Sol–Gel-Synthesized Pt, Ni and Co-Based Electrocatalyst Effects of the Support Type, Characterization, and Possible Application in AEM-URFC
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Sol–Gel Synthesis of Electrocatalysts
4.2. Electrodes Fabrication
4.3. Electrocatalyst Characterization
4.3.1. Physicochemical Characterization
4.3.2. Electrochemical Characterization
- i
- Initial OER activity testing
- ii
- ORR and OER testing with RRDE
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPT | Magnelli-phase titania |
| HER | Hydrogen evolution reaction |
| OER | Three letter acronym |
| ORR | Oxygen reduction reaction |
| HOR | Hydrogen oxidation reaction |
| LSV | Linear sweep voltammetry |
| CV | Cycling voltammetry |
| RRDE | Rotating disk electrode |
| CO2RR | CO2 electrochemical reduction |
References
- Bard, A.J.; Fox, M.A. Artificial photosynthesis: Solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar]
- Nocera, D.G. Chemistry of personalized solar energy. Inorg. Chem. 2009, 48, 10001–10017. [Google Scholar]
- Zhao, S.; Yan, L.; Luo, H.; Mustain, W.; Xu, H. Recent progress and perspectives of bifunctional oxygen reduction/evolution catalyst development for regenerative anion exchange membrane fuel cells. Nano Energy 2018, 47, 172–198. [Google Scholar]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part B: Unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell, Renew. Sustain. Energy Rev. 2017, 75, 775–795. [Google Scholar] [CrossRef]
- Gabbasa, M.; Sopian, K.; Fudholi, A.; Asim, N. A review of unitized regenerative fuel cell stack: Material, design and research achievements. Int. J. Hydrogen Energy 2014, 39, 17765–17778. [Google Scholar]
