Development, Characterization, and Stability of Margarine Containing Oleogels Based on Olive Oil, Coconut Oil, Starch, and Beeswax
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Oleogelators on Margarine Formation and Physical Properties
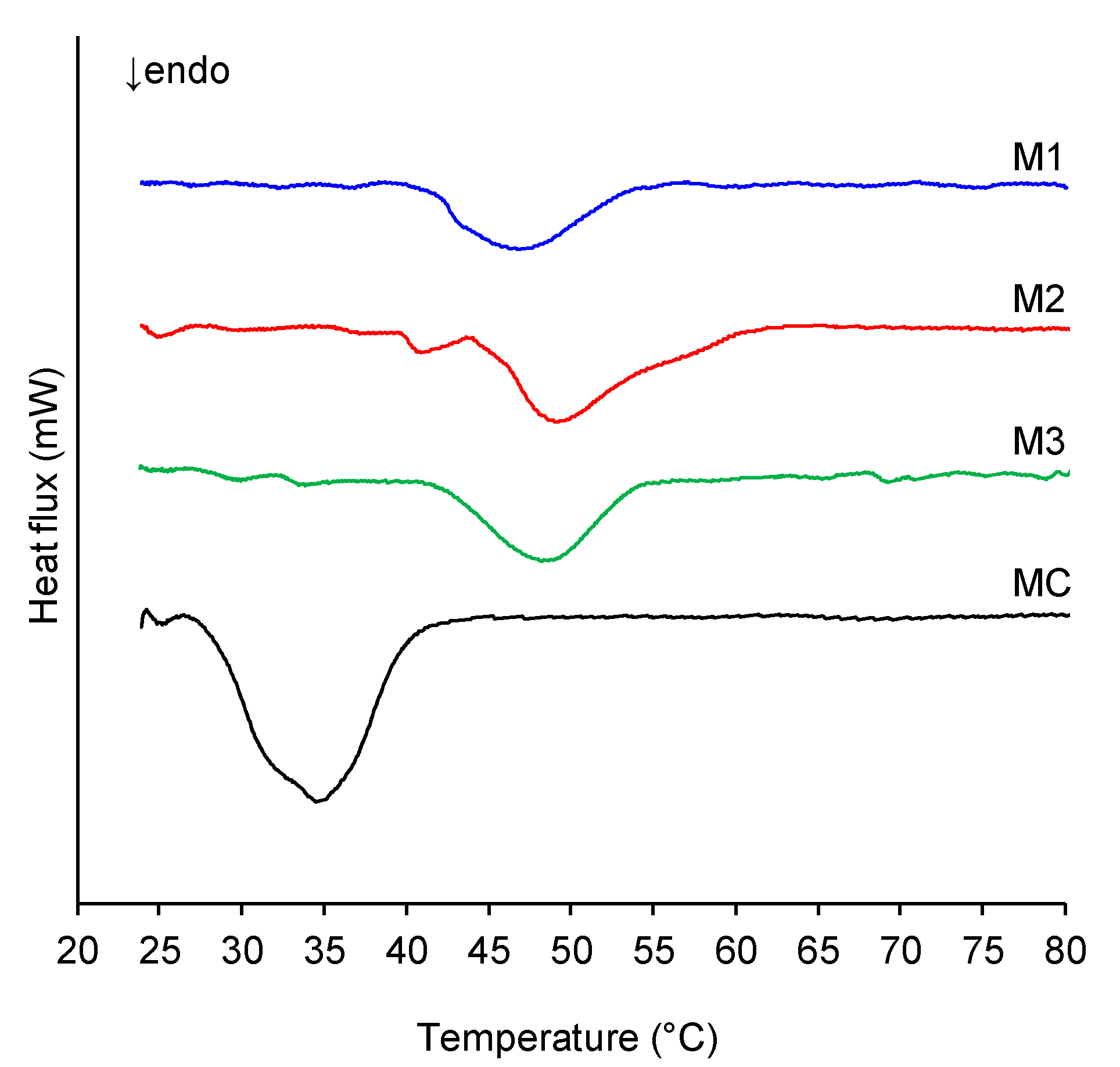
2.2. Melting Behavior
2.3. Color
2.4. Thermal Cyclization
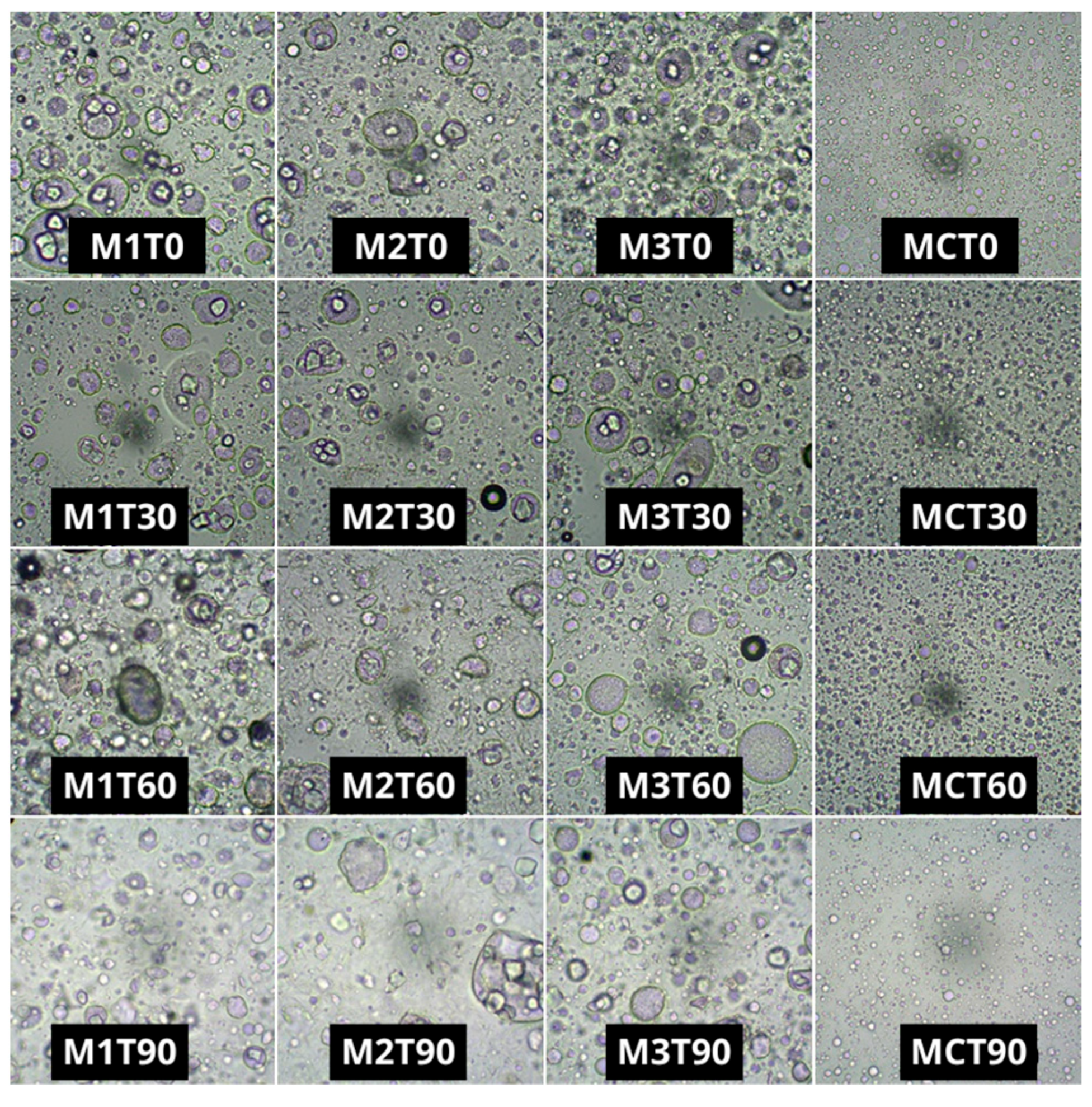
2.5. Microstructure
2.6. Oxidation Evaluation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Formulation of the Oleogel-Based Margarines
4.2.1. Experimental Design
4.2.2. Margarine Preparation
4.3. Thermal Behavior
4.4. Color Analysis
4.5. Thermal Stability by Cyclization
4.6. Microstructure Analysis
4.7. Lipid Stability and Oxidation
4.7.1. Peroxide Index
4.7.2. Acidity Index
4.7.3. Anisidine Value
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira Izar, M.C.; Lottenberg, A.M.; Giraldez, V.Z.R.; Santos Filho, R.D.D.; Machado, R.M.; Bertolami, A.; Assad, M.H.V.; Saraiva, J.F.K.; Faludi, A.A.; Moreira, A.S.B.; et al. Position statement on fat consumption and cardiovascular health-2021. Arq. Bras. Cardiol. 2021, 116, 160–212. [Google Scholar] [CrossRef]
- Federal Register: Final Determination Regarding Partially Hydrogenated Oils. Available online: https://www.federalregister.gov/documents/2015/06/17/2015-14883/final-determination-regarding-partially-hydrogenated-oils (accessed on 7 May 2025).
