Engineering Moxifloxacin-Encapsulated Liposome-Enriched Alginate Hydrogel Films
Abstract
1. Introduction
2. Results and Discussion
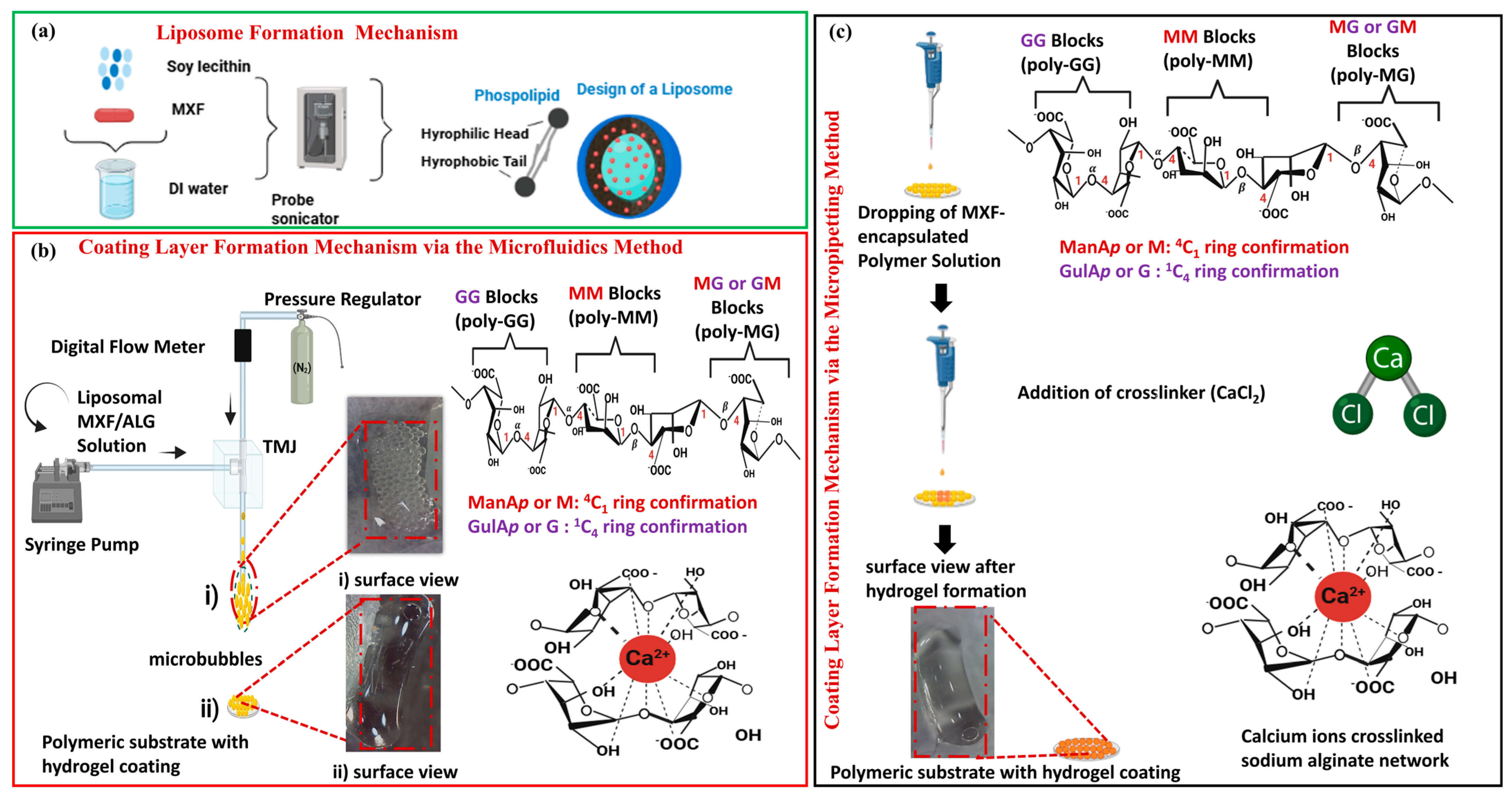
2.1. MXF-Free and MXF-Encapsulated Liposome Formation by Probe Sonication
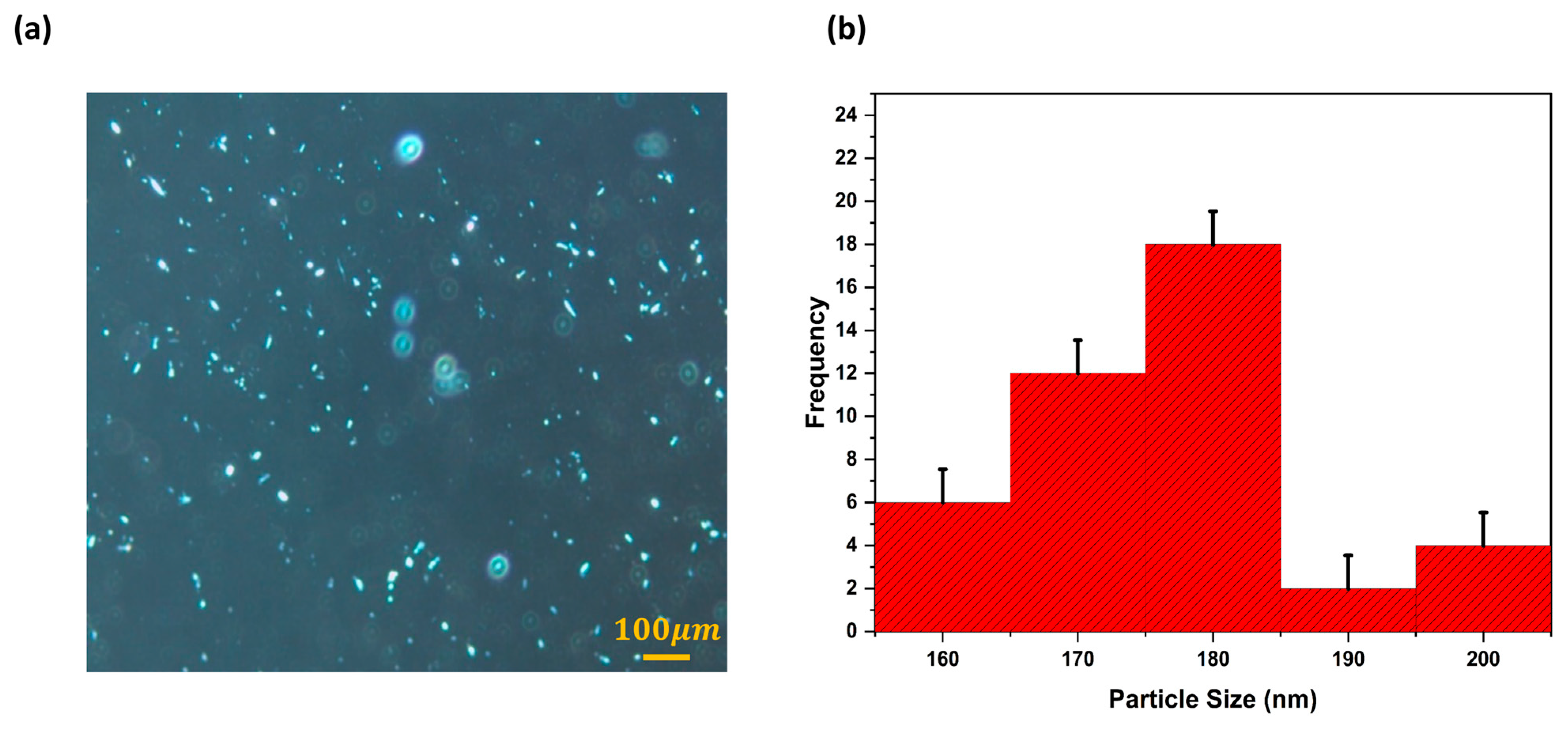
2.2. Characterization of Freeze-Dried Liposomes
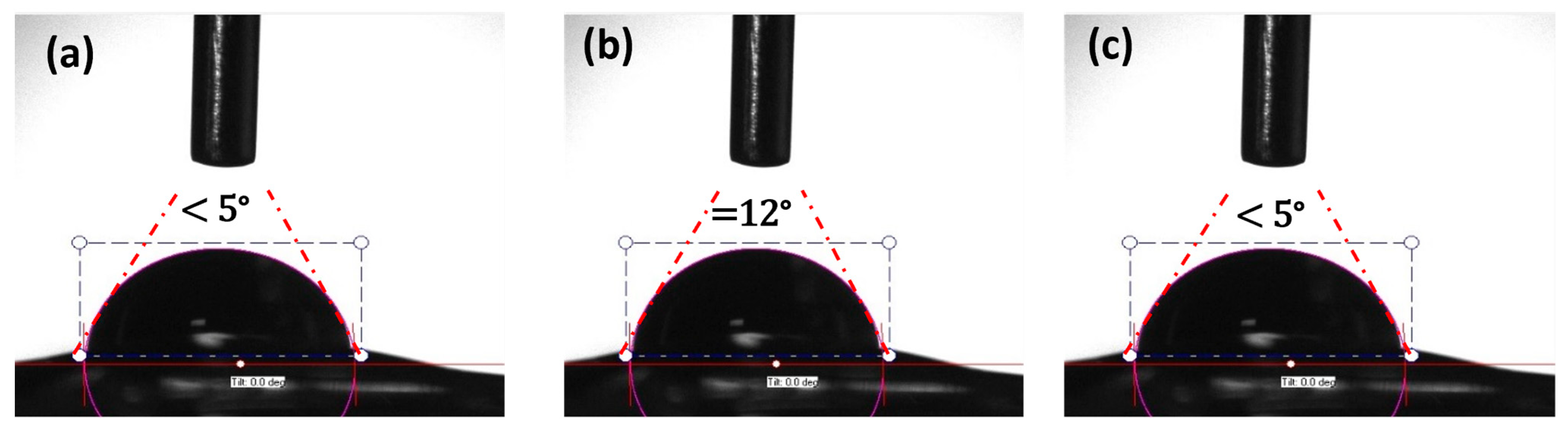
2.3. Characterization of Hydrogel Nanocomposites
2.3.1. Microbubble Formation
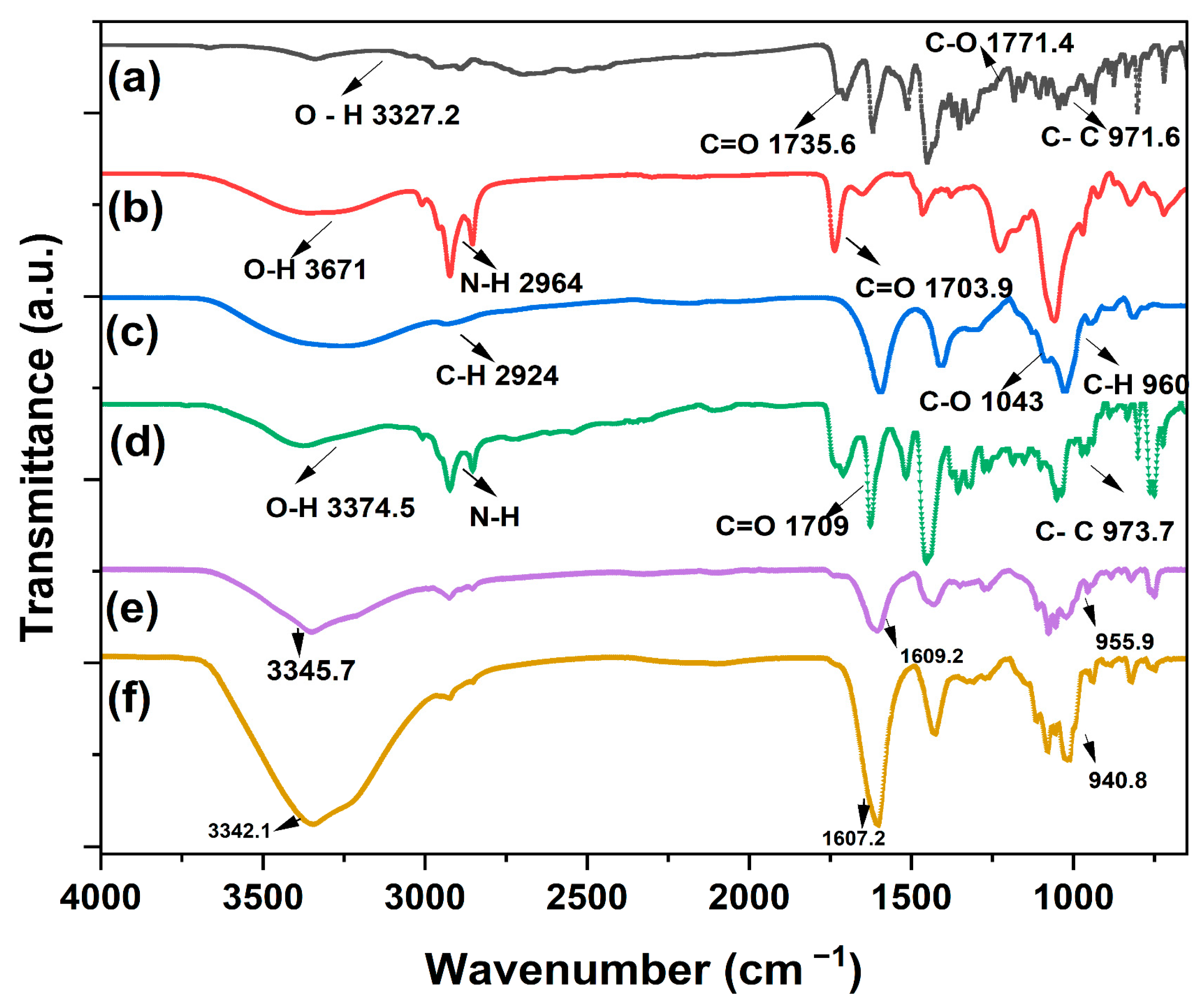
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Method
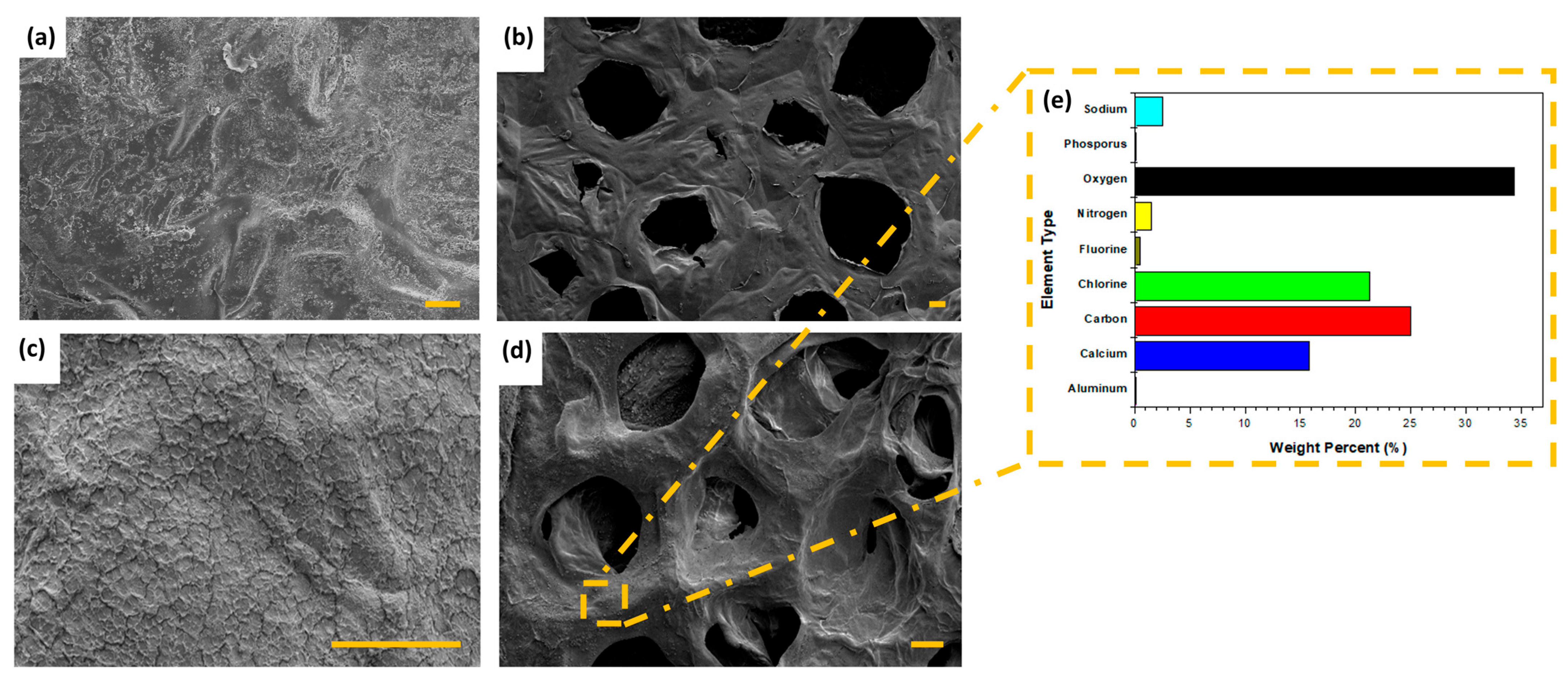
2.3.3. Scanning Electron Microscope (SEM) of Hydrogel Nanocomposites
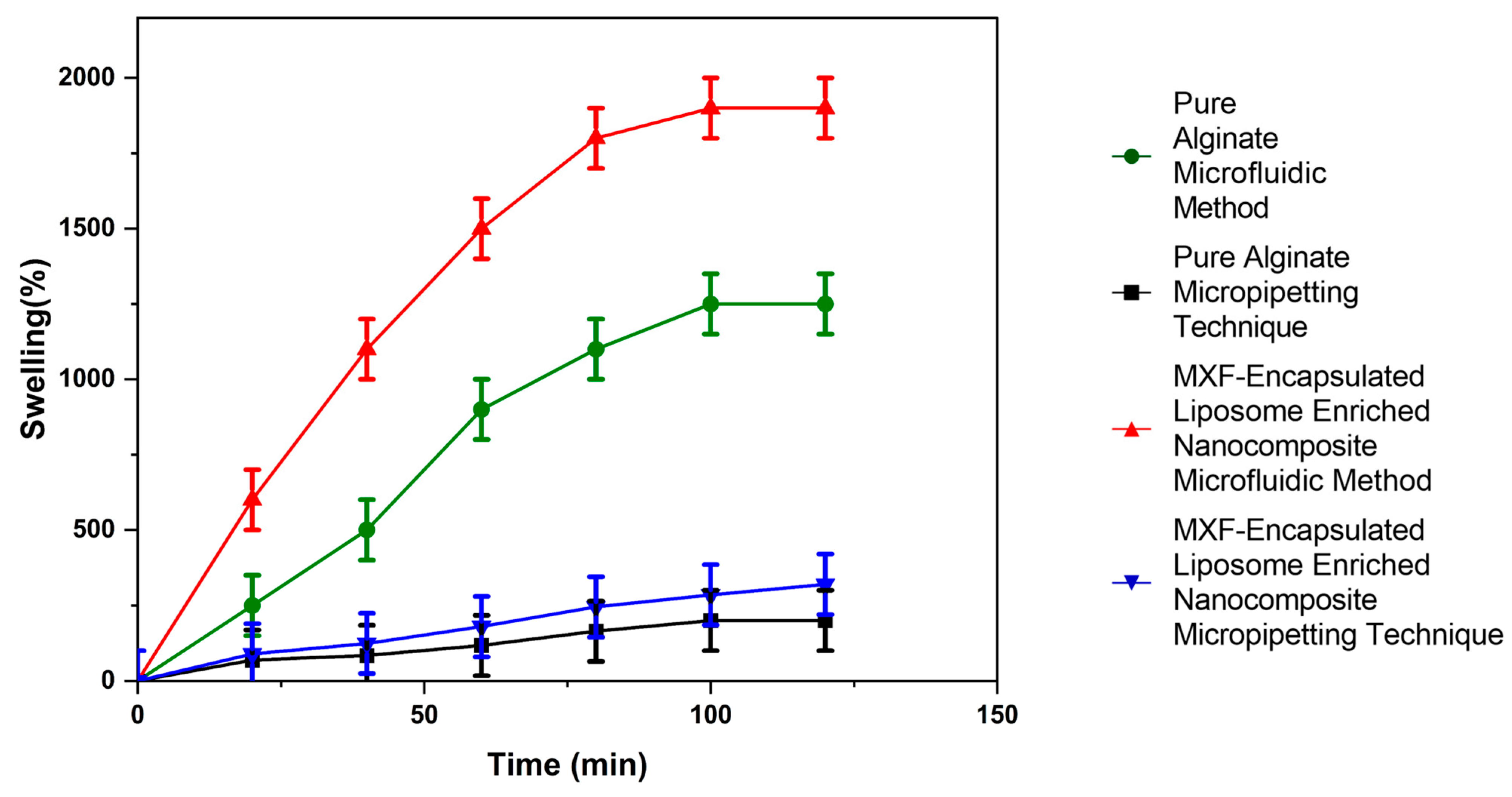
2.3.4. Investigation of Swelling Profile
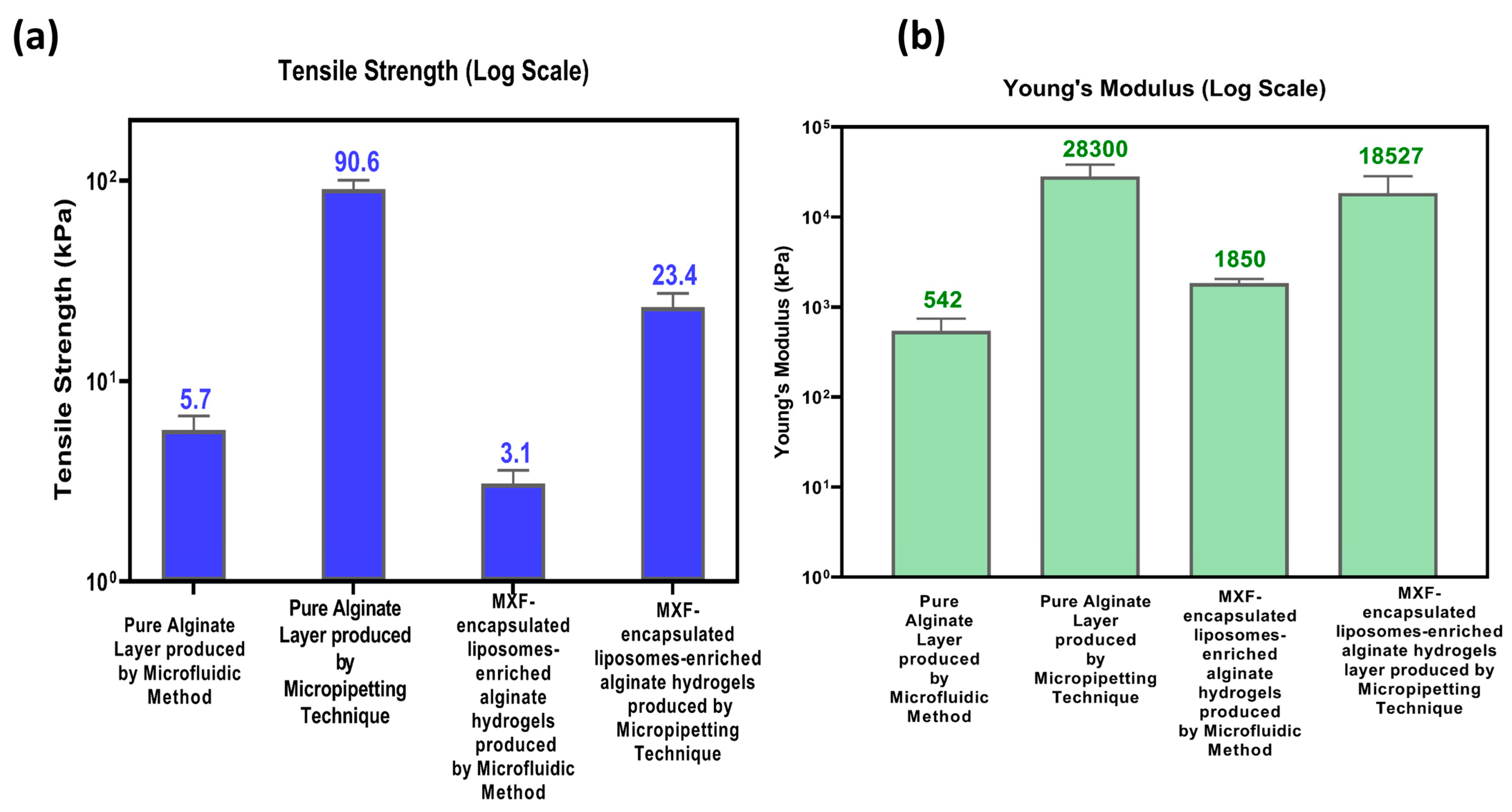
2.3.5. Mechanical Properties
2.3.6. In Vitro Moxifloxacin Release of the Alginate-Based Nanocomposite Hydrogel Coating Composites
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Moxifloxacin-Encapsulated Liposomal Alginate Hydrogel Nanocomposite Coatings
4.2.1. Preparation of Only MXF Drug Solution and MXF-Encapsulated Liposomal Nanoparticles
4.2.2. Characterization of MXF-Free and MXF-Encapsulated Liposome Nanoparticles
4.2.3. Preparation of MXF-Encapsulated Liposomes Enriched Alginate Nanocomposite Hydrogel Coatings on a Polymeric Substrate Material
4.3. Characterization of MXF-Encapsulated Liposome-Enriched Alginate Nanocomposite Hydrogels
4.3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of the Constructs
4.3.2. Morphological Characterization of the Resultant Alginate-Based Nanocomposite Hydrogel Coating Constructs
4.3.3. Analysis of Swelling Index
4.3.4. Mechanical Measurements
4.3.5. In Vitro Drug Release Performance of the Resultant Constructs
4.3.6. Evaluating Drug Release Profiles
4.3.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MXF | Moxifloxacin |
| TMJ | T-shaped Microfluidic Junction Device |
