Encapsulation of Lactobacillus reuteri in Chia–Alginate Hydrogels for Whey-Based Functional Powders
Abstract
1. Introduction
2. Results and Discussion
2.1. Encapsulation Efficiency (EE)
2.2. Micromorphology Analysis
2.2.1. Confocal Laser Scanning Microscopy (CLSM) Analysis
2.2.2. Micromorphology Analysis by SEM
2.3. Physicochemical Properties of the Functional Beverages
2.4. Determination of Moisture and Color
2.5. Structural and Molecular Characterization of Reconstituted Beverages by Raman Spectroscopy
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Chia Seed Mucilage Extraction
4.2.2. Activation and Growth of Lactobacillus reuteri DSM 17938
4.2.3. Solution Preparation
4.2.4. Preparation of Encapsulates by Electrohydrodynamic Atomization (EHDA) and Drip Mode (DM)
4.2.5. Encapsulation Efficiency (EE)
4.2.6. Lyophilization of Encapsulates
4.2.7. Microstructure Analysis
Confocal Laser Scanning Microscopy (CLSM) Analysis
Scanning Electron Microscopy (SEM)
4.3. Beverage Preparation
4.4. Physicochemical Characterization
4.4.1. Protein
4.4.2. Total Lipids
4.4.3. Fiber
4.4.4. Ash Percentage
4.4.5. Percentage of Total Carbohydrates
4.5. Color
4.6. Humidity
4.7. Raman Spectroscopy
4.8. Experimental Design
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony Forming Units |
| CLSM | Confocal Laser Scanning Microscopy |
| CM | Chia Mucilage |
| DM | Drip Mode |
| EHDA | Electrohydrodynamic Atomization |
| FTIR | Fourier Transform Infrared Spectroscopy |
| LR | Lactobacillus reuteri |
| SEM | Scanning Electron Microscopy |
References
- Mosquera-Vivas, E.; Ayala-Aponte, A.; Serna-Cock, L.; Torres-León, C.; Tirado, D.F. Viability of Lactobacillus reuteri DSM 17938 Encapsulated by Ionic Gelation during Refractance Window® Drying of a Strawberry Snack. Foods 2024, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D. (Ed.) Nutraceutical and Funcional Food Regulations in the United States and Around the Word, 2nd ed.; Elsevier: Houston, TX, USA, 2014; ISBN 978-0-12-405870-5. [Google Scholar]
- Niu, X.; Yin, X.; Wu, X.; Zhang, Q.; Jiang, Y.; He, J.; Zhao, Y.; Zhang, C.; Ren, Y.; Lai, M.; et al. Heat-killed Bifidobacterium longum BBMN68 in pasteurized yogurt alleviates mugwort pollen-induced allergic airway responses through gut microbiota modulation in a Murine model. Foods 2023, 12, 2049. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.D.L.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Valdespino-León, M.; Calderón-Domínguez, G. Liberación de glucosa oxidasa de cápsulas de mucílago de chía-alginato de sodio estresadas preparadas por electropulverización. Rev. Proces. Conserv. Aliment. 2021, 45, e15484. [Google Scholar]
- Coghetto, C.C.; Webster, K.; Silva, M.L.; Leite, B.; Castell-Palou, M.A. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. Colloids Surf. B Biointerfaces 2016, 146, 677–685. [Google Scholar] [CrossRef]
- Yang, S.; Wei, S.; Wu, Y.; Fang, Y.; Deng, Z.; Xu, J.; Zhang, H. Encapsulation techniques, action mechanisms, and evaluation models of probiotics: Recent advances and future prospects. Food Front. 2024, 5, 1212–1239. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.D.L.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Farrera-Rebollo, R.R.; Valdespino León, M.; Calderón-Domínguez, G. Effect of electrohydrodynamic atomization conditions on morphometric characteristics and mechanical resistance of chia mucilage-alginate particles. CyTA-J. Food 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Tian, Y.; Li, N.; Zheng, M.; Zhang, W.; Jiang, Y.; Zhao, Q.; Man, C. Recent advances in electrospray encapsulation of probiotics: Influencing factors, natural polymers and emerging technologies. Crit. Rev. Food Sci. Nutr. 2025, 1–18. [Google Scholar] [CrossRef]