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Auzanneau, F.I.; Rogers, M.A. Advances in edible oleogel technologies—A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical properties of rice bran wax in bulk and organogels. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 1163–1173. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Pușcas, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Facciolo, D.; Baldino, N.; Seta, L.; de Cindio, B. Effect of organogelator and fat source on rheological properties of olive oil-based organogels. Food Res. Int. 2012, 46, 177–184. [Google Scholar] [CrossRef]
- Öǧütcü, M.; Yilmaz, E. Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas Aceites 2014, 65, e040. [Google Scholar] [CrossRef]
- Zampouni, K.; Mouzakitis, C.K.; Lazaridou, A.; Moschakis, T.; Katsanidis, E. Physicochemical properties and microstructure of bigels formed with gelatin and κ-carrageenan hydrogels and monoglycerides in olive oil oleogels. Food Hydrocoll. 2023, 140, 108636. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Rocha, K.D.C.; Ferreira, M.S.; Garcia, C.E.R. Óleo de Coco: Características e aplicações fisiológicas. In Compostos Bioativos e Suas Aplicações; Mérida Publishers: Canoas, Brasil, 2021. [Google Scholar] [CrossRef]
- Cheng, J.; Kan, Q.; Cao, J.; Dudu, O.E.; Yan, T. Interfacial compositions of fat globules modulate coconut oil crystallization behavior and stability of whipped-frozen emulsions. Food Hydrocoll. 2021, 114, 106580. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Nimbkar, S.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Medium chain triglycerides (MCT): State-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 843–867. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Öʇütcü, M. Oleogels as spreadable fat and butter alternatives: Sensory description and consumer perception. RSC Adv. 2015, 5, 50259–50267. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Shabbir, M.A.; Tahir, F.; Khan, M.R.; Ahmed, W.; Yıkmış, S.; Manzoor, M.F.; Abdi, G.; Aadil, R.M. Development of oleogel by structuring the blend of corn oil and sunflower oil with beeswax to replace margarine in cookies. Food Chem. X 2024, 23, 101676. [Google Scholar] [CrossRef]
- Silva, T.J.; Barrera-Arellano, D.; Badan Ribeiro, A.P. The impact of fatty acid profile on the physicochemical properties of commercial margarines in Brazil. JAOCS J. Am. Oil Chem. Soc. 2022, 99, 469–483. [Google Scholar] [CrossRef]
- US Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services and the U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: https://odphp.health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 10 March 2024).
- Faludi, A.A.; de Oliveira Izar, M.C.; Saraiva, J.F.K.; Chacra, A.P.M.; Bianco, H.T.; Afiune Neto, A.; Bertolami, A.; Pereira, A.C.; Lottenberg, A.M.; Sposito, A.C.; et al. Update of the Brazilian guidelines on dyslipidemias and prevention of atherosclerosis-2017. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- World Health Organization. Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023; p. 117. Available online: https://www.who.int/publications/i/item/9789240073630 (accessed on 10 March 2024).
