Abstract
Strontium-added bioactive glass (SBG) has been widely used in bone tissue engineering. SBG can be prepared by conventional high-temperature melt-quenching or calcining sol-gelled powder at 700 °C or above. In the present study, the effects of calcination temperature (400–650 °C) and the amount of strontium addition (0–7 mol.%) were investigated simultaneously. The sol-gel process and post-calcination were used to prepare the Sr-added 58S bioactive glass (SBG) powders. The bioactivity of the SBG powder was assessed by immersing it in simulated body fluid, while biocompatibility and cytotoxicity were evaluated using L929 and MG63 cells, and a zebrafish animal model. The calcination temperatures were determined by thermogravimetric analysis based on the weight loss at various stages. X-ray diffraction was used to reveal the crystalline structure of calcined or SBF-immersed SBG powders. Meanwhile, the texture characteristics of SBG powders were examined by the BET method. Fourier-transformed infrared spectroscopy and scanning electron microscopy were used to investigate the absorption bands and powder morphology of SBG powders before and after SBF immersion. The experimental results showed that all SBG powders were mesoporous with a high specific surface area larger than 200 m2/g. SBG powder calcined at 650 °C with 5% Sr addition possessed a major Ca14.92(PO4)2.35(SiO4)5.65 phase, the smallest pore size of 5.86 nm, and the largest specific surface area of 233 m2/g. It was noncytotoxic and exhibited good bioactivity and biocompatibility.
1. Introduction
As the aging population increases, the number of bone defect cases has risen significantly, driving a high demand for advanced bone repair materials. These materials must be biocompatible, anti-inflammatory, and anti-infective while being biodegradable and capable of promoting bone tissue regeneration. Thus, the development of bioactive glass has become a key focus in current research and innovation. Bioactive glass (BG) was discovered by Prof. L.L. Hench’s team as Bioglass® 45S5 in 1969 [1]. It is composed of 45 wt.% SiO2, 24.5 wt.% Na2O, 24.5 wt.% CaO, and 6 wt.% P2O5 [2], and the composition can be bonded chemically with bone tissue to form a hydroxyapatite (HA) layer and promote cell growth [3]. After the invention of Bioglass® 45S5 [1,2], a series of bioactive glasses with various compositions have been investigated, such as 58S [4], 64S [5], 70S [6], 80S [7], etc. Its excellent biological performance has made bioactive glass a superior bone substitute in bone tissue engineering. The main preparation methods for bioactive glass include melt-quenching [8,9], spray drying [10,11], spray pyrolysis [12,13], and the sol-gel process [14,15].
Melt-quenching and sol-gel are conventional techniques to prepare BG powder. By melt-quenching, a mixture of starting powders is mixed, melted at high temperature, and quickly quenched to prepare bioactive glass frits. For energy conservation, the low-temperature sol-gel method is an alternative way to prepare the desired BG powder. The sol-gel process, a facile chemical route, has been widely used to prepare BG powder due to its unique processing advantages. For instance, various precursors for ion sources, adjustable parameters (aging time, calcination temperature, and time), surfactants, and incorporation of therapeutic ions (zinc, strontium, etc.) can be used to modify the so-prepared BG for the desired applications [16]. In addition, the sol-gel process can be used to prepare BG powders, films, and objects with desired shapes. This makes the sol-gel process an attractive processing technique for various applications [17]. Numerous research papers make use of the sol-gel process to prepare bioactive powders that can serve as carriers for drug delivery and additives for bioscaffolds. Doping with various functional ions can further extend the applications of bioactive glass powder. Many biologically active ions, such as zinc, magnesium, cerium, strontium, fluoride, etc., have been incorporated with BG to grant it antibacterial, anti-inflammatory, and osteoconductive properties. Pantulap et al. [18] have reviewed a wide variety of less-common ions that were introduced into the bioactive glass systems. Among the doped ions, strontium ion was the most widely investigated, followed by Zn, Ag, Mg, etc., for the past two decades.
Strontium is abundant in the human body, with the majority found in bones and teeth. Sr is an alkaline earth metal element and is in the same group as calcium, allowing it to easily substitute calcium to form an apatite phase. Its bone-stimulating and antibacterial abilities make Sr an attractive doping element in bioactive glass for bone tissue engineering [19]. Silva et al. reviewed the effects of strontium-incorporated bioactive glass on bone regeneration [20]. The preparation techniques, types of BG, and the amount of strontium concentration have been addressed. Generally, sol-gelled bioactive glass with less than 5% Sr doping exhibits an improvement in the formation of hydroxyapatite. For example, Moghanian et al. [21] evaluated the effect of 700 °C-calcined sol-gel derived 0, 5, and 10 mol.% Sr-doped BG and demonstrated that 5 mol.% Sr-BG exhibited the maximum cell proliferation and alkaline phosphatase activity, as well as antibacterial ability. Ma et al. [4] investigated 58 SiO2-(38-x)CaO-xSrO-4P2O3 with x ranging from 0 to 10 mol.% and reported that Sr-containing BG decreased the sample degradation and delayed the apatite formation. Goudarzi et al. [22] also studied 58SBG at an interval of 2 mol.% of Sr, and the HA formation rate reached a maximum with 2 mol.% Sr substitution. Another work by Macon et al. [23], who prepared 58S BG (60 SiO2-36CaO-4P2O3, mol.%) by the sol-gel process and used SrO to substitute CaO (0–100 mL.%), demonstrated that HA nucleation did not occur at high Sr concentrations (75 and 100 mol.% SrO). Bahati et al. [14] prepared 74SiO2-(26-x)CaO-xSrO, where x = 0–5, and found that the in vitro apatite-forming ability decreased with increasing Sr concentration. Hu et al. [24] studied 80SiO2-4P2O3-16(SrO: CaO), where SrO = 0, 6, 15 mol.%, and found that 6Sr-BG was a promising dental repair biomaterial. Excess Sr substitution (15 Sr-BG) decreased cell proliferation, differentiation, and mineralization abilities.
As mentioned above, sol-gel-derived bioactive glass materials were calcined at temperatures ranging from 600 to 700 °C. The substitution of calcium ions by various concentrations of strontium ions was widely investigated with different types of bioactive glasses. For instance, Table S1 summarizes a comparison of various sol-gelled 58S bioactive glass powder with Sr additions. It can be noted that the effect of calcination temperature and Sr concentration on the structure, bioactivity, and biocompatibility of bioactive glass has seldom been investigated simultaneously. As shown in Table S1, Moghanian et al. [21] prepared Sr-doped BG (0, 5, and 10 mol.%) by the sol-gel process, and calcination was performed at 700 °C for 3 h. Texture characteristics (specific surface area and pore size), optimal Sr doping around 5 mol., and the effect of calcination at lower temperatures were not investigated. In the present study, bioactive glass powder with various Sr concentrations (3, 5, and 7 mol.%) was prepared by the sol-gel process followed by calcination at relatively lower temperatures (400–650 °C). The effect of calcination temperature and the amount of strontium concentration on the crystalline structure, texture characteristics, bioactivity, and biocompatibility of Sr-BG powders was investigated. Furthermore, selected samples were examined further by using a zebrafish animal model to determine the cytotoxicity before practical application.