- Librán, C.M.; Castro, S.; Lagaron, J.M. Encapsulation by electrospray coating atomization of probiotic strains. Innov. Food Sci. Emerg. Technol. 2017, 39, 216–222. [Google Scholar] [CrossRef]
- Premjit, Y.; Mitra, J. Synthesis, characterization, and in vitro digestion of electrosprayed and freeze-dried probiotics encapsulated in soy protein isolate-sunflower oil emulsions. Food Biosci. 2023, 53, 102532. [Google Scholar] [CrossRef]
- Hua, C.; Yang, F.; Jia, X.; Lu, Y.; Li, X.; Zhao, P.; Xing, M.; Lyu, G. Multi-comparted microgels delivering human derived probiotics and deferoxamine for multidrug-resistant infection and healing. Chem. Eng. J. 2024, 483, 148432. [Google Scholar] [CrossRef]
- Perea-Flores, M.D.J.; Aguilar-Morán, H.F.; Calderón-Domínguez, G.; García-Hernández, A.B.; Díaz-Ramírez, M.; Romero-Campos, H.E.; Cortés-Sánchez, A.D.J.; Salgado-Cruz, M.d.l.P. Entrapment efficiency (EE) and release mechanism of rhodamine B encapsulated in a mixture of chia seed mucilage and sodium alginate. Appl. Sci. 2020, 13, 1213. [Google Scholar] [CrossRef]
- Silva e Alves, A.T.; Spadoti, L.M.; Zacarchenco, P.B.; Trento, F.K. Probiotic functional carbonated whey beverages: Development and quality evaluation. Beverages 2018, 4, 49. [Google Scholar] [CrossRef]
- Malos, I.G.; Ghizdareanu, A.I.; Vidu, L.; Matei, C.B.; Pasarin, D. The role of whey in functional microorganism growth and metabolite generation: A biotechnological perspective. Foods 2025, 14, 1488. [Google Scholar] [CrossRef]
- Lee, D.W.; Hwang, S.J.; Park, J.B.; Park, H.J. Preparation and release characteristics of polymer-coated and blended alginate microspheres. J. Microencapsul. 2003, 20, 179–192. [Google Scholar] [CrossRef]
- Popović, M.; Stojanović, M.; Veličković, Z.; Kovačević, A.; Miljković, R.; Mirković, N.; Marinković, A. Characterization of potential probiotic strain, L. reuteri B2, and its microencapsulation using alginate-based biopolymers. Int. J. Biol. Macromol. 2021, 183, 423–434. [Google Scholar] [CrossRef]
- Dragoni-Rosado, J.J. Micro-encapsulación de Lactobacillus Casei y el Efecto Sobre la Supervivencia Durante el Procesamiento del Yogurt y Bajo Condiciones Simuladas del Estómago Humano. Ph.D. Thesis, Universidad De Puerto Rico, San Juan, Puerto Rico, 2014. [Google Scholar]
- Huang, H.Y.; Tang, Y.J.; King, V.A.E.; Chou, J.W.; Tsen, J.H. Properties of Lactobacillus reuteri chitosan-calcium-alginate encapsulation under simulated gastrointestinal conditions. Int. Microbiol. 2015, 18, 61–69. [Google Scholar] [CrossRef]
- Gowda, H.; Ivanisevic, J.; Johnson, C.H.; Kurczy, M.E.; Benton, H.P.; Rinehart, D.; Siuzdak, G. Interactive XCMS Online: Simplifying Advanced Metabolomic Data Processing and Subsequent Statistical Analyses. Anal. Chem. 2014, 86, 6931–6939. [Google Scholar] [CrossRef]
- Nasiri, H.; Golestan, L.; Shahidi, S.A.; Darjani, P. Encapsulation of Lactobacillus casei in sodium alginate microcapsules: Improvement of the bacterial viability under simulated gastrointestinal conditions using wild sage seed mucilage. J. Food Meas. Charact. 2021, 15, 4726–4734. [Google Scholar] [CrossRef]
- Hernández San José, C. Análisis de Distribuciones de Carga Espacial Emitidas por Atomización Electrohidrodinámica (Electrospray) en Vacío. Ph.D. Thesis, Universidad Nacional de Educación a Distancia, Madrid, Spain, 2018. Portal Científico UNED. Available online: https://portalcientifico.uned.es/documentos/5f63fc8d29995274fc8e8d01 (accessed on 1 July 2025).