| EE | Encapsulation Efficiency |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-Dispersive Spectroscopy |
| PDI | Polydispersity Index |
| CA | Contact Angle |
| FDA | Food and Drug Administration |
| HPLC | High-Performance Liquid Chromatography |
References
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Arshad, M.S.; Ayub, A.; Zafar, S.; Rana, S.J.; Muhammad, S.A.; Aleem, A.; Onaiwu, E.; Nazari, K.; Chang, M.-W.; Ahmad, Z. Fabrication of miconazole nitrate solid lipid nanoparticle loaded microneedle patches for the treatment of Candida albicans biofilms. RSC Pharm. 2024, 1, 458–471. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Jurgelane, I.; Egle, K.; Grava, A.; Galkina, D.; Brante, M.; Melnichuks, M.; Skrinda-Melne, M.; Salms, G.; Dubnika, A. Exploring the effects of cannabidiol encapsulation in liposomes on their physicochemical properties and biocompatibility. Drug Deliv. 2025, 32, 2460666. [Google Scholar] [CrossRef] [PubMed]
- Mufamadi, M.S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K. A Review on Composite Liposomal Technologies for Specialized Drug Delivery. J. Drug Deliv. 2011, 2011, 939851. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, F.; He, J.; Liang, M. Ferritin versus Liposomes: A Comparative Analysis of Protein- and Lipid-Based Drug Delivery Systems. Bioconjug. Chem. 2025, 36, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Montan, P.G.; Wejde, G.; Setterquist, H.; Rylander, M.; Zetterström, C. Prophylactic intracameral cefuroxime: Evaluation of safety and kinetics in cataract surgery. J. Cataract. Refract. Surg. 2002, 28, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Bremond-Gignac, D.; Chiambaretta, F.; Milazzo, S. A European perspective on topical ophthalmic antibiotics: Current and evolving options. Ophthalmol. Eye Dis. 2011, 3, 29–43. [Google Scholar] [CrossRef]
