The Thermodynamic and Gelation Properties of Ovalbumin and Lysozyme
Abstract
1. Introduction
2. Results and Discussion
2.1. The Thermal Properties of OVA and LYZ
2.2. Effect of the Heating Rate
2.3. Effect of Protein Concentration on the Thermal Properties
2.4. Effect of pH on the Thermal Properties
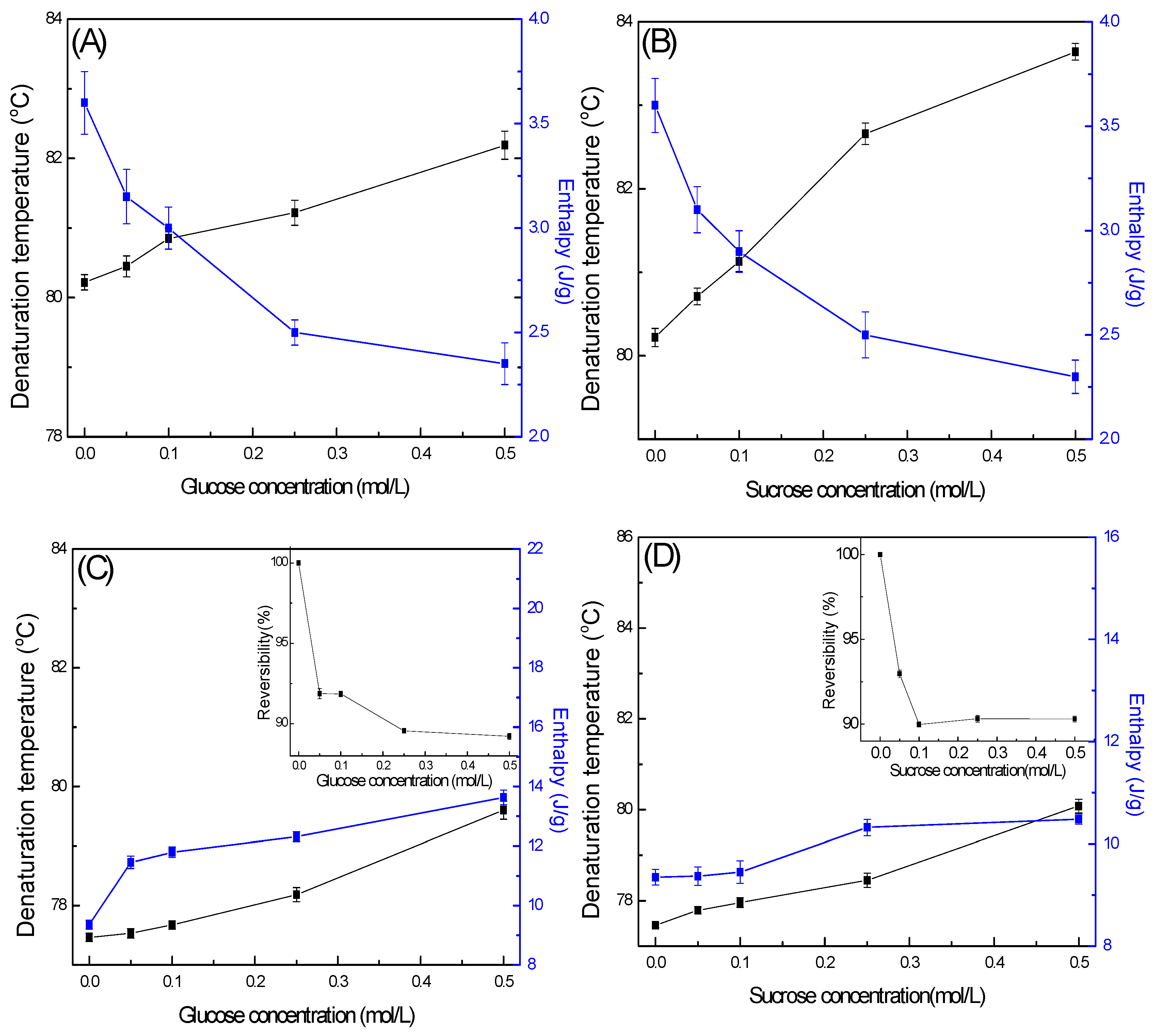
2.5. Effect of Sugars on the Thermal Properties
2.6. Effect of Salts on the Thermal Properties
2.6.1. Effect of Salts on Thermal Properties of OVA
2.6.2. Effect of Salts on Thermal Properties of LYZ
2.7. Structural Characteristics of OVA and LYZ
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.2.1. Effect of pH
4.2.2. Effect of Salts
4.2.3. Effect of Sugars
4.2.4. Effect of Heating Rate
4.2.5. Effect of Protein Concentration
4.2.6. Differential Scanning Calorimetry (DSC)
4.2.7. Circular Dichroism Spectrum
4.2.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mine, Y. Egg proteins. In Applied Food Protein Chemistry; Ustunol, Z., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 459–490. [Google Scholar]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, T.; Ren, C.; Ma, W.; Wu, D.; Xu, X.; Wang, L.S.; Du, M. Advancement of food-derived mixed protein systems: Interactions, aggregations, and functional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 627–651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Geveke, D.J. Shell egg pasteurization using radio frequency in combination with hot air or hot water. Food Microbiol. 2020, 85, 103281. [Google Scholar] [CrossRef] [PubMed]
- Keener, K.M. Shell Egg Pasteurization. In Egg Innovations and Strategies for Improvements; Academic Press: Cambridge, MA, USA, 2017; pp. 165–175. [Google Scholar]
- Georgescu, N.; Apostol, L.; Gherendi, F. Inactivation of Salmonella enterica serovar Typhimurium on egg surface, by direct and indirect treatments with cold atmospheric plasma. Food Control 2017, 76, 52–61. [Google Scholar] [CrossRef]
- Mizutani, K.; Chen, Y.; Yamashita, H.; Hirose, M.; Aibara, S. Thermostabilization of Ovotransferrin by Anions for Pasteurization of Liquid Egg White. Biosci. Biotechnol. Biochem. 2006, 70, 1839–1845. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.; Ye, T.; Wan, S.; Wang, Y.; Wang, D.; Li, B.; Wang, C. Influence of konjac glucomannan on gelling properties and water state in egg white protein gel. Food Res. Int. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Ferreira, M.; Hofer, C.; Raemy, A. A calorimetric study of egg white proteins. J. Therm. Anal. 1997, 48, 683–690. [Google Scholar] [CrossRef]
