Antifouling Mussel-Inspired Hydrogel with Furanone-Loaded ZIF-8 for Quorum Sensing-Mediated Marine Antifouling
Abstract
1. Introduction
2. Results and Discussion
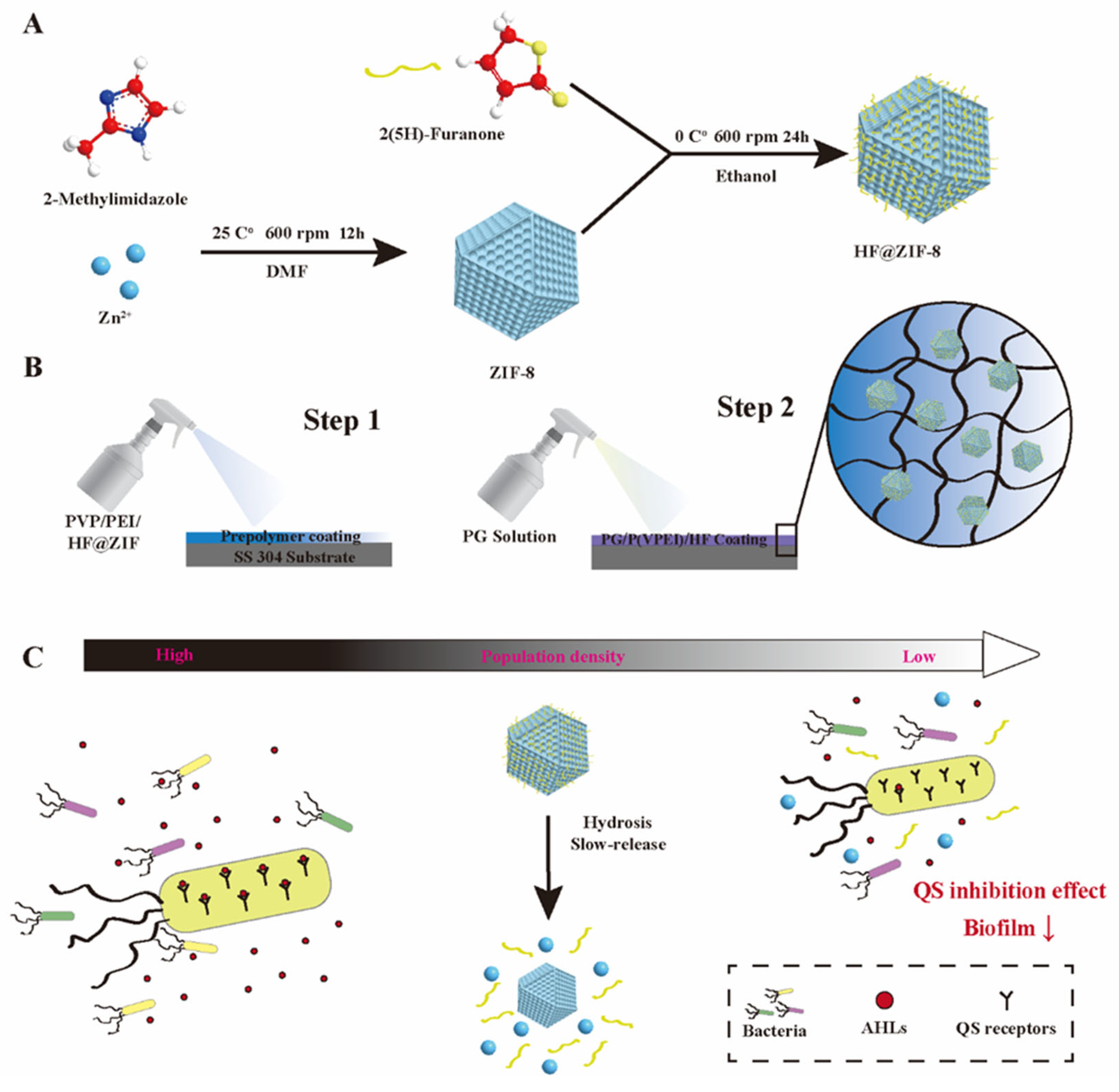
2.1. Characterization of ZIF-8 and HF@ZIF-8
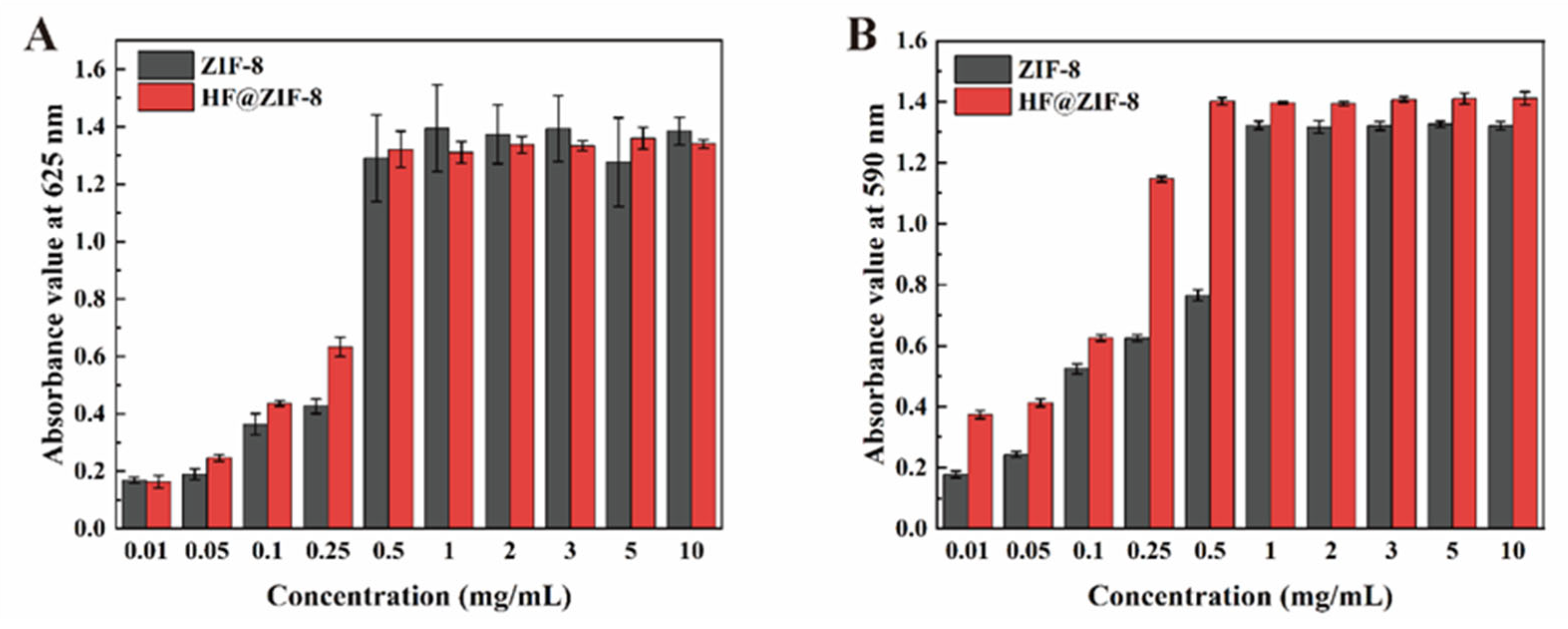
2.2. Quorum Sensing Inhibition Performance of ZIF-8 and HF@ZIF-8
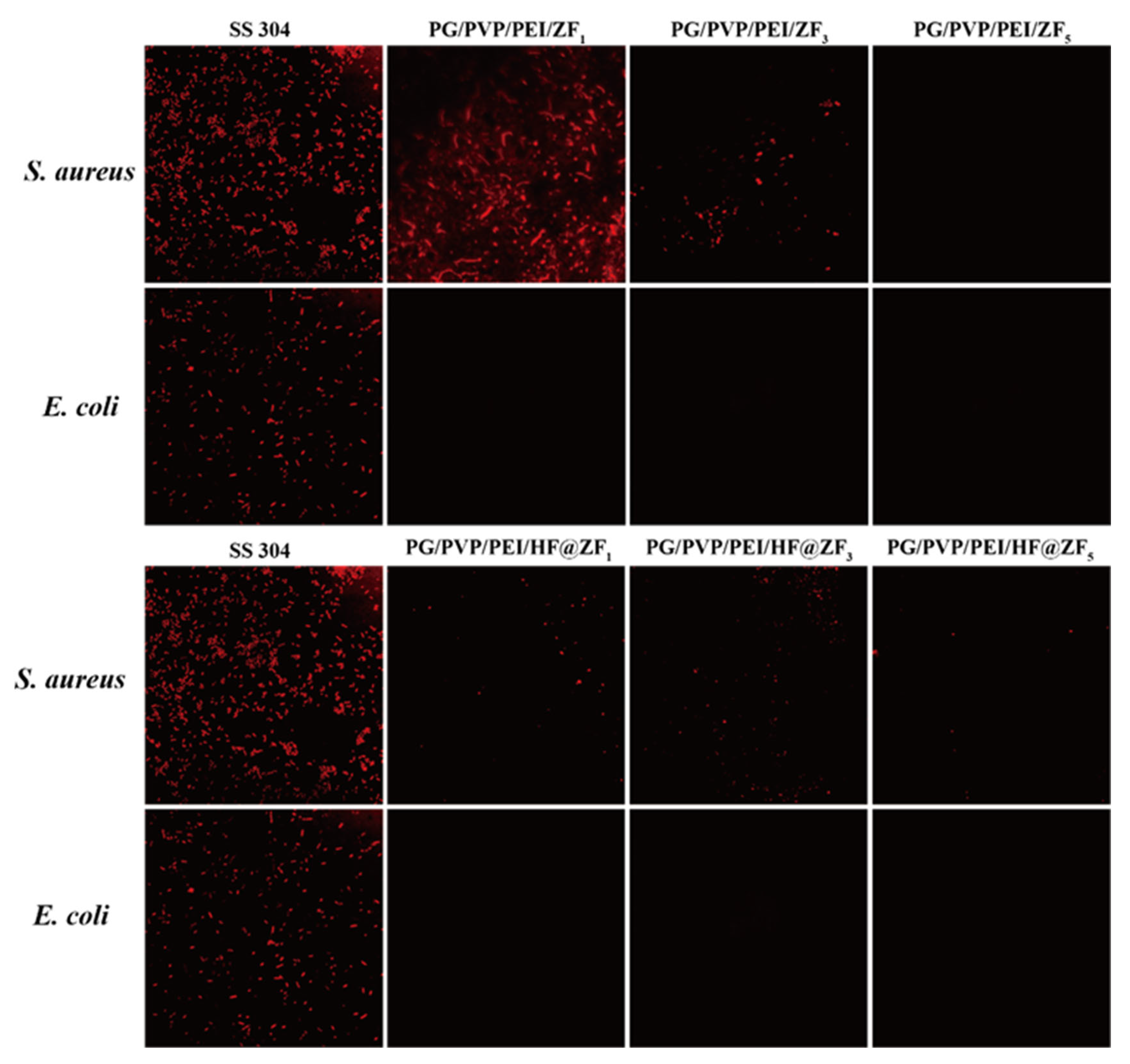
2.3. Antifouling Performance
