Morphologic Pattern Differences in Reconstructive Tissue Repair of Bone Defects Mediated by Bioactive Ceramics and Hydrogels: A Microscopic Follow-Up Evaluation of Re-Ossification
Abstract
1. Introduction
2. Results and Discussion
2.1. Results
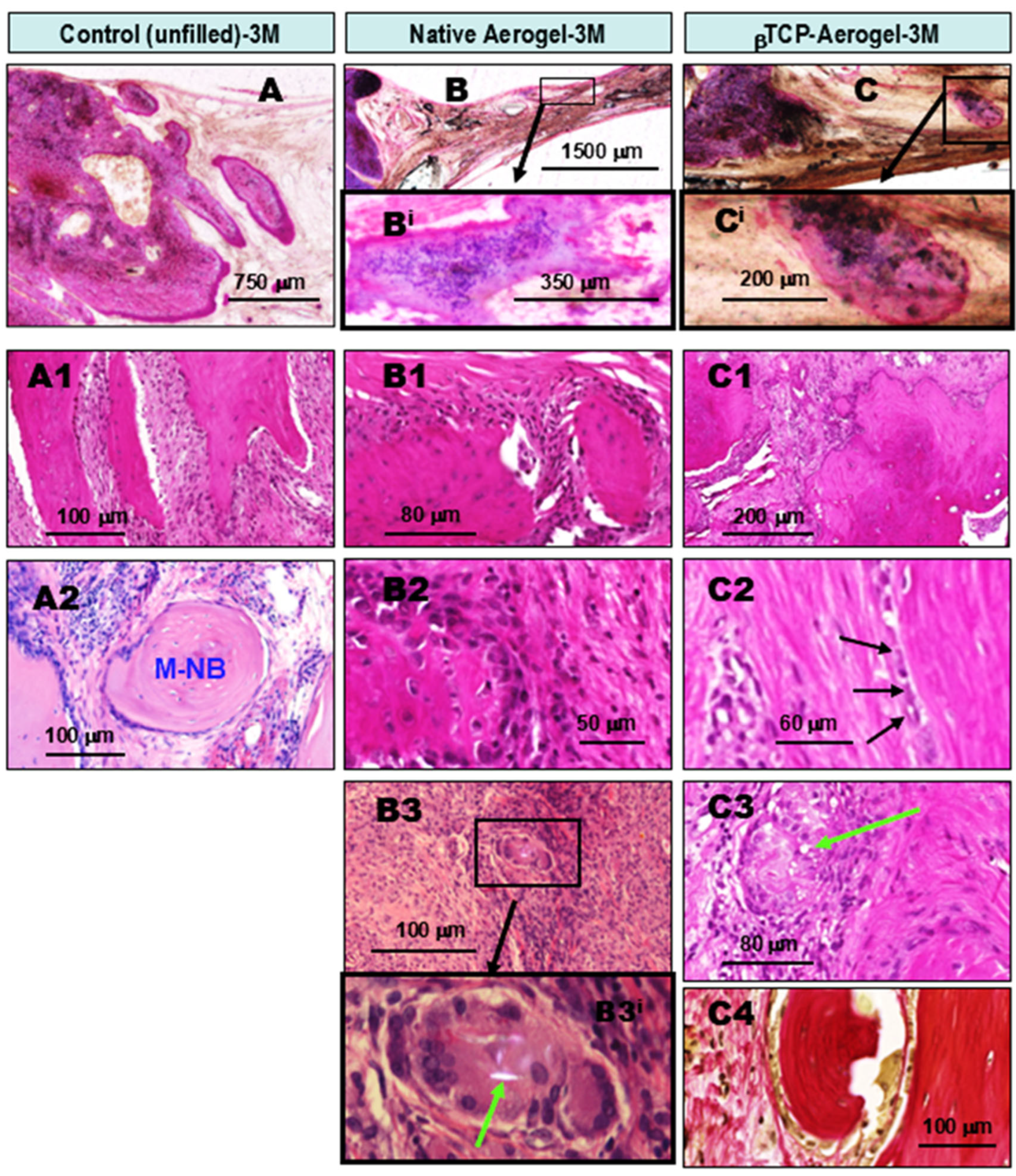
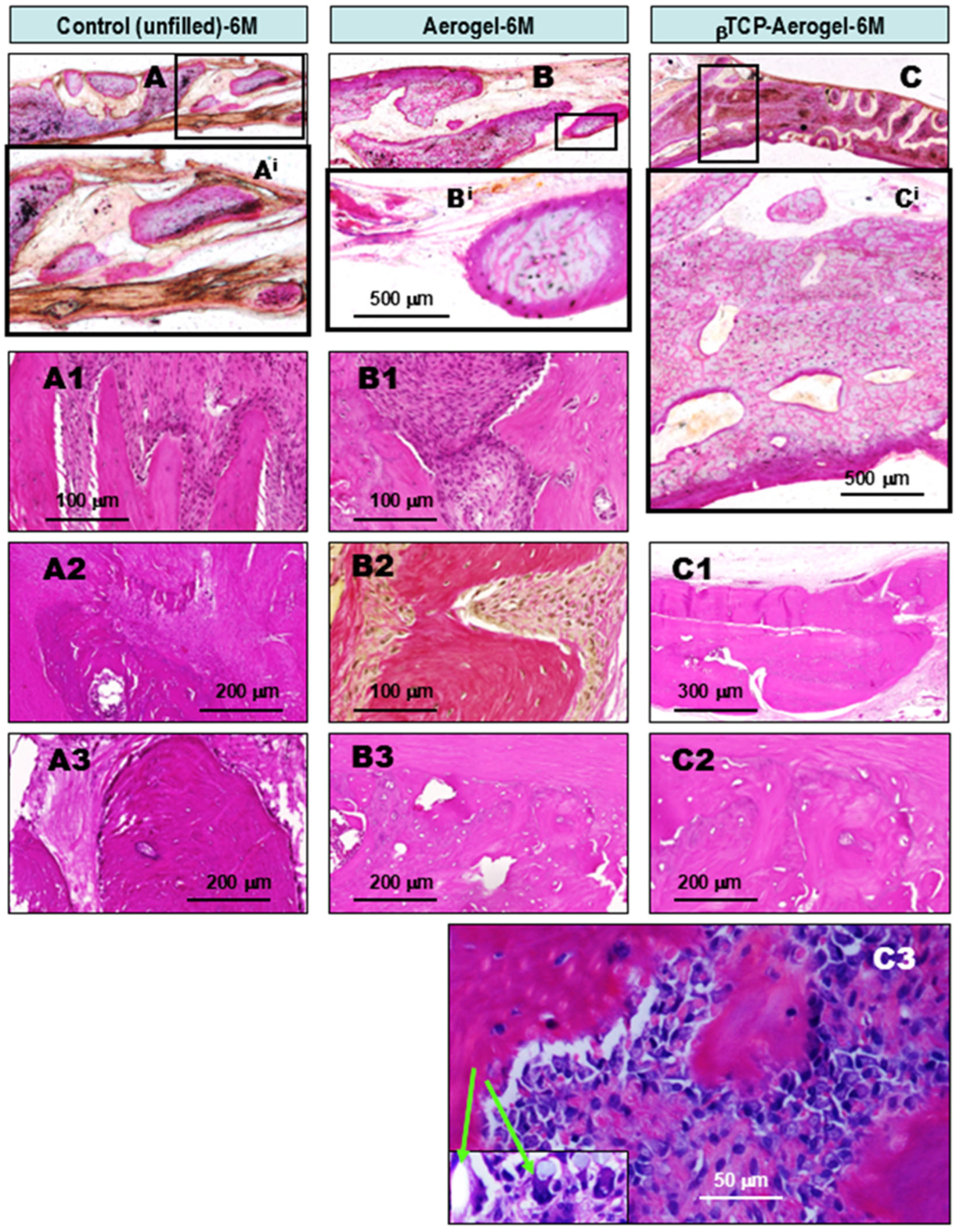
2.1.1. Comparative Chronological Histopathology of AE and βTCP-AE Scaffolds at 1, 3, and 6 Months Following Implant Treatments of Calvaria Bone Defects in Rats
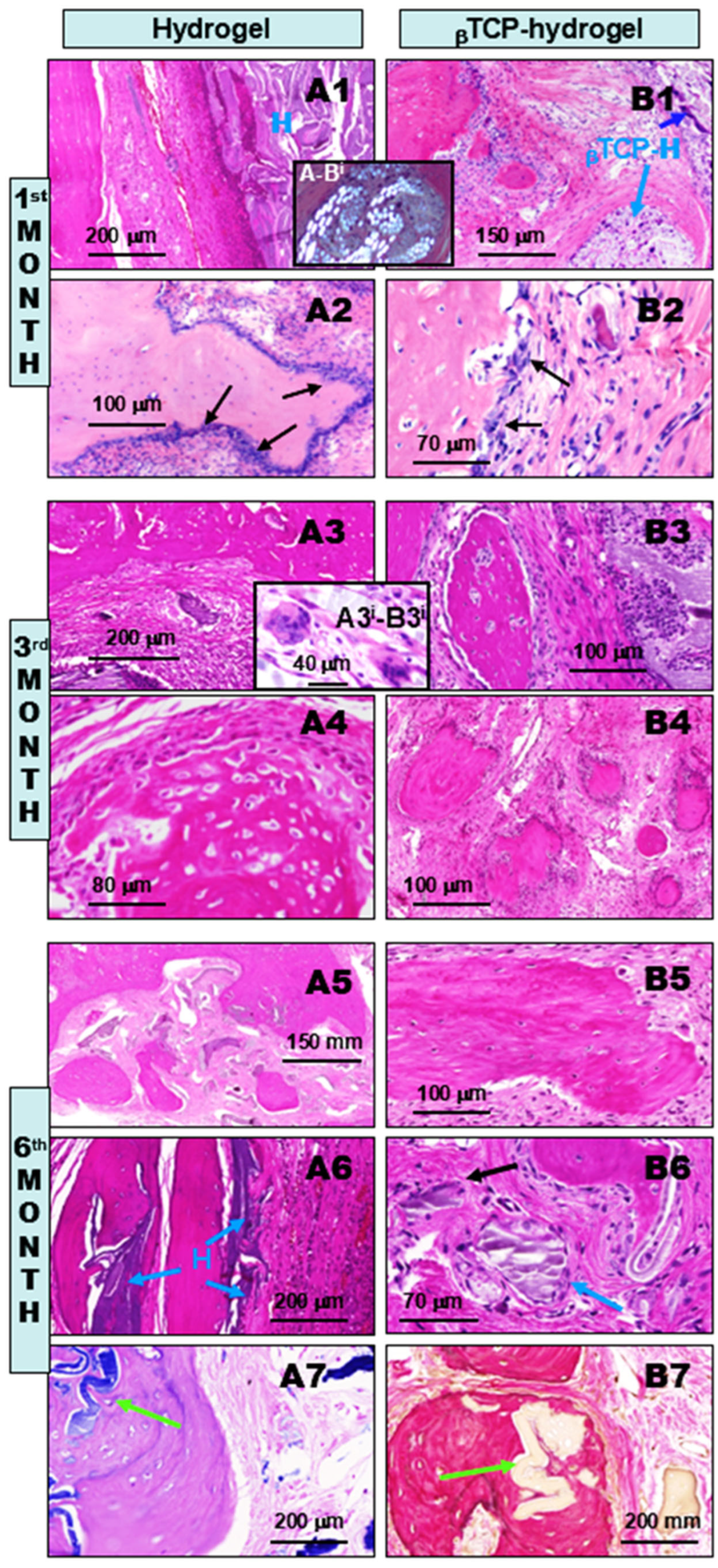
2.1.2. Comparative Chronological Histopathology of H and βTCP-H Scaffolds at 1, 3, and 6 Months Following Implant Treatments of Calvaria Bone Defects in Rats
2.1.3. Morphometric Assessment of Time-Dependent Re-Ossification in Rat Calvaria Bone Defect
2.2. Discussion
3. Conclusions
- (1)
- Compared to the untreated control cases, all of the above bone substituents demonstrated well-tolerated healing processes with significant stimulated osseous restoration. This was preceded by control-comparable injury-associated common transient non-specific inflammation, followed by capillary-rich granulation tissue formation.