- Bustamante, M.; Oomah, B.D.; Burgos-Díaz, C.; Vergara, D.; Flores, L.; Shene, C. Viability of Microencapsulated Probiotics in Crosslinked Alginate Matrices and Chia Seed Mucilage during Spray-Drying and Storage. Microorganisms 2025, 13, 1457. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; López-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT-Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Retention of Encapsulated Lactobacillus plantarum in Spray-Dried Milk–Whey Powder during Storage and Simulated Gastrointestinal Conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Chacón-Gurrola, L.R.; Chávez, A.; Rentería-Monterrubio, A.L.; Rodríguez-Figueroa, J.C. Proteínas del lactosuero: Usos, relación con la salud y bioactividades. Interciencia 2017, 42, 712–718. [Google Scholar]
- Shiby, V.K.; Radhakrishna, K.; Bawa, A.S. Development of whey-fruit-based energy drink mixes using D-optimal mixture design. Int. J. Food Sci. Technol. 2013, 48, 742–748. [Google Scholar] [CrossRef]
- Sepúlveda Valencia, J.U.; Flórez Flórez, L.E.; Peña Álvarez, C.M. Utilización de lactosuero de queso fresco en la elaboración de una bebida fermentada con adición de pulpa maracuyá (passiflora edulis) variedad púrpura y carbóximetil celulosa (cmc), enriquecida con vitaminas ay d. Rev. Fac. Nac. Agron. Medellín 2002, 2, 1633–1674. [Google Scholar]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Aziz, M.G.; Yusof, Y.A.; Blanchard, C.; Saifullah, M.; Farahnaky, A.; Scheiling, G. Material properties and tableting of fruit powders. Food Eng. Rev. 2018, 10, 66–80. [Google Scholar] [CrossRef]