- Melega, M.V.; Alves, M.; Lira, R.P.C.; da Silva, I.C.; Gil Ferreira, B.; Filho, H.L.A.; Chaves, F.R.P.; Martini, A.A.; Freire, L.M.D.; dos Reis, R.; et al. Safety and efficacy of intracameral moxifloxacin for prevention of post-cataract endophthalmitis: Randomized controlled clinical trial. J. Cataract Refract. Surg. 2019, 45, 343–350. [Google Scholar] [CrossRef]
- Bowen, R.C.; Zhou, A.X.; Bondalapati, S.; Lawyer, T.W.; Snow, K.B.; Evans, P.R.; Bardsley, T.; McFarland, M.; Kliethermes, M.; Shi, D.; et al. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: A meta-analysis. Br. J. Ophthalmol. 2018, 102, 1268–1276. [Google Scholar] [CrossRef]
- Grzybowski, A.; Brona, P.; Zeman, L.; Stewart, M.W. Commonly used intracameral antibiotics for endophthalmitis prophylaxis: A literature review. Surv. Ophthalmol. 2021, 66, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Stark, W.J.; O’brien, T.P.; Dick, J.D. Aqueous penetration and biological activity of moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% solution in cataract surgery patients. Ophthalmology 2005, 112, 1992–1996. [Google Scholar] [CrossRef]
- Solomon, R.; Donnenfeld, E.D.; Perry, H.D.; Snyder, R.W.; Nedrud, C.; Stein, J.; Bloom, A. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology 2005, 112, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Tsoka, P.; Scoulica, E.; Magkafouraki, E.; Natsaridis, E.; Antimisiaris, S.; Tsilimbaris, M.K. Novel liposomal formulation for sustained release of moxifloxacin; efficacy in experimental bacterial endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 2127-F0143. [Google Scholar]
- Ferreira, K.S.A.; dos Santos, B.M.A.; Lucena, N.d.P.; Ferraz, M.S.; Carvalho, R.d.S.F.; Júnior, A.P.D.; Magalhães, N.S.S.; Lira, R.P.C. Ocular delivery of moxifloxacin-loaded liposomes. Arq. Bras. Oftalmol. 2018, 81, 510–513. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nomura, M.; Matsuoka, T.; Koda, S. Effects of frequency and power of ultrasound on the size reduction of liposome. Chem. Phys. Lipids 2009, 160, 58–62. [Google Scholar] [CrossRef]