- (2)
- Without treatment, i.e., no bone defect substitution (control), the re-ossification exclusively started along the lateral edges of the bony defect. This regenerative osteogenesis, driven by osteoblasts, could be detected as early as the 1st month after the operation. While lateral ossification continued throughout the observational time, although with gradually decreasing intensity along with increasing scarring, by the 6th month, only moderate amounts of intralesional new bone islets had formed within the fibrous tissues, independently of the lateral ossification.
- (3)
- In contrast, all of the applied bioactive bone substituents, including AE, H, and their βTCP-containing counterparts, used to fill the bone defects not only induced robust lateral re-ossification (arising from the remaining circular bone edges of the defect), but early and rapid intralesional multifocal ossification as well. This resulted in well-suited substantial osseous bone remodeling for restoration by the end of the observation period. The facilitated osteo-induction is directly related to the presence of AE and H crystal particles in the tissue as foreign body components. These particles characteristically induce low-grade chronic protracted granulomatous inflammation to recruit T cells, alternatively activated macrophages, and multinucleated histiocytes (“foreign body giant cells”) for elimination of the “invaders”. According to our findings, this process did not appear to be complete by the 6th month of follow-up. Nevertheless, it is noteworthy that such inflammation may have some beneficial effects in certain conditions. In a bony environment, activated inflammatory cells activate fibroblasts and likely stems cells capable of osteogenic differentiation within the injured bony lesion, facilitating ossification and subsequently, bone reconstruction.
- (4)
- Among the applied scaffolds, the βTCP-containing AE and H composite materials proved to be the most powerful in terms of their osteo-inductive capacities for bone repair. However, the methacrylate component of the hydrogel, irrespective of βTCP content, showed the slowest rate of tissue clearance from the healing bone defect, potentially sustaining the persistent low-grade chronic inflammation.