- Zamani, H.; Zamani, S.; Zhang, Z.; Abbaspourrad, A. Exceptional colloidal stability of acidified whey protein beverages stabilized by soybean soluble polysaccharide. J. Food Sci. 2020, 85, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Miralles, B.; Recio, I.; López-Rubio, A. Microencapsulation of a whey protein hydrolysate within micro-hydrogels: Impact on gastrointestinal stability and potential for functional yoghurt development. J. Funct. Foods 2016, 26, 290–300. [Google Scholar] [CrossRef]
- Liang, L.I.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Sabater, C.; Ramírez, J.; Blanco, L.; Torres, A. Effect of Low-Esterified Pectin Interactions with Milk Proteins in Reduced-Syneresis Yogurts: Consequences on Carbohydrate Retention and Compositional Profile. J. Dairy Sci. 2020, 103, 2342–2353. [Google Scholar]
- Arcila, N.; Mendoza, Y. Elaboración de una bebida instantánea a base de semillas de amaranto (Amaranthus cruentus) y su uso potencial en la alimentación humana. Rev. Fac. Agron. 2006, 23, 114–124. [Google Scholar]
- Hernández-Rojas, M.; Vélez-Ruiz, J.F. Suero de leche y su aplicación en la elaboración de alimentos funcionales. Temas Sel. Ing. Aliment. 2014, 8, 13–22. [Google Scholar]
