Highly Stretchable, Low Hysteresis, and Transparent Ionogels as Conductors for Dielectric Elastomer Actuators
Abstract
1. Introduction
2. Results and Discussion
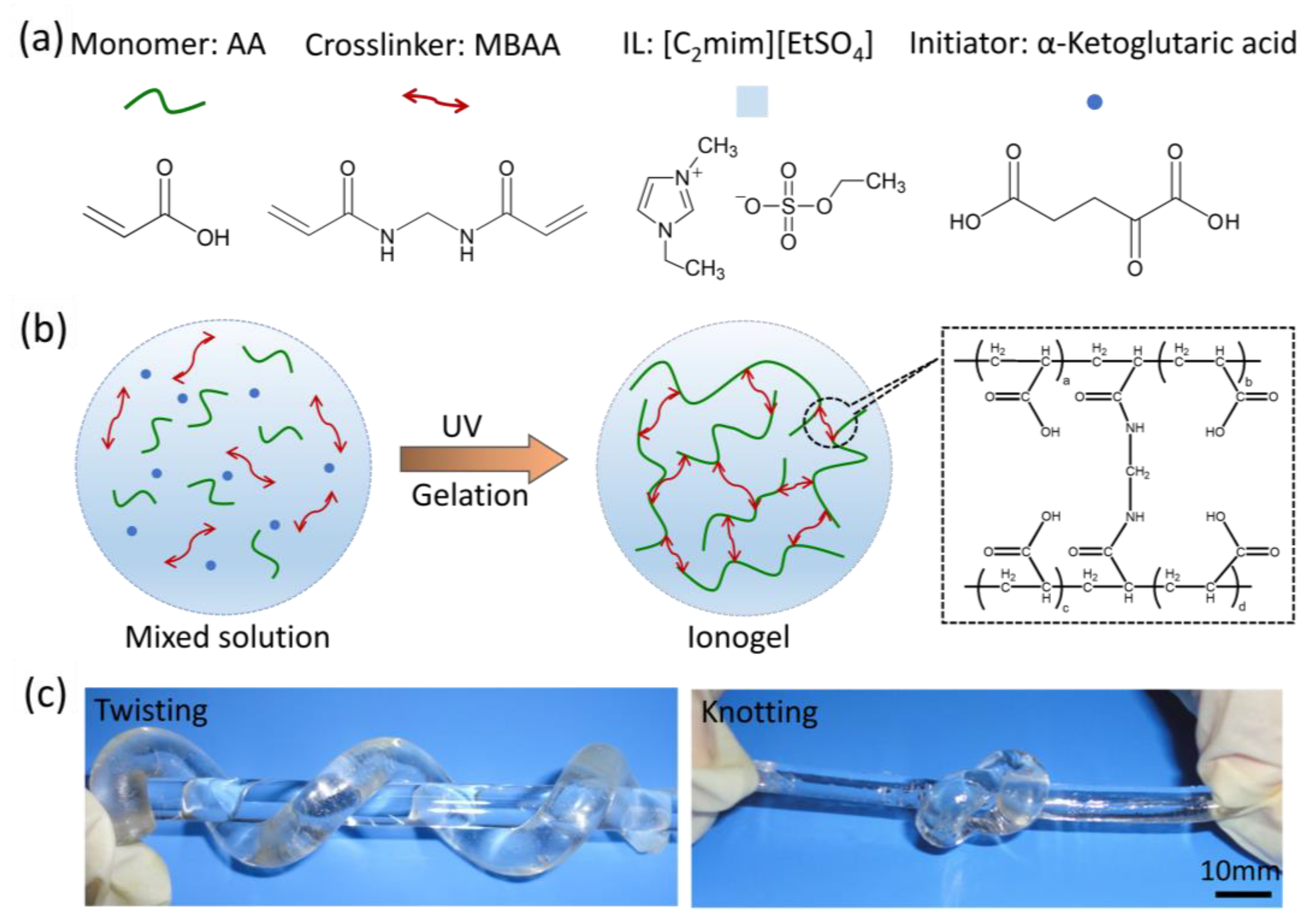
2.1. Preparation of PAA Ionogel
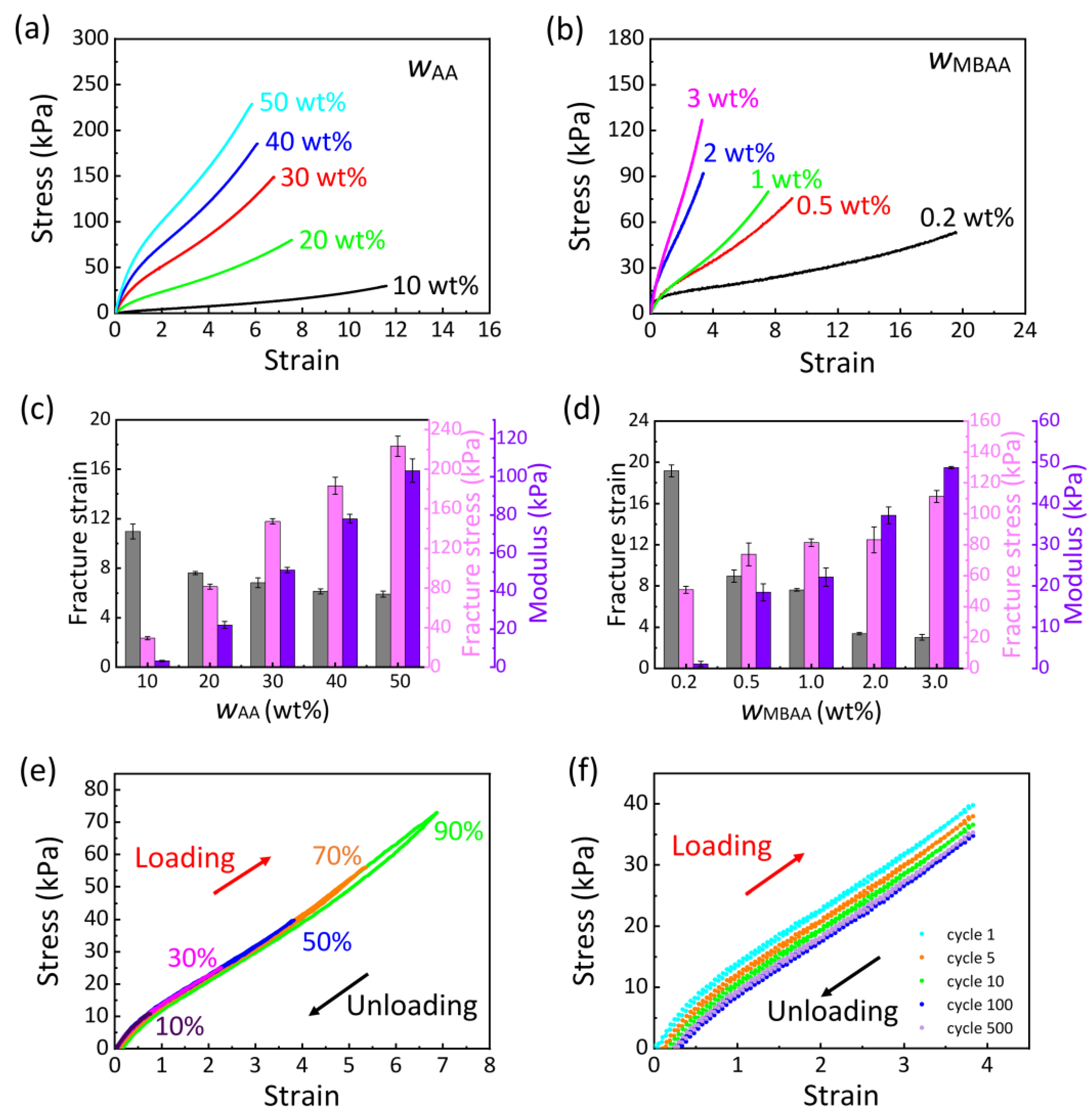
2.2. Mechanical Properties of PAA Ionogel
2.3. Multifunctional Properties of PAA Ionogel
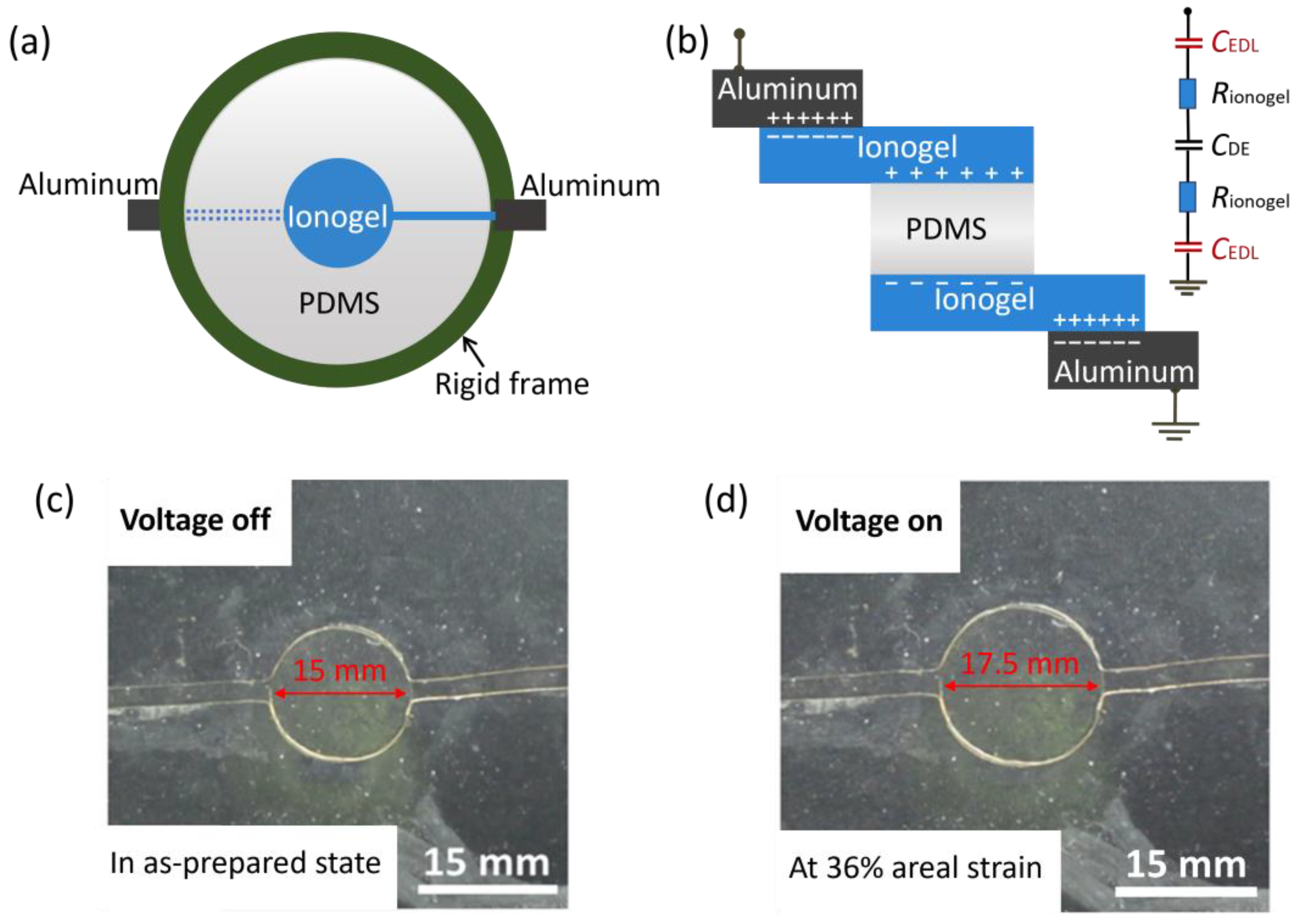
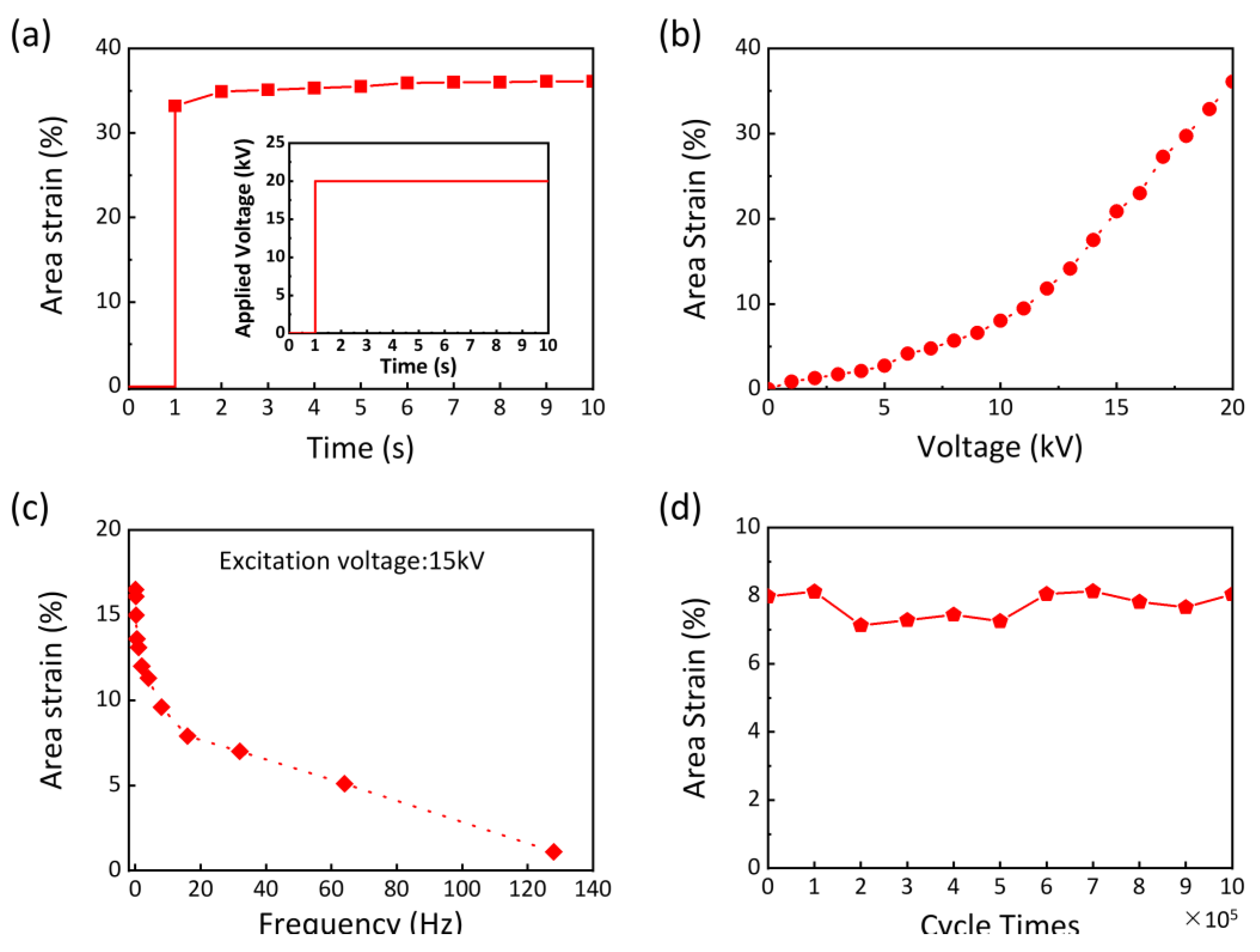
2.4. PAA Ionogel as Conductors for Dielectric Elastomer Actuators
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PAA Ionogels
4.3. Mechanical Testing
4.4. Transmittance Testing
4.5. TGA Testing
4.6. Strain-Dependent Resistance Testing
4.7. Fabrication of Dielectric Elastomer Actuator
4.8. Area Strain Testing of Dielectric Elastomer Actuator
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Xu, H.; Deng, Z.; Guo, B.; Zhang, J. Low modulus hydrogel-like elastomer sensors with ultra-fast self-healing, underwater self-adhesion, high durability/stability and recyclability for bioelectronics. Nano Today 2024, 59, 102469. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Tan, J.; Wang, M.; Ren, Y.; Ge, F.; Zhang, Q. A healable, mechanically robust and ultrastretchable ionic conductive elastomer for durably wearable sensor. Nano Res. 2023, 17, 3369–3378. [Google Scholar] [CrossRef]
- Chen, L.; Chang, X.; Wang, H.; Chen, J.; Zhu, Y. Stretchable and transparent multimodal electronic-skin sensors in detecting strain, temperature, and humidity. Nano Energy 2022, 96, 107077. [Google Scholar] [CrossRef]
