H.; Wang, C.-H.; Jeng, U.S.; Tung, S.-H.; Liu, C.-L. Mixed Ionic–Electronic Conducting Hydrogels with Carboxylated Carbon Nanotubes for High Performance Wearable Thermoelectric Harvesters. ACS Appl. Mater. Interfaces 2023, 15, 56072–56083. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Lee, L.-C.; Lin, Y.-T.; Hong, S.-H.; Wang, K.-C.; Tung, S.-H.; Liu, C.-L. Stretchable polyvinyl alcohol and sodium alginate double network ionic hydrogels for low-grade heat harvesting with ultrahigh thermopower. Mater. Today Energy 2023, 37, 101383. [Google Scholar] [CrossRef]
- Zhao, W.; Lei, Z.; Wu, P. Mechanically Adaptative and Environmentally Stable Ionogels for Energy Harvest. Adv. Sci. 2023, 10, 2300253. [Google Scholar] [CrossRef]
- Cheng, H.; He, X.; Fan, Z.; Ouyang, J. Flexible Quasi-Solid State Ionogels with Remarkable Seebeck Coefficient and High Thermoelectric Properties. Adv. Energy Mater. 2019, 9, 1901085. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Luo, M.; Qing, X.; Wang, W.; Zhong, W.; Liu, Q.; Wang, Y.; Yang, L.; Zhu, X.; et al. Wearable ionogel fiber-based ionic thermoelectric device for low-grade human body heat harvesting. Chem. Eng. J. 2024, 485, 149784. [Google Scholar] [CrossRef]
- Kim, B.; Na, J.; Lim, H.; Kim, Y.; Kim, J.; Kim, E. Robust High Thermoelectric Harvesting Under a Self-Humidifying Bilayer of Metal Organic Framework and Hydrogel Layer. Adv. Funct. Mater. 2019, 29, 1807549. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Li, Y.; Wang, S.; Li, Q.; Wang, Y.; Li, B.; Zhu, K.; Liu, W. Ionic thermoelectric gels and devices: Progress, opportunities, and challenges. EnergyChem 2024, 6, 100123. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, L.; Liang, H.; Zhong, Z. Longevous ionogels with high strength, conductivity, adhesion and thermoplasticity. Chem. Eng. J. 2024, 497, 155047. [Google Scholar] [CrossRef]
- Ye, Y. Nanocellulose-Based Gel Ionic Conductors: Design, Manufacturing, and Applications. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 2023. [Google Scholar]
- Bocchetta, P.; Othman, A.; Gupta, M.; Andriani, G.; Martin, P.; Kumar, Y.; Joly, N.; Sacco, P.; Sufyan Javed, M. Chitosan in electrochemical (bio) sensors: Nanostructuring and methods of synthesis. Eur. Polym. J. 2024, 213, 113092. [Google Scholar] [CrossRef]
- Sawangphruk, M. New Materials for Lithium–Sulfur Batteries: Challenges and Future Directions. Chem. Commun. 2025, 61, 7770–7794. [Google Scholar] [CrossRef] [PubMed]
- McKay, I.S.; Kunz, L.Y.; Majumdar, A. Electrochemical redox refrigeration. Sci. Rep. 2019, 9, 13945. [Google Scholar] [CrossRef] [PubMed]
- Mua, Y.; Quickenden, T.I. Power conversion efficiency, electrode separation, and overpotential in the ferricyanide/ferrocyanide thermogalvanic cell. J. Electrochem. Soc. 1996, 143, 2558. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Giovanelli, D.; Lawrence, N.S.; Compton, R.G. High-temperature electrochemistry: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 421–433. [Google Scholar] [CrossRef]
- Nandal, V.; Wei, Q.; Seki, K. Insight into the effect of the configuration entropy of additives on the Seebeck coefficient. Phys. Chem. Chem. Phys. 2021, 23, 14803–14810. [Google Scholar] [CrossRef]
- Bai, B.-L.; Du, S.; Li, M.-J. Photovoltaic efficiency improved by self-adaptive water uptake hydrogel evaporative cooling. Appl. Energy 2025, 383, 125367. [Google Scholar] [CrossRef]
- Zamengo, M.; Morikawa, J. Evaluation of cooling ability for a novel heat sink made of polyvinyl alcohol hydrogel. Int. J. Heat Mass Transf. 2019, 143, 118523. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wang, Z.; Sun, W.; Kong, F. Photovoltaic passive cooling via water vapor sorption-evaporation by hydrogel. Appl. Therm. Eng. 2024, 240, 122185. [Google Scholar] [CrossRef]