2. Results and Discussion
2.1. Effect of Calcination Temperature and Strontium Addition on SBG
In order to select suitable calcination temperatures, 0SBG and 5SBG as-prepared sol-gelled powders were examined using thermogravimetric analysis, and the results are shown in Figure 1. Generally, the sol-gelled powders exhibited various stages of weight loss before stabilization. For the 0SBG powder (blue curve in Figure 1), a weight loss (6.4%) before heating up to 100 °C was observed. This can be attributed to the vaporization of absorbed water within the powder. Within the temperatures ranging from 100 to 325 °C, another weight loss (18.2%) occurred, probably due to the decomposition of organic solvents. From 325 to 600 °C, organic solvents continued to decompose, and crystallization occurred, which resulted in another stage of weight loss, 18.7%. Thereafter, limited weight loss of ~2% was exhibited for temperatures ranging from 600 to 800 °C. The overall weight loss for 0SBG powder was 45.3% during thermogravimetric analysis from room temperature to 800 °C. A similar trend can be observed for 5SBG powder (red curve in Figure 1), but the magnitude of weight loss (61.7%) was larger when compared to that of 0SBG powder. The weight loss for various stages was 12.2% (RT to 100 °C), 27.2% (100–325 °C), 19.5% (325–600 °C), and 2.3% (600–800 °C), respectively. The increase in weight loss with the substitution of calcium by strontium was also reported in the literature [21,25].
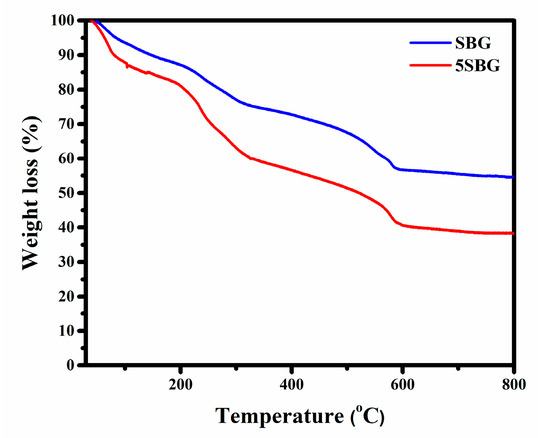
Figure 1.
Thermogravimetric analysis of 0SBG and 5SBG powders.
Based on the thermogravimetric analysis (TGA) results, limited weight loss was observed at temperatures higher than 600 °C. This suggests that calcination at 650 °C was able to remove the harmful organic residues within the bioactive glass powder. Thus, calcination was performed at 400, 500, 575, and 650 °C for 3 h, respectively. The X-ray diffraction (XRD) was used to reveal the crystalline structure of 0SBG and 5SBG powders after calcination. As shown in Figure 2a, the as-prepared 0SBG powder was amorphous without distinct diffraction peaks. After calcination at 400 °C, a few diffraction peaks appeared that corresponded to Ca9HPO4(PO4)5OH (calcium deficient hydroxyapatite or CDHAp, PDF 46-0905), Ca2SiO4 (calcium silicate, PDF 29-0369), and CaCO3 (calcium carbonate, PDF 05-0586) phases. A similar diffraction pattern with stronger diffraction intensities was observed for 0SBG powder calcined at 500 °C [26,27]. In addition, decomposition of CaCO3 and an increase in calcium silicate were also observed. Ca2SiO4 became the major phase instead of CDHAp. The complete decomposition of CaCO3 and phase transformation of Ca2SiO4 and CDHAp into oxyapatite (Ca10(PO4)6O, PDF 89-6495) occurred for 575 °C-calcined 0SBG powder [28]. When the calcination temperature was further increased to 650 °C, the calcium phosphate silicate phase (Ca14.92(PO4)2.35(SiO4)5.65, PDF 83–1494) became the major phase. Table 1 summarizes the detailed percentages of various phases for calcined 0SBG powder.
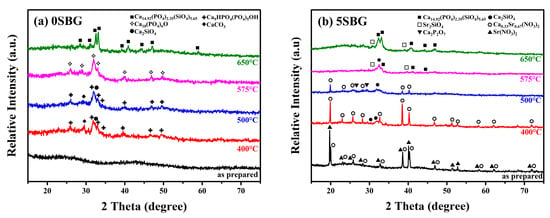
Figure 2.
X-ray diffraction patterns of as-prepared and calcined (a) 0SBG and (b) 5SBG powders.

Table 1.
Results of XRD analysis of 0SBG powder after calcination at various temperatures.
With the addition of strontium (Figure 2b), the as-prepared sol-gelled 5SBG powder exhibited a mixture of Ca0.33Sr0.67(NO3)2 phases (PDF 26-1073) and Sr(NO3)2 (PDF 25-0746). After calcining at 400 °C, Sr(NO3)2 disappeared, the crystalline peaks of Ca0.33Sr0.67(NO3)2 became more evident, and the formation of Ca2SiO4 was observed. When the calcination temperature was further increased to 500 °C, partial decomposition of the Ca0.33Sr0.67(NO3)2 phase (from 86.0 to 59.0 wt.%) was observed. In addition, a slight increase in the percentage of Ca2SiO4 (from 14.0 to 25.2%) and the formation of a calcium pyrophosphate (Ca2P2O7, PDF 33-0297) phase were observed [29]. The nitrides were decomposed completely after calcination at a temperature of 575 °C, and the formation of Ca14.92(PO4)2.35(SiO4)5.65 (PDF 83-1494) occurred [30]. For 650 °C-calcined 5SBG powder, Ca14.92(PO4)2.35(SiO4)5.65 was the major phase (87.8%), with a minor Sr2SiO4 (PDF 38-0271) phase [31]. The XRD patterns with detailed diffraction peaks of those referred phases are shown in Figure S1. The relative percentages of various phases for calcined-5SBG powders are summarized in Table 2.

Table 2.
Results of XRD analysis of 5SBG powder after calcination at various temperatures.
For testing the practical usage of bioactive glass, a calcination temperature of at least 650 °C was used in a previous study [21]. Thus, the calcination was set at 650 °C to investigate the effect of strontium concentration. Figure 3 shows 0–7SBG powder after calcination at 650 °C for 3 h. It can be noted that calcium phosphate silicate (i.e., Ca14.92(PO4)2.35(SiO4)5.65) was the major phase for all 0–7SBG powders, and its percentage decreased with increasing Sr concentration. With the addition of strontium, the formation of Sr2SiO4 was observed. Figure S2 shows the XRD patterns of 650 °C-calcined 0–7SBG powder with the referred phases. The amount of strontium silicate increased with increasing Sr addition up to 5SBG. For 7SBG with a high Sr concentration, the formation of calcium silicate was observed. Table 3 summarizes the resulting phases and the corresponding percentage of these 0–7SBG powders.