- Laurent, M.A.; Boulenguer, P. Stabilization mechanism of acid dairy drinks (ADD) induced by pectin. Food Hydrocoll. 2003, 17, 445–454. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Ren, S.; Jiménez-Flores, R.; Giusti, M.M. The interactions between anthocyanin and whey protein: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5992–6011. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment concentration of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Tadapaneni, R.K.; Banaszewski, K.; Patazca, E.; Edirisinghe, I.; Cappozzo, J.; Jackson, L.; Burton-Freeman, B. Effect of high-pressure processing and milk on the anthocyanin composition and antioxidant capacity of strawberry-based beverages. J. Agric. Food Chem. 2012, 60, 5795–5802. [Google Scholar] [CrossRef]
- Etzion, Y.; Linker, R.; Cogan, U.; Shmulevich, I. Determination of protein concentration in raw milk by mid-infrared Fourier transform infrared/attenuated total reflectance spectroscopy. J. Dairy Sci. 2004, 87, 2779–2788. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Valdespino-León, M.; Calderón-Domínguez, G. Glucose oxidase release of stressed chia mucilage-sodium alginate capsules prepared by electrospraying. J. Food Process Preserv. 2021, 45, e15484. [Google Scholar] [CrossRef]
- Castro Crespo, A. Cultivo de Lactobacillus Reuteri en Solitario y en Cocultivo con Escherichia Coli a 37 °C. Bachelor’s Thesis, Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá, Colombia, 2020. [Google Scholar]
