Preparation of Janus-Structured Evaporators for Enhanced Solar-Driven Interfacial Evaporation and Seawater Desalination
Abstract
1. Introduction
2. Results and Discussion
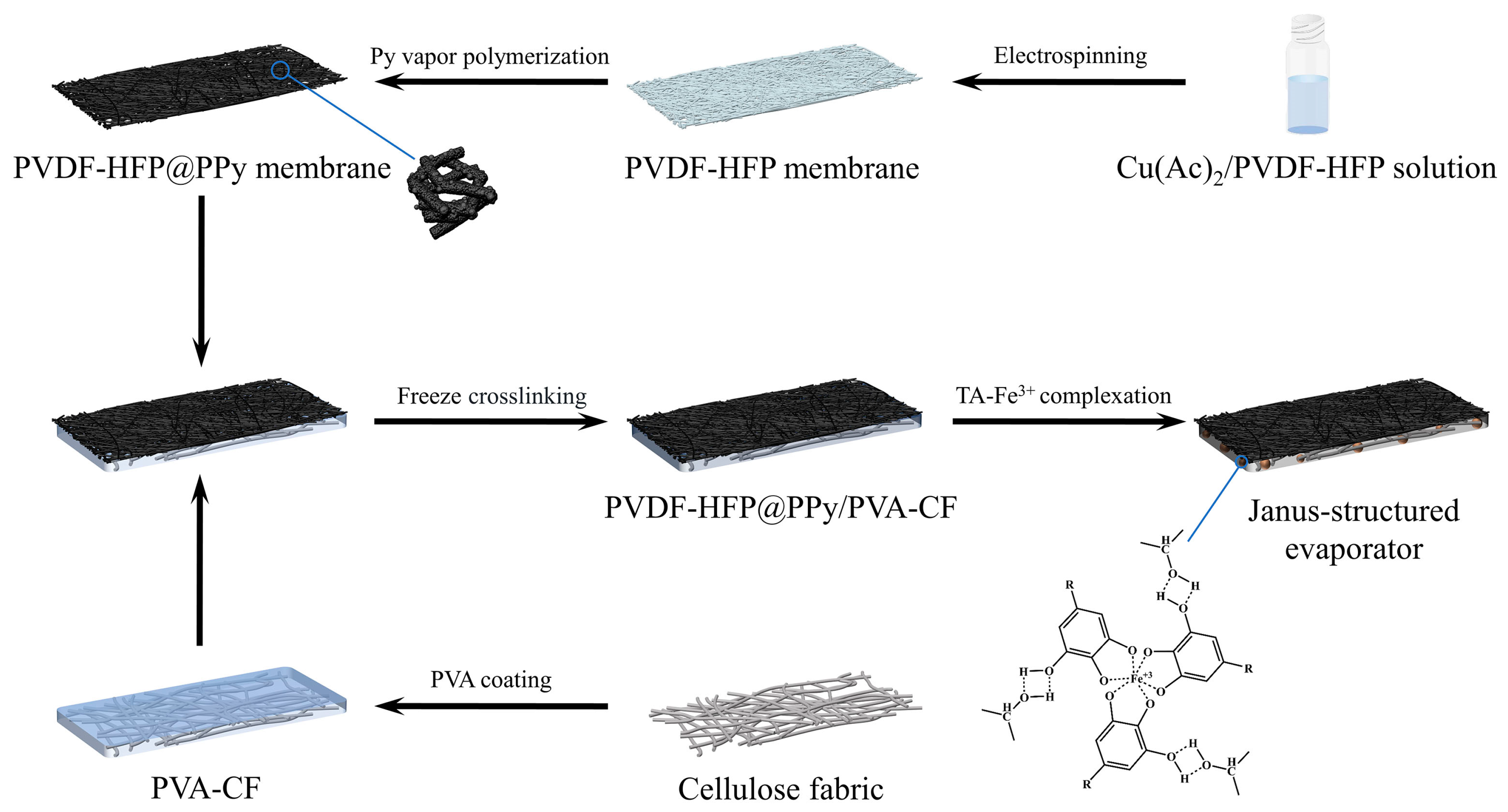
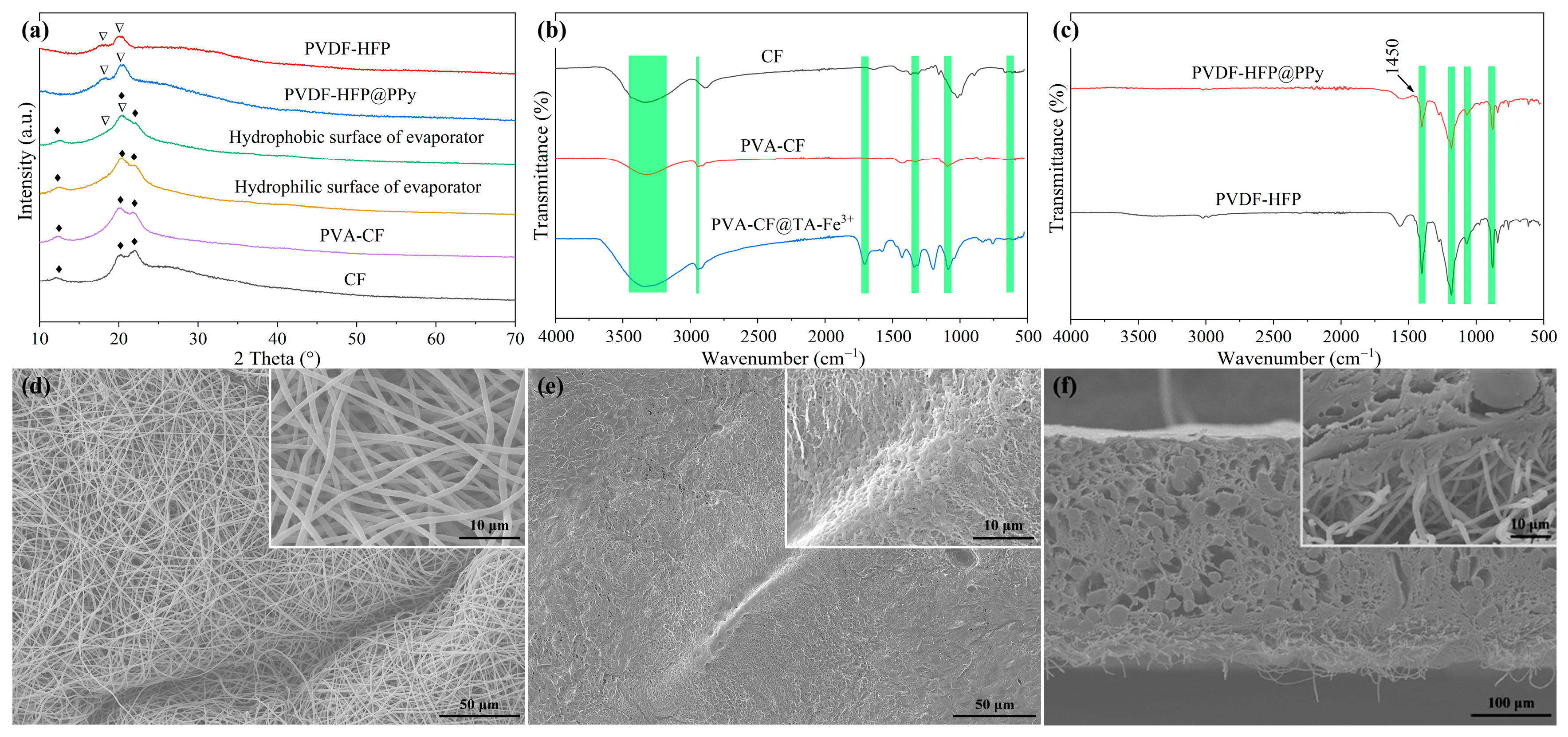
2.1. Characterization of the Janus-Structured Evaporator
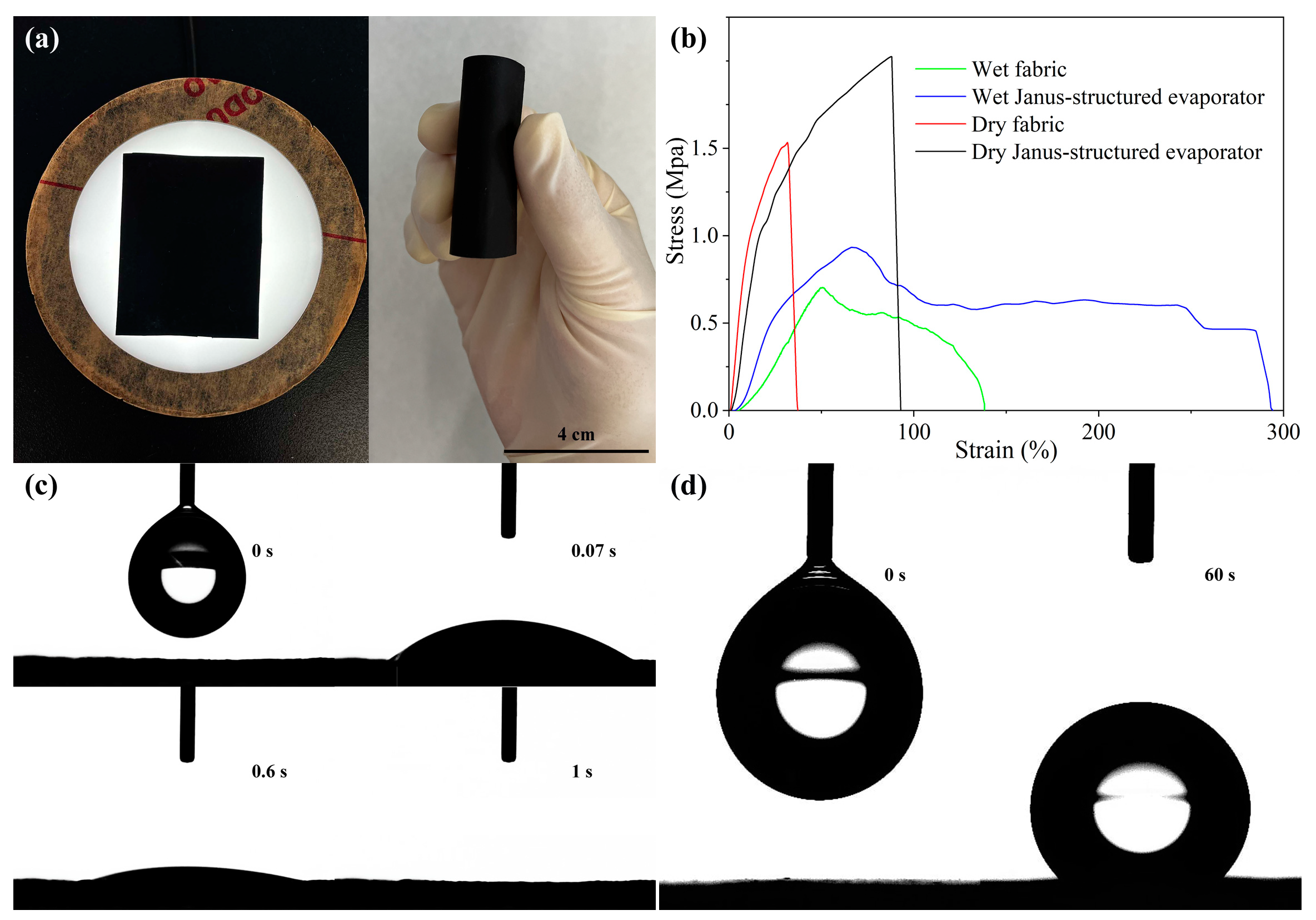
2.2. Mechanical Property and Wettability of the Janus-Structured Evaporator
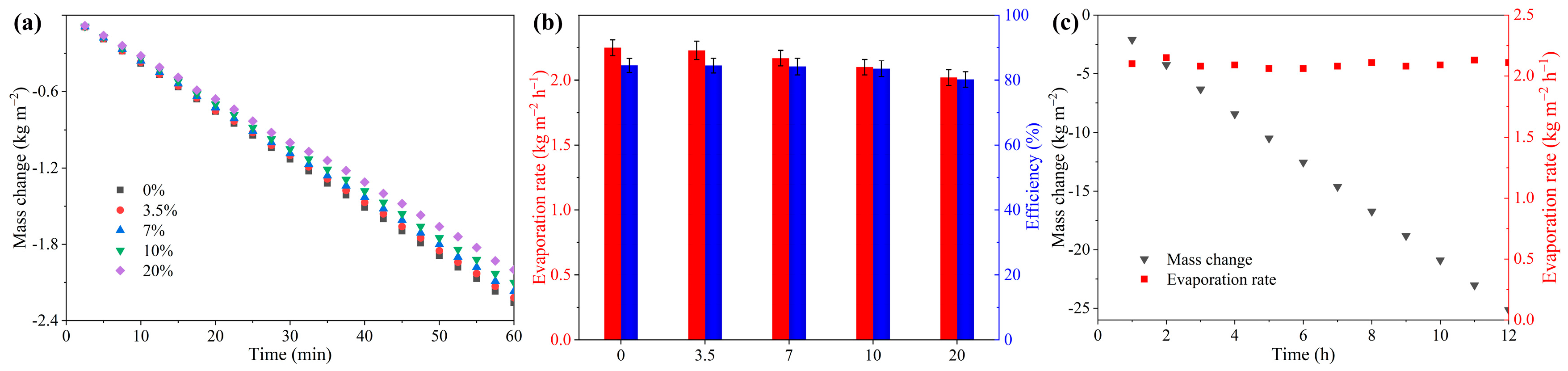
2.3. Evaporation Performance for Pure Water
2.4. Evaporation Performance for Brine
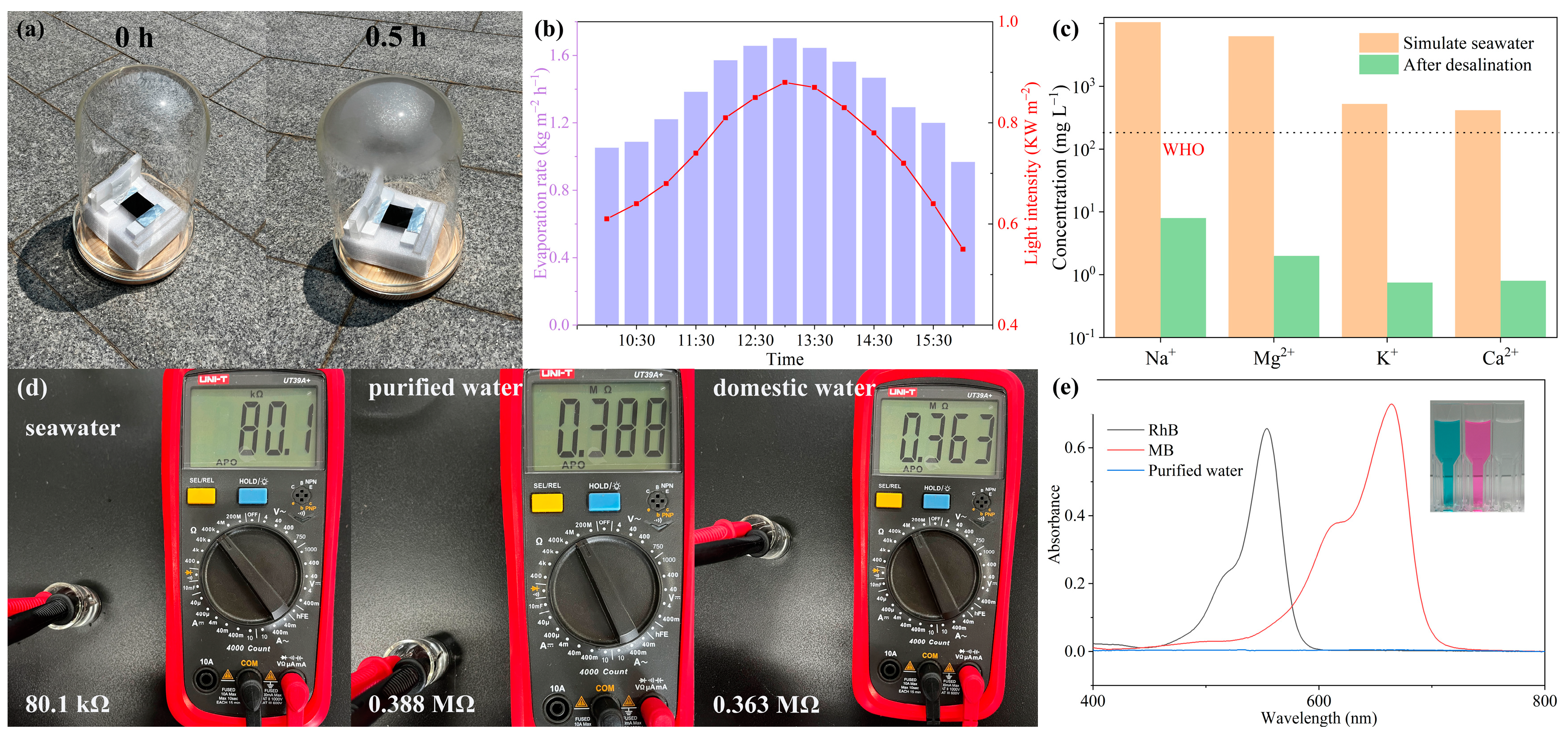
2.5. Outdoor Evaporation and Wastewater Purification Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PVA–Cellulose Fabric (PVA-CF)
4.3. Preparation of PVDF-HFP@PPy Nanofiber Membrane
4.4. Preparation of the Janus-Structured Evaporator
4.5. Characterization
4.6. Evaporation Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, S.; Yu, F.; Arshad, N.; Huang, S.J.; Tao, J.Y.; Li, C.W.; Lin, L.Y.; Qian, J.W.; Irshad, M.S.; Wang, X.B. Jellyfish–mimetic solar evaporator with polyelectrolyte skeleton for sustainable desalination under higher salinity. Desalination 2025, 593, 118209. [Google Scholar] [CrossRef]
- Miao, X.; Yang, Y.M.; Song, S.Q.; Zhu, C.Q.; Ren, G.N.; Dong, L.J.; Ge, B. The construction of composite hydrogel evaporator with interfacial heating performance and its application in water treatment. Surf. Interfaces 2023, 41, 103120. [Google Scholar] [CrossRef]