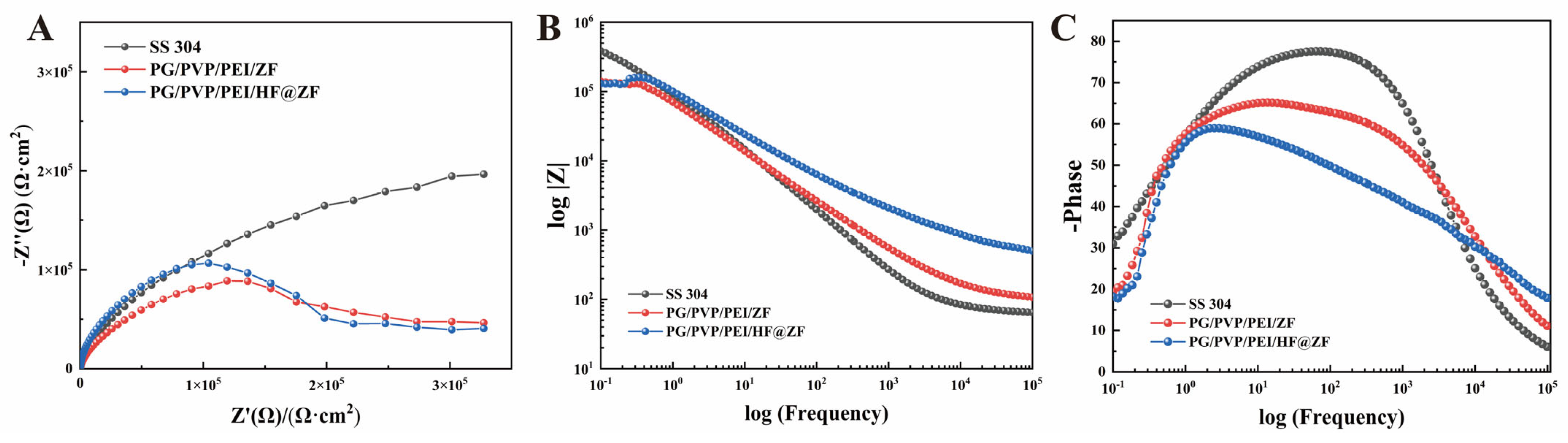
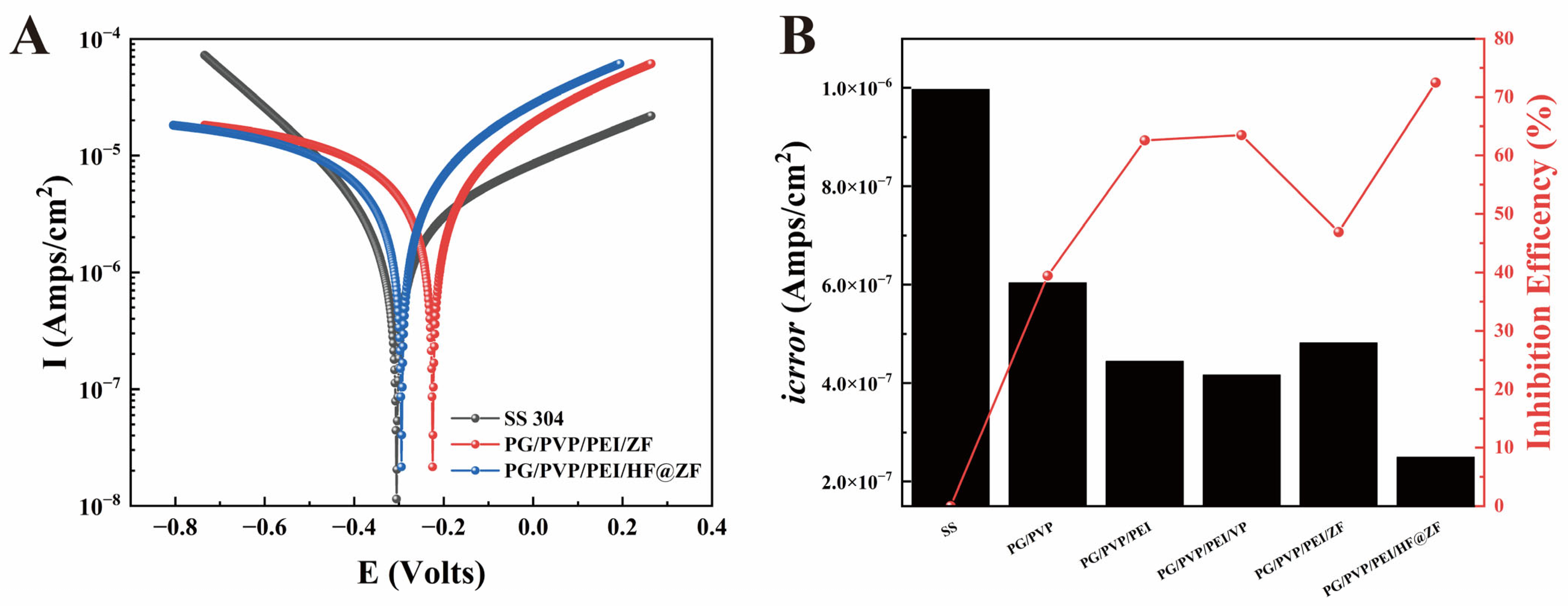
2.4. Corrosion Inhibition Analysis
3. Conclusions
- (1)
- FTIR, XPS, and TGA analyses confirmed the successful loading of HF into ZIF-8. SEM, HRTEM, BET, and XRD results indicated the effective encapsulation of ZIF-8 by HF, with no disruption to the crystalline structure of ZIF-8 before and after HF loading.
- (2)
- Antifouling performance tests using Staphylococcus aureus and Escherichia coli showed that when the concentrations of ZIF-8 in the hydrogel prepolymer were 1%, 3%, and 5%, the inhibition rates against S. aureus were 34.28%, 56.14%, and 98.25%, respectively. Replacing ZIF-8 with HF@ZIF-8 increased the inhibition rates to 65.89%, 74.23%, and 97.28%, respectively. For E. coli, all samples exhibited inhibition rates exceeding 97%.