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; Askari, G. Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Activity of cecropin P1 and FA-LL-37 against urogenital microflora. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Ramzan, R.; Zhang, R.; Zhang, M. Resistant starch-based edible coating composites for spray-dried microencapsulation of Lactobacillus acidophilus: Thermal protection, in vitro digestion, and physicochemical characteristics. Coatings 2021, 11, 587. [Google Scholar] [CrossRef]
- Cuellas, A.V.; Wagner, J.R. Elaboración de bebida energizante a partir de suero de quesería. INNOTEC 2010, 5, 54–57. [Google Scholar]
- Lai, J.; Wu, R.; Wang, J.; Wang, Y.; Zhang, X.; Zhou, L.; Zhu, Y. Effect of cooking modes on quality and flavor characteristic in Clitocybe squamulose chicken soup. Front. Nutr. 2022, 9, 1048352. [Google Scholar] [CrossRef]
- Guaman Rivera, S.A.; Guerrero-Pincay, A.E.; Ortiz-Naveda, N.R.; González-Marcillo, R.L. Prediction of the nutritional values by INRA in grass forage: Crude fiber was analyzed according to the Weende method by acid hydrolysis with 1.25% H2SO4, followed by alkaline hydrolysis with 1.25% NaOH. J. Agric. Environ. Int. Dev. 2023, 117, 117–140. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Colín-Alvarez, M.D.L.; Gaona-Sánchez, V.A.; Chalapud, M.C.; García-Hernández, A.B.; León-Espinosa, E.B.; Valdespino-León, M.; Serrano-Villa, F.S.; Calderón-Domínguez, G. Characterization and Applications of the Pectin Extracted from the Peel of Passiflora tripartita var. mollissima. Membranes 2023, 13, 797. [Google Scholar] [CrossRef]
