Investigating the Interactions of Peptide Nucleic Acids with Multicomponent Peptide Hydrogels for the Advancement of Healthcare Technologies
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of PNA Sequences
2.2. Design and Synthesis of Peptide Building Blocks
2.3. Formulation of Multicomponent Hydrogels
2.4. Rheological Characterization
2.5. Stability Assessment of Hydrogels
2.6. Swelling Rate Analysis
2.7. Multicomponent PNA-Hydrogels’ Secondary Structure Characterization
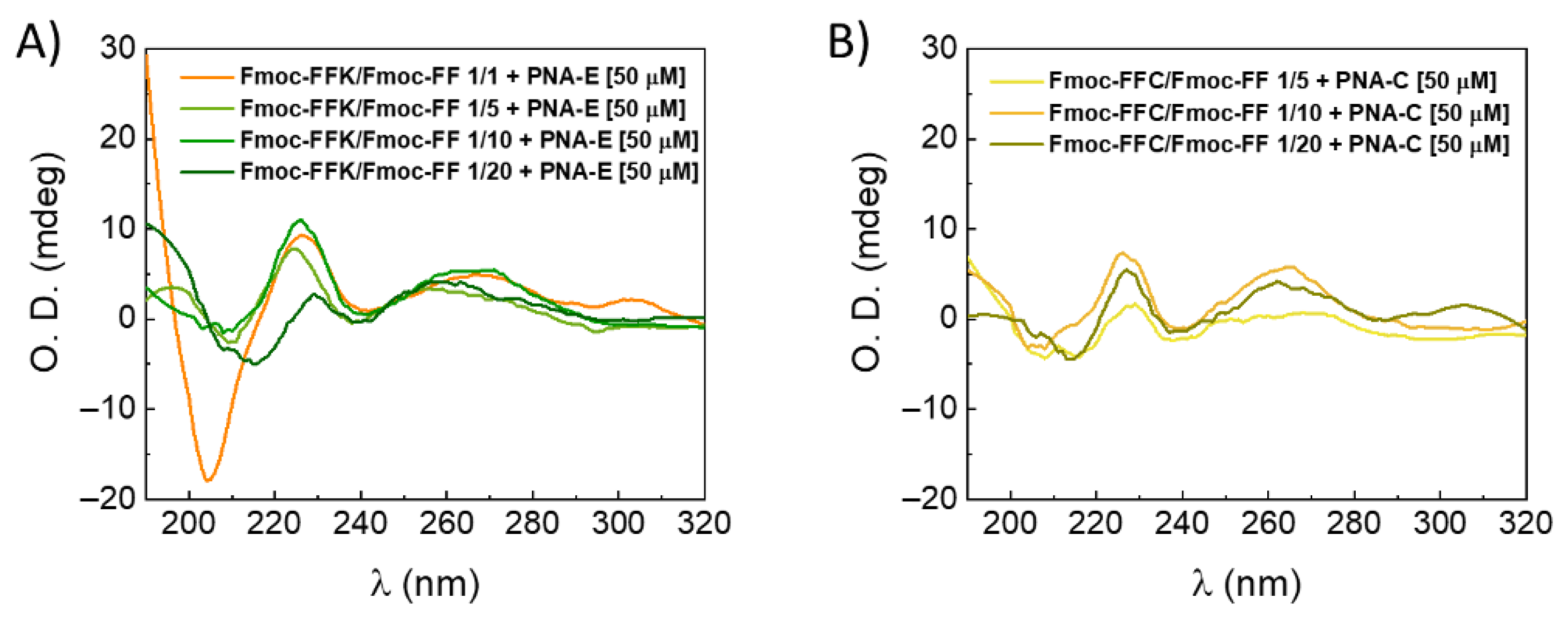
2.7.1. Circular Dichroism Spectroscopy (CD)
2.7.2. Fourier Transform Infrared Spectroscopy (FT-IR)
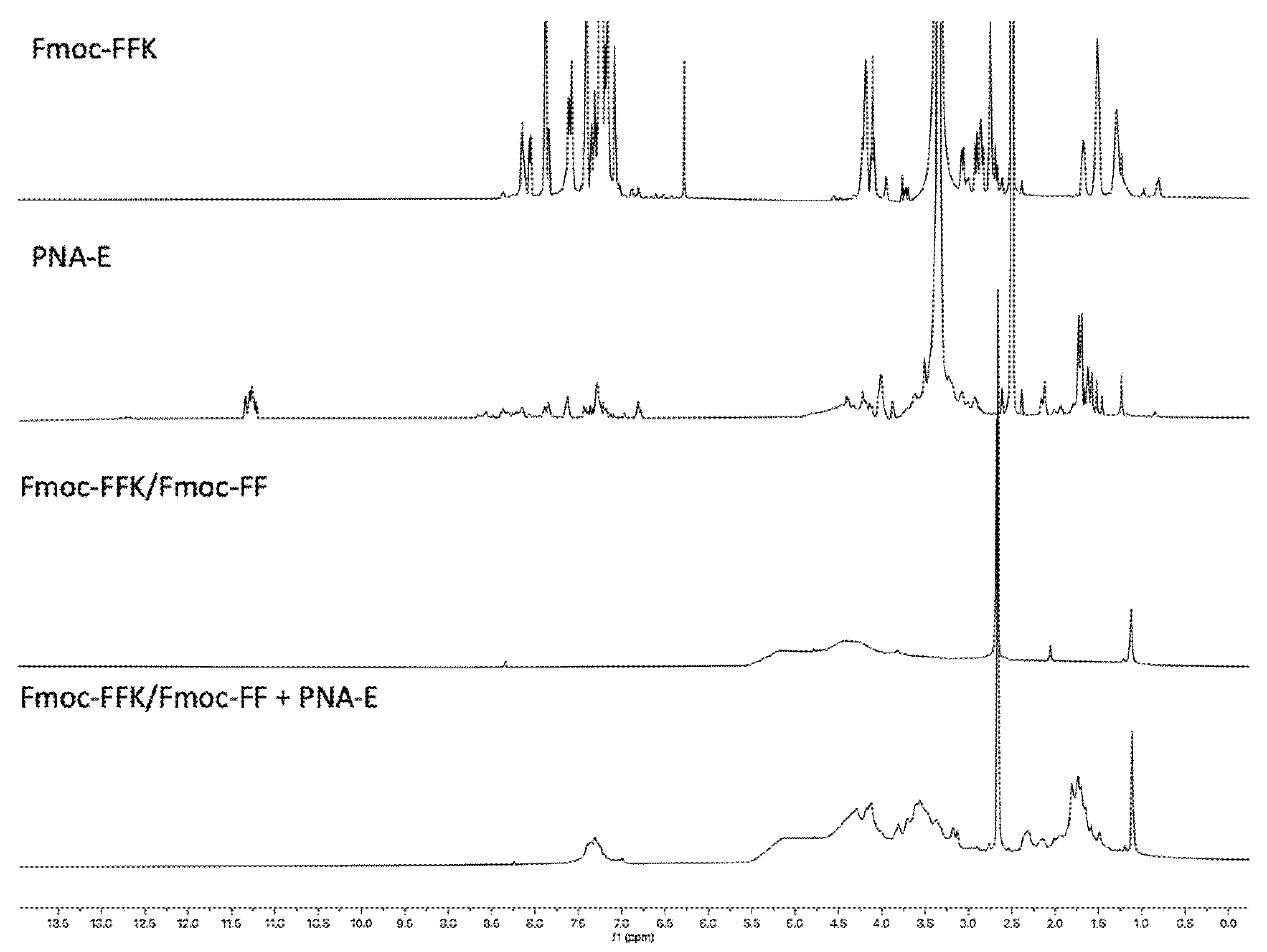
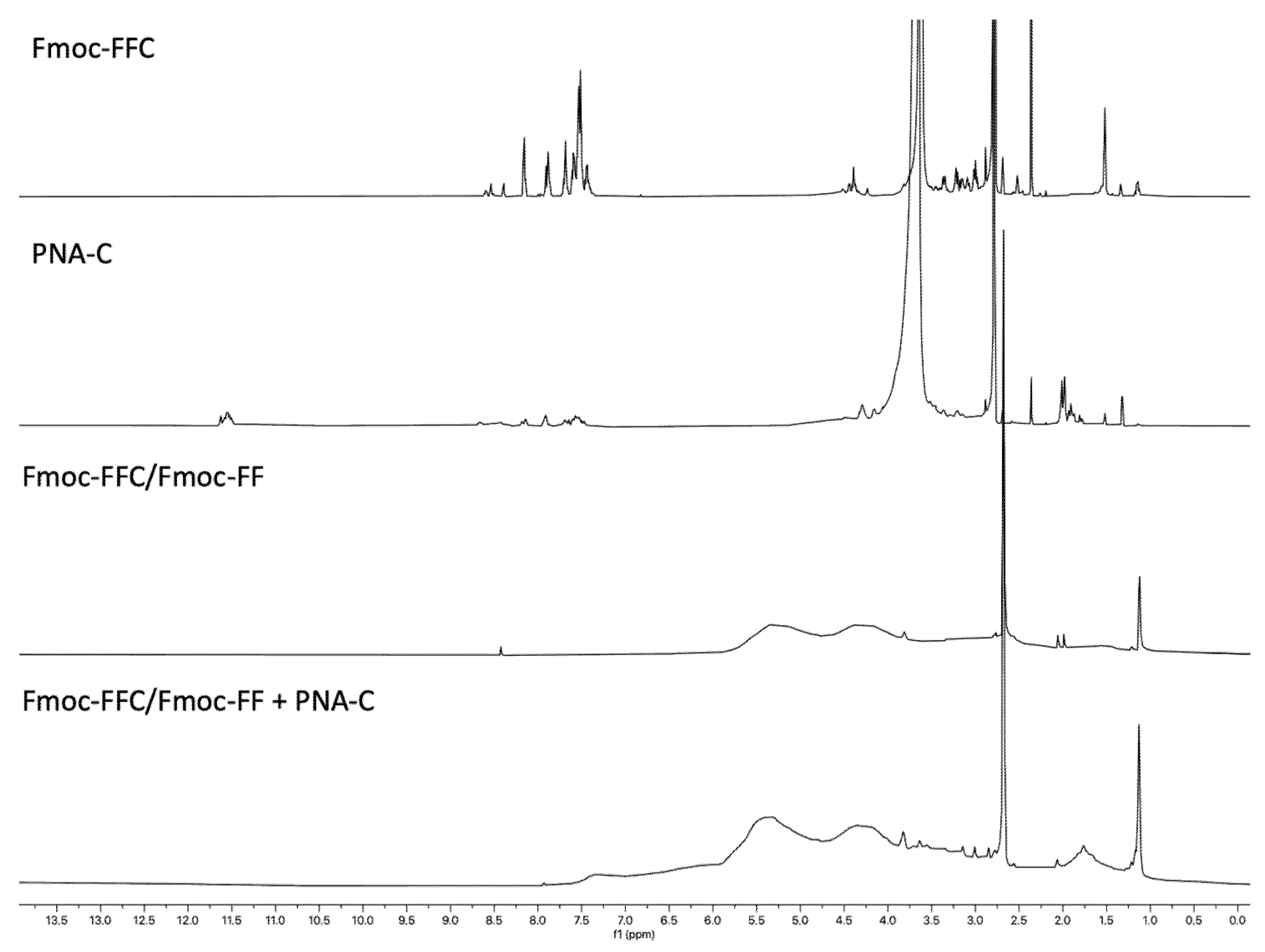
2.7.3. Peptides and PNAs 1H-NMR Spectroscopy
2.7.4. Fluorescence Spectroscopic Assay
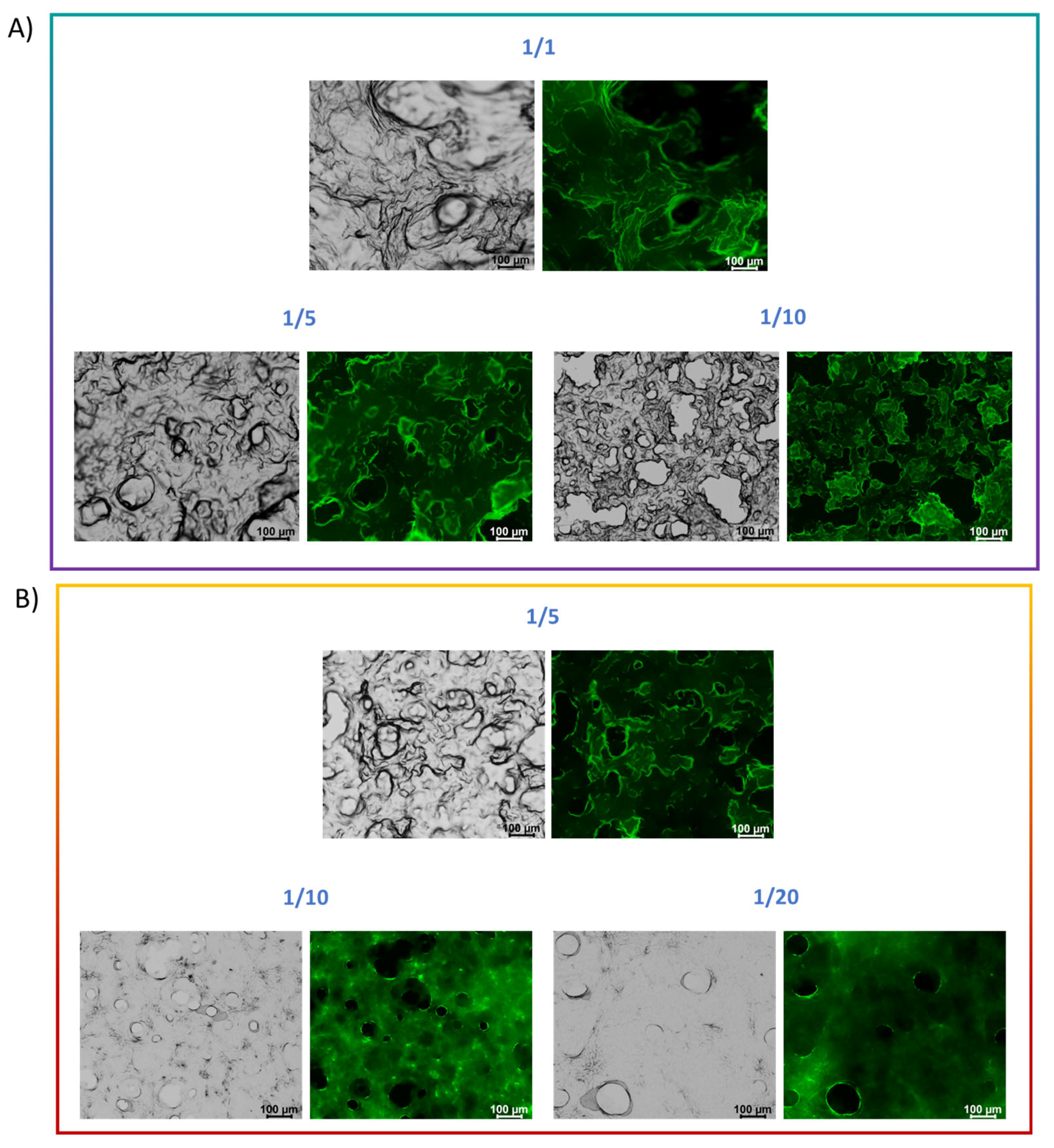
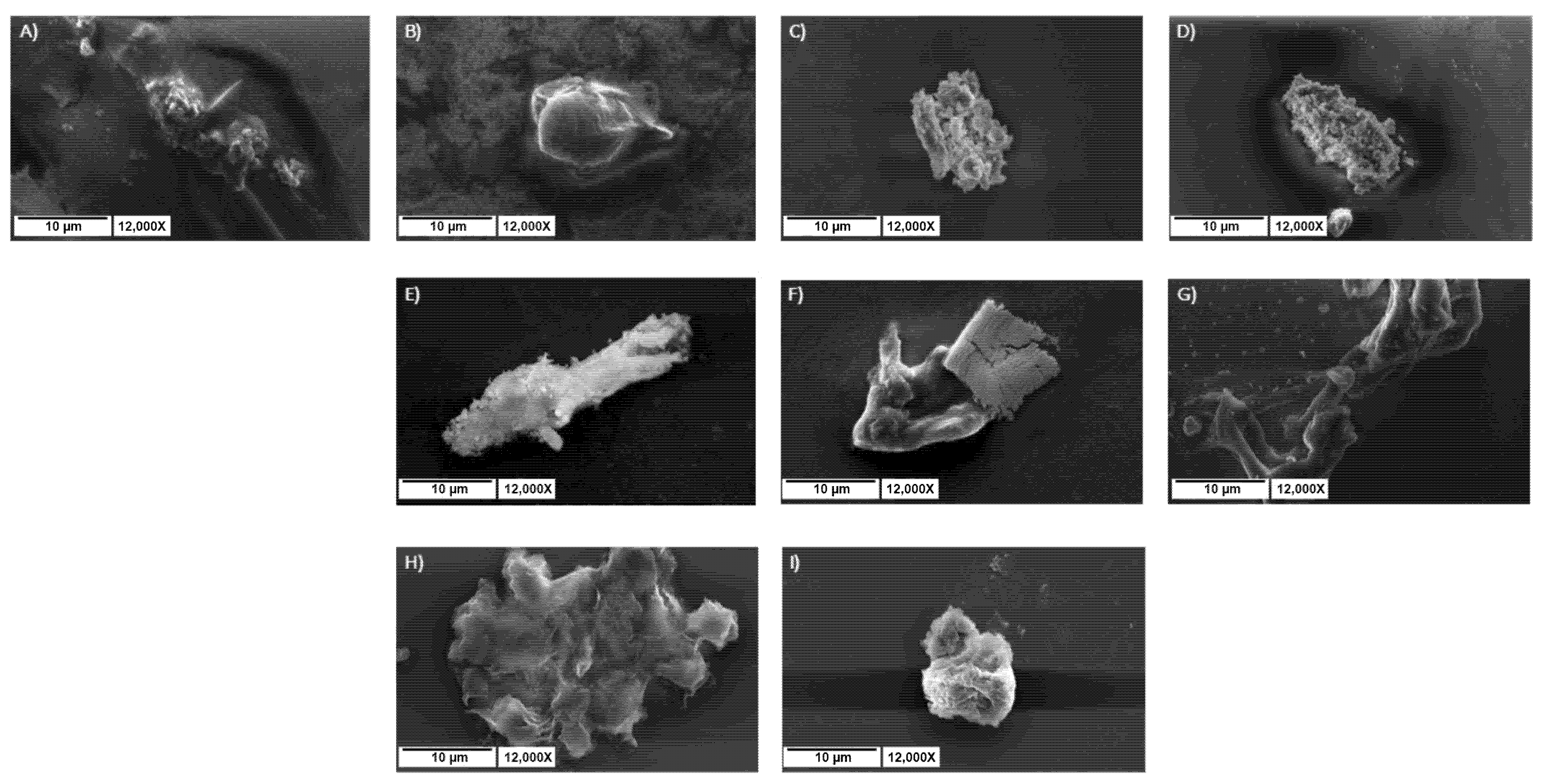
2.7.5. SEM Analysis of Hydrogel Morphology
2.8. Diffusion Studies of PNAs in Hydrogel Matrices
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. PNA and Peptides Synthesis, Purification, and Analysis
4.3. Formulation of Hydrogels
4.4. Rheology
4.5. Hydrogel Stability Studies
4.6. Swelling Kinetics
4.7. Circular Dichroism (CD) Spectroscopy
4.8. Fourier Transform Infrared Spectroscopy (FT-IR)
4.9. 1H-NMR Spectroscopy
4.10. Fluorescence Spectroscopy
4.11. Scanning Electron Microscopy (SEM)
4.12. Release and Permeation Studies of PNAs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef] [PubMed]
- Karchoubi, F.; Afshar Ghotli, R.; Pahlevani, H.; Baghban Salehi, M. New insights into nanocomposite hydrogels: A review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-based hydrogels: New materials for biosensing and biomedical applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D. Rational design of peptide-based smart hydrogels for therapeutic applications. Front. Chem. 2021, 9, 123456. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Dezhbord, M.; Lee, E.