Surface Relief Gratings of Slide-Ring Hydrogels for Label-Free Biosensing
Abstract
1. Introduction
2. Results and Discussion
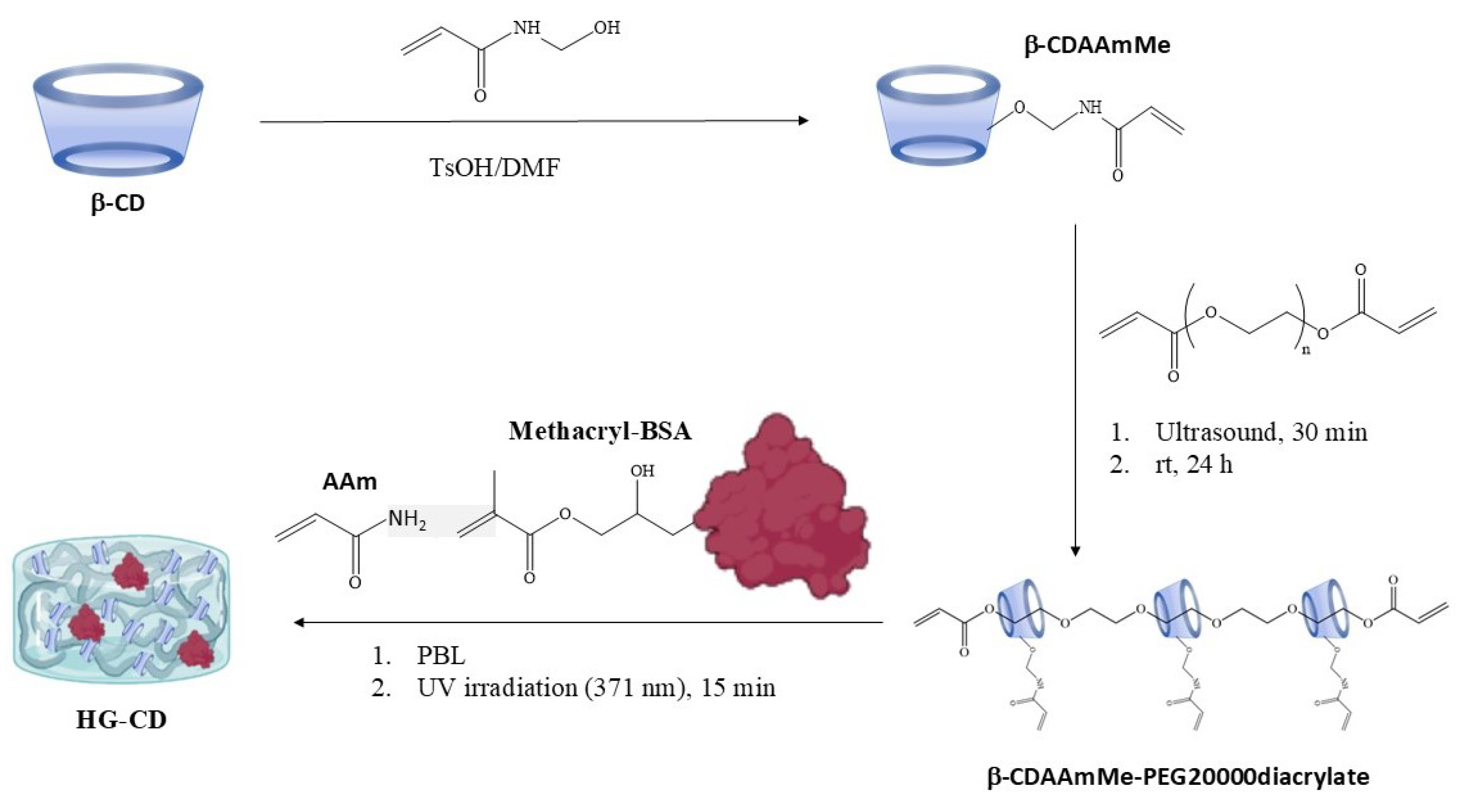
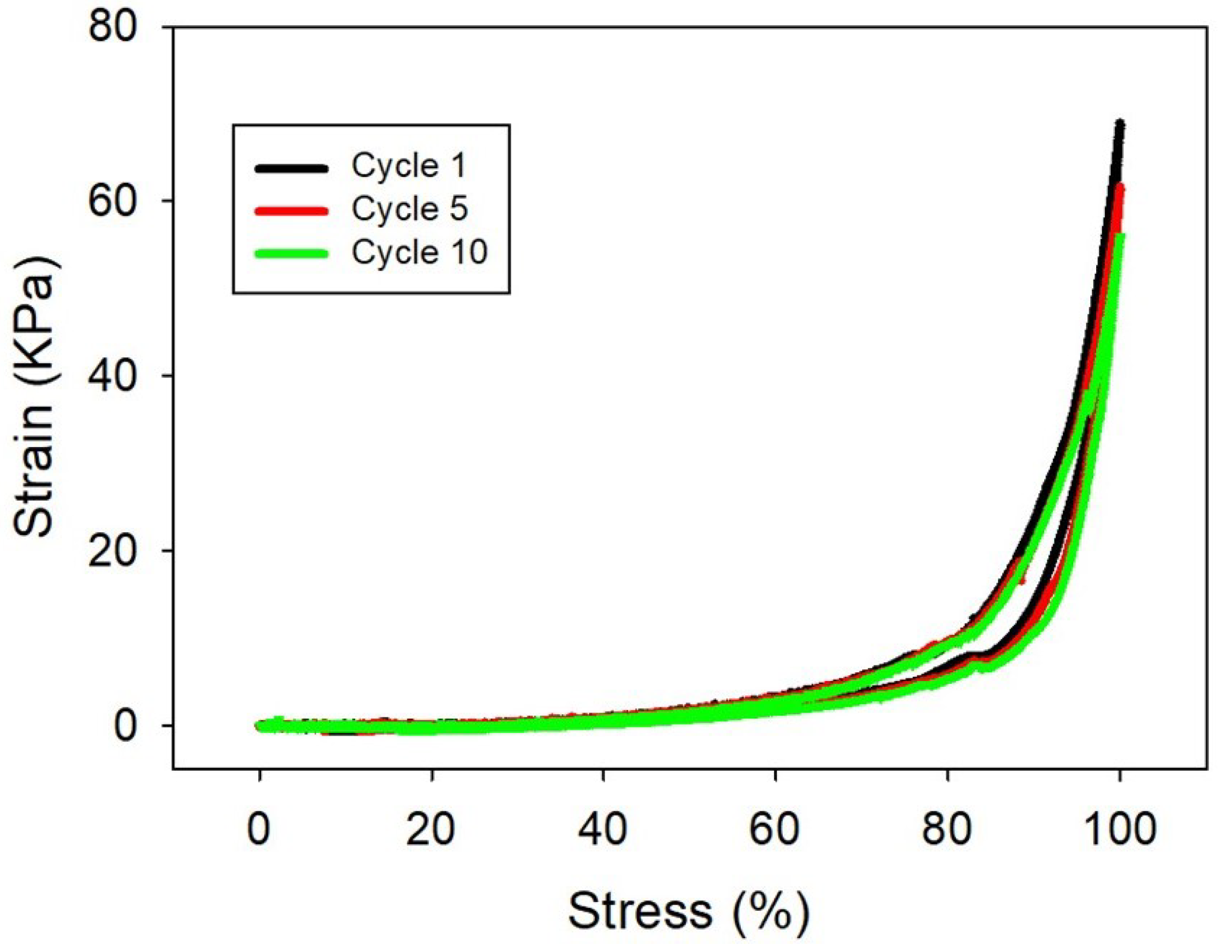
2.1. Synthesis and Characterization of Hydrogels
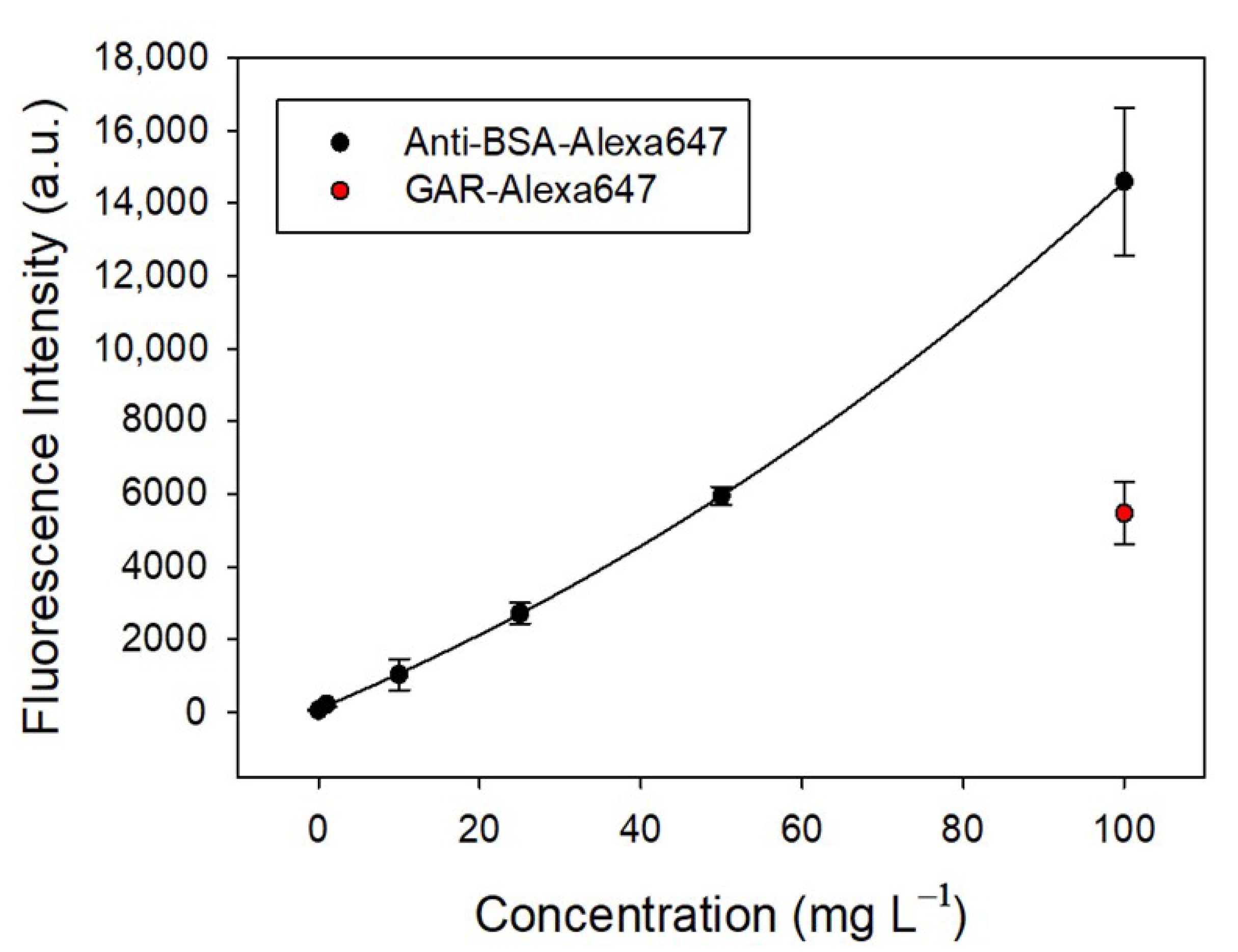
2.2. Ability to Specifically Recognize Anti-BSA by Fluorescence Assays
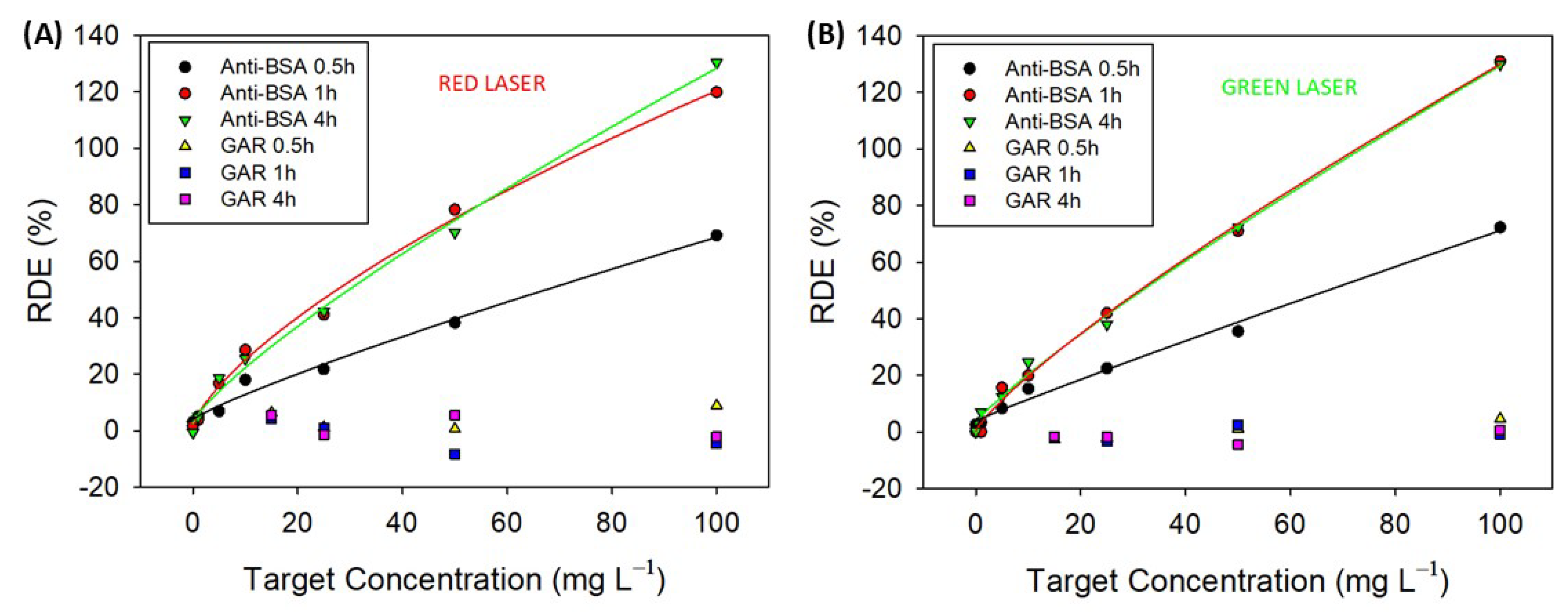
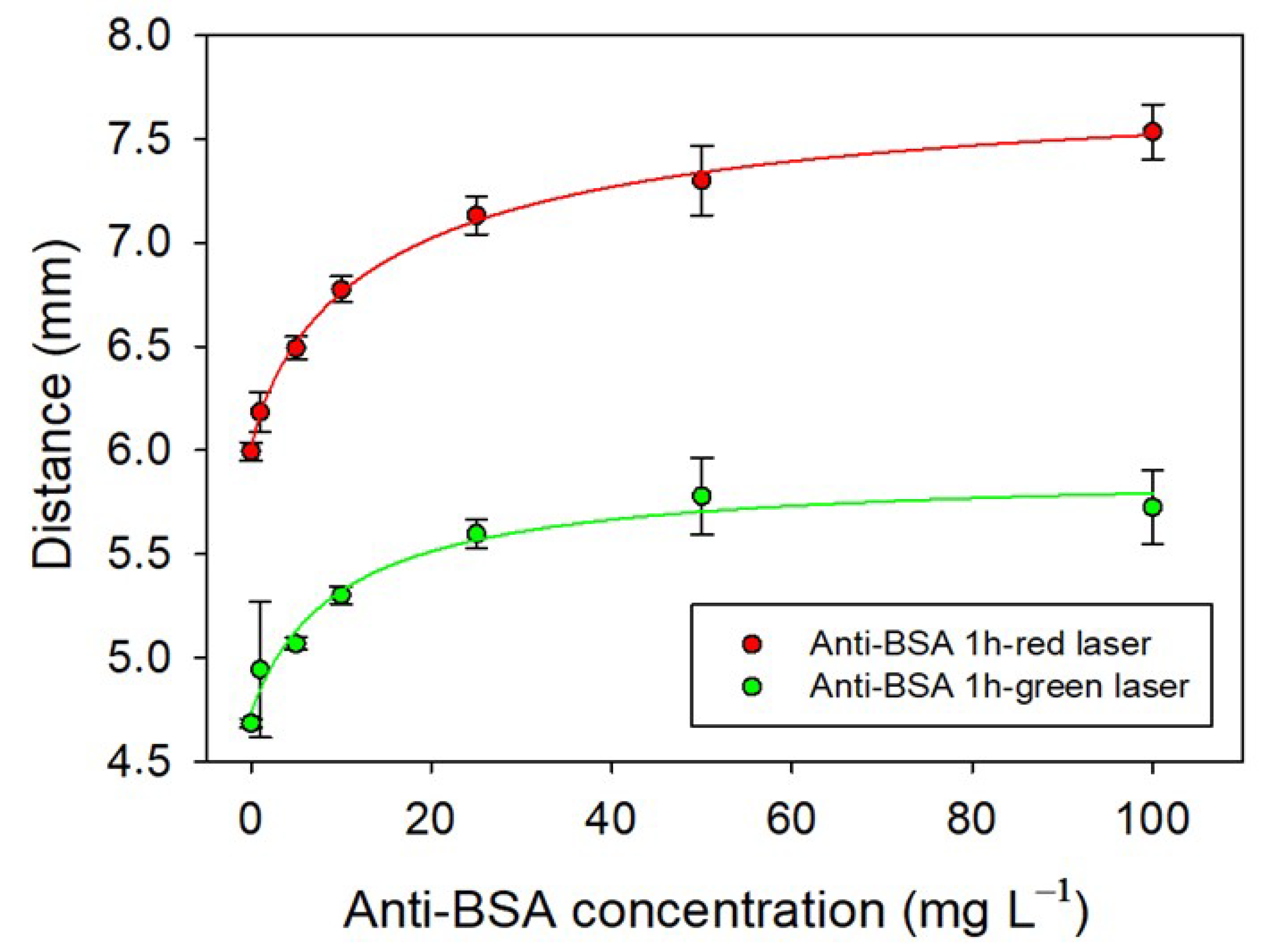
2.3. Label-Free Biosensing of Anti-BSA-Sensing Hydrogel–CD
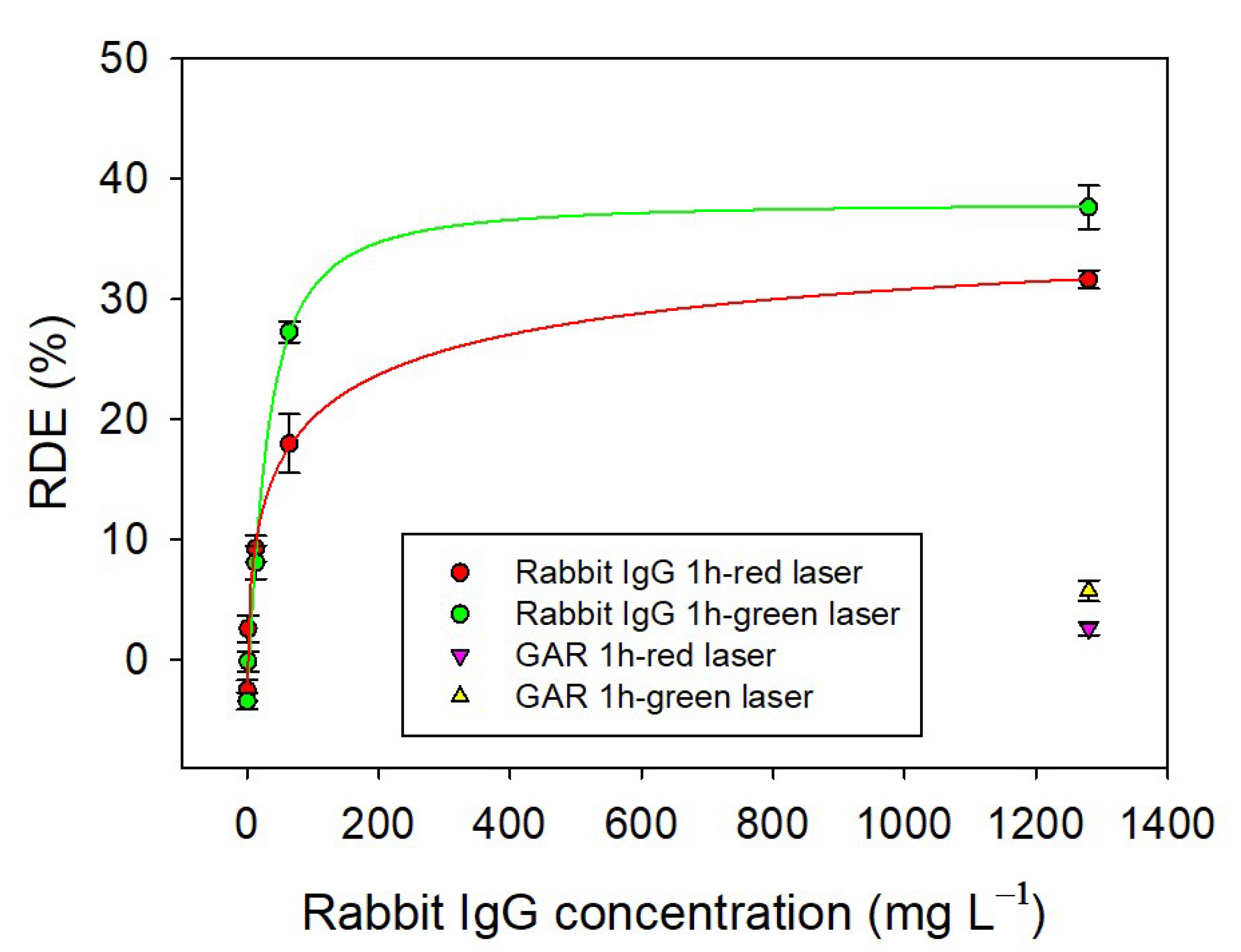
2.4. Detection of RIgG in a Rabbit Serum Sample
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Slide-Ring Hydrogels (HG-CD-6)
4.2. Fabrication of Antibody-Sensitive Hydrogel–CD SRGs
4.3. Mechanical Properties Characterization
4.4. Scanning Electron Microscopy (SEM)
4.5. Swelling Studies
4.6. Fluorescence Measurements
4.7. Diffraction Efficiency Measurements
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAm | Acrylamide |
| Alexa Fluor 647 | NHS Alexa Fluor 647 ester (succinimidyl ester) |
| anti-BSA | Anti-bovine serum albumin antibody |
| BSA | Bovine serum albumin |
| CDs | Cyclodextrins |
| DE (%) | Diffraction efficiency |
| DLIP | Direct laser interference patterning |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| GAR | Goat anti-rabbit antibody |
| GAM | Goat anti-mouse IgG (H+L) cross-adsorbed secondary antibody |
| HG-CD | Hydrogel with cyclodextrin |
| HG-CD-6 SRGs | Anti-BSA-sensitive hydrogel–CD surface relief gratings |
| IgG | Total immunoglobulin G |
| IVDs | In vitro diagnostics |
| LF | Label-free |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| PBS1X | Phosphate buffered saline ( |
| PBS-T | PBS1X containing 0.05% Tween 20 |
| PBL | Phenyl-2,4,6-lithium trimethyl-benzoylphosphinate |
| PDMS | Polydimethylsiloxane |
