Development and Optimization of Kunzea ericoides Nanoemulgel Using a Quality by Design Approach for Transdermal Anti-Inflammatory Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Analysis of Kanuka Oil
2.2. GC-MS Analysis of Kanuka Oil
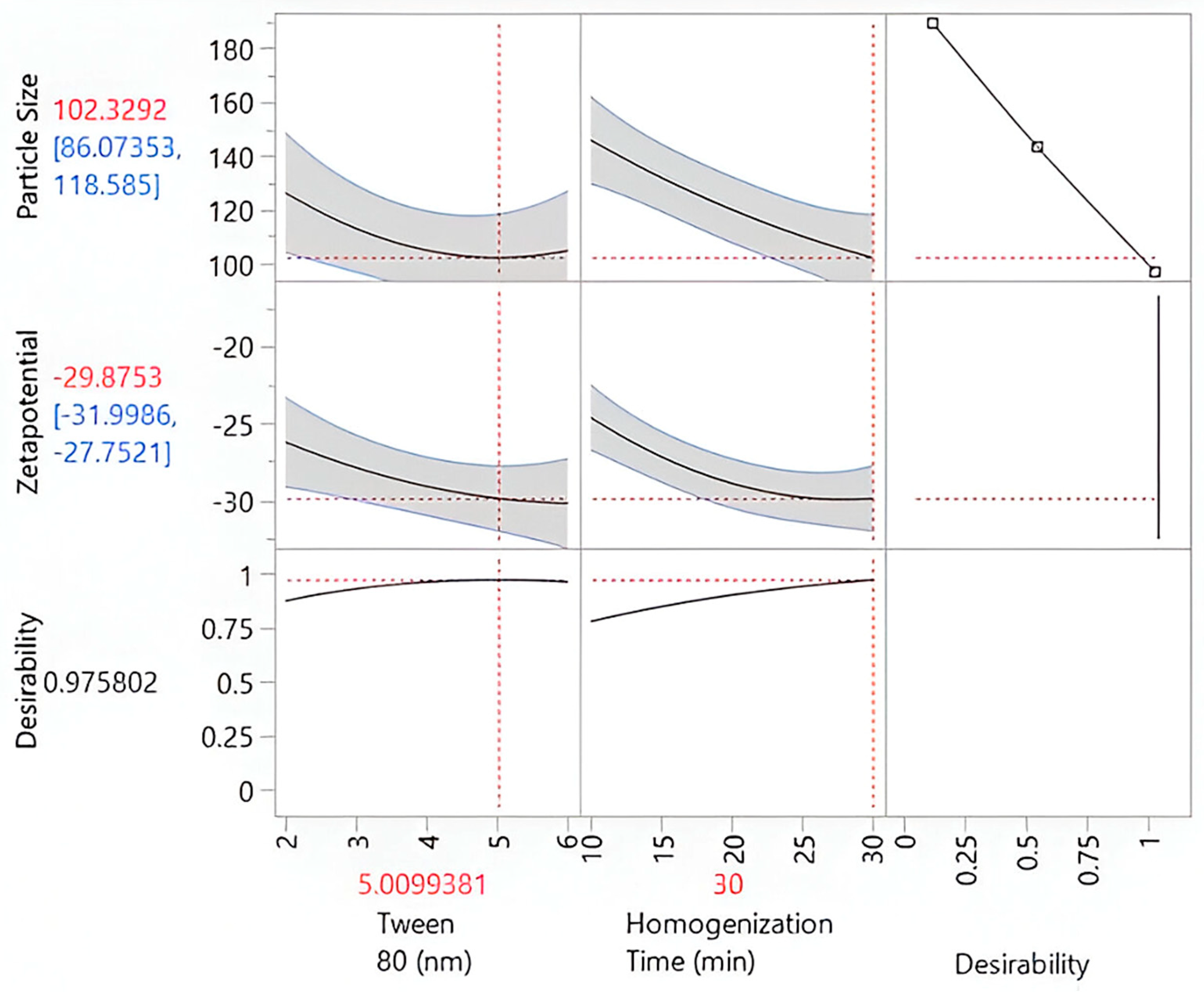
2.3. Preparation and Optimization of Kanuka Nanoemulsions
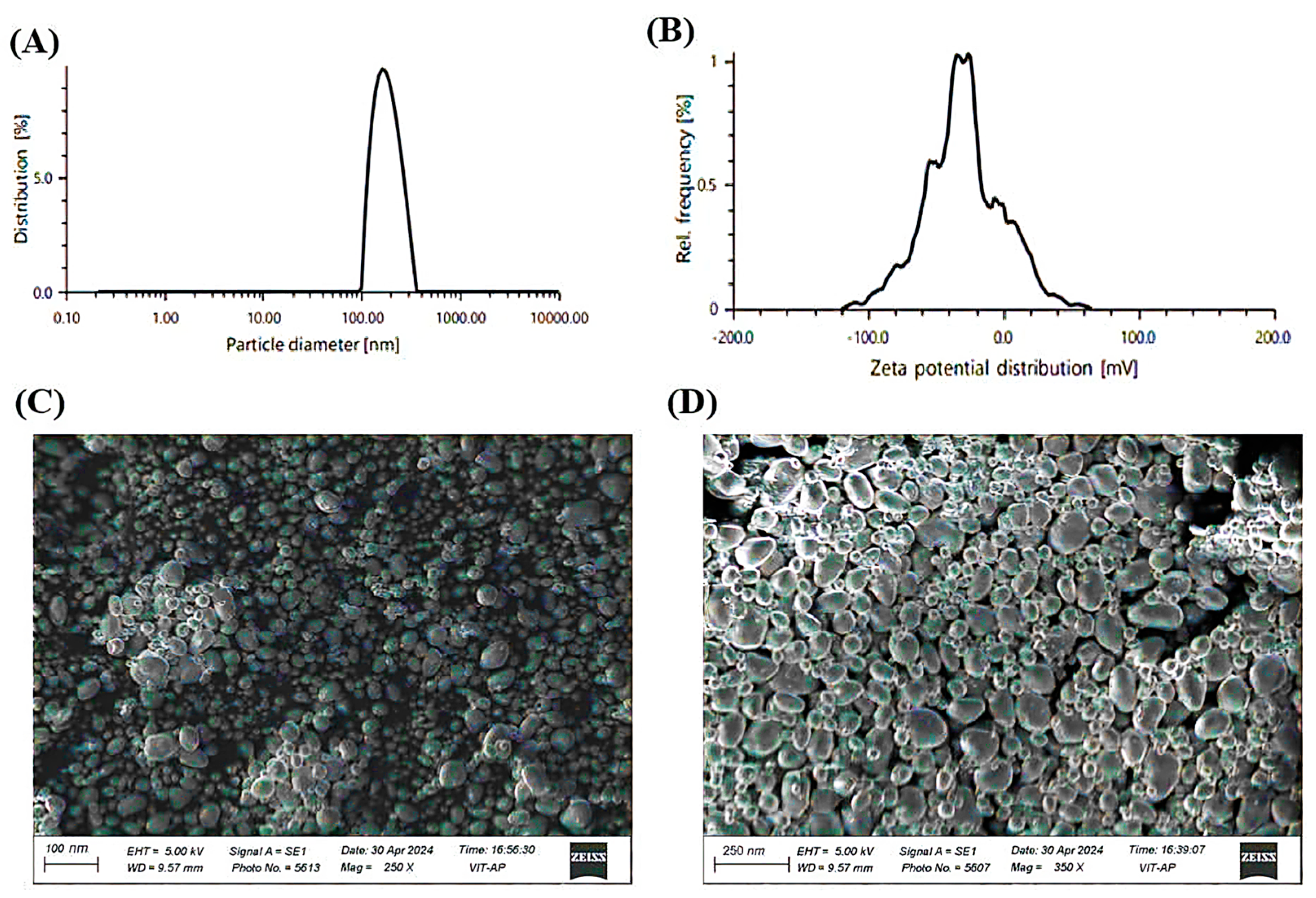
2.4. Characterization Study of Kanuka-Loaded Nanoemulsions (K-NE)
2.5. Physicochemical Properties and Morphological Evaluation
2.6. Entrapment Efficiency of Kanuka Nanoemulsion
2.7. Development and Characterization of Kanuka Nanoemulgel (K-NG)
2.8. In Vitro Drug Release and Diffusion Kinetics
2.9. Evaluation of Antibacterial Activity
2.10. Evaluation of Dermal Response in the Acute Irritation Study
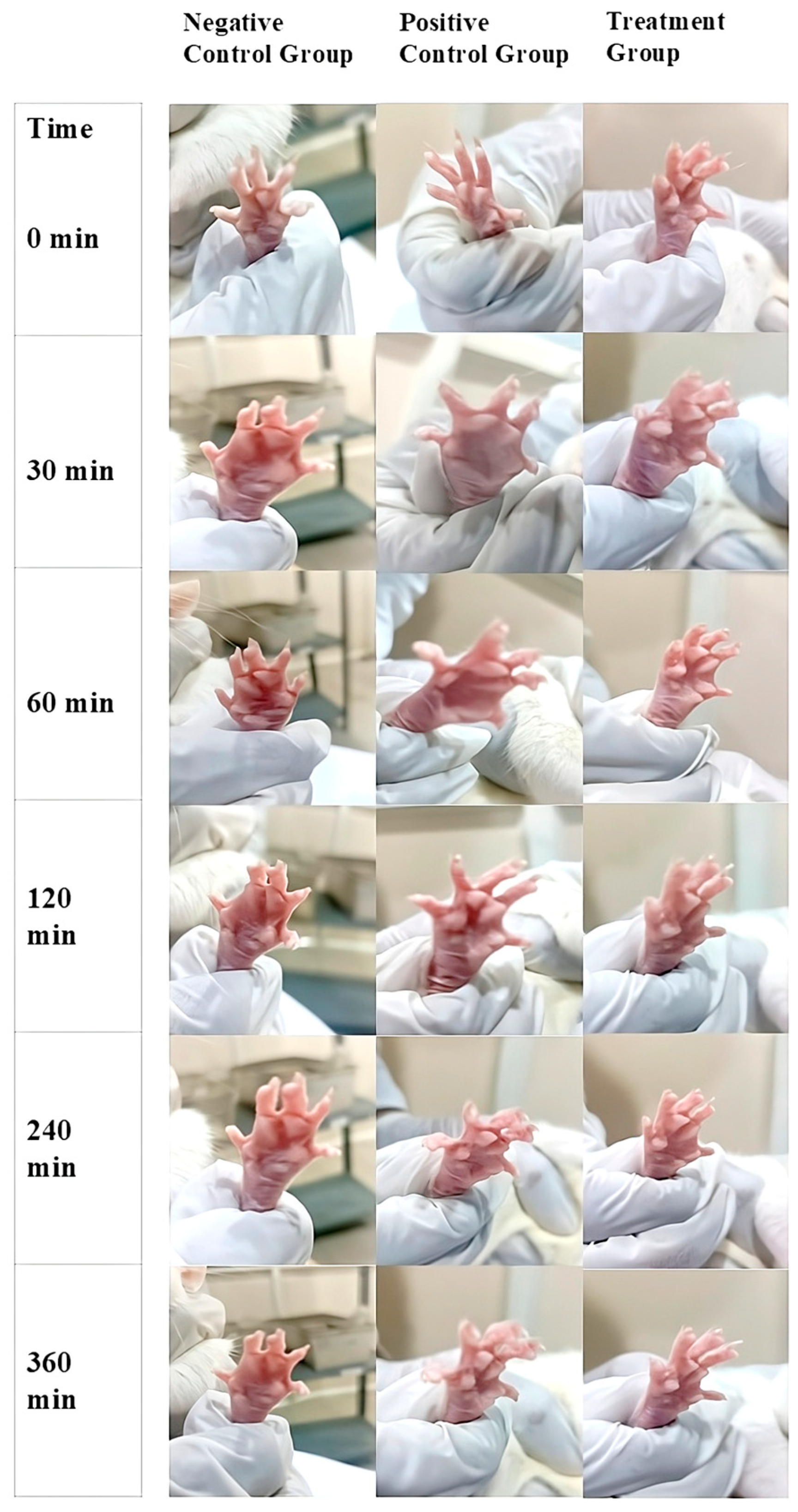
2.11. In-Vivo Anti-Inflammatory Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Screening of Surfactants and FT-IR Analysis of Kanuka Oil
4.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.4. Preparation and Optimization of Kanuka Nanoemulsion
4.5. Evaluation of Entrapment Efficiency
4.6. Physicochemical Characterization and Stability Assessment
4.7. Preparation of Kanuka Loaded Nanoemulgel
4.8. In Vitro Drug Release Study and Kinetics
4.9. Antimicrobial Activity of K-NG
4.10. In Vivo Study
4.11. Acute Dermal Irritation Study
4.12. Evaluation of In Vivo Anti-Inflammatory Activity
4.13. Statistical Analysis and Interpretation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QbD | Quality by Design |
| NE | Nanoemulsion |
| NEs | Nanoemulsions |
| K-NE | Kanuka Nanoemulsion |
| K-NEs | Kanuka Nanoemulsions |
| n-EnKan | non-encapsulated Kanuka oil |
