Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs)
Abstract
1. Introduction
2. Results and Discussion
2.1. Results
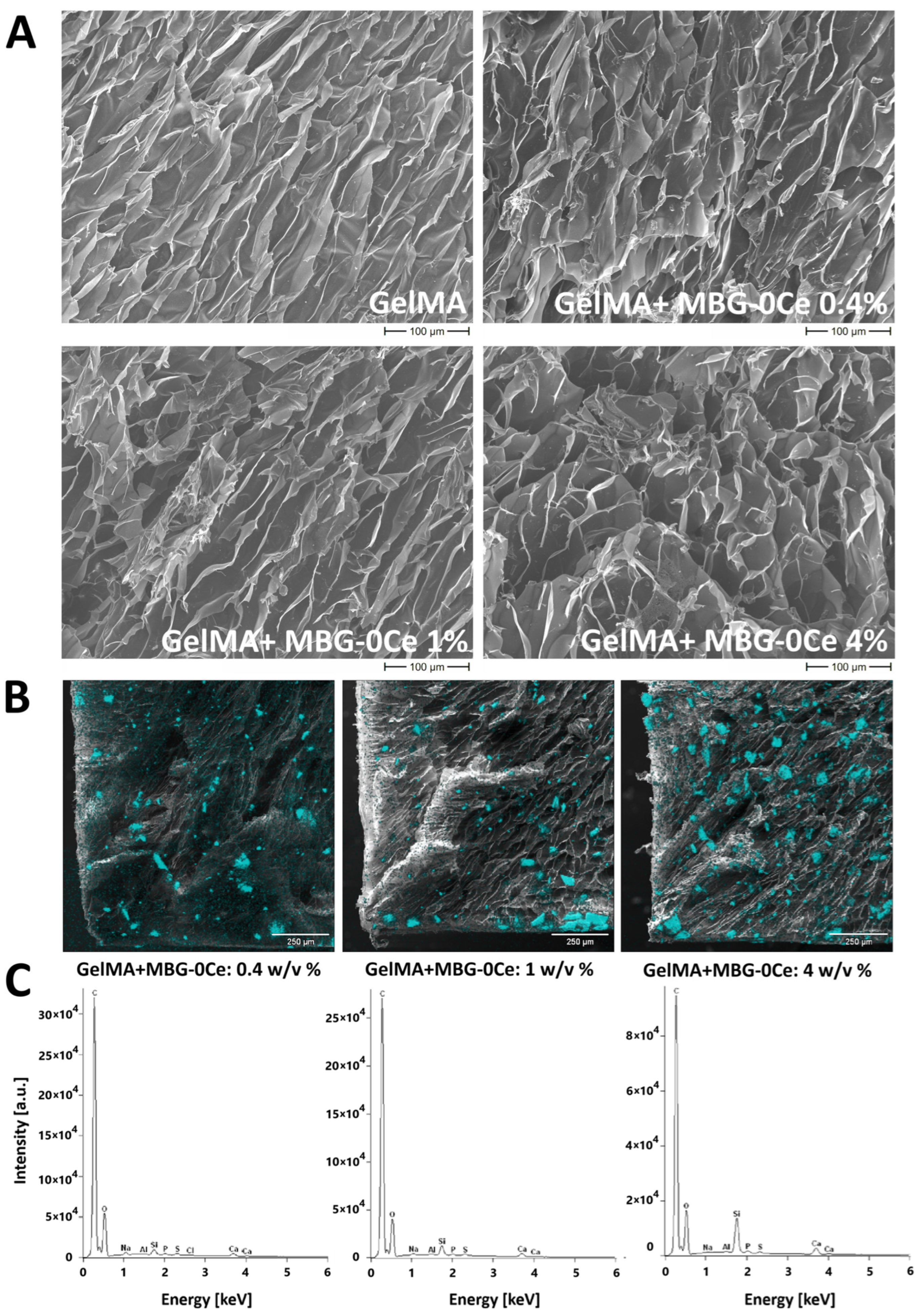
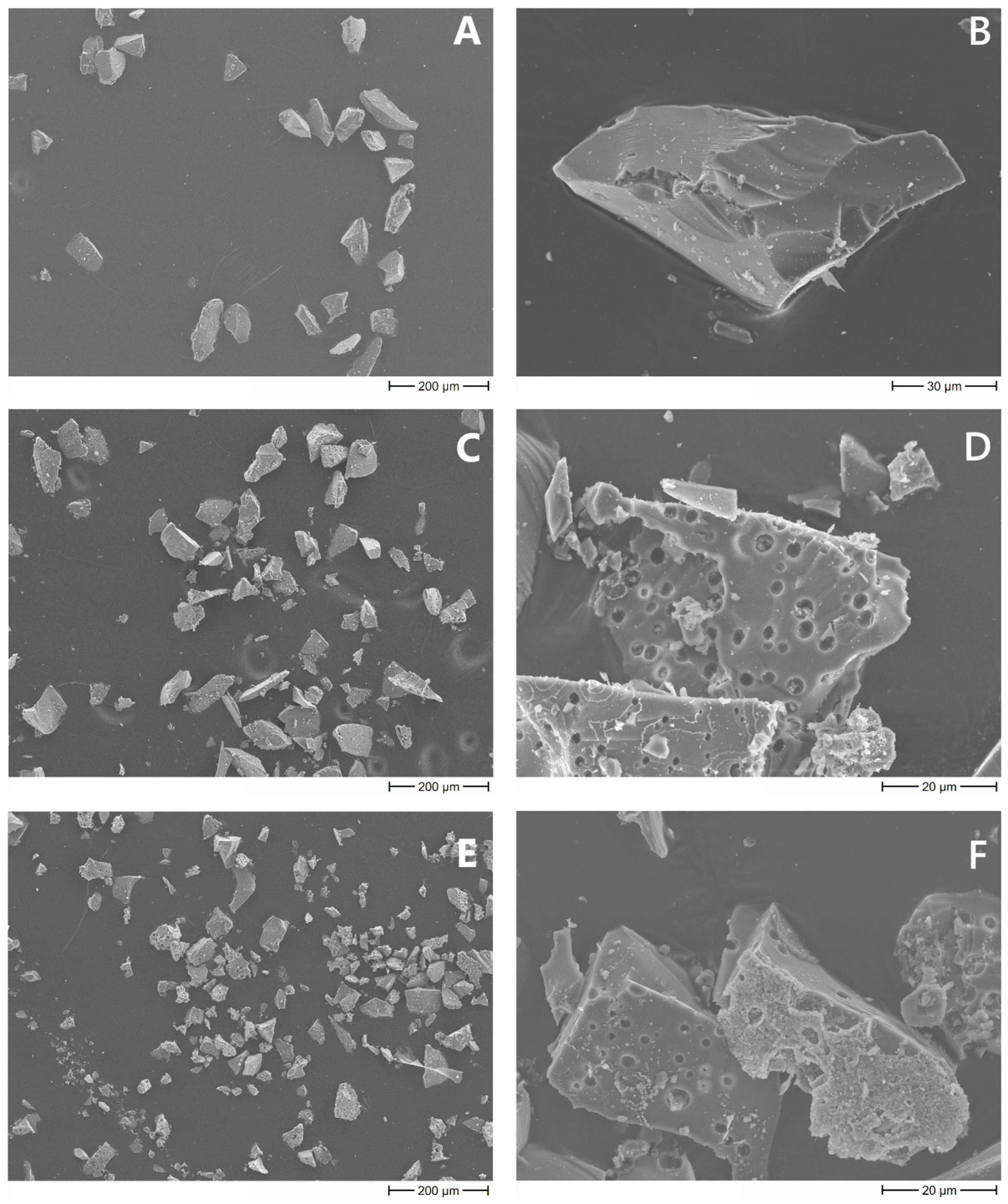
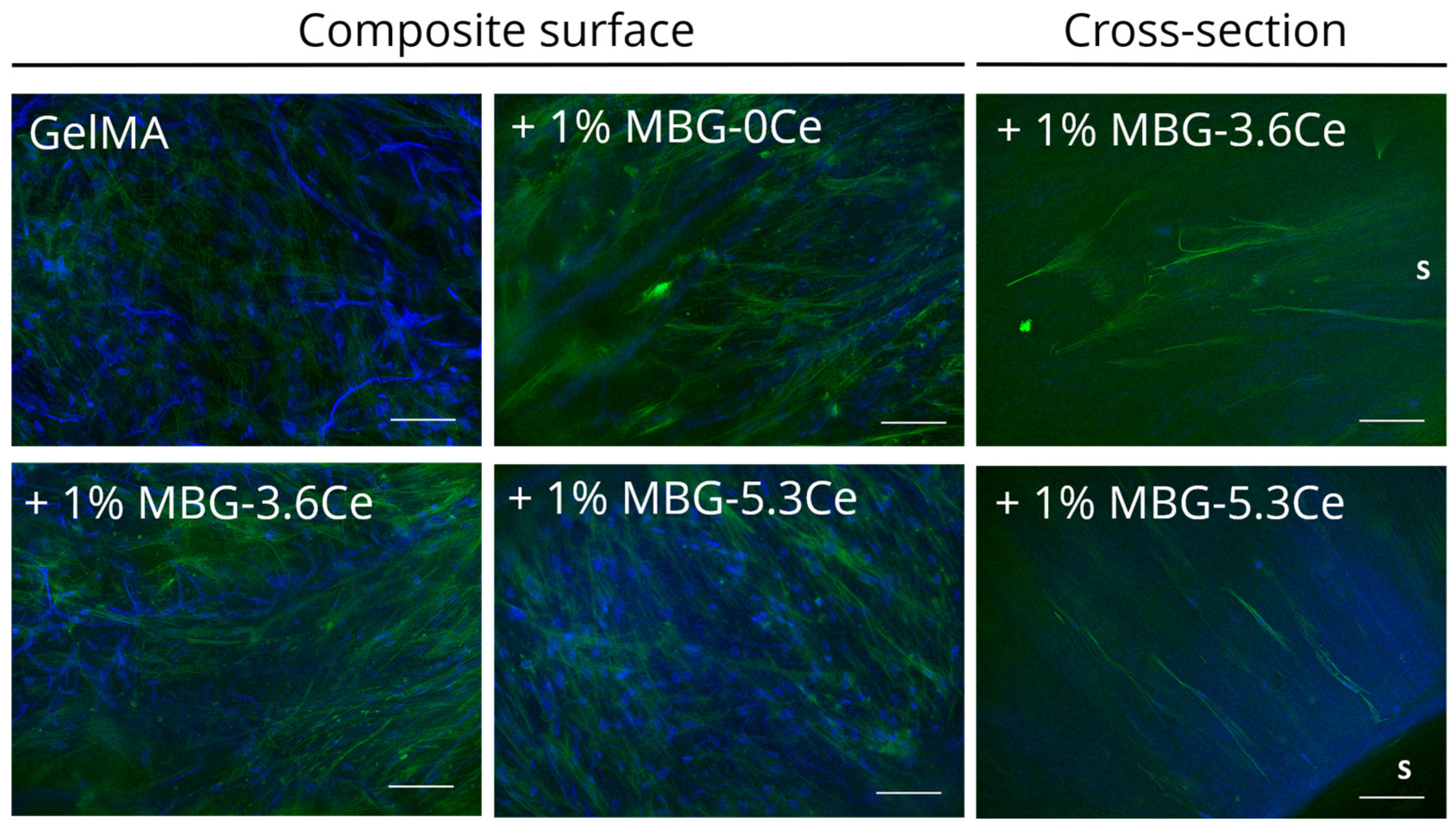
2.1.1. Intrinsic Morphology of Composites and Distribution of MBGs
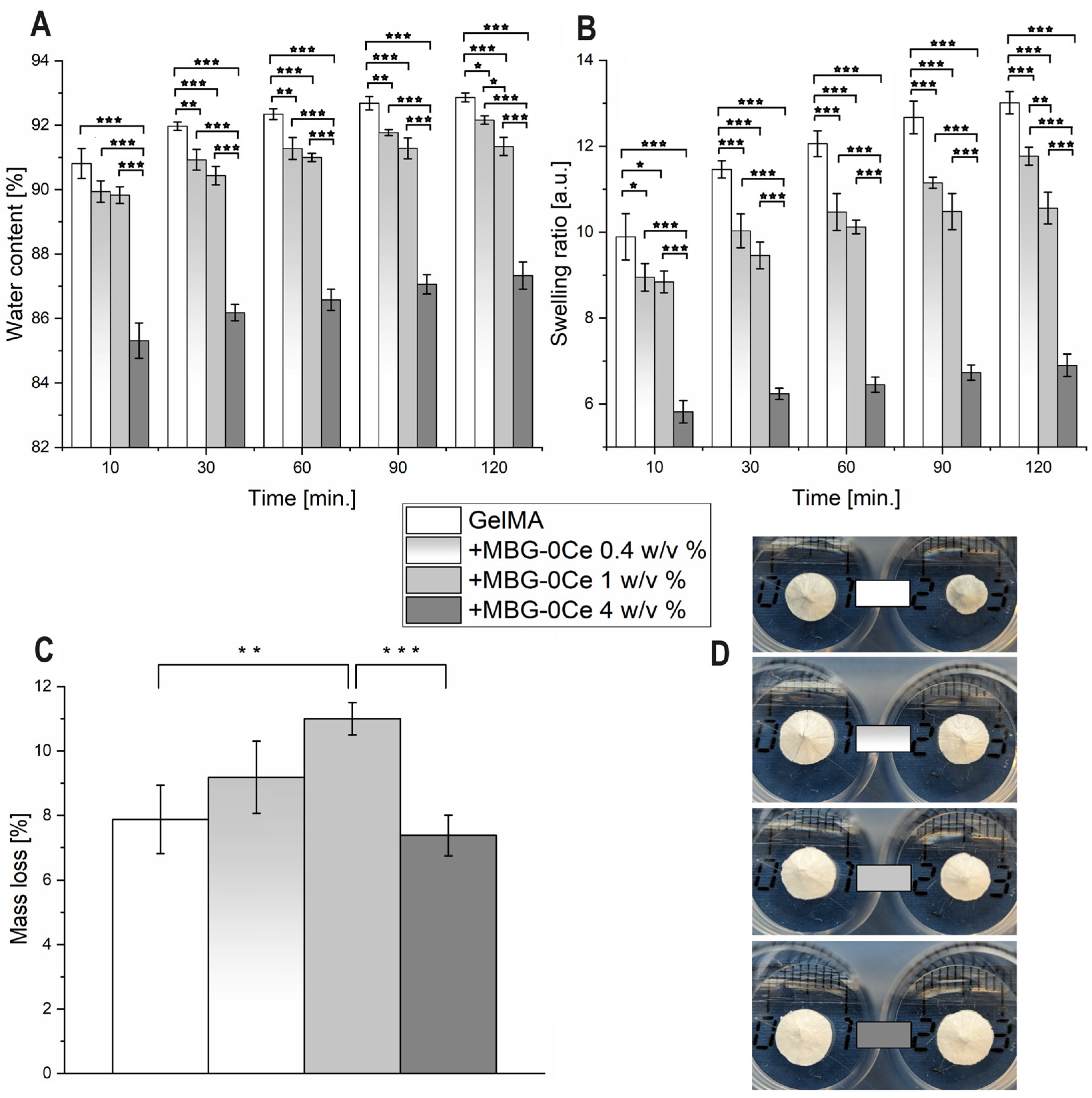
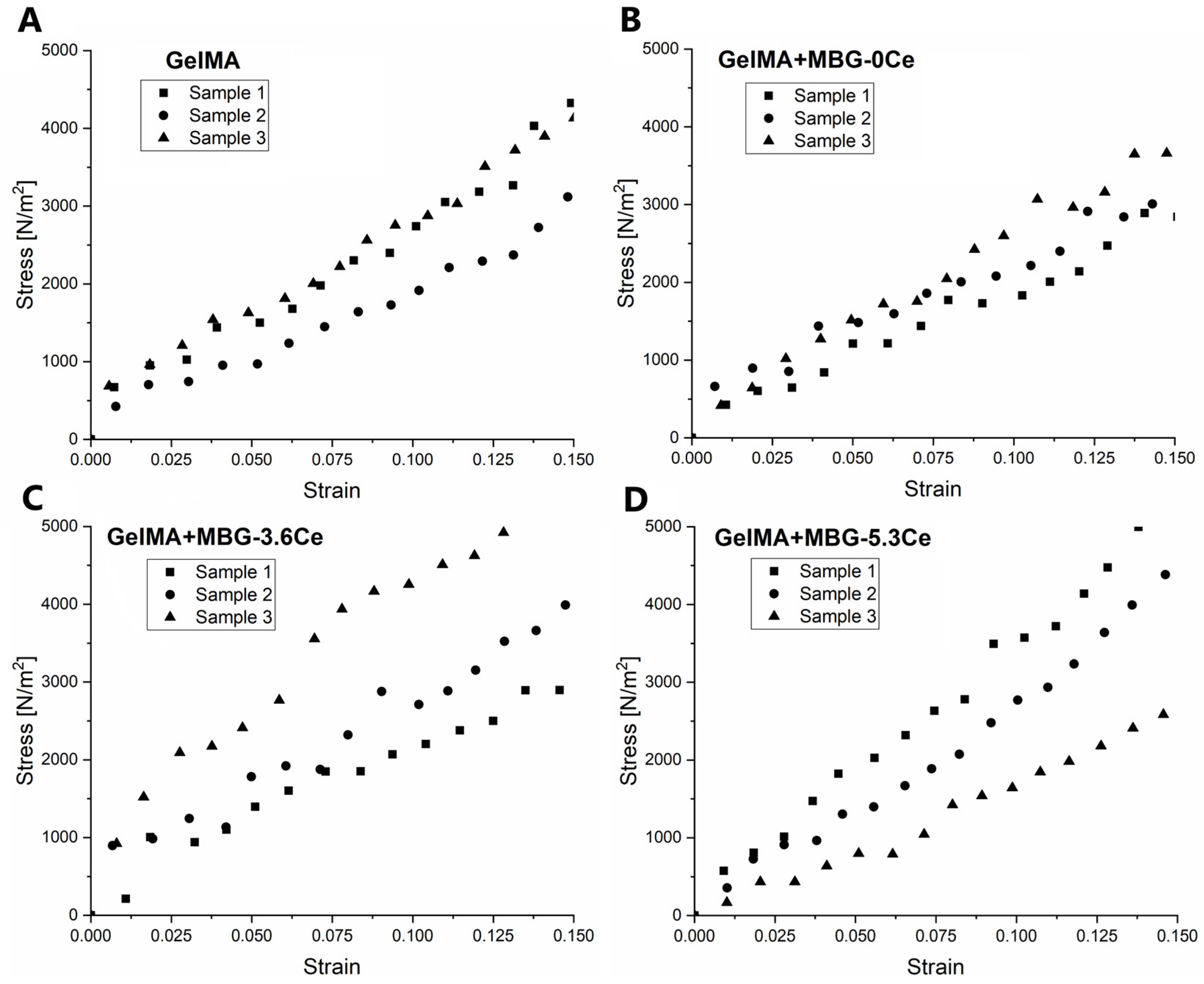
2.1.2. Swelling and Mechanical Properties of Composites
2.1.3. Stability and Bioactivity of Composites
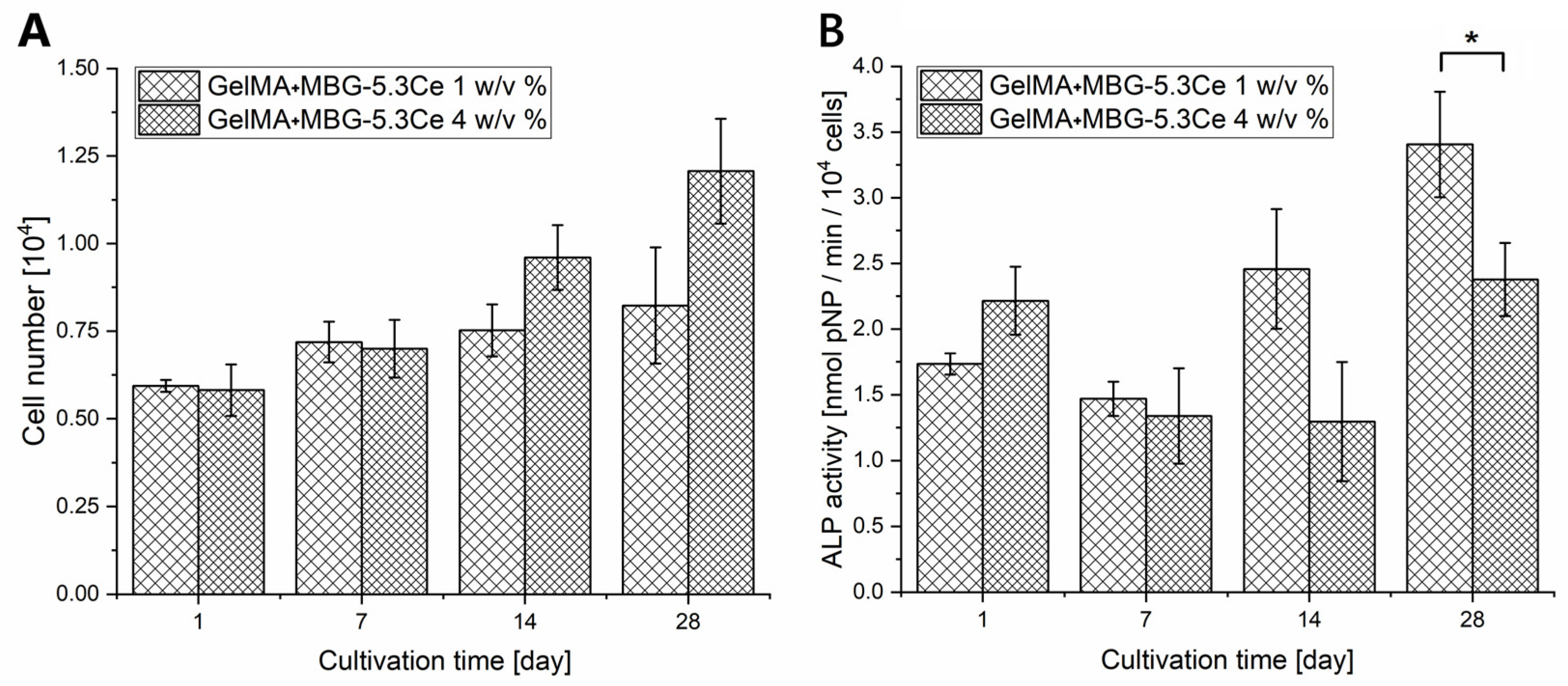
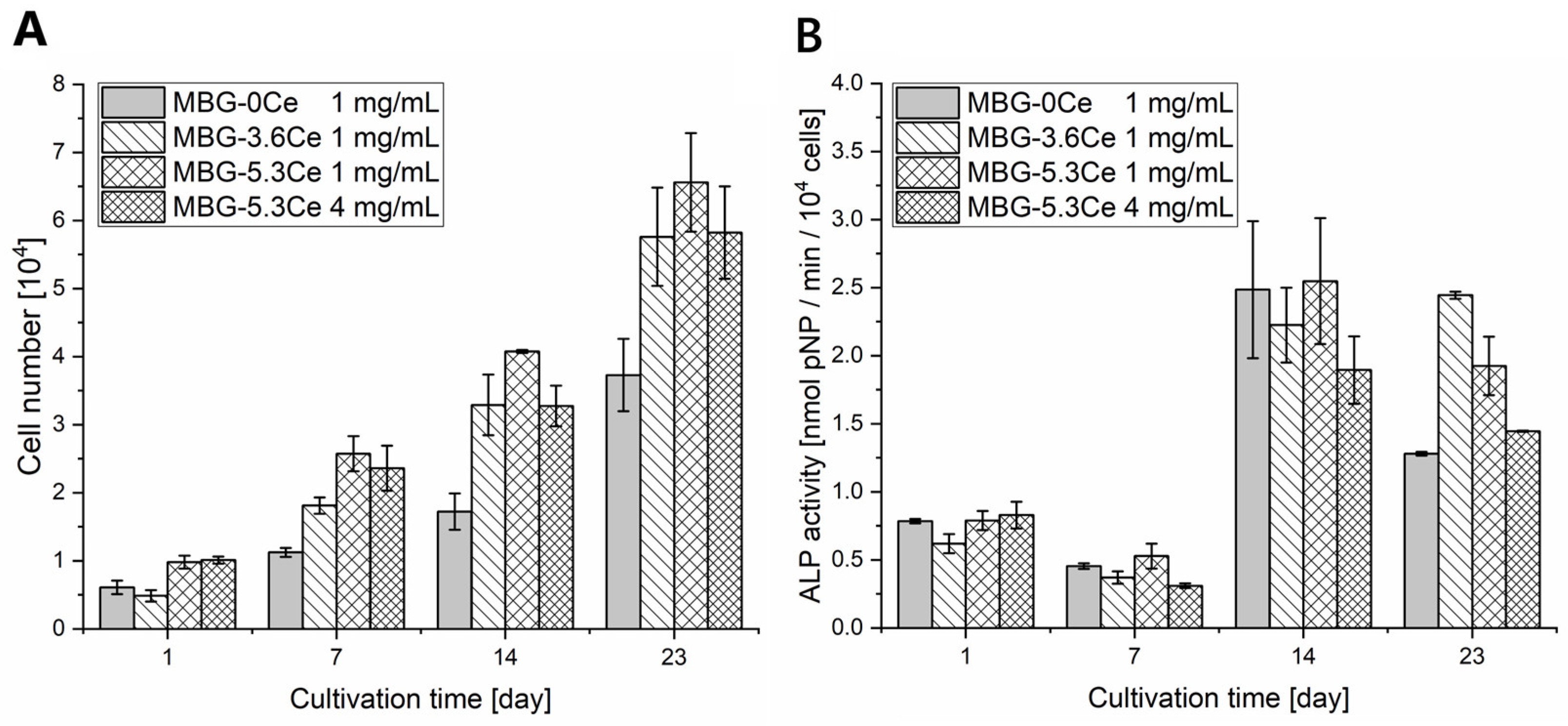
2.1.4. Cell Proliferation and Differentiation
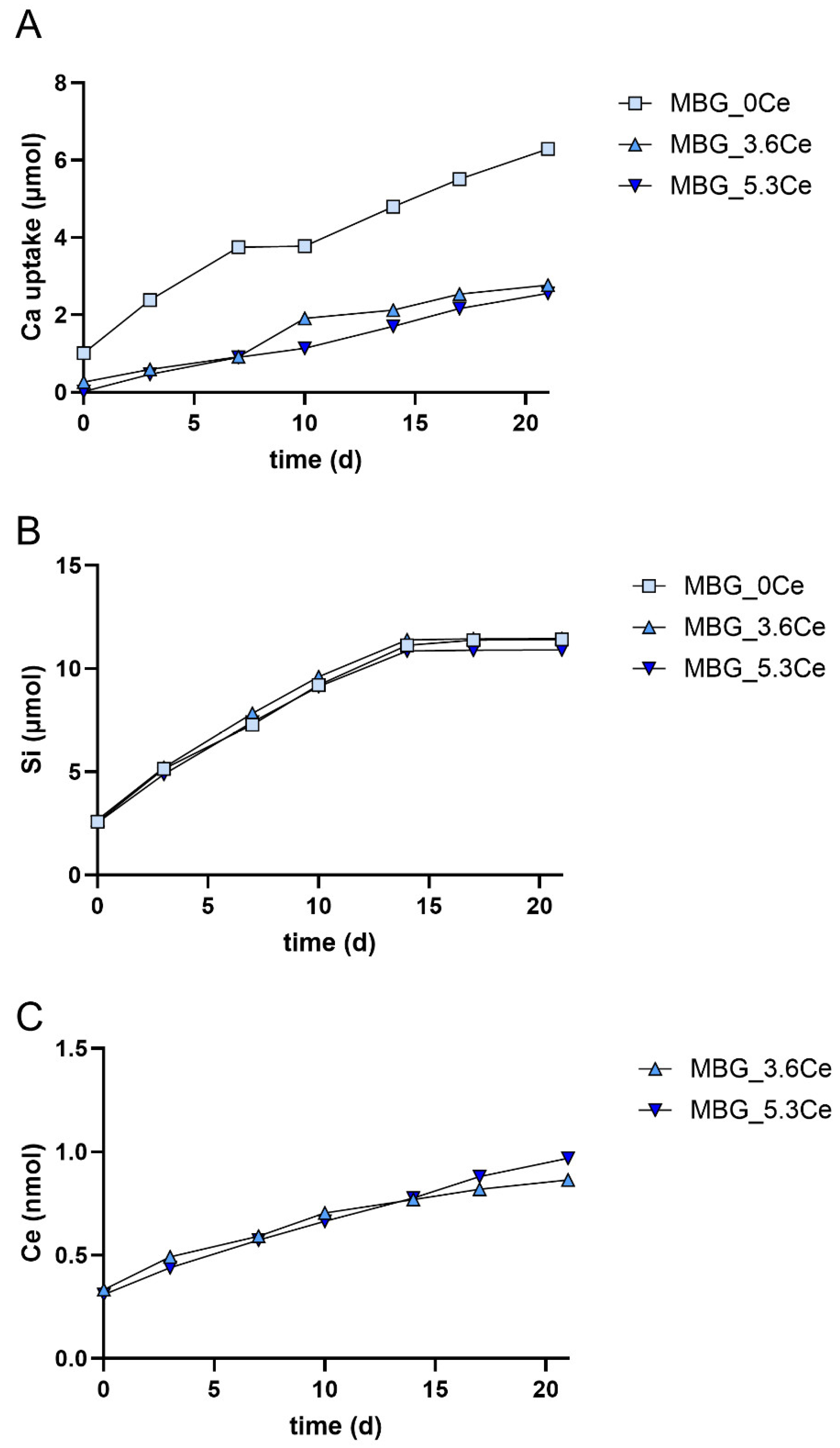
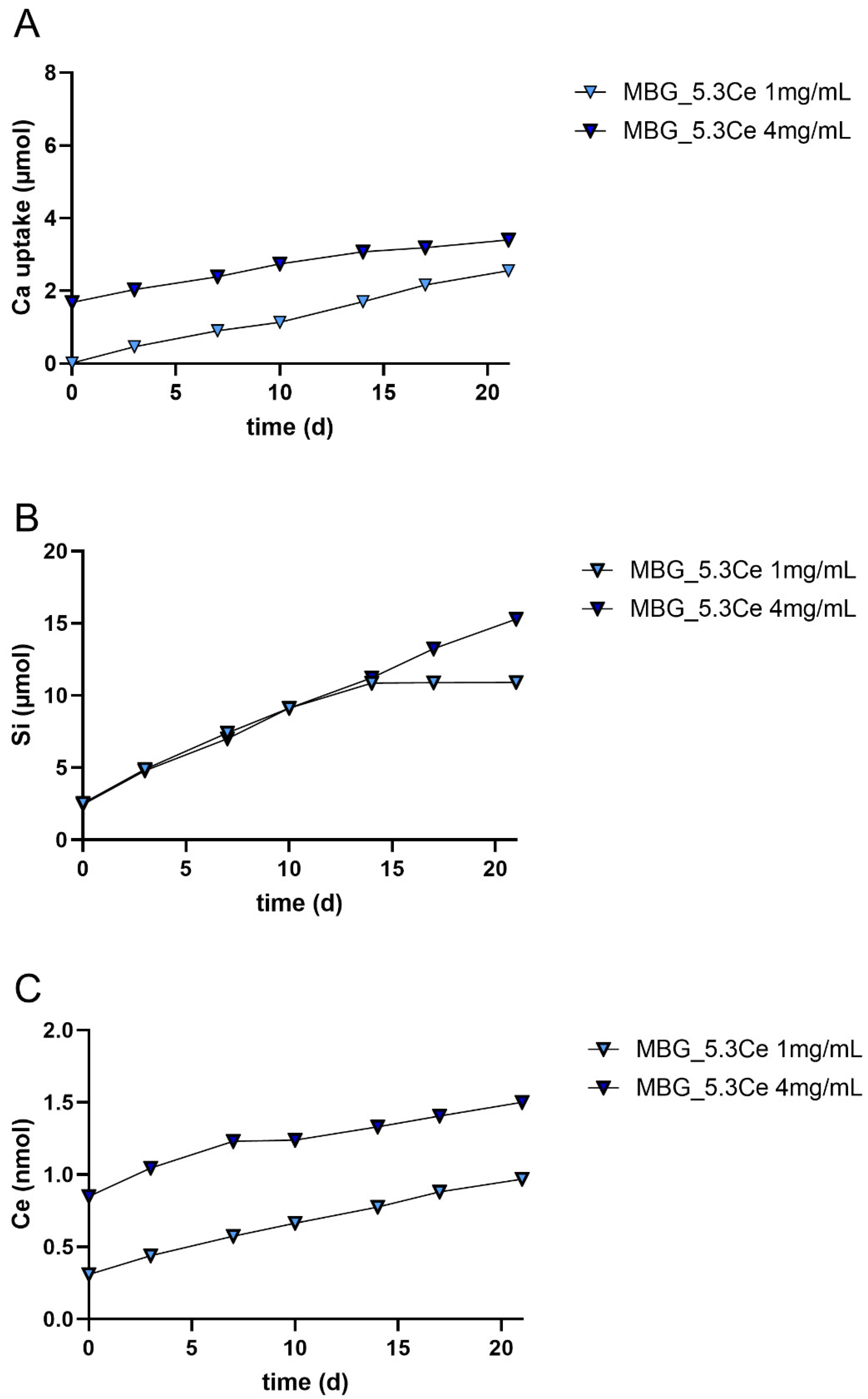
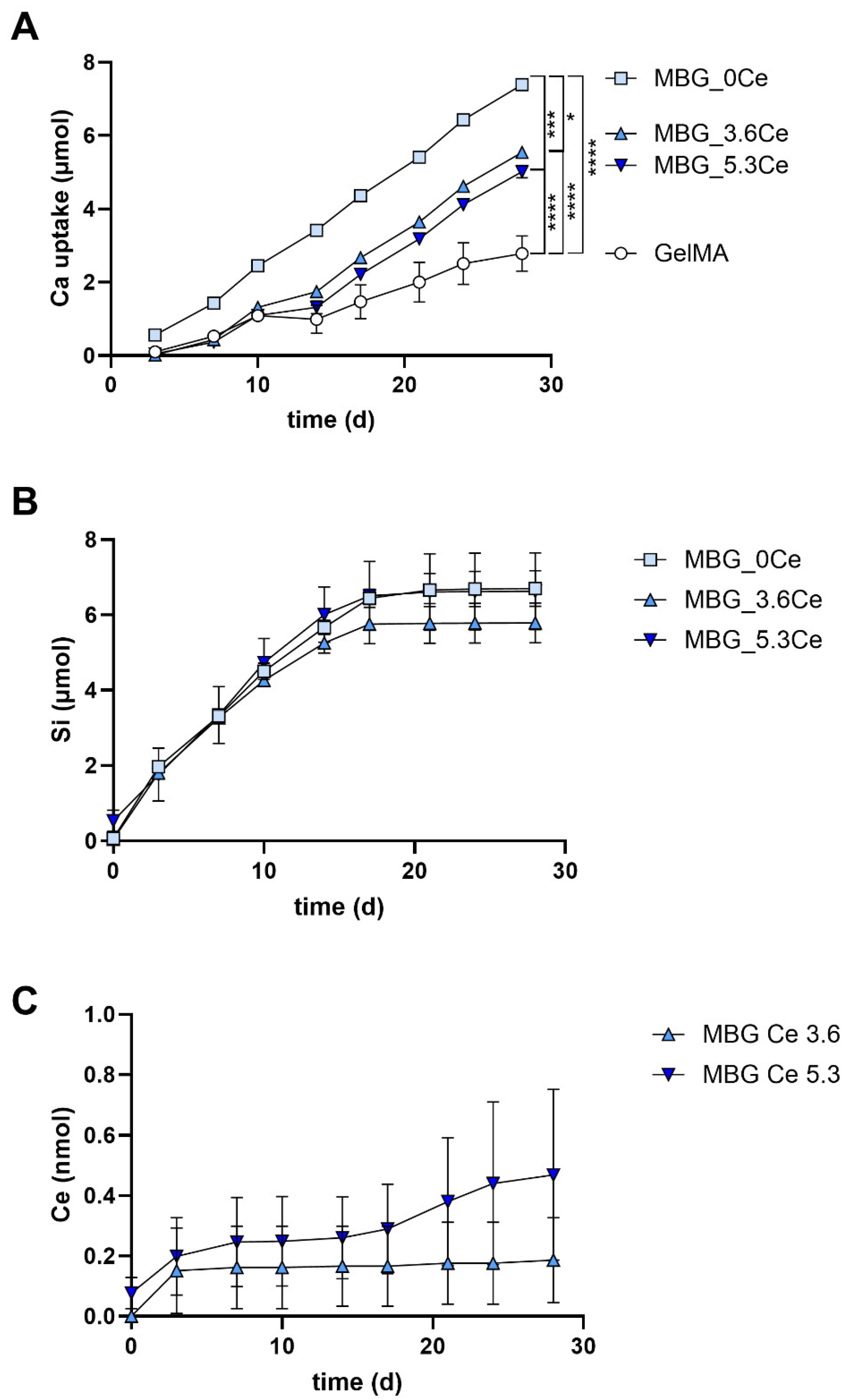
2.1.5. Release and Uptake of Ions from Composites During Cell Culture
2.2. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Composites
4.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDX)
4.4. Swelling Properties
4.5. Mechanical Characterization
4.6. Stability and Bioactivity of the Composites in PBS and Cell Culture Media
4.7. Cell Culture Experiments
4.7.1. Indirect Cell Culture
4.7.2. Direct Cell Culture
4.8. Ion Release Profiles by ICP-OES
4.9. Colorimetric Measurements
4.10. Immunofluorescence Staining of f-Actin and Nuclei
4.11. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BGs | bioactive glasses |
| BGNs | bioactive glass nanoparticles |
