Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints
Abstract
1. Introduction
2. Results and Discussion
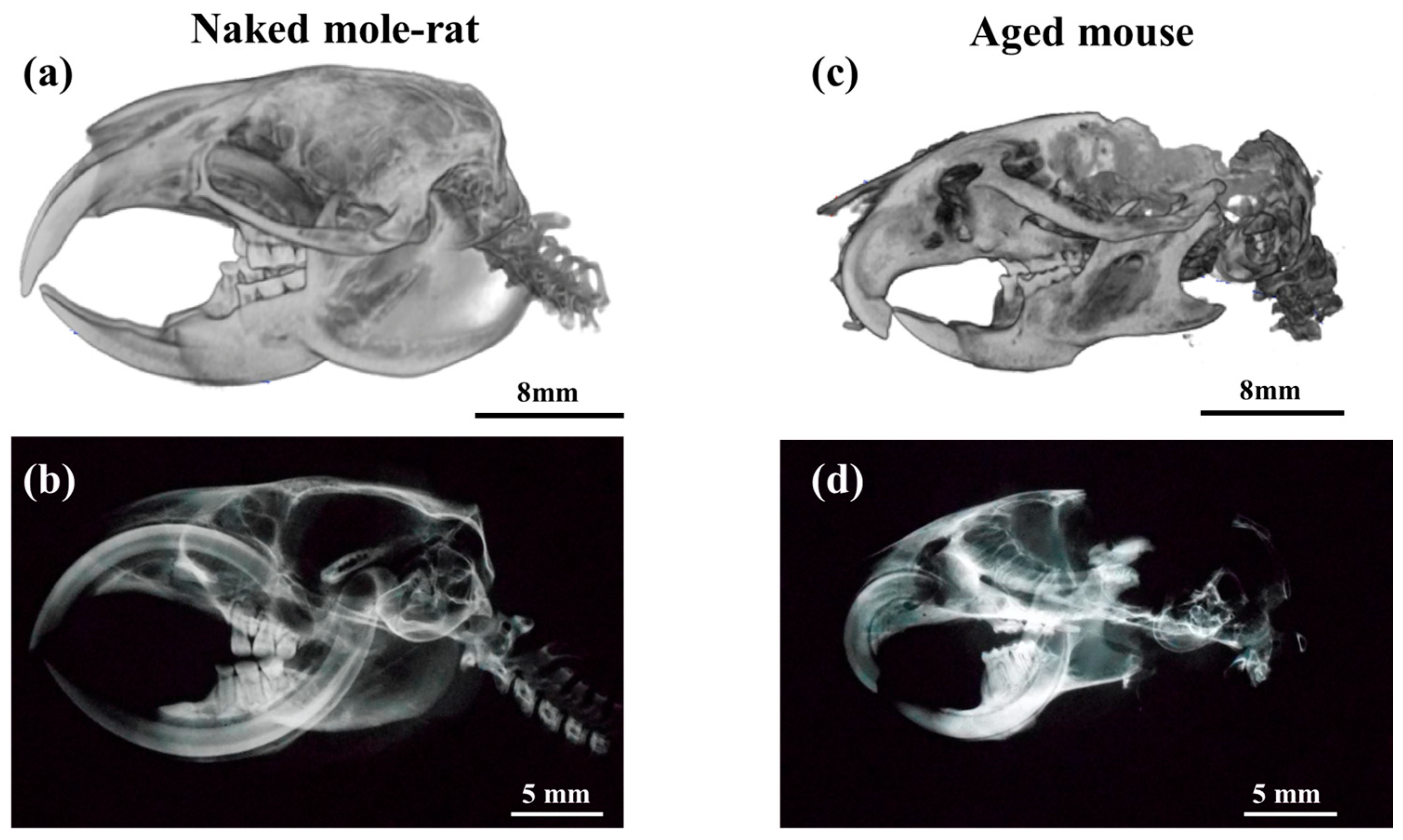
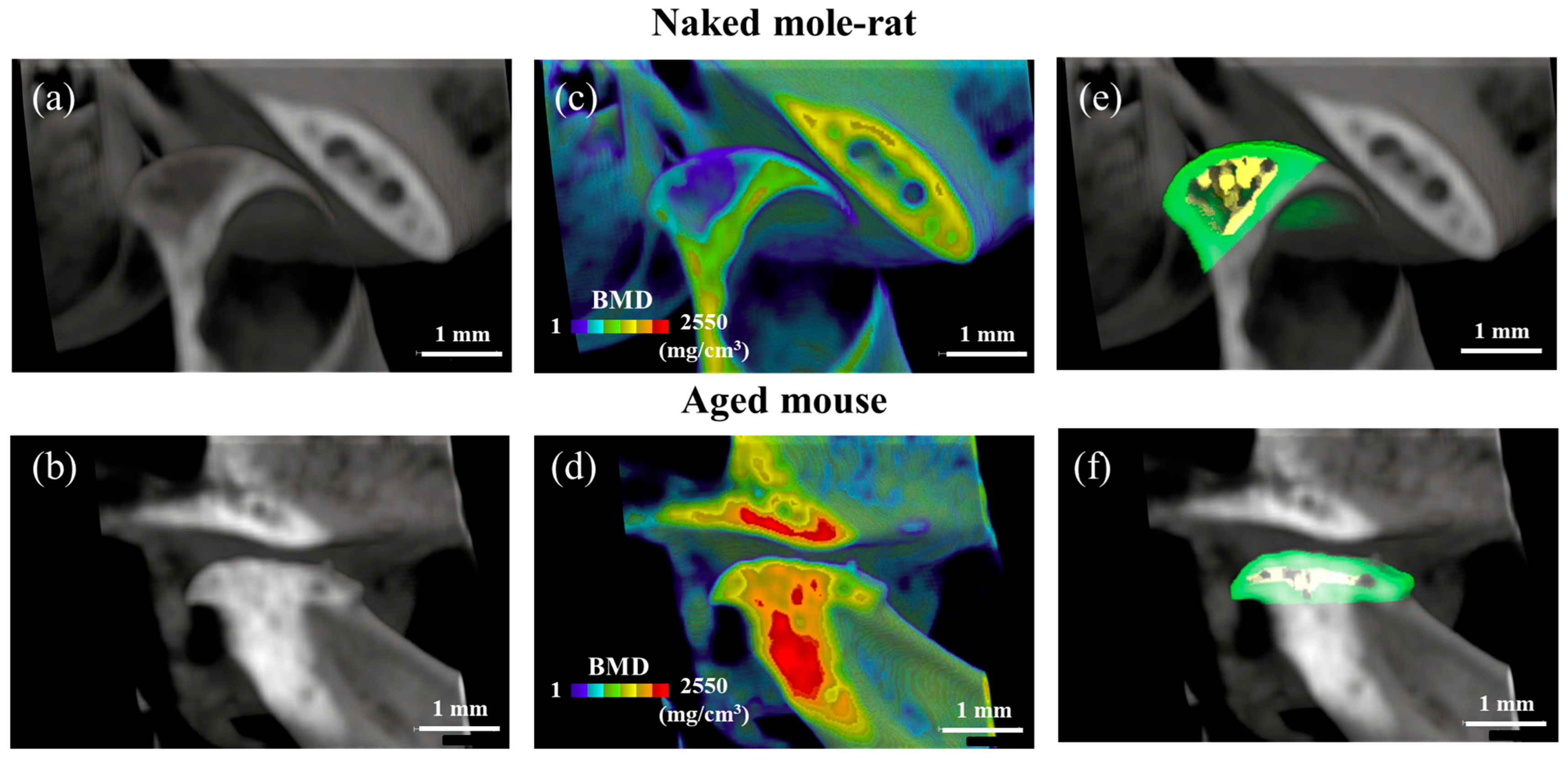
2.1. Comparative Micro-CT Analysis Reveals Age-Related Degeneration in Mouse TMJs, but Not in Naked Mole-Rats
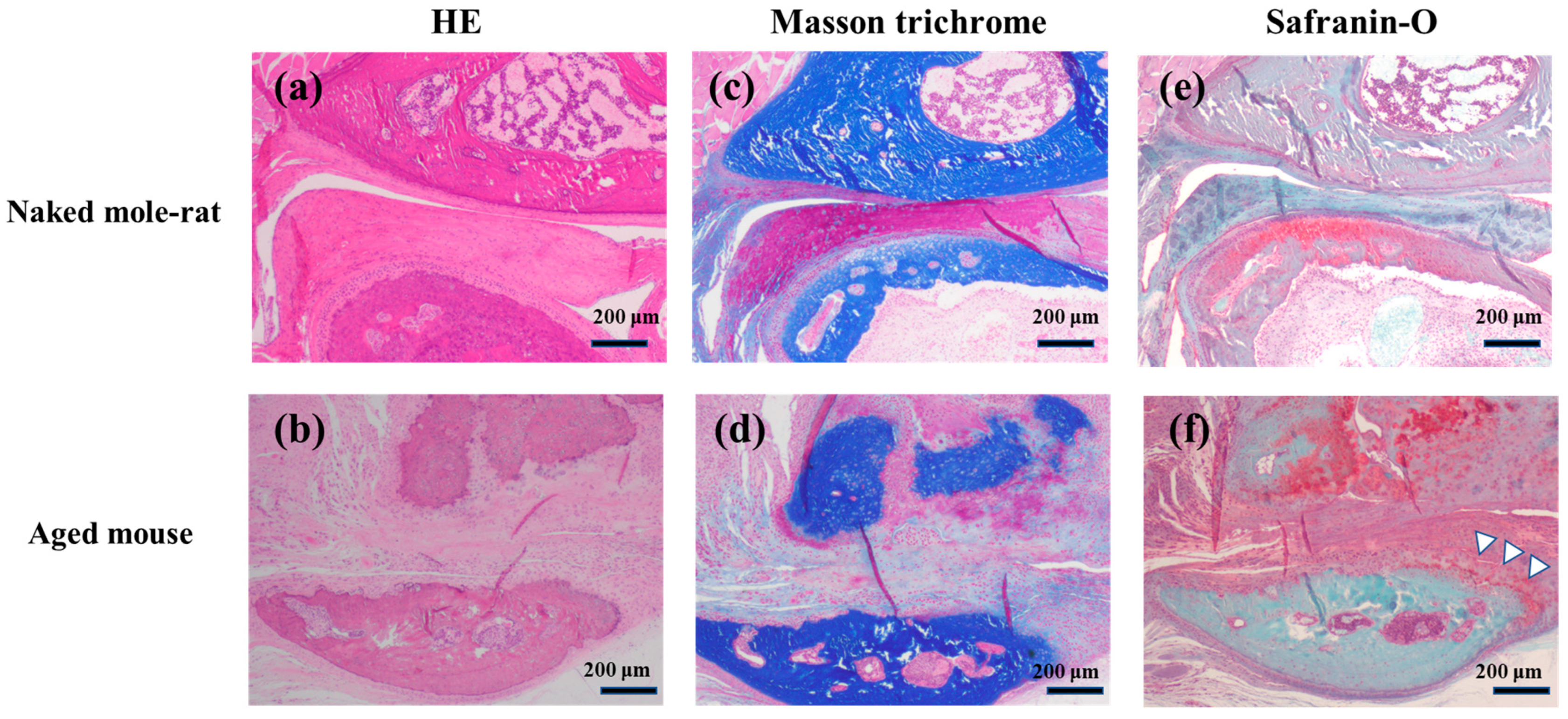
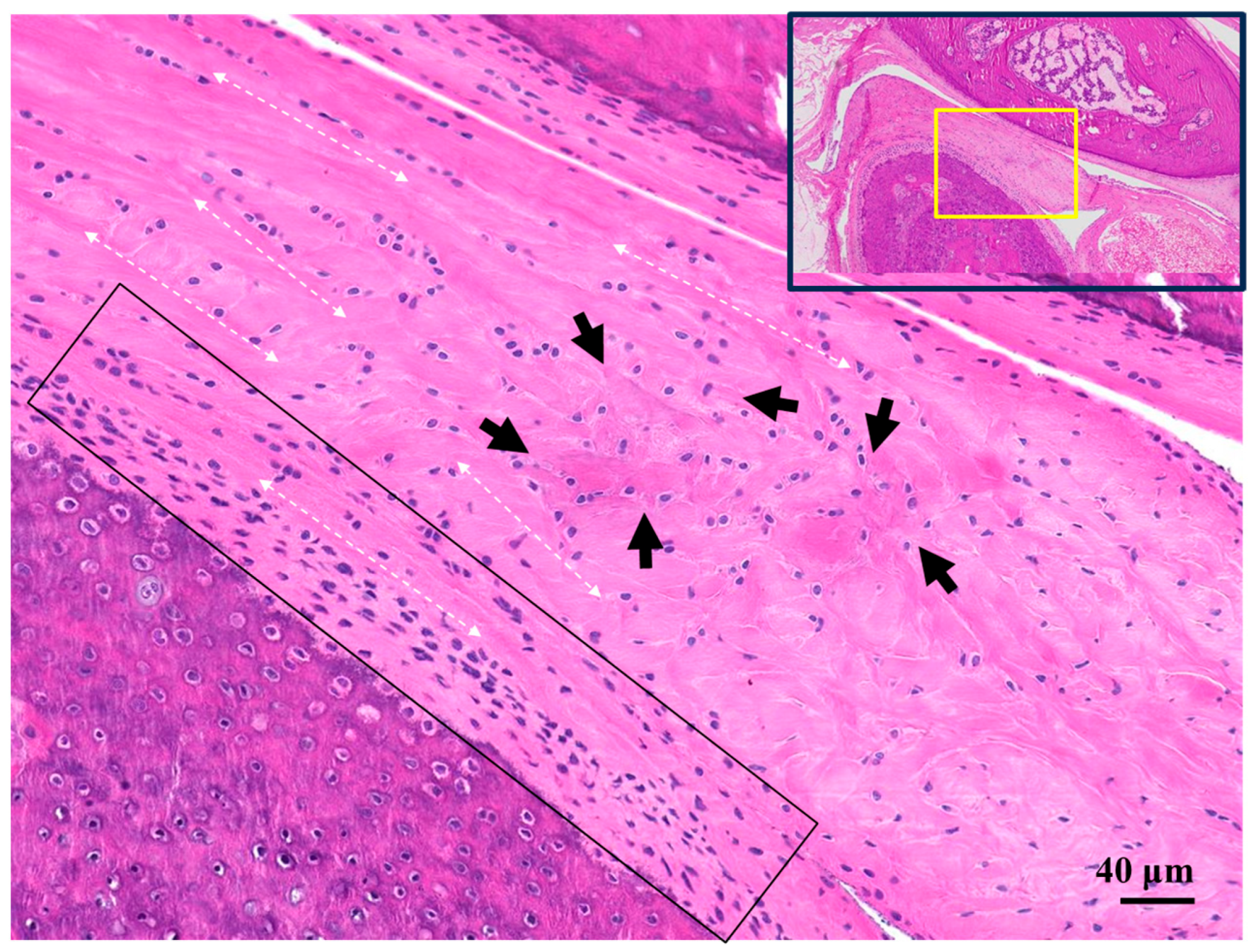
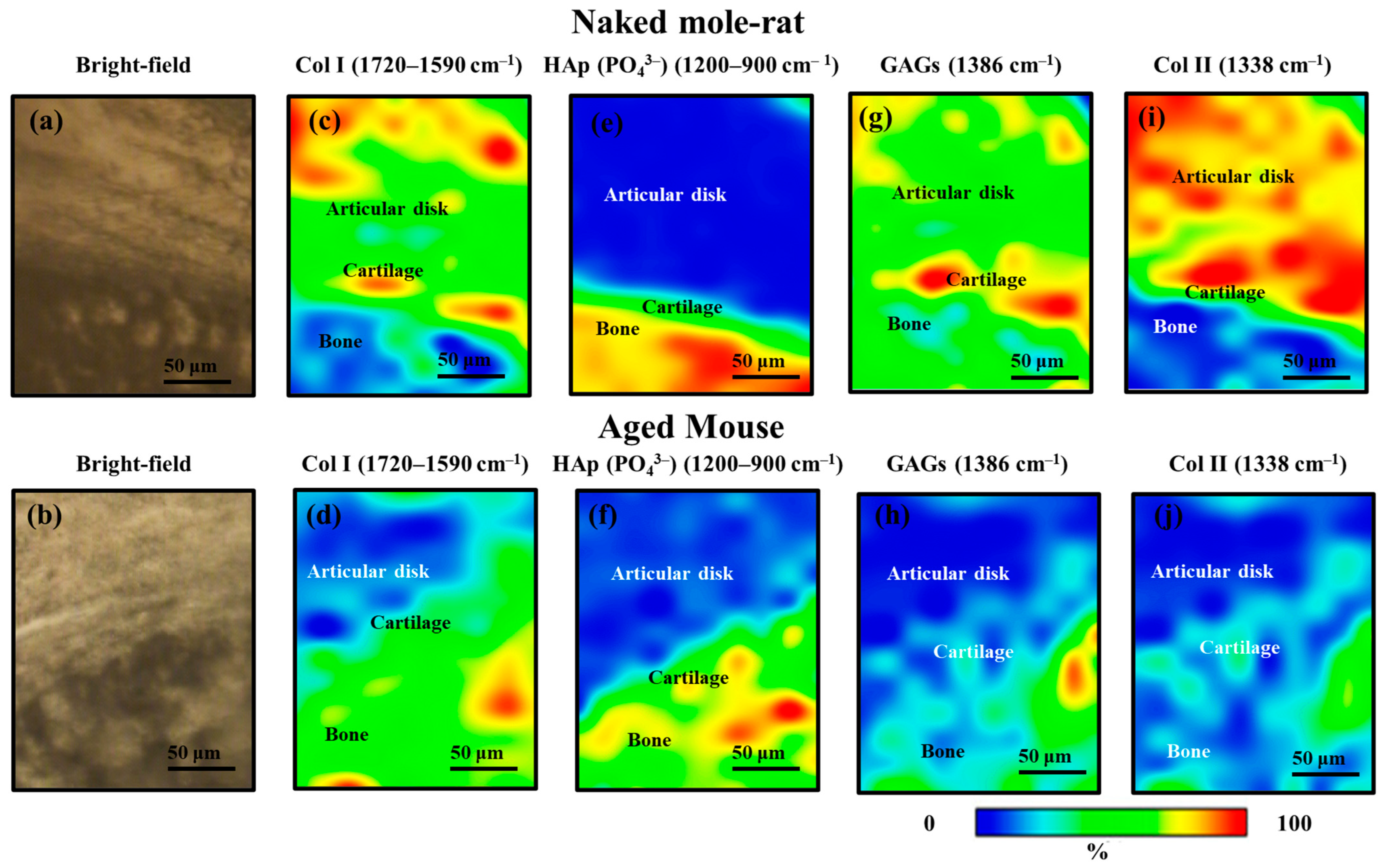
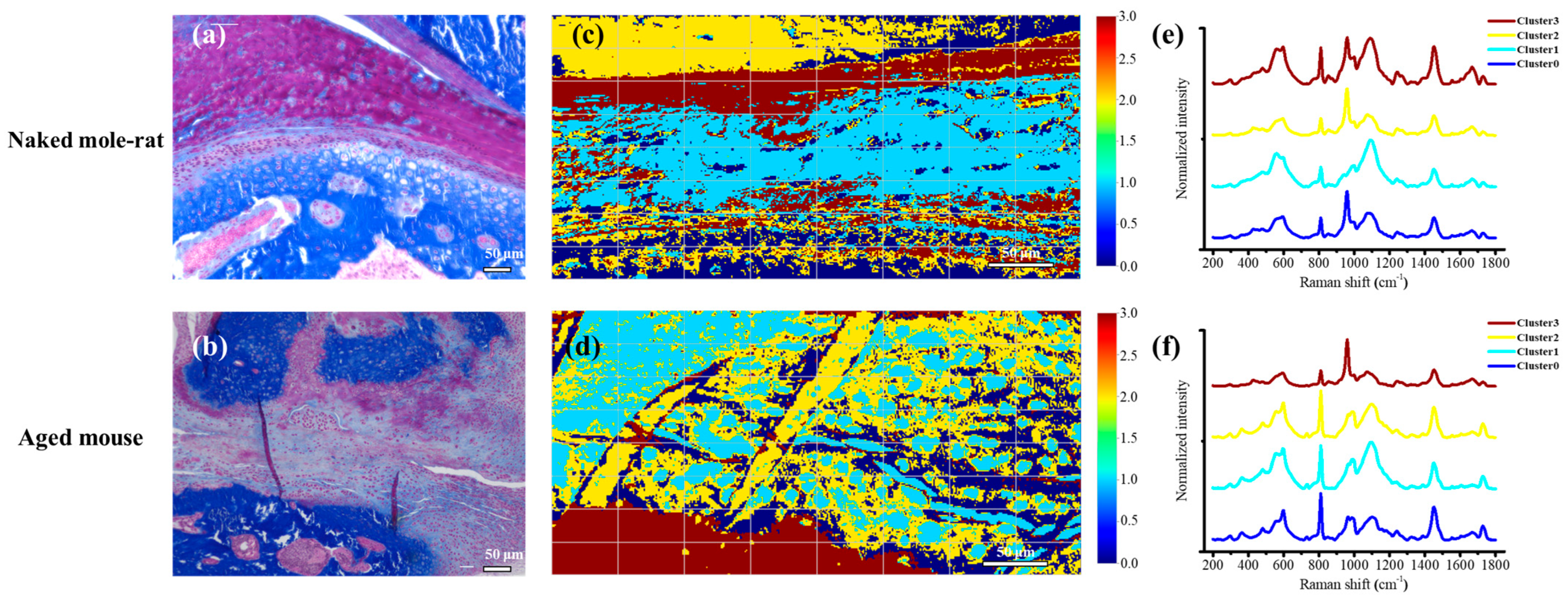
2.2. Histological Findings
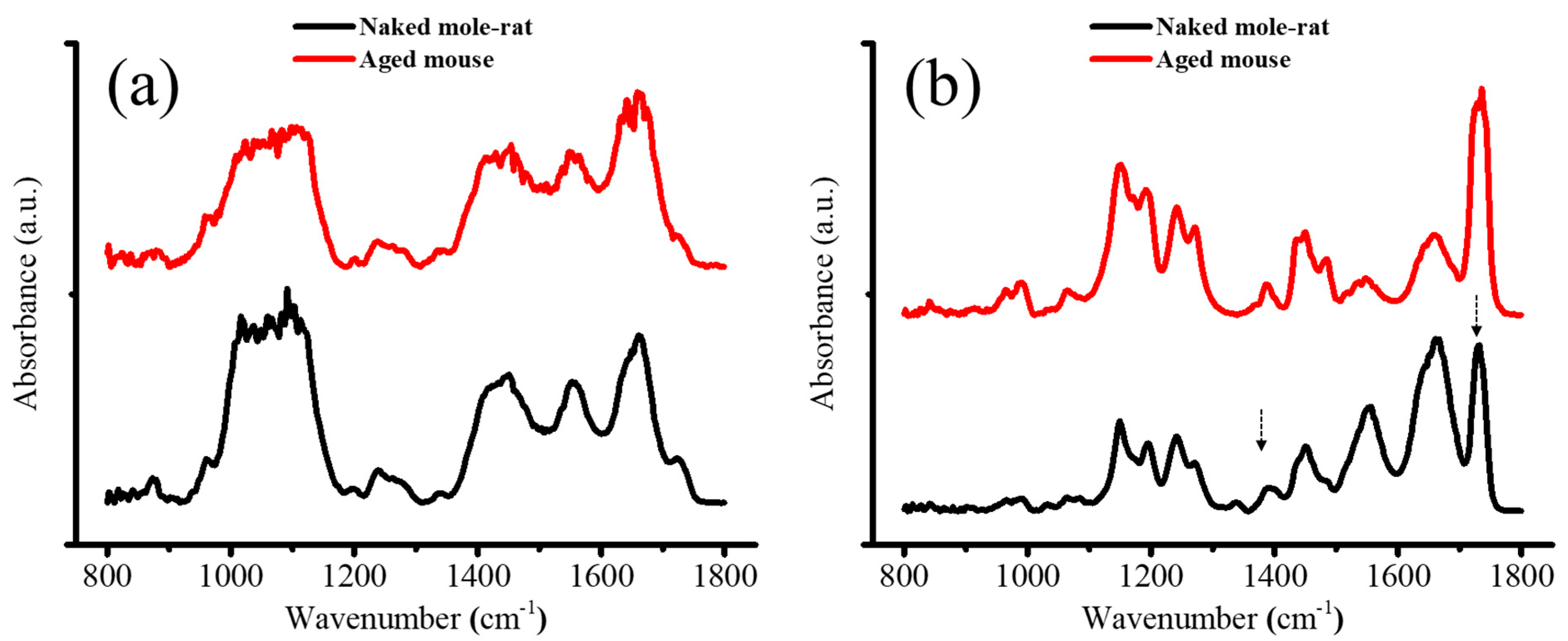
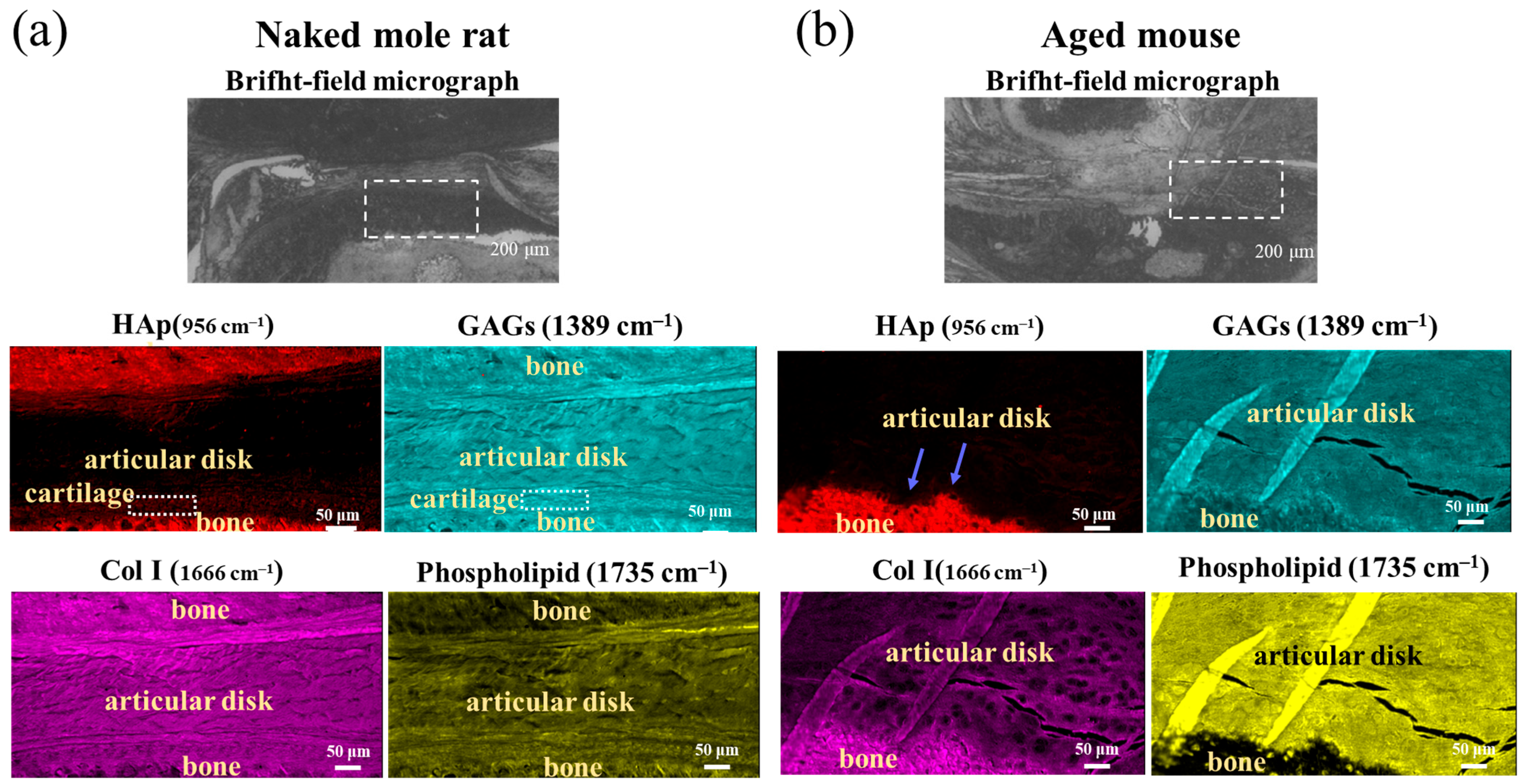
2.3. FTIR
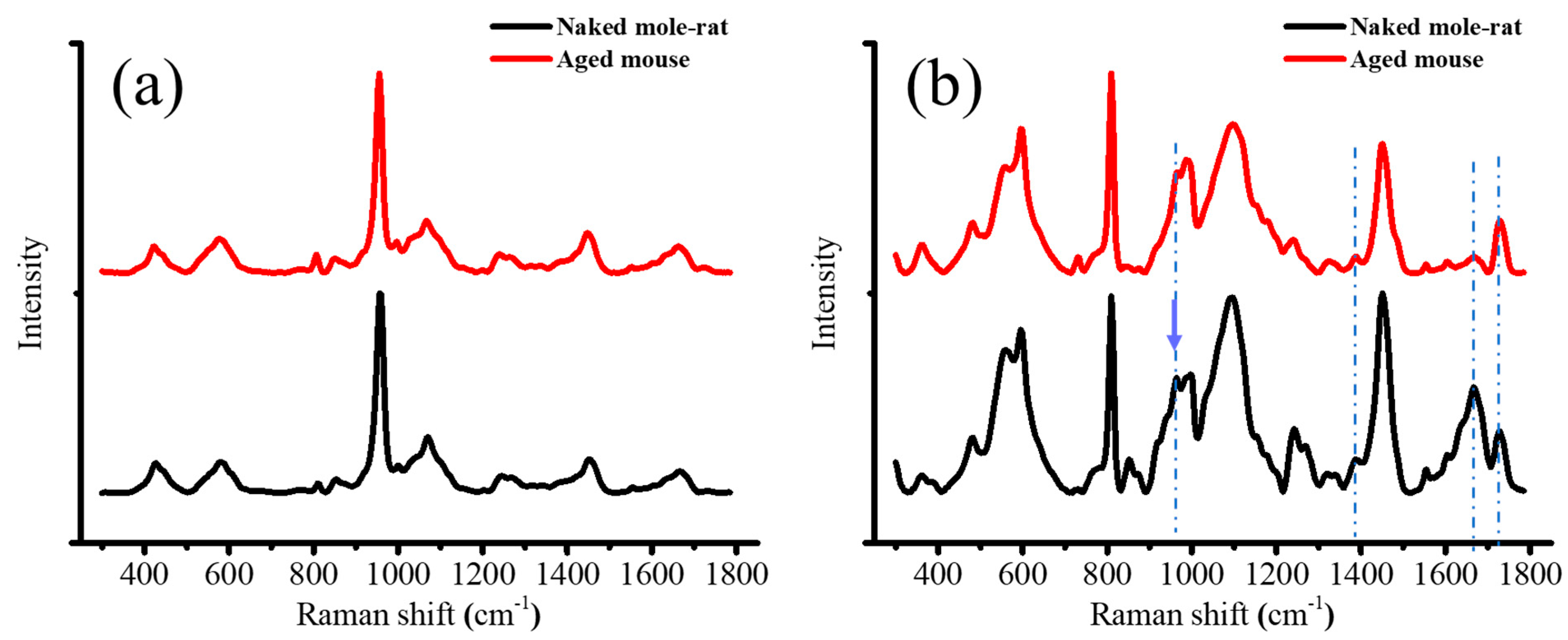
2.4. Raman Spectroscopy
2.5. Chemometric Analysis Using Unsupervised Learning
3. Conclusions
- The mandibular condyle of aged mice was worn down and calcified, and the articular disk disappeared. The morphology of the mandibular condyle and articular disk was maintained in naked mole-rats.
- The articular disk of naked mole-rats had fibers with high expression of HA (cartilage layer) and collagen fibers running in various directions, which reduced occlusal pressure.
4. Materials and Methods
4.1. Animals
4.2. Histochemical Analyses
4.3. Micro-Computed Tomography (CT) Analysis
4.4. Synchrotron Radiation-Based SR-FTIR
4.5. Raman Analyses
4.6. Chemometric Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| CCD | charge-coupled device |
| CT | computed tomography |
| ECM | extracellular matrix |
| GMM | Gaussian mixture model |
| HA | hyaluronic acid |
| HE | hematoxylin and eosin |
| MT | Masson’s trichrome |
| PCA | principal component analysis |
| SAM | senescence-accelerated mice |
| SVD | singular-value decomposition |
| TMD | tissue mineral density |
| TMJ | temporomandibular joint |
| UMAP | uniform manifold approximation and projection |
References
- Bordoni, B.; Varacallo, M. Anatomy, Head and Neck, Temporomandibular Joint; StatPearls Internet: Treasure Island, FL, USA, 2019. [Google Scholar]
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implant. 2013, 28, e393–e414. [Google Scholar] [CrossRef]