| K-NG | Kanuka Nanoemulgel |
| CCD | Central Composite Design |
| ANOVA | Analysis of Variance |
| PDI | Polydispersity Index |
| CI | Confidence Interval |
| EE | Entrapment Efficiency |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| SEM | Scanning Electron Microscopy |
| UV | Ultraviolet |
| O/W | Oil-in-Water |
| MHB | Mueller–Hinton Broth |
| MIC | Minimum Inhibitory Concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| CFU | Colony-Forming Unit |
| IAEC | Institutional Animal Ethics Committee |
References
- Mirrashed, F.; Sharp, J.; Krause, V.; Morgan, J.; Tomanek, B. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res. Technol. 2004, 10, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Asthana, G.S.; Asthana, A.; Singh, D.; Sharma, P.K. Etodolac containing topical niosomal gel: Formulation development and evaluation. J. Drug Deliv. 2016, 2016, 9323616. [Google Scholar] [CrossRef]
- Khavkin, C.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Madrid Sani, A.T.; Ramos-Rocha, K.L.V.; Sarcinelli, M.A.; Chaves, M.H.d.C.; Rocha, H.V.A.; Léo, P.; Cerize, N.N.P.; Zanin, M.H.A.; Feitosa, V.A.; Rangel-Yagui, C.d.O.; et al. Development of a dry powder formulation for pulmonary delivery of azithromycin-loaded nanoparticles. J. Pharm. Pharm. Sci. 2024, 27, 13635. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef]
- Alexander, A.; Dwivedi, S.; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef]
- Burki, I.K.; Khan, M.K.; Khan, B.A.; Uzair, B.; Braga, V.A.; Jamil, Q.A. Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech 2020, 21, 211. [Google Scholar] [CrossRef]