| PEG20000diacrylate | Poly(ethylene glycol) diacrylate |
| POC | Point-of-care |
| PRs | Polyrotaxanes |
| RDE (%) | Relative diffraction efficiency |
| RIgGs | Rabbit IgG antibodies |
| RIgGs-HG-CD SRGs | Anti-rabbit IgG-sensitive hydrogel–CD surface relief gratings |
| SEM | Scanning electron microscopy |
| SR | Slide-ring |
| SRGs | Surface relief gratings |
| β-CD | β-Cyclodextrin |
| β-CDAAmMe | β-CD modified with acrylate groups |
| β-CDAAmMe-PEG20000diacrylate | Pseudopolyrotaxane |
References
- Cagnani, G.R.; da Costa Oliveira, T.; Mattioli, I.A.; Sedenho, G.C.; Castro, K.P.R.; Crespilho, F.N. From Research to Market: Correlation between Publications, Patent Filings, and Investments in Development and Production of Technological Innovations in Biosensors. Anal. Bioanal. Chem. 2023, 415, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Valente, B.; Pinto, H.; Pereira, T.S.; Campos, R. Exploring Biosensors’ Scientific Production and Research Patterns: A Bibliometric Analysis. Sensors 2024, 24, 3082. [Google Scholar] [CrossRef]
- Li, C.Z. Special Topic: Point-of-Care Testing (POCT) and In Vitro Diagnostics (IVDs). J. Anal. Test. 2019, 3, 1–2. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-Free Optical Biosensors for Food and Biological Sensor Applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of New Label-Free Techniques for Biosensors: A Review. Crit. Rev. Biotechnol. 2016, 36, 465–481. [Google Scholar] [CrossRef]
- Lucío, M.I.; Cubells-Gómez, A.; Maquieira, Á.; Bañuls, M.J. Hydrogel-Based Holographic Sensors and Biosensors: Past, Present, and Future. Anal. Bioanal. Chem. 2021, 414, 993–1014. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X. Aptamer-Functionalized Hydrogel Diffraction Gratings for the Human Thrombin Detection. Chem. Commun. 2013, 49, 5957–5959. [Google Scholar] [CrossRef]