- Zou, W.; Ji, M.; Han, C.; Tian, E.; Mo, J. Enhancing the internal thermal conductivity of hydrogel for efficient passive heat dissipation: Experimental study of a surface simulating a cooled photovoltaic panel. Energy Convers. Manag. 2024, 306, 118328. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Q.; Chen, J.; Zhang, Q.; Zhao, X.; Qian, Y.; Zhu, C.; Yang, L.; Zhao, Y.; Kong, X.-Y.; et al. Improved Ion Transport and High Energy Conversion through Hydrogel Membrane with 3D Interconnected Nanopores. Nano Lett. 2020, 20, 5705–5713. [Google Scholar] [CrossRef]
- Dai, C.; Li, Z.; Zheng, K.; Zhang, J.-H.; Dai, R.; Luo, D.; Gao, H.; Thabet, H.K.; El-Bahy, Z.M.; Pan, L.; et al. Strategic design of porous interfacial evaporators: A comprehensive review unveiling the significant role of pore engineering. Nano Energy 2024, 131, 110244. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, C.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Zhang, L.; Hu, W.; Wilkinson, D.P.; Zhang, J. Opportunities of Flexible and Portable Electrochemical Devices for Energy Storage: Expanding the Spotlight onto Semi-solid/Solid Electrolytes. Chem. Rev. 2022, 122, 17155–17239. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.B.; Chen, F. Conductive polymer based hydrogels and their application in wearable sensors: A review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Bhuyan, M.M.; Jeong, J.-H. Single/Multi-Network Conductive Hydrogels—A Review. Polymers 2024, 16, 2030. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, T.; Xie, X.; Song, G.; Ma, X.; Mu, Q.; Huang, Z.; Liu, X.; Sun, C.; Xu, W. Advances and challenges in conductive hydrogels: From properties to applications. Eur. Polym. J. 2022, 177, 111454. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Zhao, X.; Wang, Z. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: A review. Carbohydr. Polym. 2021, 267, 118179. [Google Scholar] [CrossRef]
- Hussain, S.; Maktedar, S.S. Structural, functional and mechanical performance of advanced Graphene-based composite hydrogels. Results Chem. 2023, 6, 101029. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Li, L.; Wang, Z.; Shu, Y.; Wang, J. Ionic liquid-based gels for biomedical applications. Chem. Eng. J. 2023, 452, 139248. [Google Scholar] [CrossRef]
- Hossain, K.R.; Jiang, P.; Yao, X.; Yang, X.; Hu, D.; Wang, X. Ionic liquids for 3D printing: Fabrication, properties, applications. J. Ion. Liq. 2023, 3, 100066. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Zaidi, S.J. Hydrogel Performance in Boosting Plant Resilience to Water Stress—A Review. Gels 2025, 11, 276. [Google Scholar] [CrossRef]
- Zhang, C.W.; Si, M.; Chen, C.; He, P.; Fei, Z.; Xu, N.; He, X. Hierarchical Engineering for Biopolymer-based Hydrogels with Tailored Property and Functionality. Adv. Mater. 2025, 37, 2414897. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W. Application of hydrogel for energy storage and conversion. Next Mater. 2023, 1, 100049. [Google Scholar] [CrossRef]
- Han, H.; Zhao, L.; Wu, X.; Zuo, B.; Bian, S.; Li, T.; Liu, X.; Jiang, Y.; Chen, C.; Bi, J. Advancements in thermoelectric materials: Optimization strategies for enhancing energy conversion. J. Mater. Chem. A 2024, 12, 24041–24083. [Google Scholar] [CrossRef]
- Li, M.; Hong, M.; Dargusch, M.; Zou, J.; Chen, Z.-G. High-efficiency thermocells driven by thermo-electrochemical processes. Trends Chem. 2021, 3, 561–574. [Google Scholar] [CrossRef]