- Du, R.; Bao, T.; Zhu, T.; Zhang, J.; Huang, X.; Jin, Q.; Xin, M.; Pan, L.; Zhang, Q.; Jia, X. A Low-Hysteresis and Highly Stretchable Ionogel Enabled by Well Dispersed Slidable Cross-Linker for Rapid Human-Machine Interaction. Adv. Funct. Mater. 2023, 33, 2212888. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Liu, Z.; Shen, S.; Tat, T.; Wang, Z.; Lu, C.; Ding, W.; He, X.; Yang, J.; et al. Kirigami-Inspired Pressure Sensors for Wearable Dynamic Cardiovascular Monitoring. Adv. Mater. 2022, 34, 2202478. [Google Scholar] [CrossRef]
- Shintake, J.; Piskarev, Y.; Jeong, S.H.; Floreano, D. Ultrastretchable Strain Sensors Using Carbon Black-Filled Elastomer Composites and Comparison of Capacitive Versus Resistive Sensors. Adv. Mater. Technol. 2017, 3, 1700284. [Google Scholar] [CrossRef]
- Volkert, C.; Colucci, R.; Berger, R.; Besenius, P.; Blom, P.W.M.; Kraft, U. Transfer-printing of patterned PEDOT:PSS structures for bendable, stretchable and biodegradable electronics. J. Mater. Chem. C 2024, 12, 3865–3872. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 62. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Tang, G.; Ru, J.; Zhu, Z.; Li, B.; Guo, C.F.; Li, L.; Zhu, D. Ionic Flexible Sensors: Mechanisms, Materials, Structures, and Applications. Adv. Funct. Mater. 2022, 32, 2110417. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem.-Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Ying, B.; Chen, R.Z.; Zuo, R.; Li, J.; Liu, X. An Anti-Freezing, Ambient-Stable and Highly Stretchable Ionic Skin with Strong Surface Adhesion for Wearable Sensing and Soft Robotics. Adv. Funct. Mater. 2021, 31, 2104665. [Google Scholar] [CrossRef]
- Hu, H.; Li, D.; Salim, T.; Li, Y.; Cheng, G.; Lam, Y.M.; Ding, J. Electrically driven hydrogel actuators: Working principle, material design and applications. J. Mater. Chem. C 2024, 12, 1565–1582. [Google Scholar] [CrossRef]