- Irshad, M.S.; Wang, X.B.; Abbasi, M.S.; Arshad, N.; Chen, Z.H.; Guo, Z.Z.; Yu, L.; Qian, J.W.; You, J.; Mei, T. Semiconductive, Flexible MnO2 NWs/Chitosan Hydrogels for Efficient Solar Steam Generation. ACS Sustain. Chem. Eng. 2021, 9, 3887–3900. [Google Scholar] [CrossRef]
- Wang, B.C.; Yang, K.; Cai, B.H.; Zhang, J.T.; Wei, C.Y.; Zhou, A.J. A magnetic nanostructure PAC@Fe3O4 driven design toward Janus hydrogel achieves highly efficient solar water evaporation. Chem. Eng. J. 2023, 465, 142944. [Google Scholar] [CrossRef]
- Wu, P.; Wu, X.; Wang, Y.D.; Xu, H.L.; Owens, G. A biomimetic interfacial solar evaporator for heavy metal soil remediation. Chem. Eng. J. 2022, 435, 134793. [Google Scholar] [CrossRef]
- Li, G.X.; Chen, Z.L.; Tang, J.S.; Chen, L.; Zhao, S.H.; Zhu, Z.L. Preparation and simulation optimization of 3D modified PVA carbon-based photothermal evaporation materials with high evaporation rate and exceptional photocatalytic effects. Surf. Interfaces 2025, 56, 105632. [Google Scholar] [CrossRef]
- Qian, Y.Q.; Xue, G.F.; Chen, L.Z.; Xu, G.; Wang, G. Conductive metal–organic framework nanosheets constructed hierarchical water transport biological channel for high-performance interfacial seawater evaporation. Adv. Mater. 2024, 36, 2310795. [Google Scholar] [CrossRef]