4. Materials and Methods
4.1. Preparation of Scaffolds
4.1.1. Preparation of Mesoporous Silica Aerogel and βTCP-Aerogel
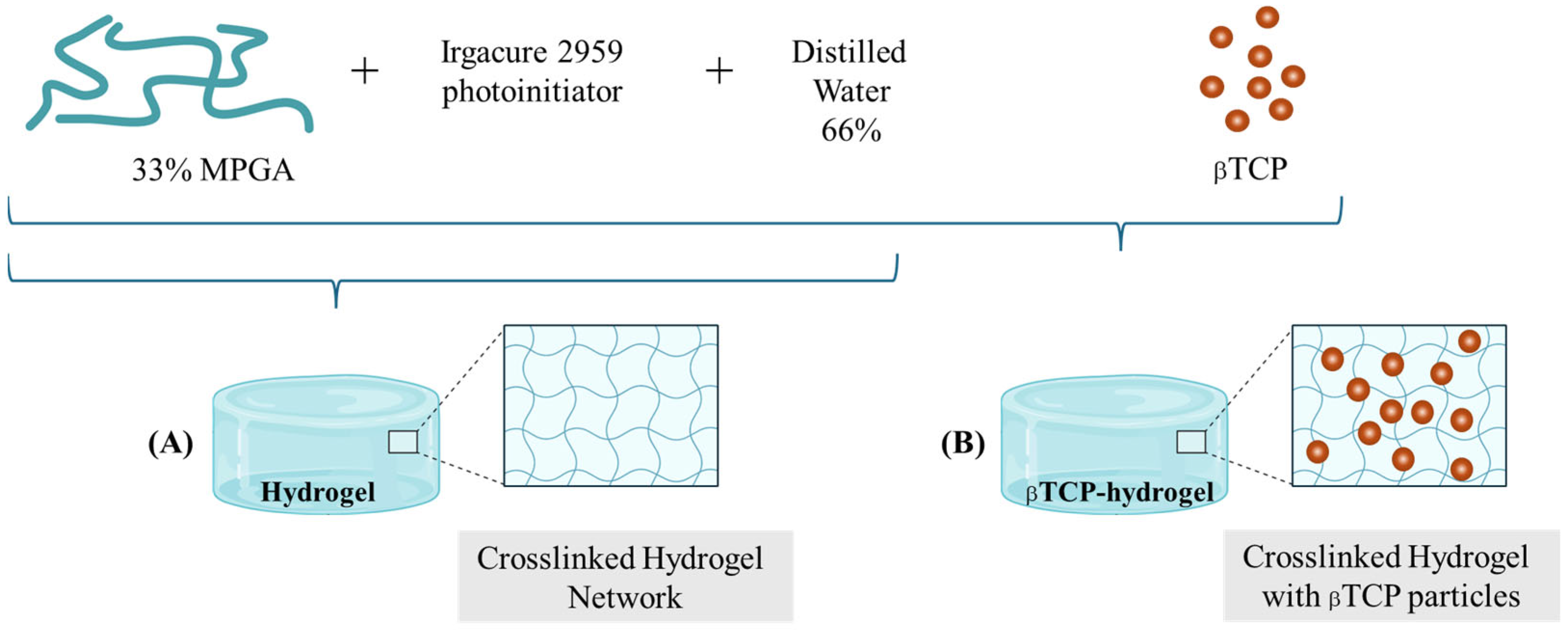
4.1.2. Preparation of Methacrylated Polyglutamic Acid-Based Hydrogel and βTCP-Hydrogel
4.2. Animals
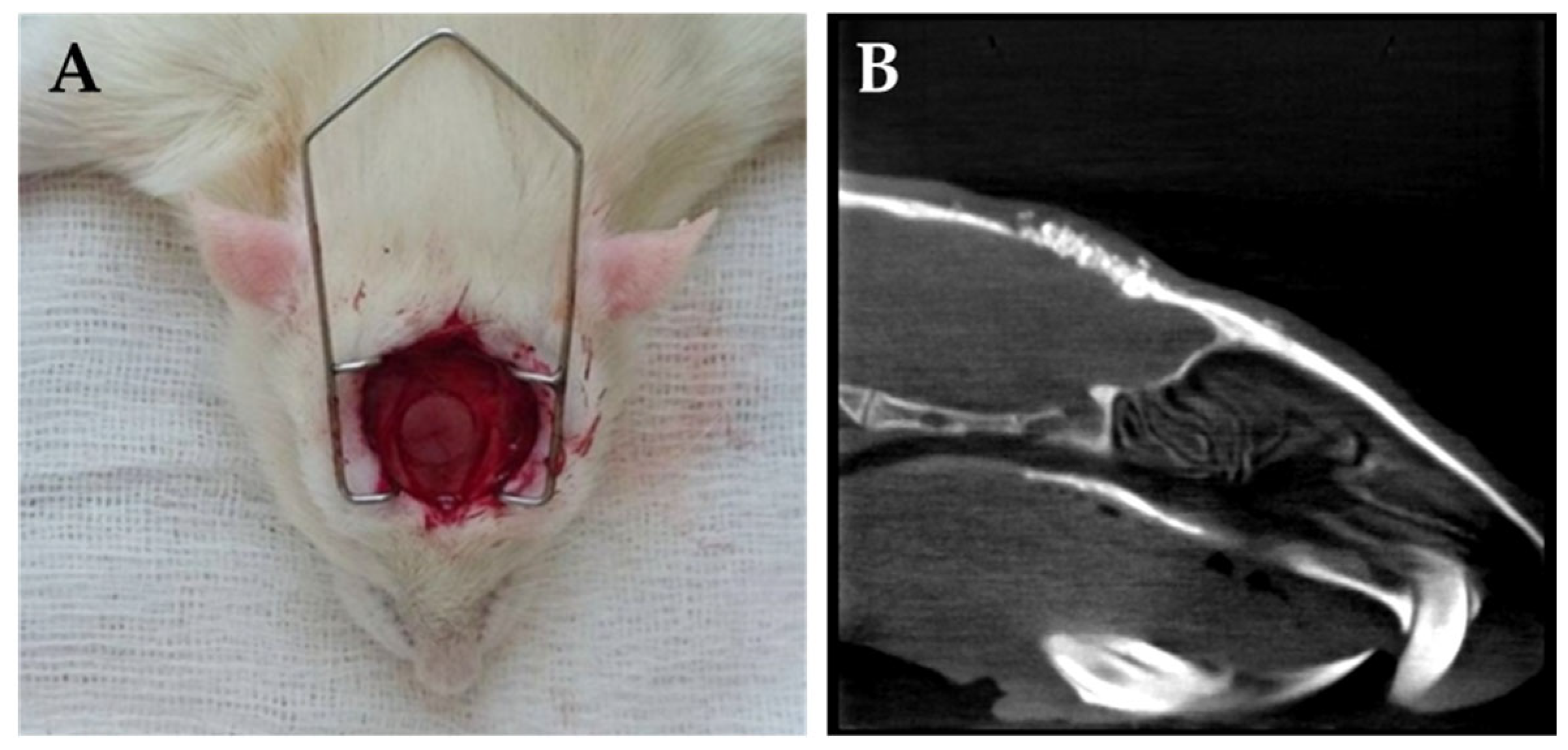
4.3. Surgical Procedures
4.4. Tissue Processing for Microscopic Analyses
4.5. Ground Section Preparation of Non-Decalcified Bone Defects
4.6. Immunohistochemistry (IHC)
4.7. Morphometric Assessment of Re-Ossification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | aerogel |
| βTCP | β-tricalcium-phosphate |
| βTCP-AE | β-tricalcium-phosphate-containing aerogel |
| βTCP-H | β-tricalcium-phosphate-containing hydrogel |
| BM | bone marrow |
| CB | compact bone |
| C-R | Congo Red |
| G | Giemsa |
| H | hydrogel |
| HE | hematoxylin–eosin |
| MPGA | methacrylated poly-γ-glutamic acid |
| MTri | Masson trichrom |
| NB | new bone |
| vG | picrosirius-based van Gieson |
| vK | von Kossa |
References
- Jin, W.; Chu, P.K. Osseointegration. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview (Chapter 7). In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 85–98. [Google Scholar]
- Khan, W.S.; Rayan, F.; Dhinsa, B.S.; Marsh, D. An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopedic surgery: How far are we? Stem Cells Int. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Pérez-Moreno, A.; Piñero, M.; Fernández-Montesinos, R.; Pinaglia-Tobaruela, G.; Reyes-Peces, M.V.; Mesa-Díaz, M.d.M.; Vilches-Pérez, J.I.; Esquivias, L.; de la Rosa-Fox, N.; Salido, M. Chitosan-Silica Hybrid Biomaterials for Bone Tissue Engineering: A Comparative Study of Xerogels and Aerogels. Gels 2023, 9, 383–402. [Google Scholar] [CrossRef]
- Lázár, I.; Čelko, L.; Menelaou, M. Aerogel-Based Materials in Bone and Cartilage Tissue Engineering—A Review with Future Implications. Gels 2023, 9, 746–785. [Google Scholar] [CrossRef] [PubMed]
- Boda, R.; Lázár, I.; Keczánné-Üveges, A.; Bakó, J.; Tóth, F.; Trencsényi, G.; Kálmán-Szabó, I.; Béresová, M.; Sajtos, Z.; Tóth, E.D.; et al. β-Tricalcium Phosphate-Modified Aerogel Containing PVA/Chitosan Hybrid Nanospun Scaffolds for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 7562–7584. [Google Scholar] [CrossRef]