-H.; Jeon, Y.; Jung, D.; Gu, S.H.; Yu, C.; Lee, S.H.; Kim, S.C.; et al. Cell-permeable peptide nucleic acid antisense oligonucleotide platform targeting human betacoronaviruses. Front. Microbiol. 2023, 14, 123456. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Gallo, E.; Morelli, G.; Accardo, A. Differently N-capped analogues of Fmoc-FF. Chem. Eur. J. 2023, 29, e202300000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Q.; Zhang, Y.; Adler-Abramovich, L.; Fan, X.; Mei, D.; Gazit, E.; Tao, K. Fmoc-diphenylalanine gelating nanoarchitectonics: A simplistic peptide self-assembly to meet complex applications. J. Colloid Interface Sci. 2023, 636, 113–133. [Google Scholar] [CrossRef]
- Choe, R.; Il Yun, S. Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery. e-Polymers 2020, 20, 458–468. [Google Scholar] [CrossRef]
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptide-based multicomponent hydrogels as scaffold materials. Soft Matter 2019, 15, 487. [Google Scholar] [CrossRef]
- Apostolidou, C.P.; Kokotidou, C.; Platania, V.; Nikolaou, V.; Landrou, G.; Nikoloudakis, E.; Charalambidis, G.; Chatzinikolaidou, M.; Coutsolelos, A.G.; Mitraki, A. Antimicrobial Potency of Fmoc-Phe-Phe Dipeptide Hydrogels with Encapsulated Porphyrin Chromophores Is a Promising Alternative in Antimicrobial Resistance. Biomolecules 2024, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Gallo, E.; Diaferia, C.; Rosa, E.; Carrese, B.; Borbone, N.; Scognamiglio, P.L.; Franzese, M.; Oliviero, G.; Accardo, A. Multicomponent peptide-based hydrogels containing chemical functional groups as innovative platforms for biotechnological applications. Gels 2023, 9, 903. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Giordano, S.; Rosa, E.; Carrese, B.; Piccialli, G.; Borbone, N.; Morelli, G.; Oliviero, G.; Accardo, A. Ultrashort cationic peptide Fmoc-FFK as hydrogel building block for potential biomedical applications. Gels 2023, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, E.; Peyman, A.; Breipohl, G.; Will, D.W. PNA: Synthetic polyamide nucleic acids with unusual binding properties. Angew. Chem. Int. Ed. 1998, 37, 2796–2823. [Google Scholar] [CrossRef]