- Yu, N.N.; Hu, H.; Xia, W.T.; Zhao, Z.P.; Cheng, H.Y. Iron diselenide/carbon black loaded mushroom-shaped evaporator for efficiently continuous solar-driven desalination. J. Colloid Interface Sci. 2024, 658, 238–246. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Qu, Y.; Yang, B.W.; Zhang, Q.; Wang, J.; Lin, Y.M.; Chen, Z.; Lu, G.P. Bio-based tannic acid as a raw material for membrane surface modification. Desalination 2023, 555, 116535. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Jiang, Y.; Zou, X.; Xing, Q.L.; Liu, W.X.; Huang, Z.Y.; Feng, Y.W.; Wang, J.K. Biomass-based ferric tannate hydrogel with a photothermal conversion function for solar water evaporation. ACS Appl. Polym. Mater. 2023, 5, 9574–9584. [Google Scholar] [CrossRef]
- Guo, X.X.; Gao, H.; Wang, S.Y.; Yin, L.F.; Dai, Y.R. Scalable, flexible and reusable graphene oxide-functionalized electrospun nanofibrous membrane for solar photothermal desalination. Desalination 2020, 488, 114535. [Google Scholar] [CrossRef]
- Jian, H.; Qi, Q.; Wang, W.; Yu, D. A Janus porous carbon nanotubes/poly (vinyl alcohol) composite evaporator for efficient solar-driven interfacial water evaporation. Sep. Purif. Technol. 2021, 264, 118459. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Zhao, F.; Bae, J.; Rosenberger, B.; Yu, G. Synergistic energy nanoconfinement and water activation in hydrogels for efficient solar water desalination. ACS Nano. 2019, 13, 7913–7919. [Google Scholar] [CrossRef]
