Clinical Safety and Efficacy of Hyaluronic Acid–Niacinamide–Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening
Abstract
1. Introduction
2. Results and Discussion
2.1. Global Aesthetic Improvement Scales
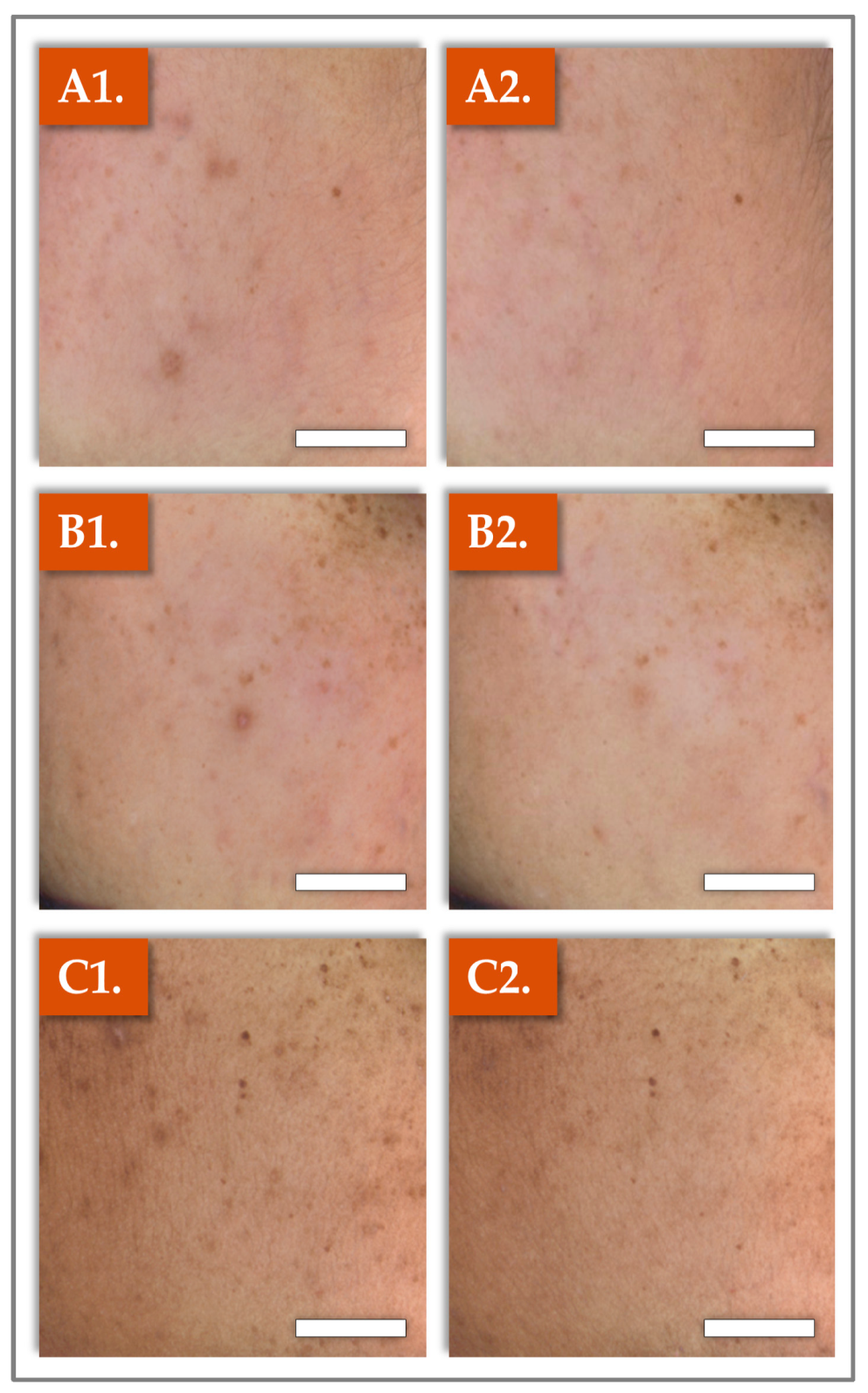
2.2. Clinical Photography Results
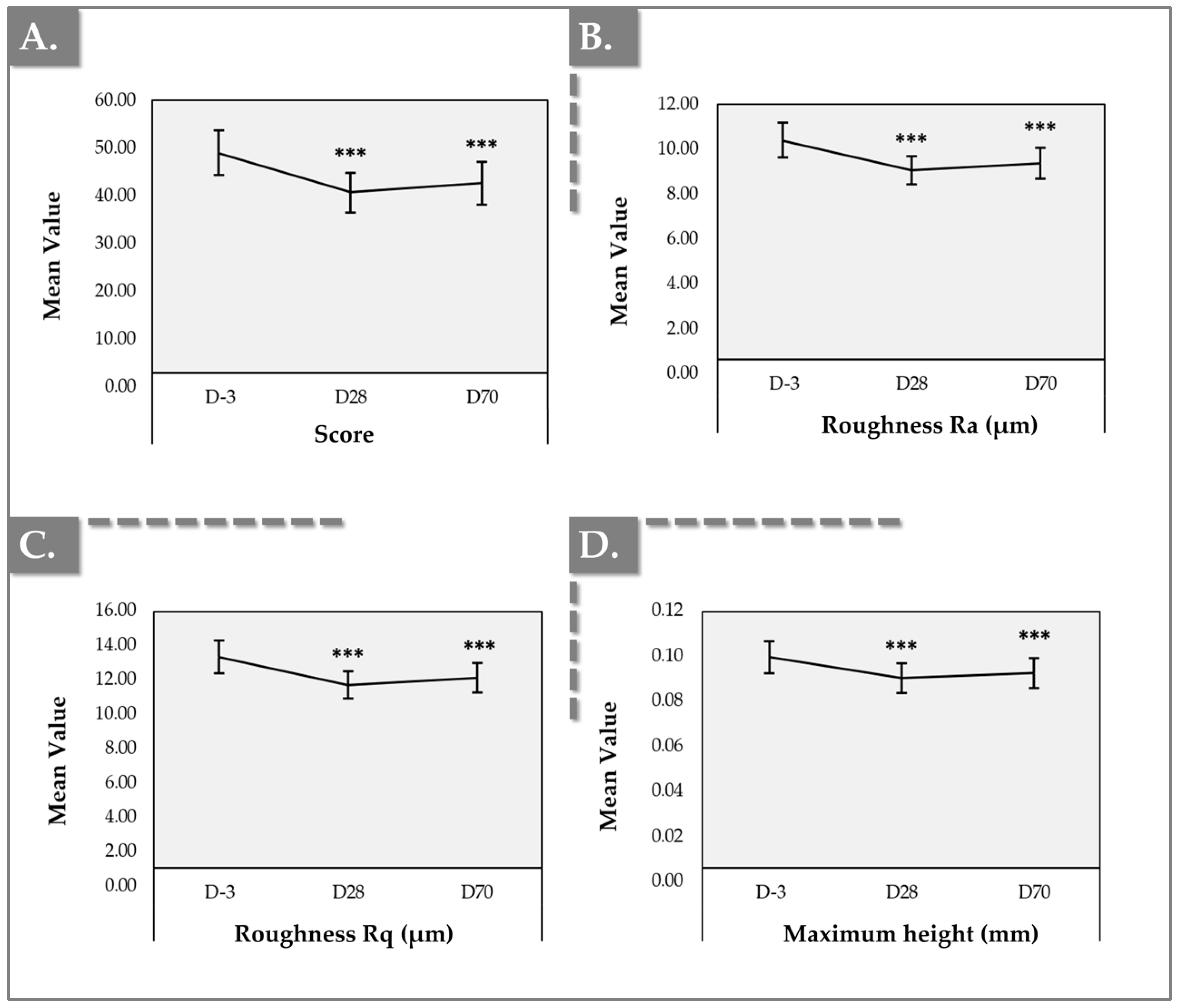
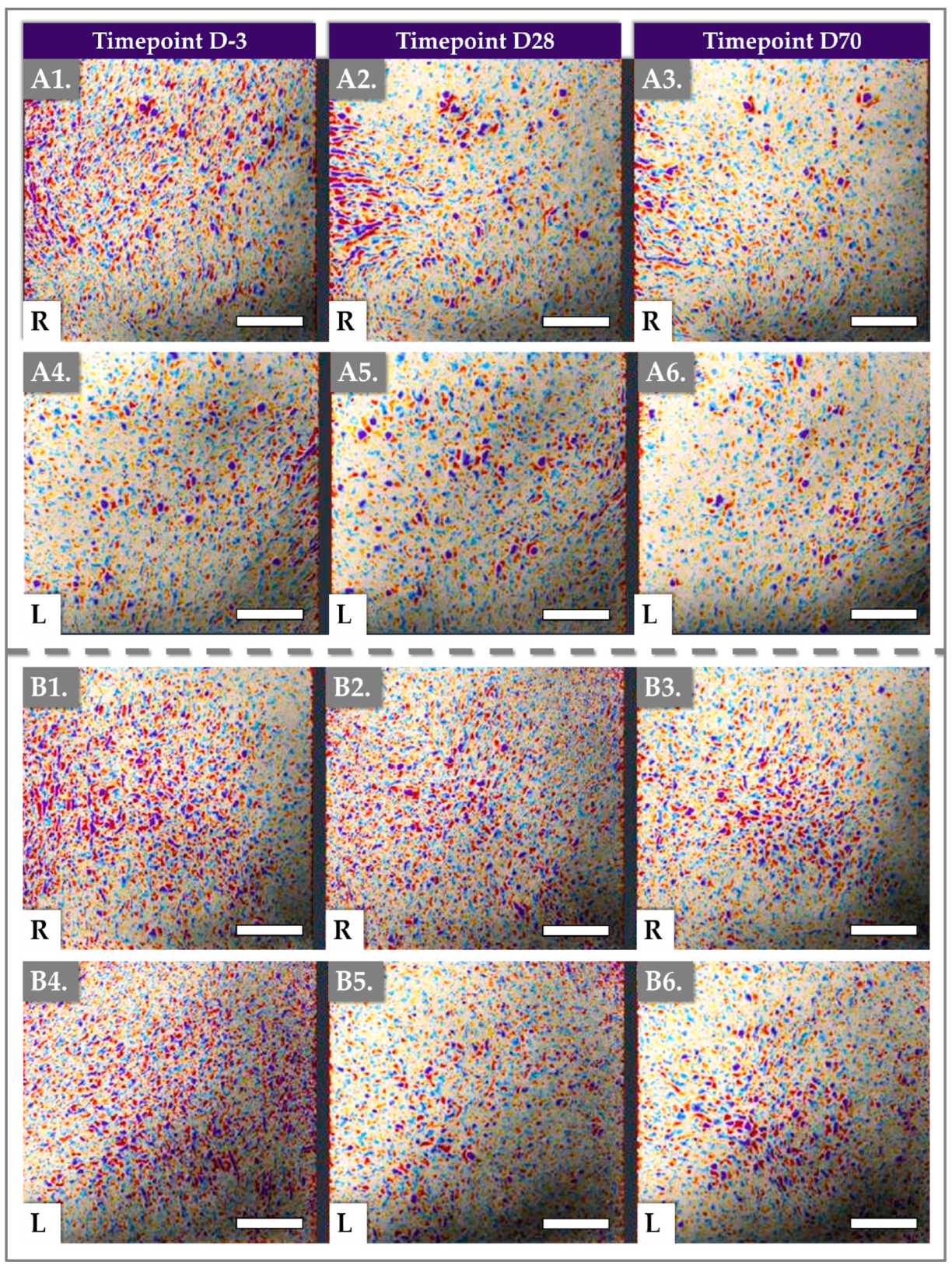
2.3. Antera 3D Results for Skin Texture and Roughness
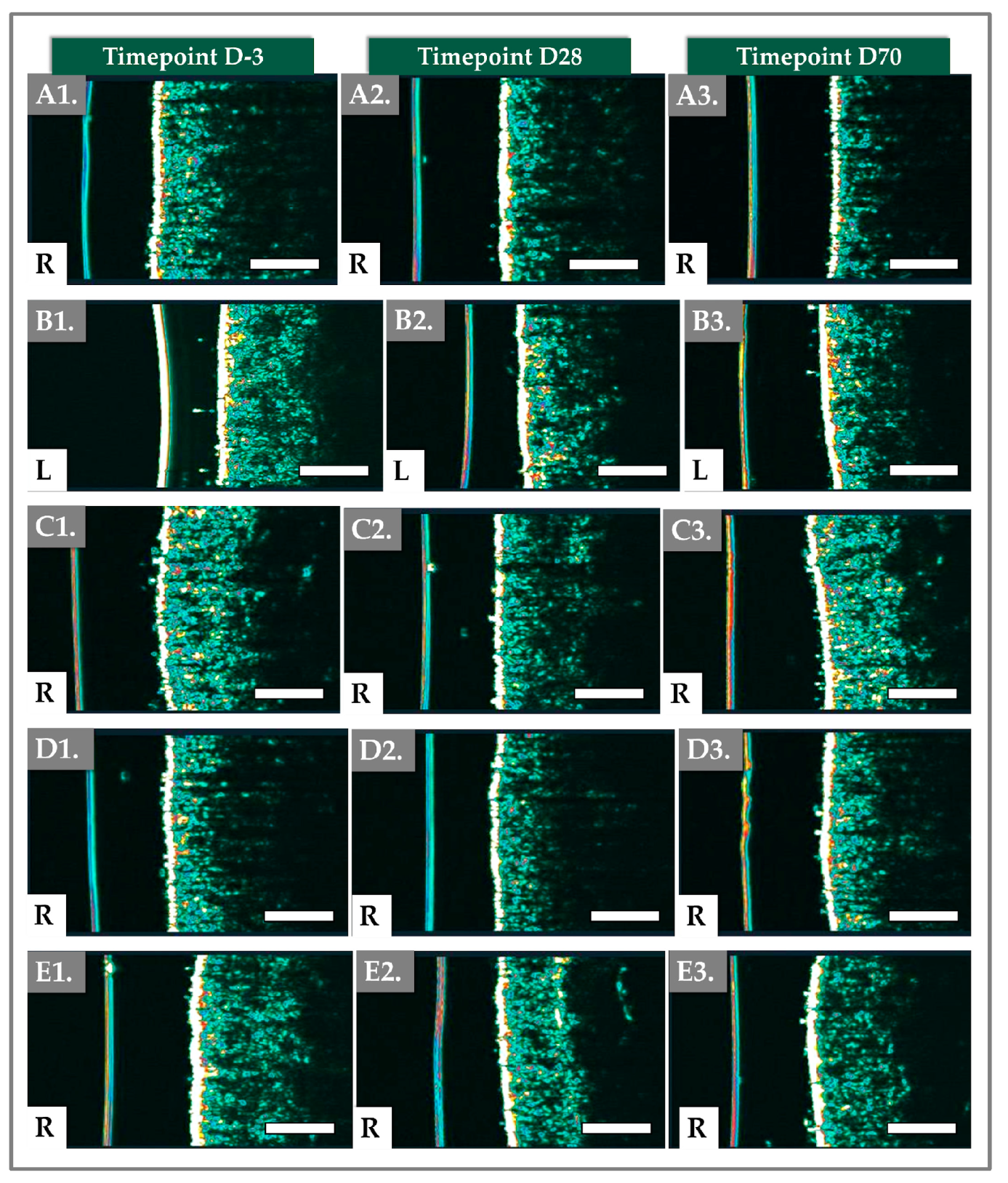
2.4. DermaScan Results for Dermal Structure and Thickness
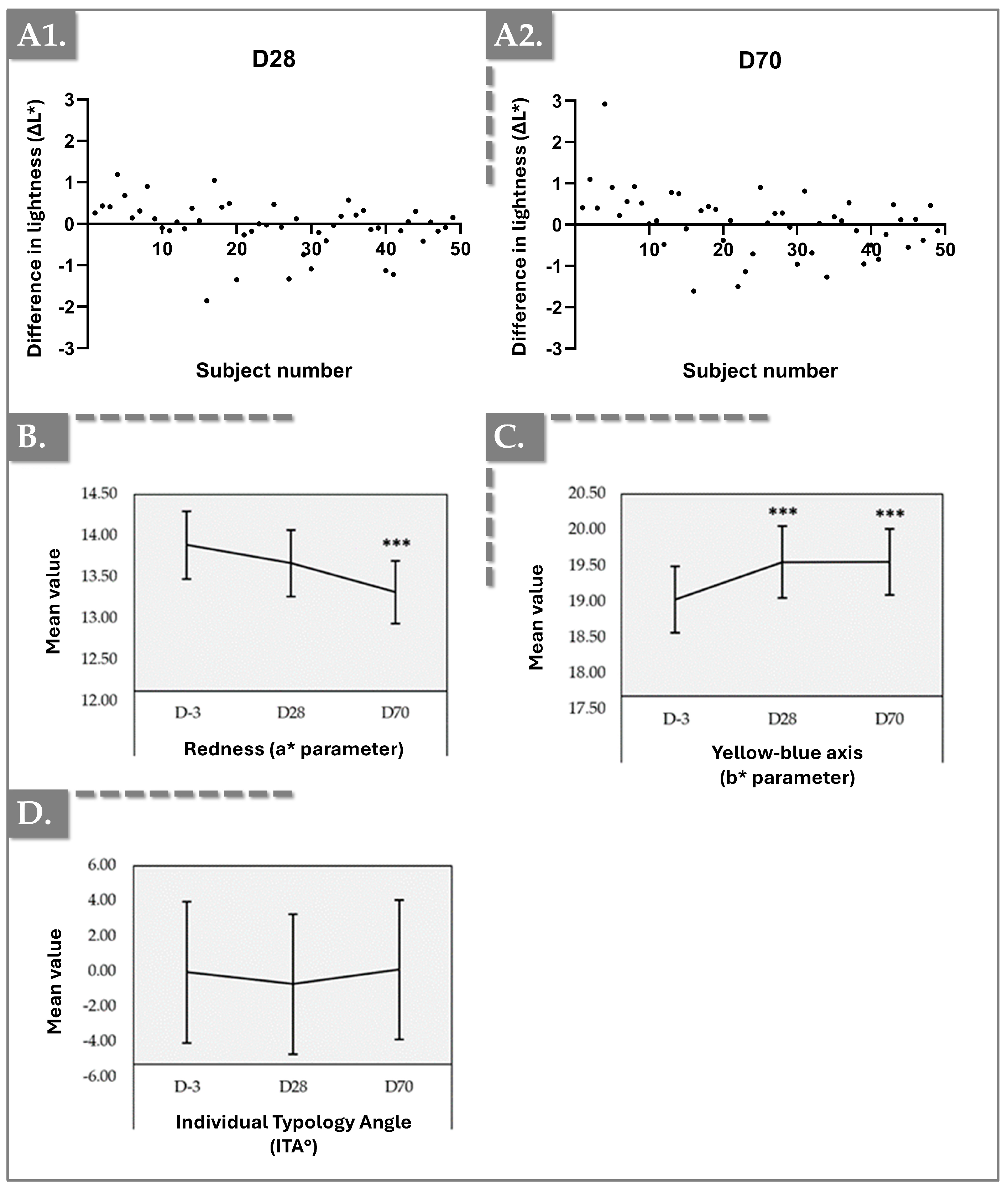
2.5. Chromameter Results for Skin Tone and Color Balance
2.6. Cutometer Results for Skin Elasticity and Firmness
2.7. Corneometer Results for Skin Hydration
2.8. Safety and Injectability Evaluation Results
2.9. General Discussion
2.9.1. Combining Injectable Ingredients for Enhanced Aesthetic Function
2.9.2. Focus on the Physical Properties of Hydragel A1
2.9.3. Linking Product Formulation Attributes with Clinical Results
2.9.4. Outlook on the Temporality of the Clinical Results
2.9.5. Considerations on Injection Depth and Dermal Targeting
2.9.6. Confirmed Safety and Injectability of Hydragel A1
2.10. Study Limitations and Future Perspectives
3. Conclusions
4. Materials and Methods
4.1. Clinical Study Design
4.2. Patient Selection Methodology
4.3. Investigational Test Item
4.4. Administration Protocol and Injection Technique
4.5. Outcome Endpoints
4.5.1. Efficacy Endpoints
- Skin roughness and texture: Antera 3D device (Miravex, Dublin, Ireland).
- Skin density: Dermascan C USB Ultrasound System (Cortex, Aalborg, Denmark).
- Skin brightness: Chromameter CR400 instrument (Konica Minolta, Tokyo, Japan).
- Skin firmness and elasticity: Cutometer® dual MPA 580 instrument (Courage + Khazaka electronic GmbH, Köln, Germany).
- Skin hydration: Corneometer CM 825 instrument (Courage + Khazaka electronic GmbH, Köln, Germany).
- Macroscopic skin images: DERMLITE lens on a Dermlite Foto Pro II, mounted on a DSLR camera, capturing small areas on both cheeks.
4.5.2. Safety and Tolerability Endpoints
4.5.3. Injector’s Assessment of Ease of Product Use
4.6. Statistical Analyses and Data Presentation
- H0: There is no difference between the two timepoints compared.
- H1: There is a difference between the two timepoints compared.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | adverse event |
| CRO | contract research organization |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| G′ | storage modulus |
| G″ | loss modulus |
| GAIS | Global Aesthetic Improvement Scale |
| GCP | good clinical practices |
| HA | hyaluronic acid |
| IGAIS | Investigator Global Aesthetic Improvement Scale |
| ISR | injection site reactions |
| ITA | individual typological angle |
| LED | light-emitting diode |
| mRNA | messenger ribonucleic acid |
| NA | non-applicable |
| ns | non-significant |
| Pa | Pascals |
| Pa·s | Pascal seconds |
| PGE2 | prostaglandin E2 |
| ROS | reactive oxygen species |