- Fiume, Z. Final Report on the Safety Assessment of Lecithin and Hydrogenated Lecithin. Int. J. Toxicol. 2001, 20, 21–45. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Duman, G.; Yıldır, I.; Macit, M.; Genç, E.; Sümer, E.; Kale, S.; Deniz, I. Development and evaluation of 3D-printed ocular insert containing liposomal moxifloxacin. J. Drug Deliv. Sci. Technol. 2024, 92, 105353. [Google Scholar] [CrossRef]
- Valentino, A.; Yazdanpanah, S.; Conte, R.; Calarco, A.; Peluso, G. Smart Nanocomposite Hydrogels as Next-Generation Therapeutic and Diagnostic Solutions. Gels 2024, 10, 689. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Haghighi, F.H.; Chronopoulou, L.; Palocci, C. Liposome–Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Begum, B.; Koduru, T.S.; Madni, S.N.; Anjum, N.F.; Seetharaman, S.; Veeranna, B.; Gupta, V.K. Dual-Self-Crosslinking Effect of Alginate-Di-Aldehyde with Natural and Synthetic Co-Polymers as Injectable In Situ-Forming Biodegradable Hydrogel. Gels 2024, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Yu, Q.; Tian, Z.; Li, G.; Yang, Y.; Chen, X.; Wang, D.; Peng, W.; Liu, R.; Gu, H.; Yue, X. Multifunctional composite capsules in drug delivery systems: Bridging pharmaceutical and biomedical applications. Adv. Compos. Hybrid Mater. 2025, 8, 118. [Google Scholar] [CrossRef]
- Zafar, S.; Rana, S.J.; Sayed, E.; Chohan, T.A.; Kucuk, I.; Nazari, K.; Arshad, M.S.; Ahmad, Z. Enhancing linezolid activity in the treatment of oral biofilms using novel chitosan microneedles with iontophoretic control. Mater. Sci. Eng. C 2024, 164, 213995. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.-P.N.; et al. Electrosprayed mesoporous particles for improved aqueous solubility of a poorly water soluble anticancer agent: In vitro and ex vivo evaluation. J. Control. Release 2018, 278, 142–155. [Google Scholar] [CrossRef]
