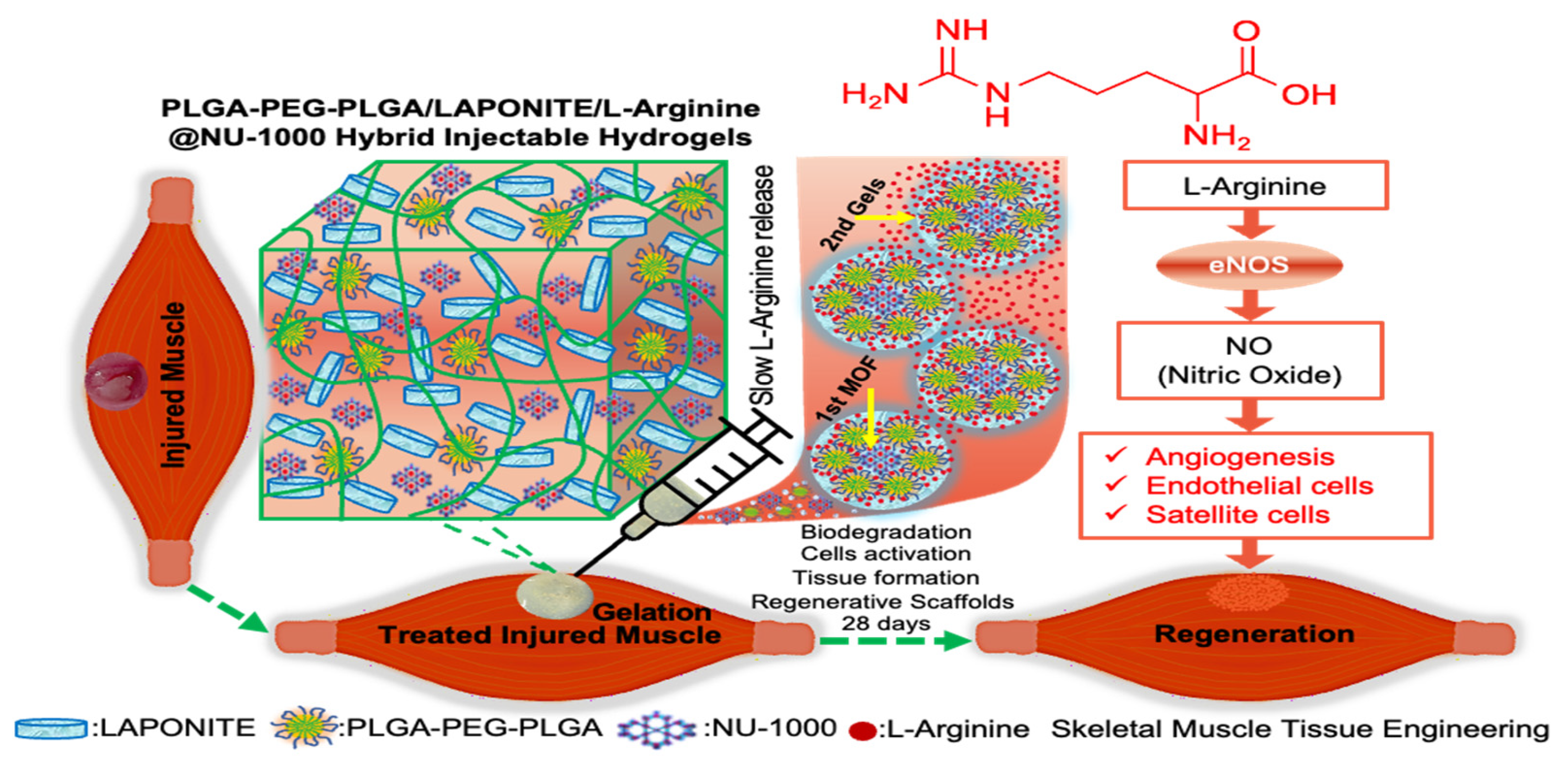
MOFs—Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of PLGA-PEG-PLGA/LAPONITE/L-Arg@NU-1000 Hydrogels
2.2. Rheological Properties of the Hydrogels
2.3. In Vitro Injectability of the Hydrogels
2.4. L-Arginine Release Profiles of the Hydrogels
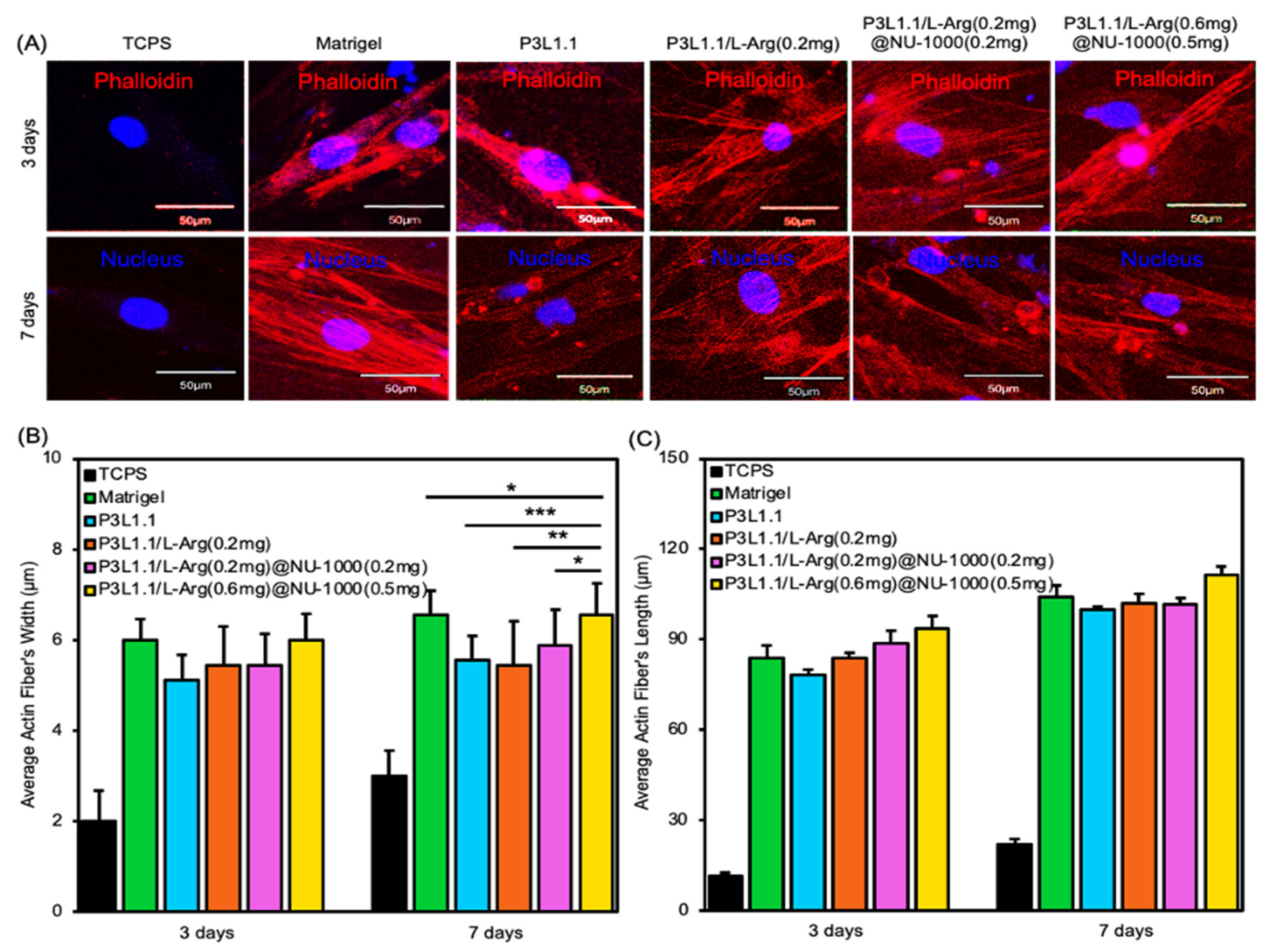
2.5. In Vitro Cell Compatibility and Proliferation of the Hydrogels
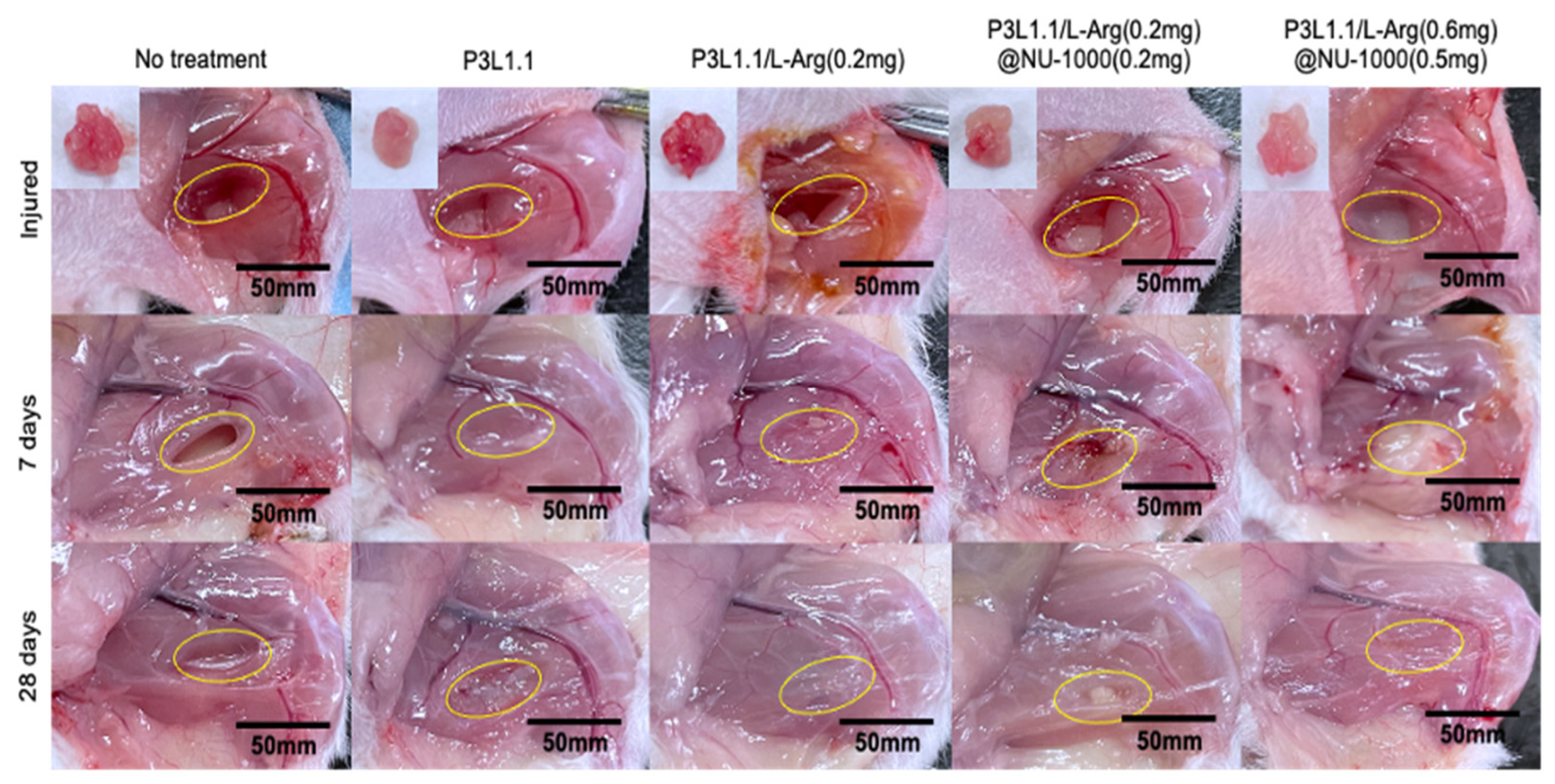
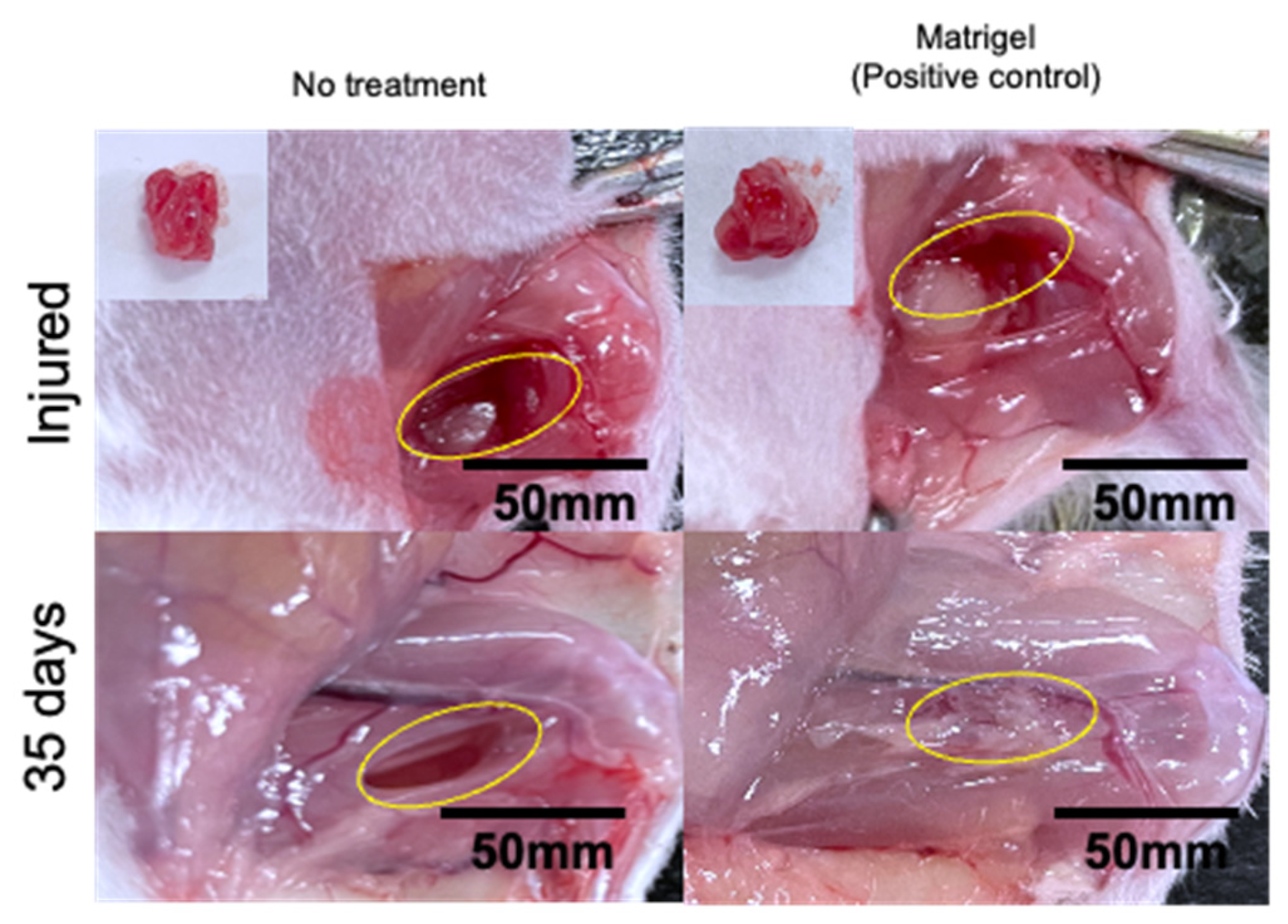
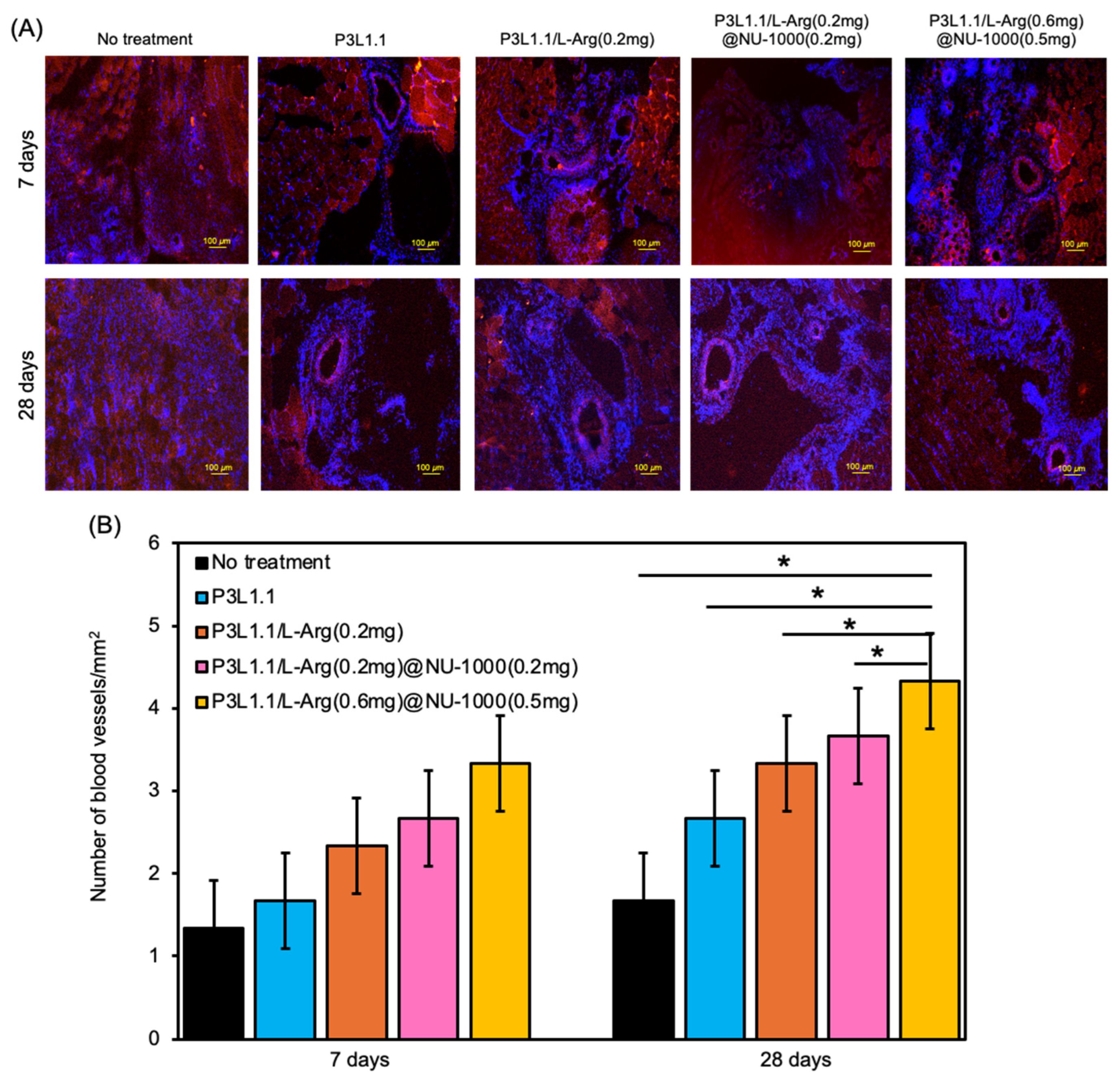
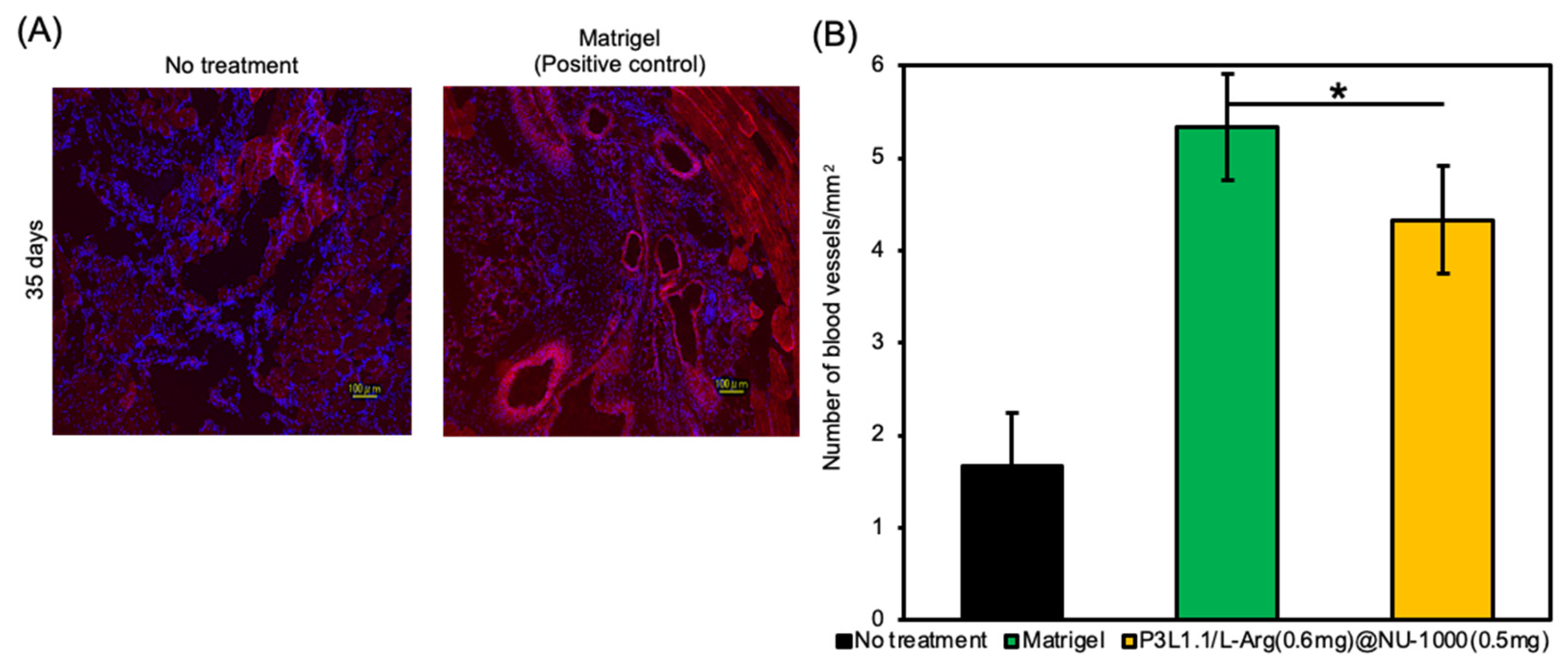
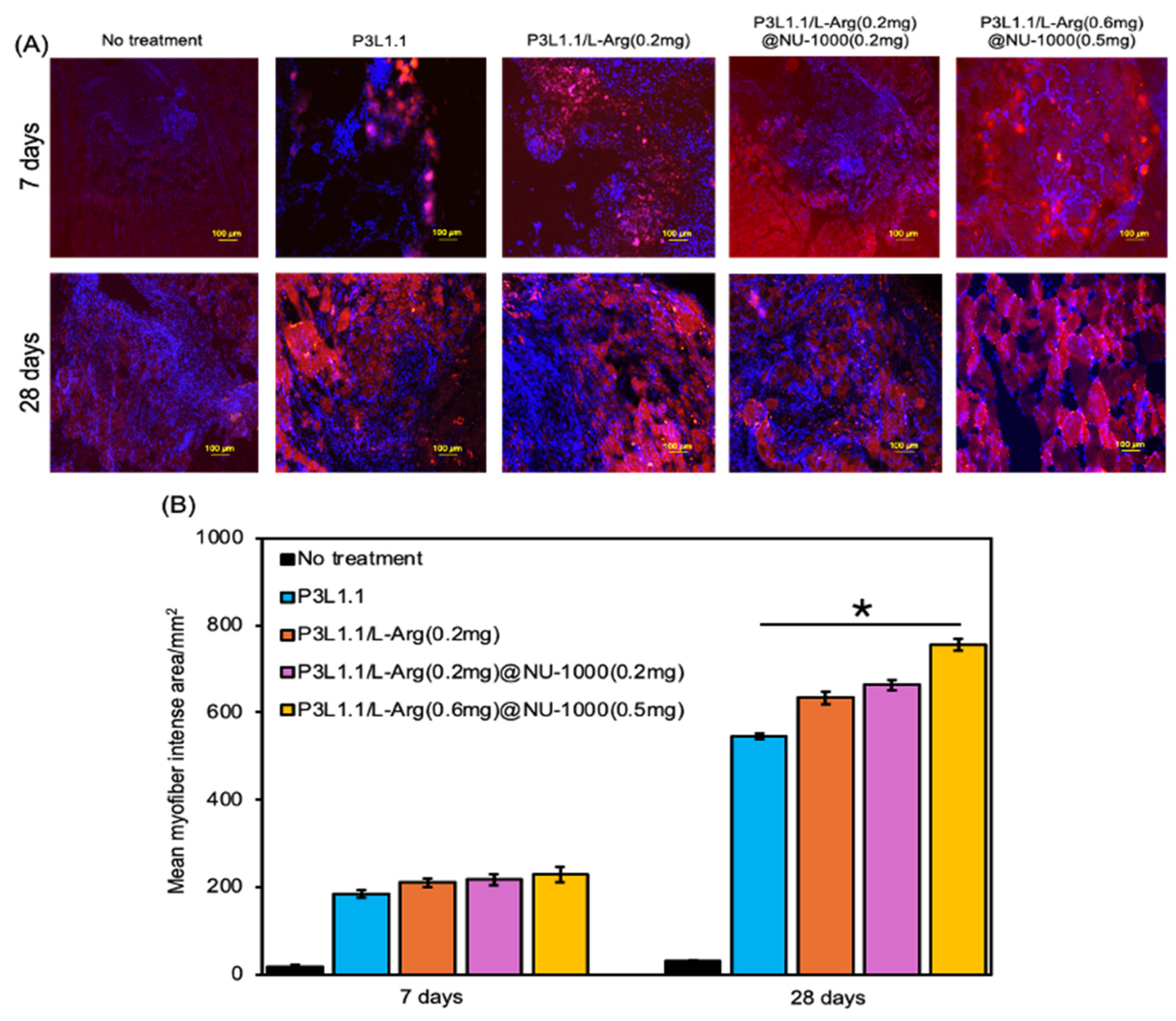
2.6. Evaluation of Skeletal Muscle Tissue Regeneration Capability of the Hydrogels by Immunostaining
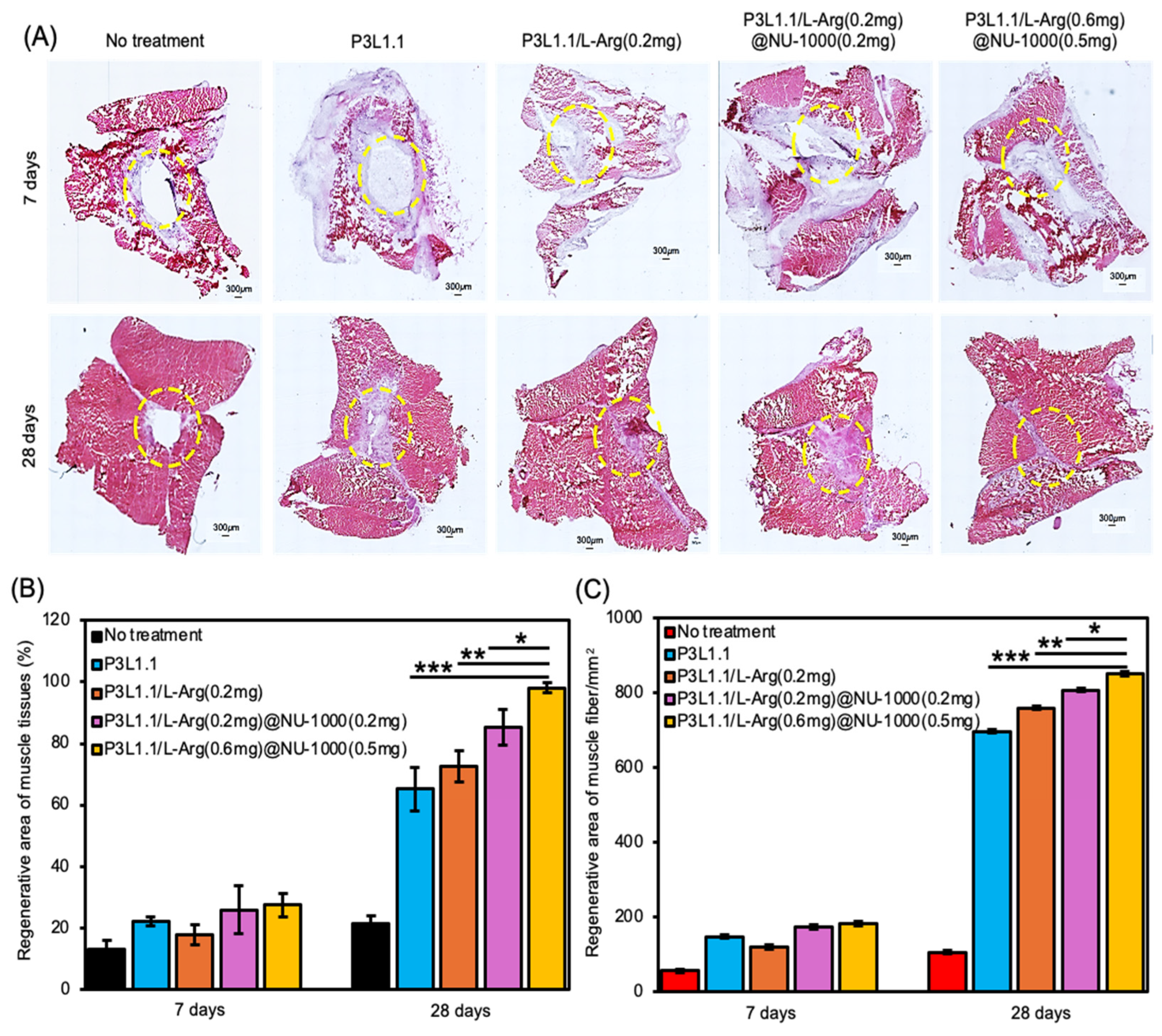
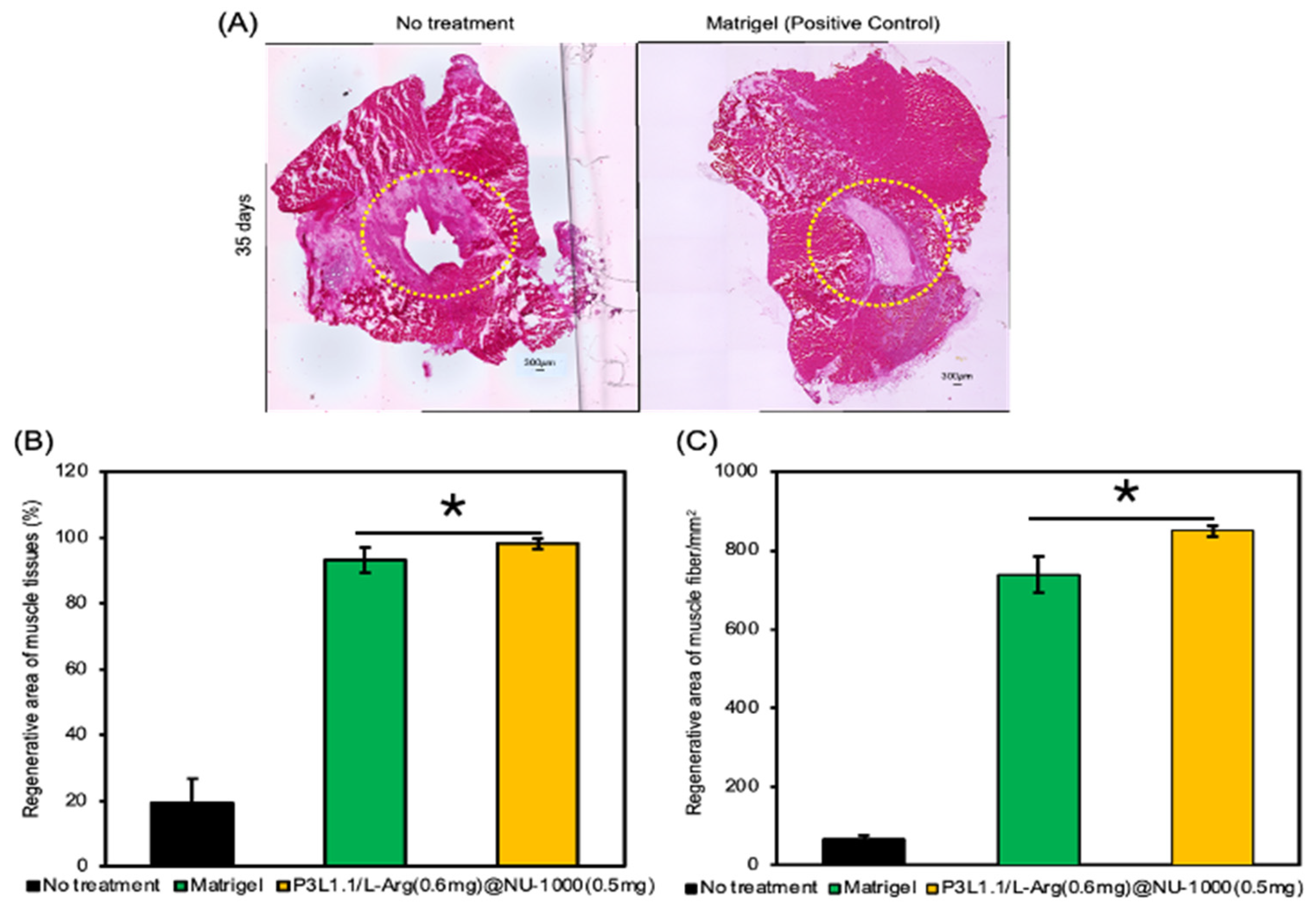
2.7. Evaluation of Skeletal Muscle Tissue Regeneration Capability of the Hydrogels by H&E and Masson’s Trichrome Staining
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of NU-1000
4.3. Preparation of L-Arginine-Loading NU-1000
4.4. Preparation and Characterization of PLGA-PEG-PLGA/LAPONITE®/L-Arg@NU-1000 Hydrogels
4.5. In Vitro Injectability Properties of PLGA-PEG-PLGA/LAPONITE®/L-Arg@NU-1000 Hydrogels
4.6. L-Arginine Release Properties of the Hydrogels
4.7. In Vitro Cytocompatibility of the Hydrogels
4.8. Phalloidin Staining of HsKMSCs Cultured with the Hydrogels
4.9. Skeletal Muscle Tissue Regeneration Test
4.10. CD31 Staining of the Tissue Section of the Reconstructed Muscle Tissues
4.11. MYH3 Staining of the Tissue Section of the Reconstructed Muscle Tissues
4.12. H&E Staining of the Tissue Section of the Reconstructed Muscle Tissues
4.13. Masson Trichrome Staining of Tissue Sections of the Reconstructed Muscle Tissues
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional porous coordination polymers. Angew. Chem. Int. Ed. Engl. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; Keeffe, M.O.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S. Future porous materials. Acc. Chem. Res. 2017, 50, 514–516. [Google Scholar] [CrossRef]