- Ye, G.; Yang, C.; Wang, X. Sensing Diffraction Gratings of Antigen-Responsive Hydrogel for Human Immunoglobulin-G Detection. Macromol. Rapid Commun. 2010, 31, 1332–1336. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wang, W.; Wang, W.; Wang, F.; Zhao, Y.; Cai, Q.W.; Xie, R.; Xie, R.; Ju, X.J.; Ju, X.J.; et al. Smart Hydrogel Grating Immunosensors for Highly Selective and Sensitive Detection of Human-IgG. Ind. Eng. Chem. Res. 2020, 59, 10469–10475. [Google Scholar] [CrossRef]
- Fuchs, Y.; Soppera, O.; Mayes, A.G.; Haupt, K. Holographic Molecularly Imprinted Polymers for Label-Free Chemical Sensing. Adv. Mater. 2013, 25, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Lucío, M.I.; Montoto, A.H.; Fernández, E.; Alamri, S.; Kunze, T.; Bañuls, M.J.; Maquieira, Á. Label-Free Detection of C-Reactive Protein Using Bioresponsive Hydrogel-Based Surface Relief Diffraction Gratings. Biosens. Bioelectron. 2021, 193, 113561. [Google Scholar] [CrossRef] [PubMed]
- Cubells-Gómez, A.; Lucío, M.I.; Bañuls, M.-J.; Maquieira, Á. Holographic Surface Relief Diffraction Gratings Made of Hydrogels for Direct Label-Free Biosensing of IgGs. Talanta 2024, 279, 126563. [Google Scholar] [CrossRef] [PubMed]
- Zezza, P.; Lucío, M.I.; Fernández, E.; Maquieira, Á.; Bañuls, M.J. Surface Micro-Patterned Biofunctionalized Hydrogel for Direct Nucleic Acid Hybridization Detection. Biosensors 2023, 13, 312. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-Links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Ito, K. Novel Entropic Elasticity of Polymeric Materials: Why Is Slide-Ring Gel so Soft? Polym. J. 2011, 44, 38–41. [Google Scholar] [CrossRef]