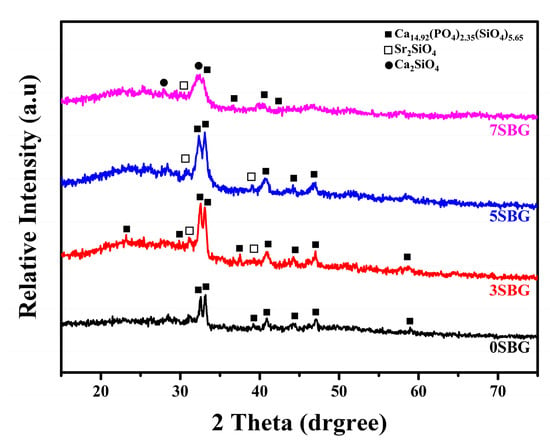
Figure 3.
X-ray diffraction patterns of 0–7SBG powder after calcination at 650 °C for 3 h.

Table 3.
Results of XRD analysis of 0–7SBG powder after calcination at 650 °C for 3 h.
In addition to the crystalline structures examined using the X-ray diffraction technique, texture characteristics, including surface area and porosity, were determined using the Barrett–Emmett–Teller (BET) method [32]. The specific surface area was 204.31 m2/g for 0SBG. It increased to 216.41 m2/g for 3SBG, reached a maximum of 233.07 m2/g for 5SBG, and slightly decreased to 232.56 m2/g for 7SBG. The addition of strontium increased the specific surface area of bioactive glass powder. In addition to the specific surface area, Figure 4 shows the nitrogen adsorption–desorption isotherms of the 0–7SBG powders. Similar hysteresis loops were observed for all 0–7SBG powders that exhibited a Type IV isotherm. It is also noted that the hysteresis loop possessed an inclined mound due to the existence of mesopores and was classified as semi-IUPAC H2 [33]. The average pore diameter was 6.04, 6.39, 5.86, and 5.92 nm for 0, 3, 5, and 7SBG, respectively. The pore size did not exhibit a monotonic trend, though 5SBG had a relatively small one. Moreover, the total pore volume was also similar. It was 0.32 cm3/g for 0 and 3SBG, and slightly decreased to 0.31 cm3/g for 5 and 7SBG. Table 4 summarizes the results of BET analysis for various 650 °C calcined-SBG powders.
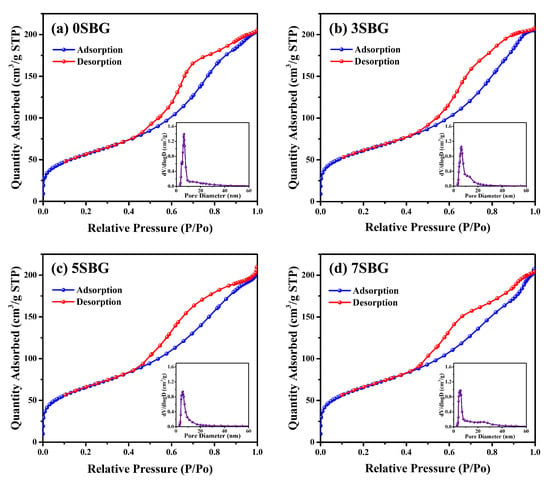
Figure 4.
BET analysis of (a) 0SBG, (b) 3SBG, (c) 5SBG, and (d) 7SBG powder after calcination at 650 °C for 3 h. The insert in each figure shows the result of pore size analysis.

Table 4.
BET results of various SBG powders after calcination at 650 °C for 3 h.
2.2. In Vitro Mineralization of SBG Powder
The bioactivities of the SBG powders were investigated by immersing them in simulated body fluid. The crystalline structure, powder morphology, and functional groups before and after immersion in simulated body fluids (SBFs) were examined using X-ray diffraction, scanning electron microscopy, and FT-IR spectroscopy. Figure 5 shows the XRD patterns of 0–7SBG powders before and after immersion in SBF for 1, 3, and 7 days, respectively. Similar behavior was observed for all SBG powders. After immersion in SBF for 1 day, almost all the crystalline peaks of the SBG powders disappeared (Figure 5a,b) or weakened significantly (Figure 5c,d). This suggests that the calcium or strontium (phosphate) silicates were dissolved into the SBF solution after immersion in SBF. For 0SBG powder with prolonged immersion to 3 or 7 days (Figure 5a), ambiguous hydroxyapatite crystalline peaks (PDF 09-0432) were observed after 7 days of SBF immersion [34]. With the addition of strontium (3SBG, Figure 5b), the formation of hydroxyapatite was observed after 1 day of SBF immersion, and the peak intensities increased with immersion time. This phenomenon became more evident for 5SBG, Figure 5c. The crystalline peaks of HA were the strongest compared to the other SBG powders. The HA phase became uncertain when the Sr concentration further increased to 7%, Figure 5d. The superfluous Sr concentration did not further improve the in vitro bioactivity but hindered the formation of hydroxyapatite. This shows a similar trend to that reported in the literature [4,23]. The crystalline phases of XRD patterns with references before and after immersion in SBF were presented in Figure S3. The SBF immersion experiments suggest that 5SBG powder exhibited optimal in vitro mineralization activity.
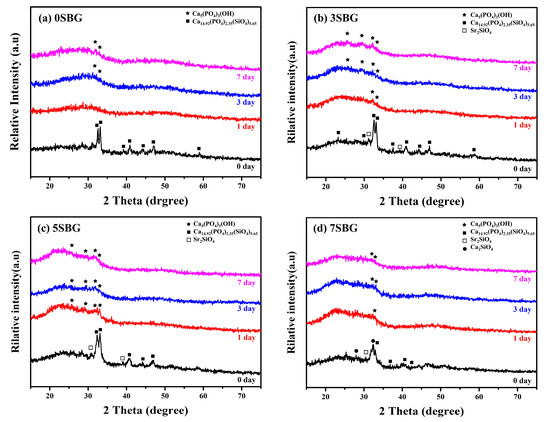
Figure 5.
XRD patterns of (a) 0SBG, (b) 3SBG, (c) 5SBG, and (d) 7SBG powder after immersion in simulated body fluid for 1, 3, and 7 days.
Figure 6 shows the FT-IR spectra of 0–7SBG powders before and after immersion in SBF for 7 days. As shown in Figure 6a, similar FT-IR spectra were observed for all SBG powders. Relatively broad bands of PO43− at 1073, 559, 517, and 469 cm−1 were observed together with CO32− (1490 and 878 cm−1), Si-O-Si (783 cm−1), and OH- (1654 cm−1) bands. After SBF immersion (Figure 6b), these bands became more evident compared to those of the so-prepared SBG powders. The vibration bands of PO43− at 1076, 563, and 468 cm−1 indicated the formation of hydroxyapatite [35,36].
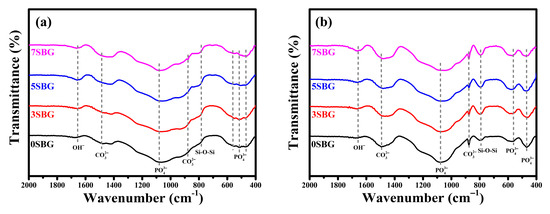
Figure 6.