- Rojas-Candelas, L.E.; Díaz-Ramírez, M.; Rayas-Amor, A.A.; Cruz-Monterrosa, R.G.; Méndez-Méndez, J.V.; Salgado-Cruz, M.D.L.P.; Calderón-Domínguez, G.; Cortes-Sanchez, A.D.J.; González-Vázquez, M. Development of Biodegradable Films Produced from Residues of Nixtamalization of Popcorn. Appl. Sci. 2023, 13, 8436. [Google Scholar] [CrossRef]
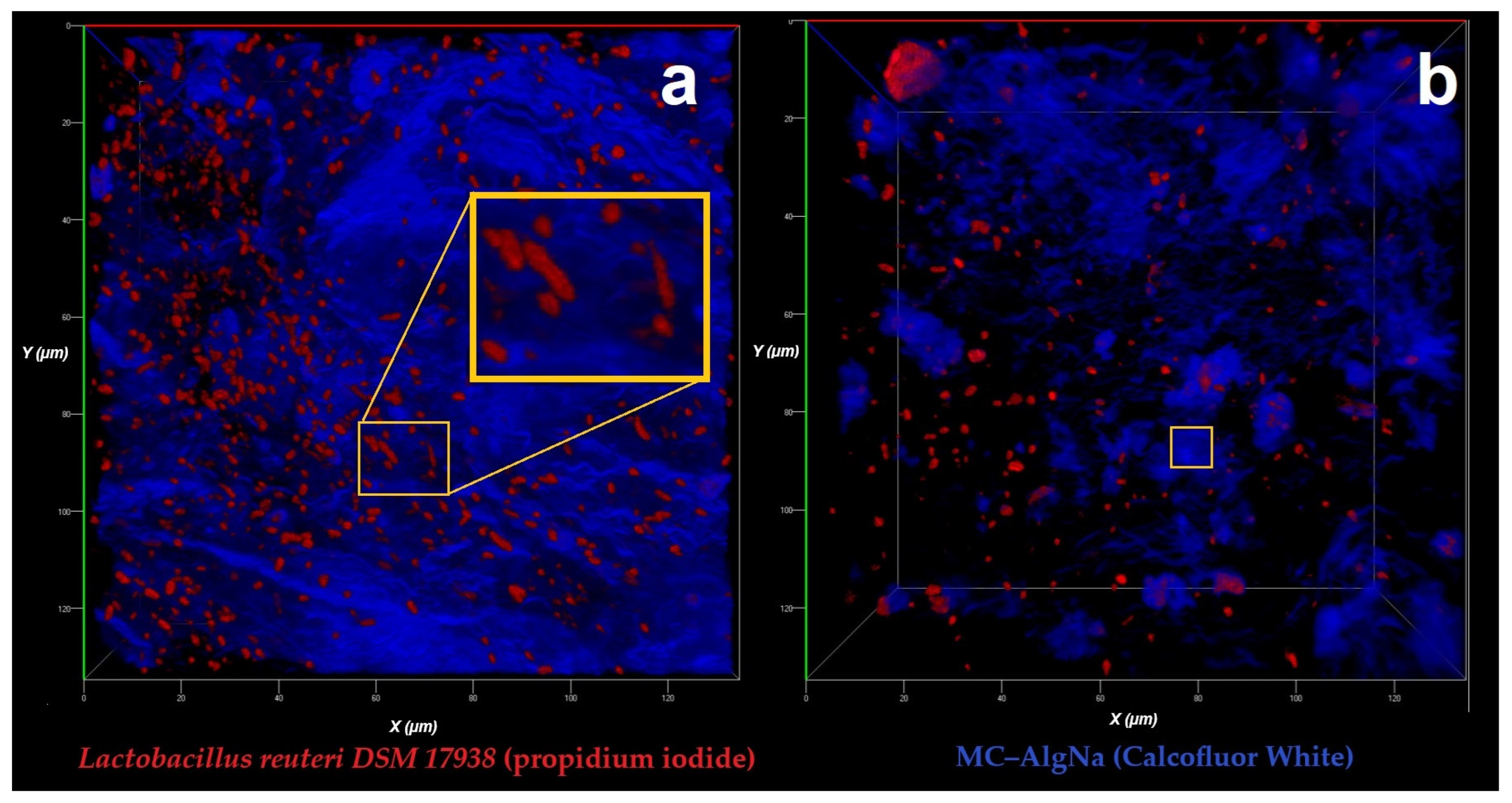
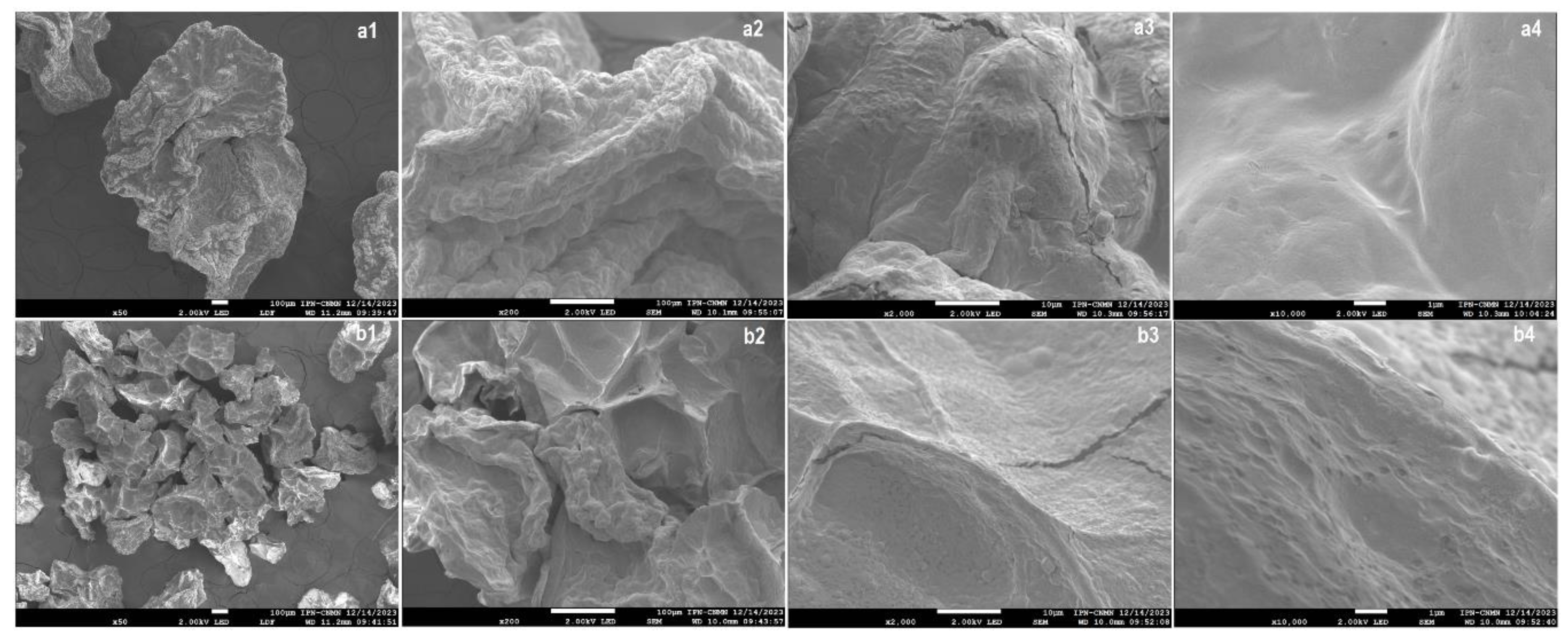
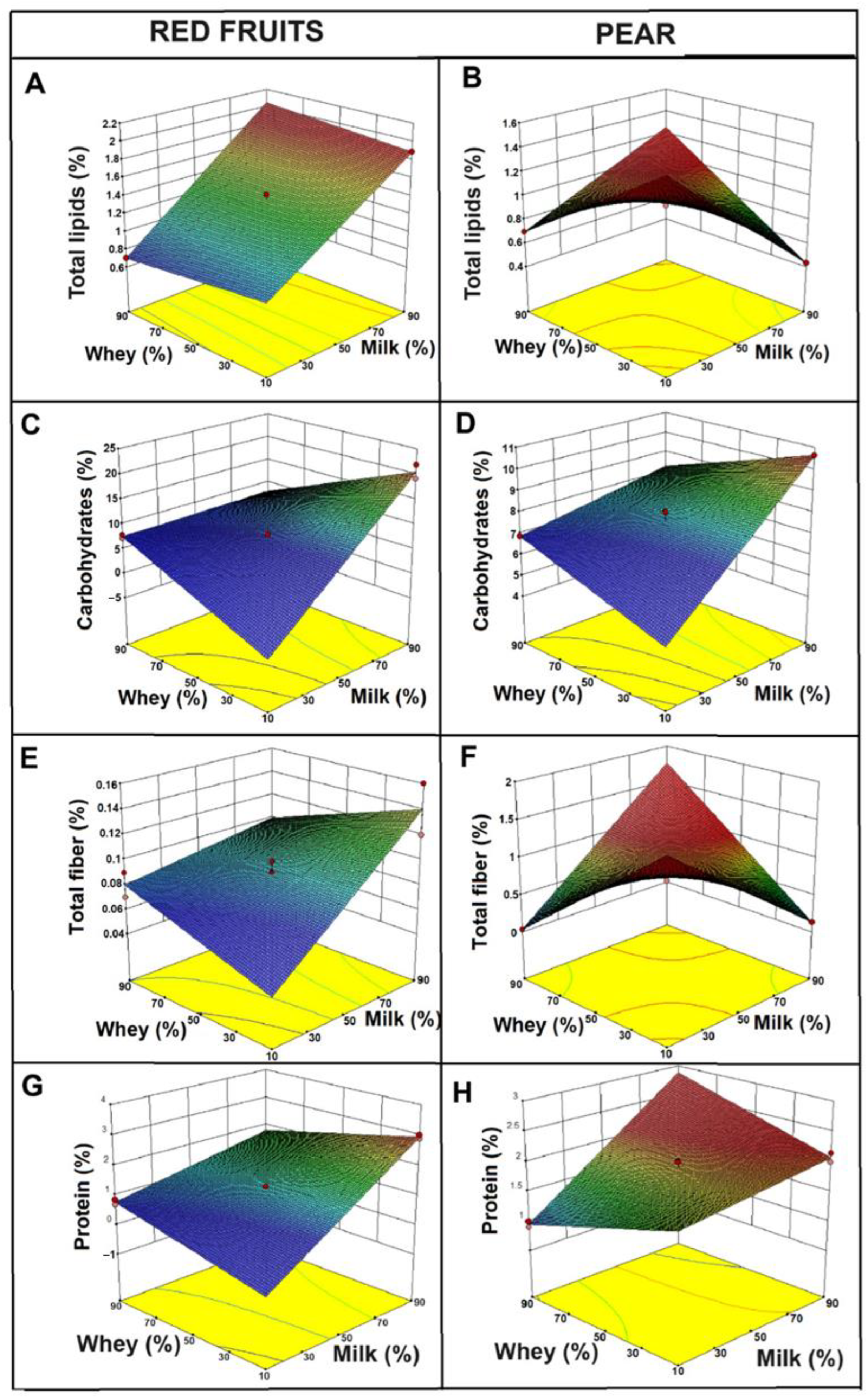
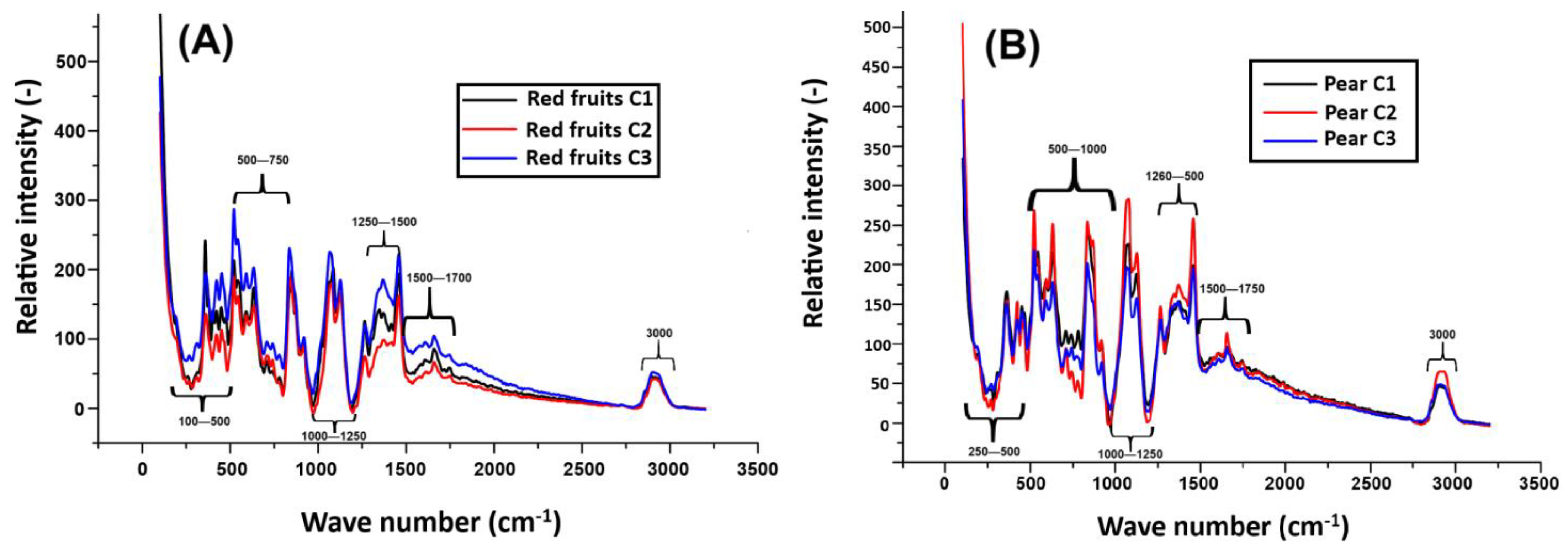
| Encapsulation Method | Viability (log CFU/mL) | EE (%) |
|---|---|---|
| Dripping mode (DM) | 9.90 ± 0.05 a | 99.00 ± 0.16 a |
| Electrohydrodynamic spraying (EHDA) | 9.91 ± 0.04 a | 99.00 ± 0.01 a |
| Parameter | Concentration (Milk/Whey) | Pear Flavor | Red Fruits |
|---|---|---|---|
| Moisture (%) | 10/90 | 4.13 ± 0.12 a | 3.6 ± 0.2 b |
| Color (L*) | 76.16 ± 10.47 a | 44.06 ± 5.44 b | |
| (a*) | 1.92 ± 0.56 b | 11.21 ± 7.27 a | |
| (b*) | 14.87 ± 11.66 a | 1.43 ± 1.44 b | |
| Moisture (%) | 50/50 | 4.6 ± 0.13 a | 3.8 ± 0.6 b |
| Color (L*) | 74.91 ± 5.70 a | 41.58 ± 5.34 b | |
| (a*) | 7.20 ± 9.00 a | 7.2 ± 8.87 a | |
| (b*) | 0.53 ± 0.79 a | 0.53 ± 0.79 a | |
| Moisture (%) | 90/10 | 3.98 ± 0.13 a | 2.53 ± 0.31 b |
| Color (L*) | 71.43 ± 7.20 a | 44.11 ± 4.07 b | |
| (a*) | 5.90 ± 4.10 b | 18.54 ± 4.57 a | |