| s | second |
| SAE | serious adverse event |
| SGAIS | Subject Global Aesthetic Improvement Scale |
| TGF | transforming growth factor |
| TXA | tranexamic acid |
| USA | United States of America |
| UV | ultraviolet |
| UVA | ultraviolet A |
| UVB | ultraviolet B |
References
- Markiewicz, E.; Karaman-Jurukovska, N.; Mammone, T.; Idowu, O.C. Post-inflammatory hyperpigmentation in dark skin: Molecular mechanism and skincare implications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Saade, D.S.; Maymone, M.B.C.; De La Garza, H.; Secemsky, E.A.; Kennedy, K.F.; Vashi, N.A. Trends in use of prescription skin lightening creams. Int. J. Environ. Res. Public Health 2021, 18, 5650. [Google Scholar] [CrossRef] [PubMed]
- Hsin, S.; Lourenço, K.; Porcello, A.; Marques, C.; Rodriguez, C.; Raffoul, W.; Scaletta, C.; Abdel-Sayed, P.; Hadjab, B.; Applegate, L.A.; et al. Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients. Cosmetics 2024, 11, 168. [Google Scholar] [CrossRef]
- Karrabi, M.; Mansournia, M.A.; Sharestanaki, E.; Abdollahnejad, Y.; Sahebkar, M. Clinical evaluation of efficacy and tolerability of cysteamine 5% cream in comparison with tranexamic acid mesotherapy in subjects with melasma: A single-blind, randomized clinical trial study. Arch. Dermatol. Res. 2021, 313, 539–547. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, K.S. Novel formulations for topical delivery of tranexamic acid: Assessing the need of epidermal targeting for hyperpigmentation disorders. Expert Opin. Drug Deliv. 2023, 20, 773–783. [Google Scholar] [CrossRef]
- Greene, J.J.; Sidle, D.M. The hyaluronic acid fillers: Current understanding of the tissue device interface. Facial Plast. Surg. Clin. N. Am. 2015, 23, 423–432. [Google Scholar] [CrossRef]
- Wu, G.T.; Kam, J.; Bloom, J.D. Hyaluronic acid basics and rheology. Facial Plast. Surg. Clin. N. Am. 2022, 30, 301–308. [Google Scholar] [CrossRef]
- Abd Elraouf, I.G.; Obaid, Z.M.; Fouda, I. Intradermal injection of tranexamic acid versus platelet-rich plasma in the treatment of melasma: A split-face comparative study. Arch. Dermatol. Res. 2023, 315, 1763–1770. [Google Scholar] [CrossRef]
- Rho, N.K.; Kim, H.S.; Kim, S.Y.; Lee, W. Injectable “Skin Boosters” in aging skin rejuvenation: A current overview. Arch. Plast. Surg. 2024, 51, 528–541. [Google Scholar] [CrossRef]
- Ghatge, A.S.; Ghatge, S.B. The effectiveness of injectable hyaluronic acid in the improvement of the facial skin quality: A systematic review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 891–899. [Google Scholar] [CrossRef]