- Daglar, H.; Gulbalkan, H.C.; Avci, G.; Aksu, G.O.; Altundal, O.F.; Altintas, C.; Erucar, L.; Keskin, S. Effects of metal-organic framework (MOF) database selection on the assessment of gas storage and separation potentials of MOFs. Angew. Chem. Int. Ed. Engl. 2021, 60, 7828–7837. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Metal-organic framework HKUST-1 promotes methane hydrate formation for improved gas storage capacity. ACS Appl. Mater. Interfaces 2020, 12, 53510–53518. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, T.; Wang, L.; Ren, Z.; Zhang, W.; Huo, F. Programmable logic in metal-organic frameworks for catalysis. Adv. Mater. 2021, 33, 2007442. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photo physics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, S.; Li, B.; Liu, B.; Shi, Y.; Cong, W.; Gao, F.; Li, J.; Wang, F.; Liu, K.; et al. DUCNP@Mn-MOF/FOE as a highly selective and bioavailable drug delivery system for synergistic combination cancer therapy. Nano Lett. 2023, 23, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Yao, X.; Chen, X.; Sun, Y.; Yang, P.; Gu, X.; Dai, X. Application of metal-organic frameworks-based functional composite scaffolds in tissue engineering. Regener. Biomater. 2024, 11, rbae009. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface modification of the Ti surface with nanoscale bio-MOF-1 for improving biocompatibility and osteointegration in vivo. J. Mater. Chem. B. 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Katayama, T.; Tanaka, S.; Tsuruoka, T.; Nagahama, K. Two-dimensional metal-organic framework-based cellular scaffolds with high protein adsorption, retention, and replenishment capabilities. ACS Appl. Mater. Interfaces 2022, 14, 34443–34454. [Google Scholar] [CrossRef]
- Huang, K.; Liu, W.; Wei, W.; Zhao, Y.; Zhuang, P.; Wang, X.; Wang, Y.; Hu, Y.; Dai, H. Photothermal hydrogel encapsulating intelligently bacteria-capturing bio-MOF for infectious wound healing. ACS Nano 2022, 16, 19491–19508. [Google Scholar] [CrossRef]
- Shyngys, M.; Ren, J.; Liang, X.; Miao, J.; Blocki, A.; Beyer, S. Metal-organic framework (MOF)-based biomaterials for tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2021, 9, 603608. [Google Scholar] [CrossRef]
- Li, X.; Shu, X.; Shi, Y.; Li, H.; Pei, X. MOFs and bone: Application of MOFs in bone tissue engineering and bone diseases. Chin. Chem. Lett. 2023, 34, 107986. [Google Scholar] [CrossRef]
