Gel Electrolytes in the Development of Textile-Based Power Sources
Abstract
1. Introduction
2. Gel Electrolytes Applied in Textile Structures
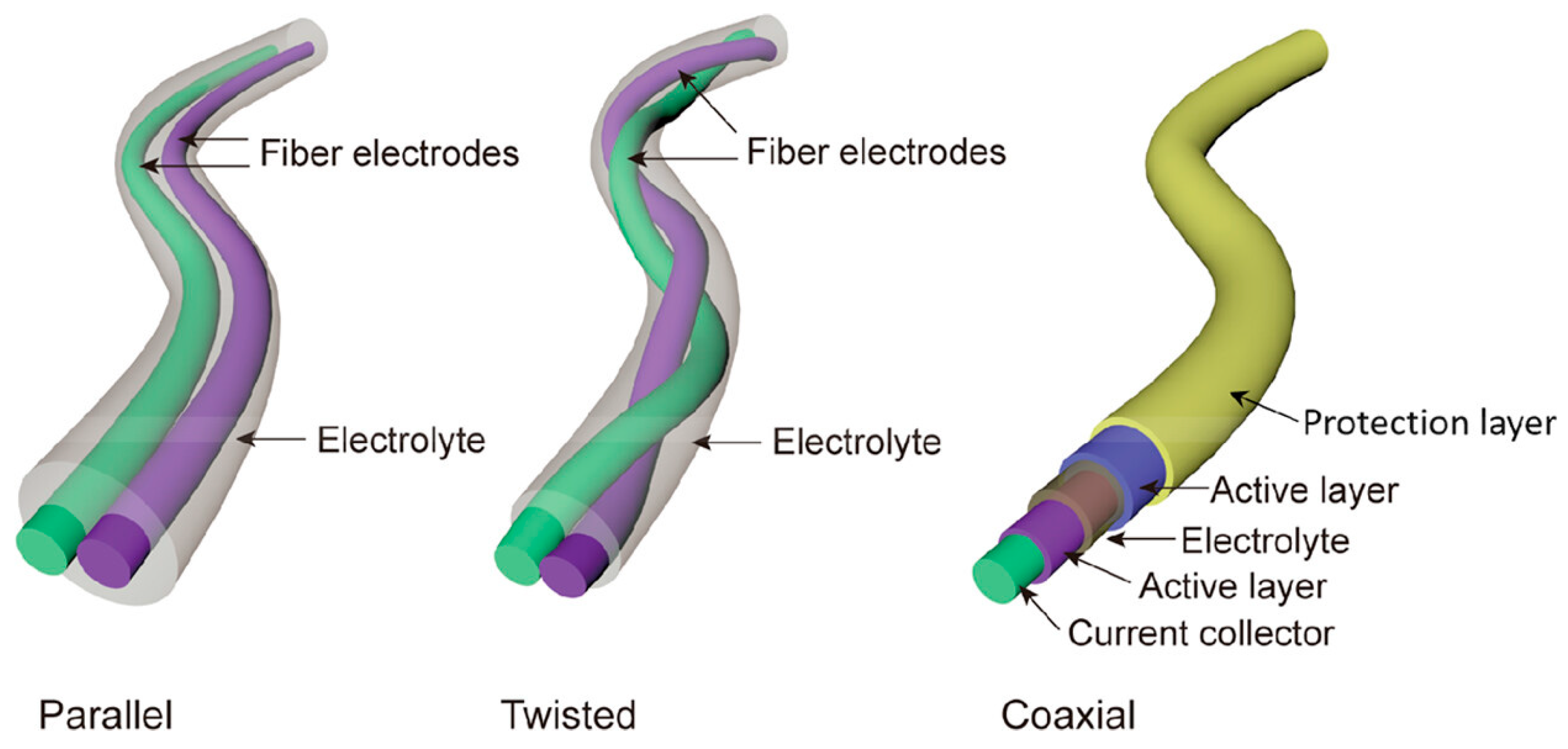
2.1. Fibers
| Gel Electrolyte | Fiber Composition | Electrodes | Preparation Method | Conductivity Tests and Results | Ref. |
|---|---|---|---|---|---|
| PVDF/EMIMBF4 | CNT | PANI–graphene–CNT | Dry-spinning CNT fibers; CNT/rGO via chemical vapor deposition; PANI nanorod coating; gel electrolyte coating | Energy density of 1.3 × 10 Wh/kg; power density of 1.4 × 103 W/kg; 98.4% capacitance retention after 2000 cycles | [21] |
| H2SO4 | GO | rGO | GO wet spinning; gel electrolyte coating and two-fiber twisting | Excellent performance and fast reponse rate (500 V/s); cycling stability; stable performance at different angles | [28] |
| KOH | CNT-GO | CNT-rGO | CNT-GO wet spinning; hydrothermal activation; gel electrolyte coating | Volumetric capacitance of 6.1 × 104 mF/cm3; volumetric energy density of 4.8 × 10−3 Wh/cm3; 94% stability after 10,000 cycles; stable performance at different angles | [29] |
| ZnSO4-filled PAA | CNT-GO | CNT/rGO and Zn/graphite | Hydrothermally assemble CNT/rGO; coating of Zn fiber with graphite by electrodeposition; gel electrolyte coating | Ionic conductivity of 21.7 mS/cm; stretchability up to 2500; energy density of 48.5 mWh/cm3; volumetric capacitance of 1.0 × 105 mF/cm3; 98.5% stability after 10,000 cycles | [30] |
| H3PO4 | GO | rGO-MoS2 | Wet spinning of MoS2-GO, followed by chemical reduction; gel electrolyte coating | Volumetric capacitance of 2.2 × 105 mF/cm3; three fibers connected—capacitance of 2.4 × 102 mF/cm2; energy density of 3.2 × 10−2 Wh/cm3; 72% stability after bending 500 times at different angles | [31] |
| H3PO4 | Carbon nanobranches | Carbon nanobranches/PU | Carbon nanobranches covered with carbon dots by pyrolysis of starch; microfluidic spinning for carbon nanobranches/PU fibers; twisting of fibers and gel electrolyte coating | Specific capacitance of 2.0 × 102 mF/cm2; energy density of 4.5 μWh/cm2; 98% stability after 10,000 cycles; powered 19 LEDs | [32] |
| H3PO4 | CNT/GO/PANI | CNT/GO/PANI | CNT film by chemical vapor deposition; immersion in a GO/PANI mixture solution; over-twisting of strips of the film into two helical fibers; gel electrolyte coating; intertwining fibers and gel electrolyte coating | CNT/GO/PANI film with a specific capacitance of 5.1 × 102 mF/cm2; specific capacitance of 1.8 × 102 mF/cm2; 80% stability after 5000 cycles; stability after bending for 500 cycle at 180° | [36] |
| H2SO4 | PAN | Ti/PEDOT | Electrospun PAN nanofibers on a Ti wire with glycerol; sip-coating with PEDOT:PSS; PSS etching with H2SO4; gel electrolyte coating, twisting, and coating again with gel electrolyte | Energy density of 5.5 Wh/kg; specific capacitance of 68 F/g; power density of 9.1 × 103 W/kg; 81% stability after 10,000 cycles; stability under different deformations; powered one LED | [37] |
| LiCl | PEDOT:PSS | PEDOT:PSS | Coaxial PEDOT:PSS wet spinning; gel electrolyte coating | Electrical conductivity of 1514 S/cm; specific areal capacitance of 1.2 × 102 mF/cm2; energy density of 9 × 10−6 Wh/cm2; 81% stability after 10,000 cycles; Coulombic efficiency of ~100%; stable specific capacitance after bending 3000 times at 180° | [38] |
| H3PO4 | Kevlar® fibers | PEDOT:PSS/Kevlar® | Wet spinning of nanofibrillated Kevlar®; immersion in PEDOT:PSS gel | Capacitance of 1.1 mF; volumetric energy density of 7.1 × 10−2 Wh/cm3; 80.5% stability after 10,000 cycles; stability under different deformations; Coulombic efficiency of 99.1%; powered one LED | [39] |
| Chitosan | PEDOT:PSS/CNT | PEDOT:PSS/CNT | Wet spinning of PEDOT:PSS/CNT with internal nozzles for electrode inks and external nozzle carrying the gel electrolyte | Volumetric capacitance of 1.6 × 104 mF/cm3; 96% stability after 5000 cycles; electrochemical stability after 1 × 105 bending cycles | [40] |
| H2SO4 | PEDOT:PSS | PEDOT:PSS/V2O5 | PEDOT:PSS/V2O5 wet spinning; gel electrolyte coating | Energy density 1.4 × 10−6 Wh/cm2; specific capacitance of 6.0 × 10 mF/cm2; 94.02% stability after 4000 cycles | [41] |
| H2SO4 | PEDOT:PSS | PEDOT:PSS Mo1.33Ci-MXene and rGO fibers | PEDOT:PSS Mo1.33Ci-MXene and GO wet spinning; gel electrolyte coating | Capacitance of 105 F/g; energy density of 3.7 × 10−2 Wh/g; 94% stability after 10,000 cycles | [43] |
| PVA–LiCl | GO | MnO2/Ti3C2Tx/rGO | Ti3C2Tx/GO wet spinning; immersion in HI/CH3COOH; immersion in KMnO4 solution; twisting of fibers and gel electrolyte coating | Capacitance of 2.4 × 104 mF/cm3; energy density of 2.1 × 10−3 Wh/cm3; 92% stability after 10,000 cycles; 100% stability after bending 1000 times at 90° | [44] |
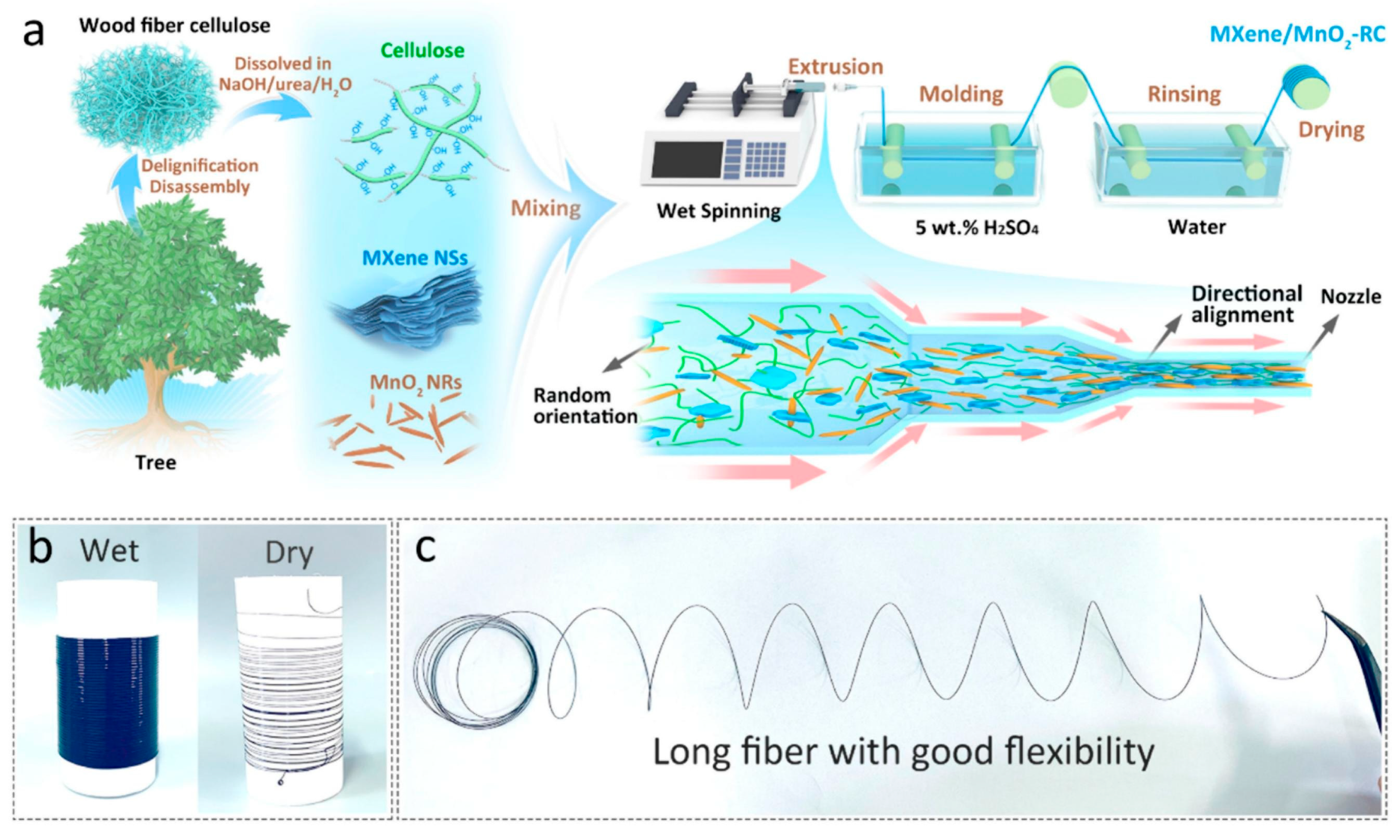
| ZnSO4/gelatin | MXene/MnO2-RC (cathode) and Zn wire (anode) | MXene/MnO2-RC fiber cathode wet spinning; gel eletrolyte coating | Volumetric capacitance of 1.1 × 102 mF/cm3; energy density of 2.2 × 10−2 Wh/cm3; 90.5% stability and 100% Coulombic efficiency after 5000 cycles | [45] | |
| PVA/H3PO4 | MXene/PEDOT:PSS/Ag/MXene/PEDOT:PSS | MXene/PEDOT:PSS ink by 3D direct-ink writing; printing of Ag on top of the electrode; printing the gel electrolyte; Coating in NOA 63 resin | Electrical conductivity of 1.6 × 103 S/cm; areal capacitance of 1.1 × 103 mF/cm2; gravimetric capacitance of 185.9 F/g; areal energy density of 9.4 × 10−5 Wh/cm2; 92% stability after 25,000 cycles; powered one LED | [46] | |
| PVA/GO/ZnSO4/MnSO4 | Carbon | Carbon wire coated with nano-MnO2 (cathode) and Zn wire (anode) | GO gel crosslinking with nano-MnO2; gel eletrolyte coating; silicone layer coating | Gel electrolyte with ion conductivity of 2.1 × 10−2 S/cm; ~230% stretchability and self-healing; energy density of 91 Wh/L; 98.0% stability after 1000 cycles | [47] |
| CMC/ZnSO4 | CNT fiber | CNT fiber coated with ZnHCF (cathode); CNT fiber coated with Zn nanosheet arrays (anode) | Roll electrodeposition of Zn arrays on CNT fibers; gel electrolyte coating comprising CNT sheets, ZnHCF, and CNT sheets | Capacity of 100.2 mAh/cm3; energy density of 2.0 × 10−1 Wh/cm3; 91.8% stability after 200 cycles; 96.8% Coulombic efficiency; 93.2% stability after bending 3000 cycles at 90°; powered one LED | [48] |
| CMC-SO4 | CNT fiber | CNT fiber coated with stitched ZVO nanosheets (cathode) and CNT fiber coated with Zn (anode) | CNT-stitched ZVO nanosheets hydrothermally; electrodeposition of Zn on CNT fiber; gel electrolyte coating; twisting of electrodes | 69.7% stability after 100 cycles; volumetric energy density of 7.2 × 10−2 Wh/cm3; 88.9% stability retention after 2000 cycles; 100% stability after bending angles from 0 to 180° | [49] |
| KOH | CNT fiber | Nitride-doped CNT fiber coated with Ag2O/PEDOT:PSS (cathode) and CNT fiber coated with Zn (anode) | Deposition of Ag2O on a nitride-doped CNT fiber and coating with PEDOT:PSS; electrodeposition of Zn on CNT fiber; gel electrolyte coating; twisting of two electrodes | Capacity of 1.05 mAh/cm2; energy density 1.6 × 10−3 Wh/cm2; power density of 1.4 × 10−3 W/cm2; 79.5% eletrochemical stabily retention after 200 cycles | [50] |
| ZnSO4 | Ag fiber Spandex fiber | Ag fiber coated with graphene and PANI (cathode) and Ag fiber coated with Zn (anode) | Coating of Ag fibers with graphene and PANI, and Zn nanoflakes by electrodeposition; gel electrolyte coating; encapsulation with PU, and twisting around a spandex fiber | Specific capacity of 32.56 mAh/cm3; energy density of 3.6 × 10−2 Wh/cm3; stability of 76.5% after 1000 cycles and 99.0, 93.6 and 91.5% during knotting, bending, and twisting; strain up to 900% | [51] |
| Al(CF3SO3)3 | CNT fiber | CNT fiber coated with MnHCF (cathode) and CNT fiber coated with GO/MoO3 (anode) | Coating of CNT fibers with MnHCF and GO/MoO3; gel electrolyte coating; assembled on a silicone rubber substrate and gel electrolyte coating | Al(CF3SO3)3 hydrogel with ionic conductivity of 2.2 × 10−2 S/cm and strain of 461%; specific capacity of 42 mAh/cm3; energy density of 3.1 × 10−2 Wh/cm3; stability of 91.6% after 100 cycles | [52] |
| PVDF/HFP/Al2O3 | LFP (cathode) and LTO (anode) | Etching of GO with H2O2 and reduction with vitamin C; PVA/PVDF adhesive in NMP; printing ink by dissolving LFP or LTO and CNTs in the PVA/PVDF solution; extrusion into a NaBO2 solution; immersion in electrolyte | Specific capacity/capacity retention rate of 153.7 mAh/g/92% and 156.5 mAh/g/86.32% after 100 cycles, LFP/HrGO and LTO/HrGO fiber electrodes; FLIB@HrGO with a discharge capacity/capacity retention rate of 142.2 mAh/g/90.4%, after 100 cycles; specific capacity of 62.42 mAh/cm3; energy density of 1.6 × 10−1 Wh/cm3 | [53] |
2.2. Yarns
2.3. Woven Fabrics
2.4. Knitted Fabrics
2.5. Non-Wovens
3. Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teixeira, J.S.; Pereira, A.M.; Pereira, C. Smart dual-functional energy storage/fluorescent textile device based on a new redox-active Mn-doped ZnS solid-gel electrolyte. Chem. Eng. J. 2021, 426, 131274. [Google Scholar] [CrossRef]
- Rafique, A.; Ferreira, I.; Abbas, G.; Baptista, A.C. Recent Advances and Challenges Toward Application of Fibers and Textiles in Integrated Photovoltaic Energy Storage Devices. Nano-Micro Lett. 2023, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Ding, D.; Tang, S.; Ma, Z.; Liu, C.; Yuan, Y. Solvent-Triggered, Ultra-Adhesive, Conductive, and Biocompatible Transition Gels for Wearable Devices. Small 2024, 20, e2310731. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mo, Z.; Liu, Z.; Hu, Y.; Du, C.; Liang, L.; Liu, Z.; Chen, G. Robust, Efficient, and Recoverable Thermocells with Zwitterion-Boosted Hydrogel Electrolytes for Energy-Autonomous and Wearable Sensing. Angew. Chem. Int. Ed. 2024, 63, e202405357. [Google Scholar] [CrossRef]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive Ionic Conductive Alginate/Gelatin Organohydrogels with Tunable Functions. Adv. Funct. Mater. 2024, 34, 2410663. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Xu, S.; Song, H. Stretchable ionic conductive gels for wearable human-activity detection. Chem. Eng. J. 2024, 489, 151231. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, J.; Yan, J.; Liu, X. Advanced Fiber Materials for Wearable Electronics. Adv. Fiber Mater. 2022, 5, 12–35. [Google Scholar] [CrossRef]