- da Silva, T.L.T.; Chaves, K.F.; Fernandes, G.D.; Rodrigues, J.B.; Bolini, H.M.A.; Arellano, D.B. Sensory and Technological Evaluation of Margarines With Reduced Saturated Fatty Acid Contents Using Oleogel Technology. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 673–685. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.A.; Hwang, H.S. Texture and flavor evaluation of peanut butter stabilized with natural waxes. J. Food Sci. 2022, 87, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.R.; Lourenço, J.; Pereira, Z.L. Desirability function approach: A review and performance evaluation in adverse conditions. Chemom. Intell. Lab. Syst. 2011, 107, 234–244. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, S. Development and evaluation of a novel oleogel system based on starch–water–wax–oil. Food Funct. 2020, 11, 7727–7735. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, S. Thermal and oxidation stability of functional oleogels formed by edible wax/starch and Schisandra chinensis oil. Food Funct. 2019, 10, 8056–8068. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, Y.; Shi, Y.; Liu, Y. Crystallization and Structural Properties of Oleogel-Based Margarine. Molecules 2022, 27, 8952. [Google Scholar] [CrossRef]
- Lomonaco, T.; da Silva, T.; Deschamps Fernandes, G.; Arellano, D.B. The combination of monoglycerides, wax and hardfat on oleogels structuration. Braz. J. Food Technol. 2022, 25, e2021137. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Bhandari, B. Effect of addition of beeswax based oleogel on 3D printing of potato starch-protein system. Food Struct. 2021, 27, 100176. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.; Felker, F.C.; Hwang, H.S. Physical Properties of Beeswax, Sunflower Wax, and Candelilla Wax Mixtures and Oleogels. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 1125–1142. [Google Scholar] [CrossRef]
- Rupini, R.V.; Nandagopal, R. A study on the influence of senses and the effectiveness of sensory branding. Afr. J. Psychiatry 2015, 18, 2. [Google Scholar] [CrossRef]
- Garcia, R.K.A.; Moreira Gandra, K.; Barrera-Arellano, D. Development of a zero-trans margarine from soybean-based interesterified fats formulated using artificial neural networks. Grasas Aceites 2013, 64, 521–530. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Y.; Lu, Q.; Jiang, Y.; Jin, H.; Li, Q.; Yu, X. Development and evaluation of a novel margarine using starch hydrogel combined edible wax oleogel bigels. J. Food Eng. 2025, 388, 112360. [Google Scholar] [CrossRef]
- da Silva, R.F.; Ascheri, J.L.R.; de Souza, J.M.L. Influência do processo de beneficiamento na qualidade de amêndoas de castanha-do-brasil. Ciência Agrotecnol. 2010, 34, 445–450. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Codex Alimentarius: Fats and oils, 2nd ed.; Food and Agriculture Organization (FAO): Geneva, Switzerland, 2001; Volume 8.
- Yılmaz, E.; Öğütcü, M. Comparative analysis of olive oil organogels containing beeswax and sunflower wax with breakfast margarine. J. Food Sci. 2014, 79, E1732–E1738. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Freitas, M.P.; Pinheiro, A.C.M.; Bastos, S.C. Chemoface: A novel free user-friendly interface for chemometrics. J. Braz. Chem. Soc. 2012, 23, 2003–2010. [Google Scholar] [CrossRef]
- AOCS—American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS—American Oil Chemists’ Society. Method Cd 18-90. P-anisidine value. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; AOCS: Champaign, IL, USA, 2011. [Google Scholar]
- Ferreira, D.F. SISVAR: A program for statistical analysis and teaching. Rev. Cient. Symp. 2008, 6, 36–41. Available online: https://des.ufla.br/~danielff/meusarquivospdf/art63.pdf (accessed on 7 May 2025).