- Vapniarsky, N.; Huwe, L.W.; Arzi, B.; Houghton, M.K.; Wong, M.E.; Wilson, J.W.; Hatcher, D.C.; Hu, J.C.; Athanasiou, K.A. Tissue Engineering Toward Temporomandibular Joint Disc Regeneration. Sci. Transl. Med. 2018, 10, eaaq1802. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.; Williams, L.A.; Leben, J.R. Health Care Utilization and Cost Among Health Maintenance Organization Members with Temporomandibular Disorders. J. Orofac. Pain. 2001, 15, 158–169. [Google Scholar]
- Catania, K.C.; Remple, M.S. Somatosensory Cortex Dominated by the Representation of Teeth in the Naked Mole-Rat Brain. Proc. Natl. Acad. Sci. USA 2002, 99, 5692–5697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tyshkovskiy, A.; Muñoz-Espín, D.; Tian, X.; Serrano, M.; de Magalhaes, J.P.; Nevo, E.; Gladyshev, V.N.; Seluanov, A.; Gorbunova, V. Naked Mole Rats Can Undergo Developmental, Oncogene-Induced and DNA Damage-Induced Cellular Senescence. Proc. Natl. Acad. Sci. USA 2018, 115, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Zhu, W.; Terai, Y.; Marin, E.; Boschetto, F.; Kawamoto, K.; Itaka, K. Raman Spectroscopic Insight into Osteoarthritic Cartilage Regeneration by mRNA Therapeutics Encoding Cartilage-Anabolic Transcription Factor Runx1. Mater. Today Bio. 2022, 13, 100210. [Google Scholar] [CrossRef]
- Chen, W.H.; Hosokawa, M.; Tsuboyama, T.; Ono, T.; Iizuka, T.; Takeda, T. Age-Related Changes in the Temporomandibular Joint of the Senescence Accelerated Mouse. SAM-P/3 as a New Murine Model of Degenerative Joint Disease. Am. J. Pathol. 1989, 135, 379–385. [Google Scholar]
- Mori, H.; Izawa, T.; Tanaka, E. Smad3 Deficiency Leads to Mandibular Condyle Degradation via the Sphingosine 1-Phosphate (S1P)/S1P3 Signaling Axis. Am. J. Pathol. 2015, 185, 2742–2756. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Shibukawa, Y.; Nagayama, M.; Decker, R.; Kinumatsu, T.; Saito, A.; Pacifici, M.; Koyama, E. TMJ Degeneration in SAMP8 Mice Is Accompanied by Deranged Ihh Signaling. J. Dent. Res. 2014, 93, 281–287. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current Understanding of Pathogenesis and Treatment of TMJ Osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef]