- Leverick, G.; Shao-Horn, Y. Controlling electrolyte properties and redox reactions using solvation and implications in battery functions: A Mini-Review. Adv. Energy Mater. 2023, 13, 2204094. [Google Scholar] [CrossRef]
- Hong, S.-H.; Hsu, C.-C.; Liu, T.-H.; Lee, T.-C.; Tung, S.-H.; Chen, H.-L.; Yu, J.; Liu, C.-L. Extremely large Seebeck coefficient of gelatin methacryloyl (GelMA)-based thermogalvanic cells by the dual effect of ion-induced crystallization and nanochannel control. Mater. Today Energy 2024, 42, 101546. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, X. Ionic polymer–metal composites: From material engineering to flexible applications. Acc. Chem. Res. 2023, 57, 131–139. [Google Scholar] [CrossRef]
- Mazaheripour, A.; Majumdar, S.; Hanemann-Rawlings, D.; Thomas, E.M.; McGuiness, C.; d’Alencon, L.; Chabinyc, M.L.; Segalman, R.A. Tailoring the seebeck coefficient of PEDOT: PSS by controlling ion stoichiometry in ionic liquid additives. Chem. Mater. 2018, 30, 4816–4822. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.; Shao, J.; Wang, W.; Si, W.; Huang, W.; Dong, X. Stretchable, transparent, and self-patterned hydrogel-based pressure sensor for human motions detection. Adv. Funct. Mater. 2018, 28, 1802576. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Li, H.; Wu, J.; Wan, Q.; Chen, T.; Liu, W.; Peng, H.; Zhang, H.; Luo, Y. Smart hydrogel sensors for health monitoring and early warning. Adv. Sens. Res. 2024, 3, 2400003. [Google Scholar] [CrossRef]
- Hill, J.; Szewczyk, R.; Woo, A.; Hollar, S.; Culler, D.; Pister, K. System architecture directions for networked sensors. ACM Sigplan Not. 2000, 35, 93–104. [Google Scholar] [CrossRef]
- Jacob, A.P.; Xie, R.; Sung, M.G.; Liebmann, L.; Lee, R.T.P.; Taylor, B. Scaling challenges for advanced CMOS devices. Int. J. High Speed Electron. Syst. 2017, 26, 1740001. [Google Scholar] [CrossRef]
- Kim, S.; Yoo, H. Active-matrix array based on thin-film transistors using emerging materials for application: From lab to industry. Electronics 2024, 13, 241. [Google Scholar] [CrossRef]
- Wu, X.; Mao, S.; Chen, J.; Huang, J. Strategies for improving the performance of sensors based on organic field-effect transistors. Adv. Mater. 2018, 30, 1705642. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Kim, D.C.; Shim, H.J.; Kim, T.H.; Kim, D.H. Flexible and stretchable smart display: Materials, fabrication, device design, and system integration. Adv. Funct. Mater. 2018, 28, 1801834. [Google Scholar] [CrossRef]
- Milgrew, M.J.; Riehle, M.O.; Cumming, D.R.S. A large transistor-based sensor array chip for direct extracellular imaging. Sens. Actuators B Chem. 2005, 111, 347–353. [Google Scholar] [CrossRef]
- Cui, S.; Xiao, J.-J.; Goldsmith, A.J.; Luo, Z.-Q.; Poor, H.V. Estimation diversity and energy efficiency in distributed sensing. IEEE Trans. Signal Process. 2007, 55, 4683–4695. [Google Scholar]
- Dai, Y.; Hu, H.; Wang, M.; Xu, J.; Wang, S. Stretchable transistors and functional circuits for human-integrated electronics. Nat. Electron. 2021, 4, 17–29. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Recent progress on stretchable electronic devices with intrinsically stretchable components. Advanced Materials 2017, 29, 1603167. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Z.; Zhao, Y.; Liu, Y.; Wang, S. Stretchable conductors for stretchable field-effect transistors and functional circuits. Chem. Soc. Rev. 2023, 52, 795–835. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.; Elias, A.L.; Chung, H.J. Flexible electronics under strain: A review of mechanical characterization and durability enhancement strategies. J. Mater. Sci. 2016, 51, 2771–2805. [Google Scholar] [CrossRef]