- Sadhasivam, T.; Dhanabalan, K.; Roh, S.H.; Kim, T.H.; Park, K.W.; Jung, S. A comprehensive review on unitized regenerative fuel cells: Crucial challenges and developments. Int. J. Hydrogen Energy 2017, 42, 4415–4433. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar]
- Gorlin, Y.; Jaramillo, T.F. A bifunctional nonprecious metal catalyst for oxygen reduction and water oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar]
- Chen, G.; Bare, S.R.; Mallouk, T.E. Development of supported bifunctional electrocatalysts for unitized regenerative fuel cells. J. Electrochem. Soc. 2002, 149, A1092–A1099. [Google Scholar]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Science 2013, 339, 1214–1218. [Google Scholar] [CrossRef]
- Rajendran, S.; Naushad, M.; Raju, K.; Boukherroub, R. Emerging Nanostructured Materials for Energy and Environmental Science; Springer: Berlin/Heidelberg, Germany, 2019; Volume 23, ISBN 978-3-030-04473-2. [Google Scholar]
- Belenov, S.; Pavlets, A.; Paperzh, K.; Mauer, D.; Menshikov, V.; Alekseenko, A.; Pankov, I.; Tolstunov, M.; Guterman, V. The PtM/C (M = Co, Ni, Cu, Ru) Electrocatalysts: Their Synthesis, Structure, Activity in the Oxygen Reduction and Methanol Oxidation Reactions, and Durability. Catalysts 2023, 13, 243. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, 1500564. [Google Scholar] [CrossRef]
- Maksimova, K.; Lefterova, E.; Slavcheva, E. Nanostructured nickel and cobalt supported on Magneli—Phase titania—Preparation, properties and catalytic efficiency toward alkaline water electrolysis. Nanosci. Nanotechnol. 2015, 15, 40. [Google Scholar]
- Kim, J.H.; Lee, S.Y.; Lee, H.J. Strategies for the Design and Synthesis of Pt-Based Nanostructured Electrocatalysts in Proton Exchange Membrane Fuel Cells (PEMFCs). ACS Eng. Au 2025, 5, 1–9. [Google Scholar] [CrossRef]