| Formulation | CS (g/100 g) | BW (g/100 g) | OO/CO (g/100 g) | Average Desirability |
|---|---|---|---|---|
| 1 | −1.00 (1.80) | −1.00 (2.80) | −1.00 (36.10/39.30) | 0.00 |
| 2 | −1.00 (1.80) | −1.00 (2.80) | 1.00 (53.90/21.50) | 0.85 |
| 3 | −1.00 (1.80) | 1.00 (5.20) | −1.00 (36.10/36.90) | 0.40 |
| 4 | −1.00 (1.80) | 1.00 (5.20) | 1.00 (53.90/19.10) | 0.82 |
| 5 | 1.00 (4.20) | −1.00 (2.80) | −1.00 (36.10/36.90) | 0.46 |
| 6 | 1.00 (4.20) | −1.00 (2.80) | 1.00 (53.90/19.10) | 0.36 |
| 7 | 1.00 (4.20) | 1.00 (5.20) | −1.00 (36.10/45.50) | 0.53 |
| 8 | 1.00 (4.20) | 1.00 (5.20) | 1.00 (53.90/16.70) | 0.46 |
| 9 | −1.68 (0.98) | 0.00 (4.00) | 0.00 (45.00/30.02) | 0.87 |
| 10 | 1.68 (5.02) | 0.00 (4.00) | 0.00 (45.00/25.98) | 0.76 |
| 11 | 0.00 (3.00) | −1.68 (1.98) | 0.00 (45.00/30.02) | 0.65 |
| 12 | 0.00 (3.00) | 1.68 (6.02) | 0.00 (45.00/25.98) | 0.81 |
| 13 | 0.00 (3.00) | 0.00 (4.00) | −1.68 (30.03/42.97) | 0.52 |
| 14 | 0.00 (3.00) | 0.00 (4.00) | 1.68 (59.97/13.03) | 0.47 |
| 15 | 0.00 (3.00) | 0.00 (4.00) | 0.00 (45.00/28.00) | 0.20 |
| 16 | 0.00 (3.00) | 0.00 (4.00) | 0.00 (45.00/28.00) | 0.10 |
| 17 | 0.00 (3.00) | 0.00 (4.00) | 0.00 (45.00/28.00) | 0.00 |
| Coefficient | Value | Error | t | p |
|---|---|---|---|---|
| b0 | 0.12 | 0.03 | 4.22 | 0.000464 |
| CS | −0.03 | 0.01 | −2.54 | 0.019896 |
| BW | 0.06 | 0.01 | 4.55 | 2.19 × 10−4 |
| OO/CO | 0.07 | 0.01 | 5.70 | 1.70 × 10−5 |
| CS × BW | −0.03 | 0.02 | −1.49 | 0.152451 |
| CS × OO/CO | −0.18 | 0.02 | −10.68 | 1.81 × 10−9 |
| BW × OO/CO | −0.05 | 0.02 | −3.02 | 7.04 × 10−3 |
| CS2 | 0.20 | 0.01 | 14.16 | 1.51 × 10−11 |
| BW2 | 0.17 | 0.01 | 12.07 | 2.35 × 10−10 |
| OO/CO2 | 0.09 | 0.01 | 6.30 | 4.79 × 10−6 |
| GL | SQ | QM | F | p-Value | |
|---|---|---|---|---|---|
| Regression | 2.04 | 8 | 0.26 | 9.90 | 4.23 × 10−6 |
| Residue | 0.64 | 25 | 0.03 | ||
| Lack of fit | 0.56 | 6 | 0.09 | 20.58 | 2.27 × 10−7 |
| Pure error | 0.09 | 19 | 0.00 | ||
| Total | 2.69 | 33 | |||
| R2 | 0.76 | ||||
| R2 adjusted | 0.68 |
| Ingredients | g/100 g |
|---|---|
| Aqueous phase 20%: | |
| Water * | 16.2 |
| Salt * | 2.0 |
| Powdered milk * | 1.8 |
| Lipid phase 80%: | |
| Extra virgin olive oil | 45–60 |
| Coconut oil | 40–55 |
| Beeswax | 4–5.2 |
| Corn starch | 1–1.8 |
| Monoacylglycerol * | 0.2 |
| Turmeric * | 0.03 |
| Butter scent * | 0.04 |
| Antioxidant * | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naves, B.V.B.; Silva, T.L.T.d.; Nunes, C.A.; Haddad, F.F.; Bastos, S.C. Development, Characterization, and Stability of Margarine Containing Oleogels Based on Olive Oil, Coconut Oil, Starch, and Beeswax. Gels 2025, 11, 513. https://doi.org/10.3390/gels11070513
Naves BVB, Silva TLTd, Nunes CA, Haddad FF, Bastos SC. Development, Characterization, and Stability of Margarine Containing Oleogels Based on Olive Oil, Coconut Oil, Starch, and Beeswax. Gels. 2025; 11(7):513. https://doi.org/10.3390/gels11070513
Chicago/Turabian StyleNaves, Bárbara Viana Barbosa, Thais Lomonaco Teodoro da Silva, Cleiton Antônio Nunes, Felipe Furtini Haddad, and Sabrina Carvalho Bastos. 2025. "Development, Characterization, and Stability of Margarine Containing Oleogels Based on Olive Oil, Coconut Oil, Starch, and Beeswax" Gels 11, no. 7: 513. https://doi.org/10.3390/gels11070513
APA StyleNaves, B. V. B., Silva, T. L. T. d., Nunes, C. A., Haddad, F. F., & Bastos, S. C. (2025). Development, Characterization, and Stability of Margarine Containing Oleogels Based on Olive Oil, Coconut Oil, Starch, and Beeswax. Gels, 11(7), 513. https://doi.org/10.3390/gels11070513








