Dosimetric Evaluation of the Sensitivity of PAGAT Gel Dosimeters Infused with Clinically Used Gadolinium-Based Contrast Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Gd-PAGAT Elaboration Viability
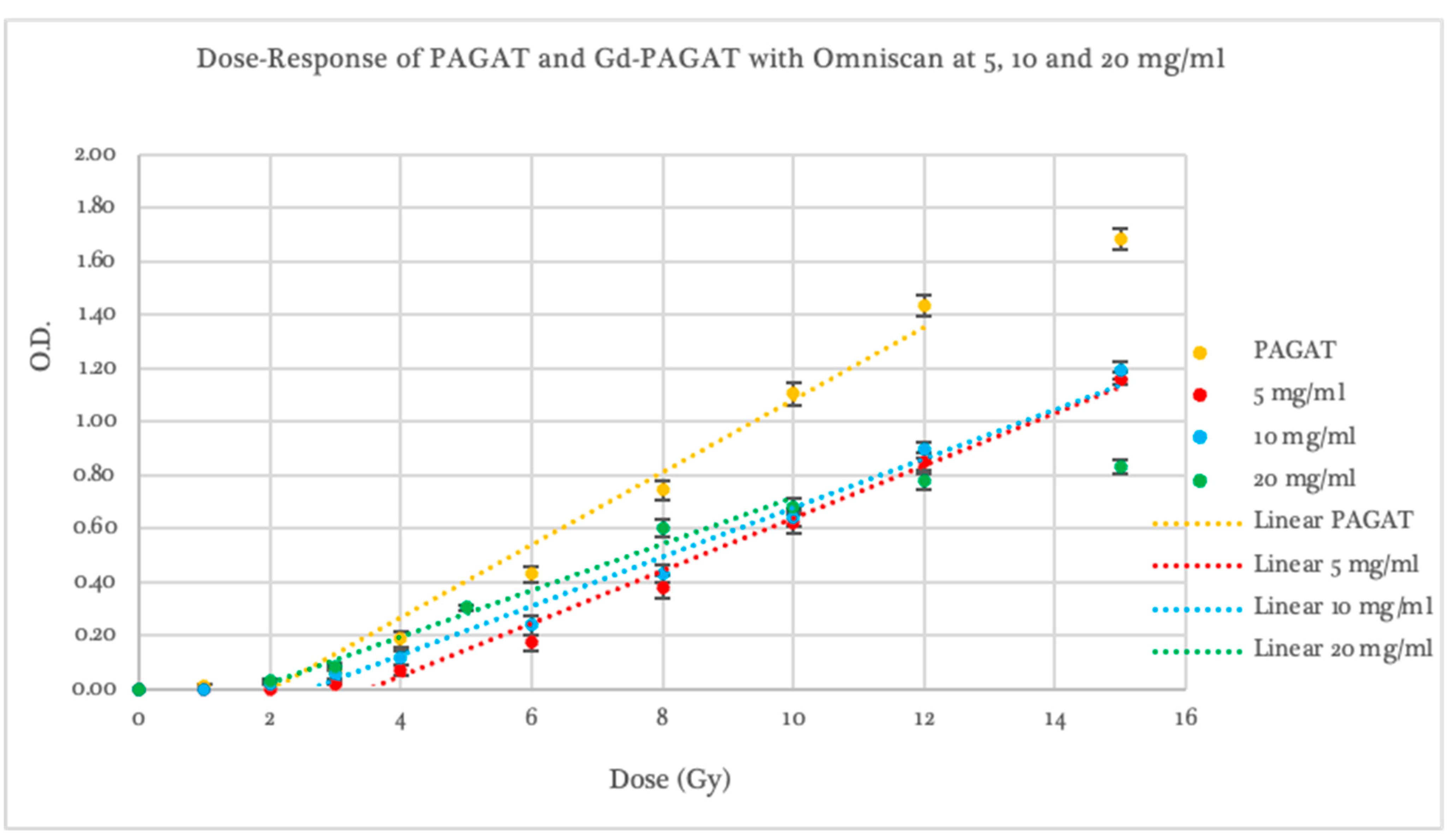
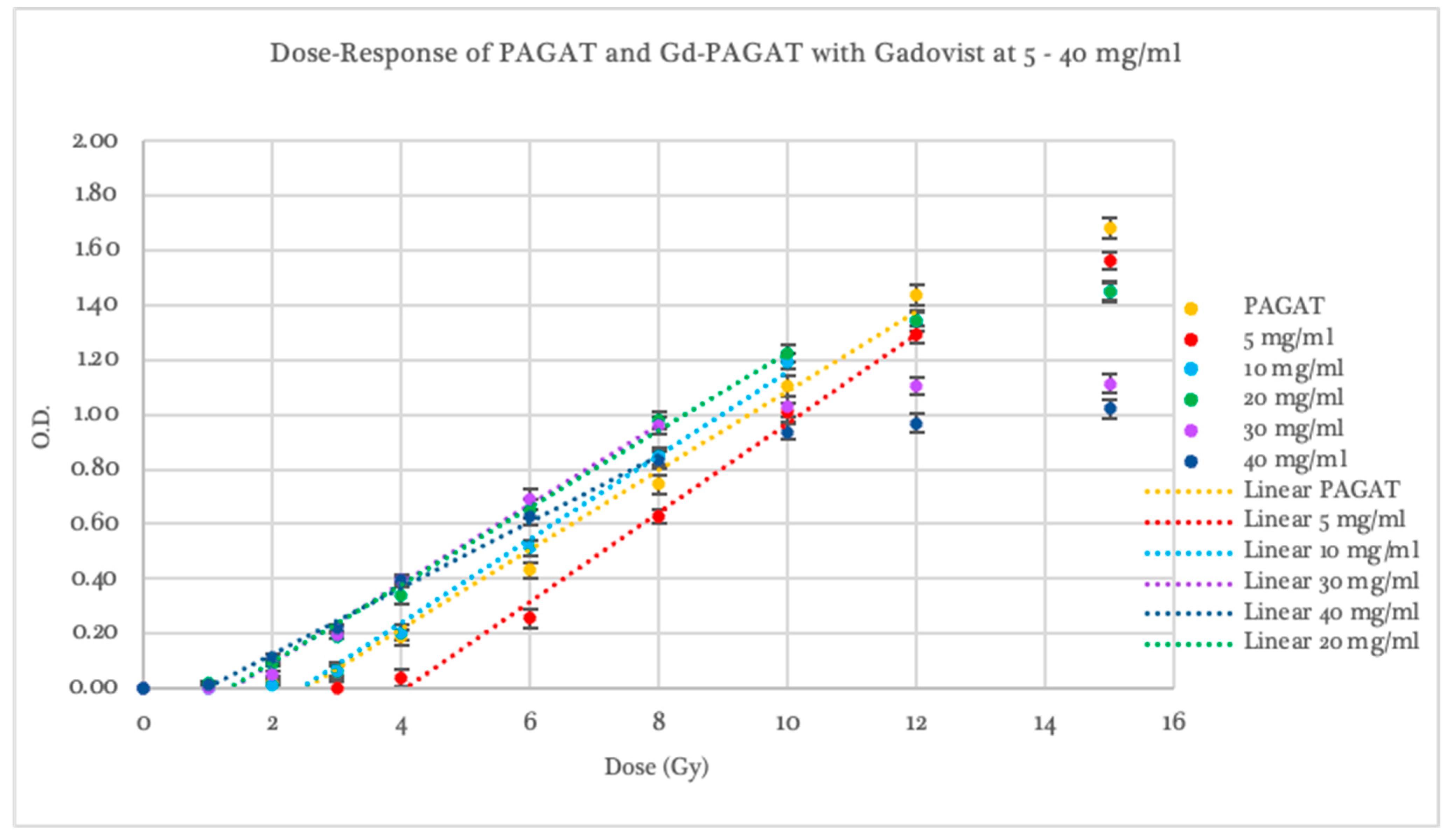
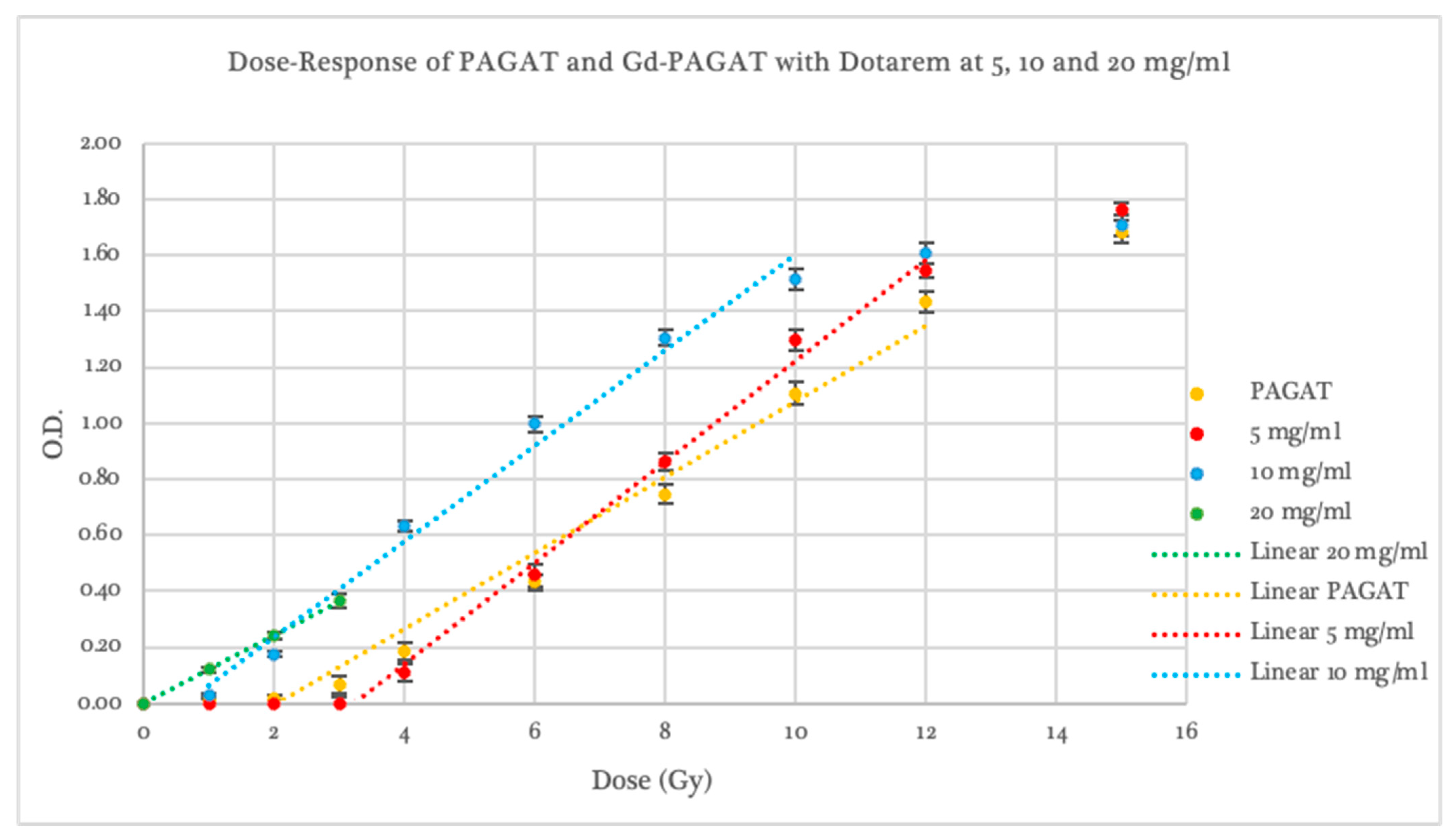
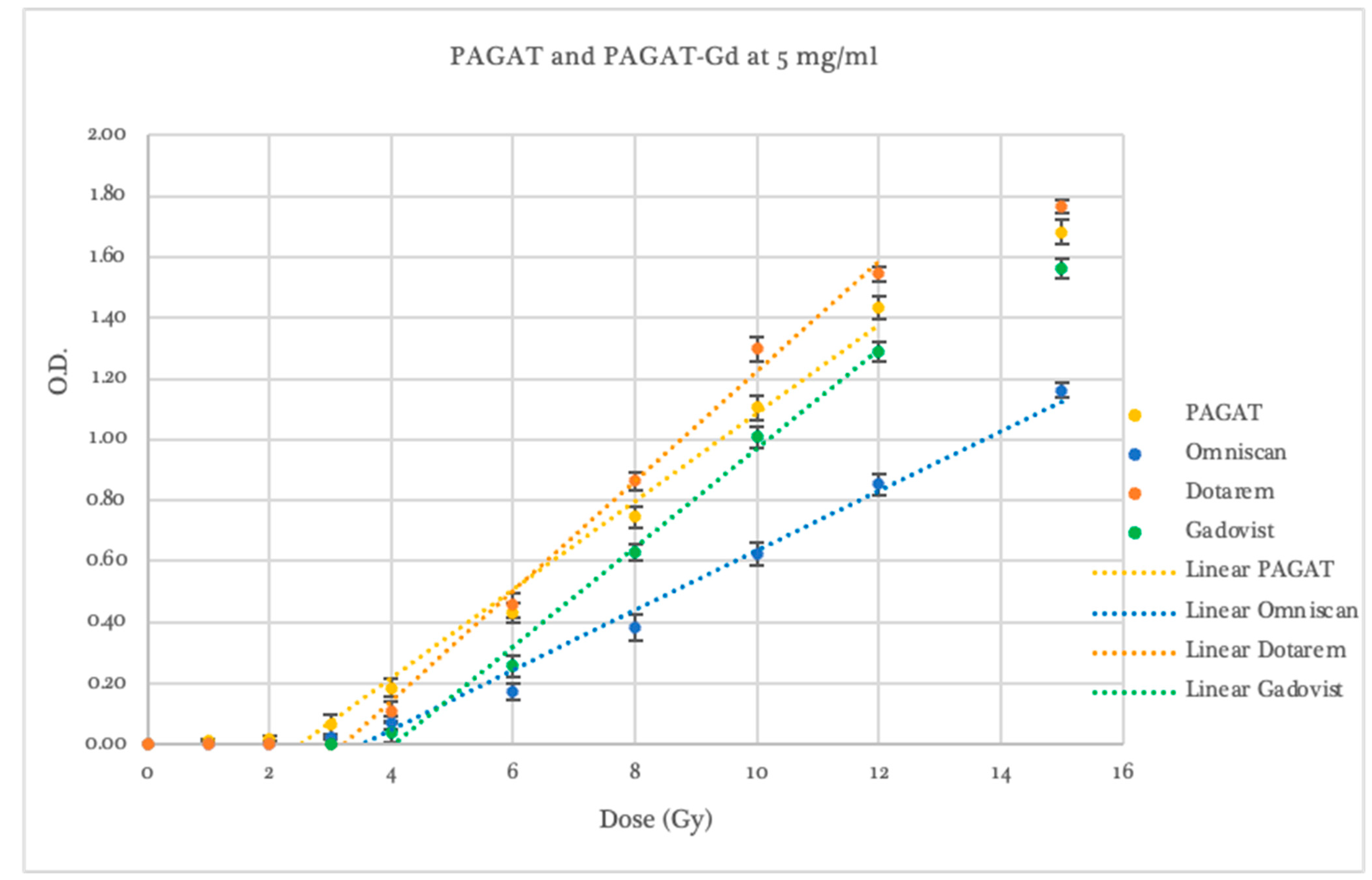
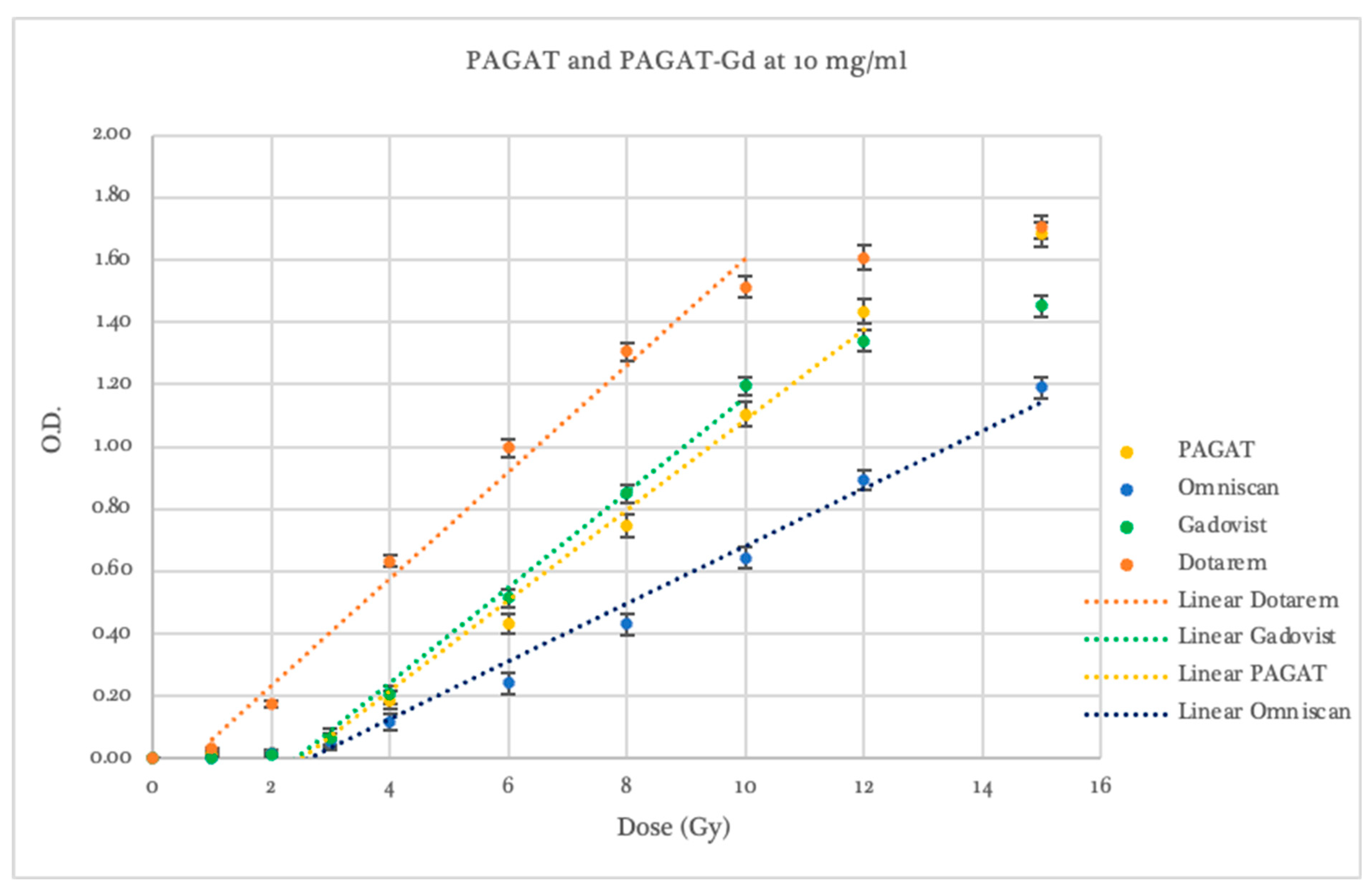
2.2. Dosimetric Evaluation According to Contrast Agent
2.3. Dosimetric Evaluation According to Gd Concentration
2.4. Radiological and Dosimetric Properties
3. Conclusions
4. Materials and Methods
4.1. Gd-PAGAT Infused Dosimeter
Gd Contrast Agent
4.2. Irradiation System
4.3. Dosimeter Processing
4.4. Radiological and Dosimetric Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, J.C.; Kang, Y.S. Measurement of Radiation Dose Distributions by Nuclear Magnetic Resonance (NMR) Imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.M.; Keil, D.C.; Does, M.D.; Gore, J.C. Polymer Gels for Magnetic Resonance Imaging of Radiation Dose Distributions at Normal Room Atmosphere. Phys. Med. Biol. 2001, 46, 3105–3113. [Google Scholar] [CrossRef]
- Deene, Y.D.; Hurley, C.; Venning, A.; Vergote, K.; Mather, M.; Healy, B.J.; Baldock, C. A Basic Study of Some Normoxic Polymer Gel Dosimeters. Phys. Med. Biol. 2002, 47, 3441–3463. [Google Scholar] [CrossRef] [PubMed]
- Macchione, M.A.; Lechón Páez, S.; Strumia, M.C.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Spěváček, V.; Pilařová, K.; Končeková, J.; Konček, O. The Influence of Antioxidant THPC on the Properties of Polymer Gel Dosimeter. Phys. Med. Biol. 2014, 59, 5141–5161. [Google Scholar] [CrossRef]
- Jirasek, A.; Hilts, M.; Shaw, C.; Baxter, P. Investigation of Tetrakis Hydroxymethyl Phosphonium Chloride as an Antioxidant for Use in X-Ray Computed Tomography Polyacrylamide Gel Dosimetry. Phys. Med. Biol. 2006, 51, 1891. [Google Scholar] [CrossRef]
- Vullo, W.J. Hydroxymethyl Replacement Reactions of Tetrakis(Hydroxymethyl)Phosphonium Chloride. IEC Prod. Res. Dev. 1966, 5, 346–349. [Google Scholar] [CrossRef]