- Nie, S.; Cai, M.; Yang, H.; Shen, L.; Wang, S.; Zhu, Y.; Song, J. Soft, stretchable thermal protective substrates for wearable electronics. npj Flex. Electron. 2022, 6, 36. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, X.; Tian, M.; Zhang, X.; Qu, L.; Fan, T.; Miao, J. Smart fibers and textiles for emerging clothe-based wearable electronics: Materials, fabrications and applications. J. Mater. Chem. A 2023, 11, 17336–17372. [Google Scholar] [CrossRef]
- Islam, M.R.; Afroj, S.; Novoselov, K.S.; Karim, N. Smart Electronic Textile-Based Wearable Supercapacitors. Adv. Sci. 2022, 9, e2203856. [Google Scholar] [CrossRef]
- Guan, L.; Yu, L.; Chen, G.Z. Capacitive and non-capacitive faradaic charge storage. Electrochim. Acta 2016, 206, 464–478. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Newby, S.; Mirihanage, W.; Fernando, A. Modern Developments for Textile-Based Supercapacitors. ACS Omega 2023, 8, 12613–12629. [Google Scholar] [CrossRef]
- Grube, A.; Shaban, M.M.; Hilger, L.; Firouzjaei, M.D.; Shamsabadi, A.A.; Demirel, Y.; Elliott, M.; Nejati, S.; Bavarian, M. Wearable Textile Supercapacitors: Material Advancements and Applications. J. Energy Storage 2024, 99, 113228. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Hao, Q.; Ma, X.; Gao, Y.; Chen, F.; Chen, X.; Qi, Y.; Li, N. Commercial carbonate based gel polymer electrolytes enable safe and stable high-voltage Li-metal batteries. Energy Storage Mater. 2024, 70, 103509. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Lei, Y.; Jaumaux, P.; Tian, H.; Wang, G. Quasi-Solid Gel Electrolytes for Alkali Metal Battery Applications. Nano-Micro Lett. 2025, 17, 194. [Google Scholar] [CrossRef]
- Duay, J.; Gillette, E.; Liu, R.; Lee, S.B. Highly flexible pseudocapacitor based on freestanding heterogeneous MnO2/conductive polymer nanowire arrays. Phys. Chem. Chem. Phys. 2012, 14, 3329–3337. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, H.; Cheng, X.; He, J.; Long, Y.; Chang, Y.; Gong, X.; Zhang, K.; Li, J.; Zhu, Z.; et al. High-performance fibre battery with polymer gel electrolyte. Nature 2024, 629, 86–91. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Zhang, S.; Liu, Y.; Ma, R.; Wallace, G.; Chen, J. Highly stretchable double-network gel electrolytes integrated with textile electrodes for wearable thermo-electrochemical cells. SusMat 2024, 4, e225. [Google Scholar] [CrossRef]
- Adusei, P.K.; Kanakaraj, S.N.; Gbordzoe, S.; Johnson, K.; DeArmond, D.; Hsieh, Y.-Y.; Fang, Y.; Mishra, S.; Phan, N.; Alvarez, N.T.; et al. A scalable nano-engineering method to synthesize 3D-graphene-carbon nanotube hybrid fibers for supercapacitor applications. Electrochim. Acta 2019, 312, 411–423. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Ma, B.; Zhang, D.; Zhao, Y. Liquid metal-integrated ultra-elastic conductive microfibers from microfluidics for wearable electronics. Sci. Bull. 2020, 65, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Hunpratub, S.; Chullaphan, T.; Chumpolkulwong, S.; Chanlek, N.; Phokha, S. Characterization and electrochemical properties of Carbon/CeO2 composites prepared using a hydrothermal method. Mater. Chem. Phys. 2023, 303, 127820. [Google Scholar] [CrossRef]
- Chen, C.; Feng, J.; Li, J.; Guo, Y.; Shi, X.; Peng, H. Functional Fiber Materials to Smart Fiber Devices. Chem. Rev. 2022, 123, 613–662. [Google Scholar] [CrossRef]
- Shalini, S.; Naveen, T.B.; Durgalakshmi, D.; Balakumar, S.; Rakkesh, R.A. Progress in flexible supercapacitors for wearable electronics using graphene-based organic frameworks. J. Energy Storage 2024, 86, 111260. [Google Scholar] [CrossRef]
- Luo, Z.; Quan, J.; Ding, T.; Xu, B.; Li, W.; Mao, Q.; Ma, W.; Li, M.; Xiang, H.; Zhu, M. Recent advances in iron oxide/graphene composites for flexible supercapacitors. J. Alloys Compd. 2024, 980, 173614. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Quan, J.; Ding, T.; Xu, B.; Li, W.; Mao, Q.; Ma, W.; Xiang, H.; Zhu, M. Oxygen defect enriched hematite nanorods @ reduced graphene oxide core-sheath fiber for superior flexible asymmetric supercapacitor. J. Colloid Interface Sci. 2024, 653, 77–84. [Google Scholar] [CrossRef]
- Gao, K.; Wang, S.; Liu, W.; Yue, Y.; Rao, J.; Su, J.; Li, L.; Zhang, Z.; Liu, N.; Xiong, L.; et al. All Fiber Based Electrochemical Capacitor towards Wearable AC Line Filters with Outstanding Rate Capability. ChemElectroChem 2019, 6, 1450–1457. [Google Scholar] [CrossRef]
- Park, H.; Ambade, R.B.; Noh, S.H.; Eom, W.; Koh, K.H.; Ambade, S.B.; Lee, W.J.; Kim, S.H.; Han, T.H. Porous Graphene-Carbon Nanotube Scaffolds for Fiber Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 9011–9022. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, Z.; Wang, C.; Yuan, Z.; Wei, L.; Pan, Y.; Mahmood, A.; Shao, Q.; Chen, Y. Flexible Zinc-Ion Hybrid Fiber Capacitors with Ultrahigh Energy Density and Long Cycling Life for Wearable Electronics. Small 2019, 15, e1903817. [Google Scholar] [CrossRef]
- Li, J.; Shao, Y.; Jiang, P.; Zhang, Q.; Hou, C.; Li, Y.; Wang, H. 1T-Molybdenum disulfide/reduced graphene oxide hybrid fibers as high strength fibrous electrodes for wearable energy storage. J. Mater. Chem. A 2019, 7, 3143–3149. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Wu, X.; Cheng, H.; Li, Q.; Chen, S. Facile synthesis of carbon nanobranches towards cobalt ion sensing and high-performance micro-supercapacitors. Nanoscale Adv. 2019, 1, 3614–3620. [Google Scholar] [CrossRef] [PubMed]
- Nayan, R.; Sinha, S.; Dixit, V.; Satnami, M.L.; Ghosh, K.K.; Pervez, S.; Deb, M.K.; Shrivas, K.; Rai, M.K.; Yenchalwar, S.G.; et al. PANI-grafted boron, nitrogen co-doped carbon fiber: An outstanding, high-performance supercapacitor electrode. J. Energy Storage 2024, 96, 112668. [Google Scholar] [CrossRef]
- Huang, H.; Abbas, S.C.; Deng, Q.; Ni, Y.; Cao, S.; Ma, X. An all-paper, scalable and flexible supercapacitor based on vertically aligned polyaniline (PANI) nano-dendrites@fibers. J. Power Sources 2021, 498, 229886. [Google Scholar] [CrossRef]
- Li, J.; Qiu, S.; Liu, B.; Chen, H.; Xiao, D.; Li, H. Strong interaction between polyaniline and carbon fibers for flexible supercapacitor electrode materials. J. Power Sources 2021, 483, 229219. [Google Scholar] [CrossRef]
- Jiang, Q.; Shang, Y.; Sun, Y.; Yang, Y.; Hou, S.; Zhang, Y.; Xu, J.; Cao, A. Flexible and multi-form solid-state supercapacitors based on polyaniline/graphene oxide/CNT composite films and fibers. Diam. Relat. Mater. 2019, 92, 198–207. [Google Scholar] [CrossRef]
- Lai, H.; Li, W.; Zhou, Y.; He, T.; Xu, L.; Tian, S.; Wang, X.; Fan, Z.; Lei, Z.; Jiao, H. Hydrophilically engineered polyacrylonitrile nanofiber aerogel as a soft template for large mass loading of mesoporous poly(3,4-ethylenedioxythiophene) network on a bare metal wire for high-rate wire-shaped supercapacitors. J. Power Sources 2019, 441, 227212. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Liu, Y.; Zhang, X.; Wang, B. Thin-walled hollow fibers for flexible high energy density fiber-shaped supercapacitors. Energy Mater. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Gibertini, E.; Magagnin, L. PEDOTS:PSS@KNF Wire-Shaped Electrodes for Textile Symmetrical Capacitor. Adv. Mater. Interfaces 2022, 9, 2200513. [Google Scholar] [CrossRef]
- Hong, Y.; Cheng, X.-L.; Liu, G.-J.; Hong, D.-S.; He, S.-S.; Wang, B.-J.; Sun, X.-M.; Peng, H.-S. One-step Production of Continuous Supercapacitor Fibers for a Flexible Power Textile. Chin. J. Polym. Sci. 2019, 37, 737–743. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, C.; Sun, S.; Zhang, K. Electrochemical properties of PEDOT: PSS /V2O5 hybrid fiber based supercapacitors. J. Phys. Chem. Solids 2019, 129, 234–241. [Google Scholar] [CrossRef]
- Wang, R.; Young Jang, W.; Zhang, W.; Venkata Reddy, C.; Kakarla, R.R.; Li, C.; Gupta, V.K.; Shim, J.; Aminabhavi, T.M. Emerging two-dimensional (2D) MXene-based nanostructured materials: Synthesis strategies, properties, and applications as efficient pseudo-supercapacitors. Chem. Eng. J. 2023, 472, 144913. [Google Scholar] [CrossRef]
- Qin, L.; Jiang, J.; Hou, L.; Zhang, F.; Rosen, J. MXene-based multifunctional smart fibers for wearable and portable electronics. J. Mater. Chem. A 2022, 10, 12544–12550. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Kang, L.; He, X.; Li, Q.; Sun, J.; Jiang, R.; Xu, H.; Shi, F.; Lei, Z.; et al. Intercalation and delamination behavior of Ti3C2Txand MnO2/Ti3C2Tx/RGO flexible fibers with high volumetric capacitance. J. Mater. Chem. A 2019, 7, 12582–12592. [Google Scholar] [CrossRef]
- Wei, S.; Wan, C.; Huang, Q.; Chai, H.; Chai, Y.; Li, X.; Wu, Y. 3D cellulose network confining MXene/MnO2 enables flexible wet spinning microfibers for high-performance fiber-shaped Zn-ion capacitors. Int. J. Biol. Macromol. 2024, 276, 134152. [Google Scholar] [CrossRef]
- Ovhal, M.M.; Lee, H.B.; Satale, V.V.; Tyagi, B.; Chowdhury, S.; Kang, J.W. One-Meter-Long, All-3D-Printed Supercapacitor Fibers Based on Structurally Engineered Electrode for Wearable Energy Storage. Adv. Energy Mater. 2023, 14, 2303053. [Google Scholar] [CrossRef]
- Xiao, X.; Xiao, X.; Zhou, Y.; Zhao, X.; Chen, G.; Liu, Z.; Wang, Z.; Lu, C.; Hu, M.; Nashalian, A.; et al. An ultrathin rechargeable solid-state zinc ion fiber battery for electronic textiles. Sci. Adv. 2021, 7, eabl3742. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Li, Q.; Pan, Z.; Sun, J.; Zhou, Z.; He, B.; Man, P.; Xie, L.; Kang, L.; et al. Flexible and High-Voltage Coaxial-Fiber Aqueous Rechargeable Zinc-Ion Battery. Nano Lett. 2019, 19, 4035–4042. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, J.; Yang, J.; Zhang, Q.; Zhang, H.; Li, X.; Kou, Z.; Zhang, Y.; Chen, H.; Yan, C.; et al. Stitching of Zn3(OH)2V2O7·2H2O 2D Nanosheets by 1D Carbon Nanotubes Boosts Ultrahigh Rate for Wearable Quasi-Solid-State Zinc-Ion Batteries. ACS Nano 2019, 14, 842–853. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Li, T.; Zhu, Z.; He, B.; Zhou, Z.; Man, P.; Li, Q.; Yao, Y. An ultra-high endurance and high-performance quasi-solid-state fiber-shaped Zn–Ag2O battery to harvest wind energy. J. Mater. Chem. A 2019, 7, 2034–2040. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Ye, X.; Zhang, X.; Qu, L.; Tian, M. Tendril-Inspired 900% Ultrastretching Fiber-Based Zn-Ion Batteries for Wearable Energy Textiles. ACS Appl. Mater. Interfaces 2021, 13, 17110–17117. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; He, B.; Zhou, T.; Wang, Z.; Wang, Z.; Xin, J.; Zhang, H.; Zhou, X.; Liu, Y.; Wei, L. Stretchable fiber-shaped aqueous aluminum ion batteries. EcoMat 2022, 4, e12218. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, R.; Liang, H.; Fang, B.; Li, R.; Wu, R.; Mo, R. Autonomous self-healing strategy for flexible fiber lithium-ion battery with ultra-high mechanical properties and volumetric energy densities. Chem. Eng. J. 2024, 496, 154153. [Google Scholar] [CrossRef]
