Engineering Gel-Based Precursors into Advanced ORR Catalysts for Zn–Air Batteries and Fuel Cells: Insights into Hydrogels, Aerogels, Xerogels, Metal–Organic Gels, and Metal Aerogels
Abstract
1. Introduction
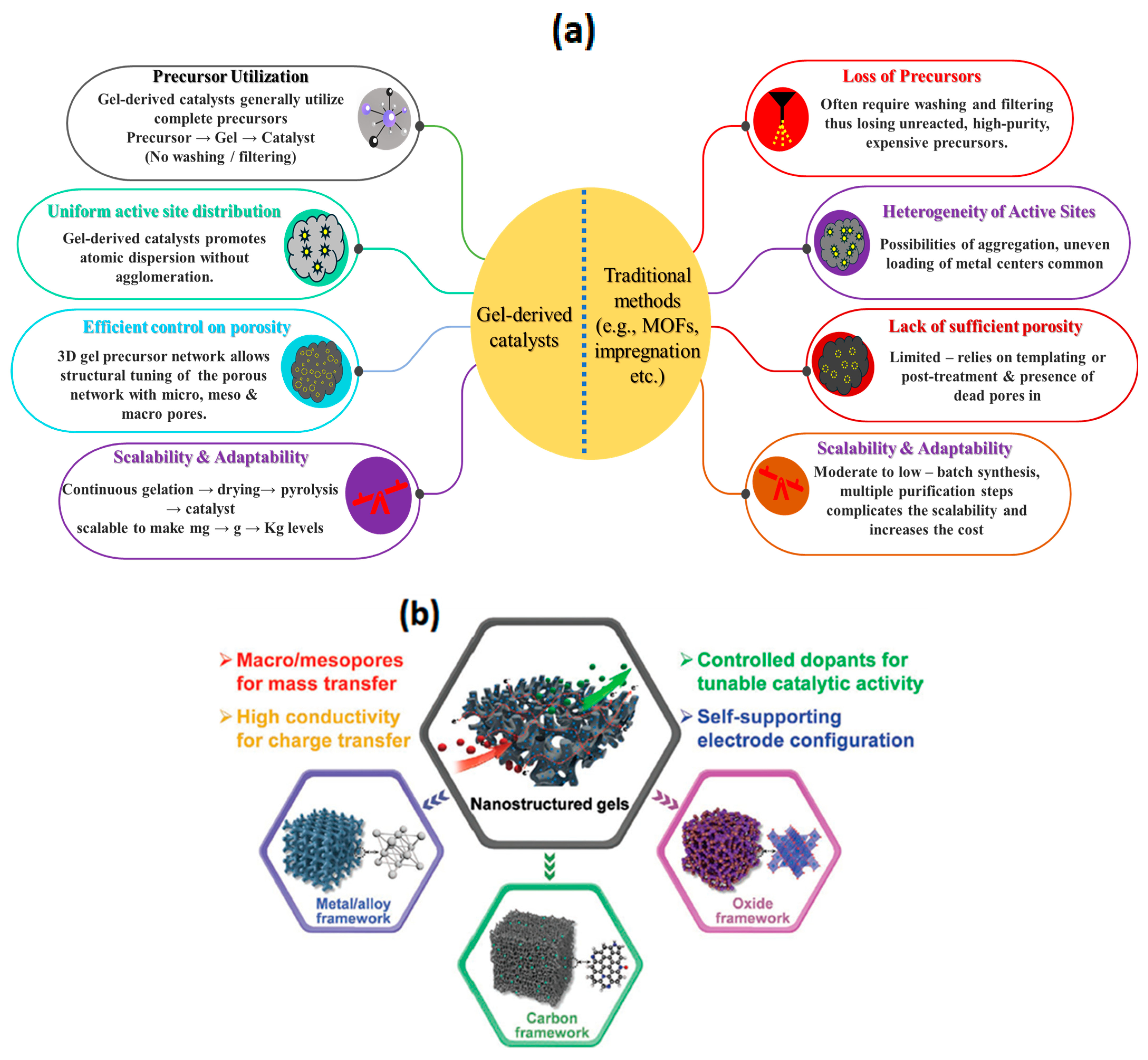
2. Gel-Derived ORR Catalysts vs. Conventional ORR Catalysts
2.1. Complete Utilization of the Precursors
2.2. Scalability
2.3. Uniform Distribution of Active Sites
2.4. Efficient Control on Surface Area and Porosity
2.5. Sustainability
2.6. Adaptability
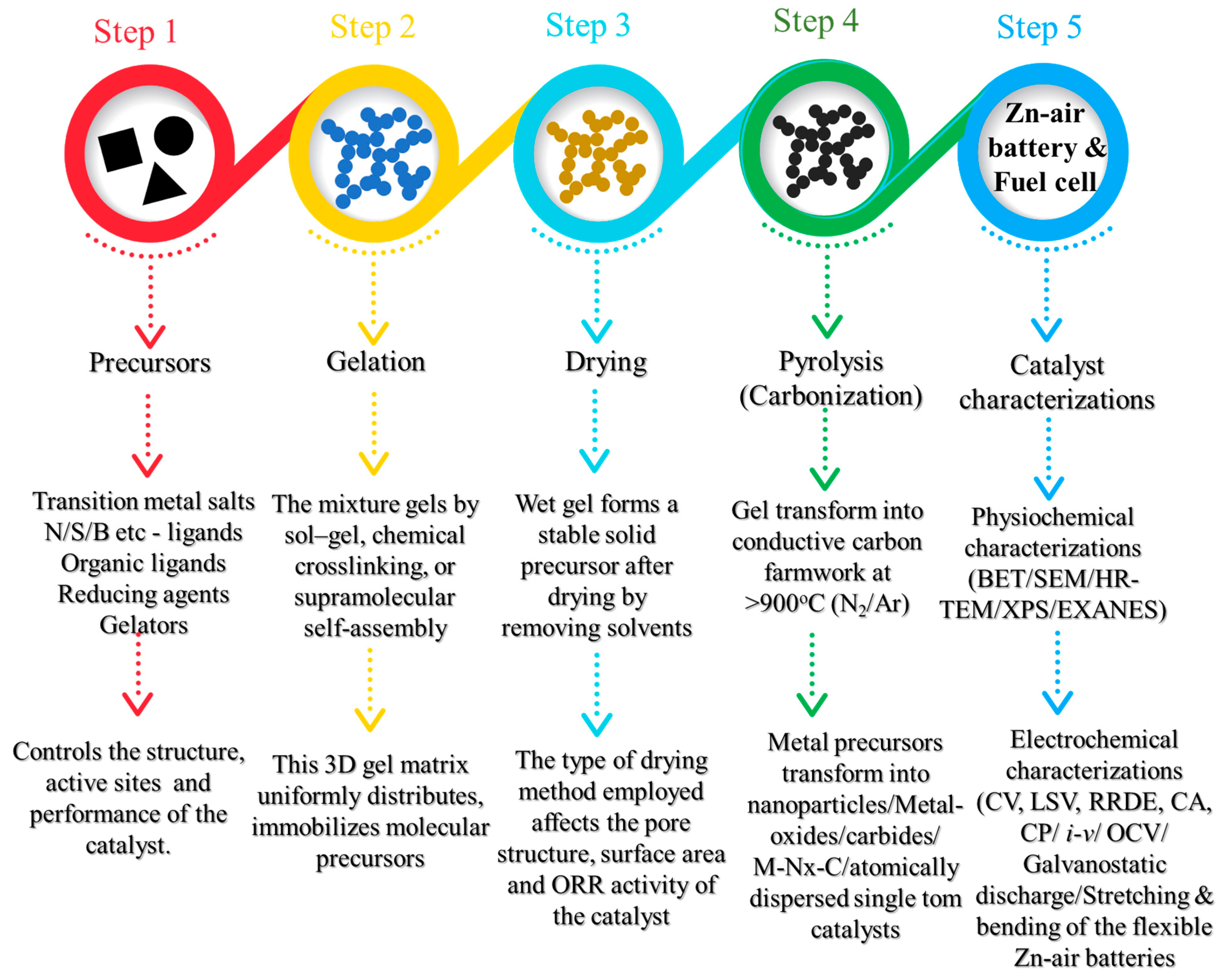
3. Gel-Derived Catalysts
3.1. Hydrogels
3.1.1. Hydrogel Synthesis and Its Gelation Chemistry
3.1.2. Hydrogel-Derived ORR Catalysts
4. Aerogels
4.1. The Sol–Gel Synthesis of the Gel
4.2. The Key to 3D Gel Structure—Gelation
4.3. Obtaining Aerogels from Wet Gels and Carbonization
4.4. Aerogel-Derived ORR Catalysts
5. Xerogel-Derived Catalyst
6. Metal–Organic Gel (MOG)- and Supramolecular Gel-Derived Catalysts
6.1. Covalent/Coordination Boned MOG-Derived Catalysts
6.2. Supramolecular MOG (SMG)-Derived Catalysts
7. Metal Aerogel-Derived Catalysts
8. Benchmarking ORR Performance Across Gel-Derived Catalyst Types
9. Key Insights
- After reviewing a number of research studies, it has been found that gel-derived catalysts show outstanding characteristics and electrocatalytic oxygen reduction reaction (ORR) activities, usually either equivalent to or better than those of commercial Pt/C catalysts.
- Exceptional porosity is a common and important characteristic of all five forms of gel-derived catalysts; it can be finely tuned from microporous to mesoporous and macroporous structures. These catalysts also have remarkably high specific surface areas—approaching or even surpassing 2000 m2 g−1, which surpasses traditional expectations.
- Achieving well-dispersed catalytic sites with minimum agglomeration depends especially on such high surface areas. This is particularly pertinent in the development of atomically scattered catalysts, where their high surface energy makes controlling the aggregation of single atomic sites intrinsically difficult.
- The abundance of surface area in gel-derived catalysts offers an ideal matrix to host a high density of single-atom catalytic sites without appreciable aggregation. On the other hand, attempts to include a high density of such sites into low-surface-area carbon supports usually lead to agglomeration.
- In particular, some studies have shown how effectively more than 15% of single-atom metallic active sites could be incorporated into gel-derived matrices without aggregation, so stressing their possible use as hosts for atomically distributed catalysts with high loading, without aggregations.
- In terms of the ORR activity, stability, porosity, and surface area among the several gel forms, hydrogels and aerogels have shown the most promise; metal–organic gels (MOGs) with a supramolecular assembly strategy and xerogels follow in second place of importance for synthesizing high densities of non-precious metal-based (M-N4-C, M = transition metal) ORR catalysts.
- For metal-aerogel-based catalysts, the proposed method has mostly produced the synthesis of noble metal alloy systems, especially supportless catalysts. These noble metal-based aerogel catalysts show remarkable electrocatalytic performance unlike transition metal-based catalysts with carbon supports, which are less commonly prepared via metal-aerogel routes. Aerogel-derived noble metal catalysts show remarkable mass and specific activities, according to several studies; in some cases, these values surpass the 2025 targets set by the U.S. Department of Energy for ORR performance. This emphasizes their great relevance for useful applications in systems of energy conversion. The scalability of these materials for mass production and their integration into useful devices like fuel cells and metal-air batteries still present major obstacles.
- For flexible Zn–air batteries, especially, it would be beneficial if these supportless metal-aerogel catalysts could be directly deposited onto flexible substrates. This would improve device commercial viability and streamline the manufacture of them. Generally speaking, aerogel-derived catalysts are a unique and quite promising class. They differ from other gel-derived catalysts in their unusual capacity to create supportless, noble metal alloy structures with extraordinary electrochemical performance. By means of ongoing research on scalable synthesis and flexible integration techniques, their acceptance in next-generation energy technologies could be accelerated.
10. Future Perspectives and Recommendations
- Although metal-aerogel catalysts have shown remarkably high oxygen reduction reaction (ORR) activity, their synthesis has been mainly limited to noble metals thus far, including platinum, palladium, and their alloys. Although these materials show great mass and specific activities—often exceeding DOE performance criteria—their great cost makes extensive commercial deployment very difficult. From an economic standpoint, then, noble metal-based aerogel catalysts are not seen as suitable for broad uses.
- A major development in ORR catalysis could come from metal-aerogel catalysts based on non-precious, earth-abundant transition metals. Given their cost-effectiveness and abundance, if such catalysts can attain mass and specific activities equivalent to their noble metal counterparts, they would present a quite appealing substitute. Moreover, the effective fabrication of non-precious metal-aerogel catalysts with high catalytic activities would open new paths for scalable production, making them feasible candidates for integration into fuel cell stacks and zinc–air batteries. Reaching this target will represent major progress in the design of next-generation, reasonably priced ORR electrocatalysts.
- Gel-based catalysts are appealing from scientific and practical perspectives due to their simple and effective synthesis paths. Precursor solutions often gel naturally and can be dried and turned into a functional catalyst without post-processing. This simplified process reduces material loss and chemical use by eliminating solvent-intensive washing, filtration, and purification. Gel-derived catalysts enable eco-friendly and affordable synthesis. Gel systems are more adaptable because they use cheap, readily available biomolecular precursors like gelatin, glycine, starch, alginate, dextrose, etc. These readily available components simplify and scale synthesis and enable gel development under moderate conditions. Gel-derived catalysts can be scaled up from laboratory to industrial levels using benign, low-cost precursors, allowing gram- to kilogram-scale production without compromising catalytic performance. Gel-based synthesis’s minimal processing, low solvent use, and sustainable precursors make it a promising electrocatalysis method.
- Several catalysts derived from hydrogels and aerogels exhibit extraordinary surface areas (approximately 2000 m2 g−1). However, when we attempted to establish a correlation between surface area and ORR activities, we did not observe any linear trends. Catalysts with the highest surface area exhibit comparable activities to those with a surface area of approximately 400 m2 g−1; therefore, we contend that a high surface area may not necessarily lead to enhanced ORR activity but facilitate the accommodation of substantial loads of metallic nanoparticles or single-atom catalysts without noticeable aggregation, which is typically challenging to achieve with conventional catalyst synthesis methods such as MOFs. Therefore, if high-surface-area catalysts can be utilized to incorporate elevated metallic loadings, it could lead to enhanced ORR and mass activities suitable for commercial applications.
- One of the primary limitations of ORR catalysts is the challenge in categorizing them as viable alternatives to Pt/C in practical fuel cells and Zn–air batteries, as only a limited number of catalysts report mass and specific activities, which are critical criteria for ORR catalysts. Due to the limitations of half-wave potential patterns, it is unlikely that their activities will be effectively translated into realistic energy storage and conversion devices. Consequently, it is recommended that the authors and other researchers also report on the mass and specific activities.
- Another limitation is that nearly 90% of the catalysts are exclusively evaluated in alkaline electrolytes, with only a few studies examining the catalytic activities in acidic electrolytes. Although the same catalysts can be utilized in both electrolytes, there is generally a substantial difference in their activities in acidic and alkaline electrolytes. On the other hand, ORR in basic electrolytes can be applied to AEM fuel cells and Zn–air batteries. PEM fuel cells, which are based on acidic conditions, are the most ideal for transportation applications, and it is essential to evaluate the ORR activity in acidic conditions and then principally in a single cell.
- The high surface area and hierarchical porosity within micro-/meso-/macropores of gel-derived catalysts comprise some of the best physicochemical properties that distinguish them from other conventional catalyst systems. Therefore, it is interesting to note the effect of the hierarchical porous structure on its pivotal role in facilitating an efficient mass flow of gases or transporting intermediate reactants, though modelling and experimental validation can solve one of the important issues in terms of catalyst thickness and ORR activity in a realistic Zn–air battery or fuel cells. It is very well known that non-precious metal catalysts require higher catalyst loading in order to deliver the desired power density, which, in turn, restricts the flow of O2 (especially when air is used as an oxidant). In this regard, gel-derived catalysts have been shown to possess balanced micro-/meso-/macropores that could reduce the mass transport resistance associated with the O2 diffusion from the bulk to the catalyst layer and quick H2O removal.