- Lin, J.; Zong, C.; Chen, B.; Wang, T.; Xu, J.; Du, J.; Lin, Y.; Gu, Y.; Zhu, J. Improvement in the healing of bone fractures using a cyclodextrin/Ni-MOF nanofibers network: The development of novel substrate to increase the surface area with desirable functional properties. RSC Adv. 2023, 13, 5600–5608. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, J.; Ma, Z.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.; Pei, X.; Wang, J.; Wan, Q. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, C.; Lei, K.; Xiao, C.; Jiang, X.; Zhang, W.; Wu, L.; Huang, J.; Li, W. Multifunctional MOF-based microneedle patch with synergistic chemo-photodynamic antibacterial effect and sustained release of growth factor for chronic wound healing. Adv. Health. Mater. 2023, 19, e2300250. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, G.; Guo, J.; Liu, Y.; Ren, J.; Kong, T.; Zhao, Y. Vitamin metal-organic framework-laden microfibers form microfluidics for wound healing. Mater. Horiz. 2018, 5, 1137–1142. [Google Scholar] [CrossRef]
- Abednejad, A.; Ghaee, A.; Nourmohammadi, J.; Mehrizi, A.A. Hyaluronic acid/carbonylated zeolitic imidazole framework film with improved mechanical and antibacterial properties. Carbohydr. Polym. 2019, 222, 115033. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Zhang, X.; Zheng, Y.; Lee, S.; Wang, D.; Yang, Y. Polyvinyl alcohol/chitosan and polyvinyl alcohol/Ag@MOF bilayer hydrogel for tissue engineering applications. Polymers 2021, 13, 3151. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, X.; Yao, M.; Han, L.; Zhang, X.; Jia, Z.; Weng, J.; Chen, W.; Fang, L.; Wang, X.; et al. Bioinspired adhesive and tumor microenvironment responsive nano MOFs assembled 3D-printed scaffold for anti-tumor therapy and bone regeneration. Nano Today 2021, 39, 101182. [Google Scholar] [CrossRef]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Wang, H.; Wan, H.; Wang, Q.; Ma, Y.; Su, G.; Cao, X.; Gao, H. Engineered multifunctional shilk fibroin/gelatin hydrogel conduit loaded with miR-29a@ZIF-8 nanoparticles for peripheral nerve regeneration. Smart Mater. Med. 2023, 4, 480–492. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF encapsulated antibacterial and degradable microneedles array for promoting wound healing. Adv. Health. Mater. 2021, 10, 2100056. [Google Scholar] [CrossRef]