FT-IR spectra of 0-7 SBG powder (a) before and (b) immersion in simulated body fluid for 7 days.
The presented FTIR curves were further deconvoluted with the observed bands with the Gaussian method [37,38,39]. The main difference of absorbed bands for HA formation fell within the wavenumber range from 1400 cm−1 to 400 cm−1. The PO43− functional groups (1073, 559, 517, and 469 cm−1), which were the characteristic IR absorption bands of HA, were calculated from their obtained fitting area percentage for semi-quantifying HA formation in SBF. Figure 7a shows the IR deconvolution curves with their fitting area. The estimated amounts of PO43− functional groups were 58.6, 50.2, 43.7, and 44.8% for 0, 3, 5, and 7SBG, respectively. After 7 days of immersion in simulated body fluid, as shown in Figure 7b, the absorbed bands of PO43− grew to 77.2, 70.3, 77.2, and 66.0% for 0, 3, 5, and 7SBG, respectively. The existence of CO32− is able to enhance the HA formation [40,41]. However, the amount of CO32- (878 cm−1) decreased after 7 days in simulated body fluid immersion from the FTIR spectra. The results suggest that the OH− in simulated body fluid (pH = 7.4) reduced the amount of CO32− on the SBG powders [42]. The amounts of PO43− and CO32− are listed in Table 5. Furthermore, the HA formation amount was calculated by the difference of the PO43− amount before and after SBF immersion to compare the HA formation ability, and the results are shown in Figure 8. It can be noted that the doping of Sr in bioactive glass improved the HA forming ability, and 5SBG exhibited the highest formation amount of HA (76.5%).
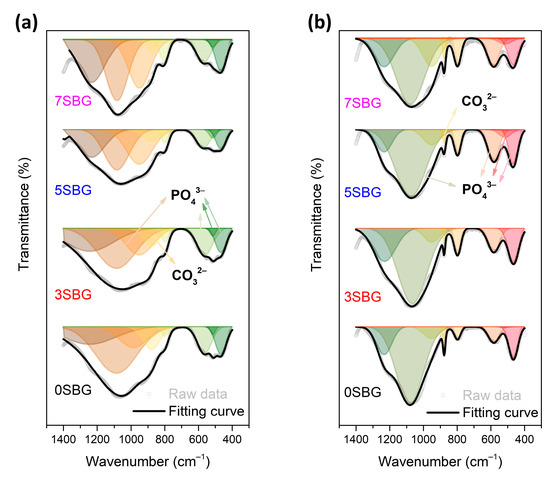
Figure 7.
The FTIR deconvolution curves (a) before and (b) after immersion in simulated body fluid for 7 days.

Table 5.
The fitting area amount of PO43− and CO32− groups for 0~7SBG before and after 7 days immersion in simulated body fluid.
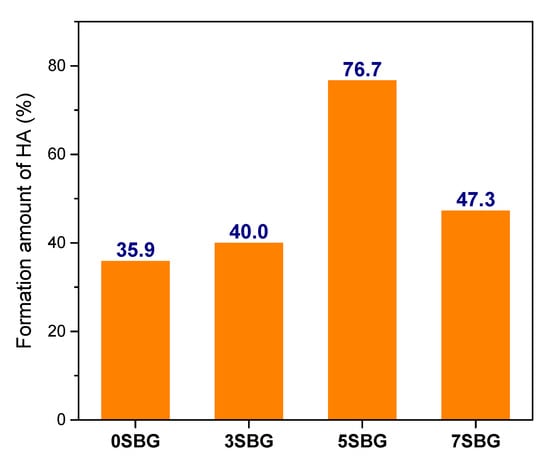
Figure 8.
The formation amount of HA calculated from the fitting area of PO43− in FTIR spectra.
The surface topography of the 0SBG and 5SBG before and after SBF immersion was examined by scanning electron microscopy, and Figure 9 shows the corresponding images. Before immersion, the 0SBF powders exhibited a plate-like microstructure covered with some small particles, as shown in Figure 9a. For the 5SBG powder shown in Figure 9b, plate-like particles with smaller particles were observed. After immersing in SBF, repetitive dissolution and precipitation of the SBG powder occurred. For 0SBG powder, precipitation of some small particles was observed, as shown in Figure 9c. This phenomenon became more evident for 5SBG powder. As shown by Figure 9d, numerous particles were precipitated on the large plate particles. Figure S4 shows the elemental mappings with Si, Ca, P, Sr, C, and O elements for 0SBG and 5SBG powders before and after immersion in SBF for 7 days, and Table S2 summarizes the corresponding EDX mapping results. A slight increase in Sr concentration after immersion was noticed. Compared with the FTIR deconvolution results, it was difficult to quantify the HA formation by EDX before and after SBF immersion, probably due to the relatively small amount of HA formation on the SBG powder. In addition to EDX, pH values after various SBF immersion times were measured, and the results are shown in Figure S5. An increase in pH value after immersion was noticed and 0SBG exhibited a slightly larger pH value than 5SBG, probably due to the release of Sr ions into the SBF solution, as shown in Figure S6.

Figure 9.
SEM images for 0SBG and 5SBG before (a,b) and after (c,d) immersion in simulated body fluid for 7 days, respectively.
As shown by the XRD, FTIR, and EDX, the deconvolution of FTIR spectra provided semi-quantitative evidence for HA formation after immersion in SBF. The amount of HA formation was 35.9% for 0SBG. It increased with Sr doping, reached a maximum of 76.7% for 5SBG, and decreased to 47.3% for 7SBG. This suggests that 5SBG powder was optimal among all SBG powders [24].
2.3. Biocompatibility and Cytotoxicity of SBG
Two types of cell lines (L929 and MG63) were used to evaluate the biocompatibility of 650 °C-calcined SBG powders by using a cell counting kit-8 (CCK8) assay. The statistical results represent three independent experiments, and n = 3 for each experiment. Figure 10 shows the cell viability of ISO-required L929 and human osteosarcoma MG63 cell lines for all 0–7SBG powders. Most of the cell viabilities for both cells were larger than 100% (except 93.94% for 3SBG tested by L929), and all of them met the ISO10993-5 standard requirement of 70% [43]. Generally, the cell viability of MG63 was higher than that of L929. It is also interesting to note that 5SBG exhibited the highest cell viability for both the L929 (117.22 ± 6.65%) and MG63 (121.13 ± 2.13%) cell lines. This indicates that, though all the SBG exhibited good biocompatibility, the 5SBG powder was the best among them. According to the result of the statistical analysis, 5SBG has significant differences with the control group (p-value = 0.0019) and 3SBG (p-value = 0.0005) for the L929 cells. In addition, 5SBG was significantly different from the control (p-value = 0.0017) and 7SBG (p-value = 0.0221) for the MG63 cells. Selected samples (0SBG and 5SBG) were examined further by using the live/dead staining assay, and the results are shown in Figure 11. Good cell proliferation and growth with limited dead cells for both the L929 (Figure 11a) and MG63 (Figure 11b) cells were observed for the 0SBG and 5SBG powders. This confirmed the excellent biocompatibility of the so-prepared SBG powders [44]. The release of Sr2+ ion from bioactive glass powder has proven to play a main role in cell proliferation and differentiation and is recognized for promoting osteoblast activity [45]. Owing to Sr2+ ions, the 5SBG powder exhibited the largest cellular activity in both the L929 and MG63 cells. The cell viability results are summarized in Table 6.