- Feng, T.; Ling, D.; Li, C.; Zheng, W.; Zhang, S.; Li, C.; Emel’yanov, A.; Pozdnyakov, A.S.; Lu, L.; Mao, Y. Stretchable on-skin touchless screen sensor enabled by ionic hydrogel. Nano Res. 2023, 17, 4462–4470. [Google Scholar] [CrossRef]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.; Suo, Z.; Vlassak, J.J. Highly Stretchable and Tough Hydrogels below Water Freezing Temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, B.; Xiang, F.; Zhou, J.; Wang, H.; Suo, Z. Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl. Phys. Lett. 2014, 105, 151903. [Google Scholar] [CrossRef]
- Xin, F.; Lyu, Q. A Review on Thermal Properties of Hydrogels for Electronic Devices Applications. Gels 2022, 9, 7. [Google Scholar] [CrossRef]
- Li, H.-N.; Zhang, C.; Yang, H.-C.; Liang, H.-Q.; Wang, Z.; Xu, Z.-K. Solid-state, liquid-free ion-conducting elastomers: Rising-star platforms for flexible intelligent devices. Mater. Horiz. 2024, 11, 1152–1176. [Google Scholar] [CrossRef]
- Niu, W.; Liu, X. Stretchable Ionic Conductors for Soft Electronics. Macromol. Rapid Commun. 2022, 43, 2200512. [Google Scholar] [CrossRef]
- Li, F.; Nguyen, G.T.M.; Vancaeyzeele, C.; Vidal, F.; Plesse, C. Photopolymerizable Ionogel with Healable Properties Based on Dioxaborolane Vitrimer Chemistry. Gels 2022, 8, 381. [Google Scholar] [CrossRef]
- Yan, S.; Liu, J.; He, Z.; Jia, H.; Chen, Z.; Zhang, Y.; Gohy, J.F. High-Performance Ionogels from Dynamic Polyrotaxane-Based Networks. Angew. Chem. Int. Ed. 2025, 64, e202503307. [Google Scholar]
- Luo, X.; Li, J.; Gu, Y.; Du, J.; Huang, Y.; Baskin, I.; Ein-Eli, Y.; Hyun, W.J. Talc Nanosheet Ionogel Electrolytes with High Lithium-Ion Conductivity for Solid-State Lithium Metal Batteries. Nano Lett. 2025, 25, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, M.; Wu, X.; Yu, J.; Li, X.; Song, P. Transparent, Stretchable, and Adherable Ionogel with High Electrical Conductivity for Flexible Strain Sensors. ACS Appl. Polym. Mater. 