- Wang, T.L.; Zhou, Z.; Liu, J.F.; Hou, X.D.; Zhou, Z.; Dai, Y.L.; Hou, Z.Y.; Chen, F.; Zheng, L.P. Donut-like MOFs of copper/nicotinic acid and composite hydrogels with superior bioactivity for rh-bFGF delivering and skin wound healing. J. Nano-Biotechnol. 2021, 19, 275. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, H.; Yan, L.; Jiang, S.; Zheng, Y.; Zhang, X.; Wang, K.; Wang, Q.; Han, L.; Lu, X. A metal-organic framework-incorporated hydrogel for delivery of immunomodulatory neobavaiso-flavone to promote cartilage regeneration in osteoarthritis. ACS Appl. Mater. Interfaces 2023, 15, 46598–46612. [Google Scholar] [CrossRef] [PubMed]
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable hyaluronic acid-co-gelatin cryogels for tissue engineering applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Y.; Pan, J.J.; Sun, Y.H.; Zhang, S.Q.; Yang, Y.; Liu, H.; Li, Y.; Xu, X.; Sui, Y.; Wei, S.C. Injectable 3D porous micro-scaffolds with a bio-engine for cell transplantation and tissue regeneration. Adv. Funct. Mater. 2018, 28, 1804335. [Google Scholar] [CrossRef]
- Mealy, J.E.; Chung, J.J.; Jeong, H.-H.; Issadore, D.; Lee, D.; Atluri, P.; Burdick, J.A. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater. 2018, 30, 1705912. [Google Scholar] [CrossRef]
- Mullen, P.; Ritchie, A.; Laangdon, S.P.; Miller, W.R. Effect of Matrigel on the tumorigenicity of human breast and ovarian carcinoma cell lines. Int. J. Cancer 1996, 67, 816–820. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Arkudas, A.; Horch, R.E.; Kneser, U. To Matrigel or not to Matrigel. Am. J. Pathol. 2008, 5, 172. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, I.; Rawal, P.; Tripathi, D.M.; Vasudevan, A. Non-Matrigel scaffolds for organoid cultures. Cancer Lett. 2021, 504, 58–66. [Google Scholar] [CrossRef]
- Capeling, M.M.; Czerwinski, M.; Huang, S.; Tsai, Y.H.; Wu, A.; Nagy, M.S.; Juliar, B.; Sundaram, N.; Song, Y.; Han, W.M.; et al. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Rep. 2019, 12, 381–394. [Google Scholar] [CrossRef]
- Stefan, W.; Anthony, J.T.; Qin, D.; Seiichi, O.; Julie, A.; Harold, F.D.; Warren, C.W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar]
- Bengt, F.; Cyrill, B.; Sonia, M.; Ester, V.; Emmanuel, F.; Florence, M.; Lauris, E.; Laury, G.; Antti, J.K.; Ulla, V.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar]
- Nagahama, K.; Oyama, N.; Ono, K.; Hotta, A.; Kawauchi, K.; Nishikata, T. Nanocomposite injectable gels capable of self-replenishing regenerative extracellular microenvironments for in vivo tissue engineering. Biomater. Sci. 2018, 6, 550. [Google Scholar] [CrossRef] [PubMed]