- Falanga, A.P.; Cerullo, V.; Marzano, M.; Feola, S.; Oliviero, G.; Piccialli, G.; Borbone, N. Peptide nucleic acid-functionalized adenoviral vectors targeting G-quadruplexes in the P1 promoter of Bcl-2 proto-oncogene: A new tool for gene modulation in anticancer therapy. Bioconjug. Chem. 2019, 30, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Hanvey, J.C.; Peffer, N.J.; Bisi, J.E.; Thomson, S.A.; Cadilla, R.; Josey, J.A.; Ricca, D.J.; Hassman, C.F.; Bonham, M.A.; Au, K.G.; et al. Antisense and antigene properties of peptide nucleic acids. Science 1992, 258, 1481–1485. [Google Scholar] [CrossRef]
- Terracciano, M.; Fontana, F.; Falanga, A.P.; D’Errico, S.; Torrieri, G.; Greco, F.; Tramontano, C.; Rea, I.; Piccialli, G.; De Stefano, L.; et al. Development of surface chemical strategies for synthesizing redox-responsive diatomite nanoparticles as a green platform for on-demand intracellular release of an antisense peptide nucleic acid anticancer agent. Small 2022, 18, 2200000. [Google Scholar] [CrossRef]
- MacLelland, V.; Kravitz, M.; Gupta, A. Therapeutic and diagnostic applications of antisense peptide nucleic acids. Mol. Ther. Nucleic Acids 2024, 35, 102086. [Google Scholar] [CrossRef]
- Sannigrahi, A.; De, N.; Bhunia, D.; Bhadra, J. Peptide nucleic acids: Recent advancements and future opportunities in biomedical applications. Bioorg. Chem. 2025, 155, 108146. [Google Scholar] [CrossRef]
- Zarrilli, F.; Amato, F.; Morgillo, C.M.; Pinto, B.; Santarpia, G.; Borbone, N.; D’Errico, S.; Catalanotti, B.; Piccialli, G.; Castaldo, G.; et al. Peptide nucleic acids as miRNA target protectors for the treatment of cystic fibrosis. Molecules 2017, 22, 1144. [Google Scholar] [CrossRef]
- Cadoni, E.; Manicardi, A.; Madder, A. PNA-Based MicroRNA Detection Methodologies. Molecules 2020, 25, 1296. [Google Scholar] [CrossRef] [PubMed]