- Gavrilov, A.A.; Potemkin, I.I. Adaptive Structure of Gels and Microgels with Sliding Cross-Links: Enhanced Softness, Stretchability and Permeability. Soft Matter 2018, 14, 5098–5105. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely Stretchable Thermosensitive Hydrogels by Introducing Slide-Ring Polyrotaxane Cross-Linkers and Ionic Groups into the Polymer Network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Katsuno, C.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Pressure-Responsive Polymer Membranes of Slide-Ring Gels with Movable Cross-Links. Adv. Mater. 2013, 25, 4636–4640. [Google Scholar] [CrossRef]
- Sakai, T.; Murayama, H.; Nagano, S.; Takeoka, Y.; Kidowaki, M.; Ito, K.; Seki, T. Photoresponsive Slide-Ring Gel. Adv. Mater. 2007, 19, 2023–2025. [Google Scholar] [CrossRef]
- Nakahata, M.; Mori, S.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Materials Formed by Cross-Linked Polyrotaxanes with Reversible Bonds. Chem 2016, 1, 766–775. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.G.; et al. Polymerizable Rotaxane Hydrogels for Three-Dimensional Printing Fabrication of Wearable Sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Sun, Y.; Qin, Y.; Zhang, H.; Yu, X.; Liu, Y. Stretchable Slide-Ring Supramolecular Hydrogel for Flexible Electronic Devices. Commun. Mater. 2022, 3, 2. [Google Scholar] [CrossRef]
- Geerligs, M.; van Breemen, L.; Peters, G.; Ackermans, P.; Baaijens, F.; Oomens, C. In Vitro Indentation to Determine the Mechanical Properties of Epidermis. J. Biomech. 2011, 44, 1176–1181. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Poh, P.S.P.; Machens, H.G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef]
- Takashima, Y.; Sawa, Y.; Iwaso, K.; Nakahata, M.; Yamaguchi, H.; Harada, A. Supramolecular materials cross-linked by host-guest inclusion complexes: The effect of side chain molecules on mechanical properties. Macormolecules 2017, 50, 3254–3261. [Google Scholar] [CrossRef]