- Carbonaceous frameworks made from gel-based materials without metallic active sites or heteroatom doping can be used as gas diffusion layers (GDLs) in electrochemical energy devices, as well as active electrocatalysts or catalyst supports. These gel-derived carbons naturally have hierarchical porous architectures with interconnected micropores and mesopores for fuel cell and metal–air battery gas transport and electrolyte access. Mesopores mimic conventional GDL materials’ ideal structures by increasing gas permeability and lowering mass transfer resistance, while micropores increase surface area and capillary condensation. By creating materials in desired shapes and thicknesses, the direct gel-to-carbon conversion technique allows GDL design and integration flexibility. Due to their low cost, scalability, and tunable porosity, these metal-free, heteroatom-free gel-derived carbons could replace commercial GDLs made from carbon cloths or carbon papers in next-generation flexible or portable electrochemical devices.
- Among different types of gels, hydrogels offer unique possibilities of synthesizing electrocatalysts from biological sources like gelatine, agar, starch, cellulose, alginate, hyaluronic acid, etc., which not only make them sustainable precursors but also impact the catalyst synthesis cost. Therefore, we recommend that more research should be conducted on hydrogel-derived catalysts for SAC synthesis and further translate the synthesis process from the lab scale to the gram level.
- Among all types of gels, the precursor toxicity is the lowest for hydrogel-derived catalysts due to the use of natural gelling agents, whereas in all other catalysts, a specific organic ligand is used that is either expensive or toxic to the environment.
- In terms of scalability, hydrogel-derived catalysts have tremendous potential, followed by xerogels and metal–organic gels. In contrast, aerogels and metal gels possess specific challenges in scalability due to their complexity in the synthesis and post-synthesis processes, such as freeze-drying or supercritical drying, and sensitivity to pH, temperature, and the use of structural directing agents.
- So far, in the hydrogel-derived catalysts, the use of heteroatom-containing ligands has not been established. It is important to note that natural gelling agents intrinsically possess some heteroatoms; however, introducing high concentrations of the different heteroatoms could synergistically improve the ORR activity of SACs. In addition, high concentrations of heteroatoms, such as N, improve the electronic conductivity and high density of M-N4-C active sites. Furthermore, other dopants such as S, P, B, and F can further optimize the polarization of the carbon matrix, which benefits from enhanced ORR activity. Therefore, we recommend the modification of the hydrogel’s synthesis by introducing heteroatom-containing ligands as novel gel synthesis routes.
- The hydrogel- and aerogel-derived catalysts could possess extremely high BET surface areas > 1000 m2 g−1; therefore, these catalysts possess extremely high possibilities of introducing high loading SACs. Therefore, we recommend exploring the hydrogel synthesis catalysts for high loading SAC studies that can be game-changing by achieving the high mass and specific activities set by DoE.
- At present, the xerogel-derived catalysts are found to be the least active in ORR. Therefore, we recommend the hybridization of xerogels with other 2D/3D advanced materials like MOF/COF/MXenes to create a hybrid catalyst for improved ORR kinetics.
- In terms of MOG catalysts, there is a need to develop alternatives/explore high-coordinating ligands to improve the coordination environment and gelation kinetics.
- One of the highly possible and anticipated research areas includes the development of metal aerogels that are made of non-precious/transition metals such as Fe, Co, Ni, and Mn. The noble metal catalysts have already evidently shown extraordinary mass and specific activities. However, metal aerogels from non-precious/transition metals such as Fe, Co, Ni, and Mn have rarely been synthesized and explored for ORR catalysts. Therefore, we highly recommend future research to explore non-noble metal aerogel catalysts for ORR catalysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Vallejo, M.C.; Alzate, C.A.C. Sustainability of hydrogen production considering alternative technologies towards a neutral carbon society. Int. J. Hydrogen Energy 2024, 64, 853–863. [Google Scholar] [CrossRef]
- Carboni, M.; Dall-Orsoletta, A.; Hawkes, A.; Giarola, S. The future of road freight transport and alternative technologies: A case study for Italy. Energy Convers. Manag. 2024, 299, 117819. [Google Scholar] [CrossRef]
- Xia, W.; Zheng, S.; Qiu, L.; Hu, H.; Chen, Y.; Wei, S.; Zhou, H. Evolution of the Innovation Network of Lithium-Ion Battery Recycling Technologies in China from the Perspective of Patents. Pol. J. Environ. Stud. 2025. [CrossRef] [PubMed]
- Xu, X.; Han, X.; Lu, L.; Wang, F.; Yang, M.; Liu, X.; Wu, Y.; Tang, S.; Hou, Y.; Hou, J.; et al. Challenges and opportunities toward long-life lithium-ion batteries. J. Power Sources 2024, 603, 234445. [Google Scholar] [CrossRef]
- Chen, F.; Huang, K.; Li, H.-Y.; Zhong, Q.; Yue, J.; Diao, J.; Wang, Z.; Huang, G.; Jiang, B.; Pan, F. Interlayer cationic defect engineering in Lamellar Vanadate Cathodes enables Ultralong-Lifespan Magnesium-Ion batteries. ACS Energy Lett. 2025, 10, 2052–2060. [Google Scholar] [CrossRef]
- Cai, X.; Yue, Y.; Yi, Z.; Liu, J.; Sheng, Y.; Lu, Y. Challenges and industrial perspectives on the development of sodium ion batteries. Nano Energy 2024, 129, 110052. [Google Scholar] [CrossRef]
- Fu, L.; Wang, J.; Fu, X.; Zhao, G. Finite-time Pade-based adaptive FNN controller implementation for microbial fuel cell with delay and multi-disturbance. Int. J. Hydrogen Energy 2024, 98, 1034–1043. [Google Scholar] [CrossRef]
- Ye, L.; Qi, S.; Cheng, T.; Jiang, Y.; Feng, Z.; Wang, M.; Liu, Y.; Dai, L.; Wang, L.; He, Z. Vanadium Redox Flow Battery: Review and perspective of 3D electrodes. ACS Nano 2024, 18, 18852–18869. [Google Scholar] [CrossRef]
- Fu, L. A Novel Robust Control for Disturbed Uncertain Microbial Fuel Cell with Noisy Output. J. New Mater. Electrochem. Syst. 2025, 28, 76–84. [Google Scholar] [CrossRef]
- Dar, M.A.; Majid, S.; Satgunam, M.; Siva, C.; Ansari, S.; Arularasan, P.; Ahamed, S.R. Advancements in Supercapacitor electrodes and perspectives for future energy storage technologies. Int. J. Hydrogen Energy 2024, 70, 10–28. [Google Scholar] [CrossRef]
- Wang, C.-J.; Zhu, Y.-L.; Zhang, T.; Tian, J.; Gao, F.; Zhao, Y.; Bu, X.-Y.; Quan, T. Competition between discharge reaction and side reaction for anode’s lithium during internal short circuit in lithium-ion batteries. J. Clean. Prod. 2024, 470, 143280. [Google Scholar] [CrossRef]
- Machín, A.; Morant, C.; Márquez, F. Advancements and Challenges in Solid-State Battery Technology: An In-Depth Review of solid electrolytes and anode innovations. Batteries 2024, 10, 29. [Google Scholar] [CrossRef]
- Meng, N.-Q.; Fan, Y.-X.; Cai, J.-S. Zn–air batteries for electric vehicles. Tungsten 2022, 6, 164–173. [Google Scholar] [CrossRef]
- Fang, T.; Vairin, C.; von Jouanne, A.; Agamloh, E.; Yokochi, A. Review of Fuel-Cell electric Vehicles. Energies 2024, 17, 2160. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Y.; Luo, D.; Qiu, W.; Wang, J.; Wang, X.; Chen, Z. Recent progress on mechanisms, principles, and strategies for high-activity and high-stability non-PGM fuel cell catalyst design. Carbon Carbon Energy 2024, 6, e426. [Google Scholar] [CrossRef]
- Fang, W.; Yu, X.; Zhao, J.; Cao, Z.; Wu, M.; Ho, D.; Hu, H. Advances in flexible zinc–air batteries: Working principles, preparation of key components, and electrode configuration design. J. Mater. Chem. A 2023, 12, 1880–1909. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.-J.; Feng, X.; Ma, Z.; Ma, Z.-F.; Xue, Y. Progress of Pt and iron-group transition metal alloy catalysts with high ORR activity for PEMFCs. J. Electroanal. Chem. 2024, 959, 118165. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Akula, S.; Asokan, A.; Moni, P.; Selvaraj, M.; Balamurugan, J.; Kim, S.O.; Liu, C.; Sahu, A.K. Carbon nanofibers as potential catalyst support for fuel cell cathodes: A review. Energy Fuels 2021, 35, 11761–11799. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, P.; Zhao, Y.; Jiang, W.; Zhao, B.; Chen, X.; Li, M. Ultradurable Pt-Based catalysts for oxygen reduction electrocatalysis. Catalysts 2024, 14, 57. [Google Scholar] [CrossRef]
- Peera, S.G.; Lee, T.G.; Sahu, A.K. Pt-rare earth metal alloy/metal oxide catalysts for oxygen reduction and alcohol oxidation reactions: An overview. Sustain. Energy Fuels 2019, 3, 1866–1891. [Google Scholar] [CrossRef]
- Letchumanan, I.; Wani, A.A.; Shaari, N.; Beygisangchin, M.; Kamarudin, S.K.; Karim, N.A. Metal–Organic Frameworks as a catalyst and catalyst support in fuel cells: From Challenges to Catalytic Application. Chem. Eng. Technol. 2024, 47, 202300580. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, S.; Zhang, H. Recent Progress in Atomically Dispersed Non-noble Metal Catalysts for Electrochemical Two-electron Oxygen Reduction Reaction. ChemElectroChem 2024, 11, e202300630. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.; Wei, Y.; Zhuang, Z.; Dou, Y.; Yang, J.; Li, W.-H.; Wang, D. From Lab-Scale to Industrialization: Atomically M-N-C catalysts for oxygen reduction reaction. Energy Environ. Sci. 2025, 18, 3462–3501. [Google Scholar] [CrossRef]
- Liu, F.; Wei, P.; Zhang, J.; Shi, M.; Hou, J.; Chen, H.; Li, Y.; Li, S. Potential-driven instability effect of carbon supports for Pt/C electrocatalysts. Carbon 2023, 216, 118562. [Google Scholar] [CrossRef]
- Park, S.; Lee, E.; Park, Y.; Kim, M.-G.; Yoo, S.J. Toward Hydrogen Mobility: Challenges and Strategies in Electrocatalyst Durability for Long-Term PEMFC Operation. JACS Au 2025, 5, 1617–1632. [Google Scholar] [CrossRef]
- Chowdury, S.K.; Park, Y.; Park, S.B.; Park, Y.-I. Degradation Mechanisms, Long-Term durability Challenges, and mitigation methods for proton exchange membranes and membrane electrode assemblies with Pt/C electrocatalysts in Low-Temperature and High-Temperature fuel Cells: A comprehensive review. J. Electroanal. Chem. 2024, 975, 118712. [Google Scholar] [CrossRef]