- (3)
- QSI analysis of HF@ZIF-8 using Chromobacterium violaceum CV026 showed that the growth inhibition of CV026 by both ZIF-8 and HF@ZIF-8 peaked at a concentration of 0.5 mg/mL. At concentrations of 0.01–0.5 mg/mL, HF@ZIF-8 demonstrated a stronger inhibition of violacein synthesis in CV026 compared to unloaded ZIF-8, with a significant inhibition of purple pigment production observed even at a sub-inhibitory concentration of 0.25 mg/mL, confirming the quorum sensing inhibition capability of HF@ZIF-8.
- (4)
- The PG/PVP/PEI/HF@ZF hydrogel coating exhibited a high corrosion inhibition rate of 72.47%, attributed to the synergistic effects of the anodic protection and physical barrier provided by the zinc-rich ZIF-8 and crosslinked HF within the hydrogel matrix.
4. Materials and Methods
4.1. Materials and Reagents
4.2. ZIF-8 Synthesis and HF Loading
4.3. Preparation of ZIF-8 and HF@ZIF-8 Composite Hydrogel Coatings
4.4. Material Characterization
4.5. Quorum Sensing Inhibition Analysis of HF@ZIF-8
4.6. Antifouling Performance Evaluation
4.7. Electrochemical Performance Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Du, W.; Su, D.; He, X.; Bai, X. Morphology and chemical state tuning of Ce-MOF with enhanced haloperoxidase-like activity for antibacterial and antifouling performances. Mater. Today Chem. 2025, 45, 102630. [Google Scholar] [CrossRef]
- Selim, M.S.; Fatthallah, N.A.; Higazy, S.A.; Madian, H.R.; Elsaeed, A.M. Non-toxic silicone-polyurethane nanocomposites filled with graphene/tungsten disulphide nanorods as hierarchical superhydrophobic marine antifouling surfaces. Prog. Org. Coat. 2025, 200, 108987. [Google Scholar] [CrossRef]
- Vuong, P.; McKinley, A.; Kaur, P. Understanding biofouling and contaminant accretion on submerged marine structures. npj Mater. Degrad. 2023, 7, 50. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Cao, P.; Tian, F.; Bai, X.Q.; Yuan, C.Q. Infused configurations induced by structures influence stability and antifouling performance of biomimetic lubricant-infused surfaces. Surf. Coat. Technol. 2019, 358, 159–166. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Tello, E.; Castellanos, L.; Arévalo-Ferro, C.; Duque, C. Disruption in quorum-sensing systems and bacterial biofilm inhibition by cembranoid diterpenes isolated from the octocoral Eunicea knighti. J. Nat. Prod. 2012, 75, 1637–1642. [Google Scholar] [CrossRef]