- Sobuj, I.S.; Dode, T.; Fukuoka, M.; Tsuruoka, T.; Nagahama, K. Metal-organic framework-injectable hydrogel hybrid scaffolds promote accelerated angiogenesis for in vivo tissue engineering. RSC Adv. 2025. under the review. [Google Scholar]
- Park, I.-S.; Kang, S.-W.; Shin, Y.-J.; Chae, K.-Y.; Park, M.-O.; Kim, M.-Y.; Wheatley, D.-N.; Min, B.-H. Arginine deiminase: A potential inhibitor of angiogenesis and tumor growth. Br. J. Cancer 2003, 89, 907–914. [Google Scholar] [CrossRef]
- Nafise, K.; Mahalati, M.J.; Yeganeh, K.; Al-Musawi, M.H.; Varshosaz, J.; Bakhtiari, S.S.E.; Tavakoli, M.; Sharifianjazi, F.; Salehi, S.; Najafinezhad, A.; et al. Core-shell nanofibers containing L-arginine stimulates angiogenesis and full thickness dermal wound repair. Int. J. Pharm. Sci. 2024, 653, 123931. [Google Scholar]
- Pan, Y.; Soheila, S.; Marzieh, N.; Reza, A.; Junkuo, G.; Alexander, M.K. Post synthetic Modification of NU-1000 for Designing a Polyoxometalate-Containing Nanocomposite with Enhanced Third Order Nonlinear Optical Performance. Inorg. Chem. 2022, 61, 18873–18882. [Google Scholar] [CrossRef]
- Satu, L.; David, A.V.M.; Anna, P.R.; Pete, L. Optimizing aerosolization of a high-dose L-arginine powder for pulmonary delivery. Asian J. Pharm. Sci. 2015, 10, 528–540. [Google Scholar]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: Protein encapsulation, protection, and release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Yang, L.; Guo, J.; Wang, L.; Tang, S.; Wang, A.; Zheng, S.; Guo, Z.; Zan, X. Transformation of the shape and shrinking the size of acid-resistant metal-organic frameworks (MOFs) for use as the vehicle of oral proteins. Biomater. Sci. 2023, 11, 3726. [Google Scholar] [CrossRef]
- Hnia, K.; Gayraud, J.; Hugo, G.; Ramonatxo, M.; Porte, S.D.L.; Matecki, S.; Mornet, D. L-Arginine decreases inflammation and modulates the nuclear factor-kβ/matrix, metallo proteinase cascade in Mds muscle Fibers. Am. J. Path. 2008, 172, 6. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Furrer, A.A.; Andrade, S.D.; Bachmann, D.; Jeker, H.; Steinmann, M.; Accart, N.; Dunbar, A.; Rausch, M.; Bono, E.; Rimann, M.; et al. Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses. Commun. Boil. 2021, 4, 1183. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Pelacho, B.; Prado, R.; Aguirre, J.J.; Sanchez, M.; Padilla, S.; Aranguren, X.L.; Abizanda, G.; Collantes, M.; Hernandez, M.; et al. Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodeling after severe hind limb ischemia. J. Control. Release 2015, 202, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kabara, M.; Kawabe, J.I.; Matsuki, M.; Hira, Y.; Minoshima, A.; Shimamura, K.; Yamauchi, A.; Aonuma, T.; Nishimura, M.; Saito, Y.; et al. Immortalized multipotent pericytes derived from the vasa vasorum in the injured vasculature. A cellular tool for studies of vascular remodeling and regeneration. Lab. Investig. 2014, 94, 1340–1354. [Google Scholar] [CrossRef]
- Grabowska, I.; Zimowska, M.; Maciejewska, K.; Jablonska, Z.; Bazga, A.; Ozieblo, M.; Streminska, W.; Bem, J.; Brzoska, E.; Ciemerych, M.A. Adipose tissue-derived stromal cells in Matrigel impact the regeneration of severely damaged skeletal muscles. Int. J. Mol. Sci. 2019, 20, 3313. [Google Scholar] [CrossRef]
- Chen, W.C.W.; Baily, J.E.; Corselli, M.; Diaz, M.E.; Sun, B.; Xiang, G.S.; Gray, G.A.; Huard, J.; Peault, B. Human myocardial pericytes: Multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 2015, 33, 557–573. [Google Scholar] [CrossRef]
- Grimaldi, A.; Banfi, S.; Gerosa, L.; Tettamanti, G.; Noonan, D.M.; Valvassori, R.; Eguileor, M.D. Identification, isolation and expansion of myoendothelial cells involved in leech muscle regeneration. PLoS ONE 2009, 4, e7652. [Google Scholar] [CrossRef]
- Shahinia, A.; Vydiamb, K.; Choudhurya, D.; Rajabiana, N.; Nguyenb, T.; Leia, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Wieszkowski, W.S. Hydrogel-based fiber biofabrication techniques for skeletal muscle tissue engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Goh, L.; Otake, K.; Goh, S.S.; Loh, X.J.; Kitagawa, S. Biomedically-relevant metal-organic framework hydrogel composites. Biomater. Sci. 2023, 11, 2661. [Google Scholar] [CrossRef]
- Oyama, N.; Minami, H.; Kawano, D.; Miyazaki, M.; Maeda, T.; Toma, K.; Hotta, A.; Nagahama, K. Nanocomposite approach to develop biodegradable thermogels exhibiting excellent cell-compatibility for injectable cell delivery. Biomater. Sci. 2014, 2, 1057–1062. [Google Scholar] [CrossRef]