- Chong, Y.-W.; Ismail, W.; Ko, K.; Lee, C.-Y. Energy harvesting for wearable devices: A review. IEEE Sens. J. 2019, 19, 9047–9062. [Google Scholar] [CrossRef]
- Portilla, L.; Loganathan, K.; Faber, H.; Eid, A.; Hester, J.G.D.; Tentzeris, M.M.; Fattori, M.; Cantatore, E.; Jiang, C.; Nathan, A. Wirelessly powered large-area electronics for the Internet of Things. Nat. Electron. 2023, 6, 10–17. [Google Scholar] [CrossRef]
- Lee, S.; Nathan, A. Subthreshold Schottky-barrier thin-film transistors with ultralow power and high intrinsic gain. Science 2016, 354, 302–304. [Google Scholar] [CrossRef]
- Liang, Y.; Yamada, T.; Zhou, H.; Kimizuka, N. Hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin–I5−complex in aqueous I−/I3−thermocells and enhancement in the Seebeck coefficient. Chem. Sci. 2019, 10, 773–780. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Wu, J. Conductive hydrogel-and organohydrogel-based stretchable sensors. ACS Appl. Mater. Interfaces 2021, 13, 2128–2144. [Google Scholar] [CrossRef]
- Moshwan, R.; Shi, X.-L.; Liu, W.-D.; Liu, J.; Chen, Z.-G. Entropy engineering: An innovative strategy for designing high-performance thermoelectric materials and devices. Nano Today 2024, 58, 102475. [Google Scholar] [CrossRef]
- Mouselly, M.; Alawadhi, H.; Senthilkumar, S.T. Current status of ferro-/ferricyanide for redox flow batteries. Curr. Opin. Electrochem. 2024, 48, 101581. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Z.; Luo, J.; Li, W.; Pei, Y. Gradient Doping Enables an Extraordinary Efficiency in Thermoelectric PbTe1−xIx. Adv. Mater. 2024, 36, 2405299. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Hu, R.; Xu, D. High-thermopower polarized electrolytes enabled by methylcellulose for low-grade heat harvesting. Sci. Adv. 2022, 8, eabl5318. [Google Scholar] [CrossRef]
- Zhou, H.; Yamada, T.; Kimizuka, N. Supramolecular Thermo-Electrochemical Cells: Enhanced Thermoelectric Performance by Host–Guest Complexation and Salt-Induced Crystallization. J. Am. Chem. Soc. 2016, 138, 10502–10507. [Google Scholar] [CrossRef]
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z.L.; et al. Thermosensitive crystallization–boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.J.; Tachikawa, N.; MacFarlane, D.R.; Pringle, J.M. Investigation of the kinetic and mass transport limitations in thermoelectrochemical cells with different electrode materials. Phys. Chem. Chem. Phys. 2014, 16, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Artyukhov, D.; Kiselev, N.; Gorshkov, N.; Kovyneva, N.; Ganzha, O.; Vikulova, M.; Gorokhovsky, A.; Offor, P.; Boychenko, E.; Burmistrov, I. Harvesting Waste Thermal Energy Using a Surface-Modified Carbon Fiber-Based Thermo-Electrochemical Cell. Sustainability 2021, 13, 1377. [Google Scholar] [CrossRef]
- Iyer, V.A.; Schuh, J.K.; Montoto, E.C.; Nemani, V.P.; Qian, S.; Nagarjuna, G.; Rodríguez-López, J.; Ewoldt, R.H.; Smith, K.C. Assessing the impact of electrolyte conductivity and viscosity on the reactor cost and pressure drop of redox-active polymer flow batteries. J. Power Sources 2017, 361, 334–344. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Sun, L.; Zhuo, K.; Chen, Y.; Du, Q.; Zhang, S.; Wang, J. Ionic liquid-based redox active electrolytes for supercapacitors. Adv. Funct. Mater. 2022, 32, 2203611. [Google Scholar] [CrossRef]