- Djezzar, B. Ionizing Radiation Effects and Applications; BoD—Books on Demand: Norderstedt, Germany, 2018; ISBN 978-953-51-3953-9. [Google Scholar]
- Sabbaghizadeh, R.; Shamsudin, R.; Deyhimihaghighi, N.; Sedghi, A. Enhancement of Dose Response and Nuclear Magnetic Resonance Image of PAGAT Polymer Gel Dosimeter by Adding Silver Nanoparticles. PLoS ONE 2017, 12, e0168737. [Google Scholar] [CrossRef]
- Silveira, M.A.; Pavoni, J.F.; Baffa, O. Three-Dimensional Quality Assurance of IMRT Prostate Plans Using Gel Dosimetry. Phys. Med. 2017, 34, 1–6. [Google Scholar] [CrossRef]
- Cosgrove, V.P.; Murphy, P.S.; McJury, M.; Adams, E.J.; Warrington, A.P.; Leach, M.O.; Webb, S. The Reproducibility of Polyacrylamide Gel Dosimetry Applied to Stereotactic Conformal Radiotherapy. Phys. Med. Biol. 2000, 45, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Ertl, A.; Berg, A.; Zehetmayer, M.; Frigo, P. High-Resolution Dose Profile Studies Based on MR Imaging with Polymer BANGTM Gels in Stereotactic Radiation Techniques. Magn. Reson. Imaging 2000, 18, 343–349. [Google Scholar] [CrossRef]
- Papagiannis, P.; Karaiskos, P.; Kozicki, M.; Rosiak, J.M.; Sakelliou, L.; Sandilos, P.; Seimenis, I.; Torrens, M. Three-Dimensional Dose Verification of the Clinical Application of Gamma Knife Stereotactic Radiosurgery Using Polymer Gel and MRI. Phys. Med. Biol. 2005, 50, 1979–1990. [Google Scholar] [CrossRef]
- Farajollahi, A.R.; Bonnett, D.E.; Ratcliffe, A.J.; Aukett, R.J.; Mills, J.A. An Investigation into the Use of Polymer Gel Dosimetry in Low Dose Rate Brachytherapy. Br. J. Radiol. 1999, 72, 1085–1092. [Google Scholar] [CrossRef]
- Wuu, C.-S.; Schiff, P.; Maryanski, M.J.; Liu, T.; Borzillary, S.; Weinberger, J. Dosimetry Study of Re-188 Liquid Balloon for Intravascular Brachytherapy Using Polymer Gel Dosimeters and Laser-Beam Optical CT Scanner. Med. Phys. 2003, 30, 132–137. [Google Scholar] [CrossRef]