| Ce-MBGs | cerium-doped mesoporous BG microparticles |
| CCM | cell culture medium |
| EDX | energy-dispersive X-ray spectroscopy |
| ICP-OES | inductively coupled plasma–optical emission spectrometry |
| IDPs | ion dissolution products |
| GelMA | methacrylated gelatin |
| hBMSCs | human mesenchymal stem cells |
| OBs | osteoblasts |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
Appendix A



References
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45 (Suppl. 2), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, H.; Tian, Y.; Xie, Y.; Li, A.; Ji, L.; Niu, Z.; Wu, D.; Qiu, D. Bioactive nanoparticle-gelatin composite scaffold with mechanical performance comparable to cancellous bones. ACS Appl. Mater. Interfaces 2014, 6, 13061–13068. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. A 2018, 106, 201–209. [Google Scholar] [CrossRef]
- Fang, X.; Xie, J.; Zhong, L.; Li, J.; Rong, D.; Li, X.; Ouyang, J. Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1070–1080. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Selective Contribution of Bioactive Glasses to Molecular and Cellular Pathways. ACS Biomater. Sci. Eng. 2020, 6, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.-W.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of catalase mimetic activity in Ce3+/Ce4+ doped bioactive glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef]
- Varini, E.; Sánchez-Salcedo, S.; Malavasi, G.; Lusvardi, G.; Vallet-Regí, M.; Salinas, A.J. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: Bioactivity, biocompatibility and reactive oxygen species activity. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 105, 109971. [Google Scholar] [CrossRef]
- Zambon, A.; Malavasi, G.; Pallini, A.; Fraulini, F.; Lusvardi, G. Cerium Containing Bioactive Glasses: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4388–4401. [Google Scholar] [CrossRef]
- Lusvardi, G.; Fraulini, F.; D’Addato, S.; Zambon, A. Loading with Biomolecules Modulates the Antioxidant Activity of Cerium-Doped Bioactive Glasses. ACS Biomater. Sci. Eng. 2022, 8, 2890–2898. [Google Scholar] [CrossRef]
- Westhauser, F.; Rehder, F.; Decker, S.; Kunisch, E.; Moghaddam, A.; Zheng, K.; Boccaccini, A.R. Ionic dissolution products of Cerium-doped bioactive glass nanoparticles promote cellular osteogenic differentiation and extracellular matrix formation of human bone marrow derived mesenchymal stromal cells. Biomed. Mater. 2021, 16, 35028. [Google Scholar] [CrossRef]
- Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2022, 14, 23. [Google Scholar] [CrossRef]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: Enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mater. Sci. 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Gao, C.; Gao, Q.; Li, Y.; Rahaman, M.N.; Teramoto, A.; Abe, K. In vitro evaluation of electrospun gelatin-bioactive glass hybrid scaffolds for bone regeneration. J. Appl. Polym. Sci. 2013, 127, 2588–2599. [Google Scholar] [CrossRef]