- Peters, L.; König, G.M.; Wright, A.D.; Pukall, R.; Stackebrandt, E.; Eberl, L.; Riedel, K. Secondary Metabolites of Flustra foliace a and Their Influence on Bacteria. Appl. Environ. Microbiol. 2003, 69, 3469–3475. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef]
- Ee, L.; Jiajia, W.; Dun, Z.; Peng, W.; Yu, W.; Ming, X.; Ce, L.; Zhihua, S.; Liyang, Z. Electron donor dependent inhibition mechanisms of D-phenylalanine on corrosion of Q235 carbon steel caused by Desulfovibrio sp. Huiquan2017. Corros. Sci. 2021, 188, 109493. [Google Scholar]
- Li, E.E.; Wu, J.; Zhang, D.; Sun, Y.; Chen, J. D-phenylalanine inhibits the corrosion of Q235 carbon steel caused by Desulfovibrio sp. Int. Biodeterior. Biodegrad. 2018, 127, 178–184. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Kim, H.; Song, Y.; Jung, D.; Kang, S.; Seo, J.-H.; Nam, S.; Lee, Y. Calcium-binding polymer-coated poly (lactide-co-glycolide) microparticles for sustained release of quorum sensing inhibitors to prevent biofilm formation on hydroxyapatite surfaces. ACS Appl. Mater. Interfaces 2019, 11, 7686–7694. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2020, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, M.; Peng, P.; Liu, J.; Lu, J.; Qian, S.; Feng, J. “Self-Defensive” antifouling zwitterionic hydrogel coatings on polymeric substrates. ACS Appl. Mater. Interfaces 2022, 14, 56097–56109. [Google Scholar] [CrossRef]
- Xue, L.; Lu, X.; Wei, H.; Long, P.; Xu, J.; Zheng, Y. Bio-inspired self-cleaning PAAS hydrogel released coating for marine antifouling. J. Colloid Interface Sci. 2014, 421, 178–183. [Google Scholar] [CrossRef]
- Yang, J.; Xue, B.; Zhou, Y.; Qin, M.; Wang, W.; Cao, Y. Spray-painted hydrogel coating for marine antifouling. Adv. Mater. Technol. 2021, 6, 2000911. [Google Scholar] [CrossRef]
- Shen, J.; Du, M.; Wu, Z.; Song, Y.; Zheng, Q. Strategy to construct polyzwitterionic hydrogel coating with antifouling, drag-reducing and weak swelling performance. RSC Adv. 2019, 9, 2081–2091. [Google Scholar] [CrossRef]
- Lin, X.; Huang, X.; Zeng, C.; Wang, W.; Ding, C.; Xu, J.; He, Q.; Guo, B. Poly (vinyl alcohol) hydrogels integrated with cuprous oxide–tannic acid submicroparticles for enhanced mechanical properties and synergetic antibiofouling. J. Colloid Interface Sci. 2019, 535, 491–498. [Google Scholar] [CrossRef]