- Mira, D.; Llorente, R.; Morais, S.; Puchades, R.; Maquieira, A.; Marti, J. High-Throughput Screening of Surface-Enhanced Fluorescence on Industrial Standard Digital Recording Media. Proc. SPIE 2004, 5617, 364–373. [Google Scholar]
- Wang, T.; Wang, L.; Ma, N.; Zhang, Y.; Liu, L.; Wan, Y.; Zhou, L.; Qian, W. Nanoporous Polystyrene Inverse Opal Materials with Optical Interference Properties for Label-Free Biosensing. Langmuir 2024. [Google Scholar] [CrossRef]
- Chain, C.Y.; Prieto, E.D.; Cisneros, J.S.; Daza Millone, M.A.; Ramirez, E.A.; Labriola, C.A.; Aniasi, V.; Villagra, A.P.M.; Franchin, L.; Paccagnella, A.; et al. Plasmonic Biosensor of Cruzipain-Antibody Complexes Based on Gold Nano-Gratings for Chagas Disease Screening. IEEE Sens. J. 2025, 25, 8230–8237. [Google Scholar] [CrossRef]
- Chen, J.K.; Zeng, X.Y.; Chang, C.J.; Chen, C.W. Poly[2-(Dimethylamino)Ethyl Methacrylate]/Gold Nanoparticle Composite Dual-Stripe Nanowire Arrays as Optical Biosensors for Label-Free Plague Diagnosis. J. Taiwan Inst. Chem. Eng. 2023, 146, 104855. [Google Scholar] [CrossRef]
- Beliaev, L.Y.; Takayama, O.; Xiao, S. Effectively Detecting Cardiac Myoglobin by Use of Bound States in the Continuum in Silicon Nitride Gratings. J. Appl. Phys. 2024, 135, 223101. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, X.; Gao, K.; Yin, Z.; Liu, W.; Zhong, B.; Kan, G.; Wang, X.; Jiang, J.; et al. Highly Absorbing Monolayer MoS2 for a Large Reflection Phase Modulation. Adv. Opt. Mater. 2024, 12, 2400429. [Google Scholar] [CrossRef]
- Li, Y.; Du, M.; He, S.; Wang, R.; Zhang, Z.; Wang, Q. Sensitive Label-Free Hemoglobin Detection Based on Polydopamine Functionalized Graphene Oxide Coated Micro-Tapered Long-Period Fiber Grating. Optik 2023, 275, 170626. [Google Scholar] [CrossRef]
- Guo, W.; Yu, Y.; Jin, G.; Xin, C. Optical Fiber Biosensor with a Bilayer Bio-Sensitive Film for Label-Free Detection of Pseudomonas Exotoxin A. Chin. Opt. Lett. 2024, 22, 111201. [Google Scholar] [CrossRef]