- Asha, P.; Mekhola, S.; Anupam, A.O.; Sukhendu, M. Water-Stable Nanoscale Zirconium-Based Metal-Organic Frameworks for the Effective Removal of Glyphosate from Aqueous Media. ACS Omega 2018, 3, 7832–7839. [Google Scholar]
- Delmas, V.T.E.; Kevin, Y.T.; Chancellin, N.P.; Sherman, L.Z.J.; Arnaud, K.T.; Ingo, D.; Anayancy, O.; Ignas, K.T.; Emmanuel, N. Amino-Functionalized Laponite Clay Material as a Sensor Modifier for the Electrochemical Detection of Quercetin. Sensors 2022, 22, 6173. [Google Scholar] [CrossRef] [PubMed]
- Saidulu, G.; Mariana, L.D.R.; Niroshani, S.A.; David, M.K.; Daryl, R.W.; Vladimir, M.; Hugo, A.; Lara, G.; Bruno, D.; Nazario, L.; et al. Functionalized NU-1000 with an Iridium Organometallic Fragment: SO2 Capture Enhancement. ACS Appl. Mater. Interfaces 2020, 12, 41758–41764. [Google Scholar]
- Ximin, Y.; Zhou, Z.; Pengcheng, X.; Zhenjia, W.; Xiao, Z.; Xiao, J.; Tianming, W.; Qing, G.; Jie, X.; Debin, S.; et al. Tough Gelatin Hydrogel for Tissue Engineering. Adv. Sci. 2023, 10, 2301665. [Google Scholar]
- Xu, P.; Yang, P.; Zhang, L.; Wu, K.; Bai, Y.S.; Yang, H.; Zhou, H.; Lin, X.; Yang, L. A multi-functional SiO32-releasing hydrogel with bioinspired mechanical properties and biodegradability for vascularized skeletal muscle regeneration. J. Mater. Chem. B 2022, 10, 7540. [Google Scholar] [CrossRef]
- Pang, S.; Wu, R.; Lv, W.; Zou, J.; Li, Y.; Li, Y.; Zhang, P.; Ma, X.; Wang, Y.; Liu, S. Use of a pH-responsive imatinib mesylate sustained-release hydrogel for the treatment of tendon adhesion by inhibiting PDGFRβ/CLDN1 pathway. Bioact. Mater. 2024, 38, 124–136. [Google Scholar] [CrossRef]
- Manoli, S.; Coppola, S.; Duranti, C.; Lulli, M.; Magni, L.; Kuppalu, N.; Nielsen, N.; Schmidt, T.; Schwab, A.; Becchetti, A.; et al. The activity of Kv 11.1 potassium channel modulates F-actin organization during cell migration of pancreatic ductal adenocarcinoma cells. Cancers 2019, 11, 135. [Google Scholar] [CrossRef]
- Gunasekara, H.; Perera, T.; Chao, C.; Bruno, J.; Saed, B.; Anderson, J.; Zhao, Z.; Hu, Y.S. Phalloidin-PAINT: Enhanced quantitative nanoscale imaging of F-actin. Biophys. J. 2024, 123, 3051–3064. [Google Scholar] [CrossRef]
- Sougata, S.; Maureen, M.M.; Fangliang, Z.; Ryan, W.D.; Farida, K.; Tatyana, S.; Alex, A.P.; John, F.D.; Anna, K. Arginylation Regulates Intracellular Actin Polymer Level by Modulating Actin Properties and Binding of Capping and Severing Proteins. Mol. Biol. Cell 2010, 21, 1350–1361. [Google Scholar]