- Mostajeran, H.; Baheiraei, N.; Bagheri, H. Effects of cerium-doped bioactive glass incorporation on an alginate/gelatin scaffold for bone tissue engineering: In vitro characterizations. Int. J. Biol. Macromol. 2024, 255, 128094. [Google Scholar] [CrossRef]
- Lu, B.; Zhu, D.-Y.; Yin, J.-H.; Xu, H.; Zhang, C.-Q.; Ke, Q.-F.; Gao, Y.-S.; Guo, Y.-P. Incorporation of cerium oxide in hollow mesoporous bioglass scaffolds for enhanced bone regeneration by activating the ERK signaling pathway. Biofabrication 2019, 11, 25012. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, F.; Zhang, W.; Mo, Y.; Zeng, L.; Li, X.; Chen, X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 89, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Akhtar, M.; Peng, P.; Bernhardt, A.; Gelinsky, M.; Ur Rehman, M.A.; Boccaccini, A.R.; Basu, B. Gelatin Methacryloyl (GelMA)—45S5 Bioactive Glass (BG) Composites for Bone Tissue Engineering: 3D Extrusion Printability and Cytocompatibility Assessment Using Human Osteoblasts. ACS Biomater. Sci. Eng. 2024, 10, 5122–5135. [Google Scholar] [CrossRef]