- Maity, S.; Yadav, M.; Patra, A.K. Polypyrrole coated textiles as photothermal material for interfacial solar evaporation. Fibers Polym. 2023, 24, 3591–3600. [Google Scholar] [CrossRef]
- Mu, X.T.; Chen, L.H.; Qu, N.N.; Yu, J.L.; Jiang, X.Q.; Xiao, C.H.; Luo, X.P.; Hasi, Q. MXene/polypyrrole coated melamine-foam for efficient interfacial evaporation and photodegradation. J. Colloid Interface Sci. 2023, 636, 291–304. [Google Scholar] [CrossRef]
- Wen, B.Y.; Zhang, X.Y.; Yan, Y.H.; Huang, Y.Q.; Lin, S.; Zhu, Y.L.; Wang, Z.P.; Zhou, B.H.; Yang, S.H.; Liu, J. Tailoring polypyrrole-based Janus aerogel for efficient and stable solar steam generation. Desalination 2021, 516, 115228. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Y.Y.; Yin, Y.; Zou, L.Q.; Chen, Q.Y.; Liu, K.; Lin, P.C.; Su, H.; Chen, Y. Janus polypyrrole nanobelt@polyvinyl alcohol hydrogel evaporator for robust solar-thermal seawater desalination and sewage purification. ACS Appl. Mater. Interfaces 2021, 13, 46717–46726. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, Z.Y.; Song, F.Y.; Huang, X.Y.; Chen, D.Y.; Nie, Z.H. Amphiphilic Janus patch-grafted hydrogels for salt-rejecting solar water desalination. J. Mater. Chem. A 2024, 12, 16933–17732. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.F.; Song, W.; Li, X.G.; Yang, L.Y.; Yan, L.G. Boosting interfacial solar steam generation by three-dimensional bilayer cellulose aerogels. J. Colloid Interface Sci. 2023, 650, 339–349. [Google Scholar] [CrossRef]