- Gaglione, M.; Milano, G.; Chambery, A.; Moggio, L.; Romanelli, A.; Messere, A. PNA-based artificial nucleases as antisense and anti-miRNA oligonucleotide agents. Mol. Biosyst. 2011, 7, 2490. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mohanty, S.K. PNA functionalized gold nanoparticles on TiO2 nanotubes biosensor for electrochemical DNA fragment detection. Adv. Mat. Interfaces 2025, 12, 2400762. [Google Scholar] [CrossRef]
- Crisci, T.; Falanga, A.P.; Casalino, M.; Borbone, N.; Terracciano, M.; Chianese, G.; Gioffrè, M.; D’Errico, S.; Marzano, M.; Rea, I.; et al. Bioconjugation of a PNA probe to zinc oxide nanowires for label-free sensing. Nanomaterials 2021, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Pooga, M.; Land, T.; Bartfai, T.; Langel, Ü. PNA oligomers as tools for specific modulation of gene expression. Biomol. Eng. 2001, 17, 183–192. [Google Scholar] [CrossRef]
- Berger, S.; Lächelt, U.; Wagner, E. Dynamic carriers for therapeutic RNA delivery. Proc. Natl. Acad. Sci. USA 2024, 121, e123456. [Google Scholar] [CrossRef]
- Gupta, A.; Bahal, R.; Gupta, M.; Glazer, P.M.; Saltzman, W.M. Nanotechnology for delivery of peptide nucleic acids (PNAs). J. Control Release 2016, 240, 302–311. [Google Scholar] [CrossRef]
- Ambasht, P.K. Use of group-specific reagents in active site functional group elucidation I: Cys, Ser, Tyr, and Trp residues. In Frontiers in Protein Structure, Function, and Dynamics; Springer: Singapore, 2020; pp. 71–94. [Google Scholar]
- Choi, H.; Go, M.; Cha, Y.; Choi, Y.; Kwon, K.-Y.; Jung, J.H. A facile method to fabricate hydrogels from DMSO polymer gels via solvent exchange. New J. Chem. 2017, 41, 4793–4796. [Google Scholar] [CrossRef]