- Laux, E.; Uhl, S.; Journot, T.; Brossard, J.; Jeandupeux, L.; Keppner, H. Aspects of Protonic Ionic Liquid as Electrolyte in Thermoelectric Generators. J. Electron. Mater. 2016, 45, 3383–3389. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Chen, J. Heterogeneous electron transfer at nanoscopic electrodes: Importance of electronic structures and electric double layers. Chem. Soc. Rev. 2014, 43, 5372–5386. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Quilty, C.D.; Wu, D.; Li, W.; Bock, D.C.; Wang, L.; Housel, L.M.; Abraham, A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.M.; Chou, S.L.; Chen, J. Recent developments on and prospects for electrode materials with hierarchical structures for lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1701415. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Zhang, S.; Wang, J.; Huang, Q.; Yu, N.; Zhu, Y.; Fu, L.; Wang, F.; Chen, Y. Three-dimensional ordered porous electrode materials for electrochemical energy storage. NPG Asia Mater. 2019, 11, 12. [Google Scholar] [CrossRef]
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; Yang, H.D.; Kihm, K.D.; Baughman, R.H.; Lee, H.H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.; Wu, D.; Chen, B.; Du, C.; Liang, L.; Huang, Y.; Li, Z.; Rao, F.; Chen, G.; et al. Constructing Flexible Film Electrode with Porous Layered Structure by MXene/SWCNTs/PANI Ternary Composite for Efficient Low-Grade Thermal Energy Harvest. Adv. Funct. Mater. 2023, 33, 2209806. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially wearable thermo-electrochemical cells for body heat harvesting: From mechanism, materials, strategies to applications. Adv. Sci. 2021, 8, 2100669. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zhao, S.; Li, D.; Tang, X.; Zhang, H.; Huang, J.; Guo, Z.; Liu, W. Mechanical Regulation of Polymer Gels. Chem. Rev. 2024, 124, 10435–10508. [Google Scholar] [CrossRef]
- Li, J.; Du, X.; Zhang, A.; Wen, J.; Shuai, L.; Li, S.; Zhu, M.; Nie, Y. Hydrogen-bonded polymeric materials with high mechanical properties and high self-healing capacity. Mater. Chem. Front. 2024, 8, 3828–3858. [Google Scholar] [CrossRef]
- Huo, H.; Xuan, Y.; Meng, T. Enhancing thermoelectric conversion efficiency of hydrogel-based supercapacitors by the three-dimensional ion channels hydration. J. Energy Storage 2024, 80, 110437. [Google Scholar] [CrossRef]
- Ren, G.; Yang, W.; Bao, J.; Shi, Y.; Sun, L.; Mo, Z.; Du, M. Conductive Polymer/Multidimensional Carbon Composite on Graphite Felt Electrodes for Liquid Thermo-Electrochemical Cells. ACS Sustain. Chem. Eng. 2025, 13, 6021–6030. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Li, J.; Wu, R.; Jin, M.; Zhao, H.; Chen, S.; Wang, H. Advanced Bacterial Cellulose Ionic Conductors with Gigantic Thermopower for Low-Grade Heat Harvesting. Nano Lett. 2022, 22, 8152–8160. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Z.; Li, M.; Yin, Z.; Butt, H.A. The synthesis, mechanisms, and additives for bio-compatible polyvinyl alcohol hydrogels: A review on current advances, trends, and future outlook. J. Vinyl Addit. Technol. 2023, 29, 939–959. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gao, J.; Xiang, Y.; Yang, J. Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers 2023, 15, 3782. [Google Scholar] [CrossRef]
- Georges, M.M.; Wang, K.; Xu, J.; Christelle, M.M.; Dominique, W.O.; Moussa, C. Investigation on the development of Novel PAM structure as high-performance clay inhibitor in HT/HP conditions by using functional groups. Chem. Phys. 2025, 590, 112517. [Google Scholar] [CrossRef]