- Hao, X.; Chen, W.; Yang, Z.H.; Zhang, X.H. TiO2 nanoparticle coated carbon nanotube sponge with high sunlight absorption and good hydrophilicity for efficient interfacial solar-powered vapor generation. Surf. Interfaces 2025, 56, 105495. [Google Scholar]
- Chen, L.F.; Su, Q.; Wu, Z.F.; Wang, J.J.; Zhang, G.Q.; Xue, H.G.; Gao, J.F. Fabric interleaved composite hydrogels for high-performance solar-enabled interfacial evaporation. Sci. China Mater. 2023, 66, 2852–2862. [Google Scholar] [CrossRef]
- Fan, X.F.; Peng, Y.L.; Lv, B.W.; Yang, Y.; You, Z.J.; Song, C.W.; Xu, Y.L. A siphon-based spatial evaporation device for efficient salt-free interfacial steam generation. Desalination 2023, 552, 116442. [Google Scholar] [CrossRef]
- Liu, G.H.; Chen, T.; Xu, J.L.; Yao, G.S.; Xie, J.; Cheng, Y.P.; Miao, Z.; Wang, K.Y. Salt-Rejecting solar interfacial evaporation. Cell Rep. Phys. Sci. 2021, 2, 100310. [Google Scholar] [CrossRef]
- Lu, S.; Xiao, C.H.; Yin, M.; Jin, Y.; Xu, S.Q.; Hasi, Q.M.; Zhang, Y.H.; Chen, L.H. Double-layered solar evaporator based on polymeric ionic liquid grafted attapulgite and MXene/PPy for efficient desalination and photodegradation. Surf. Interfaces 2024, 46, 104191. [Google Scholar] [CrossRef]