- Touni, A.; Grammenos, O.; Banti, A.; Karfaridis, D.; Prochaska, C.; Lambropoulou, D.; Pavlidou, E.; Sotiropoulos, S. Iridium oxide-nickel-coated titanium anodes for the oxygen evolution reaction. Electrochim. Acta 2021, 390, 138866. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, X.; Zhou, W.; Murakami, S.; Kikuchi, K.; Nomura, N.; Wang, L.; Jiang, W.; Kawasaki, A. Preparation of monophasic titanium sub-oxides of Magnéli phase with enhanced thermoelectric performance. J. Eur. Ceram. Soc. 2018, 38, 507. [Google Scholar]
- Liu, H.; Xiao, H.; Qiao, Y.; Luo, M.Q.; Wang, C.; Yang, L.X.; Zeng, C.L.; Fu, C. Preparation, characterization, and electrochemical behavior of a novel porous Magnéli phase Ti4O7-coated Ti electrode. Ceram. Int. 2023, 49, 20564. [Google Scholar]
- Kim, M.; Choi, J.; Lee, W.; Ahn, Y.Y.; Lee, H.; Cho, K.; Lee, J. Performance of Magnéli phase Ti4O7 and Ti 3+ self-doped TiO2 as oxygen vacancy-rich titanium oxide anodes: Comparison in terms of treatment efficiency, anodic degradative pathways, and long-term stability. Appl. Catal. B Environ. 2023, 337, 122993. [Google Scholar]
- Slavcheva, E.; Nikolova, V.; Petkova, T.; Lefterova, E.; Dragieva, I.; Vitanov, T.; Budevski, E. Electrocatalytic activity of Pt and PtCo deposited on Ebonex by BH reduction. Electrochim. Acta 2005, 50, 5444. [Google Scholar]
- Figueiredo, W.; Prakash, R.; Vieira, C.G.; Lima, D.S.; Carvalho, V.E.; Soares, E.A.; Buchner, S.; Raschke, H.; Perez-Lopez, O.W.; Baptista, D.L.; et al. New insights on the electronic factor of the SMSI effect in Pd/TiO2 nanoparticles. Appl. Surf. Sci. 2022, 574, 151647. [Google Scholar]