- Tang, C.; Smith, A.M.; Collins, R.F.; Ulijn, R.V.; Saiani, A. Fmoc-diphenylalanine self-assembly mechanism induces apparent pKa shifts. Langmuir 2009, 25, 9447–9453. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Rasouli, M.; Zebarjad, S.M. The rheological behavior of the non-Newtonian thixotropic colloidal silica gels from sodium silicate. Mater. Chem. Phys. 2021, 272, 124994. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Mullen, L.M.; Berta, M.; Chen, L.; Frith, W.J. Relationship between molecular structure, gelation behaviour and gel properties of Fmoc-dipeptides. Soft Matter 2010, 6, 1971. [Google Scholar] [CrossRef]
- Orbach, R.; Mironi-Harpaz, I.; Adler-Abramovich, L.; Mossou, E.; Mitchell, E.P.; Forsyth, V.T.; Gazit, E.; Seliktar, D. The rheological and structural properties of Fmoc-peptide-based hydrogels: The effect of aromatic molecular architecture on self-assembly and physical characteristics. Langmuir 2012, 28, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Margheritelli, S.; Toumia, Y.; Paradossi, G.; Bordi, F.; Sennato, S.; Palocci, C. Biosynthesis and characterization of cross-linked Fmoc peptide-based hydrogels for drug delivery applications. Gels 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Chin, D.-H.; Woody, R.W.; Rohl, C.A.; Baldwin, R.L. Circular dichroism spectra of short, fixed-nucleus alanine helices. Proc. Natl. Acad. Sci. USA 2002, 99, 15416–15421. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Roy, S.; Javid, N.; Duncan, K.; Aitken, L.; Ulijn, R.V. Pathway-dependent gold nanoparticle formation by biocatalytic self-assembly. Nanoscale 2017, 9, 12330–12334. [Google Scholar] [CrossRef]
- Ryan, K.; Beirne, J.; Redmond, G.; Kilpatrick, J.I.; Guyonnet, J.; Buchete, N.-V.; Kholkin, A.L.; Rodriguez, B.J. Nanoscale piezoelectric properties of self-assembled Fmoc–FF peptide fibrous networks. ACS Appl. Mater. Interfaces 2015, 7, 12702–12707. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Evaluation of the information content in infrared spectra for protein secondary structure determination. Biophys. J. 2006, 90, 2946–2957. [Google Scholar] [CrossRef]
- Zandomeneghi, G.; Krebs, M.R.H.; McCammon, M.G.; Fändrich, M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef]