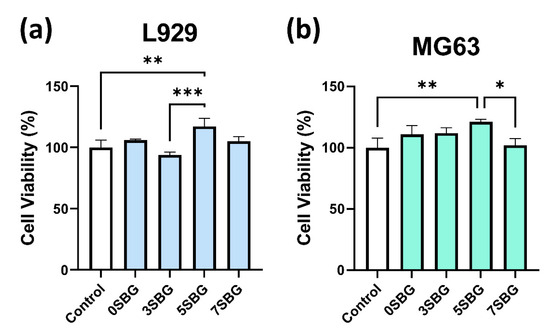
Figure 10.
Cell viabilities of (a) L929 and (b) MG63 for SBG powders were examined by using CCK-8 assay. *, **, and *** indicate that these two samples were statistically different at a 95%, 99%, and 99.9% confidence intervals, respectively.
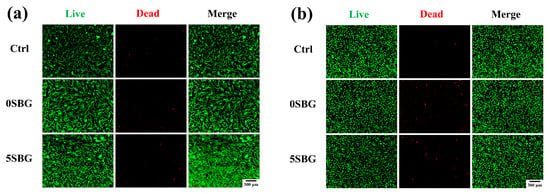
Figure 11.
Live/dead staining assay of (a) L929 and (b) MG63 cell lines for 0SBG and 5SBG powders.

Table 6.
Cell viability results of L929 and MG63 for SBG powders.
An in vitro animal test was performed using a zebrafish model (n = 30) to evaluate the cytotoxicity of the SBG powder. The hatch rate of the control group was 86.67%, and 0SBG and 5SBG had similar hatch rates of 83.33% and 86.57%. After hatching, the mortality rate was 0% for all three examined groups. Figure 12 shows the average length of the zebrafish after 72 h post-fertilization (i.e., hpf) and the images of selected ones from each group. The average length was 2.45 ± 0.08 mm for the control group, which remained the same for 0SBG (2.42 ± 0.14 mm), and slightly increased to 2.51 ± 0.13 mm for 5SBG. Only 0SBG and 5SBG were significantly different, with a p-value of 0.0294. This suggests that, in the zebrafish animal model, 5SBG did not exhibit cytotoxicity and was beneficial for the growth of zebrafish [46]. Bioactive glasses are known to release biologically active ions, such as calcium, silicon, and phosphorus, to promote skeletal development. The observed increase in body length may be attributed to the stimulatory effects of BG-derived ions on bone tissue formation and cellular proliferation [46]. Moreover, strontium is known to stimulate osteoblast activity to accelerate bone differentiation and mineralization during early development. The synergistic effect of Sr ions, alongside the release of Ca and Si from the BG matrix, [47] implies there is an osteoinductive potential in Sr-added bioactive glass when applied to zebrafish models.
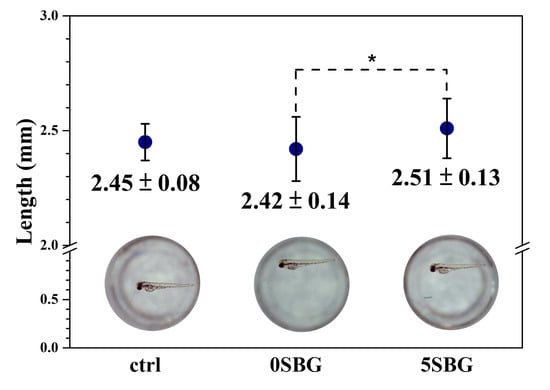
Figure 12.
The average length and a typical image of zebrafish after 72 h post-fertilization. * indicated that these two samples were statistically different at a 95% confidence interval.
The study by Moghanian et al. [21] reported bioactivity and biological studies by MC3T3-E1 of Sr-doped BG (0, 5, and 10 mol.%) prepared by the sol-gel process and calcined at 700 °C. The present study focused more on the effect of calcination temperature (400–650 °C) and the doping of Sr elements (0–7 mol.%). Biocompatibility was investigated by L929 and MG63 cells. In addition, an in vitro animal test using a zebrafish model was used for toxicological studies of SBG. From energy and cost considerations, sol-gelled bioactive glass powder calcined at 650 °C exhibited promising mineralization ability, biocompatibility, and non-cytotoxicity. Due to the limitations of the present work, further attempts to use SBG powder as bioactive materials to prepare bioscaffolds or compare the bioactive performance with commercial products are not available. The so-prepared SBG powder revealed its potential for bone regeneration applications. Based on the results, 5SBG may be the optimal additive for the preparation of bioscaffolds. Further investigations involving the preparation of SBG-added bioscaffolds, incorporation with BMP-2 growth factor, and comparison with commercially available bioscaffolds are in progress to determine whether this addition will improve bone formation in animal models. At present, the experiments in the current study demonstrate the potential clinical application of 5SBG-added composite bioscaffolds.
3. Conclusions
Sr-added 58S bioactive glass (0-7SBG) powders were prepared successfully using the sol-gel process, followed by calcination at various temperatures. Calcination at 650 °C for 3 h was able to prepare the desired bioactive glass powder. After calcination at 650 °C, all SBG powders possessed a major phase of Ca14.92(PO4)2.35(SiO4)5.65 that decreased with increasing amount of Sr addition. These SBG powders were mesoporous with an average pore size of ~6 nm. The 0SBG powder exhibited a specific surface area of 204.31 m2/g and increased to a maximum of 233.07 m2/g for 5SBG. The formation of hydroxyapatite was observed after immersing SBG powders in simulated body fluid. Deconvolution of FTIR spectra before and after immersion in SBF showed that the amount of HA formation was increased with Sr doping. The 5SBG powder exhibited good mineralization ability and a maximum of 76.7% for the amount of HA formation after 7 days of SBF immersion. All SBG powders were biocompatible, and the 5SBG powder was the best, with the highest cell viabilities of 117.22% and 121.13% for the L929 and MG63 cells, respectively. In the zebrafish animal model, the 0SBG and 5SBG powders were noncytotoxic, and the length of the zebrafish for the 5SBG group was the largest compared to that of the control or the 0SBG group. The 5SBG powder was optimal and exhibited potential to be used in bone tissue engineering.