- Liu, S.; Ban, J.; Shi, H.; Wu, Z.; Shao, G.; Cao, G.; Hu, J. Near solution-level conductivity of polyvinyl alcohol based electrolyte and the application for fully compliant Al-air battery. Chem. Eng. J. 2022, 431, 134283. [Google Scholar] [CrossRef]
- Alipoori, S.; Torkzadeh, M.M.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Performance-tuning of PVA-based gel electrolytes by acid/PVA ratio and PVA molecular weight. SN Appl. Sci. 2021, 3, 310. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Wang, Y.-F.; Yue, Y.; Bian, S.-W. Flexible polyester yarn/Au/conductive metal-organic framework composites for yarn-shaped supercapacitors. J. Electroanal. Chem. 2019, 847, 113218. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Liu, Y.; Guo, X.; Liu, Y.; Wang, C.; Li, Y.; Tian, M. Washable and anti-impact conductive cellulose yarn-based energy meta-textile against harsh environments. Appl. Mater. Today 2024, 36, 102055. [Google Scholar] [CrossRef]
- Uzun, S.; Seyedin, S.; Stoltzfus, A.L.; Levitt, A.S.; Alhabeb, M.; Anayee, M.; Strobel, C.J.; Razal, J.M.; Dion, G.; Gogotsi, Y. Knittable and Washable Multifunctional MXene-Coated Cellulose Yarns. Adv. Funct. Mater. 2019, 29, 1905015. [Google Scholar] [CrossRef]
- Du, X.; Tian, M.; Sun, G.; Li, Z.; Qi, X.; Zhao, H.; Zhu, S.; Qu, L. Self-Powered and Self-Sensing Energy Textile System for Flexible Wearable Applications. ACS Appl. Mater. Interfaces 2020, 12, 55876–55883. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Rao, W.; Fan, L.; Xia, Z.; Xu, W.; Xu, J. A high-performance all-solid-state yarn supercapacitor based on polypyrrole-coated stainless steel/cotton blended yarns. Cellulose 2018, 26, 1169–1181. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Qiang, R.; Nan, N.; You, X.; Zhou, Y.; Shao, W.; Liu, F.; Liu, R. Electrospun Conductive Nanofiber Yarn for a Wearable Yarn Supercapacitor with High Volumetric Energy Density. Materials 2019, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.T.; Cunha, I.; Coelho, J.; Fortunato, E.; Martins, R.; Pereira, L. Carbon-Yarn-Based Supercapacitors with In Situ Regenerated Cellulose Hydrogel for Sustainable Wearable Electronics. ACS Appl. Energy Mater. 2022, 5, 11987–11996. [Google Scholar] [CrossRef]
- Rani, S.; Sharma, Y. Fabrication of Binder-Free and High Energy Density Yarn Supercapacitor for Wearable Electronics. IEEE Trans. Power Electron. 2022, 37, 13022–13029. [Google Scholar] [CrossRef]
- Park, Y.; Choi, H.; Kim, M.-C.; Tran, N.A.T.; Cho, Y.; Sohn, J.I.; Hong, J.; Lee, Y.-W. Effect of ionic conductivity in polymer-gel electrolytes containing iodine-based redox mediators for efficient, flexible energy storage systems. J. Ind. Eng. Chem. 2021, 94, 384–389. [Google Scholar] [CrossRef]
- Levitt, A.; Hegh, D.; Phillips, P.; Uzun, S.; Anayee, M.; Razal, J.M.; Gogotsi, Y.; Dion, G. 3D knitted energy storage textiles using MXene-coated yarns. Mater. Today 2020, 34, 17–29. [Google Scholar] [CrossRef]
- Pal, M.; Subhedar, K.M. Biscrolled hierarchical hybrid structure of PEDOT:PSS/CNTs-NMP yarn based solid state linear supercapacitor for wearable e-textile. Carbon 2024, 229, 119552. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Liao, S.; Chen, J.; Wei, Q. Hierarchical core-shell polypyrrole@NiCo layered double hydroxide arrays grown on stainless steel yarn with high flexibility for 1D symmetric yarn-shaped supercapacitors. J. Alloys Compd. 2022, 926, 166811. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, H.-T.; Yang, S.-Y.; Yue, Y.; Bian, S.-W. Hierarchical NiCo2S4@Nickel–Cobalt Layered Double Hydroxide Nanotube Arrays on Metallic Cotton Yarns for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 30384–30390. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cong, Z.; Pu, X.; Guo, W.; Liu, T.; Li, M.; Zhang, Y.; Hu, W.; Wang, Z.L. High-Energy Asymmetric Supercapacitor Yarns for Self-Charging Power Textiles. Adv. Funct. Mater. 2019, 29, 1806298. [Google Scholar] [CrossRef]
- Al-khaykanee, A.H.; Ghorbani, S.R.; Arabi, H.; Ghanbari, R. NiMnO3-rGO nanocomposites in a cotton-based flexible yarn supercapacitor. J. Mater. Sci. Mater. Electron. 2024, 35, 141. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, Y.; Liu, T.; Liu, X.; Ni, S.; Ru, J.; Meng, F.; Li, M.; Cao, Z. A battery–capacitor hybrid yarn device with excellent flexibility and high electrochemical performance. J. Mater. Chem. A 2025, 13, 12374–12382. [Google Scholar] [CrossRef]
- Yu, Y.; Liao, Y.; Fan, J.; Ding, Y.; Fan, Y.; Cao, J.; Zhou, X.; Wang, Y.; Yan, J.; Li, H.; et al. Tip effect of NiCo-LDH with low crystallinity for enhanced energy storage performance of yarn-shaped supercapacitors. J. Colloid Interface Sci. 2025, 679, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Padmajan Sasikala, S.; Jeong, Y.; Kim, J.G.; Ha, J.-H.; Hwang, S.H.; Jeon, S.; Choi, J.; Kang, B.-H.; Ahn, J.; et al. High-Energy–Density Fiber Supercapacitors Based on Transition Metal Oxide Nanoribbon Yarns for Comprehensive Wearable Electronics. Adv. Fiber Mater. 2024, 6, 1927–1941. [Google Scholar] [CrossRef]
- Li, M.; Qiao, J.; Zhu, C.; Hu, Y.; Wu, K.; Zeng, S.; Yang, W.; Zhang, H.; Wang, Y.; Wu, Y.; et al. Gel-Electrolyte-Coated Carbon Nanotube Yarns for Self-Powered and Knittable Piezoionic Sensors. ACS Appl. Electron. Mater. 2021, 3, 944–954. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, D.; Hu, H.; Shang, J.; Chang, J.; Xie, C.; Yang, Y.; Lepró, X.; Baughman, R.H.; Zheng, Z. Additive Functionalization and Embroidery for Manufacturing Wearable and Washable Textile Supercapacitors. Adv. Funct. Mater. 2020, 30, 1910541. [Google Scholar] [CrossRef]
- Yi, H.; Ma, Y.; Zhang, S.; Na, B.; Zeng, R.; Zhang, Y.; Lin, C. Robust Aqueous Zn-Ion Fiber Battery Based on High-Strength Cellulose Yarns. ACS Sustain. Chem. Eng. 2019, 7, 18894–18900. [Google Scholar] [CrossRef]
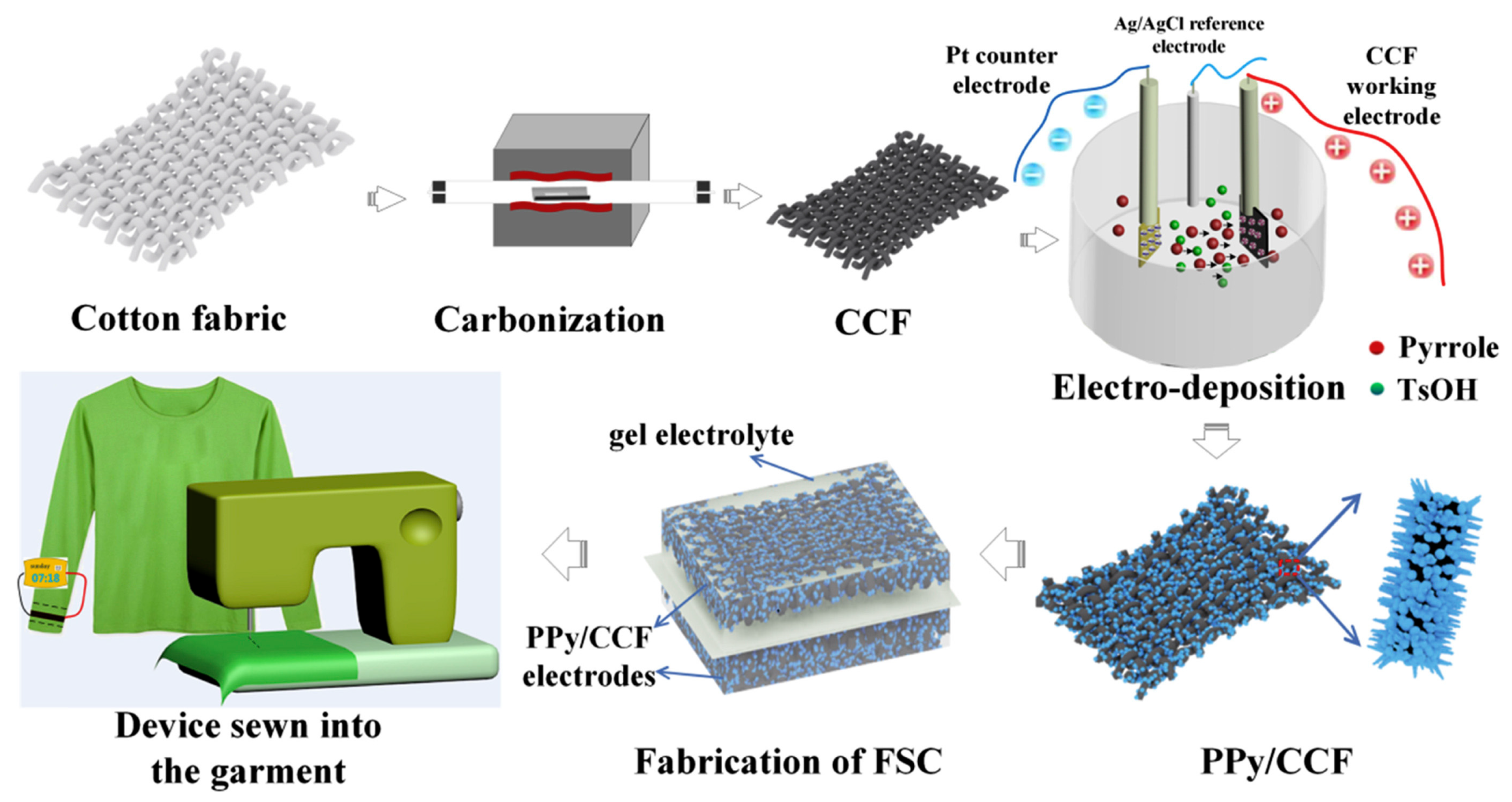
- Sun, C.; Li, X.; Cai, Z.; Ge, F. Carbonized cotton fabric in-situ electrodeposition polypyrrole as high-performance flexible electrode for wearable supercapacitor. Electrochim. Acta 2019, 296, 617–626. [Google Scholar] [CrossRef]
- Wang, D.; Sun, J.; Xue, Q.; Li, Q.; Guo, Y.; Zhao, Y.; Chen, Z.; Huang, Z.; Yang, Q.; Liang, G.; et al. A universal method towards conductive textile for flexible batteries with superior softness. Energy Storage Mater. 2021, 36, 272–278. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Xing, C.; Jia, G.; Lu, Z.; Tian, Q.; Zhang, S.; Lv, J. Polypyrrole and cotton fabric-based flexible micro-supercapacitors. J. Appl. Polym. Sci. 2022, 139, e52801. [Google Scholar] [CrossRef]
- Ramandi, S.; Entezari, M.H. Self-supporting electrode for flexible supercapacitors: NiCo-layered double hydroxide derived from metal organic frameworks wrapped on graphene/polyaniline nanotubes@cotton cloth. J. Energy Storage 2022, 56, 106106. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, J.; Hu, Q.; Nie, W.; Wang, Z.; Zou, L.; Li, C. Vapor phase polymerized conducting polymer/MXene textiles for wearable electronics. Nanoscale 2021, 13, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Hillier, N.; Yong, S.; Cruden, A.; Beeby, S. Acetonitrile-Free Organic Electrolyte for Textile Supercapacitor Applications. J. Electrochem. Soc. 2021, 168, 080520. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Q.; Yi, C.; Chen, J.; Wang, D.; Wang, Y.; Liu, X.; Li, M.; Liu, K.; Zhou, P.; et al. The construction of sea urchin spines-like polypyrrole arrays on cotton-based fabric electrode via a facile electropolymerization for high performance flexible solid-state supercapacitors. Electrochim. Acta 2020, 354, 136746. [Google Scholar] [CrossRef]
- Wang, C.; Song, Z.; Wan, H.; Chen, X.; Tan, Q.; Gan, Y.; Liang, P.; Zhang, J.; Wang, H.; Wang, Y.; et al. Ni-Co selenide nanowires supported on conductive wearable textile as cathode for flexible battery-supercapacitor hybrid devices. Chem. Eng. J. 2020, 400, 125955. [Google Scholar] [CrossRef]
- Zhai, S.; Jin, K.; Zhou, M.; Fan, Z.; Zhao, H.; Li, X.; Zhao, Y.; Ge, F.; Cai, Z. A novel high performance flexible supercapacitor based on porous carbonized cotton/ZnO nanoparticle/CuS micro-sphere. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124025. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, J.; Guo, Z.; Zhao, Y.; Cai, Z.; Ge, F. A Novel Method to Fabricate Nitrogen and Oxygen Co-Doped Flexible Cotton-Based Electrode for Wearable Supercapacitors. ChemElectroChem 2019, 6, 4049–4058. [Google Scholar] [CrossRef]
- Mallick, S.; Mondal, A.; Raj, C.R. Rationally designed mesoporous carbon-supported Ni–NiWO4@NiS nanostructure for the fabrication of hybrid supercapacitor of long-term cycling stability. J. Power Sources 2020, 477, 229038. [Google Scholar] [CrossRef]
- Hillier, N.; Yong, S.; Beeby, S. Culinary inspired electrolytes for textile supercapacitors. Energy Rep. 2021, 7, 81–86. [Google Scholar] [CrossRef]
- Chen, R.; Yang, Y.; Huang, Q.; Ling, H.; Li, X.; Ren, J.; Zhang, K.; Sun, R.; Wang, X. A multifunctional interface design on cellulose substrate enables high performance flexible all-solid-state supercapacitors. Energy Storage Mater. 2020, 32, 208–215. [Google Scholar] [CrossRef]
- Liang, C.; Zang, L.; Shi, F.; Yang, C.; Qiu, J.; Liu, Q.; Chen, Z. High-performance cotton fabric-based supercapacitors consisting of polypyrrole/Ag/graphene oxide nanocomposite prepared via UV-induced polymerization. Cellulose 2022, 29, 2525–2537. [Google Scholar] [CrossRef]
- Han, F.; Luo, J.; Pan, R.; Wu, J.; Guo, J.; Wang, Y.; Wang, L.; Liu, M.; Wang, Z.; Zhou, D.; et al. Vanadium Dioxide Nanosheets Supported on Carbonized Cotton Fabric as Bifunctional Textiles for Flexible Pressure Sensors and Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 41577–41587. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hong, H.; Hu, J.; Yan, X. Fabrication and Seamless Integration of Insensitive-Bending Fully Printed All-in-One Fabric-Based Supercapacitors Based on Cost-Effective MWCNT Electrodes. ACS Appl. Mater. Interfaces 2022, 14, 12214–12222. [Google Scholar] [CrossRef] [PubMed]