- Ramm, U.; Weber, U.; Bock, M.; Krämer, M.; Bankamp, A.; Damrau, M.; Thilmann, C.; Böttcher, H.D.; Schad, L.R.; Kraft, G. Three-Dimensional BANGTM Gel Dosimetry in Conformal Carbon Ion Radiotherapy. Phys. Med. Biol. 2000, 45, N95–N102. [Google Scholar] [CrossRef] [PubMed]
- Heufelder, J.; Stiefel, S.; Pfaender, M.; Lüdemann, L.; Grebe, G.; Heese, J. Use of BANG® Polymer Gel for Dose Measurements in a 68 MeV Proton Beam. Med. Phys. 2003, 30, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, H.; Bäck, S.Å.J.; Medin, J.; Grusell, E.; Olsson, L.E. Linear Energy Transfer Dependence of a Normoxic Polymer Gel Dosimeter Investigated Using Proton Beam Absorbed Dose Measurements. Phys. Med. Biol. 2004, 49, 3847–3855. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Gore, J.C.; Kennan, R.P.; Schulz, R.J. NMR Relaxation Enhancement in Gels Polymerized and Cross-Linked by Ionizing Radiation: A New Approach to 3D Dosimetry by MRI. Magn. Reson. Imaging 1993, 11, 253–258. [Google Scholar] [CrossRef]
- Brown, S.; Venning, A.; De Deene, Y.; Vial, P.; Oliver, L.; Adamovics, J.; Baldock, C. Radiological Properties of the PRESAGE and PAGAT Polymer Dosimeters. Appl. Radiat. Isot. 2008, 66, 1970–1974. [Google Scholar] [CrossRef]
- Sellakumar, P.; James Jebaseelan Samuel, E. Study on Energy Dependence of PAGAT Polymer Gel Dosimeter Evaluated Using X-Ray CT. Radiat. Meas. 2010, 45, 92–97. [Google Scholar] [CrossRef]
- Deene, Y.D.; Vergote, K.; Claeys, C.; Wagter, C.D. The Fundamental Radiation Properties of Normoxic Polymer Gel Dosimeters: A Comparison between a Methacrylic Acid Based Gel and Acrylamide Based Gels. Phys. Med. Biol. 2006, 51, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Chang, Y.J.; Hsieh, L.L.; Liu, M.H.; Liu, J.S.; Chu, C.H.; Hsieh, B.T. Dosimetry Study of Diagnostic X-Ray Using Doped Iodide Normoxic Polymer Gels. Radiat. Phys. Chem. 2014, 104, 414–419. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Pastorello, B.F.; Araújo, D.B.D.; Baffa, O. Formaldehyde Increases MAGIC Gel Dosimeter Melting Point and Sensitivity. J. Phys. Conf. Ser. 2009, 164, 012004. [Google Scholar] [CrossRef]