- Plancken, I.V.D.; Loey, A.V.; Hendrickx, M.E. Foaming properties of egg white proteins affected by heat or high pressure treatment. J. Food Eng. 2007, 78, 1410–1426. [Google Scholar] [CrossRef]
- Gharbi, N.; Labbafi, M. Effect of processing on aggregation mechanism of egg white proteins. Food Chem. 2018, 252, 126–133. [Google Scholar] [CrossRef]
- Iesel, V.D.P.; Van Loey, A.; Hendrickx, M.E.G. Changes in Sulfhydryl Content of Egg White Proteins Due to Heat and Pressure Treatment. J. Agric. Food Chem. 2005, 53, 5726–5733. [Google Scholar]
- Hong, T.; Iwashita, K.; Handa, A.; Shiraki, K. Arginine prevents thermal aggregation of hen egg white proteins. Food Res. Int. 2017, 97, 272. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Raikos, V.; Euston, S.R. Modification of functional properties of egg-white proteins. Nahr. Food 2003, 47, 369–376. [Google Scholar] [CrossRef]
- Iwashita, K.; Handa, A.; Shiraki, K. Co-aggregation of ovotransferrin and lysozyme. Food Hydrocoll. 2018, 89, 416–424. [Google Scholar] [CrossRef]
- Hong, T.K.J.S.A.K. Aggregation of hen egg white proteins with additives during agitation. LWT Food Sci. Technol. 2021, 146, 111378. [Google Scholar] [CrossRef]
- Handa, A.; Takahashi, K.; Kuroda, N.; Froning, G.W. Heat-induced Egg White Gels as Affected by pH. J. Food Sci. 2010, 63, 403–407. [Google Scholar] [CrossRef]
- Mine, Y.; Noutomi, T.; Haga, N. Thermally induced changes in egg white proteins. Ital. J. Zool. 1991, 38, 117–124. [Google Scholar] [CrossRef]
- Matsuda, T.; Watanabe, K.; Sato, Y. Heat-Induced Aggregation of Egg White Proteins as Studied by Vertical Flat-Sheet Polyacrylamide Gel Electrophoresis. J. Food Ence 2010, 46, 1829–1834. [Google Scholar] [CrossRef]
- Iwashita, K.; Handa, A.; Shiraki, K. Co-aggregation of ovalbumin and lysozyme. Food Hydrocoll. 2017, 67, 206–215. [Google Scholar] [CrossRef]
- Totosaus, A.; José, G.M.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2010, 37, 589–601. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces study of heat-induced myofibrillar protein gel as affected by partial substitution of NaCl with KCl, MgCl2 and CaCl2. CyTA J. Food 2016, 14, 239–247. [Google Scholar] [CrossRef]