- Bagheri, A.M.; Ranjbar, M.; Karami-Mohajeri, S.; Moshafi, M.H.; Noudeh, Y.D.; Ohadi, M.; Dehghannoudeh, G. Curcumin nanoemulgel: Characterization, optimization, and evaluation of photoprotective efficacy, anti-inflammatory properties, and antibacterial activity. J. Cluster Sci. 2024, 35, 2253–2272. [Google Scholar] [CrossRef]
- Khan, B.A.; Ahmad, N.; Alqahtani, A.; Baloch, R.; Rehman, A.U.; Khan, M.K. Formulation development of pharmaceutical nanoemulgel for transdermal delivery of febuxostat: Physical characterization and in vivo evaluation. Eur. J. Pharm. Sci. 2024, 195, 106665. [Google Scholar] [CrossRef]
- Alonso, M.M.; Martínez, V.; Rubio, L.; Parra, J.L.; Coderch, L. Antioxidant cosmeto-textiles: Skin assessment. Eur. J. Pharm. Biopharm. 2013, 84, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zacher, J.; Altman, R.; Bellamy, N.; Brühlmann, P.; Da Silva, J.; Huskisson, E.; Taylor, R. Topical diclofenac and its role in pain and inflammation: An evidence-based review. Curr. Med. Res. Opin. 2008, 24, 925–950. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-inflammatory effects of curcumin in inflammatory diseases: Status, limitations, and countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Akah, P.; Nwafor, S.; Anisiobi, A.; Ibegbunam, I.; Erojikwe, O. Anti-inflammatory activity of hexane leaf extract of Aspilia africana CD Adams. J. Ethnopharmacol. 2007, 109, 219–225. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef]
- Porter, N.G.; Wilkins, A.L. Chemical, physical and antimicrobial properties of essential oils of Leptospermum scoparium and Kunzea ericoides. Phytochemistry 1998, 407–415. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nano emulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Azami, S.J.; Teimouri, A.; Keshavarz, H.; Amani, A.; Esmaeili, F.; Hasanpour, H.; Elikaee, S.; Salehiniya, H.; Shojaee, S. Curcumin nanoemulsion as a novel chemical for the treatment of acute and chronic toxoplasmosis in mice. Int. J. Nanomed. 2018, 13, 7363–7374. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of surfactant concentrations on physicochemical properties and functionality of curcumin nanoemulsions under conditions relevant to commercial utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef]
- Singh, A.; Avupati, V.R. Development and validation of UV-spectrophotometric method for the estimation of curcumin in standardized polyherbal formulations. J. Young Pharm. 2017, 9, 491. [Google Scholar] [CrossRef]
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design development and evaluation of novel nanoemulsion formulations for transdermal potential of Celecoxib. Acta Pharm. 2007, 57, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Guleri, K.T.; Preet, K.L. Formulation and evaluation of topical gel of aceclofenac. J. Drug Deliv. Ther. 2013, 3, 51–53. [Google Scholar] [CrossRef]
- Firmansyah, F.; Muhtadi, W.K.; Indriani, S.; Ulhaq, M.D.; Auliya, S.R.; Iskandar, B.; Agistia, N.; Chabib, L. Development of novel curcumin nanoemulgel: Optimization, characterization, and ex vivo permeation. Pharm. Educ. 2022, 22, 98–103. [Google Scholar] [CrossRef]
- Manaswitha, A.; Swetha, P.S.; Devi, N.; Babu, K.N.; Shankar, K.R. Oleic acid-based emulgel for topical delivery of ofloxacin. J. Drug Deliv. Ther. 2019, 9, 183–190. [Google Scholar] [CrossRef]