- Bertella, F.; Concepción, P.; Martínez, A. TiO2 polymorph dependent SMSI effect in Co-Ru/TiO2 catalysts and its relevance to Fischer-Tropsch synthesis. Catal. Today 2017, 289, 181. [Google Scholar]
- Dogan, D.C.; Choi, J.; Seo, M.H.; Lee, E.; Jung, N.; Yim, S.-D.; Yang, T.-H.; Park, G.-G. Enhancement of Catalytic Activity and Durability of Pt Nanoparticle through Strong Chemical Interaction with Electrically Conductive Support of Magnéli Phase Titanium Oxide. Nanomaterials 2021, 11, 829. [Google Scholar] [CrossRef]
- Ekanayake, A.; Mai, H.; Chen, D.; Caruso, R.A. Recent advances in synthesis and application of Magnéli phase titanium oxides for energy storage and environmental remediation. Chem. Sci. 2025, 14, 2980–3018. [Google Scholar]
- Augustyn, V.; Simonbc, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Wu, Q.M.; Ruan, J.M.; Zhou, Z.C.; Sang, S.B. Magneli phase titanium sub-oxide conductive ceramic TinO2n−1 as support for electrocatalyst toward oxygen reduction reaction with high activity and stability. J. Cent. South Univ. 2015, 22, 1212–1219. [Google Scholar] [CrossRef]
- Gayen, P.; Saha, S.; Liu, X.; Sharma, K.; Ramani, V.K. High-performance AEM unitized regenerative fuel cell using Pt-pyrochlore as bifunctional oxygen electrocatalyst. Proc. Natl. Acad. Sci. USA 2021, 118, e2107205118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sust. Energy 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 21, 5102014. [Google Scholar] [CrossRef]
- Davis, R.J.; Mayer, J.W. Sol-Gel Synthesis of Nanostructured Catalysts. Catal. Today 2016, 116, 177–184. [Google Scholar]