- Nagamori, K.; Aoki, S.; Ikegawa, M.; Tamoto, K.; Honda, Y.; Seki, Y.; Igarashi, H.; Uchida, M. Impacts of Pt/Carbon Black Catalyst Surface Hydrophilicity on Ionomer Distribution and Durability during Water-Generating Load Cycling of Polymer Electrolyte Fuel Cells. ACS Appl. Energy Mater. 2023, 6, 11481–11496. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.-H.; Wang, K.-X.; Ye, T.-N.; Chen, J.-S. Inorganic non-carbon supported Pt catalysts and synergetic effects for oxygen reduction reaction. Energy Environ. Sci. 2023, 16, 1838–1869. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 4, 886–906. [Google Scholar] [CrossRef]
- Prithi, J.; Vedarajan, R.; Rao, G.R.; Rajalakshmi, N. Functionalization of carbons for Pt electrocatalyst in PEMFC. Int. J. Hydrogen Energy 2021, 46, 17871–17885. [Google Scholar] [CrossRef]
- Kumar, A.; Park, E.J.; Kim, Y.S.; Spendelow, J.S. Surface functionalization of carbon Black for PEM fuel cell electrodes. Macromol. Chem. Phys. 2024, 225, 2400092. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J.C.; Chen, S.; Yu, Y.; Zhang, J. Advanced noncarbon materials as catalyst supports and non-noble electrocatalysts for fuel cells and Metal–Air batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Speder, J.; Zana, A.; Arenz, M. The colloidal tool-box approach for fuel cell catalysts: Systematic study of perfluorosulfonate-ionomer impregnation and Pt loading. Catal. Today 2015, 262, 82–89. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Comprehensive insights and advancements in gel catalysts for electrochemical energy conversion. Gels 2024, 10, 63. [Google Scholar] [CrossRef]
- Yang, H.; Hu, H.; Xia, C.; You, F.; Yao, J.; Jiang, X.; Xia, B.Y. Progress on nanostructured gel catalysts for oxygen electrocatalysis. Nano Res. 2022, 15, 10343–10356. [Google Scholar] [CrossRef]
- Fang, Z.; Li, P.; Yu, G. Gel Electrocatalysts: An emerging material platform for electrochemical energy conversion. Adv. Mater. 2020, 32, 2003191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Fu, Y.; Wang, N.; Liu, Q.; Zhao, S.; Yang, H.Y.; Liu, C. Fabrication of temperature and pH dual-sensitive semi-interpenetrating network hydrogel with enhanced adhesion and antibacterial properties. Polymer 2025, 326, 128343. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for energy and water sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Luo, N.; Wang, J.; Zhang, D.; Zhao, Y.; Wei, Y.; Liu, Y.; Zhang, Y.; Han, S.; Kong, X.; Huo, P. Inorganic nanoparticle-enhanced double-network hydrogel electrolytes for supercapacitor with superior low-temperature adaptability. Chem. Eng. J. 2023, 479, 147741. [Google Scholar] [CrossRef]
- Guo, Y.; Fang, Z.; Yu, G. Multifunctional hydrogels for sustainable energy and environment. Polym. Int. 2021, 70, 1425–1432. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional hydrogels for Next-Generation batteries and supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Zhao, C.; Song, Y.; Chen, H.; Chen, H.; Li, Y.; Lei, A.; Wu, Q.; Zhu, L. Improving the performance of microbial fuel cell stacks via capacitive hydrogel bioanodes. Int. J. Hydrogen Energy 2024, 97, 708–717. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Q.; Lin, S.; Li, J. Water: The soul of hydrogels. Prog. Mater. Sci. 2025, 148, 101378. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, K.; Jiang, H.; Deng, R.; Chu, L.; Cao, Y.; Zheng, Y.; Lu, W.; Deng, Z.; Liang, B. A Three-in-One strategy: Injectable biomimetic porous hydrogels for accelerating bone regeneration via Shape-Adaptable scaffolds, controllable magnesium ion release, and enhanced osteogenic differentiation. Biomacromolecules 2021, 22, 4552–4568. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Guo, J.; An, Q.; Zhang, Y.; Zhang, J.; Li, B.; Bo, S.; Jiang, J.; Wang, W.; Liu, Q. Hydrogel-Modulated Microporous/Mesoporous Engineering on FeCo-DAC toward Efficient Oxygen Reduction. J. Phys. Chem. C 2024, 128, 9454–9461. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Hung, T.-C.; Mohamed, S.G.; Yang, C.-C.; Hung, T.-F. Two Birds with One Stone: Hydrogel-Derived Hierarchical Porous Activated Carbon toward the Capacitive Performance for Symmetric Supercapacitors and Lithium-Ion Capacitors. ACS Sustain. Chem. Eng. 2022, 10, 4717–4727. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Y.; Ma, D.; Foucher, A.C.; Xiong, L.; Zhang, J.; Stach, E.A.; Yue, Q.; Kang, Y. Atomic FE dispersed hierarchical mesoporous Fe–N–C nanostructures for an efficient oxygen reduction reaction. ACS Catal. 2020, 11, 74–81. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, Q.; Wei, Q.; Bowen, C.R.; Wong, W.-Y.; Yang, W. Non-precious metal-based single-atom catalysts for oxygen reduction reaction: Fundamentals and applications. Mater. Sci. Eng. R Rep. 2024, 160, 100822. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, N.; Shang, H.; Sun, Z.; Wei, Z.; Wang, J.; Lei, Y.; Wang, X.; Wang, D.; Zhao, Y.; et al. High-density asymmetric iron dual-atom sites for efficient and stable electrochemical water oxidation. Nat. Commun. 2024, 15, 9440. [Google Scholar] [CrossRef] [PubMed]
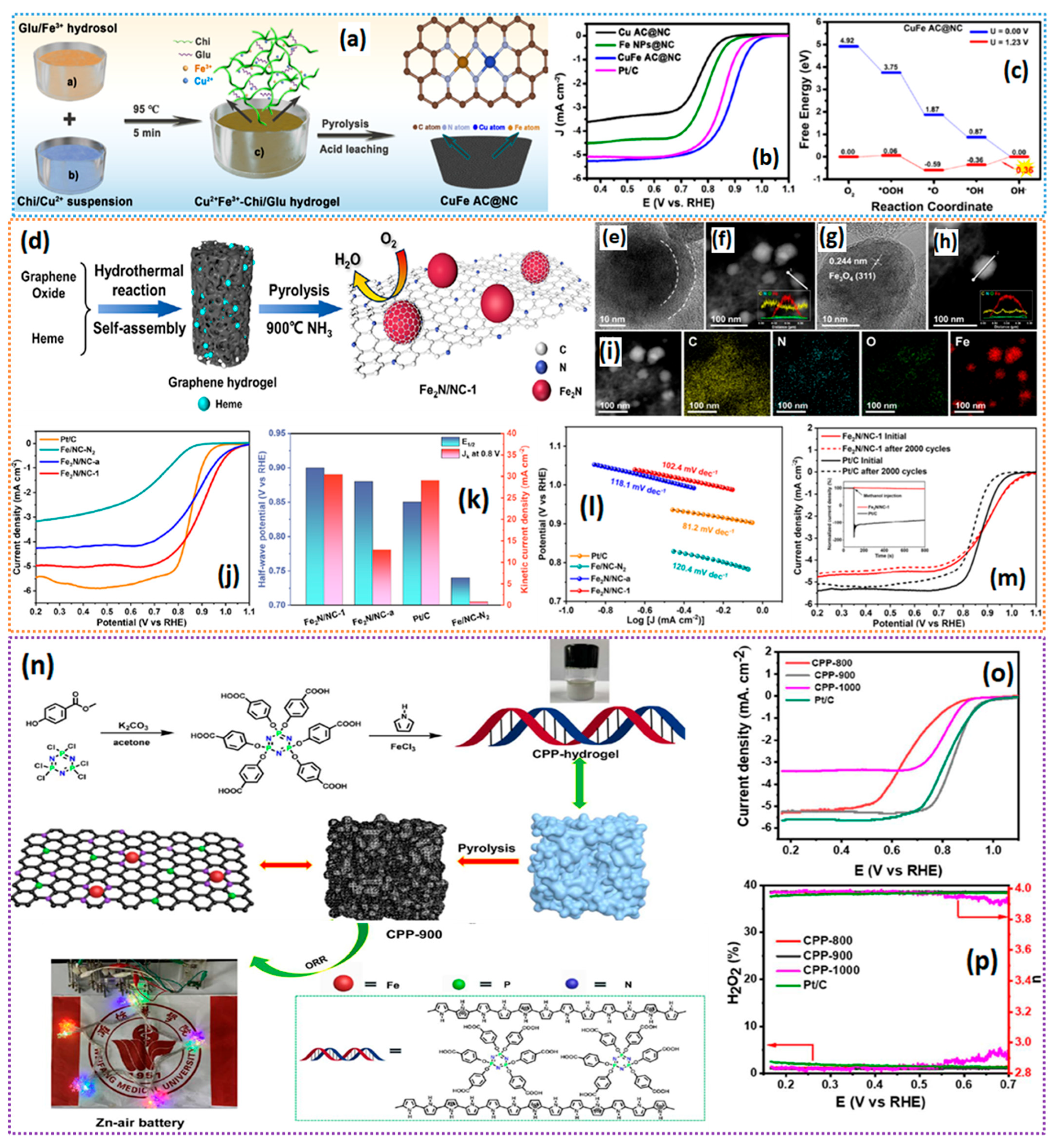
- Wang, J.; Chen, Y.; Cheng, W.; Li, H.; Liu, M.; Xu, X.; Zhao, P.; Xing, L.; Wang, D. Synergistic effect of Cu and Fe on chitosan-glutamic acid hydrogel for green synthesis of N doped carbon with CuFe dual atomic clusters for Zn-air batteries. Chem. Eng. J. 2025, 511, 161938. [Google Scholar] [CrossRef]
- Lei, G.; Wu, J.; Qin, L.; Wu, S.; Zhang, F.; Fan, X.; Peng, W.; Li, Y. Graphene hydrogel bridged pyrolysis strategy: Carbon cladded Fe2N nanoparticles in graphene matrix for efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2024, 58, 1088–1097. [Google Scholar] [CrossRef]
- Guo, W.; Teng, X.; Zhao, Q.; Zhang, B.; Yue, Q.; Tan, W.; Du, H.; Yu, J.; Zhou, B. Polypyrrole hydrogel as a universal precursor for the target preparation of heteroatom-doped hierarchical carbon with atomically distributed metal sites towards high-efficiency ORR and Zn–air batteries. Sustain. Energy Fuels 2022, 7, 515–525. [Google Scholar] [CrossRef]
- Yang, B.; Yu, H.; Jia, X.; Cheng, Q.; Ren, Y.; He, B.; Xiang, Z. Atomically dispersed isolated FE–CE Dual-Metal-Site catalysts for Proton-Exchange membrane fuel cells. ACS Appl. Mater. Interfaces 2023, 15, 23316–23327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, J.; Yang, L.; Yang, T.; Liu, Y.; Zhou, L.; Xu, Z.; Zhou, X.; Tang, J. Boosting ORR/OER bifunctional electrocatalysis by promoting electronic redistribution of Fe-N-C on CoFe-FeNC for ultra-long rechargeable Zn-air batteries. Appl. Catal. B Environ. 2024, 359, 124485. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Zhu, Z.; Zhu, R.; Zhang, J.; Yue, Y.; Li, G.; Zhou, L.; Yan, Z. Single-atom catalysts for lithium-sulfur batteries: Research progress and prospects. J. Energy Chem. 2025, 107, 440–458. [Google Scholar] [CrossRef]
- Van Tam, T.; Kang, S.G.; Kim, M.H.; Lee, S.G.; Hur, S.H.; Chung, J.S.; Choi, W.M. Novel Graphene Hydrogel/B-Doped Graphene quantum dots composites as trifunctional electrocatalysts for Zn∓Air batteries and overall water splitting. Adv. Energy Mater. 2019, 9, 1900945. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Chen, Y.; Xu, Z.; Cai, D.; Xu, M.; Tong, R. Cobalt-containing ZIF-derived catalysts for Zn–air batteries. Mater. Chem. Front. 2024, 8, 2394–2419. [Google Scholar] [CrossRef]
- Jaouen, F.; Lefèvre, M.; Dodelet, J.-P.; Cai, M. Heat-Treated FE/N/C catalysts for O2 electroreduction: Are active sites hosted in micropores? J. Phys. Chem. B 2006, 110, 5553–5558. [Google Scholar] [CrossRef]
- Pampel, J.; Fellinger, T. Opening of bottleneck pores for the improvement of nitrogen doped carbon electrocatalysts. Adv. Energy Mater. 2016, 6, 1502389. [Google Scholar] [CrossRef]
- Juvanen, S.; Sarapuu, A.; Vlassov, S.; Kook, M.; Kisand, V.; Käärik, M.; Treshchalov, A.; Aruväli, J.; Kozlova, J.; Tamm, A.; et al. Iron-Containing Nitrogen-Doped carbon nanomaterials prepared via NACL template as efficient electrocatalysts for the oxygen reduction reaction. ChemElectroChem 2021, 8, 2288–2297. [Google Scholar] [CrossRef]
- Parida, S.K.; Barik, T.; Chalke, B.A.; Amirthapandian, S.; Jena, H. Highly porous polypyrrole (PPY) hydrogel support for the design of a Co–N–C electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2023, 15, 37571–37579. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W. Application of hydrogel for energy storage and conversion. Next Mater. 2023, 1, 100049. [Google Scholar] [CrossRef]
- Zhao, F.; Bae, J.; Zhou, X.; Guo, Y.; Yu, G. Nanostructured Functional hydrogels as an emerging platform for advanced energy technologies. Adv. Mater. 2018, 30, 1801796. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Zhang, J.; Yao, J.; Liu, B.; Zhang, J.; Wang, H.; Lu, S.; Xiang, Y. Insights into the pore structure effect on the mass transfer of fuel cell catalyst layer via combining Machine learning and multiphysics simulation. Chem. Eng. Sci. 2025, 302, 120830. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Luo, Y.; Li, K.; Hu, Y.; Chen, T.; Wang, Q.; Hu, J.; Feng, J.; Feng, J. TIN as radical scavenger in Fe–N–C Aerogel oxygen reduction catalyst for durable fuel cell. Small 2024, 20, e2309822. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, B.; Hua, Y.; Lu, S.; Nong, Z.; Wang, J.; Song, Y. Ultralight MXene/rGO aerogel frames with component and structure controlled electromagnetic wave absorption by direct ink writing. Carbon 2024, 230, 119650. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Wang, B.; He, Q.; Cao, J.; Zhu, Y.; Su, K.; Yan, H.; Sun, P.; Li, R.; et al. Ultra-Bandwidth Microwave Absorption and Low Angle Sensitivity in Dual-Network Aerogels with Dual-Scale Pores. Small 2025, 2412744. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Wang, H.-L.; Hsu, C.-Y.; Wu, K.C.; Lin, Y.-F.; Tsai, D.-H. Functional nanostructured materials: Aerosol, aerogel, and de novo synthesis to emerging energy and environmental applications. Adv. Powder Technol. 2019, 31, 104–120. [Google Scholar] [CrossRef]