- Arrondo, J.L.R.; Goñi, F.M. Structure and dynamics of membrane proteins as studied by infrared spectroscopy. Prog. Biophys. Mol. Biol. 1999, 72, 367–405. [Google Scholar] [CrossRef]
- Sardaru, M.-C.; Marangoci, N.-L.; Palumbo, R.; Roviello, G.N.; Rotaru, A. Nucleic acid probes in bio-imaging and diagnostics: Recent advances in ODN-based fluorescent and surface-enhanced Raman scattering nanoparticle and nanostructured systems. Molecules 2023, 28, 3561. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.; Cheong, D.W.; Accardo, A.; Di Fabrizio, E.; Riekel, C.; Hauser, C.A.E. Aliphatic peptides show similar self-assembly to amyloid core sequences, challenging the importance of aromatic interactions in amyloidosis. Proc. Natl. Acad. Sci. USA 2013, 110, 519–524. [Google Scholar] [CrossRef]
- McFetridge, M.L.; Kulkarni, K.; Hilsenstein, V.; Del Borgo, M.P.; Aguilar, M.-I.; Ricardo, S.D. A comparison of fixation methods for SEM analysis of self-assembling peptide hydrogel nanoarchitecture. Nanoscale 2023, 15, 1431–1440. [Google Scholar] [CrossRef]
- Falcone, N.; Ermis, M.; Tamay, D.G.; Mecwan, M.; Monirizad, M.; Mathes, T.G.; Khademhosseini, A. Peptide hydrogels as immunomaterials and their use in cancer immunotherapy delivery. Adv. Healthc. Mater. 2023, 12, 2301096. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, M.; Li, W.; Li, W.; Xiao, S.; Cao, Q.; Luo, S. Sustained release of αO-conotoxin GeXIVA [1,2] via hydrogel microneedle patch for chronic neuropathic pain management. Mar. Drugs 2025, 23, 161. [Google Scholar] [CrossRef]
- Coin, I.; Beyermann, M.; Bienert, M. Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007, 2, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C.F. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef]
- Jorbágy, A.; Király, K. Chemical characterization of fluorescein isothiocyanate-protein conjugates. Biochim. Biophys. Acta Gen. Subj. 1966, 124, 166–175. [Google Scholar] [CrossRef]