- Taguchi, T.; Kotelsky, A.; Takasugi, M.; Chang, M.; Ke, Z.; Betancourt, M.; Buckley, M.R.; Zuscik, M.; Seluanov, A.; Gorbunova, V. Naked Mole-Rats Are Extremely Resistant to Post-traumatic Osteoarthritis. Aging Cell 2020, 19, e13255. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-Molecular-Mass Hyaluronan Mediates the Cancer Resistance of the Naked Mole Rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, X.; Lu, J.Y.; Boit, K.; Ablaeva, J.; Zakusilo, F.T.; Emmrich, S.; Firsanov, D.; Rydkina, E.; Biashad, S.A.; et al. Increased Hyaluronan by Naked Mole-Rat Has2 Improves Healthspan in Mice. Nature 2023, 621, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, D.; Holtze, S.; Kichler, N.; Sahm, A.; Bihin, B.; Bourguignon, V.; Dogné, S.; Szafranski, K.; Hildebrandt, T.B.; Flamion, B. Abundance and Size of Hyaluronan in Naked Mole-Rat Tissues and Plasma. Sci. Rep. 2021, 11, 7951. [Google Scholar] [CrossRef]
- Kulaberoglu, Y.; Bhushan, B.; Hadi, F.; Chakrabarti, S.; Khaled, W.T.; Rankin, K.S.; Smith, E.S.J.; Frankel, D. The Material Properties of Naked Mole-Rat Hyaluronan. Sci. Rep. 2019, 9, 6632. [Google Scholar] [CrossRef]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human Skin Penetration of Hyaluronic Acid of Different Molecular Weights as Probed by Raman Spectroscopy. Ski. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Chen, P.-J.; Dutra, E.H.; Mehta, S.; O’Brien, M.H.; Yadav, S. Age-Related Changes in the Cartilage of the Temporomandibular Joint. GeroScience 2020, 42, 995–1004. [Google Scholar] [CrossRef]
- Tanaka, E.; van Eijden, T. Biomechanical Behavior of the Temporomandibular Joint Disc. Crit. Rev. Oral Biol. Med. 2003, 14, 138–150. [Google Scholar] [CrossRef]
- Adachi, T.; Miyamoto, N.; Imamura, H.; Yamamoto, T.; Marin, E.; Zhu, W.; Kobara, M.; Sowa, Y.; Tahara, Y.; Kanamura, N.; et al. Three-Dimensional Culture of Cartilage Tissue on Nanogel-Cross-Linked Porous Freeze-Dried Gel Scaffold for Regenerative Cartilage Therapy: A Vibrational Spectroscopy Evaluation. Int. J. Mol. Sci. 2022, 23, 8099. [Google Scholar] [CrossRef]