- Hayashi, S.; Fujiwara, F.; Usui, S.; Tominaga, T. Effect of Inorganic Salt on the Dose Sensitivity of Polymer Gel Dosimeter. Radiat. Phys. Chem. 2012, 81, 884–888. [Google Scholar] [CrossRef]
- Jirasek, A.; Hilts, M.; McAuley, K.B. Polymer Gel Dosimeters with Enhanced Sensitivity for Use in X-Ray CT Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, 5269–5281. [Google Scholar] [CrossRef]
- Koeva, V.I.; Olding, T.; Jirasek, A.; Schreiner, L.J.; McAuley, K.B. Preliminary Investigation of the NMR, Optical and x-Ray CT Dose–Response of Polymer Gel Dosimeters Incorporating Cosolvents to Improve Dose Sensitivity. Phys. Med. Biol. 2009, 54, 2779–2790. [Google Scholar] [CrossRef]
- Santibáñez, M.; Guillen, Y.; Chacón, D.; Figueroa, R.G.; Valente, M. Feasibility of Dose Enhancement Assessment: Preliminary Results by Means of Gd-Infused Polymer Gel Dosimeter and Monte Carlo Study. Appl. Radiat. Isot. 2018, 141, 210–218. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Zohdiaghdam, R.; Khalkhali, H.R.; Mousavi, F. Evaluation of Gold Nanoparticle Size Effect on Dose Enhancement Factor in Megavoltage Beam Radiotherapy Using MAGICA Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2019, 9, 89–96. [Google Scholar] [CrossRef]
- Merkis, M.; Griskonis, E.; Laurikaitiene, J.; Puiso, J.; Pikas, I.; Palvanov, S.; Adliene, D. Investigation of Dose Sensitivity and Dose Enhancement Effect in Silver Nanoparticle Enriched Dose Gels. Radiat. Phys. Chem. 2023, 213, 111213. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Tadros, S.M.; Beshir, W.B.; Saad, G.R.; Gallo, S.; Ali, L.I.; Naoum, M.M. Study of Ag Nanoparticles in a Polyacrylamide Hydrogel Dosimeters by Optical Technique. Gels 2022, 8, 222. [Google Scholar] [CrossRef]
- Farahani, S.; Riyahi Alam, N.; Haghgoo, S.; Shirazi, A.; Geraily, G.; Gorji, E.; Kavousi, N. The Effect of Bismuth Nanoparticles in Kilovoltage and Megavoltage Radiation Therapy Using Magnetic Resonance Imaging Polymer Gel Dosimetry. Radiat. Phys. Chem. 2020, 170, 108573. [Google Scholar] [CrossRef]