- Huntington, J.A.; Stein, P.E. Structure and properties of ovalbumin. J. Chromatogr. B Biomed. Sci. Appl. 2001, 756, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, Y.; Kameyama, R.; Handa, A.; Takahashi, N.; Tanaka, N. Role of the N-Terminal Amphiphilic Region of Ovalbumin during Heat-Induced Aggregation and Gelation. J. Agric. Food Chem. 2013, 61, 8668–8675. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Ishibashi, J.; Hong, Y.H.; Hirose, M. Involvement of Ovotransferrin in the Thermally Induced Gelation of Egg White at around 65 °C. Biosci. Biotechnol. Biochem. 1998, 62, 593–595. [Google Scholar] [CrossRef]
- Clark, E.D.B.; Hevehan, D.; Szela, S.; Maachupalli-Reddy, J. Oxidative renaturation of hen egg-white lysozyme. Folding vs aggregation. Biotechnol. Prog. 2010, 14, 47–54. [Google Scholar] [CrossRef]
- Li-Chan, E.C.; Kim, H.O. Structure and chemical composition of eggs. In Egg Bioscience and Biotechnology; Mine, Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2008; pp. 1–95. [Google Scholar]
- Le Floch-Fouéré, C.; Pezennec, S.; Lechevalier, V.; Beaufils, S.; Desbat, B.; Pézolet, M.; Renault, A. Synergy between ovalbumin and lysozyme leads to non-additive interfacial and foaming properties of mixtures. Food Hydrocoll. 2009, 23, 352–365. [Google Scholar] [CrossRef]
- Yu, S.; Yao, P.; Jiang, M.; Zhang, G. Nanogels prepared by self-assembly of oppositely charged globular proteins. Biopolymers 2010, 83, 148–158. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Chen, L. Acid-induced gelation of thermal co-aggregates from egg white and hempseed protein: Impact of microbial transglutaminase on mechanical and microstructural properties of gels. Food Hydrocoll. 2020, 107, 105960. [Google Scholar] [CrossRef]
- Mohammadi Nafchi, A.; Tabatabaei, R.H.; Pashania, B.; Rajabi, H.Z.; Karim, A.A. Effects of ascorbic acid and sugars on solubility, thermal, and mechanical properties of egg white protein gels. Int. J. Biol. Macromol. 2013, 62, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Yi, C.; Yan, Z.; Yong-Gang, T.U.; Jian-Ke, L.I.; Xu-Ying, L.; Jun-Jie, W.; Wen-Hui, D. Research progress in the gelation mechanism of egg white proteins. Sci. Technol. Food Ind. 2014, 35, 369–373. [Google Scholar]
- Jin, H.; Chen, J.; Zhang, J.; Sheng, L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocoll. 2020, 110, 106200. [Google Scholar] [CrossRef]
- Llave, Y.; Fukuda, S.; Fukuoka, M.; Shibata-Ishiwatari, N.; Sakai, N. Analysis of color changes in chicken egg yolks and whites based on degree of thermal protein denaturation during ohmic heating and water bath treatment. J. Food Eng. 2018, 222, 151–161. [Google Scholar] [CrossRef]