- Li, P.; Su, X.; Hao, D.; Yang, M.; Gui, T.; Cong, W.; Jiang, W.; Ge, X.; Guo, X. One-pot method for preparation of capsaicin-containing double-network hydrogels for marine antifouling. RSC Adv. 2022, 12, 15613–15622. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Tan, Q.; Xiao, G.; Baeyens, J.; Haijia, S. Bioinspired bi-amino acid Ce-MOFs boosting oxidase-like activity: Dual-mode aflatoxin detection and antimicrobial activity platform. Chem. Eng. J. 2025, 512, 161977. [Google Scholar] [CrossRef]
- Li, H.; Luo, S.; Zhang, L.; Zhao, Z.; Wu, M.; Li, W.; Liu, F.-Q. Water-and acid-sensitive Cu2O@ Cu-MOF nano sustained-release capsules with superior antifouling behaviors. ACS Appl. Mater. Interfaces 2021, 14, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Zhang, X.; Li, Q.; Zhu, G.; Liu, F.-Q. A marine coating: Self-healing, stable release of Cu2+, anti-biofouling. Mar. Pollut. Bull. 2023, 195, 115524. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Dou, B.; Lin, X.; Zhao, S.; Emori, W.; Pan, J.; Hu, H.; Xiao, H. Influence of active nanofiller ZIF-8 metal-organic framework (MOF) by microemulsion method on anticorrosion of epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126836. [Google Scholar] [CrossRef]
- Li, F.; Bu, Y.; Han, G.-F.; Noh, H.-J.; Kim, S.-J.; Ahmad, I.; Lu, Y.; Zhang, P.; Jeong, H.Y.; Fu, Z.; et al. Identifying the structure of Zn-N2 active sites and structural activation. Nat. Commun. 2019, 10, 2623. [Google Scholar] [CrossRef]
- Breda, S.; Reva, I.; Fausto, R. Molecular structure and vibrational spectra of 2(5H)-furanone and 2(5H)-thiophenone isolated in low temperature inert matrix. J. Mol. Struct. 2008, 887, 75–86. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Huang, Y.; Fan, C. Composite ZIF-8 with CQDs for boosting visible-light-driven photocatalytic removal of NO. J. Alloys Compd. 2019, 802, 467–476. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, X.; Owens, G.; Chen, Z. Remediation of malachite green in wastewater by ZIF-8@ Fe/Ni nanoparticles based on adsorption and reduction. J. Colloid Interface Sci. 2021, 594, 398–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Tong, Y.; Li, X.; Guo, S.; Zhang, H.; Chen, X.; Cai, K.; Cheng, L.; He, W. Pebax mixed-matrix membrane with highly dispersed ZIF-8@ CNTs to enhance CO2/N2 separation. ACS Omega 2021, 6, 18566–18575. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Babarro, J.M.; Lahoz, F.; Sansón, M.; Martín, V.S.; Norte, M.; Fernández, J.J. From broad-spectrum biocides to quorum sensing disruptors and mussel repellents: Antifouling profile of alkyl triphenylphosphonium salts. PLoS ONE 2015, 10, e0123652. [Google Scholar] [CrossRef]