- Hegedűs, C.; Czibulya, Z.; Tóth, F.; Dezső, B.; Hegedűs, V.; Boda, R.; Horváth, D.; Csík, A.; Fábián, I.; Tóth-Győri, E.; et al. The Effect of Heat Treatment of β-Tricalcium Phosphate-Containing Silica-Based Bioactive Aerogels on the Cellular Metabolism and Proliferation of MG63 Cells. Biomedicines 2022, 10, 662–978. [Google Scholar] [CrossRef]
- Huang, G.J.; Yu, H.P.; Wang, X.L.; Ning, B.B.; Gao, J.; Shi, Y.Q.; Zhu, Y.J.; Duan, J.L. Highly porous and elastic aerogel based on ultralong hydroxyapatite nanowires for high-performance bone regeneration and neovascularization. J. Mater. Chem. B 2021, 9, 1277–1287. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, C.; Wang, X.; Yuan, Z.; Sun, B.; El-Newehy, M.; Abdulhameed, M.M.; Fang, B.; Mo, X. Aspirin-Loaded Anti-Inflammatory ZnO-SiO2 Aerogel Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 17092–17108. [Google Scholar] [CrossRef]
- Weng, L.; Boda, S.K.; Wang, H.; Teusink, M.J.; Shuler, F.D.; Xie, J. Novel 3D Hybrid Nanofiber Aerogels Coupled with BMP-2 Peptides for Cranial Bone Regeneration. Adv. Healthc. Mater. 2018, 7, 1701415–1701430. [Google Scholar] [CrossRef]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue. Eng. 2017, 8, 2041731417712073. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Xu, C.; Kang, M.; Lee, C.S.; Aghaloo, T.; Lee, M. Self-Assembled Nanocomposite Hydrogels as Carriers for Demineralized Bone Matrix Particles and Enhanced Bone Repair. Adv. Healthc. Mater. 2024, 13, 2303592–2303603. [Google Scholar] [CrossRef]
- Bakó, J.; Vecsernyés, M.; Ujhelyi, Z.; Kovácsné, I.B.; Borbíró, I.; Bíró, T.; Borbély, J.; Hegedűs, C. Composition and characterization of in situ usable light cured dental drug delivery hydrogel system. J. Mater. Sci. Mater. Med. 2013, 24, 659–666. [Google Scholar] [CrossRef]
- Győri, E.; Fábián, I.; Lázár, I. Effect of the Chemical Composition of Simulated Body Fluids on Aerogel-Based Bioactive Composites. J. Compos. Sci. 2017, 1, 15–26. [Google Scholar] [CrossRef]