- Omeiza, L.A.; Abdalla, A.M.; Wei, B.; Dhanasekaran, A.; Subramanian, Y.; Afroze, S.; Reza, M.S.; Bakar, S.A.; Azad, A.K. Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells. Energies 2023, 16, 1876. [Google Scholar] [CrossRef]
- De, A.; Kim, M.S.; Adhikari, A.; Patel, R.; Kundu, S. Sol–gel-derived nanostructured electrocatalysts for oxygen evolution reaction: A review. J. Mater. Chem. A 2024, 12, 19720–19756. [Google Scholar] [CrossRef]
- Letchumanan, I.; Yunus, R.M.; Masdar, M.S.; Karim, N.A. Advancements in electrocatalyst architecture for enhanced oxygen reduction reaction in anion exchange membrane fuel cells: A comprehensive review. Int. J. Hydrogen Energy 2025, 104, 527–546. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels 2024, 10, 63. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energ. Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Maksimova-Dimitrova, K.; Mladenova, B.; Borisov, G.; Slavcheva, E. Ni and Co Catalysts on Interactive Oxide Support for Anion Exchange Membrane Electrolysis Cell (AEMEC). Inorganics 2024, 12, 153. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Zhang, J.; Yang, J.H. Urea electrooxidation: Research progress and application of supported nickel-based catalysts. Ionics 2023, 29, 2969–2987. [Google Scholar] [CrossRef]
- Kamp, E.; Thielert, H.; von Morstein, O.; Kureti, S.; Schreiter, N.; Repke, J.U. Investigation on the simultaneous removal of COS, CS2 and O2 from coke oven gas by hydrogenation on a Pd/Al2O3 catalyst. Catal. Sci. Technol. 2020, 10, 2961–2969. [Google Scholar]
- Albertini, P.P.; Newton, M.A.; Wang, M.; Lecina, O.S.; Green, P.B.; Stoian, D.C.; Oveisi, E.; Loiudice, A.; Buonsanti, R. Hybrid oxide coatings generate stable Cu catalysts for CO2 electroreduction. Nat Mater. 2024, 23, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2. ACS Catal. 2023, 13, 948–973. [Google Scholar]