| (b*) | 1.00 ± 0.60 a | 0.63 ± 1.56 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid-Córdoba, A.Y.; Calderón-Domínguez, G.; Perea-Flores, M.d.J.; Peña-Barrientos, A.; Serrano-Villa, F.S.; Barrios-Francisco, R.; González-Vázquez, M.; Rentería-Ortega, M. Encapsulation of Lactobacillus reuteri in Chia–Alginate Hydrogels for Whey-Based Functional Powders. Gels 2025, 11, 613. https://doi.org/10.3390/gels11080613
Cid-Córdoba AY, Calderón-Domínguez G, Perea-Flores MdJ, Peña-Barrientos A, Serrano-Villa FS, Barrios-Francisco R, González-Vázquez M, Rentería-Ortega M. Encapsulation of Lactobacillus reuteri in Chia–Alginate Hydrogels for Whey-Based Functional Powders. Gels. 2025; 11(8):613. https://doi.org/10.3390/gels11080613
Chicago/Turabian StyleCid-Córdoba, Alma Yadira, Georgina Calderón-Domínguez, María de Jesús Perea-Flores, Alberto Peña-Barrientos, Fátima Sarahi Serrano-Villa, Rigoberto Barrios-Francisco, Marcela González-Vázquez, and Minerva Rentería-Ortega. 2025. "Encapsulation of Lactobacillus reuteri in Chia–Alginate Hydrogels for Whey-Based Functional Powders" Gels 11, no. 8: 613. https://doi.org/10.3390/gels11080613
APA StyleCid-Córdoba, A. Y., Calderón-Domínguez, G., Perea-Flores, M. d. J., Peña-Barrientos, A., Serrano-Villa, F. S., Barrios-Francisco, R., González-Vázquez, M., & Rentería-Ortega, M. (2025). Encapsulation of Lactobacillus reuteri in Chia–Alginate Hydrogels for Whey-Based Functional Powders. Gels, 11(8), 613. https://doi.org/10.3390/gels11080613