- Wang, F.; Do, T.T.; Smith, N.; Orringer, J.S.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Implications for cumulative and prolonged clinical improvement induced by cross-linked hyaluronic acid: An in vivo biochemical/microscopic study in humans. Exp. Dermatol. 2024, 33, e14998. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cos. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–865. [Google Scholar] [CrossRef]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Boo, Y.C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.M.; Said Abdelshafy, A.; Khattab, F.; Gharib, K. Tranexamic acid for melasma treatment: A split-face study. Dermatol. Surg. 2020, 46, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Panchal, V.S.; Patel, Y.S.; Dalal, Y.D.; Parikh, A.P.; Dalal, A.D.; Rana, D.A. Efficacy of oral, topical, and intradermal tranexamic acid in patients with melasma—A meta-analysis. Indian Dermatol. Online J. 2023, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xue, J.; Wang, Q. Tranexamic acid for the treatment of hyperpigmentation and telangiectatic disorders other than melasma: An update. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2151–2163. [Google Scholar] [CrossRef]
- Alsharif, S.H.; Alghamdi, A.S.; Alwayel, Z.A.; Alaklabi, S.N.; Alyamani, N.A.; Sabsabee, M.A.; Bu Izran, D.A.A.; Alajlan, A.M. Efficacy and best mode of delivery for tranexamic acid in post-inflammatory hyperpigmentation: A systematic review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2873–2882. [Google Scholar] [CrossRef]
- Badran, A.Y.; Ali, A.U.; Gomaa, A.S. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: A randomised clinical trial. Australas. J. Dermatol. 2021, 62, e373–e379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Yang, Y.; Gong, X.; Qian, H. The stratum corneum barrier: Impaired function in relation to associated lipids and proteins. Tissue Barriers 2024, 2361197. [Google Scholar] [CrossRef]
- Basto, R.; Andrade, R.; Nunes, C.; Lima, S.A.C.; Reis, S. Topical delivery of niacinamide to skin using hybrid nanogels enhances photoprotection effect. Pharmaceutics 2021, 13, 1968. [Google Scholar] [CrossRef]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic insights into the multiple functions of niacinamide: Therapeutic implications and cosmeceutical applications in functional skincare products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Abuyousif, H.S.; Porcello, A.; Cerrano, M.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Chemali, M.; Raffoul, W.; Hirt-Burri, N.; et al. In vitro evaluation and clinical effects of a regenerative complex with non-cross-linked hyaluronic acid and a high-molecular-weight polynucleotide for periorbital treatment. Polymers 2025, 17, 638. [Google Scholar] [CrossRef]
- Porcello, A.; Chemali, M.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Dual functionalization of hyaluronan dermal fillers with vitamin B3: Efficient combination of bio-stimulation properties with hydrogel system resilience enhancement. Gels 2024, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Calacattawi, R.; Alshahrani, M.; Aleid, M.; Aleid, F.; Basamih, K.; Alsugair, G.; Alqahtani, R.; AlKhabbaz, N.; Algaidi, Y.; Alrakayan, L.; et al. Tranexamic acid as a therapeutic option for melasma management: Meta-analysis and systematic review of randomized controlled trials. J. Dermatol. Treat. 2024, 35, 2361106. [Google Scholar] [CrossRef]