- Estabragh, M.A.R.; Pardakhty, A.; Ahmadzadeh, S.; Dabiri, S.; Afshar, R.M.; Abbasi, M.F. Successful application of alpha lipoic acid niosomal formulation in cerebral ischemic reperfusion injury in a rat model. Adv. Pharm. Bull. 2022, 12, 541. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterization of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef]
- Gharaei, S.; Ohadi, M.; Hassanshahian, M.; Porsheikhali, S.; Forootanfar, H. Isolation, optimization, and structural characterization of glycolipid biosurfactant produced by marine isolate Shewanella algae B12 and evaluation of its antimicrobial and anti-biofilm activity. Appl. Biochem. Biotechnol. 2022, 194, 1755–1774. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Vyas, S.; Raval, J.; Gajjar, A.; Shah, D. Ketorolac-dextran conjugates: Synthesis, in vitro and in vivo evaluation. Acta Pharm. 2007, 57, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development (OECD). Test No. 404: Acute Dermal Irritation/Corrosion. OECD Guidelines for the Testing of Chemicals, Section 4. 2015. Available online: https://www.oecd.org/en/publications/2015/07/test-no-404-acute-dermal-irritation-corrosion_g1g59b23.html (accessed on 20 March 2025).
- Karaman, M.; Firinci, F.; Cilaker, S.; Uysal, P.; Tugyan, K.; Yilmaz, O.; Uzuner, N.; Karaman, O. Anti-inflammatory effects of curcumin in a murine model of chronic asthma. Allergol. Immunopathol. 2012, 40, 210–214. [Google Scholar] [CrossRef] [PubMed]
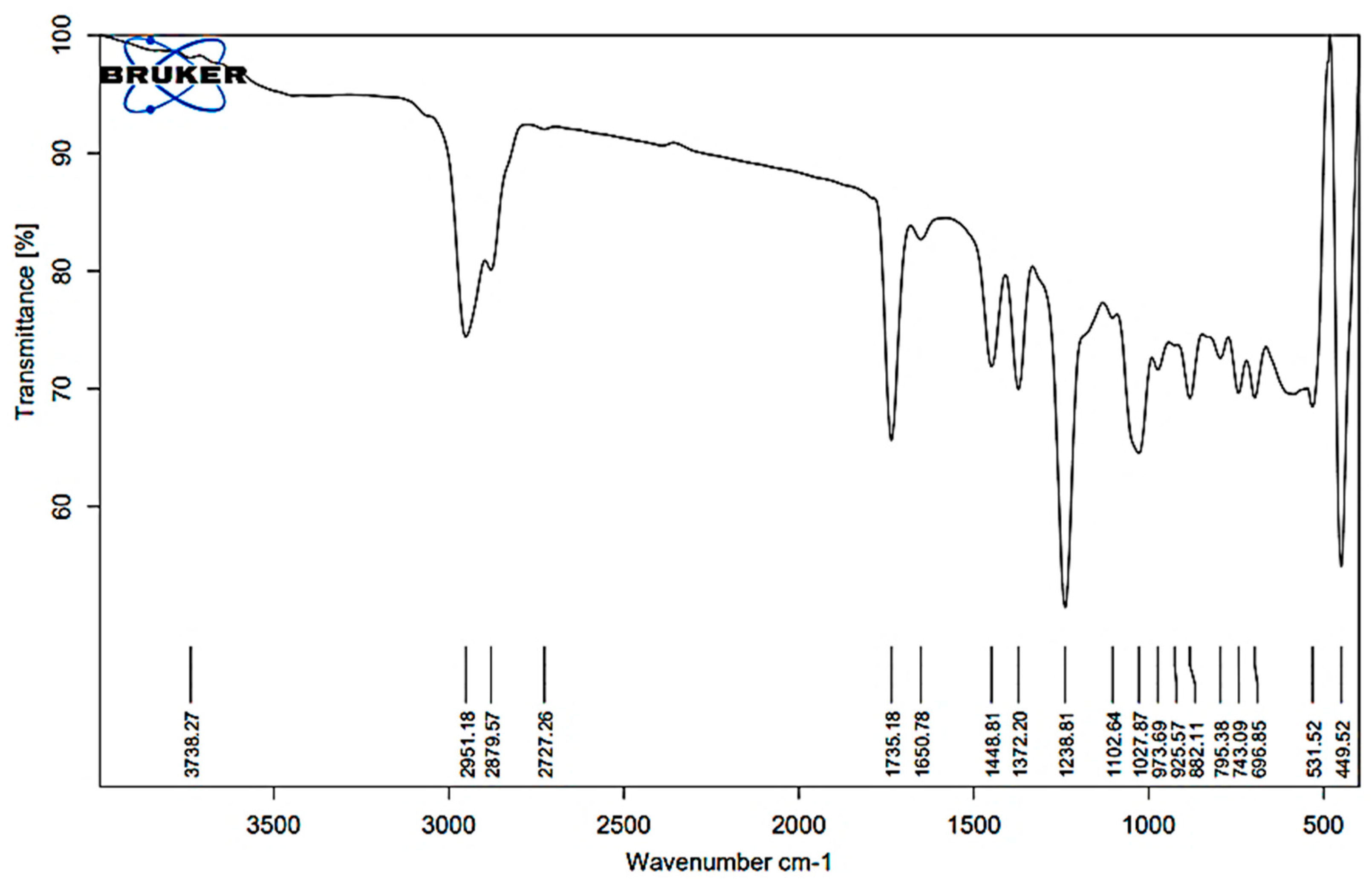

| Wavenumber (cm−1) | Functional Group | Accepted Range (cm−1) | Probable Compound Type |
|---|---|---|---|
| 3738.27 | O–H stretch | 3200–3700 | Phenols, Alcohols |
| 2951.18, 2927.57, 2727.26 | C–H stretch | 2800–3000 | Aliphatic chains (including alkanes) |
| 1735.18, 1650.78 | C=O stretch | 1650–1750 | Esters, Ketones |