- Iuliia, P.; Yuriy, R.; Adam, K.J.; Jakub, D.; Anna, W.; Galyna, P.; Marta, O.; Leoni, A.K.S.; Oleh, S.; Maria, J.R. Arginine deprivation affects glioblastoma cell adhesion, invasiveness and actin cytoskeleton organization by impairment of β-actin arginylation. Amino Acids 2015, 47, 199–212. [Google Scholar]
- Marina, K.; Marina, K.; Catherine, C.L.W.; Aaron, O.B.; John, R.Y.; Alexander, M.; Henry, Z.; Anna, K. Arginylation of b-Actin Regulates Actin Cytoskeleton and Cell Motility. Science 2006, 313, 192–196. [Google Scholar]
- Reza, A.; Soheila, S.; Majed, A.B.; Muhammad, S.J.; Peter, C.J.; Ashok, K.N.; Jinjie, Q.; Deepak, P.D. Design and Advanced Manufacturing of NU-1000 Metal–Organic Frameworks with Future Perspectives for Environmental and Renewable Energy Applications. Small 2024, 20, 2306353. [Google Scholar]
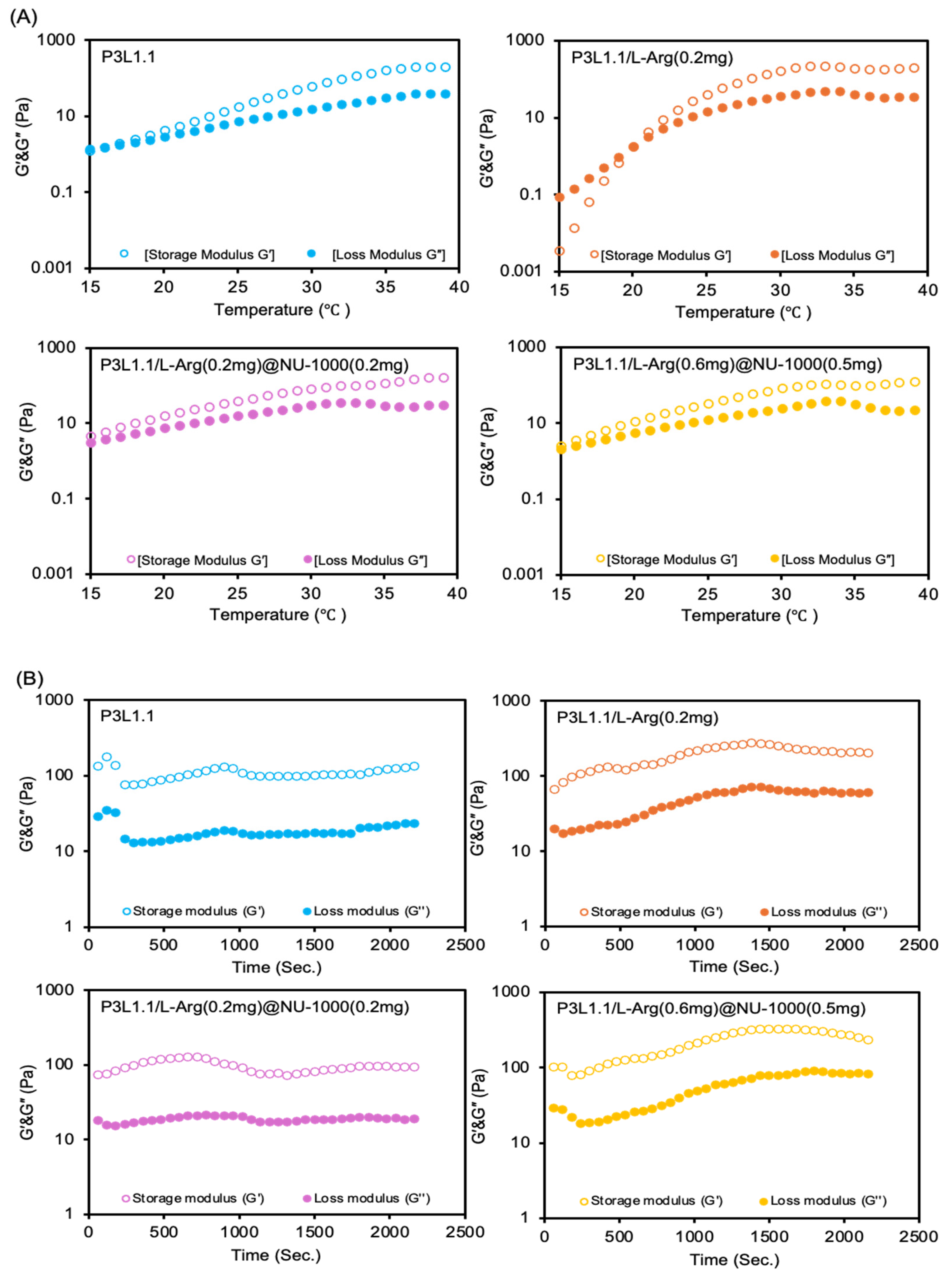
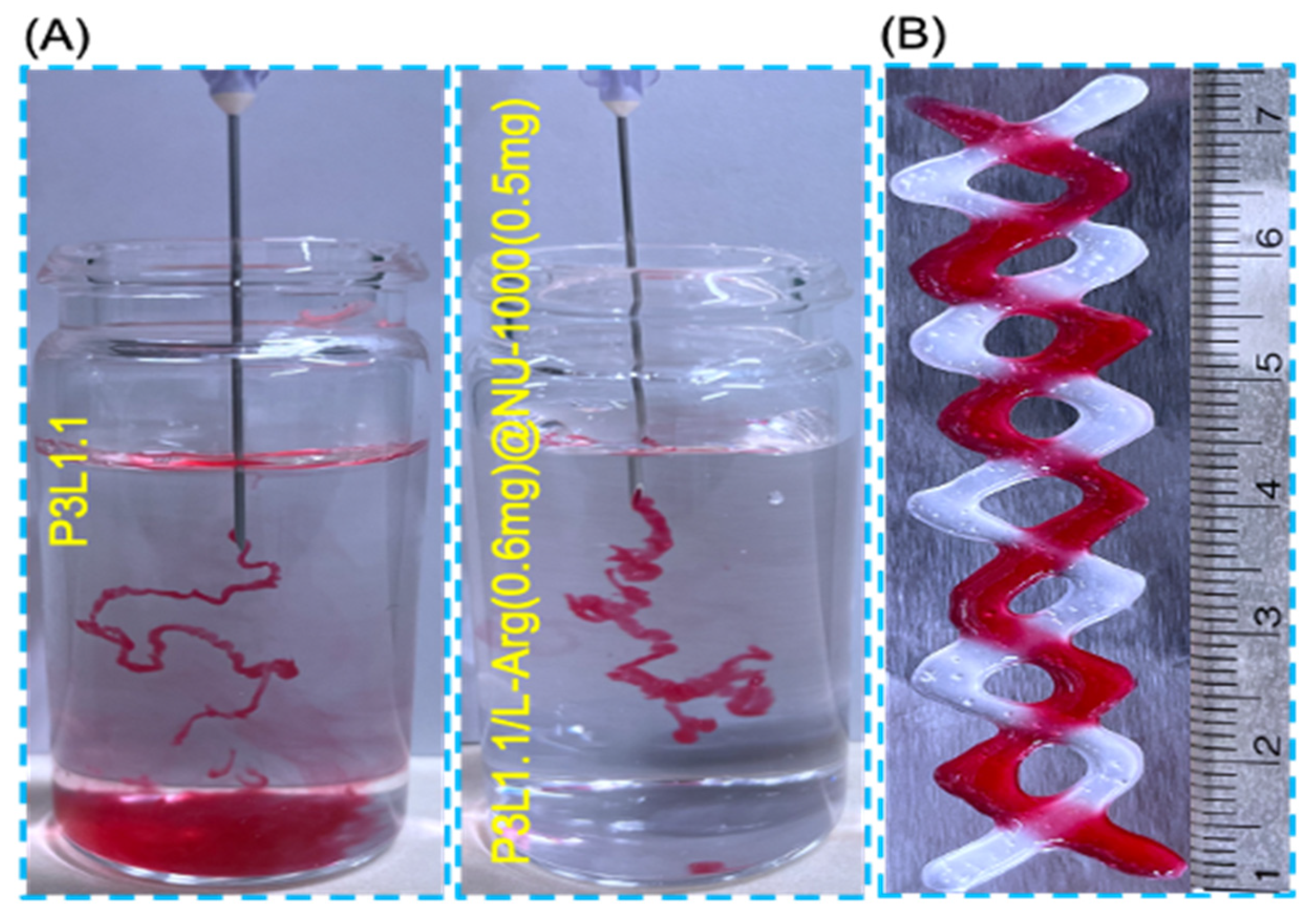

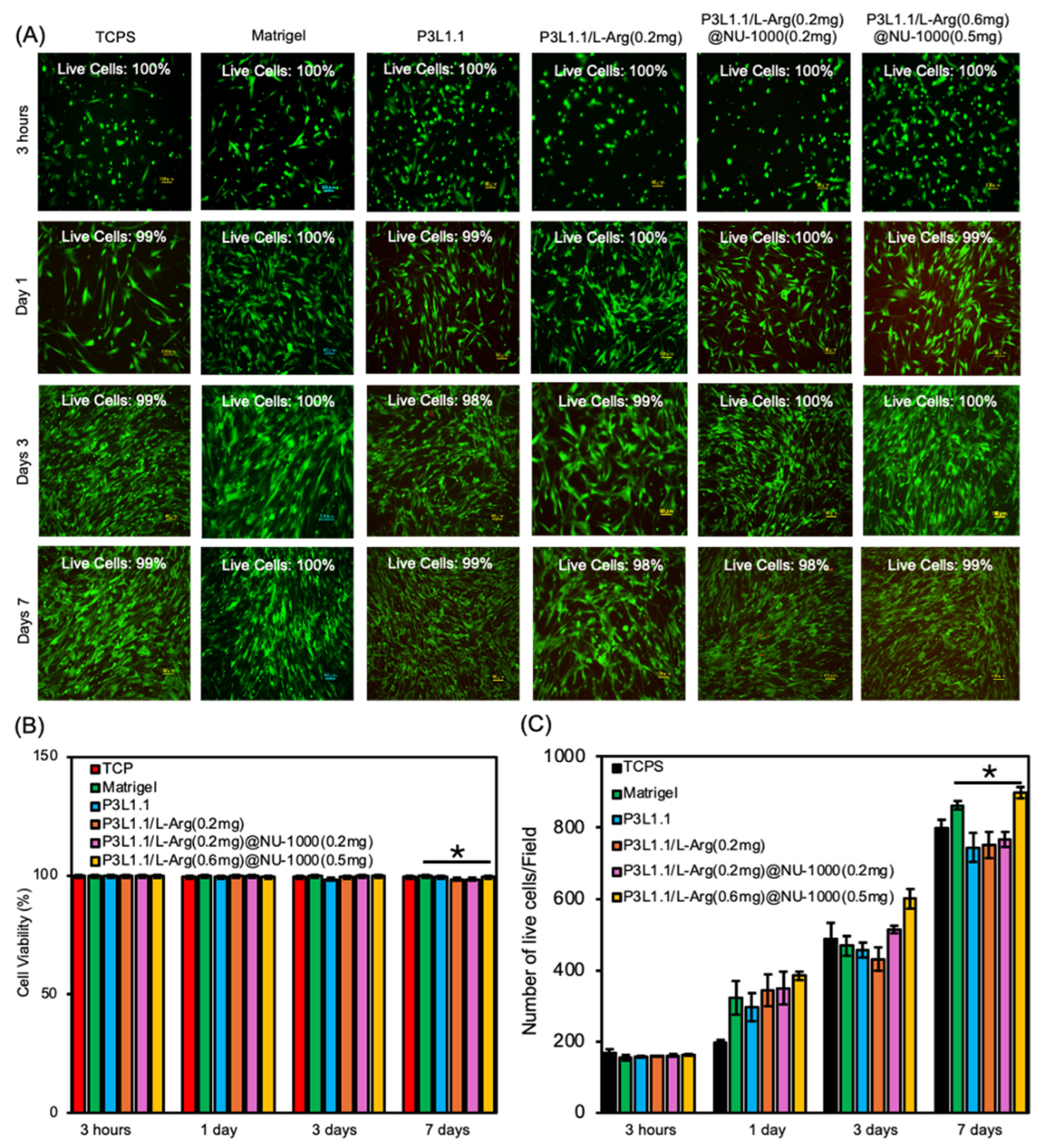



| Hydrogels | Gelation Temperature (°C) | Storage Moduli at 37 °C (Pa) | Loss Moduli at 37 °C (Pa) |
|---|---|---|---|
| P3L1.1 | 19 ± 3.6 | 141 ± 41 | 31 ± 12 |
| P3L1.1/L-Arg (0.2 mg) | 21 ± 1.0 | 181 ± 82 | 47 ± 18 |
| P3L1.1/L-Arg (0.2 mg)@NU-1000 (0.2 mg) | 15 ± 0.6 | 124 ± 49 | 30 ± 13 |
| P3L1.1/L-Arg (0.6 mg)@NU-1000 (0.5 mg) | 18 ± 3.5 | 237 ± 72 | 116 ± 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.S.; Dode, T.; Kawashima, S.; Fukuoka, M.; Tsuruoka, T.; Nagahama, K. MOFs—Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel. Gels 2025, 11, 514. https://doi.org/10.3390/gels11070514
Islam SS, Dode T, Kawashima S, Fukuoka M, Tsuruoka T, Nagahama K. MOFs—Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel. Gels. 2025; 11(7):514. https://doi.org/10.3390/gels11070514
Chicago/Turabian StyleIslam, Sobuj Shahidul, Tatsuya Dode, Soma Kawashima, Myu Fukuoka, Takaaki Tsuruoka, and Koji Nagahama. 2025. "MOFs—Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel" Gels 11, no. 7: 514. https://doi.org/10.3390/gels11070514
APA StyleIslam, S. S., Dode, T., Kawashima, S., Fukuoka, M., Tsuruoka, T., & Nagahama, K. (2025). MOFs—Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel. Gels, 11(7), 514. https://doi.org/10.3390/gels11070514