- Macchi, S.; Denmark, I.; Le, T.; Forson, M.; Bashiru, M.; Jalihal, A.; Siraj, N. Recent advancements in the synthesis and application of Carbon-Based catalysts in the ORR. Electrochem 2021, 3, 1–27. [Google Scholar] [CrossRef]
- Zhi, M.; Tang, H.; Wu, M.; Ouyang, C.; Hong, Z.; Wu, N. Synthesis and Photocatalysis of Metal Oxide Aerogels: A review. Energy Fuels 2022, 36, 11359–11379. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Y.; Yuan, J.; Zhang, X.; Yu, H.; Zhang, W.; Du, A.; Zhou, B. Synthesis, characterization and mechanism of formation of carbon aerogels incorporated with highly crystalline lanthanum oxychloride particles. RSC Adv. 2017, 7, 39635–39640. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jiang, Z.; Yuan, B.; Zhi, S.; Zhang, Y.; Li, J.; Wu, A. Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chem. Eng. Res. Des. 2021, 174, 71–78. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled graphene hydrogel via a One-Step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Potential of carbon aerogels in energy: Design, characteristics, and applications. Gels 2024, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.H.; Kamarudin, S.K.; Basri, S.; Karim, N.A. Organic aerogel as electro-catalytic support in low-temperature fuel cell. Int. J. Energy Res. 2022, 46, 16264–16280. [Google Scholar] [CrossRef]
- Xiao, H.; Lv, J.-B.; Tan, W.; He, X.; Chen, M.-H.; Zeng, K.; Hu, J.-H.; Yang, G. Ultrasound-assisted freeze-drying process for polyimide aerogels. Chem. Eng. J. 2022, 450, 138344. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, L.; Sun, J.; Wu, H.; Zhang, J.; Li, Z. High-density, high-activity and high-spin atomic Fe (Ⅲ) sites on ZIF-8-derived microporous carbon for efficient oxygen reduction reaction. J. Power Sources 2025, 646, 237264. [Google Scholar] [CrossRef]
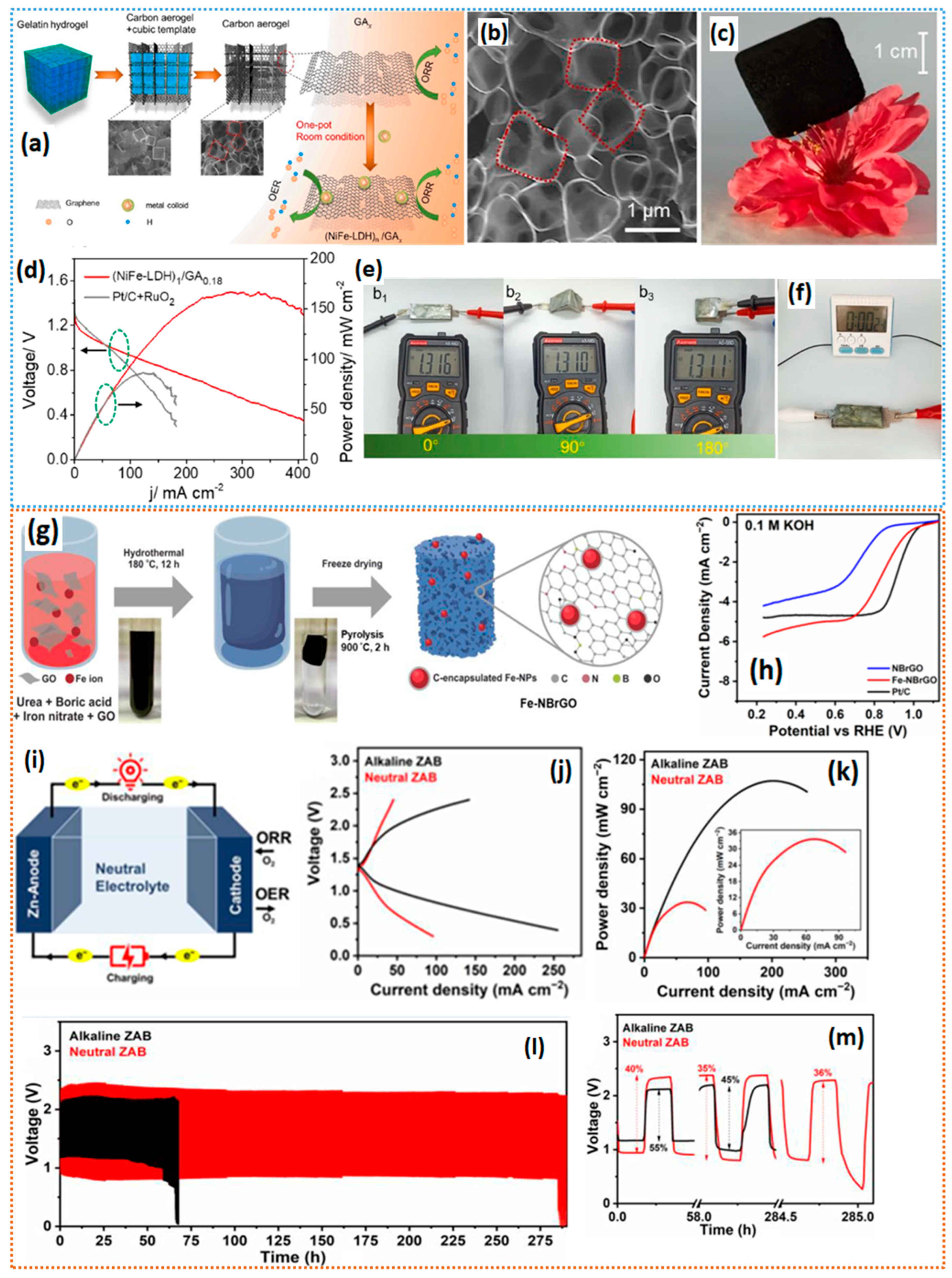
- Li, Q.; Sun, Z.; Yin, C.; Chen, Y.; Pan, D.; Yu, B.; Zhang, Y.; He, T.; Chen, S. Template-assisted synthesis of ultrathin graphene aerogels as bifunctional oxygen electrocatalysts for water splitting and alkaline/neutral zinc-air batteries. Chem. Eng. J. 2023, 458, 141492. [Google Scholar] [CrossRef]
- Li, Q.; He, T.; Jiang, X.; Lei, Y.; Liu, Q.; Liu, C.; Sun, Z.; Chen, S.; Zhang, Y. Boosting oxygen evolution activity of nickel iron hydroxide by iron hydroxide colloidal particles. J. Colloid Interface Sci. 2021, 606, 518–525. [Google Scholar] [CrossRef]
- Irmawati, Y.; Balqis, F.; Persada, P.B.; Destyorini, F.; Yudianti, R.; Iskandar, F.; Sumboja, A. Iron-Decorated Nitrogen/Boron Co-Doped Reduced Graphene Oxide Aerogel for neutral rechargeable ZN-Air batteries. Batteries 2023, 9, 356. [Google Scholar] [CrossRef]
- Bai, Y.; Hao, W.; Altaf, A.; Lu, J.; Liu, L.; Zhu, C.; Gu, X.; Wu, X.; Shen, X.; Cui, S.; et al. Construction of PDCU alloy decorated on the N-Doped carbon aerogel as a highly active electrocatalyst for enhanced oxygen reduction reaction. Gels 2025, 11, 166. [Google Scholar] [CrossRef]
- Snitkoff, R.Z.; Levy, N.; Ozery, I.; Ruthstein, S.; Elbaz, L. Imidazole decorated reduced graphene oxide: A biomimetic ligand for selective oxygen reduction electrocatalysis with Metalloporphyrins. Carbon 2018, 143, 223–229. [Google Scholar] [CrossRef]
- Bhagi-Damodaran, A.; Petrik, I.D.; Marshall, N.M.; Robinson, H.; Lu, Y. Systematic tuning of HEME redox potentials and its effects on O2 reduction rates in a designed oxidase in myoglobin. J. Am. Chem. Soc. 2014, 136, 11882–11885. [Google Scholar] [CrossRef]
- Collman, J.P.; Devaraj, N.K.; DecreéaU, R.A.; Yang, Y.; Yan, Y.-L.; Ebina, W.; Eberspacher, T.A.; Chidsey, C.E.D. A Cytochrome c Oxidase Model Catalyzes Oxygen to Water Reduction Under Rate-Limiting Electron Flux. Science 2007, 315, 1565–1568. [Google Scholar] [CrossRef]
- Persky, Y.; Kielesiński, Ł.; Reddy, S.N.; Zion, N.; Friedman, A.; Honig, H.C.; Koszarna, B.; Zachman, M.J.; Grinberg, I.; Gryko, D.T.; et al. Biomimetic Fe–CU Porphyrrole Aerogel Electrocatalyst for oxygen reduction reaction. ACS Catal. 2023, 13, 11012–11022. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Chung, D.Y.; Yoo, J.M.; Lee, H.S.; Kim, M.J.; Mun, B.S.; Kwon, S.G.; Sung, Y.-E.; Hyeon, T. Design Principle of Fe–N–C Electrocatalysts: How to optimize multimodal porous structures? J. Am. Chem. Soc. 2019, 141, 2035–2045. [Google Scholar] [CrossRef]
- Zion, N.; Douglin, J.C.; Cullen, D.A.; Zelenay, P.; Dekel, D.R.; Elbaz, L. Porphyrin Aerogel catalysts for oxygen reduction reaction in Anion-Exchange membrane fuel cells. Adv. Funct. Mater. 2021, 31, 2100963. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, T.; Yang, Z.; Zhao, H.; Zhang, J. Impact of heat treatment on the oxygen reduction reaction performance of PtCo/C(N) electrocatalyst in PEM fuel cell. ChemElectroChem 2024, 11, e202300782. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jang, I.; Sohn, Y.; Joo, E.; Jung, C.; Kim, N.D.; Jung, J.Y.; Paidi, V.K.; Lee, K.-S.; Kim, P.; et al. Effect of posttreatment on the catalytic performances of FE-N-C for oxygen reduction reactions. Int. J. Energy Res. 2023, 2023, 1–12. [Google Scholar] [CrossRef]
- Tang, Y.; Zeng, Z.; Yi, L.; Zhu, S.; Li, X.; Li, H.; Lv, N.; Xu, Y.; Zhang, Q.; Wang, Y. Heteroatom-Anchored porous carbon as efficient electrocatalyst for oxygen reduction reaction. Energy Fuels 2022, 36, 2068–2074. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Xin, Y.; Wang, L.; Zhou, Z.; Yang, L.; Jiang, J.; Zhang, D. FeNi coordination polymer based highly efficient and durable bifunction oxygen electrocatalyst for rechargeable zinc-air battery. Sep. Purif. Technol. 2022, 308, 122974. [Google Scholar] [CrossRef]
- Ha, M.-A.; Alia, S.M.; Norman, A.G.; Miller, E.M. Fe-Doped NI-Based catalysts surpass IR-Baselines for oxygen evolution due to optimal Charge-Transfer characteristics. ACS Catal. 2024, 14, 17347–17359. [Google Scholar] [CrossRef]
- Li, H.; Shu, X.; Tong, P.; Zhang, J.; An, P.; Lv, Z.; Tian, H.; Zhang, J.; Xia, H. Fe–Ni alloy nanoclusters anchored on carbon aerogels as High-Efficiency oxygen electrocatalysts in rechargeable Zn–Air batteries. Small 2021, 17, 2102002. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Zhao, X.; Schmidt, J.; Thomas, A. Active Salt/Silica-Templated 2D mesoporous FECO-Nx-Carbon as bifunctional oxygen electrodes for Zinc–Air batteries. Angew. Chem. Int. Ed. Engl. 2017, 57, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Liu, Y.; Chen, Y.; Tang, Y.; Goodenough, J.B.; Lee, J.-M. Robust N-doped carbon aerogels strongly coupled with iron–cobalt particles as efficient bifunctional catalysts for rechargeable Zn–air batteries. Nanoscale 2018, 10, 19937–19944. [Google Scholar] [CrossRef]
- Fu, G.; Liu, Y.; Wu, Z.; Lee, J.-M. 3D Robust Carbon Aerogels Immobilized with Pd3Pb Nanoparticles for Oxygen Reduction Catalysis. ACS Appl. Nano Mater. 2018, 1, 1904–1911. [Google Scholar] [CrossRef]
- Fu, G.; Chen, Y.; Cui, Z.; Li, Y.; Zhou, W.; Xin, S.; Tang, Y.; Goodenough, J.B. Novel Hydrogel-Derived Bifunctional oxygen electrocatalyst for rechargeable air cathodes. Nano Lett. 2016, 16, 6516–6522. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high-performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.H.; Yu, J.-Y.; Kim, D.-H.; Lee, S.U.; Lee, J.-H. Hierarchically designed 3D Holey C2N aerogels as bifunctional oxygen electrodes for flexible and rechargeable ZN-Air batteries. ACS Nano 2017, 12, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Dai, L.; Yao, J. Scalable fabrication of nanoporous carbon fiber films as bifunctional catalytic electrodes for flexible ZN-Air batteries. Adv. Mater. 2016, 28, 3000–3006. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Carré, B.; Caucheteux, J.; Compère, P.; Léonard, A.F.; Job, N. Development of In Situ Methods for Preparing La-Mn-Co-Based Compounds over Carbon Xerogel for Oxygen Reduction Reaction in an Alkaline Medium. Nanomaterials 2024, 14, 1362. [Google Scholar] [CrossRef]
- Fajardo-Puerto, E.; López-García, N.; Elmouwahidi, A.; Bailón-García, E.; Carrasco-Marín, F.; Ramírez-Valencia, L.D.; Pérez-Cadenas, A.F. Size control of carbon xerogel spheres as key factor governing the H2O2 selectivity in Metal-Free bifunctional Electro-Fenton catalysts for tetracycline degradation. Gels 2024, 10, 306. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Relationships between texture, surface chemistry and performance of N-doped carbon xerogels in the oxygen reduction reaction. Appl. Surf. Sci. 2021, 548, 149242. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Wang, Z.; Mo, Y.; Luo, X.; Yang, F.; Lv, M.; Li, Z.; Liu, X. Manganese-based oxide electrocatalysts for the oxygen evolution reaction: A review. J. Mater. Chem. A 2023, 11, 5476–5494. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Wei, X.; Luo, X.; Jiang, W.; Zheng, L.; Gu, W.; Zhu, C.; Yamauchi, Y. Improvement in ORR Durability of Fe Single-Atom Carbon Catalysts Hybridized with CeO2 Nanozyme. Nano Lett. 2024, 24, 9034–9041. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Lee, S.-H.; Peera, S.G. Xerogel-Derived manganese Oxide/N-Doped carbon as a Non-Precious Metal-Based oxygen reduction reaction catalyst in microbial fuel cells for energy conversion applications. Nanomaterials 2023, 13, 2949. [Google Scholar] [CrossRef] [PubMed]
- Peera, S.G.; Koutavarapu, R.; Reddy, P.S.P.; Koyyada, G.; Alodhayb, A.N.; Pandiaraj, S.; Kim, S.W.; Tamtam, M.R. Effect of N-Doped carbon on the morphology and oxygen reduction reaction (ORR) activity of a Xerogel-Derived Mn(II)O electrocatalyst. Catalysts 2024, 14, 792. [Google Scholar] [CrossRef]
- Li, L.; Han, M.; Zhang, P.; Yang, D.; Zhang, M. Recent advances in engineering Fe-N-C catalysts for oxygen electrocatalysis in ZN-Air batteries. ChemSusChem 2025, 18, e202401186. [Google Scholar] [CrossRef]
- Qu, X.; Yan, Y.; Zhang, Z.; Tian, B.; Yin, S.; Cheng, X.; Huang, R.; Jiang, Y.; Sun, S. Regulation strategies for FE−N−C and CO−N−C catalysts for the oxygen reduction reaction. Chem.—A Eur. J. 2024, 30, e20230400. [Google Scholar] [CrossRef]
- Martinez, U.; Babu, S.K.; Holby, E.F.; Chung, H.T.; Yin, X.; Zelenay, P. Progress in the development of FE-Based PGM-Free electrocatalysts for the oxygen reduction reaction. Adv. Mater. 2019, 31, 1806545. [Google Scholar] [CrossRef]