- Martinelli, D.; Grossmann, G.; Séquin, U.; Brandl, H.; Bachofen, R. Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol. 2004, 4, 25. [Google Scholar] [CrossRef]
- Morohoshi, T.; Kato, M.; Fukamachi, K.; Kato, N.; Ikeda, T. N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett. 2008, 279, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C 2014, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qin, L.; Zhao, W.; Mawignon, F.; Guo, H.; Wu, Y.; Zhang, Y.; Dong, G. Eco-friendly polysaccharide coatings for antifouling and drag-reduction and potential application for marine devices. Friction 2024, 12, 726–744. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Sun, W.; Yang, Z.; Wang, S.; Liu, G. Effect of chemical conversion induced by self-corrosion of zinc powders on enhancing corrosion protection performance of zinc-rich coatings. Corros. Sci. 2022, 194, 109942. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, B.; Li, X.; Zhao, D.; Ying, H.; Yang, W. Intelligent self-healing anti-corrosive coatings with crosslinked conducting polypyrrole hydrogel network. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132078. [Google Scholar] [CrossRef]
- Shu, H.; Cui, J.; Liu, Y.; Bai, X.; Yuan, C.; Cao, P. MAP-inspired dual crosslinked PVP-phenol sprayable hydrogel coating for stable marine antifouling applications. Friction 2025, 13, 9440946. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Qi, G. Facile in situ growth of Zif-8 nanosheets with enhanced anti-corrosion performance for carbon steel in seawater. Coatings 2022, 12, 318. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Hu, W. Preparation and corrosion resistance of ZnO@ZIF-8-SA film on carbon steel surface. Colloid Surface A 2022, 647, 129017. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Y.; Yu, D.; Yang, T.; Wu, M.; Yang, L.; Petru, M. Porous film coating enabled by Polyvinyl Pyrrolidone (PVP) for enhanced air permeability of fabrics: The effect of PVP molecule weight and dosage. Polymers 2020, 12, 2961. [Google Scholar] [CrossRef]
- Tian, R.M.; Wang, Y.; Bougouffa, S.; Gao, Z.M.; Cai, L.; Zhang, W.P.; Bajic, V.; Qian, P.Y. Effect of copper treatment on the composition and function of the bacterial community in the sponge Haliclona cymaeformis. mBio 2014, 5, 9. [Google Scholar] [CrossRef] [PubMed]