4. Materials and Methods
4.1. Preparation of SBG Powder
Tetraethyl orthosilicate (TEOS, 98.0%), triethyl phosphate (TEP, 99%), and calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, 99–103%) were used as the starting materials for the preparation of 58S bioactive glass. Partial substitution of calcium by strontium (0, 3, 5, and 7 mol%) was designed to modify the composition of bioactive glass, and strontium nitrate (Sr(NO3)2, ≥99.0%) was used as the source for strontium ions. The strontium-added bioactive glass powder was coded as 0-7SBG, respectively. Table 7 summarizes the chemical compositions of various Sr-added bioactive glass powders investigated in the present study. 1M HNO3 was added to distilled water at a ratio of 1:3 to prepare a solution of 200 mL and mixed by magnetic stirring at 300 rpm for 15 min at 37 °C. TEOS, TEP, Ca(NO3)2·4H2O, and Sr(NO3)2 were added sequentially to the HNO3-containing solution every 15 min. The final mixture solution was stirred for 4 h, kept in a 37 °C incubator for 3 days, and dried in a vacuum oven (80 °C) for 1 day. The as-prepared powders were then calcined at a temperature of 400, 500, 575, and 650 °C for 3 h, respectively.

Table 7.
Chemical compositions of various Sr-added bioactive glass powders.
4.2. Characterization of SBG Powder
To determine the suitable calcination temperature, the as-prepared dried SBG powder was examined using a thermogravimetric analyzer (TGA-50, Shimazu, Kyoto, Japan) to observe the weight change under an ambient atmosphere with a heating rate of 10 °C/min from room temperature to 800 °C. The thermogravimetric analyzer was calibrated using a certified reference standard, and the temperature error was within ±1 °C. The as-prepared and calcined SBG powders were examined using X-ray diffraction (XRD) to reveal the crystalline structure. An X-ray diffractometer (D2 PHASER, Bruker, Billerica, MA, USA) was operated at an accelerating voltage of 30 kV and a current of 10 mA. Cu Kα radiation (λ = 1.542 Å) was filtered by a Ni film and used to obtain the XRD 2θ pattern ranging from 15° to 75° at a step size of 0.05° with a scanning speed of 3.75°/min. The crystalline phases within XRD patterns were identified using an evaluation software (DIFFRAC.EVA, version 5.2., Bruker, Billerica, MA, USA). Only the top three major crystalline phases were used for calculation, and the error was less than 3%. The surface area, average pore diameter, pore size distribution, and total pore volume were measured according to the BET method using the surface area and porosity analyzer (TriStar II plus, Micromeritics, Norcross, GA, USA). The samples were degassed at 300 °C for 3 h. The analysis was performed in a N2 atmosphere, using adsorption–desorption isotherms at 77 K. The bonding of various functional groups for 0–7SBG powders was examined using Fourier transform infrared (FTIR) spectroscopy (Spectrum GX, Perkin-Elmer, Norwalk, CT, USA). The SBG powders were mixed with KBr, pressed into a disk, and examined using FT-IR to record the transmission spectra within a wavelength of 400–2000 cm−1 with a resolution of 4 cm⁻1, averaging eight scans per sample. The instrument was routinely calibrated according to the manufacturer’s instructions, and the error in peak position was within ±2 cm⁻1. The powder morphology of the SBG powder was further observed by a field emission scanning electron microscope (FE-SEM, SU-8200, Hitachi, Tokyo, Japan).
4.3. In Vitro Mineralization and Biocompatibility of SBG Powders
Selected SBG powders were immersed in simulated body fluids (SBFs) for different periods without replacing the solutions to simulate the mineralization reaction. SBF with similar ionic concentration as human plasma, composed of NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2, and Na2SO4, was prepared according to the literature [48]. All of these powders were dissolved in distilled water, and pH values were adjusted to 7.4 at 37 °C using TRIS and HCl. The immersed SBG powders were examined using XRD, FT-IR, and SEM to determine the bioactivity of SBG powders.
For the biocompatibility test, SBG powder extracts with a weight-volume ratio of 20 mg/mL were immersed in Dulbecco’s modified Eagle’s medium (DMEM, (Cytiva, Hyclone, South Logan, UT, USA) with 10% fetal bovine serum (FBS, Cytiva, Hyclone, South Logan, UT, USA) and 1% antibiotic/antimycotic solution (Cytiva, Hyclone, South Logan, UT, USA) for 24 h. Fibroblast cell L929 (ISO standard) and osteoblast cell MG 63 were seeded at 1 × 104 cells/well in 96-well plates and cultured for 24 h in a humidified atmosphere of 5% CO2 at 37 °C. After the cells were attached, DMEM was replaced with the SBG extracts. After 1 day of incubation, 10 µL of cell counting kit-8 solution (CCK-8, Sigma-Aldrich, St. Louis, MO, USA) was added into each well and incubated for another 2 h. The biocompatibility was measured at 450 nm using a Multiskan™ FC Microplate Photometer (Thermo-Fisher, Waltham, MA, USA). The relative OD450 (optical density at 450 nm) ratio was normalized to the control group, and the cell viability was presented as the mean ± SD in percentage. The live/dead cell staining of L929 and MG63 (using LIVE/DEAD™ Cell Imaging Kit (488/570), Invitrogen, Carlsbad, CA, USA) incubated with SBG extract were examined (Green—live cells, Red—dead cells) and the morphology of cells was observed using a fluorescence microscope (PAULA Smart Cell Imager, Leica Microsystems GmbH, Wetzlar, Germany).
The cytotoxicity of SBG powders was evaluated using a zebrafish animal model [46]. Adult zebrafish (Danio rerio, AB strain) were reared at 28 °C with 14 light—10 dark photoperiods. The temperature (28 ± 0.5 °C) and pH (7.0 ± 0.5) of the water were monitored and controlled. AB wild-type (WT) strains of zebrafish were provided by the Taiwan Zebrafish Core Facility of Academia Sinica (Taipei, Taiwan). The SBG powders were immersed in artificial normal water for 1 day, and 1000X extraction was prepared as an experimental medium. Three test groups (control, 0SBG, and 5SBG) were examined at the same time, and each group contained 30 embryos. The hatch rate, mortality rate, and the fish length, after 72 h post-fertilization, of each group were recorded for comparison. The experimental protocols were approved by the Taipei Medical University Animal Care and Utilization Committee (approval no.: LAC-2024-0022).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gels11060401/s1, Figure S1: X-ray diffraction patterns of as-prepared and calcined (a) 0SBG and (b) 5SBG powder; Figure S2: X-ray diffraction patterns of 0-7 SBG powder after calcination at 650 °C for 3h.; Figure S3: (a) 0SBG, (b) 3SBG, (c) 5SBG, and (d) 7SBG powder XRD patterns with their observed crystallin phases and structures after immersion in simulated body fluid for 1, 3, and 7 days; Figure S4: The mapping of Si, Ca, P, Sr, C, and O elements on (a) 0SBG, (b) 0SBG-7D, (c) 5SBG, and (d) 5SBG-7D powders; Figure S5: pH value of 0SBG (black square symbol) and 5SBG (red circle symbol) in different SBF immersion times (0, 1, 3, and 7 days); Figure S6: Sr2+ concentration of 5SBG after immersion in SBF for different period of time; Table S1: Comparison of various sol-gelled 58S bioactive glass powder with Sr addition; Table S2: EDX of 0SBG and 5SBG before and after 7 days.