- Chen, G.; Liu, P.; Liao, Z.; Sun, F.; He, Y.; Zhong, H.; Zhang, T.; Zschech, E.; Chen, M.; Wu, G.; et al. Zinc-Mediated Template Synthesis of Fe-N-C Electrocatalysts with Densely Accessible Fe-Nx Active Sites for Efficient Oxygen Reduction. Adv. Mater. 2020, 32, 1907399. [Google Scholar] [CrossRef]
- Guo, J.; Li, B.; Zhang, Q.; Liu, Q.; Wang, Z.; Zhao, Y.; Shui, J.; Xiang, Z. Highly Accessible Atomically Dispersed Fe-Nx Sites Electrocatalyst for Proton-Exchange Membrane Fuel Cell. Adv. Sci. 2021, 8, 2002249. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zhang, L.; Zhao, Y.; Chen, K.; Li, Y.; Yang, X.; Zhao, L.; Sun, S.; Zhang, J. In-Situ Silica Xerogel Assisted Facile Synthesis of Fe-N-C Catalysts with Dense Fe-Nx Active Sites for Efficient Oxygen Reduction. Small 2022, 18, 2104934. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Zhang, L.-C.; Wu, Y.; Chen, H.; Li, T.; Xu, M.; Bao, S.-J. A gel-limiting strategy for large-scale fabrication of Fe–N–C single-atom ORR catalysts. J. Mater. Chem. A 2021, 9, 7137–7142. [Google Scholar] [CrossRef]
- Zhu, C.; Aoki, Y.; Habazaki, H. Co9 S8 Nanoparticles Incorporated in Hierarchically Porous 3D Few-Layer Graphene-Like Carbon with S,N-Doping as Superior Electrocatalyst for Oxygen Reduction Reaction. Part. Part. Syst. Charact. 2017, 34, 1700296. [Google Scholar] [CrossRef]
- Peera, S.G.; Liu, C. Unconventional and scalable synthesis of non-precious metal electrocatalysts for practical proton exchange membrane and alkaline fuel cells: A solid-state co-ordination synthesis approach. Co-ord. Chem. Rev. 2022, 463, 214554. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal–nitrogen–carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, L.-C.; Chen, H.; Zhu, H.; Wu, Y.; Xu, M.; Bao, S.-J. Highly efficient Fe-N-C oxygen reduction electrocatalyst engineered by sintering atmosphere. J. Power Sources 2020, 449, 227497. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Jang, W.-J.; Shim, J.-O.; Roh, H.-S. High temperature water–gas shift without pre-reduction over spinel ferrite catalysts synthesized by glycine assisted sol–gel combustion method. Int. J. Hydrogen Energy 2016, 41, 3870–3876. [Google Scholar] [CrossRef]
- Chourashiya, M.G.; Urakawa, A. Solution combustion synthesis of highly dispersible and dispersed iridium oxide as an anode catalyst in PEM water electrolysis. J. Mater. Chem. A 2017, 5, 4774–4778. [Google Scholar] [CrossRef]
- Han, C.-G.; Zhu, C.; Sheng, N.; Aoki, Y.; Habazaki, H.; Akiyama, T. A facile one-pot synthesis of FeO/carbon/graphene composites as superior anode materials for lithium-ion batteries. Electrochim. Acta 2017, 235, 88–97. [Google Scholar] [CrossRef]
- Gao, L.; Ma, Y.; Zhang, C.; Cao, M. Nitrogen-doped carbon trapped MnMoO4 microrods toward high performance aqueous zinc-ion battery. J. Alloys Compd. 2023, 968, 172008. [Google Scholar] [CrossRef]
- Sutar, P.; Maji, T.K. Coordination polymer gels: Soft metal–organic supramolecular materials and versatile applications. Chem. Commun. 2016, 52, 8055–8074. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xu, J.; Sohrabi, S.; Zhang, J. Metal–organic gels and their derived materials for electrochemical applications. J. Mater. Chem. A 2023, 11, 11572–11606. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Shi, C.; Huo, M.; Lin, Y. Progress in Research and Application of Metal–Organic Gels: A review. Nanomaterials 2023, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Co-ord. Chem. Rev. 2013, 257, 1373–1408. [Google Scholar] [CrossRef]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef]
- Wang, H.; Chen, B.; Liu, D. Metal–Organic frameworks and Metal–Organic gels for oxygen Electrocatalysis: Structural and Compositional considerations. Adv. Mater. 2021, 33, 2008023. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Saini, H.; Scheibe, B.; Dubal, D.P.; Schneemann, A.; Jayaramulu, K. Hierarchical porous metal–organic gels and derived materials: From fundamentals to potential applications. Chem. Soc. Rev. 2022, 51, 9068–9126. [Google Scholar] [CrossRef]
- Dong, X.; Shi, W.; Wang, G.; Chen, J.; Wang, R.; Zhang, J. Dual-Ligand Strategy to Construct Metal Organic Gel Catalyst with the Optimized Electronic Structure for High-Efficiency Overall Water Splitting and Flexible Metal–Air Battery. Small 2023, 20, 2307407. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Yang, C.; Liu, J.; Shen, J. A pyridine-Fe gel with an ultralow-loading Pt derivative as ORR catalyst in microbial fuel cells with long-term stability and high output voltage. Bioelectrochemistry 2020, 131, 107370. [Google Scholar] [CrossRef]
- Guo, H.; Feng, Q.; Xu, K.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Self-Templated Conversion of Metallogel into Heterostructured TMP@Carbon Quasiaerogels Boosting Bifunctional Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1903660. [Google Scholar] [CrossRef]
- Shijina, K.; Illathvalappil, R.; Sumitha, N.S.; Sailaja, G.S.; Kurungot, S.; Nair, B.N.; Mohamed, A.P.; Anilkumar, G.M.; Yamaguchi, T.; Hareesh, U.S. Melamine formaldehyde–metal organic gel interpenetrating polymer network derived intrinsic Fe–N-doped porous graphitic carbon electrocatalysts for oxygen reduction reaction. New J. Chem. 2018, 42, 18690–18701. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Yin, F.; Chen, B.; Fan, T.; He, X. Metal-organic gel-derived Fe-Fe2O3@nitrogen-doped-carbon nanoparticles anchored on nitrogen-doped carbon nanotubes as a highly effective catalyst for oxygen reduction reaction. Electrochim. Acta 2017, 232, 114–122. [Google Scholar] [CrossRef]
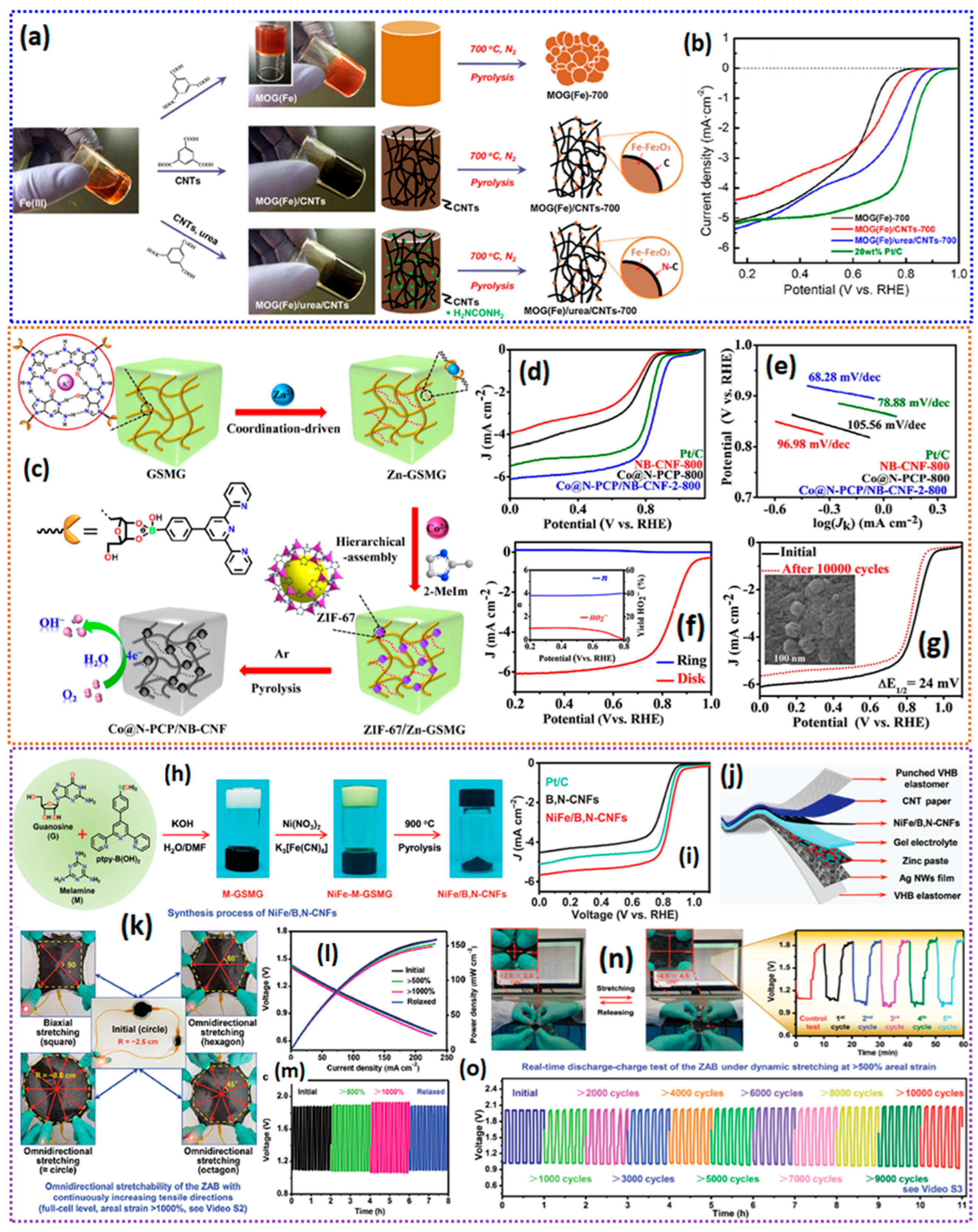
- Gu, C.; Li, J.; Liu, J.-P.; Wang, H.; Peng, Y.; Liu, C.-S. Conferring supramolecular guanosine gel nanofiber with ZIF-67 for high-performance oxygen reduction catalysis in rechargeable zinc–air batteries. Appl. Catal. B Environ. 2021, 286, 119888. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Gu, C.; Li, J.; Liang, Y.; Wang, H.; Cui, Y.; Liu, C. Supramolecular Gel-Derived Highly Efficient Bifunctional Catalysts for Omnidirectionally Stretchable Zn–Air Batteries with Extreme Environmental Adaptability. Adv. Sci. 2022, 9, 2200753. [Google Scholar] [CrossRef]
- Rizzo, C.; Marullo, S.; Billeci, F.; D’ANna, F. Catalysis in Supramolecular Systems: The Case of Gel Phases. Eur. J. Org. Chem. 2021, 2021, 3148–3169. [Google Scholar] [CrossRef]
- Ashmath, S.; Rajeshkhanna, G.; Koyyada, G.; Pandiaraj, S.; Liu, C.; Peera, S.G.; Lee, T.-G. Biomass derived Fe3C/Fe-Nx-C as cathode catalyst for oxygen reduction reaction in dual chamber microbial fuel cells. Int. J. Hydrogen Energy 2025, 137, 1286–1299. [Google Scholar] [CrossRef]
- Das, S.K.; Peera, S.G.; Kesh, A.; Varathan, P.; Sahu, A.K. Fluorine-rich Schiff base ligand derived Fe/N–C–F and Co/N–C–F catalysts for the oxygen reduction reaction: Synthesis, experimental validation, and DFT insights. Sustain. Energy Fuels 2024, 9, 231–246. [Google Scholar] [CrossRef]
- Choi, C.H.; Baldizzone, C.; Grote, J.; Schuppert, A.K.; Jaouen, F.; Mayrhofer, K.J.J. Stability of Fe-N-C catalysts in acidic medium studied by Operando Spectroscopy. Angew. Chem. Int. Ed. Engl. 2015, 54, 12753–12757. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, X.; Tsai, M.; Jin, Q.; Liang, J.; Ma, F.; Wang, T.; Zheng, S.; Hwang, B.; Huang, Y.; et al. Atomically dispersed Fe-Nx/C electrocatalyst boosts oxygen catalysis via a new Metal-Organic polymer supramolecule strategy. Adv. Energy Mater. 2018, 8, 1801226. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Ma, N.; Liu, H.; Xia, Y.; Chen, C.; Yang, D.; Yao, X. Scalable and Cost-Effective Synthesis of Highly Efficient Fe2N-Based Oxygen Reduction Catalyst Derived from Seaweed Biomass. Small 2016, 12, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Zheng, Y.; Petersen, A.S.; Wan, H.; Hübner, R.; Zhang, J.; Wang, J.; Qi, H.; Ye, Y.; Liang, C.; Yang, J.; et al. Scalable and controllable synthesis of Pt-Ni Bunched-Nanocages aerogels as efficient electrocatalysts for oxygen reduction reaction. Adv. Energy Mater. 2023, 13. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sotiriou-Leventis, C.; Mumtaz, A. Smelting in the age of nano: Iron aerogels. J. Mater. Chem. 2008, 19, 63–65. [Google Scholar] [CrossRef]
- Bigall, N.C.; Herrmann, A.; Vogel, M.; Rose, M.; Simon, P.; Carrillo-Cabrera, W.; Dorfs, D.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Hydrogels and Aerogels from Noble Metal Nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 9731–9734. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.-K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels—Synthesis, Characterization, and Application as Electrocatalysts. Accounts Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Fan, X.; Jin, X.; Hübner, R.; Hu, Y.; Eychmüller, A. Emerging Noble Metal Aerogels: State of the art and a look forward. Matter 2019, 1, 39–56. [Google Scholar] [CrossRef]
- Du, R.; Jin, X.; Hübner, R.; Fan, X.; Hu, Y.; Eychmüller, A. Engineering Self-Supported Noble Metal foams toward electrocatalysis and beyond. Adv. Energy Mater. 2019, 10, 1901945. [Google Scholar] [CrossRef]
- Li, W.; Weng, B.; Sun, X.; Cai, B.; Hübner, R.; Luo, Y.; Du, R. A Decade of Electrocatalysis with Metal Aerogels: A Perspective. Catalysts 2023, 13, 167. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, W.; Guo, K.; Liu, X.; Liu, J.; Liang, X.; Wang, X.; Gao, D.; Gan, L.; Zhu, Y.; et al. Fine-Tuning intrinsic strain in Penta-Twinned PT–CU–MN nanoframes boosts oxygen reduction catalysis. Adv. Funct. Mater. 2020, 30, 1910107. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wang, C.; Li, C.; Zeng, Y.; Hwang, S.; Li, B.; Karakalos, S.; Park, J.; Kropf, A.J.; Wegener, E.C.; et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: Performance and durability improvements. Energy Environ. Sci. 2021, 14, 4948–4960. [Google Scholar] [CrossRef]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef]
- Li, Z.; Yu, R.; Huang, J.; Shi, Y.; Zhang, D.; Zhong, X.; Wang, D.; Wu, Y.; Li, Y. Platinum–nickel frame within metal-organic framework fabricated in situ for hydrogen enrichment and molecular sieving. Nat. Commun. 2015, 6, 8248. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Yuan, K.; Shao, Y.; Shen, X.; Cui, S.; Chen, X. Modulating Electronic Structure and Atomic Insights into the Novel Hierarchically Porous PdCuFe Trimetallic Alloy Aerogel for Efficient Oxygen Reduction. Small 2023, 20, 2307243. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, Y.; Chen, S. Facile synthesis of platinum-copper aerogels for the oxygen reduction reaction. Energy Mater. 2022, 2, 200033. [Google Scholar] [CrossRef]