- Zafar, S.; Arshad, M.S.; Rana, S.J.; Patel, M.; Yousef, B.; Ahmad, Z. Engineering of clarithromycin loaded stimulus responsive dissolving microneedle patches for the treatment of biofilms. Int. J. Pharm. 2023, 640, 123003. [Google Scholar] [CrossRef]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate nanoparticles as ocular drug delivery carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Karmakar, S.; Manna, S.; Kabiraj, S.; Jana, S. Recent progress in alginate-based carriers for ocular targeting of therapeutics. Food Hydrocoll. Health 2022, 2, 100071. [Google Scholar] [CrossRef]
- Moreno-Rivas, S.C.; Ibarra-Gutiérrez, M.J.; Fernández-Quiroz, D.; Lucero-Acuña, A.; Burgara-Estrella, A.J.; Zavala-Rivera, P. pH-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water. Gels 2024, 10, 669. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Poshina, D.N.; Raik, S.V.; Urtti, A.; Skorik, Y.A. Polysaccharides in Ocular Drug Delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, J.; Shi, S.; He, J.; Liu, W.; Li, Y.; Zeng, X.; Pang, J.; Wu, C. Preparation and characterization of pH-sensitive calcium alginate hydrogel beads as delivery carriers for the controlled release of fucoxanthin. Food Hydrocoll. 2025, 163, 111106. [Google Scholar] [CrossRef]
- Ekemen, Z.; Ahmad, Z.; Stride, E.; Kaplan, D.; Edirisinghe, M. Electrohydrodynamic bubbling: An alternative route to fabricate porous structures of silk fibroin based materials. Biomacromolecules 2013, 14, 1412–1422. [Google Scholar] [CrossRef]
- Ekemen, Z.; Chang, H.; Ahmad, Z.; Bayram, C.; Rong, Z.; Denkbas, E.B.; Stride, E.; Vadgama, P.; Edirisinghe, M. Fabrication of biomaterials via controlled protein bubble generation and manipulation. Biomacromolecules 2011, 12, 4291–4300. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883. [Google Scholar] [CrossRef]