- Shibata-Ishiwatari, N.; Takagi, T.; Fukuoka, M.; Sakai, N. Kinetic Studies on the Effect of Salt on the Thermal Denaturation of Egg Constituents. Jpn. J. Food Eng. 2018, 19, 49–56. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I. Thermal denaturation of mixtures of α-lactalbumin and β-lactoglobulin: A differential scanning calorimetric study. Food Res. Int. 2000, 33, 673–682. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Wu, N.; Chen, S.; Xu, M.; Du, H.; Yao, Y.; Tu, Y. Egg white protein-based delivery system for bioactive substances: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 617–637. [Google Scholar] [CrossRef]
- Ding, L.; Xia, M.; Zeng, Q.; Zhao, Q.; Cai, Z.; Zhu, Z. Foaming properties and aggregation mechanism of egg white protein with different physical treatments. LWT 2022, 153, 112505. [Google Scholar] [CrossRef]
- Kuang, J.; Hamon, P.; Lechevalier, V.; Saurel, R. Thermal Behavior of Pea and Egg White Protein Mixtures. Foods 2023, 12, 2528. [Google Scholar] [CrossRef]
- Mine, Y. Recent advances in the understanding of egg white protein functionality. Trends Food Sci. Technol. 1995, 6, 225–232. [Google Scholar] [CrossRef]
- Hirose, M. Molten globule state of food proteins. Trends Food Sci. Technol. 1993, 4, 48–51. [Google Scholar] [CrossRef]
- Hoffmann, M.A.M.; Miltenburg, J.C.V.; Van Mil, P.J.J.M. The suitability of scanning calorimetry to investigate slow irreversible protein denaturation. Thermochim. Acta 1997, 306, 45–49. [Google Scholar] [CrossRef]
- Relkin, P. Differential scanning calorimetry: A useful tool for studying protein denaturation. Thermochim. Acta 1994, 246, 371–386. [Google Scholar] [CrossRef]
- Mession, J.L.; Sok, N.; Assifaoui, A.; Saurel, R. Thermal Denaturation of Pea Globulins (Pisum sativum L.)—Molecular Interactions Leading to Heat-Induced Protein Aggregation. J. Agric. Food Chem. 2013, 61, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Relkin, P. Thermal unfolding of beta-lactoglobulin, alpha-lactalbumin, and bovine serum albumin. A thermodynamic approach. Crit. Rev. Food Sci. Nutr. 1996, 36, 565–601. [Google Scholar] [CrossRef]
- Haug, I.J.; Skar, H.M.; Vegarud, G.E.; Langsrud, T.; Draget, K.I. Electrostatic effects on β-lactoglobulin transitions during heat denaturation as studied by differential scanning calorimetry. Food Hydrocoll. 2009, 23, 2287–2293. [Google Scholar] [CrossRef]
- Cao, X.; Li, J.; Yang, X.; Duan, Y.; Liu, Y.; Wang, C. Nonisothermal kinetic analysis of the effect of protein concentration on BSA aggregation at high concentration by DSC. Thermochim. Acta 2008, 467, 99–106. [Google Scholar] [CrossRef]