- Keawploy, N.; Venkatkarthick, R.; Wangyao, P.; Qin, J. Screen printed textile electrodes using graphene and carbon nanotubes with silver for flexible supercapacitor applications. J. Met. Mater. Miner. 2020, 30, 39–44. [Google Scholar] [CrossRef]
- Keawploy, N.; Venkatkarthick, R.; Wangyao, P.; Zhang, X.; Liu, R.; Qin, J. Eco-Friendly Conductive Cotton-Based Textile Electrodes Using Silver- and Carbon-Coated Fabrics for Advanced Flexible Supercapacitors. Energy Fuels 2020, 34, 8977–8986. [Google Scholar] [CrossRef]
- Jiang, L.; Hong, H.; Hu, J.; Yan, X. Development of flexible supercapacitors with coplanar integrated multi-walled carbon nanotubes/textile electrode and current collectors. J. Mater. Sci. Mater. Electron. 2022, 33, 5297–5310. [Google Scholar] [CrossRef]
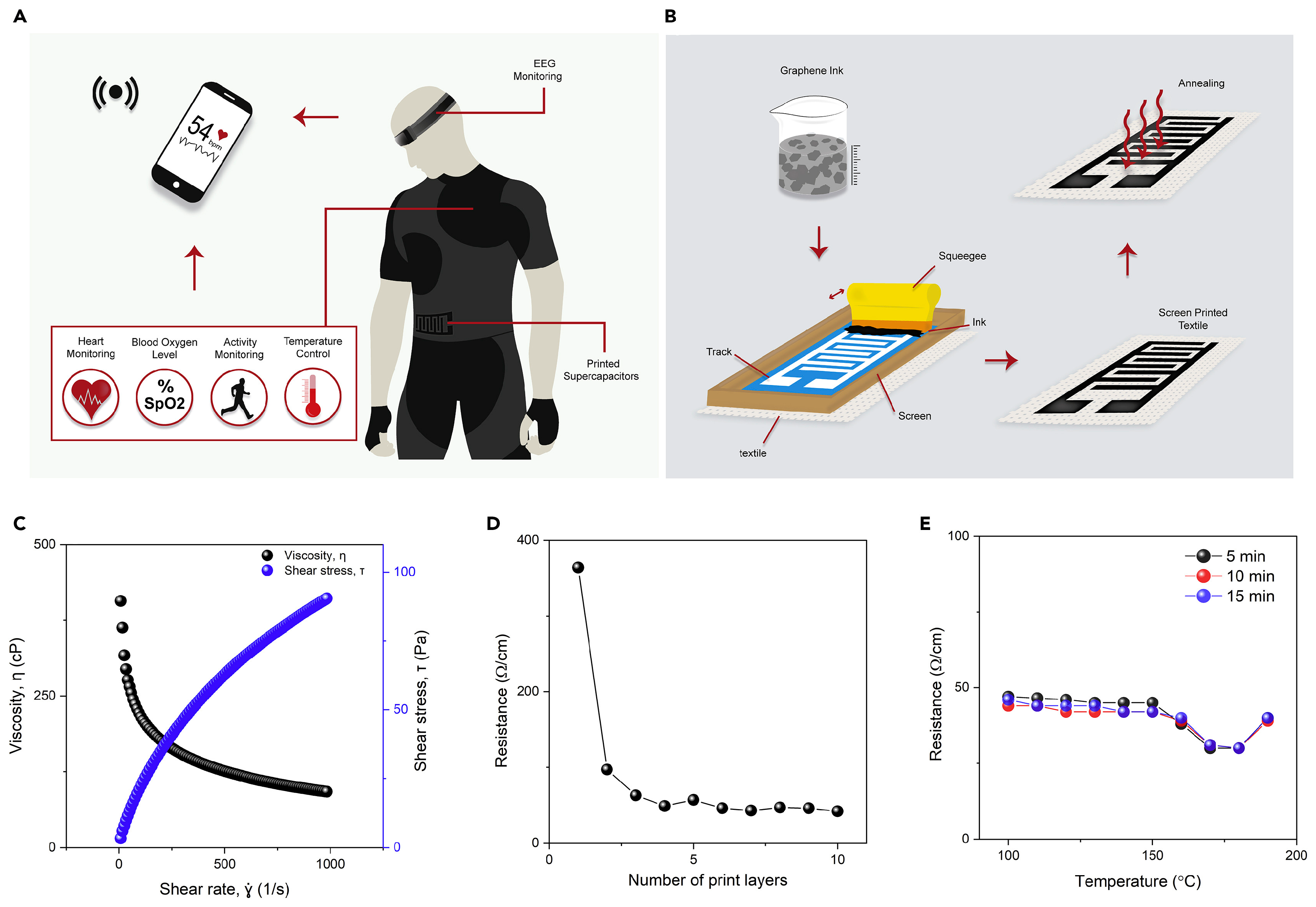
- Islam, M.R.; Afroj, S.; Beach, C.; Islam, M.H.; Parraman, C.; Abdelkader, A.; Casson, A.J.; Novoselov, K.S.; Karim, N. Fully printed and multifunctional graphene-based wearable e-textiles for personalized healthcare applications. iScience 2022, 25, 103945. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, B.; Zhao, Y.; Jin, Z.; Li, K.; Tang, L.; Cai, Z. Facile preparation of N-doped carbon/FeOx-decorated carbon cloth for flexible symmetric solid-state supercapacitors. Cellulose 2019, 27, 1591–1601. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Zhang, H.; Zhuo, L.; Huang, Q.; Zhang, W.; Chen, H.; Ling, Q. Synthesis of MXene-based nanocomposite electrode supported by PEDOT:PSS-modified cotton fabric for high-performance wearable supercapacitor. J. Colloid Interface Sci. 2024, 660, 735–745. [Google Scholar] [CrossRef]
- Costa, R.S.; Soares, O.S.G.P.; Vilarinho, R.; Moreira, J.A.; Pereira, M.F.R.; Pereira, A.; Pereira, C. Unveiling the role of oxidative treatments on the electrochemical performance of carbon nanotube-based cotton textile supercapacitors. Carbon Trends 2021, 5, 100137. [Google Scholar] [CrossRef]
- Costa, R.S.; Guedes, A.; Pereira, A.M.; Pereira, C. Fabrication of all-solid-state textile supercapacitors based on industrial-grade multi-walled carbon nanotubes for enhanced energy storage. J. Mater. Sci. 2020, 55, 10121–10141. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Xin, B.; Liu, Y.; Cui, Y.; Hu, Y. All-solid-state flexible supercapacitor of Carbonized MXene/Cotton fabric for wearable energy storage. Appl. Surf. Sci. 2020, 528, 146975. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Feng, L.-J.; Bian, S.-W. Surface Structure Construction of Fibers in a Conductive Metal–Organic Framework/Metal/Cotton Electrode for Flexible Textile Supercapacitors. ACS Appl. Electron. Mater. 2022, 4, 4595–4604. [Google Scholar] [CrossRef]
- Wan, J.; Hu, R.; Li, J.; Mi, S.; Xian, J.; Xiao, Z.; Liu, Z.; Mei, A.; Xu, S.; Fan, M.; et al. A universal construction of robust interface between 2D conductive polymer and cellulose for textile supercapacitor. Carbohydr. Polym. 2022, 284, 119230. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhou, M.; Zhao, H.; Zhai, S.; Ge, F.; Zhao, Y.; Cai, Z. Electrodeposited CuS nanosheets on carbonized cotton fabric as flexible supercapacitor electrode for high energy storage. Electrochim. Acta 2019, 295, 668–676. [Google Scholar] [CrossRef]
- Sneha, N.; Shakthivel, K.R.; Kiruthika, S. Flexible and Durable Conducting Fabric Electrodes for Next-Generation Wearable Supercapacitors. ACS Appl. Mater. Interfaces 2025, 17, 7568–7580. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Ji, S.; Wang, H.; Brett, D.J.L.; Wang, R. Design and synthesis of tremella-like Ni–Co–S flakes on co-coated cotton textile as high-performance electrode for flexible supercapacitor. J. Alloys Compd. 2020, 814, 151789. [Google Scholar] [CrossRef]
- Hekmat, F.; Tutel, Y.; Unalan, H.E. Wearable supercapacitors based on nickel tungstate decorated commercial cotton fabrics. Int. J. Energy Res. 2020, 44, 7603–7616. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Shen, L.; Wang, N.; Wang, T.; Wu, L. Design of freestanding flexible electrodes with hierarchical porous structure from cotton textiles for high-performance supercapacitors. J. Energy Storage 2025, 116, 115972. [Google Scholar] [CrossRef]
- Vashishth, E.; Raulo, A.; Srivastava, R.K.; Nandan, B. Smart Integration of Cobalt-Based Metal Organic Framework with Textile Waste for a Sustainable and Flexible High-Performance Supercapacitor. ACS Sustain. Resour. Manag. 2025, 2, 108–118. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Tao, J.; Shen, X.; Yan, Z.; Ji, Z.; Yuan, A.; Zhu, G.; Kong, L. H2SO4-assisted tandem carbonization synthesis of PANI@carbon@textile flexible electrode for high-performance wearable energy storage. Appl. Surf. Sci. 2021, 535, 147755. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Xie, M.; Tao, J.; Shen, X.; Ji, Z.; Yan, Z.; Zhai, L.; Yuan, A. Polyaniline wrapped graphene functionalized textile with ultrahigh areal capacitance and energy density for high-performance all-solid-state supercapacitors for wearable electronics. Compos. Sci. Technol. 2020, 198, 108305. [Google Scholar] [CrossRef]
- Pullanchiyodan, A.; Manjakkal, L.; Dahiya, R. Metal Coated Fabric Based Asymmetric Supercapacitor for Wearable Applications. IEEE Sens. J. 2021, 21, 26208–26214. [Google Scholar] [CrossRef]
- Pullanchiyodan, A.; Manjakkal, L.; Dervin, S.; Shakthivel, D.; Dahiya, R. Metal Coated Conductive Fabrics with Graphite Electrodes and Biocompatible Gel Electrolyte for Wearable Supercapacitors. Adv. Mater. Technol. 2020, 5, 1901107. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, R.; Wang, H.; Xie, X.; Du, C. Composite electrodes with NiCoAl-LDH coated Ti3C2Tx MXene and incorporated Ag nanowires for screen-printable in-plane hybrid supercapacitors on textiles. Appl. Surf. Sci. 2022, 598, 153796. [Google Scholar] [CrossRef]
- Kota, A.; Gogia, A.; Neidhard-Doll, A.T.; Chodavarapu, V.P. Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors. Sensors 2021, 21, 2178. [Google Scholar] [CrossRef]
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196. [Google Scholar] [CrossRef]
- Bhargava, P.; Liu, W.; Pope, M.; Tsui, T.; Yu, A. Substrate comparison for polypyrrole-graphene based high-performance flexible supercapacitors. Electrochim. Acta 2020, 358, 136846. [Google Scholar] [CrossRef]
- Afroj, S.; Tan, S.; Abdelkader, A.M.; Novoselov, K.S.; Karim, N. Highly Conductive, Scalable, and Machine Washable Graphene-Based E-Textiles for Multifunctional Wearable Electronic Applications. Adv. Funct. Mater. 2020, 30, 2000293. [Google Scholar] [CrossRef]
- Li, X.; Sun, C.; Cai, Z.; Ge, F. High-performance all-solid-state supercapacitor derived from PPy coated carbonized silk fabric. Appl. Surf. Sci. 2019, 473, 967–975. [Google Scholar] [CrossRef]
- Sim, K.; Gao, Y.; Chen, Z.; Song, J.; Yu, C. Nylon Fabric Enabled Tough and Flaw Insensitive Stretchable Electronics. Adv. Mater. Technol. 2018, 4, 1800466. [Google Scholar] [CrossRef]
- Khairy, M.; Kamal, R.; Mousa, M.A. Preparation and physical properties of conductive silk fabrics used in wearable clothes and flexible supercapacitors. J. Ind. Text. 2022, 52, 15280837221130512. [Google Scholar] [CrossRef]
- Stempien, Z.; Khalid, M.; Kozicki, M.; Kozanecki, M.; Varela, H.; Filipczak, P.; Pawlak, R.; Korzeniewska, E.; Sąsiadek, E. In-situ deposition of reduced graphene oxide layers on textile surfaces by the reactive inkjet printing technique and their use in supercapacitor applications. Synth. Met. 2019, 256, 116144. [Google Scholar] [CrossRef]
- Chen, C.; Guo, H.; Chen, L.; Wang, Y.-C.; Pu, X.; Yu, W.; Wang, F.; Du, Z.; Wang, Z.L. Direct Current Fabric Triboelectric Nanogenerator for Biomotion Energy Harvesting. ACS Nano 2020, 14, 4585–4594. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shah, S.A.; Lowery, J.L.; Oh, J.H.; Lutkenhaus, J.L.; Green, M.J. Lightweight Kevlar-Reinforced Graphene Oxide Architectures with High Strength for Energy Storage. Adv. Mater. Interfaces 2019, 6, 1900786. [Google Scholar] [CrossRef]
- Liu, M.; Pu, X.; Cong, Z.; Liu, Z.; Liu, T.; Chen, Y.; Fu, J.; Hu, W.; Wang, Z.L. Resist-Dyed Textile Alkaline Zn Microbatteries with Significantly Suppressed Zn Dendrite Growth. ACS Appl. Mater. Interfaces 2019, 11, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Sundriyal, P.; Bhattacharya, S. Textile-based supercapacitors for flexible and wearable electronic applications. Sci. Rep. 2020, 10, 13259. [Google Scholar] [CrossRef] [PubMed]
- Pullanchiyodan, A.; Manjakkal, L.; Dahiya, R. Metal Coated Fabric Based Supercapacitors. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar]
- Khudiyev, T.; Grena, B.; Loke, G.; Hou, C.; Jang, H.; Lee, J.; Noel, G.H.; Alain, J.; Joannopoulos, J.; Xu, K.; et al. Thermally drawn rechargeable battery fiber enables pervasive power. Mater. Today 2022, 52, 80–89. [Google Scholar] [CrossRef]
- Li, P.; Fang, Z.; Zhang, Y.; Mo, C.; Hu, X.; Jian, J.; Wang, S.; Yu, D. A high-performance, highly bendable quasi-solid-state zinc–organic battery enabled by intelligent proton-self-buffering copolymer cathodes. J. Mater. Chem. A 2019, 7, 17292–17298. [Google Scholar] [CrossRef]
- Cheng, P.-Y.; Cheng, H.-Y.; Huang, C.-L.; Chen, Y.-A.; Hsieh, C.-T.; Lu, S.-Y. N-Doped Hierarchical Continuous Hollow Thin Porous Carbon Nanostructure for High-Performance Flexible Gel-Type Symmetric Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 17020–17029. [Google Scholar] [CrossRef]
- Cao, X.-M.; Han, Z.-B. Hollow core–shell ZnO@ZIF-8 on carbon cloth for flexible supercapacitors with ultrahigh areal capacitance. Chem. Commun. 2019, 55, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Leng, M.; Tao, W.; Wang, Q.; Gao, Y.; Zhang, Q. Polyaniline/carbon nanotube core–shell hybrid and redox active electrolyte for high-performance flexible supercapacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 4427–4436. [Google Scholar] [CrossRef]
- Han, J.; Fan, C.; Li, D.-s.; Jiang, L. A hydrogel electrolyte with dynamically Tunable mechanical properties for wide-temperature flexible supercapacitor. J. Power Sources 2025, 631, 236217. [Google Scholar] [CrossRef]
- Shoeb, M.; Mashkoor, F.; Jeong, H.; Khan, M.N.; Jeong, C. Investigating the Effect of Carbon Nanotubes Decorated SmVO4-MoS2 Nanocomposite for Energy Storage Enhancement via VARTM-Fabricated Solid-State Structural Supercapacitors Using Woven Carbon Fiber. Small 2024, 21, e2408283. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Liao, H.; Zhao, Y.; Li, K. A physically crosslinked, self-healing hydrogel electrolyte for nano-wire PANI flexible supercapacitors. Chem. Eng. J. 2019, 367, 139–148. [Google Scholar] [CrossRef]