- Heinemann, C.; Buchner, F.; Lee, P.S.; Bernhardt, A.; Kruppke, B.; Wiesmann, H.-P.; Hintze, V. Effects of Gamma Irradiation and Supercritical Carbon Dioxide Sterilization on Methacrylated Gelatin/Hyaluronan Hydrogels. J. Funct. Biomater. 2023, 14, 317. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Ge, X.; Qi, X.; Xiang, Y.; Shi, Y.; Li, Y.; Pan, Y.; Wang, Y.; Ru, Y.; et al. Ferric Iron/Shikonin Nanoparticle-Embedded Hydrogels with Robust Adhesion and Healing Functions for Treating Oral Ulcers in Diabetes. Adv. Sci. 2024, 11, e2405463. [Google Scholar] [CrossRef]
- Ai, Y.; Dai, F.; Li, W.; Xu, F.; Yang, H.; Wu, J.; Yang, K.; Li, L.; Ai, F.; Song, L. Photo-crosslinked bioactive BG/BMSCs@GelMA hydrogels for bone-defect repairs. Mater. Today Bio. 2023, 23, 100882. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Fathi, A.; Razak, N.A.B.A.; Kadri, N.A.; Sheikhi, A.; Baino, F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021, 136, 1–36. [Google Scholar] [CrossRef]
- Qazi, T.H.; Hafeez, S.; Schmidt, J.; Duda, G.N.; Boccaccini, A.R.; Lippens, E. Comparison of the effects of 45S5 and 1393 bioactive glass microparticles on hMSC behavior. J. Biomed. Mater. Res. A 2017, 105, 2772–2782. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Li, Y.; Sun, J.; Wang, P.; Di, K.; Zhao, Y. Effect of cerium ion on the proliferation, differentiation and mineralization function of primary mouse osteoblasts in vitro. J. Rare Earths 2010, 28, 138–142. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Xiao, Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Reis, S.; Castro, A.; Fernandes, M.H. Bone Anabolic Effects of Soluble Si: In Vitro Studies with Human Mesenchymal Stem Cells and CD14+ Osteoclast Precursors. Stem Cells Int. 2016, 2016, 5653275. [Google Scholar] [CrossRef] [PubMed]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Mahajan, A.; Alexander, L.S.; Seabolt, B.S.; Catrambone, D.E.; McClung, J.P.; Odle, J.; Pfeiler, T.W.; Loboa, E.G.; Stahl, C.H. Dietary calcium restriction affects mesenchymal stem cell activity and bone development in neonatal pigs. J. Nutr. 2011, 141, 373–379. [Google Scholar] [CrossRef]
- González-Vázquez, A.; Planell, J.A.; Engel, E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014, 10, 2824–2833. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T.; Sasaki, J.-I.; Egusa, H.; Lee, K.Y.; Nakano, T.; Sohmura, T.; Nakahira, A. Effect of calcium ion concentrations on osteogenic differentiation and hematopoietic stem cell niche-related protein expression in osteoblasts. Tissue Eng. Part A 2010, 16, 2467–2473. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Franco, A.; Van Durme, B.; Van Vlierberghe, S.; Dupont-Gillain, C. Misleading Pore Size Measurements in Gelatin and Alginate Hydrogels Revealed by Confocal Microscopy. Tissue Eng. Part C. Methods 2024, 30, 307–313. [Google Scholar] [CrossRef]