- Xia, Y.; Hou, Q.F.; Jubaer, H.S.; Li, Y.; Kang, Y.; Yuan, S.; Liu, H.Y.; Woo, M.; Gao, L.L.; Wang, H.T.; et al. Spatially isolating salt crystallisation from water evaporation for continuous solar steam generation and salt harvesting. Energy Environ. Sci. 2019, 12, 1840–1847. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhong, Y.; Leroy, A.; Xu, Z.; Zhao, L.; Wang, E.N. Highly efficient and salt rejecting solar evaporation via a wick-free confined water layer. Nat. Commun. 2022, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Li, H.; Gao, L.F.; Chen, C.J.; Shi, X.W.; Du, Y.M.; Deng, H.B. Janus fibrous mats based suspended type evaporator for salt resistant solar desalination and salt recovery. Small 2022, 18, 2107156. [Google Scholar] [CrossRef]
- Yang, K.J.; Pan, T.T.; Dang, S.C.; Gan, Q.Q.; Han, Y. Three-dimensional open architecture enabling salt-rejection solar evaporators with boosted water production efficiency. Nat. Commun. 2022, 13, 6653. [Google Scholar] [CrossRef]
- Yuan, P.; Men, C.L.; Zhao, L.M.; Cao, P.; Yang, Z.P.; Niu, Y.T.; Zhang, Y.Y.; Yu, Y.Y.; Li, Q.W. Spontaneous salt-preventing solar–thermal water evaporator with a high evaporation efficiency through dual-mode water transfer. ACS Appl. Mater. Interfaces 2022, 14, 15549–15557. [Google Scholar] [CrossRef]