- Shavronskaya, D.O.; Nazarova, E.A.; Krivoshapkina, E.F. Optical Bi-Enzyme-Titania Biosensor System: A New Way to Detect Lactose. Biosens Bioelectron. X 2023, 14, 100347. [Google Scholar] [CrossRef]
- Han, P.; Li, Y.; Zhao, B.; Li, H.; Wang, Z.; Liu, X.; Meng, W. Visual and Label-Free Detection of Urea Based on Amorphous Photonic Films with Non-Iridescent Structural Colors. Anal. Chim. Acta 2025, 1345, 343731. [Google Scholar] [CrossRef]
- Sinha, S.; Panda, T.K.; Sarkar, P.; Palai, G.; Pal, M.; Kumar, B.A.; Mohanty, S.K. Designing Efficient Photonic Waveguides for Glucose Detection in Human Urine for Diabetic Management. J. Opt. 2025, 1–13. [Google Scholar] [CrossRef]
- Hasler, R.; Cattozzo Mor, D.; Aktug, G.; Fossati, S.; Vu, V.T.; Tamayo, A.; Giordani, E.; Ricciardi, E.; Giacomini, P.; Perutka, J.; et al. Surface Plasmon Resonance Biosensor with Anti-Crossing Modulation Readout. Sens. Actuators B Chem. 2024, 417, 136163. [Google Scholar] [CrossRef]
- Laffont, E.; Valour, A.; Crespo-Monteiro, N.; Berini, P.; Jourlin, Y. Biosensing in the Optical Switch Configuration on Strong Plasmonic Gratings Enabling Differential Referenced Detection. Sens. Biosensing Res. 2024, 45, 100681. [Google Scholar] [CrossRef]
- Tselikov, G.I.; Danilov, A.; Shipunova, V.O.; Deyev, S.M.; Kabashin, A.V.; Grigorenko, A.N. Topological Darkness: How to Design a Metamaterial for Optical Biosensing with Ultrahigh Sensitivity. ACS Nano 2023, 17, 19338–19348. [Google Scholar] [CrossRef]
- Bae, M.; Choi, S.; Kim, J.; Seo, G.; Lee, Y.W. Temperature-Insensitive Label-Free SARS-CoV-2 Spike Protein Detection Based on Complementary Refractive Index and Temperature Dependence of Multi-Mode Interference and Grating Resonance. Talanta 2024, 266, 125091. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Rajpal, S.; Kar, D.; Devinder, S.; Pandey, S.; Mishra, P.; Joseph, J. Guided Mode Resonance Immunosensor for Label-Free Detection of Pathogenic Bacteria Pseudomonas Aeruginosa. Biosens. Bioelectron. 2023, 241, 115695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Gong, W.; Bu, D.; Zhou, J.; Wu, Z.; Yu, R. Stable Liquid Crystal-Infiltrated Photonic Crystal Sensing Film for Facile Detection of Streptomycin. Biosens. Bioelectron. 2025, 275, 117225. [Google Scholar] [CrossRef]
- Labella, E.; Gupta, R. An Optofluidic Young Interferometer for Electrokinetic Transport-Coupled Biosensing. Micromachines 2024, 15, 861. [Google Scholar] [CrossRef]
- Li, X.; Jia, M.; Yu, L.; Li, Y.; He, X.; Chen, L.; Zhang, Y. An Ultrasensitive Label-Free Biosensor Based on Aptamer Functionalized Two-Dimensional Photonic Crystal for Kanamycin Detection in Milk. Food Chem. 2023, 402, 134239. [Google Scholar] [CrossRef]
- Occhicone, A.; Sinibaldi, A.; Chiappetta, D.; Di Matteo, P.; Pileri, T.; Danz, N.; Sonntag, F.; Munzert, P.; Allegretti, M.; De Pascale, V.; et al. Detection of Anti-SARS CoV-2 Antibodies in Human Serum by Means of Bloch Surface Waves on 1D Photonic Crystal Biochips. Biosens. Bioelectron. X 2023, 15, 100413. [Google Scholar] [CrossRef]
- Oliveira, N.C.L.; Khoury, G.E.; Versnel, J.M.; Moghaddam, G.K.; Leite, L.S.; Lima-Filho, J.L.; Lowe, C.R. A Holographic Sensor Based on a Biomimetic Affinity Ligand for the Detection of Cocaine. Sens. Actuators B Chem. 2018, 270, 216–222. [Google Scholar] [CrossRef]
- Juste-Dolz, A.; Delgado-Pinar, M.; Avella-Oliver, M.; Fernández, E.; Pastor, D.; Andrés, M.V.; Maquieira, Á. BIO Bragg Gratings on Microfibers for Label-Free Biosensing. Biosens. Bioelectron. 2021, 176, 112916. [Google Scholar] [CrossRef]