- Deyhimihaghighi, N.; Noor, N.M.; Soltani, N.; Jorfi, R.; Haghir, M.E.; Adenan, M.Z.; Saion, E.; Khandaker, M.U. Contrast Enhancement of Magnetic Resonance Imaging (MRI) of Polymer Gel Dosimeter by Adding Platinum Nano- Particles. J. Phys. Conf. Ser. 2014, 546, 012013. [Google Scholar] [CrossRef]
- Rajaee, A.; Wang, S.; Zhao, L.; Wang, D.; Liu, Y.; Wang, J.; Ying, K. Multifunction Bismuth Gadolinium Oxide Nanoparticles as Radiosensitizer in Radiation Therapy and Imaging. Phys. Med. Biol. 2019, 64, 195007. [Google Scholar] [CrossRef] [PubMed]
- Meesat, R.; Jay-Gerin, J.-P.; Khalil, A.; Lepage, M. Evaluation of the Dose Enhancement of Iodinated Compounds by Polyacrylamide Gel Dosimetry. Phys. Med. Biol. 2009, 54, 5909. [Google Scholar] [CrossRef]
- Santibáñez, M.; Fuentealba, M.; Vedelago, J.; Chacón, D.; Mattea, F.; Valente, M. Experimental Characterization and Monte Carlo Simulations of the Dose Enhancement on the Millimeter Scale of PAGAT Infused with Gadolinium. Radiat. Phys. Chem. 2021, 186, 109533. [Google Scholar] [CrossRef]
- Fiegel, V.; Berthon, C.; Costagliola, A.; Blain, G.; Vandenborre, J.; Vermeulen, J.; Saint-Louis, G.; Guerin, L.; Sauvage, T.; Fattahi-Vanani, M.; et al. Alpha Radiolysis of DOTA Ligand in Aqueous Solutions with Helium Ion Beams. Radiat. Phys. Chem. 2019, 165, 108409. [Google Scholar] [CrossRef]
- Venning, A.J.; Brindha, S.; Hill, B.; Baldock, C. Preliminary Study of a Normoxic PAG Gel Dosimeter with Tetrakis (Hydroxymethyl) Phosphonium Chloride as an Anti-Oxidant. J. Phys. Conf. Ser. 2004, 3, 155–158. [Google Scholar] [CrossRef]
- Carrasco Muñoz, S.; Calles Blanco, C.; Marcin, J.; Fernández Álvarez, C.; Lafuente Martínez, J. Contrastes basados en gadolinio utilizados en resonancia magnética. Radiología 2014, 56, 21–28. [Google Scholar] [CrossRef]
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI Contrast Agents: Basic Chemistry and Safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071. [Google Scholar] [CrossRef]