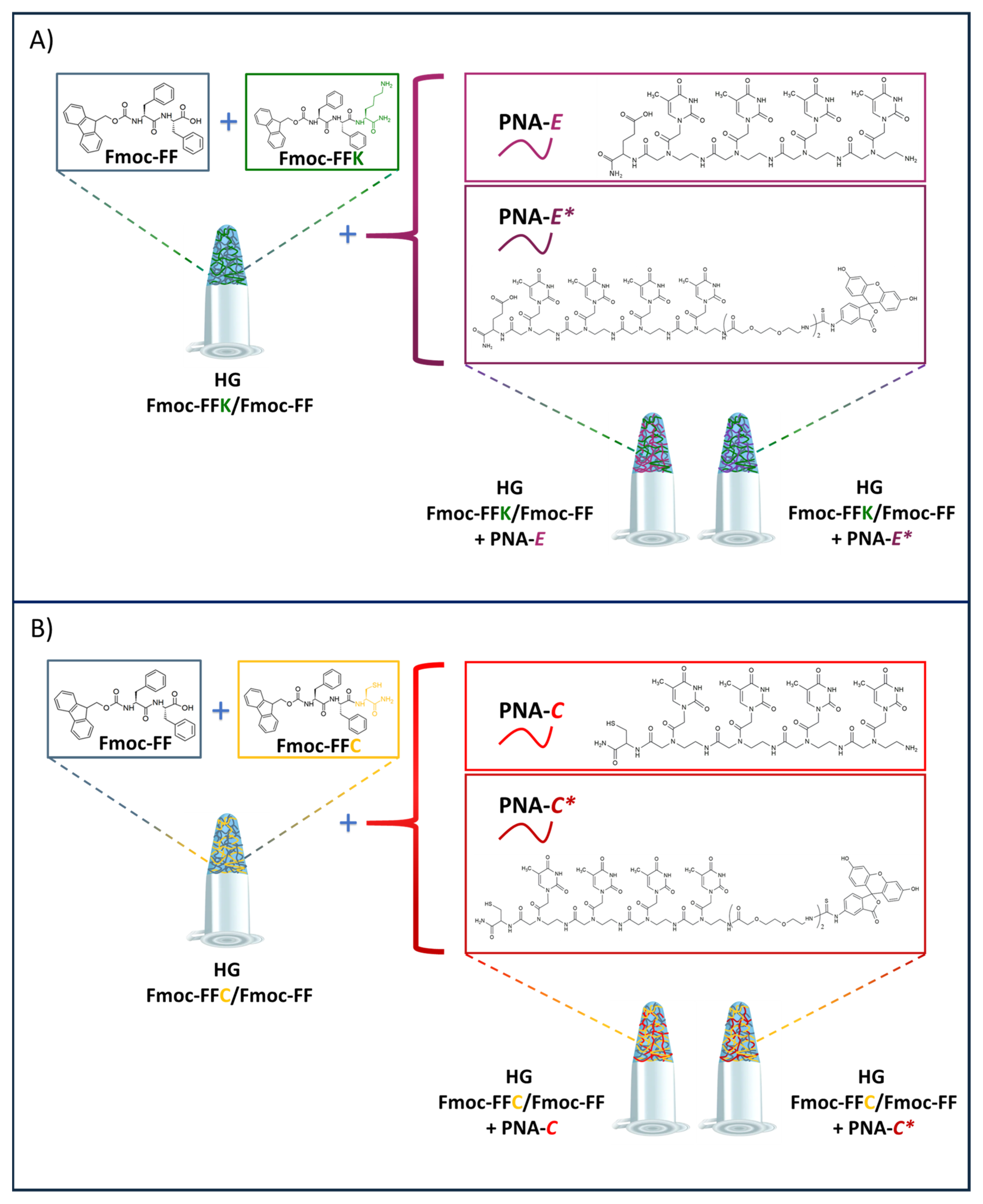



| Sample | Sequence (C → N) | Chemical Formula | M.W. calc. (a.m.u.) | M.W. det. (a.m.u.) | Rt (min) |
|---|---|---|---|---|---|
| PNA-E | C49H65N17O20 | 1211.46 | |||
| PNA-E* | C82H98N20O31S | 1891.64 | |||
| PNA-C | C47H64N18O17S | ||||
| PNA-C* | C80H97N21O28S2 | 1865.63 |
| Peptide | Chemical Formula | M.W. calc. (a.m.u.) | M.W. det. (a.m.u.) |
|---|---|---|---|
| Fmoc-FFC | C36H36N4O5S | 636.7 | |
| Fmoc-FFK | C39H42N4O6 | 662.7 |
| System (HGs) | Ratio with Fmoc-FF (w/w) | Gelation Time (min) | Gelation Time of Solely HGs (min) |
|---|---|---|---|
| Fmoc-FFK/Fmoc-FF + PNA-E [50µM] | 1/1 1/5 1/10 1/20 | 26 112 164 34 | 10 78 138 216 |
| Fmoc-FFC/Fmoc-FF + PNA-C [50µM] | 1/5 1/10 1/20 | 80 25 10 | 75 4 2 |
| Fmoc-FFK/Fmoc-FF + PNA-E | Fmoc-FFC/Fmoc-FF + PNA-C | ||||||
|---|---|---|---|---|---|---|---|
| Ratio with Fmoc-FF | G′ (Pa) | G″ (Pa) | tan δ | Ratio with Fmoc-FF | G′ (Pa) | G″ (Pa) | tan δ |
| 1/1 | 140 | 20 | 0.143 | ||||
| 1/5 | 19320 | 1770 | 0.0920 | 1/5 | 648 | 112 | 0.173 |
| 1/10 | 6243 | 444 | 0.0710 | 1/10 | 6941 | 1019 | 0.147 |
| 1/20 | 8711 | 486 | 0.0557 | 1/20 | 22471 | 2371 | 0.105 |
| Fmoc-FFK/Fmoc-FF | Fmoc-FFC/Fmoc-FF | ||||||
|---|---|---|---|---|---|---|---|
| Ratio with Fmoc-FF | W0 (g) | Wt (g) | ΔW (%) | Ratio with Fmoc-FF | W0 (g) | Wt (g) | ΔW (%) |
| 1/1 | 1.3438 | 1.3263 | 1 | ||||
| 1/5 | 1.3411 | 1.3200 | 2 | 1/5 | 1.3362 | 1.3229 | 1 |
| 1/10 | 1.3320 | 1.3290 | 0 | 1/10 | 1.3429 | 1.3298 | 1 |
| 1/20 | 1.3363 | 1.2979 | 3 | 1/20 | 1.3417 | 1.3393 | 0 |
| Fmoc-FFK/Fmoc-FF + PNA-E | Fmoc-FFC/Fmoc-FF + PNA-C | ||||||
|---|---|---|---|---|---|---|---|
| Ratio with Fmoc-FF | W0 (g) | Wt (g) | ΔW (%) | Ratio with Fmoc-FF | W0 (g) | Wt (g) | ΔW (%) |
| 1/1 | 1.3023 | 1.2849 | 1 | ||||
| 1/5 | 1.3280 | 1.3089 | 1 | 1/5 | 1.2802 | 1.2244 | 4 |
| 1/10 | 1.3266 | 1.3034 | 2 | 1/10 | 1.2927 | 1.2401 | 4 |
| 1/20 | 1.3306 | 1.2869 | 3 | 1/20 | 1.3015 | 1.2519 | 4 |