- Hegedüs, V.; Kerényi, F.; Boda, R.; Horváth, D.; Lázár, I.; Tóth-Győri, E.; Dezső, B.; Hegedüs, C. β-Tricalcium phosphate silica aerogel as an alternative bioactive ceramic for the potential use in dentistry. Adv. Appl. Ceram. 2018, 117, 476–484. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; de Groot, K. Osteoinductive biomaterials--Properties and relevance in bone repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Duan, W.; Zhu, B.; Chen, Y.; Zhu, Y.; Martin-Saldaña, S.; Xiao, Z.; Liu, X.; Feng, L.; Ren, Y.; et al. Nanozyme-Engineered Hyaluronic Acid Adhesives Loading Platelet-Rich Plasma for Multilayered Osteoarthritis Treatment with Pain-Relief Effect. Adv. Funct. Mater. 2025, 35, 2418660. [Google Scholar] [CrossRef]
- Luo, L.; Gong, Y.; Yan, L.; Bu, Y. Sponge as Scaffolds in Bone and Cartilage Tissue Engineering. Chem. Res. Chin. Univ. 2024, 40, 786–797. [Google Scholar] [CrossRef]
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782–2815. [Google Scholar] [CrossRef]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary concepts in osteointegration of dental Implants. BioMed Res. Int. 2022, 1, 6170452. [Google Scholar] [CrossRef]
- Lázár, I.; Bereczki, F.H.; Manó, S.; Daróczi, L.; Deák, G.; Fábián, I.; Csernátony, Z. Synthesis and study of new functionalized silica aerogel poly(methyl methacrylate) composites for biomedical use. Polym. Compos. 2015, 36, 348–358. [Google Scholar] [CrossRef]
- Lázár, I.; Fábián, I. A continuous extraction and pumpless supercritical CO2 drying system for laboratory-scale aerogel production. Gels 2017, 2, 26–40. [Google Scholar] [CrossRef]
- Lazar, I.; Fabian, I. Process for the Preparation of Composite Silica Alcogels, Aerogels and Xerogels, Apparatus for the Continuous Implementation of the Process, and New Composite Silica Alcogels, Aerogels and Xerogels. WO2013061104, 2 May 2011. [Google Scholar]
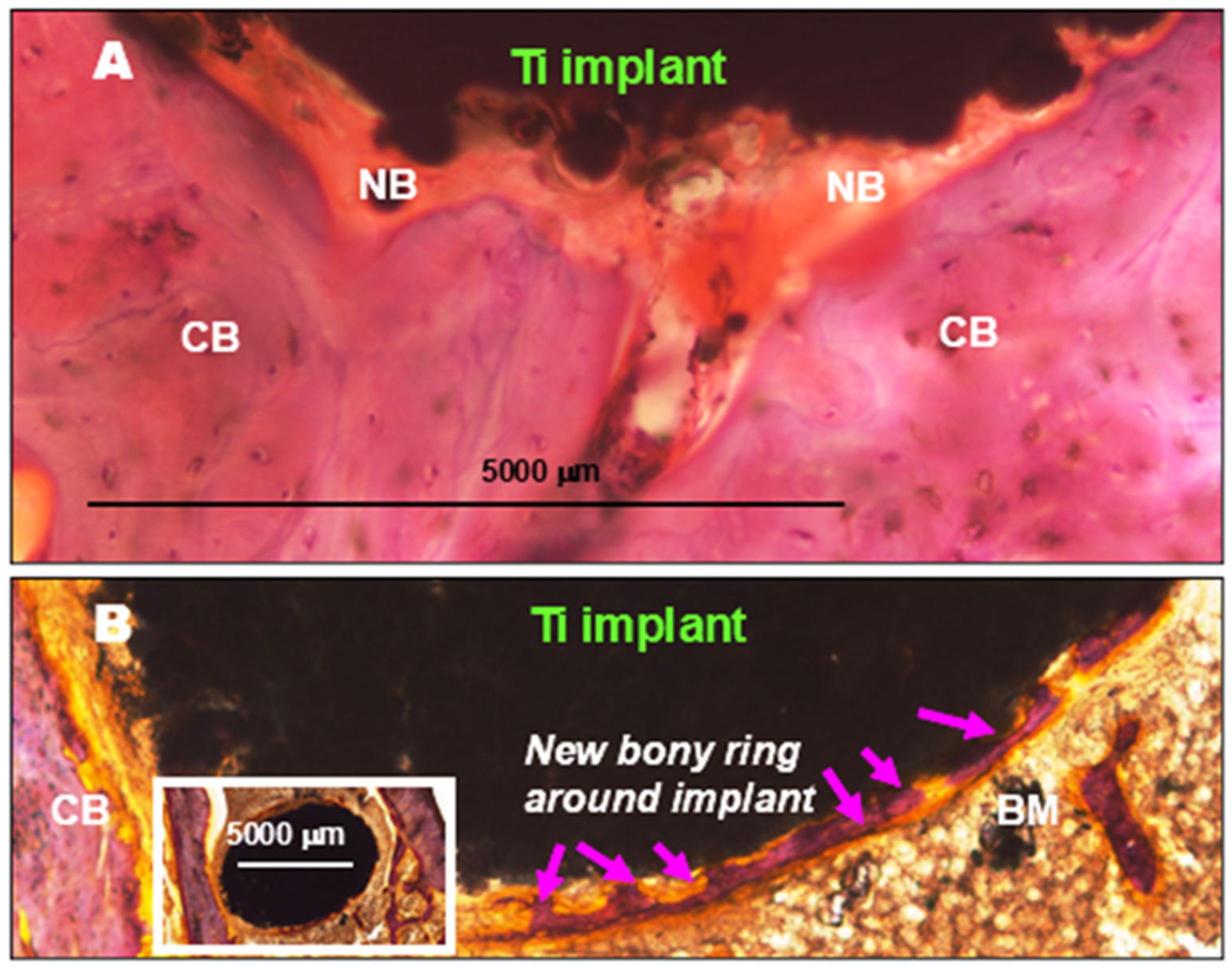
- Kovács, Á.É.; Csernátony, Z.; Csámer, L.; Méhes, G.; Szabó, D.; Veres, M.; Braun, M.; Harangi, B.; Serbán, N.; Zhang, L. Comparative Analysis of Bone Ingrowth in 3D-Printed Titanium Lattice Structures with Different Patterns. Materials 2023, 16, 3861–3876. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Orsini, E.; Trire, A.; Quaranta, M.; Martini, D.; Piccari, G.G.; Ruggeri, A.; Ottani, V. Osteogenesis and morphology of the peri-implant bone facing dental implants. Sci. World J. 2004, 4, 1083–1095. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontology 2000, 79, 178–189. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration--Communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, K.F.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 529. [Google Scholar] [CrossRef]
- Yi, H.; Ur Rehman, F.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Tsakiris, I.; Torocsik, D.; Gyongyosi, A.; Dozsa, A.; Szatmari, I.; Szantó, A.; Soos, G.; Nemes, Z.; Igali, L.; Marton, I.; et al. Carboxypeptidase-M is regulated by lipids and CSFs in macrophages and dendritic cells and expressed selectively in tissue granulomas and foam cells. Lab. Invest. 2012, 92, 345–361. [Google Scholar] [CrossRef][Green Version]
- Sellamuthu, R.; Umbright, C.; Roberts, J.R.; Young, S.H.; Richardson, D.; McKinney, W.; Chen, B.T.; Li, S.; Kashon, M.; Joseph, P. Molecular mechanisms of pulmonary response progression in crystalline silica exposed rats. Inhal. Toxicol. 2017, 2, 53–64. [Google Scholar] [CrossRef]
- Kistler, S. S Coherent expanded aerogel and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Lázár, I.; Szabó, H.J. Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing silica aerogels. Gels 2018, 4, 55. [Google Scholar] [CrossRef]
- Kuttor, A.; Szalóki, M.; Rente, T.; Kerényi, F.; Bako, J.; Fabian, I.; Lazar, I.; Jenei, A.; Hegedus, C. Preparation and application of highly porous aerogel-based bioactive materials in dentistry. Front. Mater. Sci. 2014, 8, 46–52. [Google Scholar] [CrossRef]
- Jókay, I.; Soós, G.; Répássy, G.; Dezső, B. Apoptosis in the human inner ear. Detection by in situ end-labeling of fragmented DNA and correlation with other markers. Hear. Res. 1998, 117, 131–139. [Google Scholar] [CrossRef]
- Szántó, A.; Bálint, B.L.; Nagy, Z.; Barta, E.; Dezső, B.; Pap, A.; Széles, L.; Póliska, S.; Oros, M.; Evans, R.M.; et al. STAT6 Transcription Factor is a Facilitator of the Nuclear Receptor PPARγ-Regulated Gene Expression in Macrophages and Dendritic Cells. Immunity 2010, 3, 699–712. [Google Scholar] [CrossRef]