- Luo, X.; Liang, Y.; Weng, W.; Hu, Z.; Zhang, Y.; Yang, J.; Yang, L.; Zhu, M. Polypyrrole-coated carbon nanotube/cotton hybrid fabric with high areal capacitance for flexible quasi-solid-state supercapacitors. Energy Storage Mater. 2020, 33, 11–17. [Google Scholar] [CrossRef]
- Sun, P.; Qiu, M.; Li, M.; Mai, W.; Cui, G.; Tong, Y. Stretchable Ni@NiCoP textile for wearable energy storage clothes. Nano Energy 2019, 55, 506–515. [Google Scholar] [CrossRef]
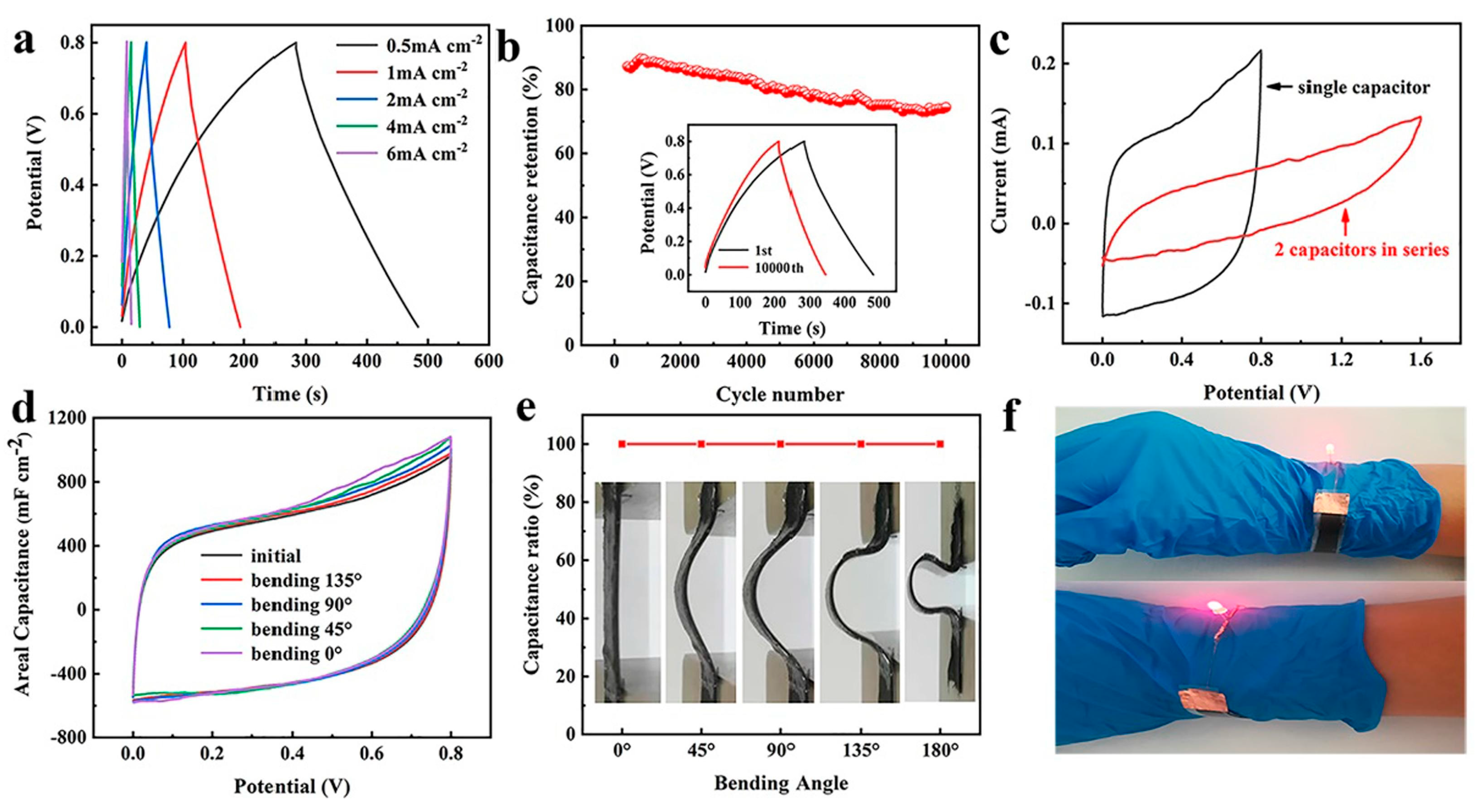
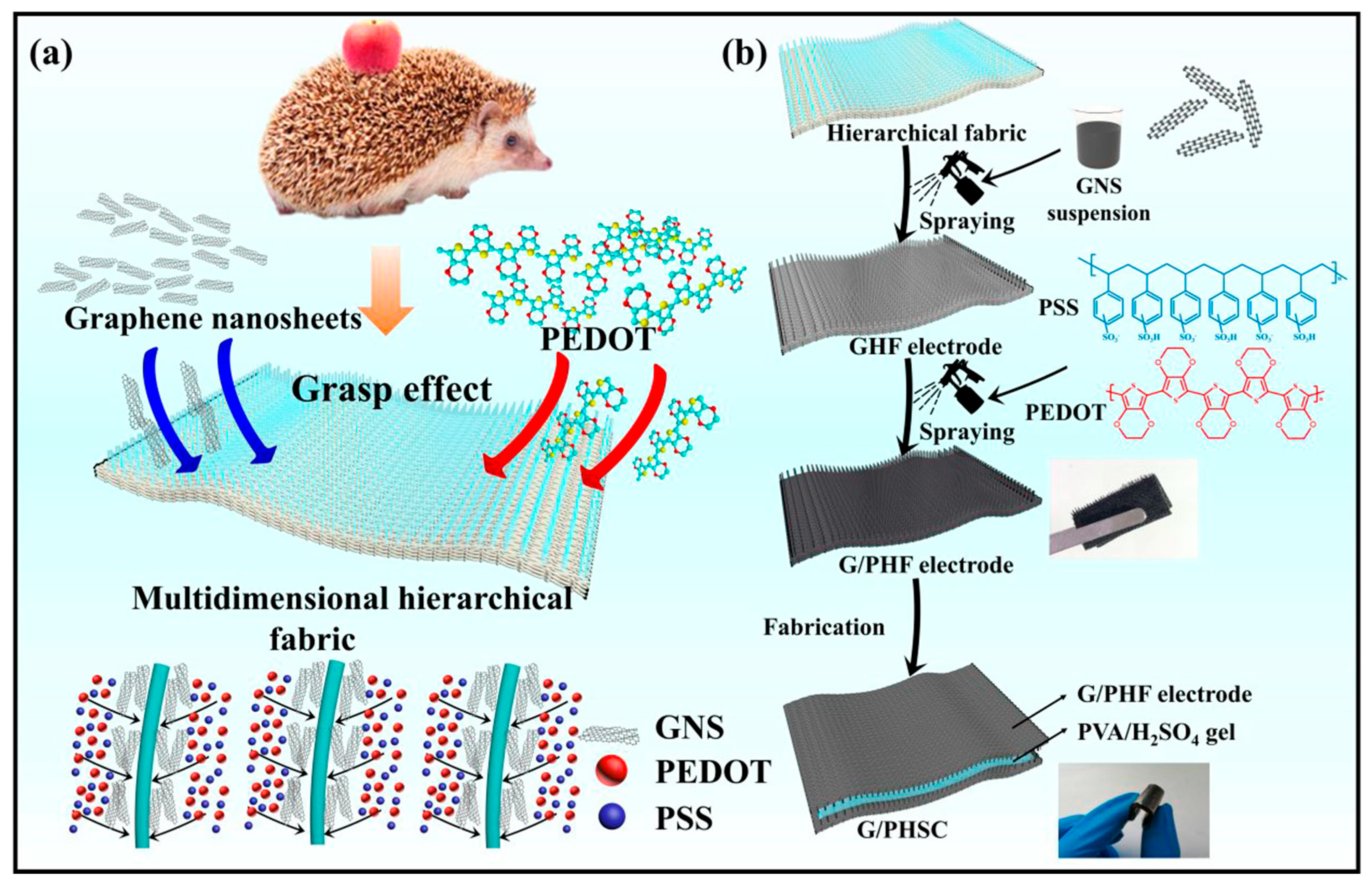
- Li, Z.; Ma, Y.; Wang, L.; Du, X.; Zhu, S.; Zhang, X.; Qu, L.; Tian, M. Multidimensional Hierarchical Fabric-Based Supercapacitor with Bionic Fiber Microarrays for Smart Wearable Electronic Textiles. ACS Appl. Mater. Interfaces 2019, 11, 46278–46285. [Google Scholar] [CrossRef]
- Su, C.; Shao, G.; Yu, Q.; Huang, Y.; Jiang, J.; Shao, H.; Chen, N. A flexible, lightweight and stretchable all-solid-state supercapacitor based on warp-knitted stainless-steel mesh for wearable electronics. Text. Res. J. 2022, 92, 1807–1819. [Google Scholar] [CrossRef]
- Cong, Z.; Guo, W.; Guo, Z.; Chen, Y.; Liu, M.; Hou, T.; Pu, X.; Hu, W.; Wang, Z.L. Stretchable Coplanar Self-Charging Power Textile with Resist-Dyeing Triboelectric Nanogenerators and Microsupercapacitors. ACS Nano 2020, 14, 5590–5599. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.W.; Hong, S.Y.; Lee, G.; Lee, H.; Song, C.; Keum, K.; Jeong, Y.R.; Jin, S.W.; Kim, D.S.; et al. Dynamically Stretchable Supercapacitor for Powering an Integrated Biosensor in an All-in-One Textile System. ACS Nano 2019, 13, 10469–10480. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, W.; Gu, P.; Fan, L.; Yin, Y.; Wang, C. A stretchable and hydrophobic polypyrrole/knitted cotton fabric electrode for all-solid-state supercapacitor with excellent strain capacitance. Electrochim. Acta 2019, 297, 794–804. [Google Scholar] [CrossRef]
- Yavuz, A.; Faal, H. Alloy coating from an ionic liquid on graphite filaments and knitted graphite fabrics for stretchable supercapacitor applications. Mater. Sci. Eng. B 2025, 317, 118227. [Google Scholar] [CrossRef]
- Schneidmadel, S.; Koch, M.; Bruchmüller, M. Effects of fiber orientation on the electrical conductivity of filled plastic melt. AIP Conf. Proc. 2016, 1779, 030007. [Google Scholar]
- Yu, L.; Tatsumi, D.; Morita, M. Relationship between Viscoelasticity and Electrical Conductivity of Carbonized Cellulose Fiber Networks. Nihon Reoroji Gakkaishi 2014, 41, 331–336. [Google Scholar] [CrossRef]
- Guan, T.; Shen, S.; Cheng, Z.; Wu, G.; Bao, N. Microfluidic-assembled hierarchical macro-microporous graphene fabrics towards high-performance robust supercapacitors. Chem. Eng. J. 2022, 440, 135878. [Google Scholar] [CrossRef]
- Qiu, H.; Qu, X.; Zhang, Y.; Chen, S.; Shen, Y. Robust PANI@MXene/GQDs-Based Fiber Fabric Electrodes via Microfluidic Wet-Fusing Spinning Chemistry. Adv. Mater. 2023, 35, e2302326. [Google Scholar] [CrossRef]
- Shao, F.; Hu, N.; Su, Y.; Yao, L.; Li, B.; Zou, C.; Li, G.; Zhang, C.; Li, H.; Yang, Z.; et al. Non-woven fabric electrodes based on graphene-based fibers for areal-energy-dense flexible solid-state supercapacitors. Chem. Eng. J. 2020, 392, 123692. [Google Scholar] [CrossRef]
- Liu, X.; Li, K.; Hou, C.; Li, H.; Chen, P.; Zhang, Q.; Li, Y.; Wang, H. Poly-ε-caprolactone nanofibrous mats as electrolyte host for tailorable flexible electrochromic devices. Mater. Sci. Eng. B 2019, 241, 36–41. [Google Scholar] [CrossRef]
- Singh, R.; Janakiraman, S.; Khalifa, M.; Anandhan, S.; Ghosh, S.; Venimadhav, A.; Biswas, K. An electroactive β-phase polyvinylidene fluoride as gel polymer electrolyte for magnesium–ion battery application. J. Electroanal. Chem. 2019, 851, 113417. [Google Scholar] [CrossRef]
- Yang, T.; Shu, C.; Hou, Z.; Zheng, R.; Hei, P.; Li, M.; Ran, Z.; Zhang, Q.; Long, J. 3D porous network gel polymer electrolyte with high transference number for dendrite-free LiO2 batteries. Solid State Ion. 2019, 343, 115088. [Google Scholar] [CrossRef]
- Li, Z.; Tian, M.; Sun, X.; Zhao, H.; Zhu, S.; Zhang, X. Flexible all-solid planar fibrous cellulose nonwoven fabric-based supercapacitor via capillarity-assisted graphene/MnO2 assembly. J. Alloys Compd. 2019, 782, 986–994. [Google Scholar] [CrossRef]
- Shao, D.; Wang, X.; Li, X.; Luo, K.; Yang, L.; Liu, L.; Liu, H. Internal in situ gel polymer electrolytes for high-performance quasi-solid-state lithium ion batteries. J. Solid State Electrochem. 2019, 23, 2785–2792. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent Advances in Biopolymer-Based Hydrogel Electrolytes for Flexible Supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2024, 35, 2416390. [Google Scholar] [CrossRef]
- Jia, R.; Wei, C.; Ma, B.; Li, L.; Yang, C.; Wang, B.; Tan, L.; Feng, J. Biopolymer-Based Gel Electrolytes for Advanced Zinc Ion Batteries: Progress and Perspectives. Adv. Funct. Mater. 2024, 35, 2417498. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zeng, J.; Chen, Y.; Huang, Y.; Liu, J.; Gan, J.; Li, T.; Zhang, H.; Zhong, L.; et al. Biopolymer-based gel electrolytes for electrochemical energy Storage: Advances and prospects. Prog. Mater. Sci. 2024, 144, 101264. [Google Scholar] [CrossRef]

| Gel Electrolyte | Yarn Composition | Electrodes | Preparation Method | Conductivity Tests and Results | Ref |
|---|---|---|---|---|---|
| PVA/KCl | PET | Au/Ni-MOF@ carbon yarn | PET dip-coating in dopamine; PET/PDA electroless plating; dip-coating in Ni-Ni3HHTP2 solution | Length capacitance of 1.1 × 102 mF/cm; energy density of 3.9 × 10−6 Wh/cm; power density of 2.5 × 10−4 W/cm | [57] |
| PVA/ZnSO4 | Stainless-steel CO blend | G/Zn-MnO2 | MnO2 (cathode) or Zn (anode) electrodeposition PVA/ZnSO4 coating | Specific capacity of 43.53 mAh/g; energy density of 5.2 × 10−2 Wh/g | [58] |
| PVA/H2SO4 | Cellulose | Ti3C2 MXene | Mxene dip-coating, followed by gel electrolyte coating | Conductivity up to 440.3 ± 0.9 S/cm; specific capacitance of 7.6 × 102 mF/cm at 2 mV/s; 2000-cycle stability at ~14% compression strain | [59] |
| PVA/H2SO4 | CO/Stainless steel | PEDOT:PSS | PEDOT in situ polymerization; SC assembled and woven | Areal specific capacitance reached a max. 1.6 × 102 mF/cm2; Areal energy density 1.0 × 10−5 Wh/cm2; 81.9% capacitance retention after 10,000 cycles | [60] |
| PVA/H2SO4 | Stainless steel/CO | PPy | PPY electrochemical deposition; gel electrolyte dip-coating | High areal capacitance (up to 3.4 × 102 mF/cm2); high cycle stability (apx. 93% capacitance retention)) | [61] |
| PVA/H3PO4 | CO | PAN/PEDOT:PSS | Ni dip-coating; PAN electroless deposition; PEDOT:PSS dip-coating | Volumetric capacitance 2.7 × 104 mF/cm3; energy density 9.6 × 10−3 Wh/cm3; power density 8.3 × 10−1 W/cm3 | [62] |
| PVA/H3PO4 | CO/Carbon fiber | Carbon fiber yarn | CO + carbon fiber twisting; yarns fixed into ABS mold; material coated with cellulose-based ionic hydrogel and gel electrolyte | Specific energy density 1.7 × 10−8 Wh/cm2; specific power density 5.3 × 10−4 W/cm2; 92% capacitance retention | [63] |
| PVA/H3PO4 | MWCNT/ TiO2 fibers | MWCNT/ TiO2 | TiO2 nanofibers (NFs) produced by electrospinning; TiO2 NF electrophoretic deposition in MWCNT; D gel electrolyte dip-coating; yarn twisting of 2 electrodes + gel electrolyte dip-coating | Good capacitance (3.7 × 10 mF/cm at 0.1 mA/cm); capacitance retention; good energy (1.2 × 10−5 Wh/cm) and power (3.7 × 10−4 W/cm) densities | [64] |
| PVA/H3PO4/KI | Carbon fibers | Carbon fibers | gel electrolyte dip-coating; yarn twisting of 2 electrodes; second dip-coating in gel electrolyte | Volumetric capacitance 363.9 F/L; energy density 5.1 × 10−2 Wh/L; specific capacitance (13.9 mF) using a 7.5 mM KI solution | [65] |
| PVA/H3PO4 | CO and Nylon® | Ti3C2Tx MXene | MXene yarn coating of CO and Nylon®; yarn knitting in fabrics; PET yarn knitted in between; gel electrolyte coating | Capacitance up to 7.1 × 102 mF/cm2 and 5.2 × 102 mF/cm2, in 1 M H3PO4 and H3PO4 gel electrolyte; delivery energy 2.5 × 10−5; power densities 4.7 × 10−7 Wh/cm2; cycling stability | [66] |
| PVA/H3PO4 | Carbon nanotubes (CNT) | PEDOT:PSS/CNT- NMP | CNT forest and sheet by chemical vapor deposition; NMP drop casting in CNT forest; yarn electrode production via biscrolling process; gel electrolyte coating | Capacitance 112.76 F/g; maximum power and energy density 9.8 × 102 W/kg (1.2 Wh/kg) and 3.8 Wh/kg (1.5 × 102 W/kg); cycling stable performance after 5000 cycles | [67] |
| PVA/KOH | PET | PPy@NiCo-double hydroxide@stainless-steel electrodes | NiCo-layer double hydroxide via hydrothermal process; PPU electrochemical deposition; gel electrolyte coating | Good specific capacitance 1196 F/g; energy density 6.6 mWh/cm3; power density 1.6 × 10−1 mW/cm3; good cycle performance (85.7% capacity retained) | [68] |
| PVA/KOH | Au/CO | NiCo2S4@Ni−Co LDH | Hydrothermal synthesis of NiCO2S4 nanotubes on Au/CO yarns; Ni−Co LDH nanosheets electrodeposition | Capacitance 5.7 × 103 mF/cm; good areal energy density 3.5 × 10−6 Wh/cm2; stable cycling performance small capacitance retention decrease (9%) | [69] |
| PVA/KOH | PET | rGO/CNT and NiCo-BOH | Ni electroless deposition on PET yarns + Cu electrodeposition; co-self-assembled rGO/CNT hydrothermal reaction on Ni/Cu-plated PET yarns; gel electrolyte dip-coating | Energy density apx. 7.8 × 10−5 Wh/cm2; high power density 1.4 × 104 W/cm2; stable cycling performance 82.7% capacity retention | [70] |
| PVA/KOH | Co wire + CO yarn | NiMnO3-rGO@CO-Cu | NiMnO3-rGO nanocomposites via hydrothermal reaction; CO yarns dip-coating in graphite and PVDF; Cu wire and CO yarns are woven; Co-Cu fibers dip-coating in NiMnO3-rGO; electrode dip-coating in gel electrolyte | Maximum specific capacitance 4.0 × 102 mF/cm2; maximum specific capacitance 8.3 × 10 mF/cm2; specific energy density 17.5 µWh/cm2 | [71] |
| PVA/KOH | Carbon-based yarns (CBY) | CoNi-layered double hydroxides@CBY and S-dopes carbon nanoparticles@CBY | CoNi-layered double hydroxide nanosheets; in situ growth via hydrothermal reactions; yarn anchoring assembly | Specific surface area 655 m2/g1 of CoNi-layered double hydroxides@CBY; voltage operating range 1.4 V; capacitance 2.3 × 102 mF/cm2; energy density (6.2 × 10−5 Wh/cm2); stable cycle performance | [72] |