- Sujan, M.I.; Sarkar, S.D.; Sultana, S.; Bushra, L.; Tareq, R.; Roy, C.K.; Azam, M.S. Bi-functional silica nanoparticles for simultaneous enhancement of mechanical strength and swelling capacity of hydrogels. RSC Adv. 2020, 10, 6213–6222. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Wu, B.; Jia, W.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. High-Performance Ionic Thermoelectric Supercapacitor for Integrated Energy Conversion-Storage. Energy Environ. Mater. 2022, 5, 954–961. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Akib, A.A.; Sarkar, S.D.; Khan, M.A.R.; Uddin, M.M.; Nasrin, D.; Roy, C.K. Polysaccharide-Based Hydrogels for Advanced Biomedical Engineering Applications. ACS Polym. Au 2024, 4, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, B.; Wang, Z.; Sun, X.; Liu, Y.; Sun, H.; Li, J.; Chen, L.; Zhu, X.; Huang, L.; et al. Rarely negative-thermovoltage cellulose ionogel with simultaneously boosted mechanical strength and ionic conductivity via ion-molecular engineering. J. Mater. Chem. A 2023, 11, 2145–2154. [Google Scholar] [CrossRef]
- Han, C.-G.; Qian, X.; Li, Q.; Deng, B.; Zhu, Y.; Han, Z.; Zhang, W.; Wang, W.; Feng, S.-P.; Chen, G.; et al. Giant thermopower of ionic gelatin near room temperature. Science 2020, 368, 1091–1098. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, L.; Cheng, H.; Chen, J.; Ouyang, J. Great enhancement in the ionic thermopower of ionogels by cationic doping. Chem. Eng. J. 2023, 477, 147257. [Google Scholar] [CrossRef]
- Fang, Y.; Cheng, H.; He, H.; Wang, S.; Li, J.; Yue, S.; Zhang, L.; Du, Z.; Ouyang, J. Stretchable and Transparent Ionogels with High Thermoelectric Properties. Adv. Funct. Mater. 2020, 30, 2004699. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, H.; Li, Y.; Rao, J.; Yue, S.; Le, Q.; Qian, Q.; Liu, Z.; Ouyang, J. Quasi-solid conductive gels with high thermoelectric properties and high mechanical stretchability consisting of a low cost and green deep eutectic solvent. J. Mater. Chem. A 2022, 10, 4222–4229. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Huang, T.; Bojórquez, D.I.S.; Tonmoy, T.H.; Pané, S.; Makarov, D.; Baraban, L. Design and Development of Transient Sensing Devices for Healthcare Applications. Adv. Sci. 2024, 11, 2307232. [Google Scholar] [CrossRef]
- Ramesh, S. Stimuli-Responsive Nano-and Micro-Composites for Smart Fabrics and Therapeutics Delivery; North Carolina State University: Raleigh, NC, USA, 2022. [Google Scholar]
- Meng, X.; Guo, Y.; Wang, Y.; Fan, S.; Wang, K.; Han, W. A systematic review of photolysis and hydrolysis degradation modes, degradation mechanisms, and identification methods of pesticides. J. Chem. 2022, 2022, 9552466. [Google Scholar] [CrossRef]
- Bartzoka, E.D.; Crestini, C.; Lange, H. Biomass derived and biomass inspired polymers in pharmaceutical applications. In Handbook of Polymers for Pharmaceutical Technologies: Biodegradable Polymers; Thakur, V.K., Thakur, M.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 3, pp. 127–203. [Google Scholar]
- Das, N. Biodegradable Hydrogels for Controlled Drug Delivery. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1433–1472. [Google Scholar]

| Polymer Gel Materials | Particulars | Thermopower (S) [mVK−1] | Electrical Conductivity (σ) [Sm−1] | Thermo Electric Power Factor (S2σ) [mWm−1 K−2] | Ref. |
|---|---|---|---|---|---|
| Cellulose-Benzyltrimethyl ammonium hydroxide | Controllable Seebeck coefficient | 2.61 | 3.8 | 0.42 | [58] |
| PVA-NaOH | Hydration connections and synergistic coordination to produce huge negative thermopower | −37.61 | 7.36 × 10−3 | - | [59] |
| Poly(3,4-ethylenedioxythiophene), Ionic poly(2-acrylamido-2-methyl-1-propanesulfonic acid) | Self-healing, wearable, and transparent thermoelectric | −25.1 | 15.9 | 9.94 | [60] |