- Murice-Lambert, E.; Banford, A.B.; Folger, R.L. Histological preparation of implanted biomaterials for light microscopic evaluation of the implant-tissue interaction. Stain Technol. 1989, 64, 19–24. [Google Scholar] [CrossRef]
- Szabo, K.; Papp, G.; Dezso, B.; Zeher, M. The histopathology of labial salivary glands in primary Sjogren’s syndrome: Focusing on follicular helper T cells in the inflammatory infiltrates. Mediat. Inflamm. 2014, 2014, 631787. [Google Scholar] [CrossRef]




| Control (Untreated) (±SD) | Aerogel (±SD) | βTCP-Aerogel (±SD) | Hydrogel (±SD) | βTCP-Hydrogel (±SD) | |
|---|---|---|---|---|---|
| 1 Month | 11.8% ± 3.9 | 15.0% ± 5.7 | 19.4% ± 6.3 | 26.7% ± 7.1 | 30.0% ± 5.0 |
| 3 Months | 23.1% ± 8.8 | 40.0% ± 13.5 | 53.9% ± 14.1 | 60.5% ± 13.8 | 65.0% ± 9.0 |
| 6 Months | 57.0% ± 9.5 | 72.8% ± 10.6 | 80.5% ± 11.6 | 76.9% ± 12.5 | 82.8% ± 10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boda, R.; Hegedűs, V.; Manó, S.; Keczánné-Üveges, A.; Dezső, B.; Hegedűs, C. Morphologic Pattern Differences in Reconstructive Tissue Repair of Bone Defects Mediated by Bioactive Ceramics and Hydrogels: A Microscopic Follow-Up Evaluation of Re-Ossification. Gels 2025, 11, 529. https://doi.org/10.3390/gels11070529
Boda R, Hegedűs V, Manó S, Keczánné-Üveges A, Dezső B, Hegedűs C. Morphologic Pattern Differences in Reconstructive Tissue Repair of Bone Defects Mediated by Bioactive Ceramics and Hydrogels: A Microscopic Follow-Up Evaluation of Re-Ossification. Gels. 2025; 11(7):529. https://doi.org/10.3390/gels11070529
Chicago/Turabian StyleBoda, Róbert, Viktória Hegedűs, Sándor Manó, Andrea Keczánné-Üveges, Balázs Dezső, and Csaba Hegedűs. 2025. "Morphologic Pattern Differences in Reconstructive Tissue Repair of Bone Defects Mediated by Bioactive Ceramics and Hydrogels: A Microscopic Follow-Up Evaluation of Re-Ossification" Gels 11, no. 7: 529. https://doi.org/10.3390/gels11070529
APA StyleBoda, R., Hegedűs, V., Manó, S., Keczánné-Üveges, A., Dezső, B., & Hegedűs, C. (2025). Morphologic Pattern Differences in Reconstructive Tissue Repair of Bone Defects Mediated by Bioactive Ceramics and Hydrogels: A Microscopic Follow-Up Evaluation of Re-Ossification. Gels, 11(7), 529. https://doi.org/10.3390/gels11070529