- Lindgren, A.L.; Austin, A.H.; Welsh, K.M. The use of tranexamic acid to prevent and treat post-inflammatory hyperpigmentation. J. Drugs Dermatol. 2021, 20, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.S.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Skin depigmenting agents in anti-aging cosmetics: A medicinal perspective on emerging ingredients. Appl. Sci. 2022, 12, 775. [Google Scholar] [CrossRef]
- Konisky, H.; Balazic, E.; Jaller, J.A.; Khanna, U.; Kobets, K. Tranexamic acid in melasma: A focused review on drug administration routes. J. Cosmet. Dermatol. 2023, 22, 1197–1206. [Google Scholar] [CrossRef]
- Khatri, K.A.; Abdullah, N.A.; Chia, S.; Ng, E.; Thibroni, N. Efficacy, safety, satisfaction, adherence to treatment with nano-formulated cysteamine tranexamic acid cream to treat melasma. J. Drugs Dermatol. 2024, 23, 529–537. [Google Scholar] [PubMed]
- Guo, J.; Fang, W.; Wang, F. Injectable fillers: Current status, physicochemical properties, function mechanism, and perspectives. RSC Adv. 2023, 13, 23841–23858. [Google Scholar] [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The rheology and physicochemical characteristics of hyaluronic acid fillers: Their clinical implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef] [PubMed]
- de la Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and physicochemical characteristics of hyaluronic acid fillers: Overview and relationship to product performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef]
- Fagien, S.; Bertucci, V.; von Grote, E.; Mashburn, J.H. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast. Reconstr. Surg. 2019, 143, 707e–720e. [Google Scholar] [CrossRef]
- Wongprasert, P.; Dreiss, C.A.; Murray, G. Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol. Ther. 2022, 35, e15453. [Google Scholar] [CrossRef] [PubMed]
- Michaud, T. Rheology of hyaluronic acid and dynamic facial rejuvenation: Topographical specificities. J. Cosmet. Dermatol. 2018, 17, 736–743. [Google Scholar] [CrossRef]
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking hyaluronic acid soft-tissue fillers: Current status and perspectives from an industrial point of view. Exp. Rev. Med. Devices 2021, 18, 1175–1187. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. The potential of lysine to extend the shelf life of soybean oil evidenced by 1Hnuclear magnetic resonance. LWT-Food Sci. Technol. 2019, 105, 169–176. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro- and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Jiang, P.; Fu, C.; Wang, J.; Meng, X. L-lysine enhances pork color through antioxidant activity and myoglobin conformational changes. Food Res. Int. 2024, 197, 115148. [Google Scholar] [CrossRef]