- Wu, X.; Ni, C.; Man, J.; Shen, X.; Cui, S.; Chen, X. A strategy to promote the ORR electrocatalytic activity by novel engineering bunched three-dimensional Pd-Cu alloy aerogel. Chem. Eng. J. 2022, 454, 140293. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, H.; Yan, J.; Zhang, X. Iron-chelated hydrogel-derived bifunctional oxygen electrocatalyst for high-performance rechargeable Zn–air batteries. Nano Res. 2017, 10, 4436–4447. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, H.; Karakalos, S.; Hwang, S.; Xue, J.; Chen, M.; Su, D.; Wu, G. 3D polymer hydrogel for high-performance atomic iron-rich catalysts for oxygen reduction in acidic media. Appl. Catal. B Environ. 2017, 219, 629–639. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Li, B.; Ma, L.; Shi, L.; Xiong, Y.; Xu, H. Novel Iron/Cobalt-Containing Polypyrrole Hydrogel-Derived trifunctional electrocatalyst for Self-Powered overall water splitting. Adv. Funct. Mater. 2017, 27, 1606497. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.; Yen, C.H.; Wai, C.M.; Wang, C.; Yang, J.; Lin, Y. Synthesis of an excellent electrocatalyst for oxygen reduction reaction with supercritical fluid: Graphene cellular monolith with ultrafine and highly dispersive multimetallic nanoparticles. J. Power Sources 2017, 347, 69–78. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Du, F.; Jiang, L. Iron/Nitrogen/Phosphorus Co-Doped Three-Dimensional porous carbon as a highly efficient electrocatalyst for oxygen reduction reaction. J. Electrochem. Soc. 2019, 166, F935–F941. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Qian, Y.; Fang, Z.; Xiao, D.; Yu, G. Probing Enhanced Site Activity of Co–Fe Bimetallic Subnanoclusters Derived from Dual Cross-Linked Hydrogels for Oxygen Electrocatalysis. ACS Energy Lett. 2019, 4, 1793–1802. [Google Scholar] [CrossRef]
- Zhou, Q.; Ai, L.; Li, Q.; Hou, S.; Xu, L.; Sun, D.; Pang, H.; Huang, K.; Tang, Y. A “Ship-in-a-Bottle” strategy to anchor CoFe nanoparticles inside carbon nanowall-assembled frameworks for high-efficiency bifunctional oxygen electrocatalysis. Chem. Eng. J. 2021, 417, 127895. [Google Scholar] [CrossRef]
- Li, H.; Yin, J.; Meng, Y.; Liu, S.; Jiao, T. Nickel/Cobalt-Containing polypyrrole hydrogel-derived approach for efficient ORR electrocatalyst. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124221. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, D.; Wang, Y.; Li, X.; Chen, Z.; Li, K.; Li, Z.; Zheng, L.; Zuo, X. Single Atoms Anchored on Cobalt-Based Catalysts Derived from Hydrogels Containing Phthalocyanine toward the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2020, 8, 8338–8347. [Google Scholar] [CrossRef]
- Miao, Z.; Xia, Y.; Liang, J.; Xie, L.; Chen, S.; Li, S.; Wang, H.; Hu, S.; Han, J.; Li, Q. Constructing Co–N–C catalyst via a double crosslinking hydrogel strategy for enhanced oxygen reduction catalysis in fuel cells. Small 2021, 17, 2100735. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, T.; Luo, H.; Dou, J.; Bian, W.; Pan, Z.; Zheng, A.; Zhou, B. Ferrocene-crosslinked polypyrrole hydrogel derived Fe–N-doped hierarchical porous carbon as an efficient electrocatalyst for pH universal ORR and Zn–air batteries. New J. Chem. 2021, 45, 10002–10011. [Google Scholar] [CrossRef]
- Niu, H.-J.; Lin, S.-Y.; Chen, Y.-P.; Feng, J.-J.; Zhang, Q.-L.; Wang, A.-J. Hydrogel derived FeCo/FeCoP embedded in N, P-codoped 3D porous carbon framework as a highly efficient electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2021, 536, 147950. [Google Scholar] [CrossRef]
- Liu, J.; Maimaitiyiming, X.; Kuerban, Z. Oxygen reduction performance study of Fe/Ni doped PAAM/Agar double net hydrogel carbon materials. ChemistrySelect 2023, 8, e202302746. [Google Scholar] [CrossRef]
- Velayutham, S.; Parida, S.K.; Jena, H. A hybrid hydrogel approach for the design of N, S Dual-Doped porous carbon electrocatalyst for oxygen reduction reaction. ACS Appl. Energy Mater. 2024, 7, 2469–2477. [Google Scholar] [CrossRef]
- Jia, F.; Huan, W.; Zhu, P.; Zhao, X.; Liu, S. Hydrogel derived N, P co-doped porous defective carbon framework anchored with Co2P nanoparticles as robust electrocatalysts for Zn-air battery. J. Energy Storage 2024, 81, 110440. [Google Scholar] [CrossRef]
- Zong, L.; Chen, X.; Liu, S.; Fan, K.; Dou, S.; Xu, J.; Zhao, X.; Zhang, W.; Zhang, Y.; Wu, W.; et al. Ultrafine Fe/Fe3C decorated on Fe-N -C as bifunctional oxygen electrocatalysts for efficient Zn-air batteries. J. Energy Chem. 2020, 56, 72–79. [Google Scholar] [CrossRef]
- Fu, X.; Choi, J.-Y.; Zamani, P.; Jiang, G.; Hoque, A.; Hassan, F.M.; Chen, Z. Co–N decorated hierarchically porous graphene aerogel for efficient oxygen reduction reaction in acid. ACS Appl. Mater. Interfaces 2016, 8, 6488–6495. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Yan, X.; Chen, Y.; Xu, L.; Sun, D.; Lee, J.; Tang, Y. Boosting Bifunctional Oxygen Electrocatalysis with 3D Graphene Aerogel-Supported Ni/MnO Particles. Adv. Mater. 2017, 30, 1704609. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal–Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qian, Z.; Li, Y.; Luo, Y.; Liu, C.; Cui, J. N, B, F-Engineered nanocellulose—Cornstalks Aerogels for High-Performance ORR/OER materials. Adv. Sustain. Syst. 2024, 8, 2300594. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, R.; Hou, C.; Wang, Z. Porous Fe−N−C Aerogels Derived from Metal-Organic Aerogels as Electrocatalysts for the Oxygen Reduction Reaction. Chempluschem 2022, 87, e202200312. [Google Scholar] [CrossRef]
- Wang, H.; Pang, Y.; Mo, Z.; Wang, X.; Ren, J.; Wang, R. Performance evaluation of functionalized carbon aerogel as oxygen reduction reaction electrocatalyst in zinc-air cell. J. Power Sources 2021, 511, 230458. [Google Scholar] [CrossRef]
- Lei, G.; Ma, J.; Zhao, M.; Wu, S.; He, H.; Qi, H.; Peng, W.; Fan, X.; Zhang, G.; Zhang, F.; et al. Nitrogen−carbon materials base on pyrolytic graphene hydrogel for oxygen reduction. J. Colloid Interface Sci. 2021, 602, 274–281. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.; Wu, D. Porous nitrogen-doped reduced graphene oxide gels as efficient supercapacitor electrodes and oxygen reduction reaction electrocatalysts. Chin. J. Chem. 2020, 38, 1123–1131. [Google Scholar] [CrossRef]
- Jin, H.; Lin, D.; Zhou, L.; Zha, G.; Wu, H.; Li, S.; Jiang, M.; Huang, P.; Xie, H. In situ construction of MOF derived CoNC anchored on N-doped carbon xerogel sphere as efficient bifunctional ORR/OER electrocatalyst for Zn-air batteries. Sci. Rep. 2025, 15, 3480. [Google Scholar] [CrossRef]
- Álvarez-Manuel, L.; Alegre, C.; Sebastián, D.; Napal, P.F.; Moreno, C.; Bailón-García, E.; Carrasco-Marín, F.; Lázaro, M.J. Effect of carbon xerogel activation on Fe−N−C catalyst activity in fuel cells. ChemElectroChem 2023, 11, e202300549. [Google Scholar] [CrossRef]
- Álvarez-Manuel, L.; Alegre, C.; Sebastián, D.; Lázaro, M.J. Organic xerogels combined with iron and nitrogen as PGM-free catalysts for the oxygen reduction reaction. Int. J. Hydrogen Energy 2023, 52, 1076–1089. [Google Scholar] [CrossRef]
- Yuan, B.; Li, P.; Tian, M.; Qin, Q.; Liu, X. Preparation of a High Performance Electrocatalyst for Oxygen Reduction Reaction by Suppressing the Agglomeration of the Carbon Material with RbCl. ChemCatChem 2018, 10, 5190–5193. [Google Scholar] [CrossRef]
- Shijina, K.; Illathvalappil, R.; Kurungot, S.; Nair, B.N.; Mohamed, A.P.; Yamaguchi, T.; Anilkumar, G.M.; Hareesh, U.S.; Sailaja, G.S. Chitosan intercalated metal organic gel as a green precursor of FE entrenched and Fe distributed N-Doped mesoporous graphitic carbon for oxygen reduction reaction. ChemistrySelect 2017, 2, 8762–8770. [Google Scholar] [CrossRef]
- Zhu, T.; Feng, Q.; Liu, S.; Zhang, C. Metallogel-derived 3D porous carbon nanosheet composites as an electrocatalyst for oxygen reduction reaction. Compos. Commun. 2020, 20, 100376. [Google Scholar] [CrossRef]
- Shang, N.; Li, S.; Zhou, X.; Gao, S.; Gao, Y.; Wang, C.; Wu, Q.; Wang, Z. Co/nitrogen-doped carbon nanocomposite derived from self-assembled metallogels as efficient bifunctional oxygen electrocatalyst for Zn-air batteries. Appl. Surf. Sci. 2021, 537, 147818. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Han, L.; Lin, R.; Liu, H.; Xin, H.L.; Wang, D. Supramolecular gel-assisted synthesis of double shelled Co@CoO@N–C/C nanoparticles with synergistic electrocatalytic activity for the oxygen reduction reaction. Nanoscale 2016, 8, 4681–4687. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Shen, H.; Liu, Y.; Li, W.; Li, J. Supramolecular gel-assisted synthesis Co2P particles anchored in multielement co-doped graphene as efficient bifunctional electrocatalysts for oxygen reduction and evolution. Electrochim. Acta 2017, 231, 344–353. [Google Scholar] [CrossRef]
- Wu, Z.; Song, M.; Wang, J.; Liu, X. Supramolecular gel assisted synthesis of Co2P nanosheets as an efficient and stable catalyst for oxygen reduction reaction. New J. Chem. 2018, 42, 8800–8804. [Google Scholar] [CrossRef]
- Du, R.; Jin, W.; Hübner, R.; Zhou, L.; Hu, Y.; Eychmüller, A. Engineering multimetallic aerogels for pH-Universal HER and ORR electrocatalysis. Adv. Energy Mater. 2020, 10, 1903857. [Google Scholar] [CrossRef]
- Cai, B.; Hübner, R.; Sasaki, K.; Zhang, Y.; Su, D.; Ziegler, C.; Vukmirovic, M.B.; Rellinghaus, B.; Adzic, R.R.; Eychmüller, A. Kern-Schale-Strukturierung rein metallischer Aerogele für eine hocheffiziente Nutzung von Platin für die Sauerstoffreduktion. Angew. Chem. 2017, 130, 3014–3018. [Google Scholar] [CrossRef]
- Song, T.; Xue, H.; Sun, J.; Guo, N.; Sun, J.; Wang, Q. Solvent assistance induced surface N-modification of PtCu aerogels and their enhanced electrocatalytic properties. Chem. Commun. 2021, 57, 7140–7143. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Bi, C.; Xia, H.; Engelhard, M.H.; Du, D.; Lin, Y. Intermetallic Pd3Pb nanowire networks boost ethanol oxidation and oxygen reduction reactions with significantly improved methanol tolerance. J. Mater. Chem. A 2017, 5, 23952–23959. [Google Scholar] [CrossRef]