- Kucuk, I.; Edirisinghe, M. Microfluidic preparation of polymer nanospheres. J. Nanopart. Res. 2014, 16, 2626. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hashimoto, M.; Dang, T.T.; Hoare, T.; Kohane, D.S.; Whitesides, G.M.; Langer, R.; Anderson, D.G. Preparation of Monodisperse Biodegradable Polymer Microparticles Using a Microfluidic Flow-Focusing Device for Controlled Drug Delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Segneanu, A.-E.; Bejenaru, C.; Bradu, I.A.; Vlase, T.; Herea, D.-D.; Văruţ, M.C.; Bălăşoiu, R.M.; Biţă, A.; Radu, A.; et al. Thermoresponsive Gels with Rosemary Essential Oil: A Novel Topical Carrier for Antimicrobial Therapy and Drug Delivery Applications. Gels 2025, 11, 61. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.B.; Cvjetan, N.; Ayache, J.; Walde, P. Multivesicular Vesicles: Preparation and Applications. ChemSystemsChem 2021, 3, e2000049. [Google Scholar] [CrossRef]
- Aizik, G.; Ostertag-Hill, C.A.; Chakraborty, P.; Choi, W.; Pan, M.; Mankus, D.V.; Lytton-Jean, A.K.; Kohane, D.S. Injectable hydrogel based on liposome self-assembly for controlled release of small hydrophilic molecules. Acta Biomater. 2024, 183, 101–110. [Google Scholar] [CrossRef]
- Setiadi, S.; Hidayah, N. The effect of papain enzyme dosage on the modification of egg-yolk lecithin emulsifier product through enzymatic hydrolysis reaction. Int. J. Technol. 2018, 9, 380–389. [Google Scholar] [CrossRef]
- Dsugi, N.F.A.; Elbashir, A.A. Supramolecular interaction of Moxifloxacin and β-cyclodextrin spectroscopic characterization and analytical application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Su, T.; Zhao, J.; Wu, Z.; Wang, D.; Zhang, W.-N.; Wu, Q.-X.; Chen, Y. Fabrication of polysaccharides-based hydrogel films for transdermal sustained delivery of Ibuprofen. Cellulose 2020, 27, 10277–10292. [Google Scholar] [CrossRef]