- Adachi, T.; Boschetto, F.; Miyamoto, N.; Yamamoto, T.; Marin, E.; Zhu, W.; Kanamura, N.; Tahara, Y.; Akiyoshi, K.; Mazda, O.; et al. In Vivo Regeneration of Large Bone Defects by Cross-Linked Porous Hydrogel: A Pilot Study in Mice Combining Micro Tomography, Histological Analyses, Raman Spectroscopy and Synchrotron Infrared Imaging. Materials 2020, 13, 4275. [Google Scholar] [CrossRef]
- Heath, S.; Han, Y.; Hua, R.; Roy, A.; Jiang, J.; Nyman, J.S.; Wang, X. Assessment of glycosaminoglycan content in bone using Raman spectroscopy. Bone 2023, 171, 116751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-T.; Liu, M.-T.; Liu, H.-N.; Lin, S.-Y. Micro-Raman spectroscopy used to identify and grade human skin pilomatrixoma. Microsc. Res. Tech. 2005, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.T.; Martinho, H. First-Principles Calculations of Raman Vibrational Modes in the Fingerprint Region for Connective Tissue. Biomed. Opt. Express 2018, 9, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Ingle, I.; Kerns, J.G.; Shepherd, R.F. Multivariate analysis of Raman spectra for discriminating human collagens: In vitro identification of extracellular matrix collagens produced by an osteosarcoma cell line. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 328, 125434. [Google Scholar] [CrossRef]
- AI Pioneers Win 2024 Nobel Prizes. Nat. Mach. Intell. 2024, 6, 1271. [CrossRef]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near Real-Time Intraoperative Brain Tumor Diagnosis Using Stimulated Raman Histology and Deep Neural Networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid Intraoperative Histology of Unprocessed Surgical Specimens via Fibre-Laser-Based Stimulated Raman Scattering Microscopy. Nat. Biomed. Eng. 2017, 1, 0027. [Google Scholar] [CrossRef]
- Shiota, M.; Naya, M.; Yamamoto, T.; Hishiki, T.; Tani, T.; Takahashi, H.; Kubo, A.; Koike, D.; Itoh, M.; Ohmura, M.; et al. Gold-Nanofève Surface-Enhanced Raman Spectroscopy Visualizes Hypotaurine as a Robust Anti-Oxidant Consumed in Cancer Survival. Nat. Commun. 2018, 9, 1561. [Google Scholar] [CrossRef]