- Ben Messaoud, G.; Aveic, S.; Wachendoerfer, M.; Fischer, H.; Richtering, W. 3D Printable Gelatin Methacryloyl (GelMA)-Dextran Aqueous Two-Phase System with Tunable Pores Structure and Size Enables Physiological Behavior of Embedded Cells In Vitro. Small 2023, 19, e2208089. [Google Scholar] [CrossRef]
- Fraulini, F.; Raimondi, S.; Candeliere, F.; Ranieri, R.; Zambon, A.; Lusvardi, G. Ce-MBGs Loaded with Gentamicin: Characterization and In Vitro Evaluation. J. Funct. Biomater. 2023, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Rother, S.; Galiazzo, V.D.; Kilian, D.; Fiebig, K.M.; Becher, J.; Moeller, S.; Hempel, U.; Schnabelrauch, M.; Waltenberger, J.; Scharnweber, D.; et al. Hyaluronan/Collagen Hydrogels with Sulfated Hyaluronan for Improved Repair of Vascularized Tissue Tune the Binding of Proteins and Promote Endothelial Cell Growth. Macromol. Biosci. 2017, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawi, S.; Rother, S.; Halfter, N.; Fiebig, K.M.; Moritz, J.; Moeller, S.; Schnabelrauch, M.; Kirkpatrick, C.J.; Sader, R.; Wiesmann, H.-P.; et al. Covalent linkage of sulfated hyaluronan to the collagen scaffold Mucograft® enhances scaffold stability and reduces proinflammatory macrophage activation in vivo. Bioact. Mater. 2022, 8, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Kroschwald, L.M.; Allerdt, F.; Bernhardt, A.; Rother, S.; Zheng, K.; Maqsood, I.; Halfter, N.; Heinemann, C.; Möller, S.; Schnabelrauch, M.; et al. Artificial Extracellular Matrices Containing Bioactive Glass Nanoparticles Promote Osteogenic Differentiation in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 12819. [Google Scholar] [CrossRef]