- Shah, A.; Ashames, A.A.; Buabeid, M.A.; Murtaza, G. Synthesis, in vitro characterization and antibacterial efficacy of moxifloxacin-loaded chitosan-pullulan-silver-nanocomposite films. J. Drug Deliv. Sci. Technol. 2020, 55, 101366. [Google Scholar] [CrossRef]
- Pentak, D.; Kozik, V.; Zieba, A.; Paździor-Heiske, M.; Szymczyk, A.; Jampilek, J.; Bak, A. Preparing a Liposome-Aided Drug Delivery System: The Entrapment and Release Profiles of Doxorubicin and 9-(N-Piperazinyl)-5-methyl-12(H)-quino [3,4-b][1,4]benzothiazinium Chloride with Human Serum Albumin. Pharmaceutics 2025, 17, 202. [Google Scholar] [CrossRef]
- Mutlu, B.; Farhan, M.; Kucuk, I. T-shaped microfluidic junction processing of porous alginate-based films and their characteristics. Polymers 2019, 11, 1386. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; He, L.; Yang, B.; Zhu, S.; Yao, M. Thermal-responsive poly(N-isopropyl acrylamide)/sodium alginate hydrogels: Preparation, swelling behaviors, and mechanical properties. Colloid Polym. Sci. 2016, 294, 1959–1967. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Design of polymer-free Vitamin-A acetate/cyclodextrin nanofibrous webs: Antioxidant and fast-dissolving properties. Food Funct. 2020, 11, 7626–7637. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.-M.; Barhalescu, M.L.; Agop, M.; Ochiuz, L. Theoretical Modeling of Long-Time Drug Release from Nitrosalicyl-Imine-Chitosan Hydrogels through Multifractal Logistic Type Laws. Comput. Math. Methods Med. 2019, 2019, 4091464. [Google Scholar] [CrossRef] [PubMed]
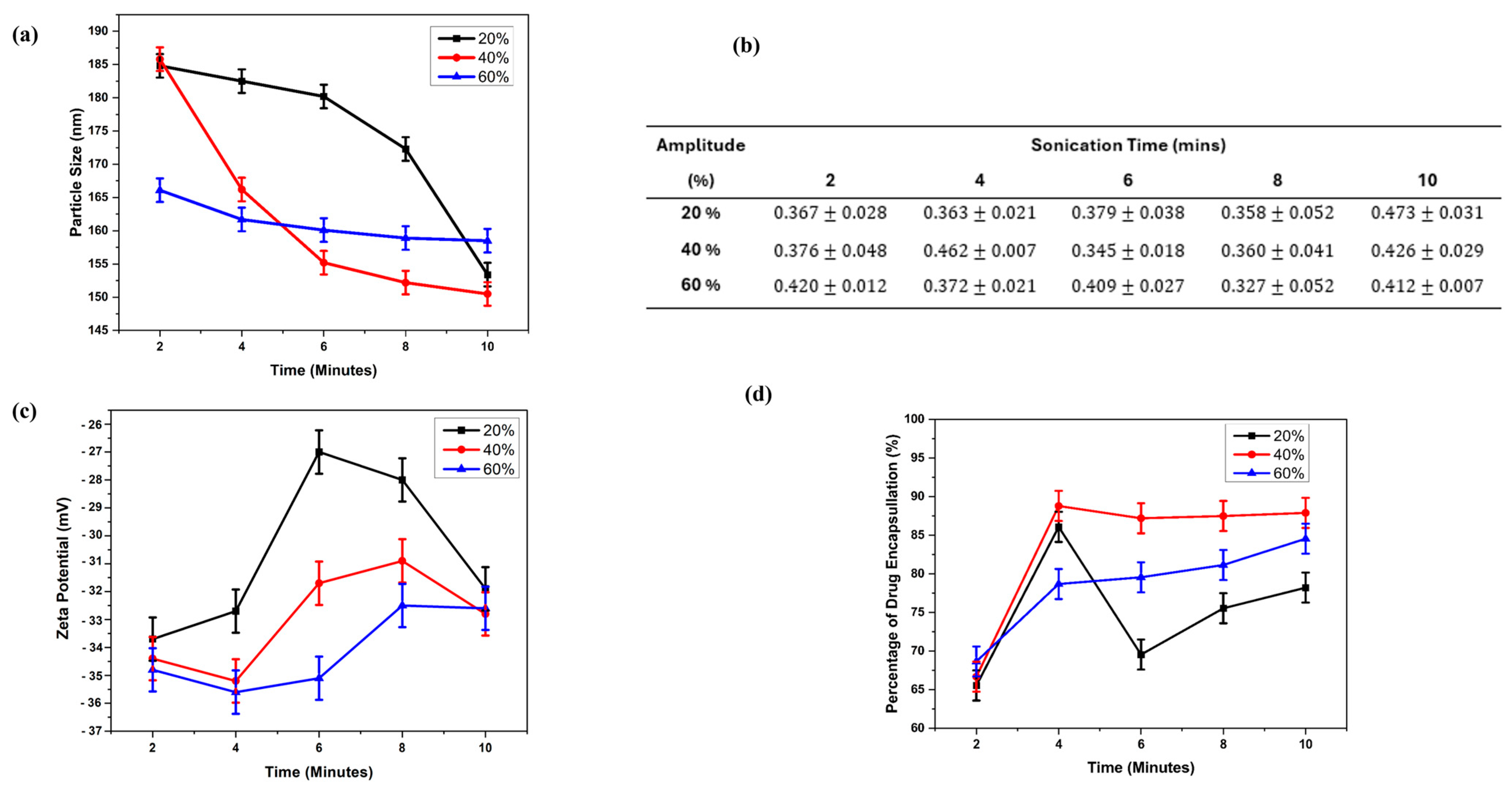


| Amplitude | Sonication Time (Min) | ||||
|---|---|---|---|---|---|
| (%) | 2 | 4 | 6 | 8 | 10 |
| 20 | L1 | L2 | L3 | L4 | L5 |
| 40 | L6 | L7 | L8 | L9 | L10 |
| 60 | L11 | L12 | L13 | L14 | L15 |
| (a) | ||||
|---|---|---|---|---|
| Formulation | R2 Value Observed by Applying Different Dissolution Models | |||
| First-Order | Higuchi | Korsmeyer–Peppas | Hixson–Crowell | |