- Liu, W.; Rodriguez, P.; Borchardt, L.; Foelske, A.; Yuan, J.; Herrmann, A.; Geiger, D.; Zheng, Z.; Kaskel, S.; Gaponik, N.; et al. Bimetallic aerogels: High-Performance electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2013, 52, 9849–9852. [Google Scholar] [CrossRef]
- Cai, B.; Hübner, R.; Sasaki, K.; Zhang, Y.; Su, D.; Ziegler, C.; Vukmirovic, M.B.; Rellinghaus, B.; Adzic, R.R.; Eychmüller, A. Core–Shell Structuring of Pure Metallic Aerogels towards Highly Efficient Platinum Utilization for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. Engl. 2017, 57, 2963–2966. [Google Scholar] [CrossRef]
- Chattot, R.; Le Bacq, O.; Beermann, V.; Kühl, S.; Herranz, J.; Henning, S.; Kühn, L.; Asset, T.; Guétaz, L.; Renou, G.; et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 2018, 17, 827–833. [Google Scholar] [CrossRef]




| Type of Gel | Characteristics | Synthesis Route and Scalability | Advantages | Limitations |
|---|---|---|---|---|
| Hydrogel |
|
|
| Pre-drying is required before pyrolysis |
| Aerogel |
| Gel formation from solution (Sol–gel) → freeze/supercritical drying Scalability: Moderate to High |
|
|
| Xerogel | Poor retention of the porosity | Gel formation from solution (Sol–gel) → hot air oven drying Scalability: High |
|
|
| Metal–organic gel (MOG) | Hybrid catalysts synthesized from organic ligand/polymer with metal salt precursors | Coordination reaction with organic ligands and metal precursors to form gel Scalability: Moderate to high |
|
|
| Metal aerogel | Only metallic nanoparticles network Support less catalysts 3D network of porosity | Simple reduction in the metallic precursors with reducing agents Scalability: Moderate to low |
|
|
| Catalyst | a Surface Area (m2 g−1) b Porosity (nm) c Pore Volume (cm3/g) | ORR Active Site | Half-Wave Potentials (V vs. RHE) 0.1 M KOH a 0.1 M HClO4 b Pt/C Standard c | a Tafel Slope (mV dec−1) b No. of Electrons from K-L and RRDE c % of H2O2/H2O− | a Number of Potential Cycles/loss in E1/2 (mV) b Chrono-Amperometry Current Retention | a Fuel Cell b Zn–Air Battery Performance c Specific Capacity | Ref. |
|---|---|---|---|---|---|---|---|
| Hydrogel-derived catalysts | |||||||
| HP/FeCo-NC-2 | a 771, b 4 nm | Atomically dispersed Fe/C-Nx | a 0.8651 c 0.86 | a 95 b 23 c 3.87−3.94, d 7% | b 90%/15 h | NR | [47] |
| CuFe AC@NC | a 289, b 4 nm | Fe-N4-C and Co-N4-C | a 0.887 c 0.853 | a 78 b 3.83–3.91 | b 95%/12,000 s | c 806.2 mAh g−1 | [52] |
| Fe2N/NC-1 | a 216 | Fe2N NPs @NC | a 0.90 & 1.01 with NH3 treatment c 0.85 | a 102 b 3.81/3.91–3.99 c 5% | NR | NR | [53] |
| Co−N−C-0.02 | a 493 c 1.49–1.60 | CoN4-C | a 0.825 b 0.691 c 0.825 | a 36 b 3.65−4.00 c ≈17.4–0.00% | a 5000/11 b 25 h/72% | NR | [63] |
| CPP-900 | a 1002 b 0.837 | N, P doping Fe SACs | a 0.848 c 0.982 | a 81 b 3.94, c <1.72% | b 20,000/91% | b 204 mW cm−2 c 811 mAh gZn−1 | [54] |
| NPMC-1000 | a 1663 b <10 nm c 1.10 | N and P dopants | a 0.85 | b ∼4.0/3.85 c 8% | NR | b 55 mW cm−2c 735 mAh g−1 @5 mA cm−2 | [67] |
| C-Fe-UFR | a 433 b 1.144 | Metallic Fe and Fe-Nx | a 0.86 | b 3.93–3.98 c 6% | a 10,000/22 | b 142 mW cm−2 c 467 mAh g−1 @5 mA cm−2 | [168] |
| PANI-EN- hydrogel | a 1400 b <2 | Fe-N4 | b 0.83 | a 118 b ~4, c <1% | a 10,000/14 | NR | [169] |
| Ppy/FeTCPP/Co | a 472 b micro- and mesopores | Fe–N–C Co–Nx–C and Co o | a 0.86 b 0.72 c 0.82 | a 61 b 4/3.93 c 2% | b 95%/10 h | Rechargeable Zn–air battery round-trip efficiency 62% | [170] |
| PtFeCo/GCM | a 728 b 5.6 c 0.65 | PtFeCo alloy | b 0.916 | NR | a 20,000/25 | NR | [171] |
| PANI-Fe/PA -N1050 | NR | N doping and Fe-Nx | a 0.84 c 0.88 | b 3.3 | b 1000/14 | NR | [172] |
| CoFe-PPy | NR | N doping and CoNx, and Fe | a ~0.85 c ~0.85 | a 60 b ~4 | 5000 0.85 | NR | [173] |
| CoFe@N-CNWF | a 233 Mesopores | N doping and Fe and Co | a 0.80 c 0.84 | b 3.68–3.87 c <15% | b 20,000 s/11.7% | b 90 mW cm−2 c 806 mA hgZn−1 | [174] |
| NiPcTs/Co/Py | NR | N doping, Ni and Co | a ~0.79 c ~0.80 | b 3.83 | b 35,000 s/75% | NR | [175] |
| CoOx/Co−N−C (800) | a 786 b 2–6 nm c 0.144 | N doping, Cox and Co-Nx | a 0.88 c 0.88 | a 61.7 b 3.97/3.80 c <10% | b 20,000 s/83.8 | NR | [176] |
| P(AA-AM)(5-1)-Co-N | a 1397 | N doping and Co–Nx/C | b 0.820 c 0.854 | a 60.8 b ~3.9 c 17% | a 5000/4 | a 0.66 W cm−2 (H2-O2) 0.28 (H2-Air) | [177] |
| PF-800 | a 370 b 0.5, 5.4, c 0.76 | N doping, Fe and Fe-Nx | a 0.79 | b 3.75–3.99 c 9.1 | b 20,000 s/89% | b 131 mW cm−2 c 748 mAh gZn−1 | [178] |
| FeCo/FeCoP@ NP-CF | 542 a 3.98 | FeCo, Fe2P, Co2P | a 0.85 c 0.84 | a 107 b 3.98 | b 15 h/91% | NR | [179] |
| Fe- Ni-NC | NR | N-doping, Ni and FeNx | a 0.66 | a 93.2 b 3.93 c 3.31% | b 1000 s/81% | NR | [180] |
| CNS-900 | NR | N and S doping | a 0.80 c 0.823 | a 37 b 3.9 | a 5000/16 | NR | [181] |
| Co2P/H-NPC | a 208 b 19.6 c 0.15 | N,P doping Co-O, Co-P | a 0.83 | a 47 b 3.7 | a 10,000/49 | b 120 mW cm−2 c 847 mAh gZn−1 | [182] |
| Fe/Fe3C@Fe-Nx-C-950 | a 535 b 5–50 nm | N doping Fe3C, FeNx-C | a 0.90 | a 56 b 3.74/c <3% | b 40,000 s/no loss | b 120 mW cm−2 | [183] |
| Aerogel-derived catalysts | |||||||
| Fe─N─C/TiN | a 540 b 5, 10, 50 | Fe SACs TiN | b 0.806 | b 4 c 1–4% | a 30,000/15 | a H2─O2 0.90 W cm−2 | [68] |
| (NiFe-LDH)n/GAx | a 344 b <2 | N doping Ni2+ to Fe3+ | a 0.840 c 0.831 | a 78 b 3.97/c 6.4% | a 5000/8 | b 230 mW cm−2 c 49 mAh gzn−1 | [85] |
| Fe-NBrGO | a 553 b 2–4 | B and N Fe3O4, Fe3C | a 0.826 | b 3.8 | NR | a 107 mW cm−2 | [87] |
| Pd3Cu@NC | a 96 | Pd3Cu alloy NPs | a 0.925 | a 90 b 4.12 c 2–3% | NR | NR | [88] |
| HT800-FeP | NR | N and Fe-N4 SACs | a 0.86 | NR | NR | a 580 mW cm−2 H2-O2 AEMFC | [94] |
| Fe-N/P/C-850 | a 615 b 0.52 | N, P doping Fe-Nx | a 0.86 c 0.84 | a 64.7 | b 30,000/95.5% | NR | [97] |
| Fe–Ni ANC@NSCA | a 241 c 0.24 | N,S doping Fe-Nx, Ni-Nx, | a 0.891 c 0.876 | a 70 c 4 | a 10,000/no loss | b 140 mW cm−2 c 750 mA.h.gZn−1 | [100] |
| FeCo/N-DNC | a 260 | N doping Fe-Nx | a 0.81 c 0.84 | b 3.92 | b 10,000 s/19.7% | b 115 mW cm−2 c 804 mA.h.gZn−1 | [102] |
| Pd3Pb/rGO-CNTs aerogel | a 134 b 22–50 | Pd0/Pd2+ Pb0/Pb2+ | b 0.862 c 0.841 | b 3.84/c 8% | b 10,000/17.6% | NR | [103] |
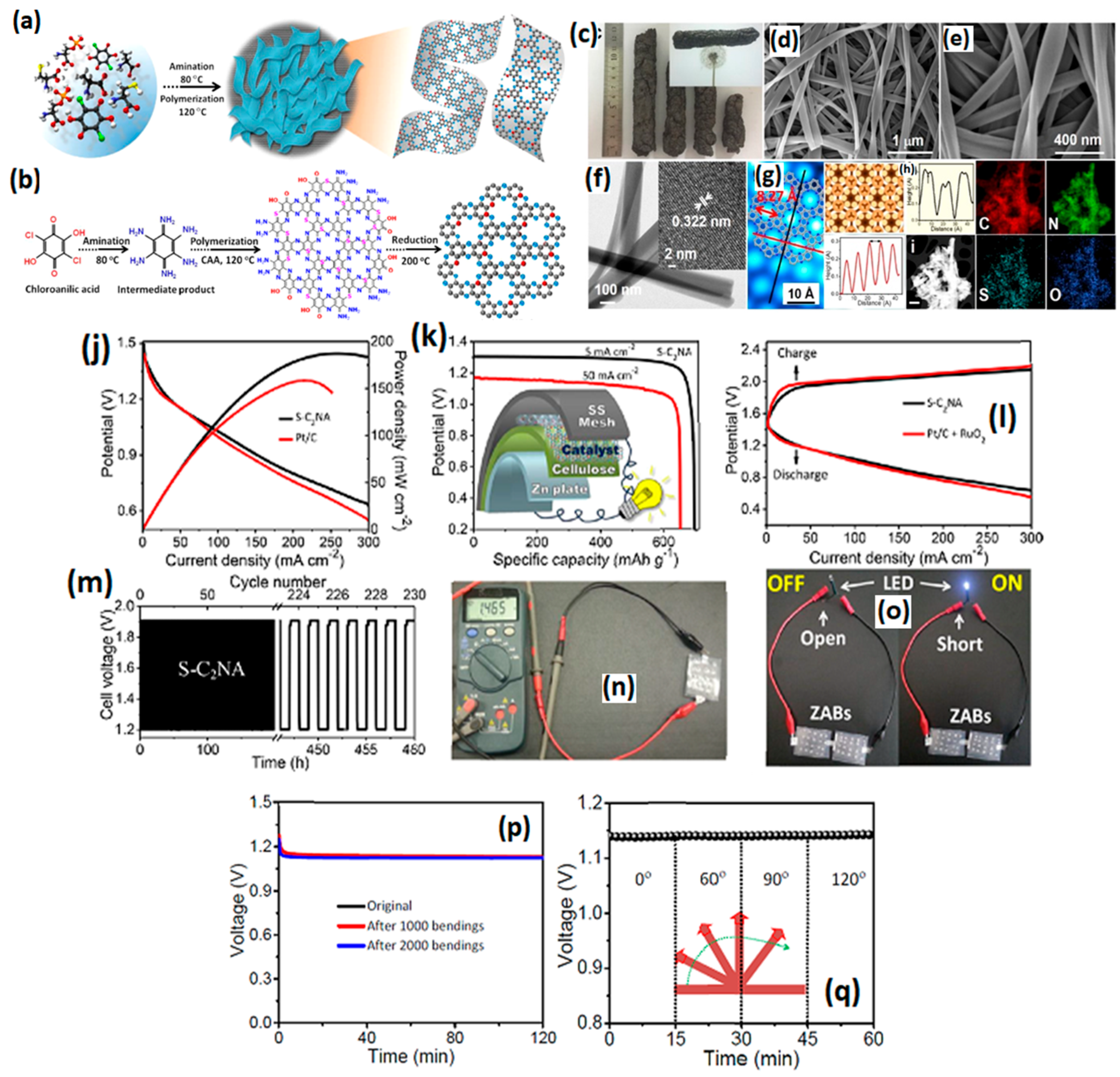
| S-C2 NA | a 1943 b 3 nm c 1.56 | N, P and S doping | a 0.88 c 0.85 | a 54 b 3.98 to 4.02/c 6% | a 5000/no loss b 10 h/no loss | b 209 mW cm−2 c 863 mA.h.gZn−1 | [106] |
| Co−N−GA | a 485 c 0.71 | N doping Co and Co-N | a 0.73 b 0.85 | b 3.75−3.85/13 b 3.94−3.97/2.26 | a 5000/15 | NR | [184] |
| Ni-MnO/rGO | a 109 b 13.5 | Mn2+/Mn Ni2+/Ni | a 0.78 c 0.84 | b 85 c 4 | b 10,000/93% | b 123 mW cm−2 c 758m A.h.gZn−1 | [185] |
| CoOx/NG-A | a 814 b 5 | N doping and CoOx | a 0.872 | b 3.8 | a 3000/26 | NR | [186] |
| N, B, F@Co-CNF | a 718 | N, B, F Co-Nx | a 0.845 c 0.834 | a 69 | b 20,000/85% | NR | [187] |
| Fe-N-C aerogel | a 292 | N doping Fe-Nx | a 0.79 | a 92 b 4/c 2% | NR | NR | [188] |
| Ce/Fe/NCG-2 | a 699 b 2–7 nm | N doping Fe-Nx | a 0.842 c 0.857 | a 58.4 b ~4 | a 3000/24 | 100.7 | [189] |
| GH-N-C-900 | a 786 c 0.76 | N doping | a 0.830 | b 3.53/3.58–3.82 c 20% | NR | NR | [190] |
| N-GA-4-900 | a 205 c 0.278 | N doping | a 0.84 c 0.84 | a 92.5 b 3.98 | b 18,000 s/92% | NR | [191] |
| Xerogel-derived catalysts | |||||||
| MnO/N-CC-2-900-2 | a 259 b 3.28 | N doping Mn-O | b 0.69 | b 3.94 | a 20,000/10 | NR | [113] |
| MnO/N-CC-5 | NR | N doping Mn-O | a 0.78 b 0.81 | a 150 b 3.95 | a 5000/10 b 25 h/97.5 | NR | [114] |
| ISG Fe-N-C | a 704 b 3.2 | Fe-Nx Fe SACs | a 0.91 b 0.74 c 0.85 | a 64 b 4 c <5% | a 5000/8 b 50,000/93% | b 259 mW cm−2 c 763 mA.h.gZn−1 | [121] |
| Fe-Ac-2 | a 950 c 0.77 | N doping Fe-Nx | a 0.87 c 0.85 | a 81 | a 12 h/94% | 153 | [122] |
| Co9S8@NS-C | a 409 | N, S doping Co-O/Co-S | a 0.85 c 0.87 | a 3.84–3.98 | b 36,000 s/94% | NR | [123] |
| CoNC@NCXS-800 | NR | N doping and CoNx | a 0.78 c 0.80 | a 137 b 3.9 c <15% | a 1000/21 | b 67 mW cm−2 c 710 mA.h.gZn−1 | [192] |
| Fe-N-CXG-H2O | a 1267 b 1.15 c 0.54 | N doping, Fe-Nx | b 0.65 c 0.820.54 | a 51 b 4.0 | 56%, Current loss after 20 h at 0.5 V in fuel cell | a 200 mWcm−2 | [193] |
| Fe-N-CXG-5.8-2-T2 | a 445b 8.8 c 0.45 | N doping, Fe-Nx | b 0.54 | a 75 b 3.53 | NR | NR | [194] |
| Metal–organic gel-derived catalysts | |||||||
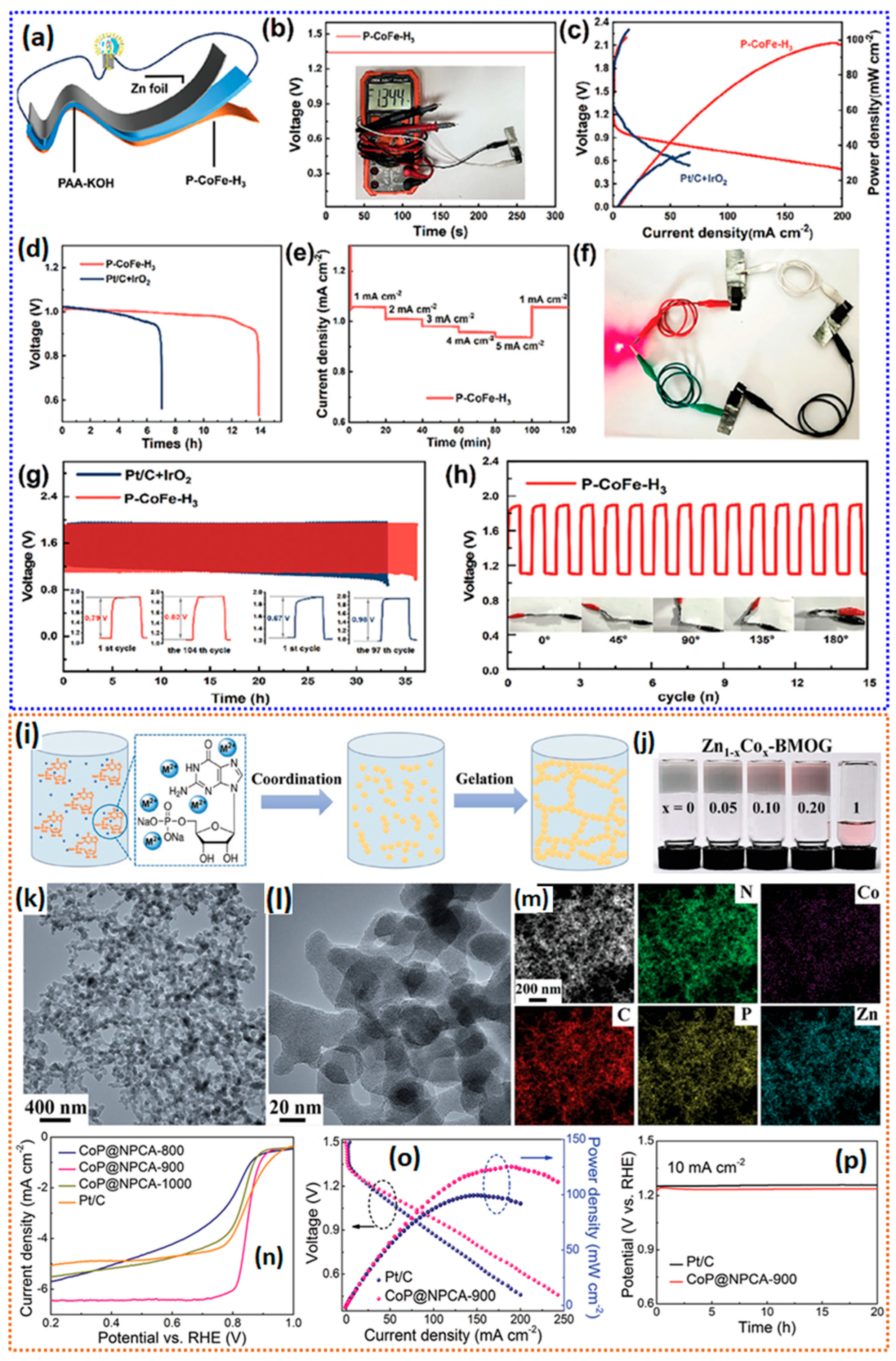
| P-CoFe-H3 | a 89 b 3.7 | N, P doping Co-Nx, Fe-Nx | a 0.80 c 0.86 | NR | NR | b 98 mW cm−2 | [139] |
| CoP@NPCA-900 | a 683 c 1.44 | N, P doping Co-P | a 0.85 | b 3.99 | NR | b 125 mW cm−2 c 668 mA.h.gZn−1 | [141] |
| Fe-MOG-MFN-C | a 950 c 0.10 | N and Fe-Nx | a 0.91(onset) c 0.91(onset) | a 68.5 b 3.6/c 20% | a 5000/31 | NR | [142] |
| MOG(Fe)/urea/CNTs-700 | a 150 c 0.27 | N, Fe and FeNx | a 0.72 | a 51 b 3.51–3.92 c <25% | b 20,000/91.7% | NR | [143] |
| Co@N-PCP/NB-CNF-2-800 | a 228 b 5.8 | N, B doping Co and Co-Nx | a 0.85 c 0.83 | a 68.28 b 3.7 c <10% | a 10,000/24 | b 143.8 mW cm−2 c 700 mA.h.gZn−1 | [144] |
| NiFe/B,N-CNFs | a 125 | N doping, Fe, Ni-Nx | a 0.84 c 0.82 | a 3.77 | NR | b 159 mW cm−2 137h stable | [145] |
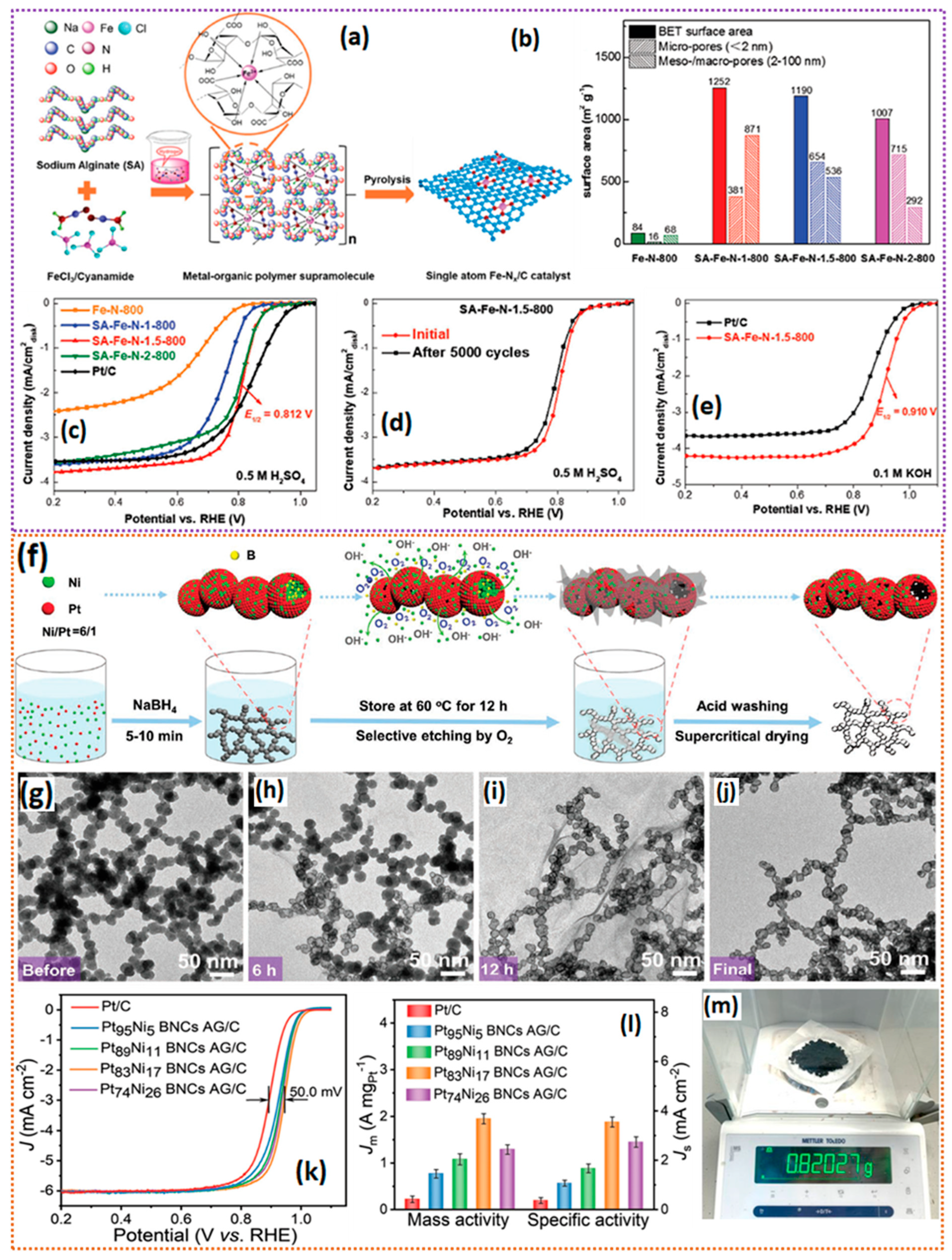
| SA-Fe-N-2-800 | a 1007 b 2–100 | N doping and Fe SACs | a 0.910 b 0.812 | a 72 b 3.9, c ~5% | a 5000/10 | NR | [150] |
| PON/C-“Rb” | a 1380 | N and P doping | a 0.87 c 0.83 | b 3.93–3.95 c <5% | b 20 h/85% | c 705 mA.h.gZn−1 | [195] |
| CHI-TMA-Fe-CW-M1 | a 565 | N and Fe-N, Fe2O3 | a 0.78 c 0.83 | a 90.9 b 3.8 c 7.8–13.8 | a 5000/24 | NR | [196] |
| Co/N@PCS-900-1 | a 742 c 0.445 | N, doping Co | a 0.82 c 0.79 | b 3.98~4.00 | b 50,000 s/94.7% | NR | [197] |
| CoNC-MOG-9 | a 351 | N doping and CoNx | a 0.851 c 0.83 | a 78 b 3.92 c <15% | a 5000/no loss | 63 | [198] |
| CoO@Co@N/C | NR | N doping Co, CoO | a 0.81 b 0.83 | b ~3.9 c ~5% | b 20,000/98% | NR | [199] |
| Co2P@CoNPG-900 | a 93.8 b 2.8 c 0.258 | N doping Co-Nx, Co-O | a 0.81 c 0.82 | a 69 b 3.96 | b 12,000/91.6% | NR | [200] |
| Co2P/C | NR | N, P doping Co-P | a ~0.81 | b ~4 c <20% | b 20,000/no loss | NR | [201] |
| Metal-gel-derived catalysts | |||||||
| Pt83Ni17 BNCs AGs/C | a 58.4 b 5–7 | Pt-Ni alloy | b 0.94 c 0.89 | b ~4 | a 20,000/6.1 | NR | [153] |
| Pd3CuFe0.5 | a 75 b 15.29 | Pd-Cu-Fe alloy | a 0.93 b 0.86 | a 96 b ~4 | b 16,000/95% | b 93.2 mW cm−2 | [165] |
| PtCu aerogel | a 43.6 | PtCu alloy | b 0.926 c 0.888 | NR | a 5000/20 | NR | [166] |