Author Contributions
Conceptualization, K.A., C.-Y.S. and C.-K.L.; methodology, P.-J.C., K.A., C.-Y.S. and C.-K.L.; software, P.-J.C., J.-Y.C. and C.-H.C., validation, P.-J.C., K.A. and C.-K.L.; investigation, P.-J.C., J.-Y.C. and C.-H.C.; data curation, P.-J.C., K.A. and C.-Y.S.; writing—original draft preparation, P.-J.C. and J.-Y.C.; writing—review and editing, C.-Y.S. and C.-K.L.; visualization, P.-J.C., J.-Y.C. and C.-H.C.; supervision, C.-Y.S. and C.-K.L.; project administration, C.-Y.S. and C.-K.L.; funding acquisition, C.-Y.S. and C.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Council, grant numbers NSTC 113-2622-E-038-002 and 113-2813-C-038-017-E.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Taipei Medical University approval no.: LAC-2024-0022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge the Precision Research and Analysis Center at NTUT for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.Z.; Rezabeigi, E.; Drew, R.A. Crystallization behavior of combeite in 45S5 Bioglass® via controlled heat treatment. J. Non-Cryst. Solids 2018, 502, 176–183. [Google Scholar] [CrossRef]
- Gavinho, S.; Graça, M.; Prezas, P.; Kumar, J.S.; Melo, B.; Sales, A.; Almeida, A.; Valente, M. Structural, thermal, morphological and dielectric investigations on 45S5 glass and glass-ceramics. J. Non-Cryst. Solids 2021, 562, 120780. [Google Scholar] [CrossRef]
- Ma, J.; Wu, L.; Liu, X.; Wang, C.; Huang, B.; Zhao, X.; Ban, C.; Hao, X. Influence of the substitution of CaO by SrO on the structure, degradation and in vitro apatite formation of sol–gel derived SiO2–CaO–SrO–P2O5 system bioactive glasses. Ceram. Int. 2024, 50, 55906–55919. [Google Scholar] [CrossRef]
- Wu, X.L.; Meng, G.L.; Wang, S.L.; Wu, F.; Huang, W.X.; Gu, Z.W. Zn and Sr incorporated 64S bioglasses: Material characterization, in-vitro bioactivity and mesenchymal stem cell responses. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 52, 242–250. [Google Scholar] [CrossRef]
- Akhtach, S.; Tabia, Z.; Bricha, M.; El Mabrouk, K. Structural characterization, in vitro bioactivity, and antibacterial evaluation of low silver-doped bioactive glasses. Ceram. Int. 2021, 47, 29036–29046. [Google Scholar] [CrossRef]
- Taye, M.B.; Ningsih, H.S.; Shih, S.-J. Antibacterial and in vitro bioactivity studies of silver-doped, cerium-doped, and silver–cerium co-doped 80S mesoporous bioactive glass particles via spray pyrolysis. Appl. Sci. 2023, 13, 12637. [Google Scholar] [CrossRef]
- Pawar, V.; Shinde, V. Bioglass and hybrid bioactive material: A review on the fabrication, therapeutic potential and applications in wound healing. Hybrid Adv. 2024, 6, 100196. [Google Scholar] [CrossRef]
- Owoeye, S.S.; Folorunso, D.O.; Aramide, F.; Olaniran, O.; Okotie, B. Microwave irradiation melt-quenching preparation of 45S5 bioglass using biogenic wastes as alternative materials. Biomed. Mater. Devices 2024, 3, 463–473. [Google Scholar] [CrossRef]
- Ningsih, H.S.; Liu, Y.-C.; Chen, J.-W.; Chou, Y.-J. Effects of strontium dopants on the in vitro bioactivity and cytotoxicity of strontium-doped spray-dried bioactive glass microspheres. J. Non-Cryst. Solids 2022, 576, 121284. [Google Scholar] [CrossRef]
- Nakanishi, A.; Ningsih, H.S.; Putra, D.F.A.; Moriga, T.; Shih, S.-J. Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying. J. Compos. Sci. 2024, 8, 111. [Google Scholar] [CrossRef]
- Workie, A.B.; Sefene, E.M. Ion-doped mesoporous bioactive glass: Preparation, characterization, and applications using the spray pyrolysis method. RSC Adv. 2022, 12, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-Y.; Tsai, P.-Y.; Chen, M.-S.; Mine, Y.; Wu, S.-H.; Chen, C.-Y.; Lin, D.-J.; Lin, C.-K. Mesoporous properties of bioactive glass synthesized by spray pyrolysis with various polyethylene glycol and acid additions. Polymers 2021, 13, 618. [Google Scholar] [CrossRef]
- Bahati, D.; Bricha, M.; El Mabrouk, K. Synthesis, characterization, and in vitro apatite formation of strontium-doped sol-gel-derived bioactive glass nanoparticles for bone regeneration applications. Ceram. Int. 2023, 49, 23020–23034. [Google Scholar] [CrossRef]
- Moghanian, A.; Tajer, M.H.M.; Zohourfazeli, M.; Miri, Z.; Yazdi, M. Sol-gel derived silicate-based bioactive glass: Studies of synergetic effect of zirconium and magnesium on structural and biological characteristics. J. Non-Cryst. Solids 2021, 554, 120613. [Google Scholar] [CrossRef]
- Taye, M.B.; Ningsih, H.S.; Shih, S.-J. Exploring the advancements in surface-modified bioactive glass: Enhancing antibacterial activity, promoting angiogenesis, and modulating bioactivity. J. Nanoparticle Res. 2024, 26, 28. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol–gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Silva, A.V.; Gomes, D.d.S.; Victor, R.d.S.; Santana, L.N.d.L.; Neves, G.A.; Menezes, R.R. Influence of strontium on the biological behavior of bioactive glasses for bone regeneration. Materials 2023, 16, 7654. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Characterization, in vitro bioactivity and biological studies of sol-gel synthesized SrO substituted 58S bioactive glass. Ceram. Int. 2017, 43, 14880–14890. [Google Scholar] [CrossRef]
- Goudarzi, Z.; Ijadi, A.; Bakhriari, A.; Eskandarinezhad, S.; Azizabadi, N.; Asgari Jazi, M. Sr-doped bioactive glasses for biological applications. J. Compos. Compd. 2020, 2, 105–109. [Google Scholar] [CrossRef]
- Maçon, A.L.; Lee, S.; Poologasundarampillai, G.; Kasuga, T.; Jones, J.R. Synthesis and dissolution behaviour of CaO/SrO-containing sol–gel-derived 58S glasses. J. Mater. Sci. 2017, 52, 8858–8870. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, W.H.; Chen, X.F.; Li, Y.L.; Liang, Q.M. The effects of Sr concentration on physicochemical properties, bioactivity and biocompatibility of sub-micron bioactive glasses spheres. Adv. Powder Technol. 2017, 28, 2713–2722. [Google Scholar] [CrossRef]