| MXF-encapsulated liposome (micropipetting method) | 0.981 | 0.878 | 0.943 n = 0.294 | 0.977 |
| MXF-encapsulated liposome (microfluidic method) | 0.996 | 0.915 | 0.997 n = 0.864 | 0.995 |
| Nanocomposite hydrogel (micropipetting method) | 0.960 | 0.754 | 0.996 n = 1.282 | 0.966 |
| Nanocomposite hydrogels (microfluidic method) | 0.959 | 0.754 | 0.998 n = 1.370 | 0.962 |
| (b) | ||||
| Formulation | R2 Value Observed by Applying Different Dissolution Models | |||
| First Order | Higuchi | Korsmeyer–Peppas | Hixson–Crowell | |
| MXF-encapsulated liposome (micropipetting method) | 0.676 | −262.951 | 0.974 | −135.750 |
| MXF-encapsulated liposome (microfluidic method) | 0.976 | 0.961 | 0.978 n = 0.441 | 0.967 |
| Nanocomposite hydrogel (micropipetting method) | 0.965 | 0.944 | 0.945 n = 0.515 | 0.994 |
| Nanocomposite hydrogels (microfluidic method) | 0.974 | 0.949 | 0.958 n = 0.556 | 0.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bal, I.; Macit, M.; Alasiri, A.; Namli, O.C.; Arshad, M.S.; Ahmad, Z.; Duman, G.; Kucuk, I. Engineering Moxifloxacin-Encapsulated Liposome-Enriched Alginate Hydrogel Films. Gels 2025, 11, 448. https://doi.org/10.3390/gels11060448
Bal I, Macit M, Alasiri A, Namli OC, Arshad MS, Ahmad Z, Duman G, Kucuk I. Engineering Moxifloxacin-Encapsulated Liposome-Enriched Alginate Hydrogel Films. Gels. 2025; 11(6):448. https://doi.org/10.3390/gels11060448
Chicago/Turabian StyleBal, Ismail, Meltem Macit, Ali Alasiri, Onur Cem Namli, Muhammad Sohail Arshad, Zeeshan Ahmad, Gulengul Duman, and Israfil Kucuk. 2025. "Engineering Moxifloxacin-Encapsulated Liposome-Enriched Alginate Hydrogel Films" Gels 11, no. 6: 448. https://doi.org/10.3390/gels11060448
APA StyleBal, I., Macit, M., Alasiri, A., Namli, O. C., Arshad, M. S., Ahmad, Z., Duman, G., & Kucuk, I. (2025). Engineering Moxifloxacin-Encapsulated Liposome-Enriched Alginate Hydrogel Films. Gels, 11(6), 448. https://doi.org/10.3390/gels11060448