| PVA/KOH | CNT-coated CO (CCY) | NiCo-LDH@CCY | CO yars dip-coated in CNT dispersion; CCY dip-coating to produce NiCo-LDH@CCY; supercapacitor assembled by coating two yarns in a PET substrate with PVA/KOH or via PVA/KOH solution dip-coating | Good areal capacitance 1.24 × 102 mF/cm2, current density 0.2 mA/cm2 and energy density 3.9 × 10−5 Wh/cm2 | [73] |
| PVA/KOHNa2SO4 | Transition metal oxide (TMO)-Ni-TMO trilayer nanoribbon yarn | (TMO)-Ni-TMO trilayer nanoribbon | TMO-Ni-TMO nanoimprinting in the mold; nanoribbon array delamination; twisting the nanoribbon array to form yarns; production of YSC, coated with gel electrolyte, using two TMO yarns or one TMO yarn + graphene fiber | Maximum energy density 7.6 × 10−2 Wh/cm3 achieved for graphene–CoNixOy@Ni (G-CNO) YSCs; power density 2.4 × 10−1 Wh/cm3 achieved with G-CNO YSCs; 94.2% initial capacitance retention after 10,000 cycles | [74] |
| PVA/H2SO4, PVA/KOH, PAAm/NaCl | MWCNT | MWCNT | Floating catalyst chemical vapor deposition to obtain MWCNT, followed by twisting; insulators prepared by connecting two Ag wires to the MWCNT yarns; gel electrolyte added to the coils | High-recognition voltage signals (4–15 mV) with low noise (0.024 mV); high voltage amplitude sensitivity to tensile stretches in multiple frequencies (0.1–10 Hz) and strains (1–80%) | [75] |
| PVA/LiCl | CO | rGO | Polymer-assisted metal deposition (PAMD) of Ni on CO; rGO electrochemical deposition; yarn embroidering | Capacitance 1.6 × 10 mF/cm2 for in-plane MWNT/Ni-CO@fabric with PVA/LiCl at 0.8V; cycling performance—97% capacitance retention | [76] |
| ZnCl2/NH4Cl/CMC | Cellulose | PANI (cathode) Zn (anode) | Cellulose yarn electrospinning; PANI in situ polymerization; conductive slurry coating to obtain cathodes; Zn electrochemical deposition in conductive slurry-coated cellulose yarn to obtain anodes; cathode CMC coating, followed by twisting with anode; gel electrolyte coating by CMC swelling | Energy density 153.2 and 6.1 × 10 Wh/Kg; good cyclic stability, specific capacity maintained at 109.7 mAh/g | [77] |
| Gel Electrolyte | Fabric Composition | Electrodes | Preparation Method | Conductivity Tests and Results | Ref. |
|---|---|---|---|---|---|
| PVA/H3PO4- commercial fluorescent pigment of ZnS-Mn | CO | CNT | Dip–pad–dry process with CNT; redox-active additive in a solid-gel electrolyte | 100% stability after 8000 cycles; energy density of 1.6 Wh/kg; potency density of 6.4 × 102 W/kg; high specific capacitance of 4.37 F/g | [1] |
| PVA/LiCl | CO | PPy; TsOH | Carbonization of the fabric; in situ electrochemical deposition with PPy; immersion in electrolyte gel | Specific capacity of 5.0 × 102 mF/cm2; 73.6% stability after 1000 cycles; energy density range of 1.18–0.68 mWh/cm3; high power density of 1.8–8.4 × 10−3 W/cm3; breaking strength of 5.27 N | [78] |
| Nanofibrillated cellulose/polyacrylamide; sodium polyacrylate | CO | Co; Cu; Ag; Ni | CO activated with PdCl2 (loading catalyst); electroless deposition with Co, Ni, Cu, and Ag | Conductivity of ~200 kS/m; capacity of 1.0 mAh/cm2; high energy density of 1.7 m × 10−3 Wh/cm2; power density of 8.5 × 10−3 W/cm2; discharge capacity of 132 mAh | [79] |
| PVA/H2SO4 | CO | PPy; PU; water repellent; 5-sulfosalicylate; ferric chloride | Spray-coating of CO fabric with PU and thickener; oxide polymerization with PPy; gel electrolyte coating | Stability of 85% after 3000 cycles; energy density of 9.0 × 10−8 Wh/cm2; power density of 1.9 × 10−5 W/cm2 | [80] |
| PVA/KOH | CO | Graphene; Co(NO3)2.6H2O; Ni(NO3)2.6H2O; PANI; cetyl trimethyl ammonium bromide; PANI nanotubes | Chemical deposition with graphene/PANI nanotubes solution; immersion into NiCo/cetyl trimethyl ammonium bromide solution; gel electrolyte coating | Specific capacitance of 4.3 × 102 mF/cm2; 84.05% stability after 10,000 cycles; 15.95% deterioration after 10,000 cycles; specific surface area of 30.63 m2/g and pore volume of 0.176 cm3/g; surface roughness of 98.7 nm; energy density of 8.0 × 10−5 Wh/cm3 | [81] |
| PVA/H2SO4 | CO | PEDOT; Ti3C2Tx MXene | Vapor phase polymerization with PEDOT; spray-coating with MXene dispersion; immersion into gel electrolyte | Sheet resistance of 3.6 Ω/sq; specific capacitance of 1.0 × 103 mF/cm2; areal energy density of 1.3 × 10−5 Wh/cm2; strong joule heating performance of 193.1 ºC; 36.62 dB EMI shielding effectiveness; high sensitivity; 25.7% capacitance retention | [82] |
| TEABF4-polyacrylamide | CO | Carbon black; activated carbon; PVA; 1,2,4-Trichlorobenzene | Spraying with ink formulation (carbon black, activated carbon, poly(ethylene-co-vinyl alcohol), and 1,2,4-Trichlorobenzene); impregnation with gel electrolyte | Specific capacitance of 3.4 × 10 mF/cm2; energy and power densities of 18.9 μW h/cm2 and 2.4 × 10−4 W/cm2; specific surface area of 1874.2 m2/g and pore size of 1.6–2.7 nm; 48% capacitance retention after 2 mouths of aging; 88% Coloumb efficiency for 2 mouths of aging assay | [83] |
| PVA/H2SO4 | CO | PPy; PVA-co-ethylene nanofiber suspension | Spray-coated on the fabric with PVA-co-etthylene nanofiber suspension; in situ chemical polymerization with PPy layers; gel electrolyte coating | 100% capacitance retention after 10,000 cycles; 98.17% stability over 1000 bending cycles; specific capacitance of 6.7 × 102 mF/cm2; areal energy density of 6.0 × 10−5 Wh/cm2; 90–100% Coloumb efficiency | [84] |
| PVA/KOH | CO | NiCl2·6H2O; CoCl2·6H2O; Fe3C | Electroless plating process (CO fabric/Ni:Co); sandwiched with gel electrolyte and silk fabric as separator | High specific capacitance of 113.7 C/g; 80% stability after 4000 cycles; energy and power densities of 4.7 × 10 Wh/kg and 1.5 × 103 W/kg | [85] |
| PVA/KOH | CO | ZnO nanoparticles; CuS microsphere | Dyeing of CO fabric; atomic layer deposition of ZnO nanoparticles; carbonization process; hydrothermal reaction with CuS; gel electrolyte immersion | Specific capacitance of 1.8 × 103 mF/cm2; 85.2% stability after 5000 cycles; energy and power densities of 0.27 Wh/cm2 and 4.3 × 10 W/cm2 | [86] |
| PVA/KOH | CO | Procion reactive dye; cellulose and polyethylene terephthalate films | Dyeing and carbonization of CO fabrics; dipping in the electrolyte; supercapacitor sandwiched with cellulose and polyethylene terephthalate films | Specific surface areas of 448.2–622.1 m2/g and pore volumes of 0.22–0.39 cm3/g; specific capacitance of 1.1 × 103–1.8 × 103 mF/cm2; 79.3% capacitance retention; sheet resistance of ∼30–45 Ω/sq; 92.2% stability after 5000 cycles; volumetric specific capacitance of 1.8 × 104 mF/cm3; energy density of 5.7 × 10−3 Wh/cm3 at the power density of 4.5 × 10−3 W/cm3 | [87] |
| PVA/KOH | CO | NiCl2·6H2O; Na2WO4·2H2O | Thermal annealing with NiCl2·6H2O and Na2WO4·2H2O; sandwiched with gel electrolyte | High specific capacitance of 60.61 F/g; 95% stability after 3000 cycles; 99.7% stability after bending; energy and power densities of 2.3 × 10 Wh/kg and 8.3 × 102 W/kg | [88] |
| LoSalt®/ Grenade Energy®/Shopper isotonic drink + agar and k/carrageenan | CO | Carbon black; activated carbon; ethylene-vinyl acetate; 1,2,4-Trichlorobenzene | Spray-coating with ink formulation; gel electrolyte impregnation | Specific capacitance of 2.3 × 10 mF/cm2; energy and power densities of 2.3 × 10−6 W h/cm2 and 2.0 × 10−4 W/cm2 | [89] |
| PVA/LiCl | Cellulosic | Dopamine; GO | Dopamine in situ polymerization; immersion on GO solution; carbonization; gel electrolyte immersion | Specific capacitance of 1.2 × 103 mF/cm2; specific area of 347.6 m2/g; 6% stability after 4000 bending cycles | [90] |
| PVA/H2SO4 | CO | PPy; GO; Ag | Vacuum filtration of GO; addition of PPy and Ag+ through UV-induced in situ polymerization; gel electrolyte immersion | Specific capacitance of 1.7 × 103 mF/cm2; capacitance retention of 46.9%; energy potency of 1.1 × 10−3 Wh/cm2; 90.5% stability after 10,000 cycles; 89.7% stability at 180º after 10,000 bending cycles | [91] |
| PVA/Zn(OTf)2 | CO | VO2; Zn nanosheets; CNT film; H2C4O4·2H2O; H2O2; ethanol; PDMS | Carbonization of fabrics; O2 plasma treatment on fabric; VO2 mixture with carbonized CO fabric; pressure sensors production; gel electrolyte immersion; sandwich structure containing Zn/CNT film (anode) and VO2/carbonized CO (cathode) | Flexible pressure sensor: electrical conductivity of ∼95.8 Ω/sq; tensile stress of ∼6.7 MPa; tensile strain of 155%; sensibility of 0.07–7.12 kPa−1; response/recovery time of 12 and 8 ms Aqueous Zn-ion batteries: specific capacity 301.5 mAh/g; 99.8% Coulombic efficiency; 88.7% stability after 5000 cycles | [92] |
| PVA/KOH | CO | MWCNT; thermoplastic PU | Gel electrolyte applied through dip–pad–dry method; screen-printing with MWCNT ink; multiple layers of electrodes and fabric separators were sandwiched | High resistance of ∼120 Ω; specific capacitance of 4.17 mF/cm2; high bending strain of 20%/s; ∼97.4% stability after 1000 cycles | [93] |
| PVA/KOH | CO | Ag; CNT; graphene | Screen-printing with coating ink (Ag, CNT/graphene, and textile ink); gel electrolyte immersion | Specific capacitance of 6.8 × 102 mF/cm2; 80% stability after 3000 cycles | [94] |
| PVA/KOH | CO | Ag; activated carbon; graphene; CNT | Screen-printing with coating ink (Ag and activated carbon); gel electrolyte immersion | Specific capacitance of 3.3 × 103 mF/cm2; ∼130% stability after 10,000 cycles; high energy and power densities of 5.1× 10−4 Wh/cm2 and 1.5 × 10−1 W/cm2 | [95] |
| PVA/KOH | CO | MWCNT; thermoplastic PU; NMP | Textile electrodes printed using MWCNT ink; gel electrolyte coating | Specific capacitance of 1.4 × 10 mF/cm2; high bending strain of 20%/s; 90% stability after 2000 cycles | [96] |
| PVA/H2SO4 | CO | Graphene; carboxymethyl celulose; PU | Printing of graphene ink onto textiles; gel electrolyte drop-casting | Specific capacitance of ∼3.2 mF/cm2; resistance of 30 Ω/cm; energy and power densities of 2.8 × 10−4 Wh/cm2 and 3.0 × 10−3 W/cm2; stability ∼10,000 cycles; durability of 3.5-time higher resistance after 10 washing cycles; device retention of 95% after 10,00 cycles | [97] |
| PVA/LiCl | CO | N-doped carbon; FeO4; PPy | Impregnation with PPy; oxidative polimerization with FeCl3; carbonization; gel electrolyte immersion | Specific capacitance of 135 F/g; moderate specific surface areas (700.8 m2/g); rate capability of 1–10 mV/s (44.4%); 88.4% stability after 1000 cycles; 92.3% Coulombic efficiency after 1000 cycles | [98] |