- Tabata, K.; Kawagoe, H.; Taylor, J.N.; Mochizuki, K.; Kubo, T.; Clement, J.-E.; Kumamoto, Y.; Harada, Y.; Nakamura, A.; Fujita, K.; et al. On-the-Fly Raman Microscopy Guaranteeing the Accuracy of Discrimination. Proc. Natl. Acad. Sci. USA 2024, 121, e2304866121. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Ishibashi, S.; Inoko, A.; Oka, Y.; Leproux, P.; Kano, H. Coherent Raman Microscopy Visualizes Ongoing Cellular Senescence Through Amide I Peak Shifts Originating from β Sheets in Disordered Nucleolar Proteins. Sci. Rep. 2024, 14, 27584. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.W.; Buffenstein, R.; Hulbert, A.J. Membrane Phospholipid Composition May Contribute to Exceptional Longevity of the Naked Mole-Rat (Heterocephalus glaber): A Comparative Study Using Shotgun Lipidomics. Exp. Gerontol. 2007, 42, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Oka, K.; Semba, T.; Takamori, M.; Sugiura, Y.; Yamasaki, R.; Suzuki, Y.; Chujo, T.; Nagase, M.; Oiwa, Y.; et al. Cellular Senescence Induction Leads to Progressive Cell Death via the INK4a-RB Pathway in Naked Mole-Rats. EMBO J. 2023, 42, e111133. [Google Scholar] [CrossRef]
- Liu, S.; Wu, C.; Yao, Y.; Dong, X.; Xu, L. Single monoiodoacetic acid injection reveals toll-like receptor, oestrogen, oxidative stress, and altered energy metabolism as key drivers of temporomandibular joint osteoarthritis in female rats. Arch. Oral Biol. 2025, 174, 106233. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Kanno, N.; Kato, S.; Ohkuma, M.; Matsui, M.; Iwasaki, W.; Shigeto, S. Machine learning-assisted single-cell Raman fingerprinting for in situ and nondestructive classification of prokaryotes. iScience 2021, 24, 102975. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.M.; Moffett, B.C., Jr. The Postnatal Development of the Human Temporomandibular Joint. Am. J. Anat. 1974, 141, 235–249. [Google Scholar] [CrossRef]
- Karakasis, D.; Tsaknakis, A. Aging Changes in the Articular Disk of the Temporomandibular Joint in the Guinea Pig. J. Dent. Res. 1976, 55, 262–265. [Google Scholar] [CrossRef]