- Saleh, F.Y.; Abdel-Azim, E.S.; Ragaie, M.H.; Guendy, M.G. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: Clinical, histopathologic, and immunohistochemical study. J. Egypt Women Dermatol. Soc. 2019, 16, 89. [Google Scholar]
- Sharma, R.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R.; Shiny, T.N. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: A comparative study. Clin. Exp. Dermatol. 2017, 42, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, G.; Garvey, D.R.; Singh, C.K.; Mintie, C.A.; Ahmad, N. Effects and mechanism of nicotinamide against UVA- and/or UVB-mediated DNA damages in normal melanocytes. Photochem. Photobiol. 2019, 95, 331–337. [Google Scholar] [CrossRef]
- Torres-Moral, T.; Tell-Martí, G.; Bague, J.; Rosés-Gibert, P.; Calbet-Llopart, N.; Mateu, J.; Pérez-Anker, J.; Potrony, M.; Alejo, B.; Iglesias, P.; et al. Evaluation of the Biological Effect of a Nicotinamide-Containing Broad-Spectrum Sunscreen on Photo-Damaged Skin. Dermatol. Ther. 2024, 14, 3321–3336. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic acid: A powerful biomolecule with wide-ranging applications-A comprehensive review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Li, M.; Xu, A.; Zhuo, F. Recent applications and molecular mechanisms of hyaluronic acid in skin aging and wound healing. Med. Nov. Technol. Devices 2024, 23, 100320. [Google Scholar] [CrossRef]
- Quan, T.; Wang, F.; Shao, Y.; Rittié, L.; Xia, W.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J. Investig. Dermatol. 2013, 133, 658–667. [Google Scholar] [CrossRef]
- Maeda, K. Mechanism of action of topical tranexamic acid in the treatment of melasma and sun-induced skin hyperpigmentation. Cosmetics 2022, 9, 108. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yamate, Y.; Sugiyama, D.; Matsuda, K.; Iizuka, Y.; Yamaguchi, T. Effect of tranexamic acid in improving the lifespan of naturally aging mice. Inflammopharmacology 2019, 27, 1319–1323. [Google Scholar] [CrossRef]
- Hseu, J.H.; Chan, C.I.; Vadivalagan, C.; Chen, S.J.; Yen, H.R.; Hseu, Y.C.; Yang, H.L.; Wu, P.Y. Tranexamic acid improves psoriasis-like skin inflammation: Evidence from in vivo and in vitro studies. Biomed. Pharmacother. 2023, 166, 115307. [Google Scholar] [CrossRef]
- Endo, K.; Niki, Y.; Ohashi, Y.; Masaki, H. Tranexamic acid improves the disrupted formation of collagen and fibrillin-1 fibers produced by fibroblasts repetitively irradiated with UVA. Biol. Pharm. Bull. 2021, 44, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Eltania, F.; Lesmana, R.; Sudigdoadi, S.; Sudigdoadi, S.; Khairani, A.F.; Goenawan, H.; Citrawan, A.; Armina Yuniarti, R.; Wahyudianingsih, R.; Gunadi, J.W.; et al. Tranexamic acid cream protects ultraviolet B-induced photoaging in Balb/c mice skin by increasing mitochondrial markers: Changes lead to improvement of histological appearance. Photochem. Photobiol. 2020, 96, 863–869. [Google Scholar] [CrossRef]