| Pd3Cu aerogel | a 44 b 8.77 | PdCu alloy | a 0.90 b 0.85 | a 50 | b 1700/13 | NR | [167] |
| Au-Pt aerogel | a 95.8 c 0.339–0.640 | Au-Pt alloy | a 0.91 b 0.86 | a 73 b 3.9–4.0 c 1–4% | a 1000/12 (0.1 M KOH b 1000/9 (0.1 M HClO4) | NR | [202] |
| Pd20Au aerogel | a 83–105 | PdAu alloy | b 0.922 | b 4 | a 10,000/no loss | NR | [203] |
| PtCu aerogel | NR | PtCu alloy | b 0.932 c 0.865 | b 4 c <1% | a 30,000/no loss | NR | [204] |
| IM-Pd3Pb NNs | a 23.3 | PdPb alloy | a 0.95 | a 56.3 b ~4 | b 10,000/16 | NR | [205] |
| Catalyst | Precursor Ratio | Gelation Time | Pyrolysis Temperature (°C)—Time | Ref. |
|---|---|---|---|---|
| Hydrogels | ||||
| HP/FeCo-NC-2 | Fe:Co:Melamine:Salicylic acid:2-Methylimidazole:Zn = 1.5:1:41:45:70:51 | 30 min | 950—2 h | [47] |
| CuFe AC@NC | Glutamic acid:Fe:Chitoson:Cu = 3.2:1.8:5.3:1 | ~5 min | 900 °C—2 h | [52] |
| Fe2N/NC-1 | GO:Heme = 2.6:1 | ~12 h (hydrothermal) | 900 °C, 1 h, N2 + NH3, 5 °C/min | [53] |
| Co−N−C-0.02 | Polypyrrole:SDS:APS:Co(acac)3 = 3.6:5:12:1 | 12 h (polymerization) | 800 °C/2 h Before, after acid leaching | [63] |
| NPMC-1000 | Aniline: Phytic acid:Ammonium persulphate: 5 mL:20 mL:0.96 g | Overnight | 1000 °C/2 h | [67] |
| C-Fe-UFR | Fe:Formaldehyde:Urea: 1.21 g:3.6 mL:1.8 g | 20 s | 900 °C/1 h | [168] |
| PANI-EN- hydrogel | Aniline:APS:FeCl3: 3.54:3.54:7.1 (mmol) | 20 min | 900 °C, 1 h Before, after acid leaching | [169] |
| Ppy/FeTCPP/Co | Pyrrole:FeTCPP:NaOH:APS:Co(NO3)2 (immersed in): 42 µL:14 mg FeTCPP:2.7 mg:137 mg:0.1 M | Instantly | 800 °C for 4 h | [170] |
| PANI-Fe/PA -N1050 | Aniline:FeCl3:pyretic acid: ammonium peroxysulfate (APS): 450 µL:20 mg:50 µL:286 mg | After several minutes | 1050/2 h | [172] |
| CoFe-PPy | Pyrrole:Co(II)(bpdc)3 (or Fe(II)(bpdc)3:APS: 42 µL:1 mL:0.6 mmol | 2 h | 800 °C for 4 h | [173] |
| NiPcTs/Co/Py | Pyrrole:APS:NiPcTs:Co (NO3)2: 42 µL:0.137 g:0.0154 g:0.1 M Co (NO3)2 | Instantly, hydrogel was immersed in Co2+ ions for 48 h | 800 °C for 4 h | [175] |
| CoOx/Co−N−C (800) | CoPc:Chitosan:acq. GO solution: 0.06 g:3% (w/v):5 mg/mL | Overnight | 800 °C for 2 h | [176] |
| P(AA-AM)(5-1)-Co-N | Acrylic acid:APS:BIS:CoCl2:cyanamide: 0.95 mL:0.19:0.2:2.8 mL | 2 h | 800 °C for 1 h | [177] |
| FeCo/FeCoP@ NP-CF | Fe:Co:PAM:pyritic acid (PA):melamine: 1.39:1.0:0.24:0.45:3.27 | 24 h | 800 °C for 2 h | [179] |
| Fe- Ni-NC | Agar:acrylamide:MBAA (cross linker):Irgacure 2959 (Initiator):Fe:Ni: 0.18:1.00:0.0018:0.064:0.5 M:0.5 M | 30 min @ 4 °C | 800 °C for 1.5 h | [180] |
| Co2P/H-NPC | Polyinosinic acid:starch:NH4Cl:Co acetate: 1.00:4.00:3.00:0.25 | 5 min @ 110 °C | 900 °C for 2 h | [182] |
| Fe/Fe3C@Fe-Nx-C-950 | Fe:EDTA: 0.550 g:1.9 g Fe-EDTA (complex):Glucose:NaNO3:Melamine: 1.00:7.20:8.40:10.08 | Following RT mixing and stirring | 950 °C for 2 h | [183] |
| Aerogels | ||||
| Fe─N─C/TiN | Resorcinol:Formaldehyde:TiO2 sol:Fe:propylene oxide: 1.00:0.57:1.96:0.098:0.204 | 5 h at 60 °C | 950 °C for 1 h and NH3 gas | [68] |
| (NiFe-DH)n/GAx | Gelation:Ni:Fe: 2.5 g:1 M:1 M | 2 h @ 4 °C | 900 °C for 2 h | [85] |
| Fe-NBrGO | GO:Urea:Boric acid:Iron nitrate:NH3 solution: 1.00:20.83:4.17:0.60 | 12 h—autoclave @ 180 °C | 900 °C for 2 h | [87] |
| Pd3Cu@NC | (i) Resorcinol:Urea:Formaldehyde: 1:0.24:2.13 → NC gel (ii) Pd:Cu:Na2CO3, Glyoxylic acid:NC: 3:1:371 mg:46 mg | Not Specified | (i) 900 °C for 2 h (ii) No pyrolysis | [88] |
| Co−N−GA | GO solution:Co:PANI: 2 mg mL−1:15 mg:80 mg | 12 h at 180 °C—hydrothermal | 900 °C for 1 h | [184] |
| Ni–MnO/rGO Aerogels | Mn:Ni:GO:PVA: 6.0 mg:25.8 mg:8 mg mL−1:16 mg mL−1 | 5 min | 600 °C (10% H2/Ar)—15 h | [185] |
| Pd3Pb/rGO-CNTs | Pd:Pb:GO:CNTs:PVA: 8.0 mg:5.0 mg:4 mg mL−1:4 mg mL−1:16 mg mL−1 | 5 min | 600 °C (10% H2/Ar)—12 h | [103] |
| FeCo/NDNC aerogels | K4Fe(CN)6:K3Co(CN)6:Chitosan:GO: 1 mL (0.5 mmol):1 mL (0.5 mmol):10 mg mL−1), 20 mg | 5 min | 600 °C for 3 h H2 | [102] |
| Fe–Ni ANC@NSCA | Fe:Ni:aniline:Tannic acid:APS: 1:2.47:9.38:0.69:0.63:15 | 12 h | 800 °C for 3 h | [100] |
| Xerogels | ||||
| MnO/N-CC-2-900-2 | KMnO4:glucose:melamine:N-doped carbon: 1.0 g:4.78 g:1 g:100 mg | 2–3 min | 900 °C—1 h | [113] |
| MnO/N-CC-5 | KMnO4:glucose:melamine:N-doped carbon: 1.0 g:4.78 g:1 g:400 mg | 2–3 min | 900 °C—1 h | [114] |
| ISG Fe-N-C | Glucosamine-HCL:ferrous gluconate:ammonia:TEOS: 1.5 g:0.75 g:50 µL:10 mL | 3 h at 60 °C | 900 °C—2 h | [121] |
| Fe-AC-2 | FeCl3:agarose:activated carbon: 1:3:3 | 2 h at 70 °C | 800 °C for 2 h | [122] |
| gl45-900 | Co:Mg:thiourea:glycise: 5 mmol:15 mmol:10 mmol:45 mmol | Not Specified | 900 °C for 2 h | [123] |
| CoNC@NCXS-800 | (i) NH3:ethanol:H2O:resorcinol: 0.1 mL:8 mL:20 mL:0.2 g (ii) (i) + ZIF-67:formaldehyde: 0.5 g:0.28 mL | (i) 30 min (ii) 100 °C—24 h (hydrothermal) | 800 °C for 2 h | [192] |
| Metal–Organic Gel | ||||
| P-CoFe-H3 | Co:Fe:Phytic acid:H3TATAB: 20 mM:20 mM:528 mg:20 mM | 30 min at 80 °C | Not Specified | [139] |
| N3/Fe/C-Pt | 2-aminopyridine:Fe: 0.1g mL−1:50 mg mL−1 | Not Specified | 900 °C for 2 h | [140] |
| Zn0.90Co0.10-BMOG | GMP:ZnCl2:CoCl2: 2.04 g:0.90 mM:0.10 mM | 5 min stirring stand still—12 h | 900 °C for 2 h | [141] |
| Fe-MOG-MF IPN | Trimesic acid:FeCl3: 1:3 melamine and formaldehyde: 1:3 naphthalene (10% w/w) | overnight | 900 °C for 3 h | [142] |
| MOG(Fe)/urea/CNTs-700 | Trimesic acid:Fe:CNTs:Urea: 1:1:75 mg:125 mg | several seconds | 700 °C for 5 h | [143] |
| CoNC-MOG-9 | FA-Co gels:Folic acid:NaOH:CoCl2:NaCl: 530 mg:96 mg:142.2 mg:5 g | 5 min stirring stand still—4 h | 900 °C for 1 h | [198] |
| NiFe/B,N-CNFs | ptpy-B(OH)2:melamine:guanosine:K3Fe[(CN)6]–Ni(NO3)2: 24.7 mg:20 mg: 0 mg:1:1 molar ratio | 5 min | 900 °C for 2 h | [145] |
| SA-Fe-N | Sodium alginate:FeCl3:cynamide:certain amount: 1 g:1.0 mL (50% solution) | 3 h at 60 °C | 800 °C for 1 h | [150] |
| Metal gels | ||||
| Pt83Ni17 BNCs AG | 5 mL of 18-mM NiCl2 and 67 μL of 0.445-M H2PtCl6, 60-mM NaBH4 | 10 min | No pyrolysis | [153] |
| Pd3CuFe0.5 | Na2CO3 (0.2968 g), glycolic acid monohydrate (0.0368 g), PdCl2 (3 mm), CuCl2·2H2O (1 mm), and FeCl3·6H2O (0.5 mm) | 10 min at 60 °C | No pyrolysis | [165] |
| PtCu | Na2PtCl4 (685 μL, 5 × 10−5 mol) CuCl2 (200 μL, 5 × 10−5 mol) NaBH4 (5 mL, 2 × 10−3 mol) | Several seconds | No pyrolysis | [166] |
| Au–Pt | Trisodium citrate dehydrate (400 × 10−3 M, 25 μL), HAuCl4·3H2O (32.5 × 10−3 M, 15.4 μL), K2PtCl4 (32.5 × 10−3 m, 15.4 μL), and NaBH4 (200 × 10−3 m, 20 μL) NH4F (1 M, 555 μL) | ~6 h | No pyrolysis | [202] |
| PtCu | 70 μL CuCl2·2H2O (0.6 mol), 2 mL H2PtCl6·6H2O (20 mmol), 10 mg NaOH, 5 mL NMP | 180 °C for 8 h autoclave | No pyrolysis | [204] |
| Catalyst | Zn–Air Battery Cycling Performance | Ref. |
|---|---|---|
| CuFe AC@NC | Galvanostatic charge–discharge research demonstrated that ZABs maintain a steady voltage gap of 0.80 V for approximately 450 cycles, with each cycle lasting 10 min and a current density of 5 mA cm−2. | [52] |
| C-Fe-UFR | The discharge–charge testing of Zn–air batteries at 10 mA·cm–2 (20 min/cycle) show minor voltage change after 100 cycles (approx. 34 h). | [168] |
| PPy/FeTCPP/Co | Cycling performance at 5 mA cm−2 and 0.75 V charge–discharge voltage gap yields ≈ 62% round-trip efficiency, with a voltage gap increase of ≈0.1 V, after 24 h (20 min each charge and discharge session) | [170] |
| CoFe@N-CNWF | After 200 charge/discharge cycles at 10 mA cm−2, 20 min per cycle, the voltaic efficiency drops to 52.5% from 62.2%. | [174] |
| CPP-900 | CPP-900 has outstanding endurance, enduring over 1000 cycles at 10 mA cm−2 in a recurrent discharge–charge cycle system. | [54] |
| Co2P/H-NPC | The initial ΔV at 2 mA cm−2 is 0.78 V. After 300 h (600 cycles), ΔV barely rises by 100 mV to 0.88 V. | [182] |
| Fe/Fe3C@Fe-Nx-C | Excellent stability over 200 cycles at 5 mA cm−2 with a narrow discharge/charge voltage gap of ~0.87 V | [183] |
| (NiFe-LDH)1/ GA0.18 | No decay for over 340 h. For the long-period cycling (2 h per cycle), ZABs for over 100 h, with stable charge/discharge voltage up to 53 cycles. | [85] |
| Fe-NBrGO | Discharge–charge cycling test at a current density of 10 mA cm−2—284 h cycling test, 5% reduction in performance, stable voltaic efficiency (~35%) (neutral ZABs) | [87] |
| Ni-MnO/r-GO | Discharge–charge cycling test at a current density of 10 mA cm−2—20 min each cycle—100 cycles—small overpotential increase. Round-trip overpotentials reduced voltaic efficiency by 9.1% from 0.73 V to 0.98 V at the 100th cycle. | [185] |
| FeCo/N-DNC | The battery has a longer cycle life (100 charge/discharge cycles) compared to the combined Pt/C + RuO2 battery (30 cycles). | [102] |
| Fe-Ni ANC@NSCA | Discharge current density of 5 mA cm−2 for 500 h—negligible voltage drop on both charge/discharge segments | [100] |
| ISG Fe-N-C | After 360 cycles for 120 h, the overpotential decreases to 0.77 V with an efficiency of 60.9%; after 660 cycles for 220 h, the overpotential increases to 0.93 V with an efficiency of 56.1%. | [121] |
| P-CoFe-H3 | Initial voltaic efficiency of 57.89%; at 104th cycle, 56.54%. | [139] |
| CoNC-MOG-9 | 110 h at current density of 10 mA cm–2 (10 min each cycle)—negligible voltage loss. | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peera, S.G.; Byun, M. Engineering Gel-Based Precursors into Advanced ORR Catalysts for Zn–Air Batteries and Fuel Cells: Insights into Hydrogels, Aerogels, Xerogels, Metal–Organic Gels, and Metal Aerogels. Gels 2025, 11, 479. https://doi.org/10.3390/gels11070479
Peera SG, Byun M. Engineering Gel-Based Precursors into Advanced ORR Catalysts for Zn–Air Batteries and Fuel Cells: Insights into Hydrogels, Aerogels, Xerogels, Metal–Organic Gels, and Metal Aerogels. Gels. 2025; 11(7):479. https://doi.org/10.3390/gels11070479
Chicago/Turabian StylePeera, Shaik Gouse, and Myunghwan Byun. 2025. "Engineering Gel-Based Precursors into Advanced ORR Catalysts for Zn–Air Batteries and Fuel Cells: Insights into Hydrogels, Aerogels, Xerogels, Metal–Organic Gels, and Metal Aerogels" Gels 11, no. 7: 479. https://doi.org/10.3390/gels11070479
APA StylePeera, S. G., & Byun, M. (2025). Engineering Gel-Based Precursors into Advanced ORR Catalysts for Zn–Air Batteries and Fuel Cells: Insights into Hydrogels, Aerogels, Xerogels, Metal–Organic Gels, and Metal Aerogels. Gels, 11(7), 479. https://doi.org/10.3390/gels11070479