2024, 6, 10635–10643. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Zhao, Z.; Liang, Y.; Wang, L.; Liu, Y. Highly Stretchable, Transparent and Adhesive Ionogel Based on Chitosan-Poly(acrylic acid) Double Networks for Flexible Strain Sensors. Gels 2022, 8, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, D.; Dong, T.; Das, R.; Pan, D.; Sun, C.; Wu, Z.; Zhang, Q.; Liu, C.; Guo, Z. Overview of Ionogels in Flexible Electronics. Chem. Rec. 2020, 20, 948–967. [Google Scholar] [CrossRef]
- Fan, X.; Liu, S.; Jia, Z.; Koh, J.J.; Yeo, J.C.C.; Wang, C.-G.; Surat’man, N.E.; Loh, X.J.; Le Bideau, J.; He, C.; et al. Ionogels: Recent advances in design, material properties and emerging biomedical applications. Chem. Soc. Rev. 2023, 52, 2497–2527. [Google Scholar] [CrossRef]
- Zhou, R.; Jin, Y.; Li, Y.; Jin, H.; Zeng, W.; Mei, J.; Liu, Y. In-situ phase separation constructing robust hydrophobic ionogels with multifunction. Chem. Eng. J. 2023, 476, 146840. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Y.; Jiang, L.; Xie, W.; Davis, B.; Wang, M.; Zhang, L.; Liu, Y.; Xing, S.; Dickey, M.D.; et al. Highly Stretchable, Tissue-like Ag Nanowire-Enhanced Ionogel Nanocomposites as an Ionogel-Based Wearable Sensor for Body Motion Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 46538–46547. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, E.; Hao, S.; Yang, X.; Li, T.; Lou, C.; Run, M.; Song, H. 3D Printable, ultra-stretchable, Self-healable, and self-adhesive dual cross-linked nanocomposite ionogels as ultra-durable strain sensors for motion detection and wearable human-machine interface. Chem. Eng. J. 2022, 431, 133949. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Li, S.; Liu, X.; Sun, J. Mechanically Robust, Elastic, and Healable Ionogels for Highly Sensitive Ultra-Durable Ionic Skins. Adv. Mater. 2020, 32, 2002706. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Dickey, M.D. Tough Ionogels: Synthesis, Toughening Mechanisms, and Mechanical Properties-A Perspective. JACS Au 2022, 2, 2645–2657. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, X.; Shen, K.; Cheng, Y.; Ma, L.; Ming, X. Hydrogen Bonds Reinforced Ionogels with High Sensitivity and Stable Autonomous Adhesion as Versatile Ionic Skins. ACS Appl. Mater. Interfaces 2024, 16, 4035–4044. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, M.; Chen, S.; Zhang, Y.; Gu, H.; Deng, Y.; Yang, G.; Fei, C.; Chen, B.; Lin, Y.; et al. Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Sci. Adv. 2021, 7, eabi8249. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Zhou, H.; Jin, Z.; Li, S.; Liu, H.; Jin, X.; Luo, C.; Ma, A.; Chen, W. Highly Stretchable, Fatigue-Resistant, Electrically Conductive, and Temperature-Tolerant Ionogels for High-Performance Flexible Sensors. ACS Appl. Mater. Interfaces 2019, 11, 26412–26420. [Google Scholar] [CrossRef]
- Wang, W.; Bai, J.; Xu, W.; Xia, Y.; Wu, M.; Wu, M. Baking-inspired process for fully physically crosslinked polyacrylic acid ionic gels with excellent moldability and versatility assiated by ionic liquid. Polymer 2025, 325, 128284. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, P.; Shamsi, M.; Thelen, J.L.; Qian, W.; Truong, V.K.; Ma, J.; Hu, J.; Dickey, M.D. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 2022, 21, 359–365. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Lu, G.; Sun, J.; Tang, L. Highly Stretchable, Transparent, and Self-Adhesive Ionic Conductor for High-Performance Flexible Sensors. ACS Appl. Polym. Mater. 2021, 3, 1610–1617. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Soni, M.; Escobedo, P.; Dahiya, R. PEDOT:PSS Microchannel-Based Highly Sensitive Stretchable Strain Sensor. Adv. Electron. Mater. 2020, 6, 2000445. [Google Scholar] [CrossRef]
- Li, Y.; Yu, P.; Wen, J.; Sun, H.; Wang, D.; Liu, J.; Li, J.; Chu, H. Nanozyme-Based Stretchable Hydrogel of Low Hysteresis with Antibacterial and Antioxidant Dual Functions for Closely Fitting and Wound Healing in Movable Parts. Adv. Funct. Mater. 2021, 32, 2110720. [Google Scholar] [CrossRef]
- Lam, T.N.; Lee, G.S.; Kim, B.; Dinh Xuan, H.; Kim, D.; Yoo, S.I.; Yoon, J. Microfluidic preparation of highly stretchable natural rubber microfiber containing CNT/PEDOT:PSS hybrid for fabric-sewable wearable strain sensor. Compos. Sci. Technol. 2021, 210, 108811. [Google Scholar] [CrossRef]
- Xu, K.; Shen, K.; Yu, J.; Yang, Y.; Wei, Y.; Lin, P.; Zhang, Q.; Xiao, C.; Zhang, Y.; Cheng, Y. Ultradurable Noncovalent Cross-Linked Hydrogels with Low Hysteresis and Robust Elasticity for Flexible Electronics. Chem. Mater. 2022, 34, 3311–3322. [Google Scholar] [CrossRef]
- Wang, M.X.; Chen, Y.M.; Gao, Y.; Hu, C.; Hu, J.; Tan, L.; Yang, Z. Rapid Self-Recoverable Hydrogels with High Toughness and Excellent Conductivity. ACS Appl. Mater. Interfaces 2018, 10, 26610–26617. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yuan, W.; Brochu, P.; Gruner, G.; Pei, Q. Highly stretchable, conductive, and transparent nanotube thin films. Appl. Phys. Lett. 2009, 94, 161108. [Google Scholar] [CrossRef]