- Zou, M.M.; Zhang, Y.Z.; Cai, R.; Li, C.X.; Sun, Z.Y.; Yu, C.L.; Dong, Z.C.; Wu, L.; Song, Y.L. 3D printing a biomimetic bridge-arch solar evaporator for eliminating salt accumulation with desalination and agricultural applications. Adv. Mater. 2021, 33, 2102443. [Google Scholar] [CrossRef]
- Morciano, M.; Fasano, M.; Boriskina, S.V.; Chiavazzo, E.; Asinari, P. Solar passive distiller with high productivity and Marangoni effect-driven salt rejection. Energy Environ. Sci. 2020, 13, 3646–3655. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.P.; Chen, X.; Liu, M.Z.; Liu, J.; Jia, T.; Zhao, X.H. A three-dimensional arched solar evaporator based on hydrophilic photothermal fibers inspired by hair for eliminating salt accumulation with desalination application. J. Mater. Chem. A. 2022, 10, 21004–21012. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Xiong, H.T.; Qu, H.; Koh, J.J.; Nandakumar, D.K.; Wang, J.; Tan, S.C. Manipulating unidirectional fluid transportation to drive sustainable solar water extraction and brine-drenching induced energy generation. Energy Environ. Sci. 2020, 13, 4891–4902. [Google Scholar] [CrossRef]
- Gao, C.; Li, Y.M.; Lan, L.Z.; Wang, Q.; Zhou, B.G.; Chen, Y.; Li, J.C.; Guo, J.S.; Mao, J.F. Bioinspired asymmetric polypyrrole membranes with enhanced photothermal conversion for highly efficient solar evaporation. Adv. Sci. 2024, 11, 2306833. [Google Scholar] [CrossRef]