- Gallo, S.; Locarno, S.; Brambilla, E.; Lenardi, C.; Pignoli, E.; Veronese, I. Dosimetric Characterization of Double Network Fricke Hydrogel Based on PVA-GTA and Phenylalanine Peptide Derivative. J. Phys. Appl. Phys. 2023, 57, 075303. [Google Scholar] [CrossRef]
- Un, A. Water and Tissue Equivalency of Some Gel Dosimeters for Photon Energy Absorption. Appl. Radiat. Isot. 2013, 82, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Konefał, A.; Bakoniak, M.; Orlef, A.; Maniakowski, Z.; Szewczuk, M. Energy Spectra in Water for the 6 MV X-Ray Therapeutic Beam Generated by Clinac-2300 Linac. Radiat. Meas. 2015, 72, 12–22. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, S.B.; Kapur, A.; Li, J.; Pawlicki, T.; Ma, C.-M. Photon Beam Characterization and Modelling for Monte Carlo Treatment Planning. Phys. Med. Biol. 2000, 45, 411–427. [Google Scholar] [CrossRef]
- Shivaramu; Vijayakumar, R.; Rajasekaran, L.; Ramamurthy, N. Effective Atomic Numbers for Photon Energy Absorption of Some Low-Z Substances of Dosimetric Interest. Radiat. Phys. Chem. 2001, 62, 371–377. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 meV for Elements z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; National Institute of Standards and Technology, Ionizing Radiation Division: Gaithersburg, MD, USA, 1995. [Google Scholar]



| Compounds | Absorbance (a.u.) | SD |
|---|---|---|
| PAGAT | 0.060 | 0.003 |
| Omniscan | 0.005 | 0.001 |
| Dotarem | 0.007 | 0.001 |
| Gadovist | 0.007 | 0.001 |
| Omniscan 5 mg/mL | 0.061 | 0.005 |
| Omniscan 10 mg/mL | 0.055 | 0.006 |
| Omniscan 20 mg/mL | 0.072 | 0.003 |
| Dotarem 5 mg/mL | 0.069 | 0.004 |
| Dotarem 10 mg/mL | 0.108 | 0.023 |
| Dotarem 20 mg/mL | 1.455 | 0.136 |
| Gadovist 5 mg/mL | 0.063 | 0.005 |
| Gadovist 10 mg/mL | 0.062 | 0.009 |
| Gadovist 20 mg/mL | 0.051 | 0.005 |
| Gadovist 30 mg/mL | 0.040 | 0.005 |
| Gadovist 40 mg/mL | 0.035 | 0.003 |
| Dosimeter | Threshold (Gy) | p-Value Compared to PAGAT | Slope | p-Value Compared to PAGAT | Linear Range (Gy) | Saturation (Gy) |
|---|---|---|---|---|---|---|