- Wang, J.; Yan, C.; Cai, G.; Cui, M.; Eh, A.L.S.; Lee, P.S. Extremely Stretchable Electroluminescent Devices with Ionic Conductors. Adv. Mater. 2015, 28, 4490–4496. [Google Scholar] [CrossRef]
- Jiang, E.; Jamali, A.; List, M.; Mishra, D.B.; Sheikholeslami, S.A.; Goldschmidtboeing, F.; Woias, P.; Baretzky, C.; Fischer, O.; Zimmermann, B.; et al. Organic photovoltaic mini-module providing more than 5000 V for energy autonomy of dielectric elastomer actuators. Nat. Commun. 2025, 16, 2048. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Lu, T.; Wang, T.J. Response time and dynamic range for a dielectric elastomer actuator. Sens. Actuators A 2016, 239, 8–17. [Google Scholar] [CrossRef]
- Feng, W.; Sun, L.; Jin, Z.; Chen, L.; Liu, Y.; Xu, H.; Wang, C. A large-strain and ultrahigh energy density dielectric elastomer for fast moving soft robot. Nat. Commun. 2024, 15, 4222. [Google Scholar] [CrossRef]
- Lee, J.; Tan, M.W.M.; Parida, K.; Thangavel, G.; Park, S.A.; Park, T.; Lee, P.S. Water-Processable, Stretchable, Self-Healable, Thermally Stable, and Transparent Ionic Conductors for Actuators and Sensors. Adv. Mater. 2019, 32, 1906679. [Google Scholar] [CrossRef]
- Bai, Y.; Jiang, Y.; Chen, B.; Chiang Foo, C.; Zhou, Y.; Xiang, F.; Zhou, J.; Wang, H.; Suo, Z. Cyclic performance of viscoelastic dielectric elastomers with solid hydrogel electrodes. Appl. Phys. Lett. 2014, 104, 062902. [Google Scholar] [CrossRef]
- Chen, B.; Lu, J.J.; Yang, C.H.; Yang, J.H.; Zhou, J.; Chen, Y.M.; Suo, Z. Highly Stretchable and Transparent Ionogels as Nonvolatile Conductors for Dielectric Elastomer Transducers. ACS Appl. Mater. Interfaces 2014, 6, 7840–7845. [Google Scholar] [CrossRef]