- Takahashi, M.; Shibata, S.; Shibui, T.; Takechi, M.; Irie, K. Comparison of the Structural Features Between Chicken Quadratomandibular Joint and Rat Temporomandibular Joint with Reference to Ligament and Articular Meniscus/Disc. J. Oral Biosci. 2025, 67, 100623. [Google Scholar] [CrossRef]
- Stegenga, B.; de Bont, L.G.; Boering, G.; van Willigen, J.D. Tissue Responses to Degenerative Changes in the Temporomandibular Joint: A Review. J. Oral Maxillofac. Surg. 1991, 49, 1079–1088. [Google Scholar] [CrossRef]
- Arnett, G.W.; Milam, S.B.; Gottesman, L. Progressive Mandibular Retrusion—Idiopathic Condylar Resorption. Part I. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 8–15. [Google Scholar] [CrossRef] [PubMed]
- de Bont, L.G.; Boering, G.; Havinga, P.; Liem, R.S. Spatial Arrangement of Collagen Fibrils in the Articular Cartilage of the Mandibular Condyle: A Light Microscopic and Scanning Electron Microscopic Study. J. Oral Maxillofac. Surg. 1984, 42, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, D.W. The Process of Lubrication Impairment and Its Involvement in Temporomandibular Joint Disc Displacement: A Theoretical Concept. J. Oral Maxillofac. Surg. 2001, 59, 36–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Hu, S.; Wang, C.; Bian, X.; Kang, H.; Zhou, P.; Bao, G. In vitro differentiation of human induced pluripotent stem cells into temporomandibular joint disc like cells. Heliyon 2023, 10, e23937. [Google Scholar] [CrossRef]
- Miyawaki, S.; Kawamura, Y.; Oiwa, Y.; Shimizu, A.; Hachiya, T.; Bono, H.; Koya, I.; Okada, Y.; Kimura, T.; Tsuchiya, Y.; et al. Tumour resistance in induced pluripotent stem cells derived from naked mole-rats. Nat. Commun. 2016, 7, 11471. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Mizutani, Y.; Abe, M.; Fukuda, S.; Higashiyama, S.; Inoue, S. Naked Mole-Rat TMEM2 Lacks Physiological Hyaluronan-Degrading Activity. Arch. Biochem. Biophys. 2024, 759, 110098. [Google Scholar] [CrossRef]
- Farias-Neto, A.; Martins, A.P.V.B.; Sánchez-Ayala, A.; Rabie, A.B.M.; Novaes, P.D.; Rizzatti-Barbosa, C.M. The Effect of Posterior Tooth Loss on the Expression of Type II Collagen, IL-1β and VEGF in the Condylar Cartilage of Growing Rats. Arch. Oral Biol. 2012, 57, 1551–1557. [Google Scholar] [CrossRef]