| 1448.81, 1322.20 | C–H bending | 1300–1500 | Aliphatic hydrocarbons |
| 1123.89, 1102.64 | C–O stretch | 1000–1300 | Ethers, Alcohols |
| 1027.69, 787.17, 882.51 | C=C bending | 650–1000 | Terpenes, Phenolics |
| 531.52, 449.52 | Fingerprint region | <600 | Specific spectral features |
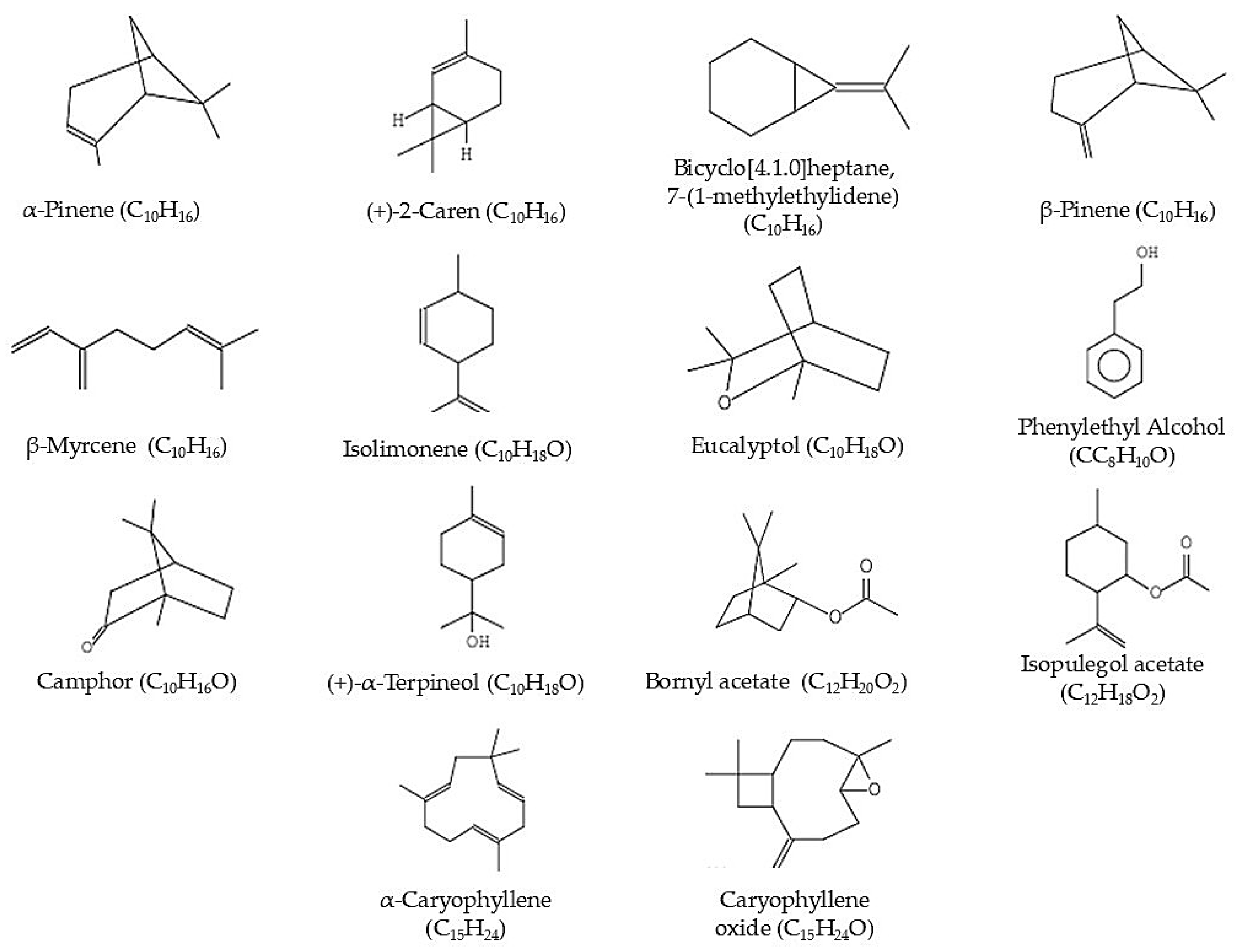
| Peak | RT (min) | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Peak Area (%) |
|---|---|---|---|---|---|
| 5 | 4.085 | α-Pinene | C10H16 | 136.24 | 0.06% |
| 6 | 4.361 | (+)-2-Caren | C10H16 | 136.24 | 0.04% |
| 12 | 5.158 | Bicyclo[4.1.0]heptane, 7-(1-methylethylidene) | C10H16 | 136.24 | 9.31% |
| 14 | 5.559 | β-Pinene | C10H16 | 136.24 | 1.63% |
| 16 | 5.811 | β-Myrcene | C10H16 | 134.24 | 0.27% |
| 22 | 6.439 | Isolimonene | C10H16 | 136.24 | 9.76% |
| 23 | 6.464 | Eucalyptol | C10H18O | 154.25 | 1.32% |
| 32 | 7.834 | Phenylethyl Alcohol | C8H10O | 122.16 | 2.45% |
| 36 | 8.195 | Camphor | C10H16O | 152.23 | 2.32% |
| 41 | 8.956 | (+)-α-Terpineol | C10H18O | 154.25 | 6.32% |
| 47 | 9.625 | Bornyl acetate | C12H20O2 | 196.29 | 0.18% |
| 49 | 9.736 | Isopulegol acetate | C12H18O2 | 194.27 | 0.45% |
| 69 | 12.445 | α-Caryophyllene | C15H24 | 204.36 | 0.04% |
| 82 | 13.896 | Caryophyllene oxide | C15H24O | 220.35 | 0.21% |
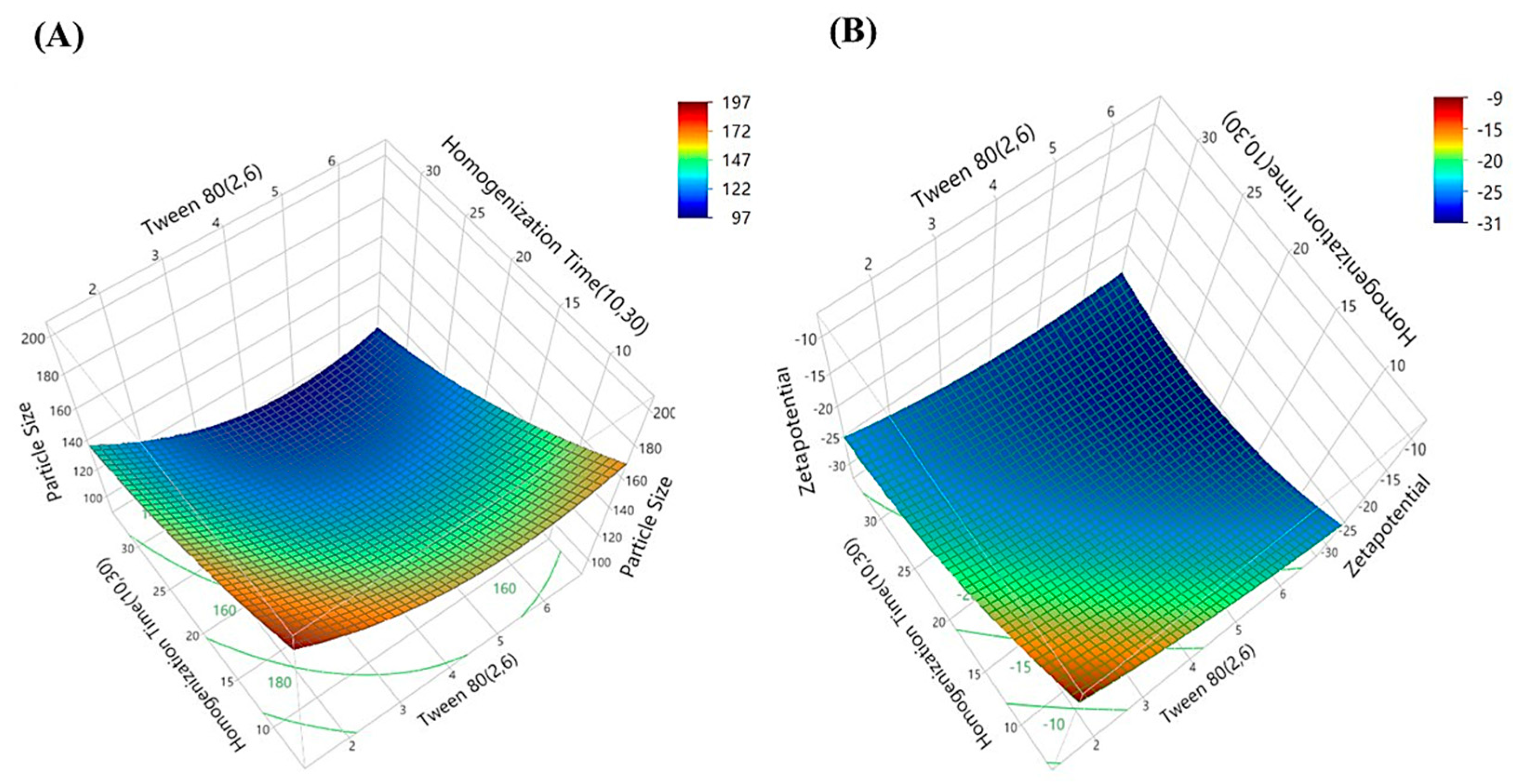
| Formulations | X1 (Coded Level) | X2 (Coded Level) | Tween 80 (%) X1 | Homogenization Time (min) X2 | Particle Size (nm) | Zetapotential |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 4.0 | 20.0 | 136.67 | −28.5 |
| 2 | + | + | 6.0 | 30.0 | 101.76 | −29.9 |
| 3 | − | − | 2.0 | 10.0 | 184.90 | −16.9 |
| 4 | 0 | 0 | 4.0 | 20.0 | 119.78 | −29.1 |
| 5 | a | 0 | 1.2 | 20.0 | 145.87 | −19.4 |