- Wang, L.; Gu, C.; Ge, X.; Zhang, J.; Zhu, H.; Tu, J. Anchoring Ni2P Sheets on NiCo2O4 Nanocone Arrays as Optimized Bifunctional Electrocatalyst for Water Splitting. Adv. Mater. Interfaces 2017, 4, 1700481–1700490. [Google Scholar]
- Singh, R.N.; Singh, J.P.; Lal, B.; Thomas, M.J.; Bera, S. New NiFe2−xCrxO4 Spinel Films for O2 Evolution in Alkaline Solutions. Electrochim. Acta 2006, 51, 5515–5523. [Google Scholar]
- Li, M.; Xiong, Y.; Liu, X.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile Synthesis of Electrospun MFe2O4 (M = Co, Ni, Cu, Mn) Spinel Nanofibers with Excellent Electrocatalytic Properties for Oxygen Evolution and Hydrogen Peroxide Reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar]
- Koza, J.A.; He, Z.; Miller, A.S.; Switzer, J.A. Electrodeposition of Crystalline Co3O4—A Catalyst for the Oxygen Evolution Reaction. Chem. Mater. 2012, 24, 3567–3573. [Google Scholar]
- Al-Mamun, M.; Su, X.; Zhang, H.; Yin, H.; Liu, P.; Yang, H.; Wang, D.; Tang, Z.; Wang, Y.; Zhao, H. Strongly Coupled CoCr2O4/Carbon Nanosheets as High Performance Electrocatalysts for Oxygen Evolution Reaction. Small 2016, 12, 2866–2871. [Google Scholar] [CrossRef]
- Putra, R.P.; Horino, H.; Rzeznicka, I.I. An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via In Situ Electrochemical Transformation. Catalysts 2020, 10, 233. [Google Scholar] [CrossRef]
- Dure, A.A.; Nazir, N.A.; Haider, A.; Iqbal, M.; Alwadai, N.; Kausar, A.; Ahmad, A. Fabrication of Efficient Electrocatalysts for Electrochemical Water Oxidation Using Bimetallic Oxides. ACS Omega 2023, 8, 9539–9546. [Google Scholar]