- Yu, J.; Mursu, E.; Typpö, M.; Laaksonen, S.; Voipio, H.-M.; Pesonen, P.; Raustia, A.; Pirttiniemi, P. MMP-3 and MMP-8 in Rat Mandibular Condylar Cartilage Associated with Dietary Loading, Estrogen Level, and Aging. Arch. Oral Biol. 2019, 97, 238–244. [Google Scholar] [CrossRef]
- Watanabe, H.; Iori, T.; Lee, J.-W.; Kajii, T.S.; Takakura, A.; Takao-Kawabata, R.; Kitagawa, Y.; Maruoka, Y.; Iimura, T. Association Between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females. Int. J. Mol. Sci. 2023, 24, 2775. [Google Scholar] [CrossRef]
- Ariyoshi, W.; Okinaga, T.; Knudson, C.B.; Knudson, W.; Nishihara, T. High Molecular Weight Hyaluronic Acid Regulates Osteoclast Formation by Inhibiting Receptor Activator of NF-κB Ligand Through Rho Kinase. Osteoarthr. Cartil. 2014, 22, 111–120. [Google Scholar] [CrossRef]
- Ariyoshi, W.; Takahashi, T.; Kanno, T.; Ichimiya, H.; Takano, H.; Koseki, T.; Nishihara, T. Mechanisms Involved in Enhancement of Osteoclast Formation and Function by Low Molecular Weight Hyaluronic Acid. J. Biol. Chem. 2005, 280, 18967–18972. [Google Scholar] [CrossRef] [PubMed]
- Mata-Miranda, M.M.; Martinez-Cuazitl, A.; Guerrero-Robles, C.I.; Noriega-Gonzalez, J.E.; Garcia-Hernandez, J.S.; Vazquez-Zapien, G.J. Biochemical Similarity Between Cultured Chondrocytes and In Situ Chondrocytes by Chemometric Analysis from FTIR Microspectroscopy. Biotechnol. Rep. 2019, 24, e00391. [Google Scholar] [CrossRef] [PubMed]
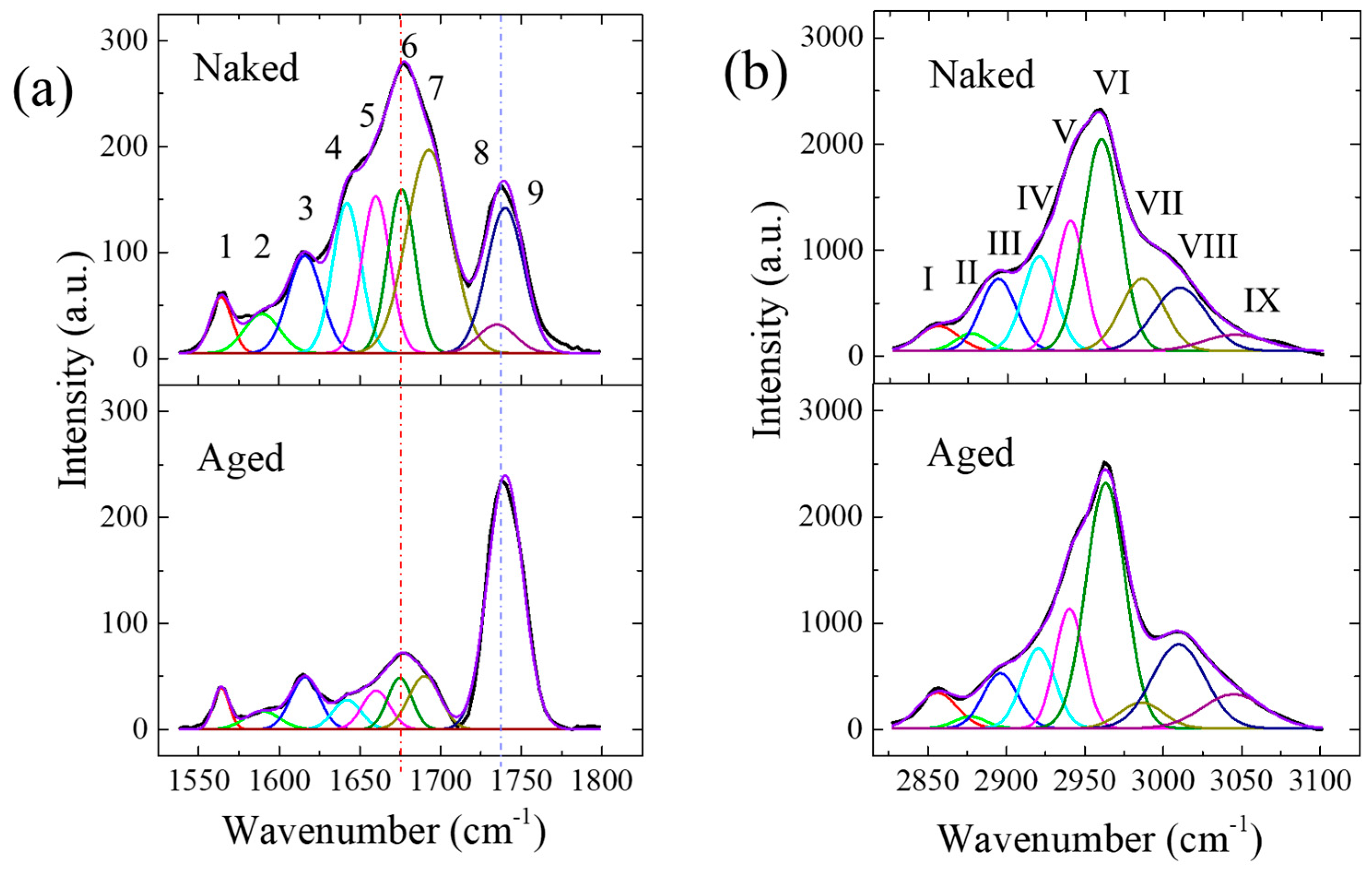
| Band Label | Position (cm−1) | Principal Assignment | Percent (%) | |
|---|---|---|---|---|
| Aged | Naked | |||
| Band 1 Red | 1564 | C=C, N–H deformation; C–N stretching (Amide II) | 23.4 | 20.7 |
| Band 2 Light green | 1589 | ν(C=C), olefinic stretching in lipid and ν(C–N) in hydroxyproline (collagen) | 22.9 | 23.6 |
| Band 3 Blue | 1616 | ν(aromatic C=C) in protein (tyrosine, tryptophan) | 53.7 | 55.8 |
| Band 4 Light blue | 1642 | Amide I, v(C=O) in protein segment | 17.2 | 19.1 |
| Band 5 Purple | 1660 | Amide I, v(C=O) in α-helix; v(C=C) in unsaturated fatty acids | 22.2 | 19.9 |
| Band 6 Green | 1675 | Amide I, v(C=O) in β sheet | 25.5 | 20 |