- Lee, K.H.; Kang, M.S.; Zhang, S.; Gu, Y.; Lodge, T.P.; Frisbie, C.D. “Cut and Stick” Rubbery Ion Gels as High Capacitance Gate Dielectrics. Adv. Mater. 2012, 24, 4457–4462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, K.; Wang, J.; Zhao, J.; Tang, J.; Hu, J. Stretchable and transparent ionogel-based heaters. Mater. Horiz. 2022, 9, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Chortos, A.; Hajiesmaili, E.; Morales, J.; Clarke, D.R.; Lewis, J.A. 3D Printing of Interdigitated Dielectric Elastomer Actuators. Adv. Funct. Mater. 2019, 30, 1907375. [Google Scholar] [CrossRef]
- Gu, H. Comprehensive review of recent research trends in dielectric elastomer transducers: Applications, materials, and fabrication. Mater. Res. Express 2024, 11, 112001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, H.; Li, Z.; Pan, W.; Men, Y.; Zhang, N.; Xu, J.; Liu, X. Highly Stretchable, Low Hysteresis, and Transparent Ionogels as Conductors for Dielectric Elastomer Actuators. Gels 2025, 11, 369. https://doi.org/10.3390/gels11050369
Zhang L, Li H, Li Z, Pan W, Men Y, Zhang N, Xu J, Liu X. Highly Stretchable, Low Hysteresis, and Transparent Ionogels as Conductors for Dielectric Elastomer Actuators. Gels. 2025; 11(5):369. https://doi.org/10.3390/gels11050369
Chicago/Turabian StyleZhang, Limei, Hong Li, Zhiquan Li, Weimin Pan, Yi Men, Niankun Zhang, Jing Xu, and Xuewei Liu. 2025. "Highly Stretchable, Low Hysteresis, and Transparent Ionogels as Conductors for Dielectric Elastomer Actuators" Gels 11, no. 5: 369. https://doi.org/10.3390/gels11050369
APA StyleZhang, L., Li, H., Li, Z., Pan, W., Men, Y., Zhang, N., Xu, J., & Liu, X. (2025). Highly Stretchable, Low Hysteresis, and Transparent Ionogels as Conductors for Dielectric Elastomer Actuators. Gels, 11(5), 369. https://doi.org/10.3390/gels11050369