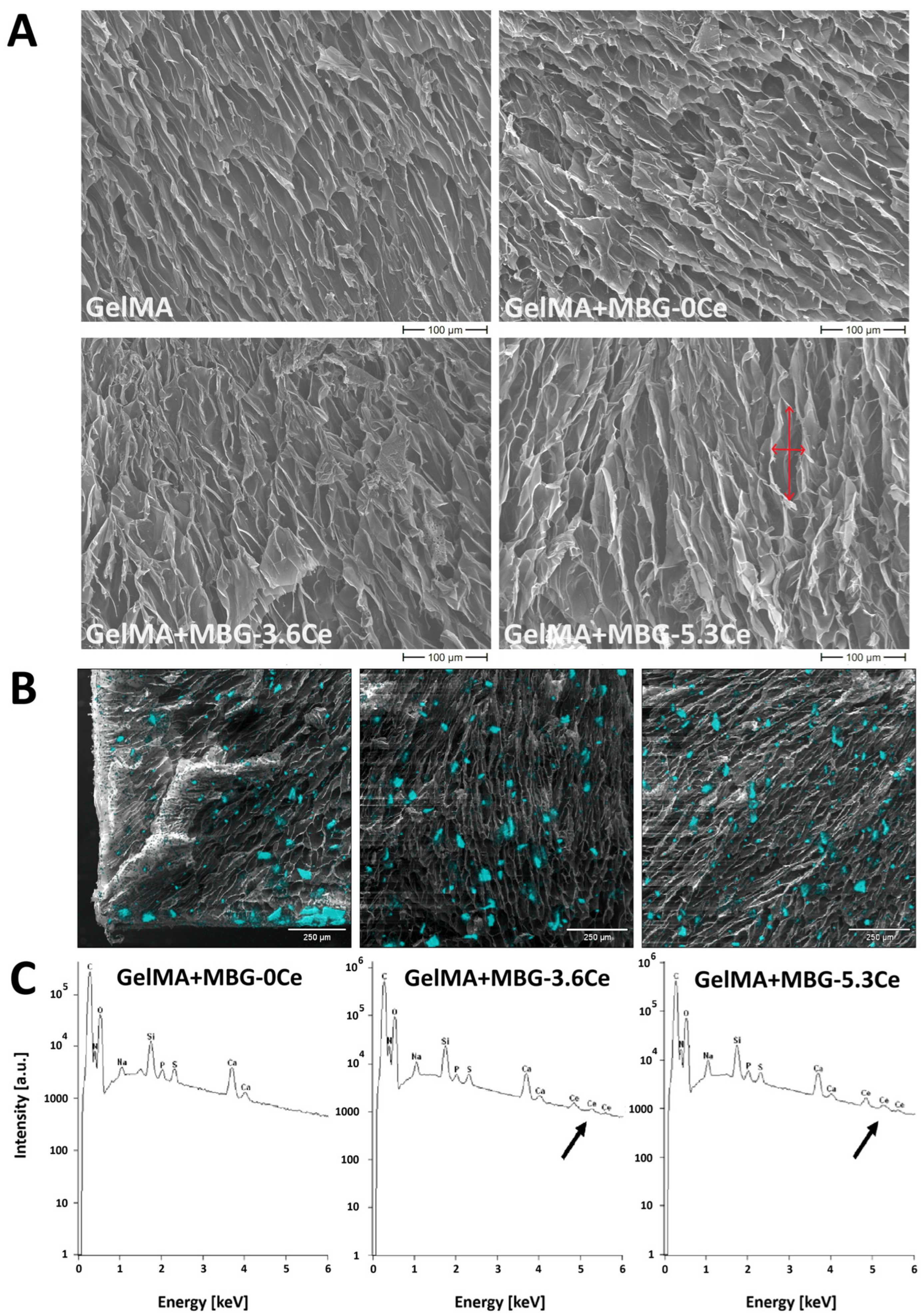
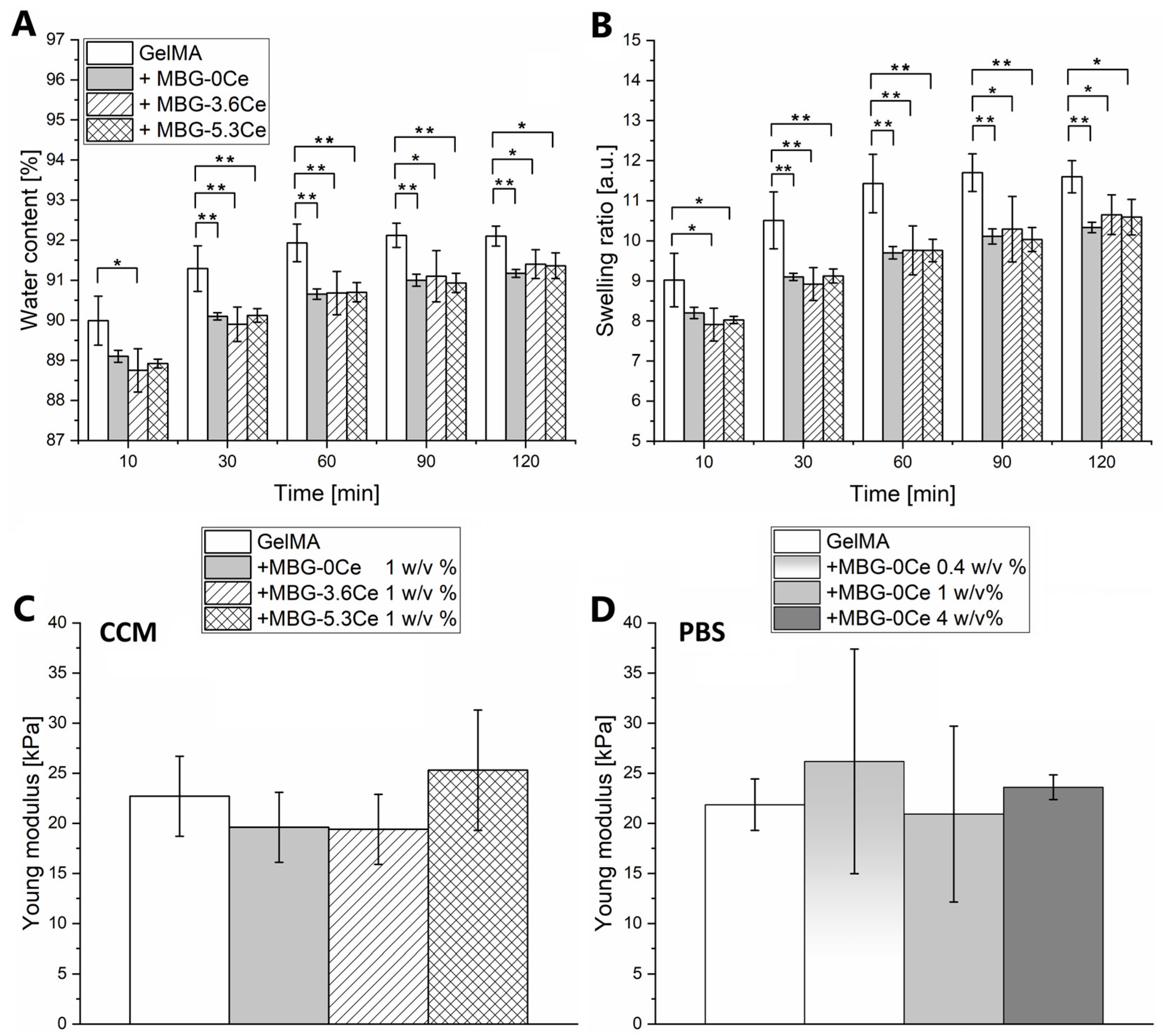
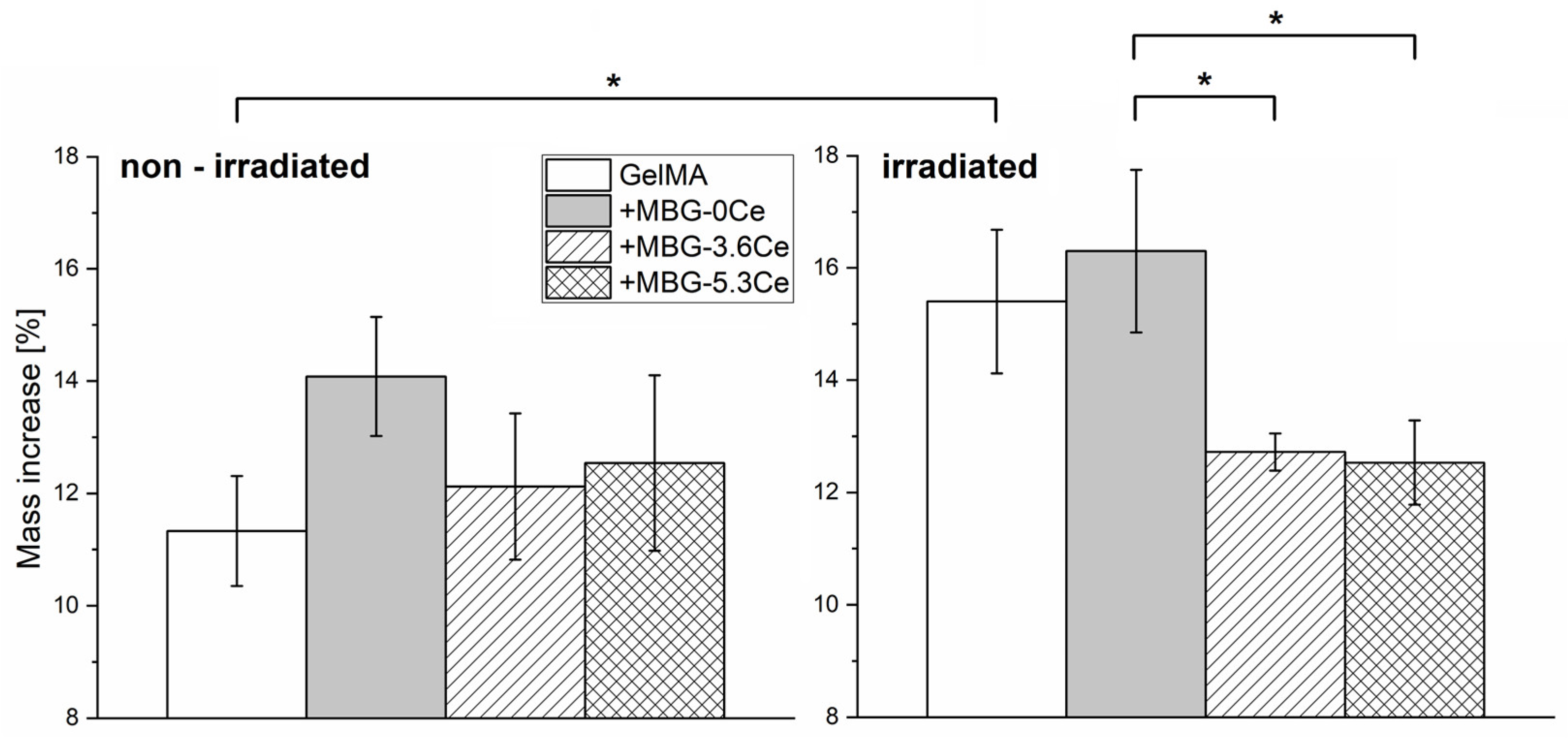

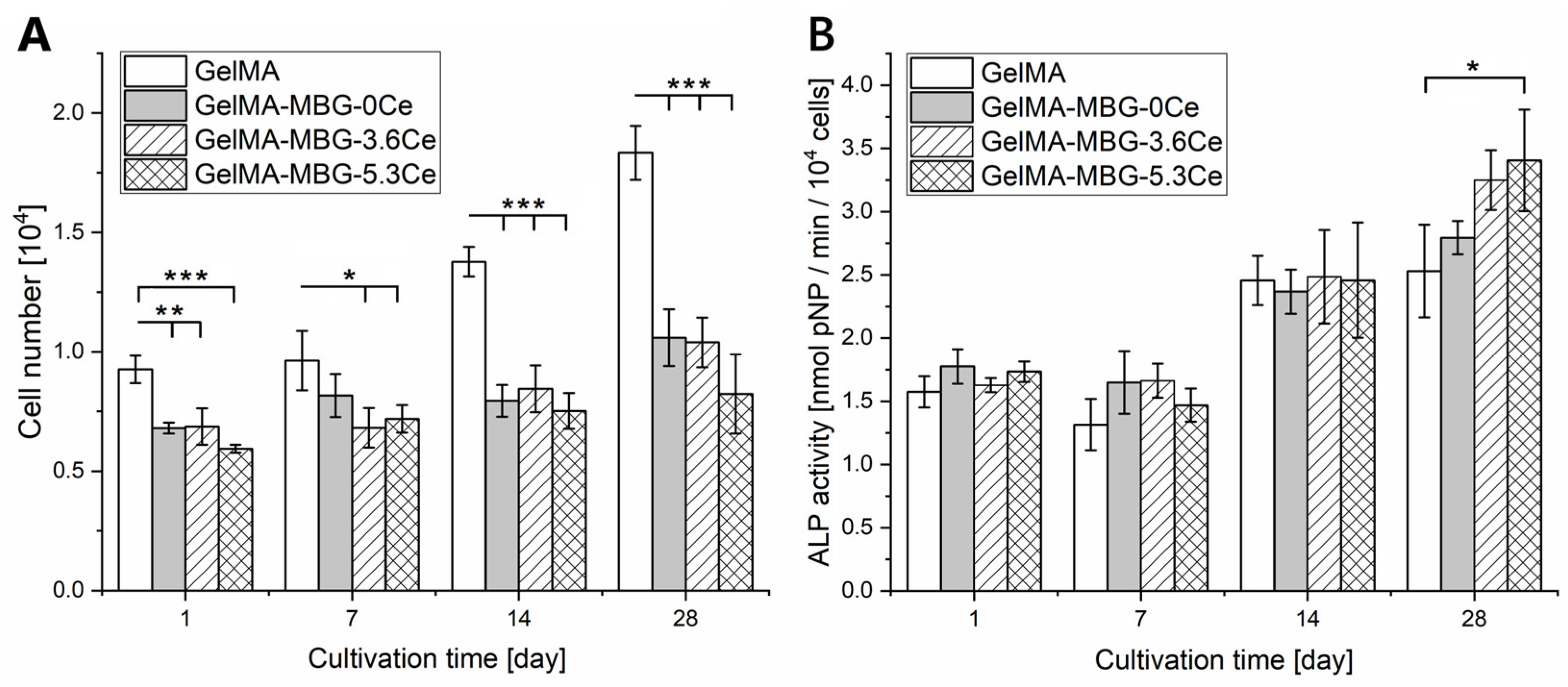
| Glass | SiO2 | CaO | P2O5 | CeO2 |
|---|---|---|---|---|
| MBG-0Ce | 80.0 | 15.0 | 5.0 | - |
| MBG-3.6Ce | 77.1 | 14.5 | 4.8 | 3.6 |
| MBG-5.3Ce | 75.8 | 14.2 | 4.7 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iodchik, A.; Lusvardi, G.; Zambon, A.; Lee, P.S.; Wiesmann, H.-P.; Bernhardt, A.; Hintze, V. Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs). Gels 2025, 11, 425. https://doi.org/10.3390/gels11060425
Iodchik A, Lusvardi G, Zambon A, Lee PS, Wiesmann H-P, Bernhardt A, Hintze V. Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs). Gels. 2025; 11(6):425. https://doi.org/10.3390/gels11060425
Chicago/Turabian StyleIodchik, Andrey, Gigliola Lusvardi, Alfonso Zambon, Poh Soo Lee, Hans-Peter Wiesmann, Anne Bernhardt, and Vera Hintze. 2025. "Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs)" Gels 11, no. 6: 425. https://doi.org/10.3390/gels11060425
APA StyleIodchik, A., Lusvardi, G., Zambon, A., Lee, P. S., Wiesmann, H.-P., Bernhardt, A., & Hintze, V. (2025). Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs). Gels, 11(6), 425. https://doi.org/10.3390/gels11060425