| Band 7 Moss green | 1690 | Amide I, v(C=O) in disordered structure and collagen type II | 35 | 41 |
| Band 8 Indigo | 1735 | v(O-C=O) of COOH in HA | ||
| Band 9 Dark purple | 1740 | v(O-C=O) of ester group, phospholipid | ||
| Band Label | Position (cm−1) | Principal Assignment | Percent (%) | |
|---|---|---|---|---|
| Aged | Naked | |||
| Band I Red | 2856 | vs(CH2) in lipid (liquid) | 5.1 | 3.7 |
| Band II Light green | 2877 | vs(CH3) in lipid (hexagonal) and protein | 1.4 | 2.1 |
| Band III Blue | 2896 | v(CH) in protein | 7.1 | 9.7 |
| Band IV Light blue | 2920 | vas(CH2) in lipid & v(CH) in HA | 9.8 | 11.9 |
| Band V Purple | 2940 | vas (CH2) in protein | 12.7 | 14.3 |
| Band VI Green | 2963 | vas (CH3) in lipid (out-of-plane chain end) | 35.5 | 30.0 |
| Band VII Moss green | 2985 | vas (CH3) in ester and HA | 4.1 | 11.8 |
| Band VIII Indigo | 3010 | v(C=C–H) in lipid chains | 16.4 | 12.7 |
| Band IX Dark purple | 3050 | v(=C–H) aromatic stretching in lipids | 7.9 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, T.; Imamura, H.; Yaji, T.; Mochizuki, K.; Zhu, W.; Shindo, S.; Shibata, S.; Adachi, K.; Yamamoto, T.; Oseko, F.; et al. Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints. Gels 2025, 11, 414. https://doi.org/10.3390/gels11060414
Adachi T, Imamura H, Yaji T, Mochizuki K, Zhu W, Shindo S, Shibata S, Adachi K, Yamamoto T, Oseko F, et al. Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints. Gels. 2025; 11(6):414. https://doi.org/10.3390/gels11060414
Chicago/Turabian StyleAdachi, Tetsuya, Hayata Imamura, Toyonari Yaji, Kentaro Mochizuki, Wenliang Zhu, Satoru Shindo, Shunichi Shibata, Keiji Adachi, Toshiro Yamamoto, Fumishige Oseko, and et al. 2025. "Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints" Gels 11, no. 6: 414. https://doi.org/10.3390/gels11060414
APA StyleAdachi, T., Imamura, H., Yaji, T., Mochizuki, K., Zhu, W., Shindo, S., Shibata, S., Adachi, K., Yamamoto, T., Oseko, F., Mazda, O., Miura, K., Kawai, T., & Pezzotti, G. (2025). Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints. Gels, 11(6), 414. https://doi.org/10.3390/gels11060414