- Hiramoto, K.; Sugiyama, D.; Takahashi, Y.; Mafune, E. The amelioration effect of tranexamic acid in wrinkles induced by skin dryness. Biomed. Pharmacother. 2016, 80, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Kim, S.; Kim, J.; Kim, J.; Chung, K.B.; Lee, J.H. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. J. Cosmet. Dermatol. 2021, 20, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, D.R.; Iqbal, J.; Hussain, M.M.; Cramer, E.B. Fibrillar collagen type I stimulation of apolipoprotein B secretion in Caco-2 cells is mediated by beta1 integrin. Biochim. Biophys. Acta 2009, 1791, 1144–1154. [Google Scholar] [CrossRef]
- Kang, H.T.; Lee, H.I.; Hwang, E.S. Nicotinamide extends replicative lifespan of human cells. Aging Cell 2006, 5, 423–436. [Google Scholar] [CrossRef]
- Abbas, D.B.; Lavin, C.V.; Fahy, E.J.; Griffin, M.; Guardino, N.; King, M.; Chen, K.; Lorenz, P.H.; Gurtner, G.C.; Longaker, M.T.; et al. Standardizing dimensionless cutometer parameters to determine in vivo elasticity of human skin. Adv. Wound Care 2022, 11, 297–310. [Google Scholar] [CrossRef]
- Dobrev, H.P. A study of human skin mechanical properties by means of Cutometer. Folia Medica 2002, 44, 5–10. [Google Scholar]
- Kerscher, M.; Bayrhammer, J.; Reuther, T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol. Surg. 2008, 34, 720–726. [Google Scholar] [CrossRef]
- Sirithanabadeekul, P.; Srieakpanit, R. Intradermal tranexamic acid injections to prevent post-inflammatory hyperpigmentation after solar lentigo removal with a Q-switched 532-nm Nd: YAG laser. J. Cosmet. Laser Ther. 2018, 20, 398–404. [Google Scholar] [CrossRef]
- Lueangarun, S.; Sirithanabadeekul, P.; Wongwicharn, P.; Namboonlue, C.; Pacharapakornpong, S.; Juntongjin, P.; Tempark, T. Intradermal tranexamic acid injection for the treatment of melasma: A pilot study with 48-week follow-up. J. Clin. Aesthet. Dermatol. 2020, 13, 36–39. [Google Scholar] [PubMed]
- Zerbinati, N.; Capillo, M.C.; Sommatis, S.; Maccario, C.; Alonci, G.; Rauso, R.; Galadari, H.; Guida, S.; Mocchi, R. Rheological investigation as tool to assess physicochemical stability of a hyaluronic acid dermal filler cross-linked with polyethylene glycol diglycidyl ether and containing calcium hydroxyapatite, glycine and L-proline. Gels 2022, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Phelps, R.; Goldberg, D.J. Nonablative facial remodeling: Erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch. Dermatol. 2004, 140, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Galimberti, M.; De Pace, B.; Pellacani, G.; Bencini, P.L. Laser skin rejuvenation: Epidermal changes and collagen remodeling evaluated by in vivo confocal microscopy. Lasers Med. Sci. 2013, 28, 769–776. [Google Scholar] [CrossRef]
- Wang, F.; Garza, L.A.; Kang, S.; Varani, J.; Orringer, J.S.; Fisher, G.J.; Voorhees, J.J. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch. Dermatol. 2007, 143, 155–163. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Available online: https://patents.google.com/patent/US10335512B2/en (accessed on 19 March 2025).