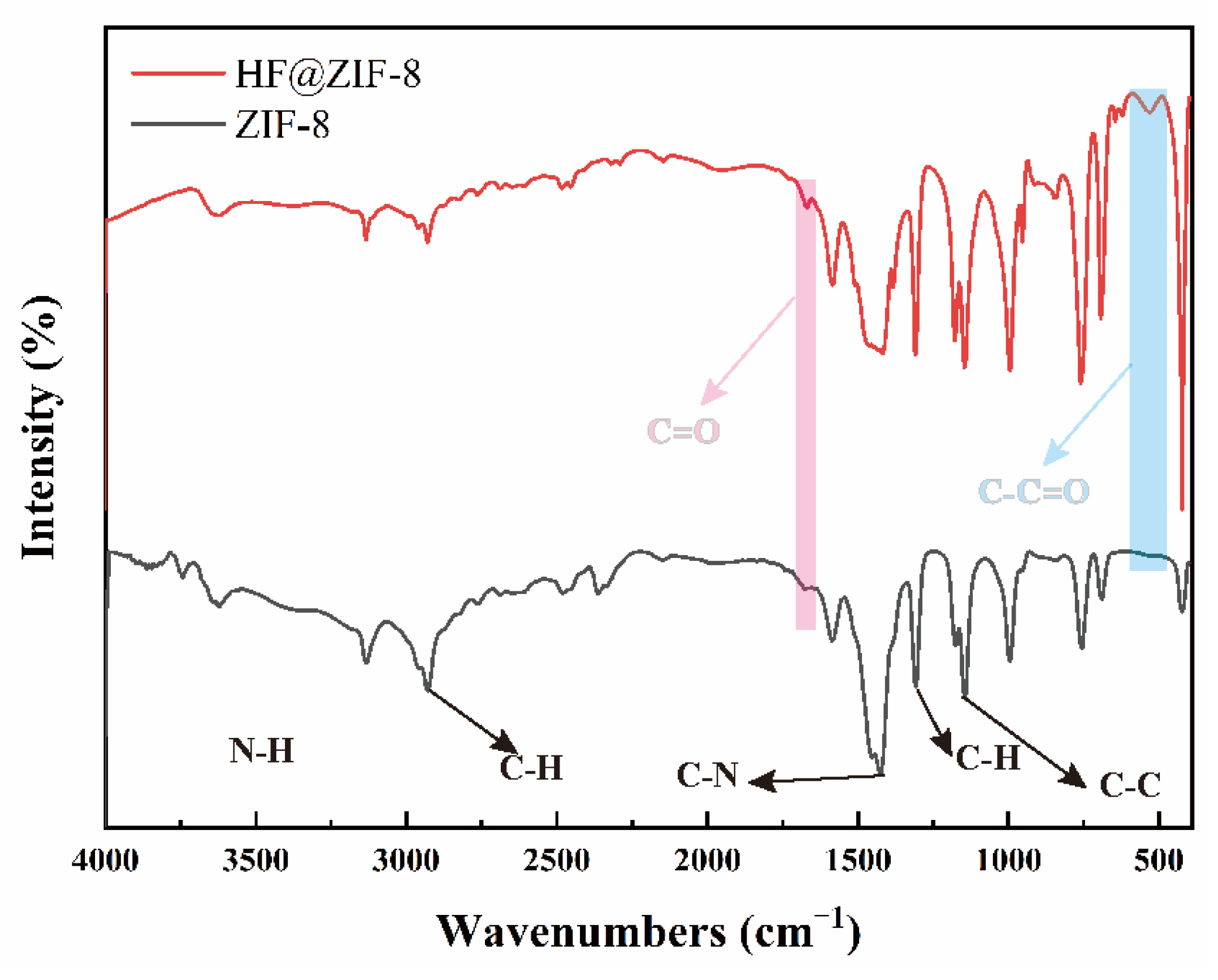
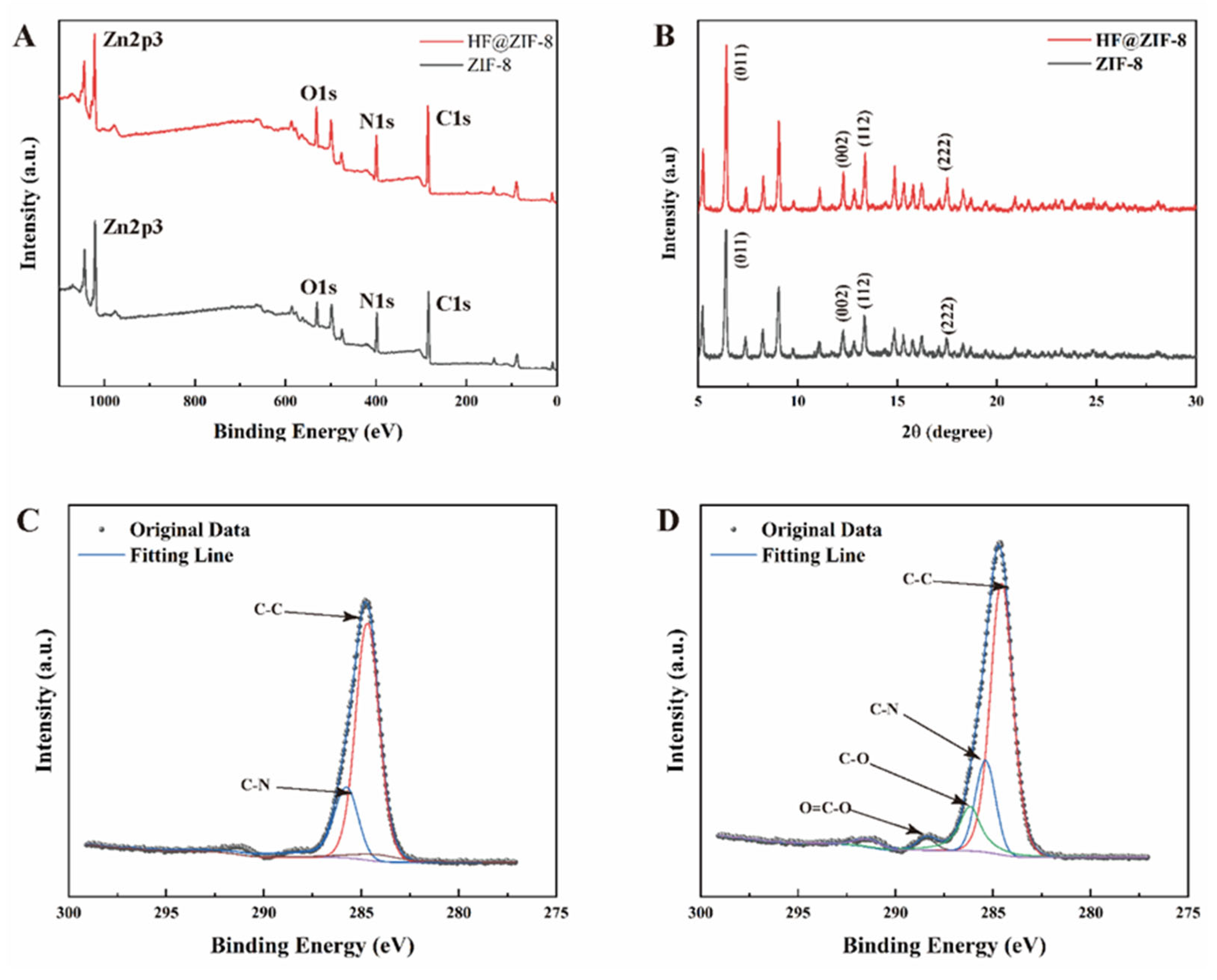
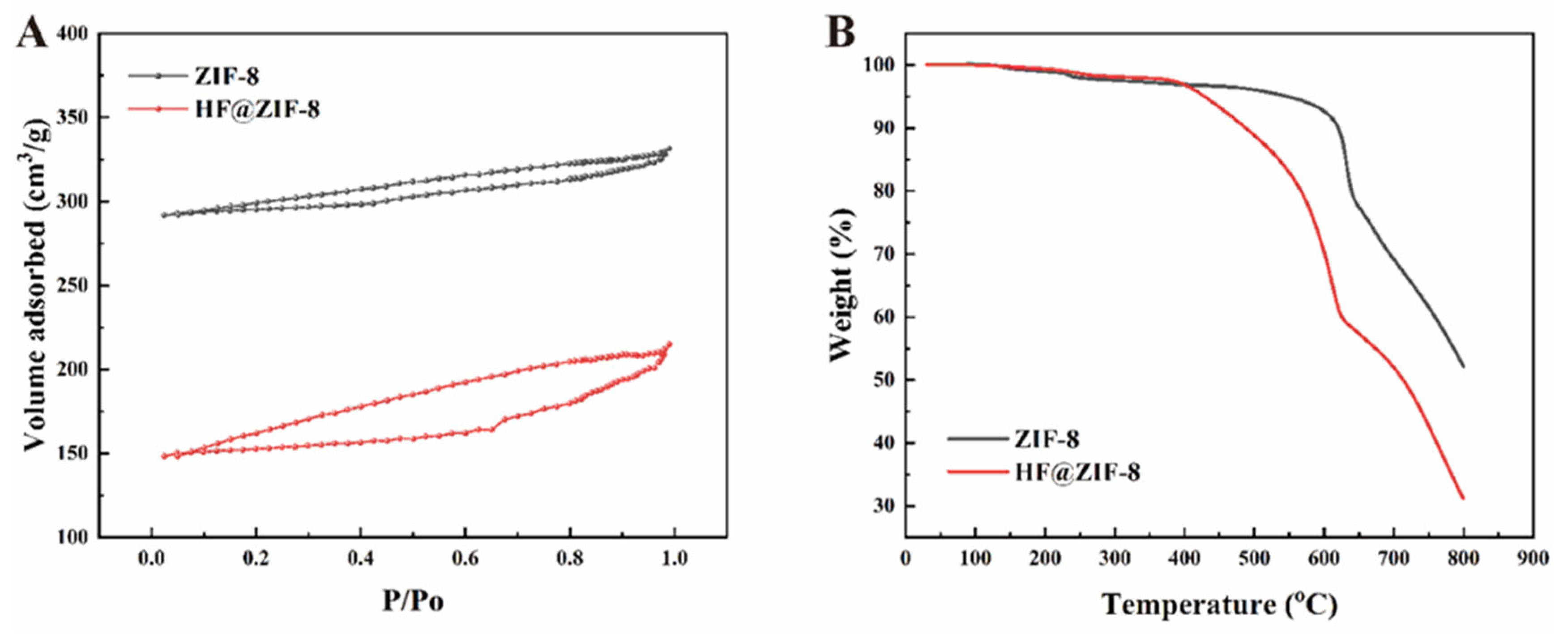
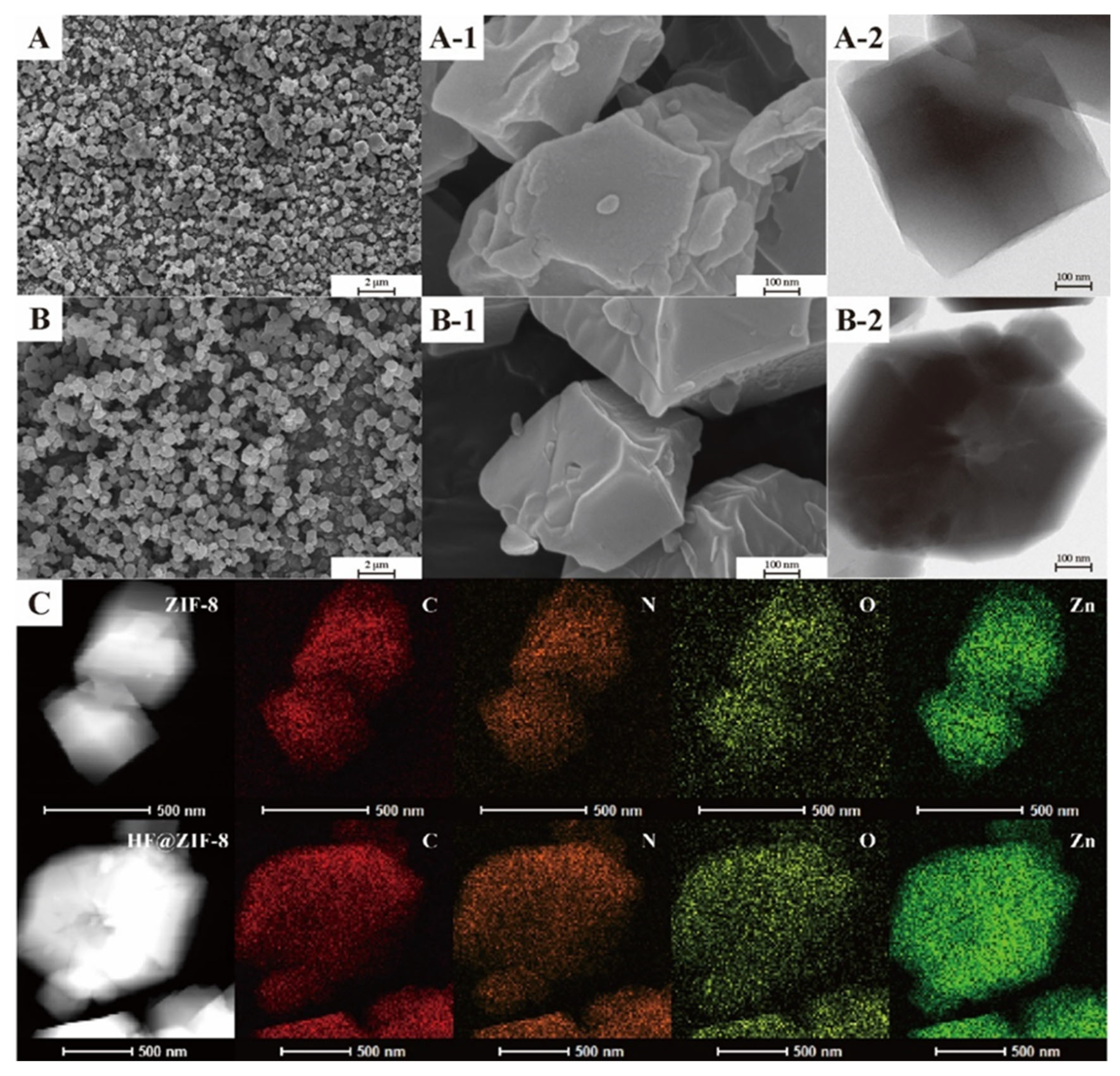

| Sample Name | C (at%) | N (at%) | O (at%) | Zn (at%) |
|---|---|---|---|---|
| ZIF-8 | 20.75 | 12.22 | 9.03 | 58.0 |
| HF@ZIF-8 | 20.95 | 11.47 | 11.72 | 55.86 |
| Sample Name | Specific Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| ZIF-8 | 1157.483 | 3.699 | 0.099 |
| HF@ZIF-8 | 613.519 | 2.971 | 0.061 |
| Sample Name | Ecorr/V | icorr/μA.cm−2 | IE% |
|---|---|---|---|
| SS | −0.305 | 0.908 | / |
| PG/PVP/PEI/ZF | −0.478 | 0.482 | 46.92% |
| PG/PVP/PEI/HF@ZF | −0.294 | 0.250 | 72.47% |
| Coating System | (IE%) | Antibacterial/Antifouling | Functions | Ref. |
|---|---|---|---|---|
| PG/PVP/PEI/HF@ZIF-8 | 72.47% | E. coli, S. aureus > 97%, C. violaceum QSI positive | Anti-corrosion, antibacterial, and QSI | This work |
| PG/PVP/PEI/VP | 63.49% | S. aureus > 99%, E. coli > 87% | Anti-corrosion and antibacterial | [36] |
| In situ ZIF-8 nanosheets | >95%, 87.8% after 168 h | N/A | Anti-corrosion | [37] |
| ZnO@ZIF-8-SA conversion | >99.99%, 95.29% after 14 d | N/A | Superhydrophobic, slow-release, and anti-corrosion | [38] |
| ZIF-8 double- layer coating | Significant improvement in Ecorr | N/A | Surface inhibits adhesion | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Cui, J.; Liu, X.; Shu, H.; Cao, P. Antifouling Mussel-Inspired Hydrogel with Furanone-Loaded ZIF-8 for Quorum Sensing-Mediated Marine Antifouling. Gels 2025, 11, 466. https://doi.org/10.3390/gels11060466
Xiong Y, Cui J, Liu X, Shu H, Cao P. Antifouling Mussel-Inspired Hydrogel with Furanone-Loaded ZIF-8 for Quorum Sensing-Mediated Marine Antifouling. Gels. 2025; 11(6):466. https://doi.org/10.3390/gels11060466
Chicago/Turabian StyleXiong, Yanbin, Junnan Cui, Xiaodan Liu, Haobo Shu, and Pan Cao. 2025. "Antifouling Mussel-Inspired Hydrogel with Furanone-Loaded ZIF-8 for Quorum Sensing-Mediated Marine Antifouling" Gels 11, no. 6: 466. https://doi.org/10.3390/gels11060466
APA StyleXiong, Y., Cui, J., Liu, X., Shu, H., & Cao, P. (2025). Antifouling Mussel-Inspired Hydrogel with Furanone-Loaded ZIF-8 for Quorum Sensing-Mediated Marine Antifouling. Gels, 11(6), 466. https://doi.org/10.3390/gels11060466