- Venkataramani, S.; Truntzer, J.; Coleman, D. Thermal stability of high concentration lysozyme across varying pH: A Fourier Transform Infrared study. J. Pharm. Bioallied Sci. 2013, 5, 148–153. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Khan, S.; Ahmed, N.; Jamal, S. Effect of pH on Diclofenac–Lysozyme Interaction: Structural and Functional Aspect. Front. Mol. Biosci. 2022, 9, 872905. [Google Scholar] [CrossRef]
- Kudou, M.; Shiraki, K.; Fujiwara, S.; Imanaka, T.; Takagi, M. Prevention of thermal inactivation and aggregation of lysozyme by polyamines. Eur. J. Biochem. 2003, 270, 4547–4554. [Google Scholar] [CrossRef]
- Nigen, M.; Croguennec, T.; Bouhallab, S. Formation and stability of a-lactalbumin-lysozyme spherical particles: Involvement of electrostatic forces. Food Hydrocoll. Oxf. 2009, 23, 510–518. [Google Scholar] [CrossRef]
- Matsuda, T.; Watanabe, K.; Sato, Y. Interaction Between Ovomucoid and Lysozyme. J. Food Sci. 1982, 47, 637–641. [Google Scholar] [CrossRef]
- Kato, Y. Modification of Ovalbumin with Glucose 6-Phosphate by Amino-Carbonyl Reaction. Improvement of Protein Heat Stability and Emulsifying Activity. J. Agric. Food Chem. 1995, 43, 301–305. [Google Scholar] [CrossRef]
- Raikos, V.; Campbell, L.; Euston, S.R. Rheology and texture of hen’s egg protein heat-set gels as affected by pH and the addition of sugar and/or salt. Food Hydrocoll. 2007, 21, 237–244. [Google Scholar] [CrossRef]
- Sydykov, B.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Hydrogen Bonding Interactions and Enthalpy Relaxation in Sugar/Protein Glasses. J. Pharm. Sci. 2017, 106, 761–769. [Google Scholar] [CrossRef]
- Timasheff, S.N. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA 2002, 99, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Satoshi, A.; Hiroki, I.; Shin-Ichi, T.; Mitsuhiro, H. Sugar-Mediated Stabilization of Protein Against Chemical or Thermal Denaturation. J. Phys. Chem. B 2018, 122, 8685–8697. [Google Scholar]
- Kulmyrzaev, A.; Cory Bryant, A.; Mcclements, D.J. Influence of Sucrose on the Thermal Denaturation, Gelation, and Emulsion Stabilization of Whey Proteins. J. Agric. Food Chem. 2000, 48, 1593. [Google Scholar] [CrossRef] [PubMed]
- Beg, I.; Minton, A.P.; Islam, A.; Hassan, M.I.; Ahmad, F. The pH Dependence of Saccharides’ Influence on Thermal Denaturation of Two Model Proteins Supports an Excluded Volume Model for Stabilization Generalized to Allow for Intramolecular Electrostatic Interactions. J. Biol. Chem. 2016, 292, 505–511. [Google Scholar] [CrossRef]