- Stelmachowski, P.; Duch, J.; Sebastián, D.; Lázaro, M.J.; Kotarba, A. Carbon-Based Composites as Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. Materials 2021, 14, 4984. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Tang, T.; Qu, Y.; Chen, Y.; Luo, H.; Pan, H.; Jiang, W.-J.; Zhang, J.; Hu, J.-S. Mitigating the Reconstruction of Metal Sulfides for Ultrastable Oxygen Evolution at High Current Density. CCS Chem. 2024, 6, 137–148. [Google Scholar] [CrossRef]
- Cheng, W.Z.; Liang, J.L.; Yin, H.-B.; Wang, Y.-J.; Yan, W.-F.; Zhang, J.-N. Bifunctional iron-phtalocyanine metal–organic framework catalyst for ORR, OER and rechargeable zinc–air battery. Rare Met. 2020, 39, 815–823. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Zhu, K.; Wang, J.; Cheng, Z.; Li, G.; Yang, L.; Bai, Z. Hybrid Co/CoO/Ce-Doped WO3 Nanoparticles on a ZIF-L Framework as Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc–Air Batteries. ACS Publ. J. Contrib. 2023, 6, 14353–14363. [Google Scholar] [CrossRef]
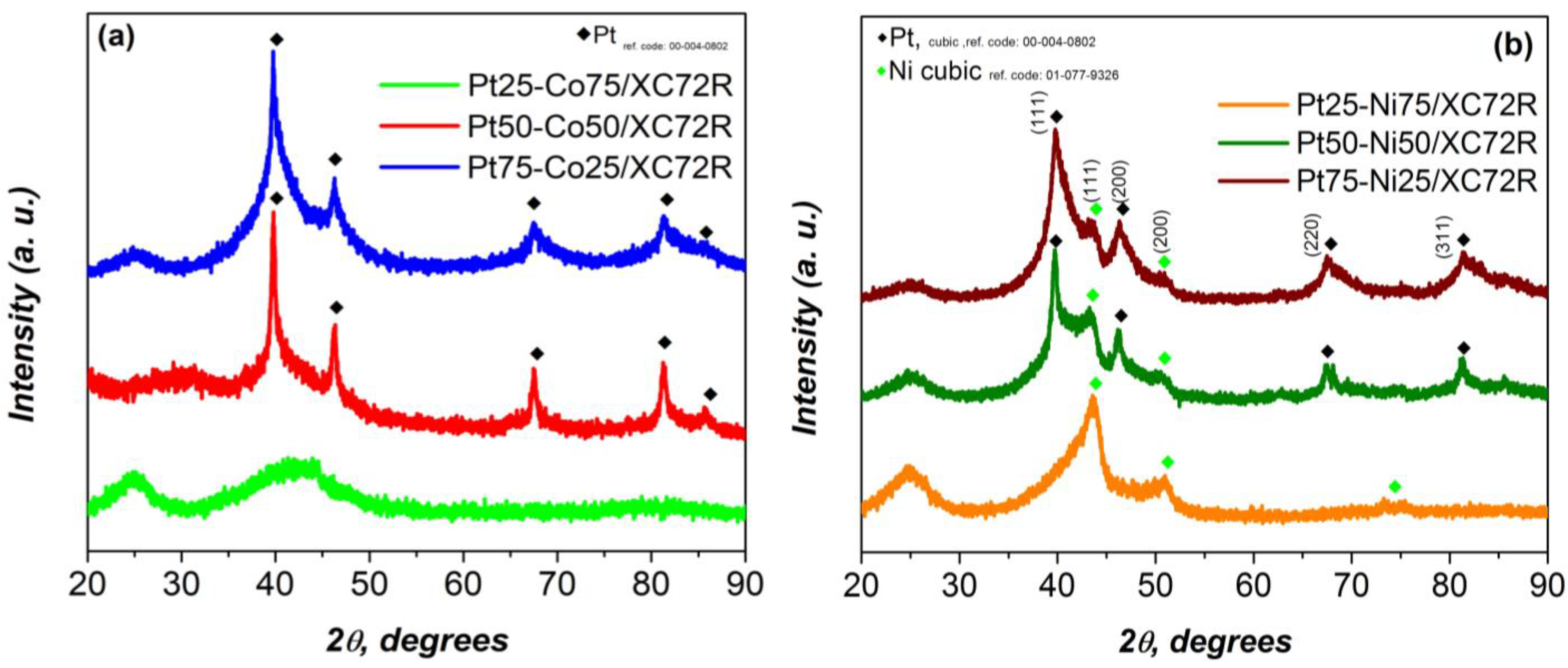
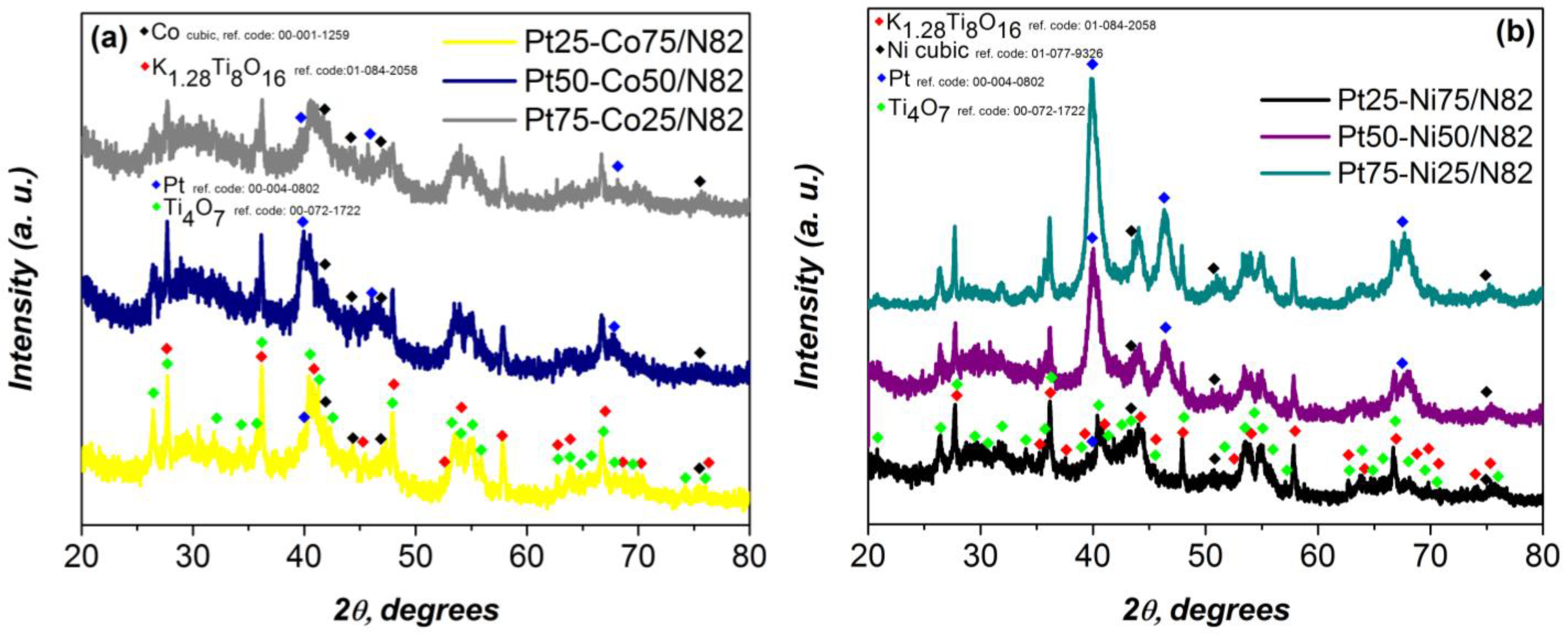
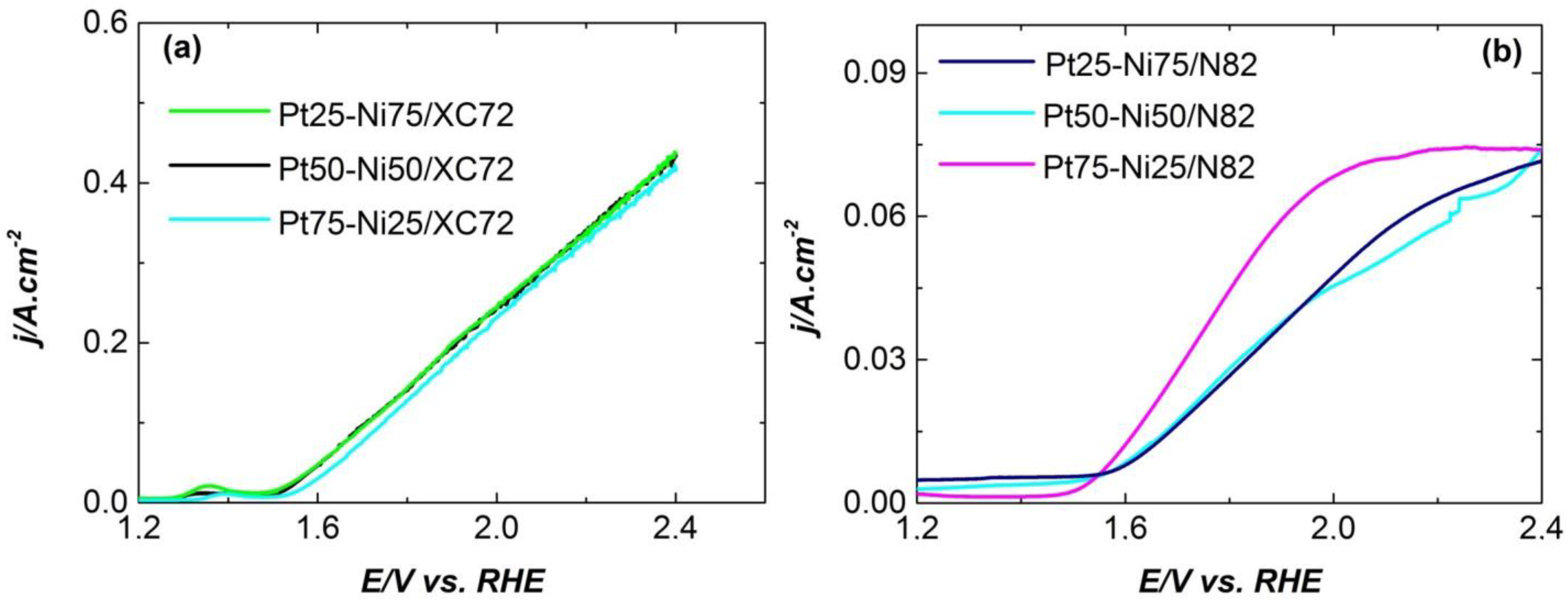

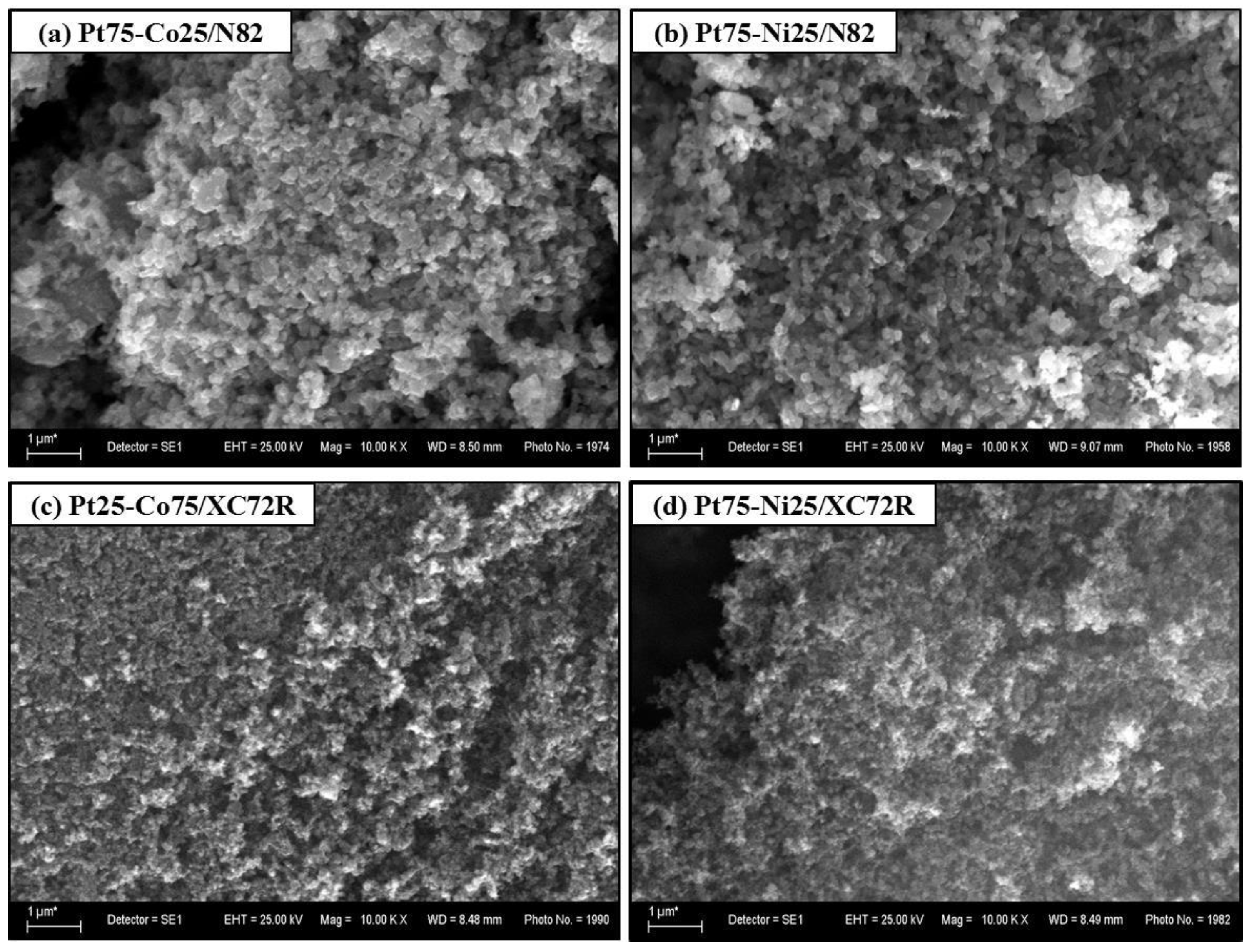
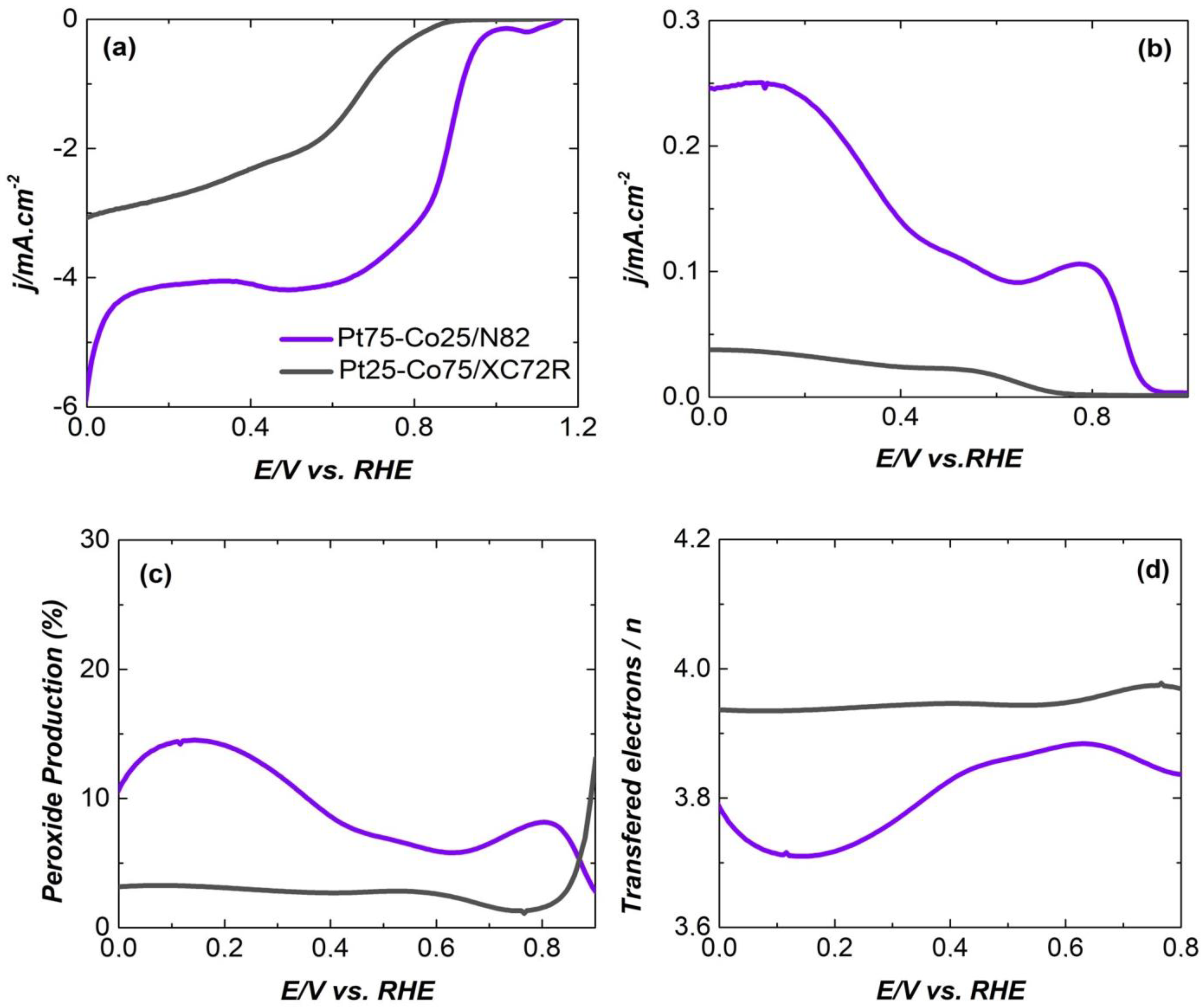
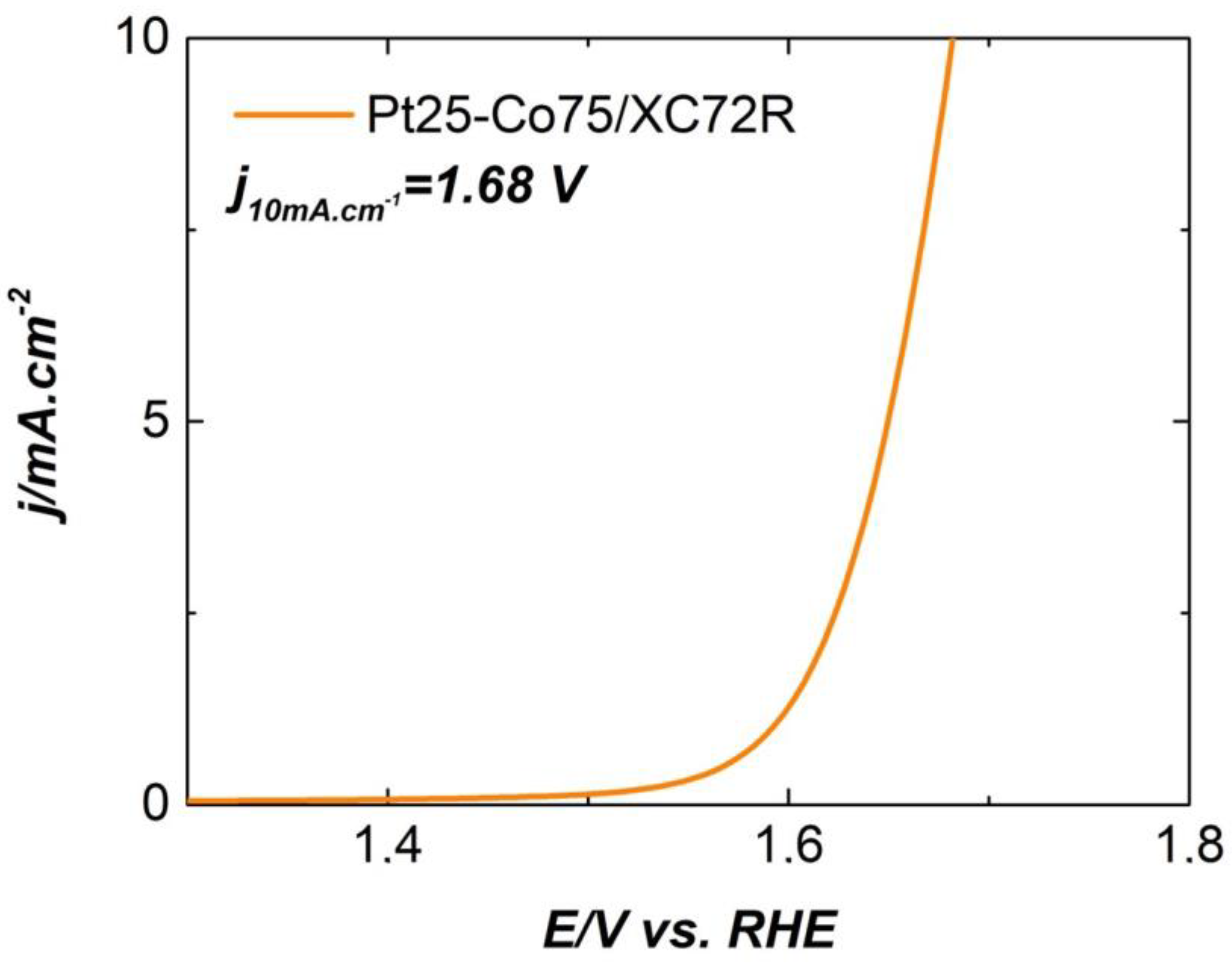
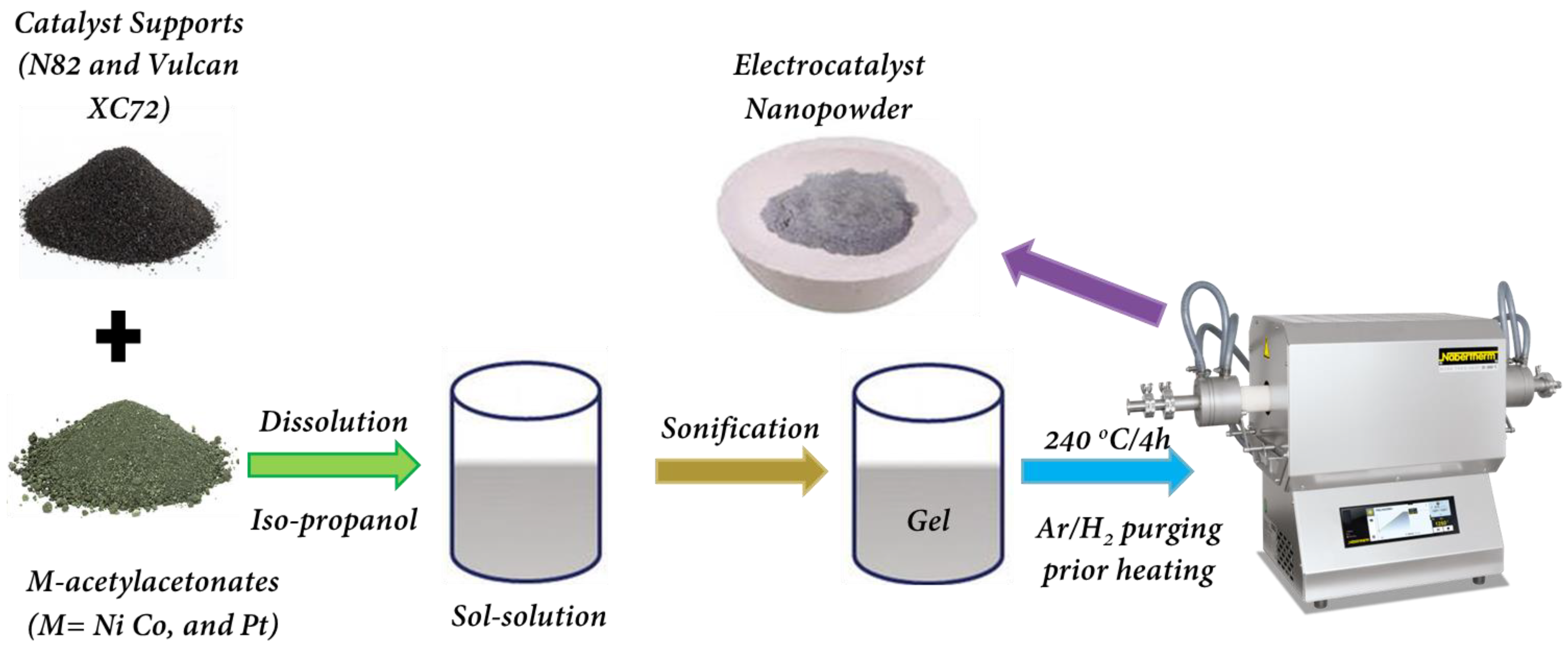

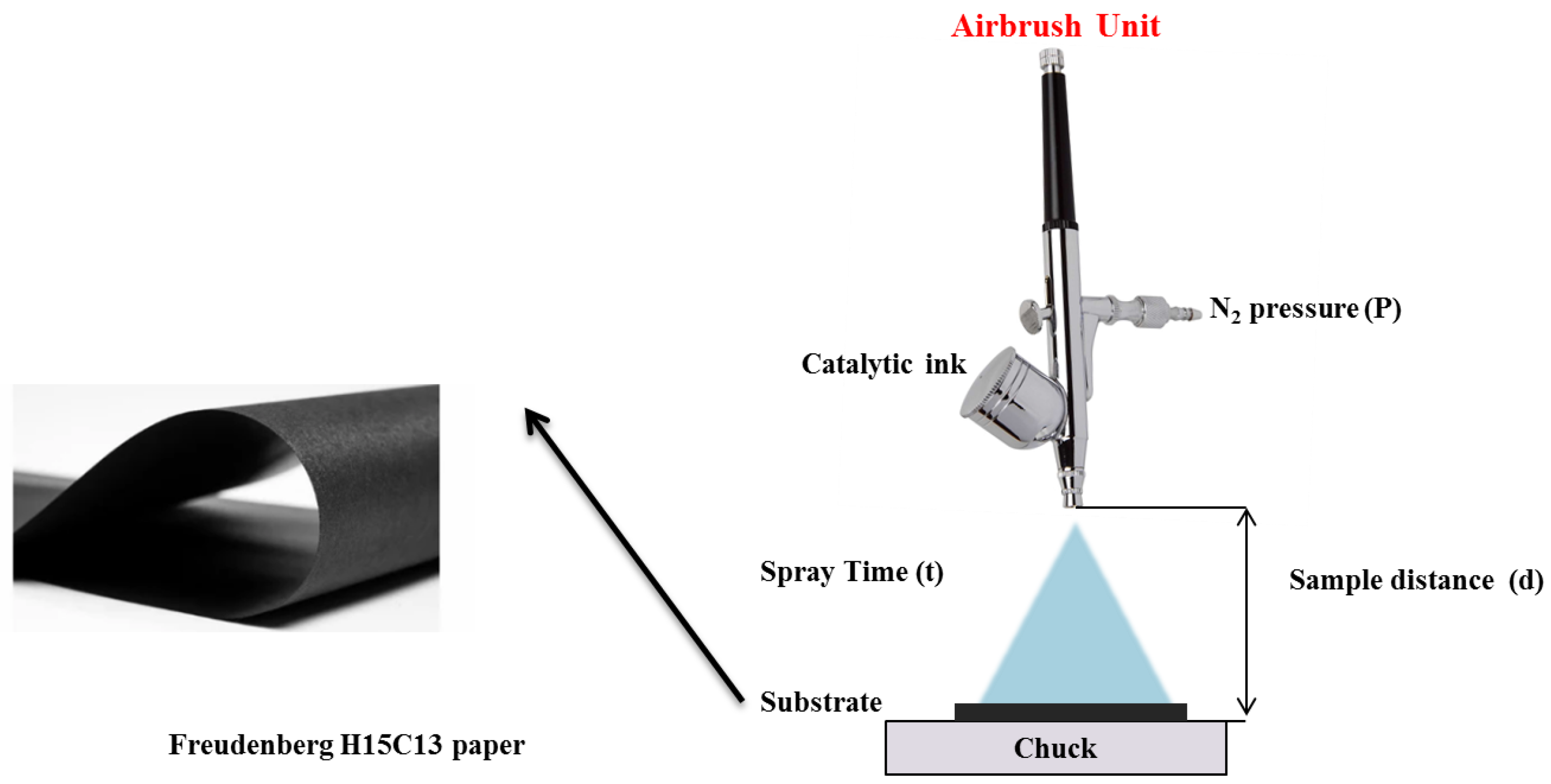
| Electrocatalysts Composition | Overpotential (V) | Reference |
|---|---|---|
| Pt25-Co75/XC72R(deposited on Freudenberg FCCTKG H2315) | 0.350 | In this work |
| Pt75-Co25/N82(deposited on Freudenberg FCCTKG H2315 | 0.591 | In this work |
| NiCo2O4 (deposited on Ni foam) | 0.250 | [46] |
| NiFeCrO4 (deposited on Ni plate) | 0.285 | [47] |
| CoFe2O4 (deposited on glassy carbon electrode) | 0.370 | [48] |