- El Baakili, S.; El Mabrouk, K.; Bricha, M. Acellular bioactivity and drug delivery of new strontium doped bioactive glasses prepared through a hydrothermal process. RSC Adv. 2022, 12, 15361–15372. [Google Scholar] [CrossRef]
- MacKenzie, K.J.; Rahner, N.; Smith, M.E.; Wong, A. Calcium-containing inorganic polymers as potential bioactive materials. J. Mater. Sci. 2010, 45, 999–1007. [Google Scholar] [CrossRef]
- Raja, N.; Park, H.; Choi, Y.-J.; Yun, H.-s. Multifunctional calcium-deficient hydroxyl apatite–alginate core–shell-structured bone substitutes as cell and drug delivery vehicles for bone tissue regeneration. ACS Biomater. Sci. Eng. 2021, 7, 1123–1133. [Google Scholar] [CrossRef]
- Kuo, C.-K.; Chen, L.-G.; Tseng, C.-F.; Chou, Y.-J. Influences of acid catalysts on the microstructure, bioactivity and cytotoxicity of bioactive glass nanoparticles prepared by spray pyrolysis. J. Non-Cryst. Solids 2021, 560, 120710. [Google Scholar] [CrossRef]
- Intawin, P.; Eitssayeam, S.; Tunkasiri, T.; Pengpat, K. Crystallization kinetics and heat treatment temperature on microstructure and properties of Na2O-CaO-P2O5 bioactive glass system. Ceram. Int. 2018, 44, S203–S206. [Google Scholar] [CrossRef]
- Kaygili, O.; Keser, S.; Tatar, C.; Koytepe, S.; Ates, T. Investigation of the structural and thermal properties of Y, Ag and Ce-assisted SiO2–Na2O–CaO–P2O5-based glasses derived by sol–gel method. J. Therm. Anal. Calorim. 2017, 128, 765–770. [Google Scholar] [CrossRef]
- Solgi, S.; Khakbiz, M.; Shahrezaee, M.; Zamanian, A.; Tahriri, M.; Keshtkari, S.; Raz, M.; Khoshroo, K.; Moghadas, S.; Rajabnejad, A. Synthesis, characterization and in vitro biological evaluation of sol-gel derived Sr-containing nano bioactive glass. Silicon 2017, 9, 535–542. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Niño-Celis, V.; Giraldo, R.I. Systematic Analysis of the Nitrogen Adsorption-Desorption Isotherms Recorded for a Series of Materials Based on Microporous-Mesoporous Amorphous Aluminosilicates Using Classical Methods. J. Chem. Eng. Data 2023, 68, 2512–2528. [Google Scholar] [CrossRef]
- Jones, J.R.; Ehrenfried, L.M.; Hench, L.L. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials 2006, 27, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Moghanian, A.; Zohourfazeli, M.; Tajer, M.H.M.; Miri, Z.; Hosseini, S.; Rashvand, A. Preparation, characterization and in vitro biological response of simultaneous co-substitution of Zr+ 4/Sr+ 2 58S bioactive glass powder. Ceram. Int. 2021, 47, 23762–23769. [Google Scholar] [CrossRef]
- Maciel, P.P.; Pessôa, J.A.M.; Medeiros, E.L.G.d.; Batista, A.U.D.; Figueiredo, L.R.F.; Medeiros, E.S.d.; Duarte, D.F.d.O.; Alves, A.F.; Sousa, F.B.d.; Vieira, B.R.; et al. Use of strontium doping glass-ceramic material for bone regeneration in critical defect: In vitro and in vivo analyses. Ceram. Int. 2020, 46, 24940–24954. [Google Scholar] [CrossRef]
- Leite, A.J.; Goncalves, A.I.; Rodrigues, M.T.; Gomes, M.E.; Mano, J.F. Strontium-Doped Bioactive Glass Nanoparticles in Osteogenic Commitment. ACS Appl Mater Interfaces 2018, 10, 23311–23320. [Google Scholar] [CrossRef]
- Yadav, A.; Sathala, P.; Divate, M.; Chenkul, L.; Nupur, N.; Upmanyu, N.; Porwal, P.K. Deciphering solid state photostability of polymyxin B using deconvolution based FTIR insights. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 339, 126256. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin Analysis by HPLC and FTIR: Spectra Deconvolution and S/G Ratio Determination. In Xylem: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023; pp. 149–169. [Google Scholar]
- Gieroba, B.; Przekora, A.; Kalisz, G.; Kazimierczak, P.; Song, C.L.; Wojcik, M.; Ginalska, G.; Kazarian, S.G.; Sroka-Bartnicka, A. Collagen maturity and mineralization in mesenchymal stem cells cultured on the hydroxyapatite-based bone scaffold analyzed by ATR-FTIR spectroscopic imaging. Mater. Sci. Eng. C 2021, 119, 111634. [Google Scholar] [CrossRef]
- Copete, H.; López, E.; Baudin, C. Synthesis and characterization of B-type carbonated hydroxyapatite materials: Effect of carbonate content on mechanical strength and in vitro degradation. Boletín de la Soc. Española de Cerámica y Vidr. 2024, 63, 255–267. [Google Scholar] [CrossRef]
- Mano, T.; Akita, K.; Fukuda, N.; Kamada, K.; Kurio, N.; Ishikawa, K.; Miyamoto, Y. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1450–1459. [Google Scholar] [CrossRef]
- Cho, Y.S.; Heo, C.M.; Gebauer, D.; Yang, S.H. Control of Hydroxyapatite Mineralization in an Orthogonal Diffusion System. Cryst. Growth Des. 2025, 25, 2960–2969. [Google Scholar] [CrossRef]
- ISO10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Chen, Y.-Y.; Ma, T.-L.; Chang, P.-J.; Chiou, Y.-J.; Chang, W.-M.; Weng, C.-F.; Chen, C.-Y.; Chang, Y.-K.; Lin, C.-K. Synergistic Effect of Strontium Doping and Surfactant Addition in Mesoporous Bioactive Glass for Enhanced Osteogenic Bioactivity and Advanced Bone Regeneration. Polymers 2025, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Amudha, S.; Ramya, J.R.; Arul, K.T.; Deepika, A.; Sathiamurthi, P.; Mohana, B.; Asokan, K.; Dong, C.-L.; Kalkura, S.N. Enhanced mechanical and biocompatible properties of strontium ions doped mesoporous bioactive glass. Compos. Part B Eng. 2020, 196, 108099. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, P.; Jiang, Y.; You, J.; Xu, Z.; Yu, K.; Boccaccini, A.R.; Ma, J.; Zheng, K. Extracellular and intracellular effects of bioactive glass nanoparticles on osteogenic differentiation of bone marrow mesenchymal stem cells and bone regeneration in zebrafish osteoporosis model. Acta Biomater. 2024, 174, 412–427. [Google Scholar] [CrossRef]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 65, 188–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).