| PAGAT | 2.46 ± 0.13 | 1.0000 | 0.153 ± 0.005 | 1.0000 | 2–12 | 12 |
| Omniscan 5 mg/mL | 3.50 ± 0.53 | 0.0692 | 0.098 ± 0.005 | 0.0002 | 3–15 | -- |
| Omniscan 10 mg/mL | 3.35 ± 0.31 | 0.0247 | 0.101 ± 0.005 | 0.0002 | 2–15 | -- |
| Omniscan 20 mg/mL | 1.75 ± 0.33 | 0.0520 | 0.087 ± 0.059 | 0.1915 | 2–8 | 8 |
| Dotarem 5 mg/mL | 3.40 ± 0.42 | 0.0501 | 0.186 ± 0.008 | 0.0065 | 4–12 | 12 |
| Dotarem 10 mg/mL | 0.64 ± 0.32 | 0.0450 | 0.171 ± 0.010 | 0.0701 | 1–10 | 10 |
| Dotarem 20 mg/mL | 0.01 ± 0.01 | 0.0009 | 0.121 ± 0.0004 | 0.0077 | 0–3 | -- |
| Gadovist 5 mg/mL | 4.04 ± 0.46 | 0.0206 | 0.163 ± 0.008 | 0.1539 | 4–12 | 12 |
| Gadovist 10 mg/mL | 2.72 ± 0.07 | 0.0538 | 0.162 ± 0.003 | 0.0687 | 2–10 | 10 |
| Gadovist 20 mg/mL | 1.55 ± 0.12 | 0.0009 | 0.147 ± 0.004 | 0.1833 | 1–10 | 10 |
| Gadovist 30 mg/mL | 1.60 ± 0.11 | 0.0011 | 0.153 ± 0.004 | 1.0000 | 2–8 | 8 |
| Gadovist 40 mg/mL | 1.01 ± 0.17 | 0.0004 | 0.122 ± 0.005 | 0.0016 | 1–8 | 8 |
| Dosimeters | Density g/cm3 | ZPEAeff 1.60–1.75 MeV | ZPEAeff 3.8 MeV | ZPEAeff 5.0 MeV | μen/ρ 1.60–1.75 MeV (cm2/g) | μen/ρ 3.8 MeV (cm2/g) | μen/ρ 5.0 MeV (cm2/g) |
|---|---|---|---|---|---|---|---|
| PAGAT | 1.030 | 3.38 | 3.46 | 3.53 | 2.742 × 10−2 | 2.098 × 10−2 | 1.905 × 10−2 |
| Omniscan 5 mg/mL | 1.038 | 3.42 | 3.50 | 3.57 | 2.737 × 10−2 | 2.097 × 10−2 | 1.906 × 10−2 |
| Gadovist 5 mg/mL | 1.039 | 3.40 | 3.49 | 3.56 | 2.738 × 10−2 | 2.097 × 10−2 | 1.906 × 10−2 |
| Dotarem 5 mg/mL | 1.040 | 3.40 | 3.49 | 3.56 | 2.737 × 10−2 | 2.097 × 10−2 | 1.906 × 10−2 |
| Omniscan 10 mg/mL | 1.046 | 3.43 | 3.52 | 3.59 | 2.733 × 10−2 | 2.096 × 10−2 | 1.907 × 10−2 |
| Gadovist 10 mg/mL | 1.046 | 3.42 | 3.51 | 3.58 | 2.735 × 10−2 | 2.097 × 10−2 | 1.907 × 10−2 |
| Dotarem 10 mg/mL | 1.049 | 3.43 | 3.52 | 3.59 | 2.733 × 10−2 | 2.096 × 10−2 | 1.907 × 10−2 |
| Omniscan 20 mg/mL | 1.063 | 3.46 | 3.56 | 3.64 | 2.727 × 10−2 | 2.097 × 10−2 | 1.911 × 10−2 |
| Gadovist 20 mg/mL | 1.064 | 3.46 | 3.55 | 3.63 | 2.728 × 10−2 | 2.097 × 10−2 | 1.909 × 10−2 |
| Dotarem 20 mg/mL | 1.069 | 3.46 | 3.56 | 3.64 | 2.727 × 10−2 | 2.097 × 10−2 | 1.911 × 10−2 |