| PVA/H3PO4 | CO | PEDOT:PSS; dimethyl sulfoxide; MXene, graphene nanoscroll; PPy | PEDOT:PSS dip-dry coating; dimethyl sulfoxide immersion; one-step in situ polymerization with MXene, graphene nanoscroll, and PPy; gel electrolyte immersion | Capacitance of 2.7 × 103 mF/cm2; high energy density of 3.2 × 10−4 Wh/cm2 and power density of 4.6 × 10−4 W/cm2; 79% stability after 2500 cycles; 97.8% stability after bending; 92% waterproof property after 2 h | [99] |
| PVA/H3PO4 | CO | MWCNT; sodium dodecylbenzenesulfonate | Dip–pad–dry with oxidized MWCNT dispersion; gel electrolyte immersion | High energy density of 3.5 Wh/kg; 98% stability after 5000 cycles; specific capacitance of 3.91 F/g | [100] |
| PVA/H3PO4 | CO | MWCNT; sodium dodecylbenzenesulfonate | Dip–pad–dry with MWCNT; gel electrolyte coating | Specific capacitance of 9.2 × 103 mF/cm2; 96.3% stability after 5000 cycles; energy and power densities of 6.3 Wh/kg and 1.1 × 103 W/kg | [101] |
| PVA/LiCl | CO | MXene | Exfoliating; delaminating; impregnation with MXene suspensions; carbonization; gel electrolyte immersion | Specific capacitance of 5.0 × 102 mF/cm2; 74% stability after 10,000 cycles; hydrophilic properties; electric conductivity of 5882 S/m | [102] |
| PVA/KCl | CO | Cu-MOFs; HAuCl4; polydopamine; polyethylene terephthalate film | Dopamine hydrochloride immersion; immersion in HAuCl4; immersion in Cu-MOF solution; gel electrolyte immersion | Specific capacitance of 258 F/g; energy density of 4.3 × 10−4 Wh/cm2; 83% stability after 3000 cycles; 94% Coulombic efficiency after 3000 cycles | [103] |
| PVA/H3PO4 | CO | PPy; PANI; PEDOT; CuCl2 | CuCl2 padding; vapor phase polymerization in situ with monomer (PANI, PEDOT or PPy) in ice bath; gel electrolyte immersion | Specific capacitance of 9.0 × 102 mF/cm2; 86.5% stability after 12,000 cycles; 90% stability at 180º after 1000 bending cycles | [104] |
| PVA/KOH | CO | CuS nanosheets | Carbonization and oxidation of the CO fabric; electrodeposition with CuS nanosheets; gel electrolyte immersion | Specific capacity of 1.3 × 103 mF/cm2; 91.8% stability after 2000 cycles; ultrahigh energy density (0.96 Wh/cm2); power density of 4. 4 × 103 W/cm2 | [105] |
| PVA/H2SO4 | CO | Au nanoparticles; tetraoctylammonium bromide; PANI; PDMS | Layer-by-layer deposition of tetraoctylammonium bromide; PDMS dip-coating eletrodeposition of PANI; gel electrolyte deposition | Au/PDMS: hydrophobicity (120–140°); washing fastness after 60 cycles; resistance to high-frequency ultrasound (300 s); high breathability; corrosion resistance; self-cleaning capability for 10 cycles; 98.88% cleaning efficiency Au/PANI: areal capacitance 5.0 × 102 mF/cm2; energy 3.3 × 10−5 Wh/cm2 and power 1.1 × 10−5 Wh/cm2 density; 77% stabilty after 1000 cycles | [106] |
| PVA/KOH | CO | Cu; Ni | Sputtering (Cu); electrochemical deposition (Ni); electroplated; gel electrolyte immersion | Specific capacity of 243.9 μAh/cm2; 70% stability after 5000 cycles; energy and power densities of 4.9 × 10 Wh/kg and 3.9 × 102 W/kg | [107] |
| PVA/KOH | CO | Nickel tungstate; niquel oxide; Ni(NO3)2 | Ultrasonic spray-coating method with Ni(NO3)2; in situ chemical synthesis of a uniform nickel oxide layer; electrochemical deposition of nickel tungstate; gel electrolyte immersion | Specific capacity of 1.4 × 102 mF/cm2; high specific of energy 12 μWh/cm2; specific power of 6.9 × 10−8 W/cm2; 78% stability after 5500 cycles | [108] |
| PVA/KOH | CO | Zn(NO3)2+MOFs+CoNi + layered double hydroxides (cathode); Zn-N (anode) | Zn(NO3)2 immersion; carbonization; Co-MOFs, NiSO4⋅6H2O and layered double hydroxide deposition; gel electrolyte application | Discharge capacity retention (60.11% after 1000 cycles); 98% Coulombic efficiency; bending resistance; high specific capacitance (161.25 F/g); energy (4.7 × 10 Wh/kg) and power (2.7 × 10 Wh/kg) density | [109] |
| PVA/KOH | CO | Co-zeolitic imidazole framework-67 nanoparticles | Co-zeolitic imidazole framework-67 nanoparticles in situ deposition; carbonization; gel electrolyte application | Specific capacitance of 288.62 F/g; energy density of 1.6 × 10 Wh/kg; power density of 6.5 × 10 W/kg; bending resistance; 76.4% stability after 2000 cycles | [110] |
| PVA/H2SO4 | CO 95%; spandex 5% | PANI;carbon;textile | Tandem procedure by imersing the fabric in acidic aniline solution; reaction with ammonium persulfate and drying the fabric at 60 °C, using H2SO4 as dopand and carbonizing assistant | Specific capacitance of 3.9 × 102 mF/cm2; over 70% capacitance retention after 5000 cycles; energy density of 3.6 × 10−2 Wh/m2 at 7.5 × 10−1 W/m2 power density; stable capacitance under bending (0–180°) and stretching (up to 50% elongation) | [111] |
| PVA/H2SO4 | CO 95%; spandex 5% | PANI;graphen;textile;HCl | Dipping and drying method, followed by in situ polymerization of aniline | Specific capacitance of 1.6 × 103 mF/cm2; over 75% capacitance after 10,000 cycles; energy density of 7.6 × 10−1 Wh/m2; stable capacitive performance under bending from 0 to 180°; 77% retention over 600 bending cycles | [112] |
| PVA/KCl | Polyamide | Metal-coated textiles | Printing a graphite with ethyl cellulose as binder and terpineol as solvent; gel electrolyte sandwiched between the positive and negative electrode with piece of cellulose PET cloth as the separator | Areal capacitance of 3.2 × 10 mF/cm2 and an energy density of 2.8 × 10−6 Wh/cm2 | [113] |
| PVA/KCl | Metal-coated polyamide;PET | Metal-coated textiles | Commercial metal-coated fabrics compared with a metal-free graphite coated PET/cellulose fabric | Ni/Cu-coated PET fabric: capacitance 9.9 × 10 mF/cm2; energy density of 8.8 × 10−6 Wh/cm2 Ni/Cu/Ag-coated polyamide: capacitance 4.7 × 10 mF/cm2 at 5 mV/s; energy density of 4.2 × 10−6 Wh/cm2; stable performance over 5000 charge–discharge cycles | [114] |
| KOH | PET; CO blend | NiCoAl-LDH); Ti3C2Tx; MXene; Ag nanowires as positive electrode; active carbon (negative electrode) | Interdigital pattern obtained by printing and electroless deposition; anchoring of the battery-type material onto conductive MXene and hydrothermal treatment; active carbon inks deposited by screen-printing; flexible supercapacitor assembled by covering a layer of PVA/KOH gel electrolyte, and encapsulated by scotch tape | Positive electrode: capacity 592 C/g, excellent rate performance and cycling stability over 10,000 cycles Positive electrode and negative electrode: energy density of 2.2 × 10−5 Wh/cm2 and a power density of 3.0 × 10−3 W/cm2 | [115] |
| PEO/KOH | PET | Silver oxide | Stencils of different thicknesses used to print different layers of the battery in polyamide-nylon 6 and PET | Capacity of 0.6 mAh/cm2 with an active electrode area of 0.5 cm × 1 cm | [116] |
| PVA/H2SO4 | PET | rGO nanosheets; PPY | Dipping and drying method | Capacitance of 2.3 × 103 mF/cm2; volumetric capacitance of 5.5 × 103 mF/cm3; energy density of 1.1 × 10−5 Wh/cm2; power density of 3.0 × 10−5 W/cm2; retains 76% of its initial capacitance after 6000 cycles and mechanical stability under bending | [117] |
| PVA/Na2SO4 | Cu; Ni coated conductive PET | PPy–graphene–PPy-coated fabric | Sandwich configuration with PVA/Na2SO4 as a gel electrolyte and filter paper as a separator; electrodes and filter paper immersed in the gel electrolyte before being assembled into a supercapacitor device | Capacitance of 6.8 × 102 mF/cm2; energy density of 6.4 × 10−5 Wh/cm3; power density of 0.6 × 10−3 W /cm3; maintained 94.2% of its capacitance after 4000 cycles | [118] |
| PVA/H2SO4 | CO; PET | Graphene; microcircuit encapsulant PE773 | Pad–dry–cure method with graphene ink and encapsulation (microcircuit encapsulant PE773); immersion in gel electrolyte | Specific capacitance of ∼2.7 mF/cm2; capacitance retention of 98% after 150 cycles at 180º flexion; high domestic washing fastness (10 cycles) | [119] |
| PVA/H3PO4 | Silk | CSF; PPy | Carbonization of silk fabrics; potentiostatic electrodeposition of PPy; immersion of the electrodes in gel electrolyte; SC assembly using cellulose sandwich | Composite: capacitance of 4.0 × 103 mF/cm2 and cycling stability of 88.6%; capacitance retention after 1500 cycles. SC: areal specific capacitance of 6.7 × 102 mF/cm2; energy density of 6.9 × 10−3 Wh/cm3 | [120] |
| PVA/H3PO4 | Nylon® | CNT; Nylon® | Nylon®/CNT electrodes produced by dip-coating; Nylon®/rubber:Nylon®/CNT laminates production; SC production using a Nylon® sheet as separator | Capacitance of 117 F/g (at 2 mV/s); maximum energy density of 4.0 Wh/kg | [121] |
| PVA/H3PO4 | Silk | PANI@GO | Dip-coating of silk in the respective mixture (either GO or PANI). For GO@PANI-coated fibers, the GO-coated silk materials were dipped in the PANI mixture. | Specific capacitance of 450 F/g; capacitance of 71.2 F/g obtained with the symmetrical PANI@GO-SL/PVA/PANI@GO–silk capacitor; 87.4% capacity retention at 5000 cycles; energy and power densities of 2.5 × 10 Wh/kg and 8.0 × 103 W/kg | [122] |
| PVA/H3PO4 | PP; PET; PAN | rGO | Reactive inkjet printing of GO in the fabric with concomitant reduction; dip-casting of the gel electrolyte; all-solid-state SC assembly | PP fabric: specific capacitance of 1.3 × 10 mF/cm2; power and energy densities of 4.6 × 10−3 W/cm2 and 1.2 × 10−3 Wh/cm2; apx. 100% of its original capacitance after 5000 cycles | [123] |
| PVA/H3PO4 | Polyamide; carbon fibers | PEDOT:PSS | Weaving a polyamide warp yarn around the self-designed mode; interwoven weft yarn (polyamide yarn/Ag-coated polyamide yarn); supercapacitor assembled by using PEDOT:PSS-coated carbon fibers, coated with gel electrolyte and separated by cellulose | Capacitance 1.3 × 10 mF/cm2 (79.9 F/g) at a current density of 0.1 mA/cm2; power and energy densities of 4.6 × 10−3 W/cm2 and 1.2 × 10−3 Wh/cm2 | [124] |
| PVA/H2SO4 | Kevlar® | rGO | rGO-coated Kevlar® fibers produced by modified hydrothermal gelation; dip-coating of rGO@Kevlar cloth in gel electrolyte | rGO@Kevlar® fibers (38.1% rGO): specific strength of 1.6 MPa.m3/kg; specific capacitance of 57 F/g rGO@Kevlar® cloth SC. Withstands impact of 9.1 N and deformation of 90º. | [125] |
| PVA/KOH-Zn(Ac)2- LiOH- Ca(OH)2 | PET; polyamide 6,6 | Zn; Cu; NiCo | Kapton applied on fabric Ni and Cu deposited by electroless and electrodeposiition; 2 interdigitated Cu electrodes coated; Zn and NiCo BOH nanosheets were electrodeposited; coating with gel electrolyte | Electroplated Zn anode and a Ni cathode; energy density of 2.6 × 102 Wh/kg; power density of 1.0 × 104 W/kg; stable cycling performance of 82.7% for 1500 cycles; good mechanical reliability (bending, twisting and tailoring) | [126] |