- Arntfield, S.; Ismond, M.; Murray, E. Thermal analysis of food proteins in relation to processing effects. Therm. Anal. Foods 1990, 1990, 51–91. [Google Scholar]
- Sow, L.C.; Yang, H. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Iwashita, K.; Inoue, N.; Handa, A.; Shiraki, K. Thermal aggregation of hen egg white proteins in the presence of salts. Protein J. 2015, 34, 212–219. [Google Scholar] [CrossRef]
- Zhu, W.K.; Luo, X.G.; Lin, X.Y.; He, J. Effects of Ca2+ Concentration on Calcium Carbonate Crystallization in Egg White Protein Solution. Adv. Mater. Res. 2011, 197–198, 199–202. [Google Scholar] [CrossRef]
- Guo, F.; Friedman, J.M. Charge Density-Dependent Modifications of Hydration Shell Waters by Hofmeister Ions. J. Am. Chem. Soc. 2009, 131, 11010. [Google Scholar] [CrossRef] [PubMed]
- Vardhanabhuti, B.; Foegeding, E.A. Effects of dextran sulfate, NaCl, and initial protein concentration on thermal stability of β-lactoglobulin and α-lactalbumin at neutral pH. Food Hydrocoll. 2008, 22, 752–762. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Thanassoulas, A.; Nounesis, G. The effect of cations on reversibility and thermodynamic stability during thermal denaturation of lysozyme. J. Chem. Thermodyn. 2018, 118, 331–337. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef]
- Schwierz, N.; Horinek, D.; Netz, R.R. Reversed anionic Hofmeister series: The interplay of surface charge and surface polarity. Langmuir Acs J. Surf. Colloids 2010, 26, 7370–7379. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.J. Practical analysis of complex coacervate systems. J. Colloid Interface Sci. 1990, 140, 227–238. [Google Scholar] [CrossRef]
- Schneider, C.P.; Shukla, D.; Trout, B.L. Arginine and the Hofmeister Series: The role of ion-ion interactions in protein aggregation suppression. J. Phys. Chem. B 2011, 115, 7447–7458. [Google Scholar] [CrossRef]
- Sheng, L.; Ye, S.; Han, K.; Zhu, G.; Ma, M.; Cai, Z. Consequences of phosphorylation on the structural and foaming properties of ovalbumin under wet-heating conditions. Food Hydrocoll. 2019, 91, 166–173. [Google Scholar] [CrossRef]
- Jin, H.; Li, P.; Jin, Y.; Sheng, L. Effect of sodium tripolyphosphate on the interaction and aggregation behavior of ovalbumin-lysozyme complex. Food Chem. 2021, 352, 129457. [Google Scholar] [CrossRef]
- Tanaka, F.; Forster, L.S.; Pal, P.K.; Rupley, J.A. The circular dichroism of lysozyme. J. Biol. Chem. 1975, 250, 6977–6982. [Google Scholar] [CrossRef]
- Kelly, S.; Jess, T.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.V.P. Sustained Stability and Activity of Lysozyme in Choline Chloride Against pH Induced Denaturation. ACS Sustain. Chem. Eng. 2017, 5, 8344–8355. [Google Scholar] [CrossRef]