| PVA-HCl | Achieved huge value of H+ transport | 38.20 | 1.887 | - | [61] |
| Polyacrylamide/poly(vinyl alcohol)/cellulose nanofiber | Outstanding performance of wearable electronics | 1.69 | 1.68 | 4.79 × 10−2 | [62] |
| Polyacrylamide/[Fe(CN)63−/Fe(CN)64−] | Stretchable thermoelectric hydrogel | 4.5 | 9.1 | 2.22 | [63] |
| PVA/[Fe(CN)63−/Fe(CN)64−] | Stretchable, high-strength, and quasi-solid thermocell | 6.5 | 2.6 | 15.6 × 10−2 | [64] |
| Tellurium-nanowire-doped poly(3,4-ethylenedioxythiophene (PEDOT): polystyrenesulfonate (PSS)/PVA | Outstanding Seebeck coefficient and high stretchability | 78.7 × 10−2 | 1.5 | 6.81 × 10−4 | [65] |
| Poly(acrylic acid)/LiCl | Outstanding capacity for self-regeneration, freezing resistance, including elevated thermoelectric characteristics | 11.3 | 5.98 | - | [66] |
| Gelatin/[Fe(CN)63−/Fe(CN)64−]/I−/I3− | Double sandwich structure created by combining two asymmetric gels for excellent thermoelectric performance | 5.2 | 45.0 × 10−2 | 5.2 | [67] |
| Polyethylene oxide/lithium bis(trifluoromethanesulfonyl)imide/ 1-Ethyl-3-methyl imidazolium chloride | Robust, self-healing ionogel with adjustable thermoelectric characteristics | 13 | 0.3 | 9.7 × 10−2 | [68] |
| Gelatin/polyacrylamide | Adhesion triggered by skin temperature, and detachment initiated by low temperature | 10.4 | 8.3 | 0.4 | [69] |
| Poly(acrylic acid-co-Nisopropylacrylamide) nanoparticles | Good Seebeck coefficient and extremely effective thermoelectric conversion | −9.5 | 2 | 4.8 × 10−4 | [70] |
| Poly(acrylic acid/Xanthan gum/Bi2Se0.3Te2.7 | Excellent self-healing and stretchy performance | −0.45 | 5 | - | [71] |
| Polyacrylamide/polydopamine/carboxylated carbon nanotubes/polyaniline | Advanced wearable technology that can capture waste heat | 18.6 | 17.53 | 6.06 | [72] |
| PVA/Sodium alginate/polyethylene glycol/ | Superb stretchy gel with a huge ionic Seebeck value | 66.7 | - | 13.96 | [73] |
| Poly(methyl methacrylate-co-methyl acrylate)/Mxene | Stable in the environment and mechanically adaptable | −8.8 | - | 2.5 × 10−2 | [74] |
| Poly(vinylidene fluoride-cohexafluoropropylene) | Crucial to harness the Earth’s vast supply of low-grade thermal energy | 26.1 | - | ≈0.46 | [75] |
| Poly (vinylidene fluoride-co-hexafluoropropylene)/1-ethyl-3-methylimidazolium dicyanamide | Outstanding mechanical quality | 22.9 | - | 87.026 × 10−2 | [76] |
| Metal organic framework/ poly(3,4-ethylenedioxythiophene)s with poly(styrene sulfonate) | Sustainable in terms of the environment | 16.2 | 0.03 | 7.6 | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumon, M.M.H.; Rahman Khan, M.M.; Amin, M.K. Design, Synthesis, and Morphological Behavior of Polymer Gel-Based Materials for Thermoelectric Devices: Recent Progress and Perspectives. Gels 2025, 11, 508. https://doi.org/10.3390/gels11070508
Rumon MMH, Rahman Khan MM, Amin MK. Design, Synthesis, and Morphological Behavior of Polymer Gel-Based Materials for Thermoelectric Devices: Recent Progress and Perspectives. Gels. 2025; 11(7):508. https://doi.org/10.3390/gels11070508
Chicago/Turabian StyleRumon, Md. Mahamudul Hasan, Mohammad Mizanur Rahman Khan, and Md Khairul Amin. 2025. "Design, Synthesis, and Morphological Behavior of Polymer Gel-Based Materials for Thermoelectric Devices: Recent Progress and Perspectives" Gels 11, no. 7: 508. https://doi.org/10.3390/gels11070508
APA StyleRumon, M. M. H., Rahman Khan, M. M., & Amin, M. K. (2025). Design, Synthesis, and Morphological Behavior of Polymer Gel-Based Materials for Thermoelectric Devices: Recent Progress and Perspectives. Gels, 11(7), 508. https://doi.org/10.3390/gels11070508