| PVA/LiCl | Bamboo | MnO2–NiCo2O4; rGO | Printing of Ni-Co + printing of KMnO4 for anode; printing with rGO + hydrazine reduction for cathode; device prepared using an anode and cathode sheets, separated by a bamboo fabric sheet, and coated with gel electrolyte | MnO2–NiCo2O4/rGO device shows stable performance within a 0–1.6 V range; capacitance of 2.1 × 103 mF/cm2; energy density of 3.8 × 10−2 W/cm3; power density of 2.7 W/cm3; 92% of capacitance retention after 5000 cycles and low charge transfer resistance 3.2 Ω | [127] |
| PVA/KCl | Ag-coated polyamide (Berlin fabric) | Ag-coated polyamide; graphite | Printing coated electrodes and gel electrolyte | Areal capacitance 1.3 × 10 mF/cm2 | [128] |
| PVDF/LiTFSI | PP-based satin | LFP/LTO;carbon black | Thermally drawn fibers comprising anode (LTO), cathode (LFP), gel electrolyte (PVDF/LiTFSI) and conductive polymer (carbon black). The fibers produced are then woven into the satin. | Battery discharge capacity of apx. 123 mAh and discharge energy of apx. 2.2 × 10−1 Wh. Woven: 96% of capacity retention after 1000 bending cycles. | [129] |
| Gel Electrolyte | Textile Composition | Electrodes | Preparation Method | Conductivity Tests and Results | Ref. |
|---|---|---|---|---|---|
| PVA/NH4Cl-ZnCl | 3D bicontinuous porous carbon-sheathed carbon cloth (CC–PC) | CCPC@PANAC (cathode); CC@Zn NP (anode) | PANAC cathode produced by the copolymerization of PANI with a redox-active phenothiazine derivative. The anode constructed by depositing Zn nanoplate arrays onto CC by the electrochemical method. The gel electrolyte is placed between the electrodes. | Energy density of 3.5 × 102 Wh/kg; specific capacity of 306.3 mAh/g; capacitance retention of 86.6% after 2000 bending cycles | [130] |
| LiCl/PVA | CC | NHPCN@CC (electrodes) | NHPCN@CC was obtained by self-assembly of a sol–gel MSS template onto CC, followed by PDA coating and subsequent carbonization. The electrodes were immersed in a LiCl/PVA gel electrolyte. | Energy density of 1.0 × 10 Wh/kg (at 1.0 × 104 W/kg) and 2.4 × 10 Wh/kg (at 5.0 × 102 W/kg); capacitance retention of 85% (for 8000 cycle) | [131] |
| PVA/KCl | CC | Flexible conductive porous electrode; PANI/ZnO@ZIF-8-CC | PANI/ZnO@ZIF-8-CC electrode: in situ growth of hollow ZnO spheres on activated CC; core–shell structure, created by coating the ZnO core with a ZIF-8 shell; aniline electropolymerization used to deposit a homogeneous PANI coating on both the inner and outer surfaces of ZnO@ZIF-8-CC | Areal capacitance of 4.8 × 103–4.0 × 103 mF/cm2 (at 5–30 mA/cm2); energy density of 1.4 × 10−4–8.9 × 10−5 Wh/cm3; equivalent series resistance of 1.22 Ω; specific capacitance of 4.3 × 103 mF/cm2; capacitance retention of 7% for 10,000 cycles | [132] |
| PVA/H2SO4-Fe3+-Fe2+ | CC | PANI; CNTs core–shell (hybrid electrode) | PANI/CNTs@CC electrodes were obtained by the deposition of CNTs onto CC via LPCVD, followed by PANI coating through electropolymerization. The gel electrolyte was prepared using H2SO4, PVA, FeSO4·7H2O, and Fe2(SO4)3. The SCs were assembled by pressing. | Diffusion resistance of 0.236 Ω (PANI/CNTs); specific capacitance of 6.0 × 102 mF/cm2 (at 5 mV/s); energy density of 2.3 × 10 Wh/kg (at a power density of 7.0 × 102 W/kg); capacitance retention of 97% (after 2000 cycles) | [133] |
| NaClO4 /PVA | CC | MnO2 nanowires/CC and activated carbon | MnO2 nanowires/CC electrode prepared on CC by dip-coating and autoclave; the supercapacitor is assembled using both MnO2 nanowires/CC and activated carbon fibers, coated with the gel electrolyte. | Capacitance retention of 81% after 25,000 cycles while exposed directly to axternal enrivronment; consistent and stable charge storage between −40 and 40 ºC. | [134] |
| PVA/Na2SO4; PVA: EMIBF4 | CC | Sm-Mo-C5 woven carbon fibers (WCFs) | SmVO4 nanoparticles were synthesized via hydrothermal techniques, using Sm(NO3)3·6H2O and NH4VO3. The SmVO4-MoS2 and CNT-SmVO4-MoS2 nanocomposites were synthesized by the same method. The supercapacitor was assembled by Vacuum-Assisted Resin Transfer Molding. | Specific capacitance of 1.0 × 103 mF/cm2 (current density of 2.187 mA/cm2) in a three-electrode system. The SCs show specific capacitance of 2.9 × 102 mF/cm2 (at a current density of 2 A/cm2). Capacitance retention: 72.5% to 50.000 cycles; maximum energy density of 8.0 × 10 Wh/Kg (at a power density of 1.0 × 103 W/Kg). | [135] |
| FeDPCL (self-healing hydrogel electrolyte) | CC | PANI-CC | PANI@CWF electrodes were obtained by in situ electropolymerization of aniline onto carbon fabric. Hydrogel was prepared using AA, CTAB, C18, Fe(NO3)3·6H2O, and potassium persulfate. The gels were immersed in H2SO4. The eletrodes were directly paved on hydrogel eletrolyte without separator. | Energy density of 1.9 × 10 Wh/kg (at power densities of 6.7 × 102 W/kg); capacitance retention of 86% (after healing behavior). | [136] |
| Gel Electrolyte | Structure and Composition | Electrodes | Preparation Method | Conductivity Tests and Results | Ref. |
|---|---|---|---|---|---|
| PVA/H2SO4 | CO;CNT | CNT; PPy | Dip-coating pyrrole; supercapacitor assembled with two fabrics as electrodes, a CO fabric as a separator, and PVA/H2SO4 as the gel electrolyte | Areal capacitance of electrode of 4.1 × 103 mF/cm2; quasi-rectangular curve cyclic voltametry; 93% Coulombic efficiency; 89% under bending | [137] |
| PAM/KOH | Spandex knitted fabric | Ni@NiCoP; SWCNT ST | Ni@NiCoP@SWCNT ST dip-coated in Ni@NiCoP ST into SWCNT ink; PAM-based hydrogel immersed in KOH; a layer of Ni@NiCoP@SWCNT ST and a layer of Ni@NiCoP ST attached to asymmetric electrodes in the supercapacitor; in between PVA/H2SO4 gel electrolyte | Eletrode conductivity 532 S/cm; areal capacitance 8.8 × 102 mF/cm2; gravimetric capacitance 713 F/g; 101% retention after 6000 cycles | [138] |
| PVA/H2SO4 | Cellulose | GNS; PEDOT:PSS | Sprayed GNS and PEDOT; gel electolyte coating | Specific areal capacitance of 2.4 × 102 mF/cm2; energy density of 2.2 × 10−5 Wh/cm2; 83.9% capacitance after 10,000 cycles | [139] |
| PVA/H3PO4 | Diamond-shaped warp knitting grid structure (stainless-steel mesh-SSM) | PEDOT; RGO; SSM | Two-step electrodeposition of PEDOT/RGO@SSM process in; PVA/H3PO4 gel electrolyte; | Areal capacitance of 5.3 × 10 mF/cm2; ~73% capacitance after 5000 cycles; rate capability of 3.6 × 10 mF/cm2; ~78% capacitance retention at 10% strain for 500 stretching cycles | [140] |
| PVA/LiCl | PET 90%; spandex 10% | Ni/rGO | Ni-coated textile was immersed in a GO dispersion for a hydrothermal reaction; PVA/LiCl gel electrolyte coating | Areal capacitance of 5.1 × 10 mF/cm2; no degradation at 50% of tensile strain; capacitance retention of 85.3% retention after charging/discharging for 5000 cycles; slightly slopped rectangular shape ciclic voltametry | [141] |
| ACN/PC/PMMA/LiClO4 | Knitted Fabric (Nylon® 82%; spandex 18%) | MWCNT; MoO3 | MWCNT/MoO3 nanocomposite spray-coated over knitted textile; gel electrolyte | 100% Coulombic efficiency; 86 and 76% capacitance after 5000 and 10,000 charge/discharge cycles; quasi-rectangular-shaped cyclic voltametry; areal capacitance of 3.4 × 10 mF/cm2 | [142] |
| PVA/ H2SO4 | CO | PPy | Dip-coating for in situ polymerization of PPy with, iron nitrate, and 5-sulfosalicylic acid e; gel electrolyte coating; device assembled simetrically | Areal capacitance of 4.5 × 102 mF/cm2; energy density of 0.4 Wh; 30% capacitance after 500 cycles; ~84–160% retention rate during 1000 stretching procedures | [143] |
| KOH/Na2SO4 | Knitted graphite fabric | Graphite and Mn-Cu alloys | Graphite fibers coated with Mn–Cu alloys by electrodeposition in an electrolyte solution | Specific capacitance of 9.3 × 104 mF/cm2; areal capacitance of 9000 mF/cm2 | [144] |
| Gel Electrolyte | Non-Woven Composition | Electrodes | Preparation Method | Conductivity Tests and Results | Ref. |
|---|---|---|---|---|---|
| PVA/H2SO4 | Graphene | Porous graphene fiber-assembled fabric | Micro wet spinning | Capacitance of 1.2 × 103 mF/cm2; 100% for 60,000 cycles; energy density of 1.2 × 10−4 Wh/cm2 | [147] |
| PVA/H2SO4 | MXene-based fiber fabrics | MXene (Ti3C2Tx) graphene quantum dots | Micro wet spinning | Capacitance of 1.8 × 106 mF/cm3; 100% stable for 5000 cycles; energy density of 2.1 × 10−2 Wh/cm3 | [148] |
| PVA/H2SO4 | rGO enveloped with PANI | rGO enveloped with PANI | Wet spinning | Capacitance of 3.8 × 103 mF/cm2; 100% for 10,000 cycles; energy density of 4.9 × 10−3 W/cm2 | [149] |
| Lithium perchlorate-PCL | PCL nanofibers containg silicon dioxide nanoparticles | PEDOT:PSS screen-printing on indium tin oxide coated polyethylene terephthalate | Electrospinning | Ionic conductivity of 5.2 × 10−3 S/cm; 100% for 100 cycles | [150] |
| Polyvinylidene fluoride/MgClO4/propylene carbonate | PVDF nanofibers | PVDF nanofibers | Electrospinning | Capacitance of 3832 mAh/cm3; ionic conductivity of 1 × 10−3 S/cm | [151] |
| Poly (ethylene oxide) | Poly (ethylene oxide) | Li-ions | Solvent casting and dipping | Run stably > 750 h at 0.5 mA/cm2; good security; 300 cycles with 1 mA/cm2 | [152] |
| PVA/H2SO4/KOH | Pure cellulose | Carbon paper | Dipping | Capacitance of 1.4 × 102 mF/cm2; 100% stable for 1000 cycles at 180° bending | [153] |
| PEGDA; ETPTA; liquid electrolyte; AIBN | PEGDA; ETPTA; liquid electrolyte; AIBN | Li (anode); LiPF6 (cathode) | Injection | Ionic conductivity 0.87 mS/cm; 100% for 200 cycles; resistance of 275 Ω | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.I.; Alves, C.; Fernandes, M.; Abreu, J.; Pedroso de Lima, F.; Padrão, J.; Zille, A. Gel Electrolytes in the Development of Textile-Based Power Sources. Gels 2025, 11, 392. https://doi.org/10.3390/gels11060392
Ribeiro AI, Alves C, Fernandes M, Abreu J, Pedroso de Lima F, Padrão J, Zille A. Gel Electrolytes in the Development of Textile-Based Power Sources. Gels. 2025; 11(6):392. https://doi.org/10.3390/gels11060392
Chicago/Turabian StyleRibeiro, Ana Isabel, Cátia Alves, Marta Fernandes, José Abreu, Fábio Pedroso de Lima, Jorge Padrão, and Andrea Zille. 2025. "Gel Electrolytes in the Development of Textile-Based Power Sources" Gels 11, no. 6: 392. https://doi.org/10.3390/gels11060392
APA StyleRibeiro, A. I., Alves, C., Fernandes, M., Abreu, J., Pedroso de Lima, F., Padrão, J., & Zille, A. (2025). Gel Electrolytes in the Development of Textile-Based Power Sources. Gels, 11(6), 392. https://doi.org/10.3390/gels11060392