- Juste-Dolz, A.; Fernández, E.; Micó, G.; Bru, L.A.; Muñoz, P.; Avella-Oliver, M.; Pastor, D.; Maquieira, Á. Surface Bragg Gratings of Proteins Patterned on Integrated Waveguides for (Bio)Chemical Analysis. Microchim. Acta 2024, 191, 63. [Google Scholar] [CrossRef]
- Calvo-Lozano, O.; Sierra, M.; Soler, M.; Estévez, M.C.; Chiscano-Camón, L.; Ruiz-Sanmartin, A.; Ruiz-Rodriguez, J.C.; Ferrer, R.; González-López, J.J.; Esperalba, J.; et al. Label-Free Plasmonic Biosensor for Rapid, Quantitative, and Highly Sensitive COVID-19 Serology: Implementation and Clinical Validation. Anal. Chem. 2022, 94, 975–984. [Google Scholar] [CrossRef]
- Adi, W.; Biswas, D.; Shelef, M.A.; Yesilkoy, F. Multiplexed COVID-19 Antibody Quantification from Human Sera Using Label-Free Nanoplasmonic Biosensors. Biomed. Opt. Express 2022, 13, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Lobry, M.; Hassan, E.M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 Breast Cancer Biomarker Detection Using a Sandwich Optical Fiber Assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubells-Gómez, A.; Lucío, M.I.; Bañuls, M.-J.; Maquieira, Á. Surface Relief Gratings of Slide-Ring Hydrogels for Label-Free Biosensing. Gels 2025, 11, 415. https://doi.org/10.3390/gels11060415
Cubells-Gómez A, Lucío MI, Bañuls M-J, Maquieira Á. Surface Relief Gratings of Slide-Ring Hydrogels for Label-Free Biosensing. Gels. 2025; 11(6):415. https://doi.org/10.3390/gels11060415
Chicago/Turabian StyleCubells-Gómez, Aitor, María Isabel Lucío, María-José Bañuls, and Ángel Maquieira. 2025. "Surface Relief Gratings of Slide-Ring Hydrogels for Label-Free Biosensing" Gels 11, no. 6: 415. https://doi.org/10.3390/gels11060415
APA StyleCubells-Gómez, A., Lucío, M. I., Bañuls, M.-J., & Maquieira, Á. (2025). Surface Relief Gratings of Slide-Ring Hydrogels for Label-Free Biosensing. Gels, 11(6), 415. https://doi.org/10.3390/gels11060415