| 6 | 0 | 0 | 4.0 | 20.0 | 113.23 | −24.7 |
| 7 | − | + | 2.0 | 30.0 | 132.50 | −27.4 |
| 8 | 0 | 0 | 4.0 | 20.0 | 125.38 | −27.6 |
| 9 | 0 | A | 4.0 | 34.1 | 100.65 | −28.3 |
| 10 | 0 | a | 4.0 | 05.9 | 148.23 | −19.5 |
| 11 | 0 | 0 | 4.0 | 20.0 | 118.34 | −26.8 |
| 12 | A | 0 | 6.8 | 20.0 | 129.45 | −31.5 |
| 13 | + | − | 6.0 | 10.0 | 155.40 | −25.6 |
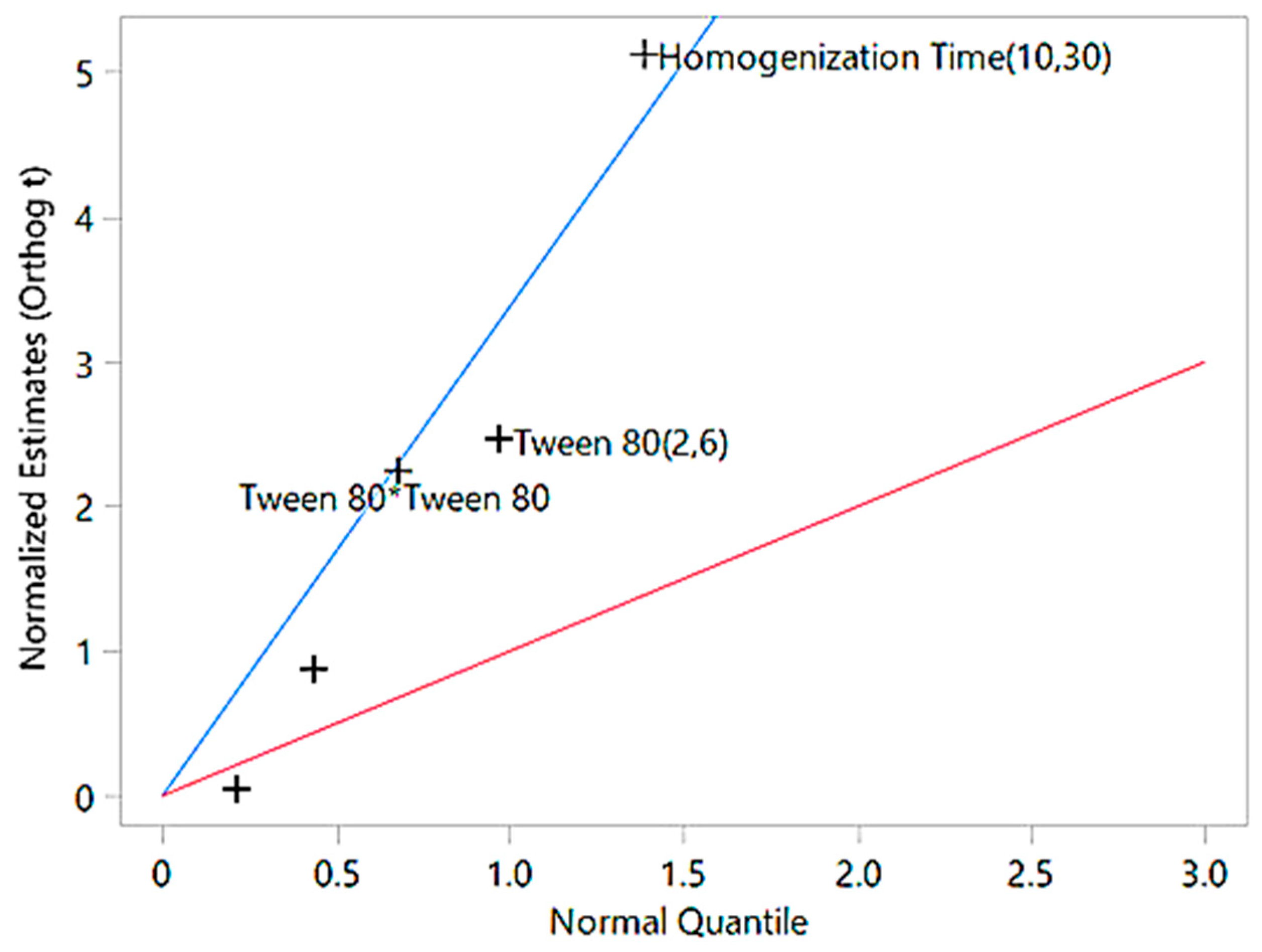
| Factor | df | Total Sum of Squares | Average Square | F-Ratio | Probability Value | Significance |
|---|---|---|---|---|---|---|
| Model | 5 | 5462.94 | 1092.59 | 7.68 | 0.0092 | Significant |
| Error | 7 | 995.58 | 142.23 | - | - | - |
| Total | 12 | 6458.53 | - | - | - | - |
| Intercept | - | - | 122.68 | 23 | <0.0001 | Highly significant |
| Tween 80 | 1 | - | −10.43 | −2.47 | 0.0426 | Significant |
| Homogenization Time | 1 | - | −21.67 | −5.14 | 0.0013 | Highly significant |
| Tween 80 × Homogenization Time | 1 | - | −0.31 | −0.05 | 0.96 | Not significant |
| Tween 80 × Tween 80 | 1 | - | 10.64 | 2.35 | 0.0509 | Marginally significant |
| Homogenization Time × Time | 1 | - | 4.03 | 0.89 | 0.4027 | Not significant |
| R2 | - | 0.8462 | - | - | - | Indicates strong model fit |
| Adjusted R2 | - | 0.7942 | - | - | - | Adjusted for predictors |
| Time Point | Kanuka Nanoemulgel 1 | Positive Control (Formalin) 1 | Negative Control (Blank Gel) 1 | p-Value 2 |
|---|---|---|---|---|
| 1 h | 1.0 ± 0.5 | 2.0 ± 0.5 | 0.0 ± 0.0 | 0.0319 |
| 24 h | 0.0 ± 0.0 | 2.0 ± 0.0 | 0.0 ± 0.0 | 0.0183 |
| 48 h | 0.0 ± 0.0 | 2.0 ± 0.5 | 0.0 ± 0.0 | 0.0211 |
| 72 h | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | 0.0183 |