| Co3O4 (deposited on Au electrode) | 0.400 | [49] |
| CoCr2O4/CNT (deposited on glassy carbon electrode) | 0.326 | [50] |
| Cu(dto)/C | 0.400 | [51] |
| NiCoO (deposited on FTO) | 0.460 | [52] |
| N-doped multi-walled carbon nanotubes (NMWNT) | 0.320 | [53] |
| N and S-doped graphene on graphite foam (SNG@GF) | 0.330 | [53] |
| S-NiFeOOH@Fe-Ni3S2 on NiFe foam | 0.242 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkucheva, E.S.; Mladenova, B.; Muhyuddin, M.; Dimitrova, M.; Borisov, G.R.; Santoro, C.; Slavcheva, E. Sol–Gel-Synthesized Pt, Ni and Co-Based Electrocatalyst Effects of the Support Type, Characterization, and Possible Application in AEM-URFC. Gels 2025, 11, 229. https://doi.org/10.3390/gels11040229
Petkucheva ES, Mladenova B, Muhyuddin M, Dimitrova M, Borisov GR, Santoro C, Slavcheva E. Sol–Gel-Synthesized Pt, Ni and Co-Based Electrocatalyst Effects of the Support Type, Characterization, and Possible Application in AEM-URFC. Gels. 2025; 11(4):229. https://doi.org/10.3390/gels11040229
Chicago/Turabian StylePetkucheva, Elitsa Stanislavova, Borislava Mladenova, Mohsin Muhyuddin, Mariela Dimitrova, Galin Rusev Borisov, Carlo Santoro, and Evelina Slavcheva. 2025. "Sol–Gel-Synthesized Pt, Ni and Co-Based Electrocatalyst Effects of the Support Type, Characterization, and Possible Application in AEM-URFC" Gels 11, no. 4: 229. https://doi.org/10.3390/gels11040229
APA StylePetkucheva, E. S., Mladenova, B., Muhyuddin, M., Dimitrova, M., Borisov, G. R., Santoro, C., & Slavcheva, E. (2025). Sol–Gel-Synthesized Pt, Ni and Co-Based Electrocatalyst Effects of the Support Type, Characterization, and Possible Application in AEM-URFC. Gels, 11(4), 229. https://doi.org/10.3390/gels11040229