| Sample (Empty HGs) | Ratio with Fmoc-FF | q (%) |
|---|---|---|
| Fmoc-FFK/Fmoc-FF | 1/1 | 36.1 |
| 1/5 | 36.8 | |
| 1/10 | 35.0 | |
| 1/20 | 34.8 | |
| Fmoc-FFC/Fmoc-FF | 1/5 | 38.7 |
| 1/10 | 37.2 | |
| 1/20 | 36.2 |
| Sample (Loaded HGs) | Ratio with Fmoc-FF | q (%) |
|---|---|---|
| Fmoc-FFK/Fmoc-FF + PNA-E | 1/1 | 37.7 |
| 1/5 | 37.7 | |
| 1/10 | 37.3 | |
| 1/20 | 37.9 | |
| Fmoc-FFC/Fmoc-FF + PNA-C | 1/5 | 36.7 |
| 1/10 | 36.9 | |
| 1/20 | 37.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, S.; Terracciano, M.; Gallo, E.; Diaferia, C.; Falanga, A.P.; Accardo, A.; Franzese, M.; Salvatore, M.; Piccialli, G.; Borbone, N.; et al. Investigating the Interactions of Peptide Nucleic Acids with Multicomponent Peptide Hydrogels for the Advancement of Healthcare Technologies. Gels 2025, 11, 367. https://doi.org/10.3390/gels11050367
Giordano S, Terracciano M, Gallo E, Diaferia C, Falanga AP, Accardo A, Franzese M, Salvatore M, Piccialli G, Borbone N, et al. Investigating the Interactions of Peptide Nucleic Acids with Multicomponent Peptide Hydrogels for the Advancement of Healthcare Technologies. Gels. 2025; 11(5):367. https://doi.org/10.3390/gels11050367
Chicago/Turabian StyleGiordano, Sabrina, Monica Terracciano, Enrico Gallo, Carlo Diaferia, Andrea Patrizia Falanga, Antonella Accardo, Monica Franzese, Marco Salvatore, Gennaro Piccialli, Nicola Borbone, and et al. 2025. "Investigating the Interactions of Peptide Nucleic Acids with Multicomponent Peptide Hydrogels for the Advancement of Healthcare Technologies" Gels 11, no. 5: 367. https://doi.org/10.3390/gels11050367
APA StyleGiordano, S., Terracciano, M., Gallo, E., Diaferia, C., Falanga, A. P., Accardo, A., Franzese, M., Salvatore, M., Piccialli, G., Borbone, N., & Oliviero, G. (2025). Investigating the Interactions of Peptide Nucleic Acids with Multicomponent Peptide Hydrogels for the Advancement of Healthcare Technologies. Gels, 11(5), 367. https://doi.org/10.3390/gels11050367