- Relkin, P.; Launay, B.; Eynard, L. Effect of Sodium and Calcium Addition on Thermal Denaturation of Apo-α-Lactalbumin: A Differential Scanning Calorimetric Study. J. Dairy Sci. 1993, 76, 36–47. [Google Scholar] [CrossRef]
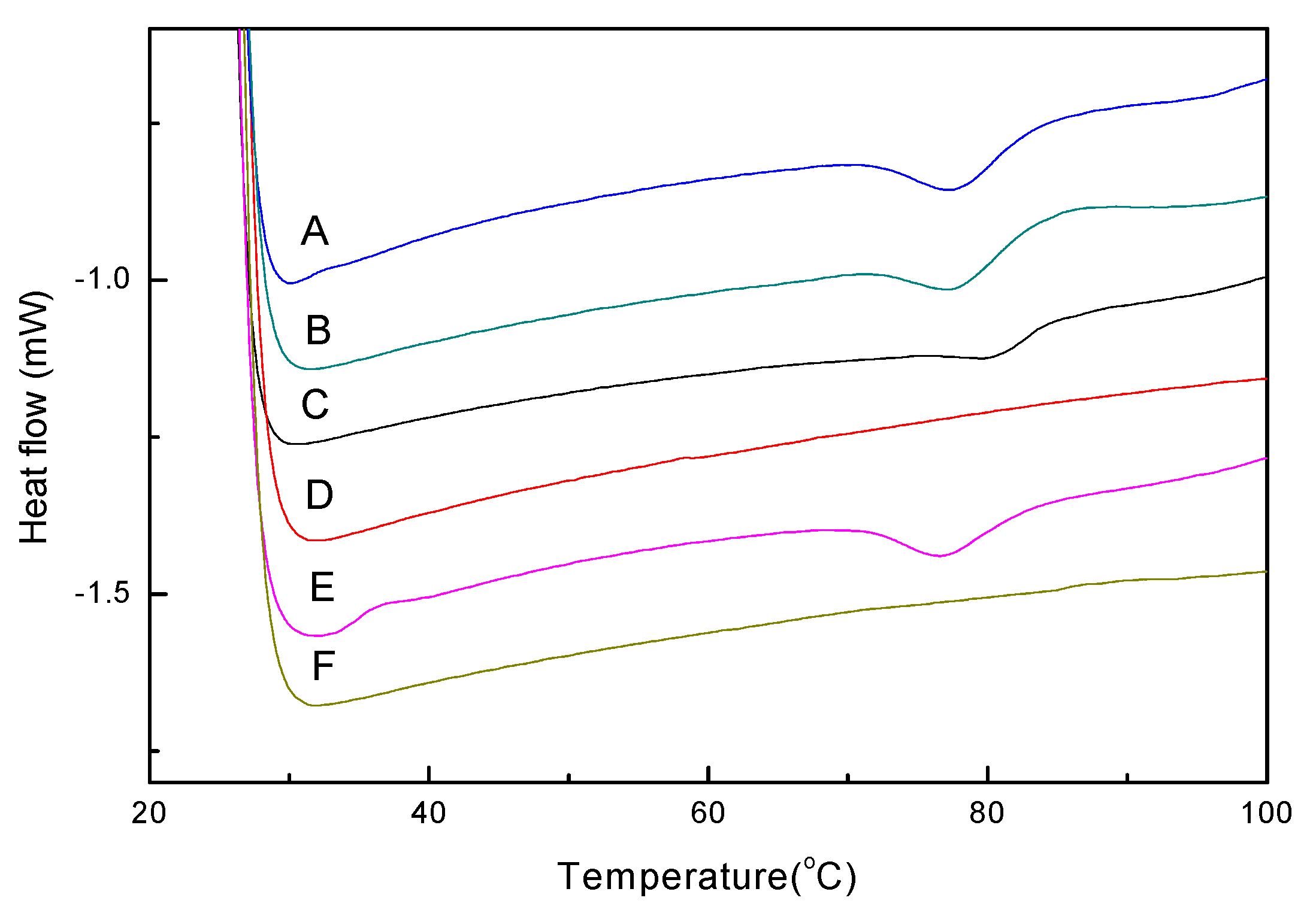




| Rate (°C/min) | OVA | LYZ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Td (°C) | Endset (°C) | ΔH (J/g) | Rd (%) | Onset (°C) | Td (°C) | Endset (°C) | ΔH (J/g) | Rd (%) | |
| 2.5 | 75.06 ± 0.15 a | 78.06 ± 0.19 a | 81.39 ± 0.21 a | 3.5 ± 0.2 a | 0 | 66.65 ± 0.13 a | 76.18 ± 0.12 a | 79.60 ± 0.16 a | 7.0 ± 0.1 a | 100 |
| 5 | 75.68 ± 0.17 b | 80.22 ± 0.11 b | 84.38 ± 0.15 b | 3.5 ± 0.1 b | 0 | 71.50 ± 0.18 b | 77.46 ± 0.05 b | 82.45 ± 0.04 b | 7.3 ± 0.1 b | 100 |
| 10 | 77.13 ± 0.18 c | 81.59 ± 0.19 c | 85.51 ± 0.17 c | 3.7 ± 0.1 b | 0 | 71.55 ± 0.11 b | 77.68 ± 0.12 c | 83.40 ± 0.13 c | 7.4 ± 0.1 b | 100 |
| Protein mg/mL | OVA | LYZ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Td (°C) | Endset (°C) | ΔH (J/g) | Rd (%) | Onset (°C) | Td (°C) | Endset (°C) | ΔH (J/g) | Rd (%) | |
| 50 | 75.68 ± 0.17 a | 80.22 ± 0.11 a | 84.38 ± 0.15 a | 3.5 ± 0.1 a | 0 | 71.50 ± 0.18 a | 77.46 ± 0.05 a | 82.45 ± 0.04 a | 7.3 ± 0.1 a | 100 |
| 100 | 74.06 ± 0.12 b | 80.05 ± 0.03 b | 83.73 ± 0.13 b | 3.2 ± 0.1 b | 0 | 70.55 ± 0.22 b | 76.51 ± 0.07 b | 81.97 ± 0.11 b | 7.2 ± 0.1 a | 100 |
| 200 | 73.63 ± 0.11 c | 79.93 ± 0.06 b | 83.57 ± 0.03 c | 3.0 ± 0.1 c | 0 | 68.93 ± 0.16 c | 74.53 ± 0.03 c | 79.90 ± 0.13 c | 7.1 ± 0.1 a | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, R.; Lv, S.; Liu, Y.; Fang, S.; Zang, J.; Qing, M.; Chi, Y. The Thermodynamic and Gelation Properties of Ovalbumin and Lysozyme. Gels 2025, 11, 470. https://doi.org/10.3390/gels11060470
Wang L, Li R, Lv S, Liu Y, Fang S, Zang J, Qing M, Chi Y. The Thermodynamic and Gelation Properties of Ovalbumin and Lysozyme. Gels. 2025; 11(6):470. https://doi.org/10.3390/gels11060470
Chicago/Turabian StyleWang, Lifeng, Rongcheng Li, Siyi Lv, Yulin Liu, Shuaifu Fang, Jingnan Zang, Mingmin Qing, and Yujie Chi. 2025. "The Thermodynamic and Gelation Properties of Ovalbumin and Lysozyme" Gels 11, no. 6: 470. https://doi.org/10.3390/gels11060470
APA StyleWang, L., Li, R., Lv, S., Liu, Y., Fang, S., Zang, J., Qing, M., & Chi, Y. (2025). The Thermodynamic and Gelation Properties of Ovalbumin and Lysozyme. Gels, 11(6), 470. https://doi.org/10.3390/gels11060470