- Andrade Del Olmo, J.; Pérez-Álvarez, L.; Sáez Martínez, V.; Benito Cid, S.; Pérez González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Drug delivery from hyaluronic acid-BDDE injectable hydrogels for antibacterial and anti-inflammatory applications. Gels 2022, 8, 223. [Google Scholar] [CrossRef]
- Linming, F.; Wei, H.; Anqi, L.; Yuanyu, C.; Heng, X.; Sushmita, P.; Yiming, L.; Li, L. Comparison of two skin imaging analysis instruments: The VISIA® from Canfield vs the ANTERA 3D® CS from Miravex. Skin Res. Technol. 2018, 24, 3–8. [Google Scholar] [CrossRef]
- Ikuta, K.; Fukuoka, K.; Suyama, Y.; Morita, M.; Kimura, Y.; Umeda, R.; Kanayama, H.; Ohga, M.; Nakagaki, M.; Yagi, S. Comparison of Antera 3D® and TcPO2 for evaluation of blood flow in skin. Yonago Acta Med. 2023, 66, 146–152. [Google Scholar] [CrossRef]
- Messaraa, C.; Metois, A.; Walsh, M.; Hurley, S.; Doyle, L.; Mansfield, A.; O’Connor, C.; Mavon, A. Wrinkle and roughness measurement by the Antera 3D and its application for evaluation of cosmetic products. Skin Res. Technol. 2018, 24, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Chen, M.; Frankowski, G.; Sinkgraven, R.; Hund, M.; Rzany, B.; Sterry, W.; Lademann, J. In Vivo Determination of Skin Surface Topography Using an Optical 3D Device. Skin Res. Technol. 2004, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Correa, J.A.; Rachelska, G.; Armour, A.; LaSalle, L. Quantitative measurement of hypertrophic scar: Interrater reliability and concurrent validity. J. Burn Care Res. 2008, 29, 501–511. [Google Scholar] [CrossRef]
- Crisan, D.; Crişan, M.; Moldovan, M.; Lupsor Platon, M.; Badea, R. Ultrasonographic assessment of the cutaneous changes induced by topical flavonoid therapy. Clin. Cosmet. Investig. Dermatol. 2012, 5, 7–13. [Google Scholar]
- Krzysztof Mlosek, R.; Malinowska, S. Ultrasound image of the skin, apparatus and imaging basics. J. Ultrason. 2013, 13, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research techniques made simple: Cutaneous colorimetry: A reliable technique for objective skin color measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Skin Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef]
- Sagiv, A.E.; Marcus, Y. The connection between in vitro water uptake and in vivo skin moisturization. Skin Res. Technol. 2003, 9, 306–311. [Google Scholar] [CrossRef]
- Clarys, P.; Clijsen, R.; Taeymans, J.; Barel, A.O. Hydration measurements of the stratum corneum: Comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (Skicon-200EX®). Skin Res. Technol. 2012, 18, 316–323. [Google Scholar] [CrossRef]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Toro, C.; Markarian, B.; Mayrovitz, H.N. Breast cancer-related lymphedema assessed via tissue dielectric constant measurements. Cureus 2024, 16, e59261. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Cherel, M.; Schoop, R.; Rawlings, A.V. A comprehensive comparison of facial skin hydration based on capacitance and conductance measurements in Chinese women. Int. J. Cosmet. Sci. 2022, 44, 703–718. [Google Scholar] [CrossRef] [PubMed]



| Parameter | D–3 | D28 | p-Value | D70 | p-Value |
|---|---|---|---|---|---|
| Total intensity (%) | 14.90 ± 3.20 | 13.46 ± 2.56 | 0.003 (Paired t-test) | 13.09 ± 2.97 | 0.002 (Wilcoxon) |
| Thickness of epidermis and dermis (mm) | 1.75 ± 0.19 | 1.89 ± 0.21 | <0.001 (Wilcoxon) | 1.70 ± 0.18 | 0.016 (Paired t-test) |
| Parameter | Face Side | Timepoint | 0: None | 1: Light | 2: Moderate | 3: Severe | Absence (0) | Presence (1, 2, 3) |
|---|---|---|---|---|---|---|---|---|
| Redness | Right | D0 (after injection) | 6 (12.2%) | 43 (87.8%) | 0 (0.0%) | 0 (0.0%) | 6 (12.2%) | 43 (87.8%) |
| D14 | 6 (12.2%) | 43 (87.8%) | 0 (0.0%) | 0 (0.0%) | 6 (12.2%) | 43 (87.8%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Left | D0 (after injection) | 6 (12.2%) | 43 (87.8%) | 0 (0.0%) | 0 (0.0%) | 6 (12.2%) | 43 (87.8%) | |
| D14 | 6 (12.2%) | 43 (87.8%) | 0 (0.0%) | 0 (0.0%) | 6 (12.2%) | 43 (87.8%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Pain/Sensitivity | Right | D0 (after injection) | 22 (44.9%) | 24 (49.0%) | 3 (6.1%) | 0 (0.0%) | 22 (44.9%) | 27 (55.1%) |
| D14 | 21 (42.9%) | 27 (55.1%) | 1 (2.0%) | 0 (0.0%) | 21 (42.9%) | 28 (57.1%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Left | D0 (after injection) | 22 (44.9%) | 24 (49.0%) | 3 (6.1%) | 0 (0.0%) | 22 (44.9%) | 27 (55.1%) | |
| D14 | 21 (42.9%) | 27 (55.1%) | 1 (2.0%) | 0 (0.0%) | 21 (42.9%) | 28 (57.1%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Hardening/Firmness | Right | D0 (after injection) | 31 (63.3%) | 17 (34.7%) | 1 (2.0%) | 0 (0.0%) | 31 (63.3%) | 18 (36.7%) |