| Time (min) | Negative Control Group (mL) 1 | Positive Control Group (mL) 1 | Treatment Group (mL) 1 | Inhibition of Edema (%) | Group Comparisons | Mean Difference (mL) | p-Value 4 |
|---|---|---|---|---|---|---|---|
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | - | - |
| 30 | 0.39 ± 0.005 | 0.30 ± 0.004 | 0.22 ± 0.003 | 23.1 2/ 43.6 3 | Control vs. Standard | −0.9 | 0.038 |
| 60 | 0.38 ± 0.004 | 0.28 ± 0.003 | 0.16 ± 0.002 | 26.3 2/ 57.9 3 | Control vs. Treatment | −2.2 | 0.005 |
| 120 | 0.37 ± 0.003 | 0.21 ± 0.002 | 0.08 ± 0.001 | 43.2 2/ 78.4 3 | Standard vs. Treatment | −1.3 | 0.089 |
| 240 | 0.36 ± 0.002 | 0.07 ± 0.001 | 0.02 ± 0.001 | 80.5 2/ 94.4 3 | - | - | - |
| 360 | 0.35 ± 0.001 | 0.00 ± 0.00 | 0.00 ± 0.00 | 100 2,3 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yetukuri, K.; Umashankar, M.S. Development and Optimization of Kunzea ericoides Nanoemulgel Using a Quality by Design Approach for Transdermal Anti-Inflammatory Therapy. Gels 2025, 11, 400. https://doi.org/10.3390/gels11060400
Yetukuri K, Umashankar MS. Development and Optimization of Kunzea ericoides Nanoemulgel Using a Quality by Design Approach for Transdermal Anti-Inflammatory Therapy. Gels. 2025; 11(6):400. https://doi.org/10.3390/gels11060400
Chicago/Turabian StyleYetukuri, Koushik, and Marakanam Srinivasan Umashankar. 2025. "Development and Optimization of Kunzea ericoides Nanoemulgel Using a Quality by Design Approach for Transdermal Anti-Inflammatory Therapy" Gels 11, no. 6: 400. https://doi.org/10.3390/gels11060400
APA StyleYetukuri, K., & Umashankar, M. S. (2025). Development and Optimization of Kunzea ericoides Nanoemulgel Using a Quality by Design Approach for Transdermal Anti-Inflammatory Therapy. Gels, 11(6), 400. https://doi.org/10.3390/gels11060400