| Gadovist 30 mg/mL | 1.080 | 3.48 | 3.58 | 3.67 | 2.723 × 10−2 | 2.097 × 10−2 | 1.913 × 10−2 |
| Gadovist 40 mg/mL | 1.100 | 3.52 | 3.63 | 3.72 | 2.716 × 10−2 | 2.096 × 10−2 | 1.915 × 10−2 |
| Gelatin (% w/w) | Water (% w/w) | BIS (% w/w) | Acrylamide (% w/w) | Gd (% w/w) | |
|---|---|---|---|---|---|
| PAGAT | 5.0 | 88.7 | 3.0 | 3.0 | 0.0 |
| Omniscan 5 mg/mL | 5.0 | 80.7 | 3.0 | 3.0 | 8.1 |
| Omniscan 10 mg/mL | 5.0 | 74.0 | 3.0 | 3.0 | 14.7 |
| Omniscan 20 mg/mL | 3.9 | 62.0 | 2.3 | 2.3 | 29.2 |
| Gadovist 5 mg/mL | 5.0 | 84.2 | 3.0 | 3.0 | 4.5 |
| Gadovist 10 mg/mL | 5.0 | 81.3 | 3.0 | 3.0 | 7.4 |
| Gadovist 20 mg/mL | 5.0 | 73.6 | 3.0 | 3.0 | 15.1 |
| Gadovist 30 mg/mL | 3.9 | 69.3 | 2.3 | 2.3 | 22.0 |
| Gadovist 40 mg/mL | 3.9 | 61.9 | 2.3 | 2.3 | 29.3 |
| Dotarem 5 mg/mL | 5.0 | 81.3 | 3.0 | 3.0 | 7.5 |
| Dotarem 10 mg/mL | 5.0 | 73.9 | 3.0 | 3.0 | 14.9 |
| Dotarem 20 mg/mL | 3.9 | 61.6 | 2.3 | 2.3 | 29.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentealba, M.; Vallejos, C.; Díez, S.; Santibáñez, M. Dosimetric Evaluation of the Sensitivity of PAGAT Gel Dosimeters Infused with Clinically Used Gadolinium-Based Contrast Agents. Gels 2025, 11, 416. https://doi.org/10.3390/gels11060416
Fuentealba M, Vallejos C, Díez S, Santibáñez M. Dosimetric Evaluation of the Sensitivity of PAGAT Gel Dosimeters Infused with Clinically Used Gadolinium-Based Contrast Agents. Gels. 2025; 11(6):416. https://doi.org/10.3390/gels11060416
Chicago/Turabian StyleFuentealba, Melani, Carolina Vallejos, Sergio Díez, and Mauricio Santibáñez. 2025. "Dosimetric Evaluation of the Sensitivity of PAGAT Gel Dosimeters Infused with Clinically Used Gadolinium-Based Contrast Agents" Gels 11, no. 6: 416. https://doi.org/10.3390/gels11060416
APA StyleFuentealba, M., Vallejos, C., Díez, S., & Santibáñez, M. (2025). Dosimetric Evaluation of the Sensitivity of PAGAT Gel Dosimeters Infused with Clinically Used Gadolinium-Based Contrast Agents. Gels, 11(6), 416. https://doi.org/10.3390/gels11060416