| D14 | 25 (51.0%) | 24 (49.0%) | 0 (0.0%) | 0 (0.0%) | 25 (51.0%) | 24 (49.0%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Left | D0 (after injection) | 31 (63.3%) | 17 (34.7%) | 1 (2.0%) | 0 (0.0%) | 31 (63.3%) | 18 (36.7%) | |
| D14 | 25 (51.0%) | 24 (49.0%) | 0 (0.0%) | 0 (0.0%) | 25 (51.0%) | 24 (49.0%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Swelling | Right | D0 (after injection) | 32 (65.3%) | 17 (34.7%) | 0 (0.0%) | 0 (0.0%) | 32 (65.3%) | 17 (34.7%) |
| D14 | 20 (40.8%) | 29 (59.2%) | 0 (0.0%) | 0 (0.0%) | 20 (40.8%) | 29 (59.2%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Left | D0 (after injection) | 32 (65.3%) | 17 (34.7%) | 0 (0.0%) | 0 (0.0%) | 32 (65.3%) | 17 (34.7%) | |
| D14 | 20 (40.8%) | 29 (59.2%) | 0 (0.0%) | 0 (0.0%) | 20 (40.8%) | 29 (59.2%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Bruising | Right | D0 (after injection) | 48 (98.0%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 48 (98.0%) | 1 (2.0%) |
| D14 | 48 (98.0%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 48 (98.0%) | 1 (2.0%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| Left | D0 (after injection) | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | |
| D14 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) | ||
| D28 | 48 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 48 (100.0%) | 0 (0.0%) | ||
| D70 | 49 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 49 (100.0%) | 0 (0.0%) |
| Parameter | Timepoint | Very Satisfied | Satisfied | Neither Satisfied nor Dissatisfied | Dissatisfied | Very Dissatisfied |
|---|---|---|---|---|---|---|
| Ease of extraction | D0 (after injection) | 33 (67.3%) | 16 (32.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| D14 | 40 (81.6%) | 9 (18.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Ease of injection | D0 (after injection) | 41 (83.7%) | 8 (16.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| D14 | 35 (71.4%) | 14 (28.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Immediate result | D0 (after injection) | 9 (18.4%) | 40 (81.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| D14 | 9 (18.4%) | 40 (81.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Study Phase/Activities | D-30 | D-3 | D0 | D14 | D28 | D70 |
|---|---|---|---|---|---|---|
| Participant inclusion | Yes | / | / | / | / | / |
| Medical examination | Yes | / | / | / | / | / |
| Hydragel A1 injection | / | / | Yes | Yes | / | / |
| IGAIS | / | / | Yes | Yes | Yes | Yes |
| SGAIS | / | / | Yes | Yes | Yes | Yes |
| ISR by the injector | / | / | Yes | Yes | Yes | Yes |
| Photographs of the cheek area using Dermlite | / | Yes | / | / | Yes | Yes |
| Antera 3D measurement | / | Yes | / | / | Yes | Yes |
| Cutometer measurement | / | Yes | / | / | Yes | Yes |
| DermaScan measurement | / | Yes | / | / | Yes | Yes |
| Corneometer measurement | / | Yes | / | / | Yes | Yes |
| Chromameter measurement | / | Yes | / | / | Yes | Yes |
| Recording of AE and SAE | / | Yes | / | Yes | Yes | Yes |
| Key Ingredient | Percentage in Finished Product |
|---|---|
| Hyaluronic acid | 1.00% |
| Tranexamic acid | 1.00% |
| Niacinamide | 1.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsin, S.; Lourenço, K.; Porcello, A.; Chemali, M.; Marques, C.; Raffoul, W.; Cerrano, M.; Applegate, L.A.; Laurent, A.E. Clinical Safety and Efficacy of Hyaluronic Acid–Niacinamide–Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening. Gels 2025, 11, 495. https://doi.org/10.3390/gels11070495
Hsin S, Lourenço K, Porcello A, Chemali M, Marques C, Raffoul W, Cerrano M, Applegate LA, Laurent AE. Clinical Safety and Efficacy of Hyaluronic Acid–Niacinamide–Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening. Gels. 2025; 11(7):495. https://doi.org/10.3390/gels11070495
Chicago/Turabian StyleHsin, Sarah, Kelly Lourenço, Alexandre Porcello, Michèle Chemali, Cíntia Marques, Wassim Raffoul, Marco Cerrano, Lee Ann Applegate, and Alexis E. Laurent. 2025. "Clinical Safety and Efficacy of Hyaluronic Acid–Niacinamide–Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening" Gels 11, no. 7: 495. https://doi.org/10.3390/gels11070495
APA StyleHsin, S., Lourenço, K., Porcello, A., Chemali, M., Marques, C., Raffoul, W., Cerrano, M., Applegate, L. A., & Laurent, A. E. (2025). Clinical Safety and Efficacy of Hyaluronic Acid–Niacinamide–Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening. Gels, 11(7), 495. https://doi.org/10.3390/gels11070495