- Yang, Z.H.; Liu, Y.Y.; Xue, K.C.; Fu, P.; Du, F.P.; Zhang, Y.F. PEI-GOs/PVA photothermal sponge with enhanced interfacial solar steam generation and seawater desalination. Mater. Today Commun. 2023, 35, 106195. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, Q.; Liu, Q.; Qiu, J.X.; Li, J.H.; Zheng, W.Q.; Cao, J.; Wang, L.N.; Wang, W.; Yuan, S.T.; et al. Full-spectrum-responsive Ti4O7-PVA nanocomposite hydrogel with ultrahigh evaporation rate for efficient solar steam generation. Desalination 2024, 577, 117400. [Google Scholar] [CrossRef]
- Jing, X.Y.; Liu, F.F.; Abdiryim, T.; Liu, X. Hydrogels as promising platforms for solar-driven water evaporators. Chem. Eng. J. 2024, 479, 147519. [Google Scholar] [CrossRef]
- Wang, S.Q.; Tong, W.S.; Li, Y.N.; Zhang, P.P.; Liu, Y.L.; Chen, Y.Y.; Zhang, Y.H. Contributions of piezoelectricity and triboelectricity to a hydroxyapatite/PVDF–HFP fiber-film nanogenerator. Nano Energy 2023, 105, 108026. [Google Scholar] [CrossRef]
- Huang, W.H.; Wang, S.; Zhang, X.X.; Kang, Y.F.; Zhang, H.B.; Deng, N.; Liang, Y.; Pang, H. Universal F4-Modified strategy on metal–organic framework to chemical stabilize pvdf-hfp as quasi-solid-state electrolyte. Adv. Mater. 2023, 35, 2310147. [Google Scholar] [CrossRef]
- Huang, J.X.; Wu, C.W.; Yu, X.G.; Li, H.; Ding, S.W.; Zhang, W. Biocompatible autonomic self-healing PVA-TA hydrogel with high mechanical strength. Macromol. Chem. Phys. 2021, 222, 2100061. [Google Scholar] [CrossRef]
- Reddy, B.R.; Ahn, J.; Ahn, H.; Cho, G.; Cho, K. Low-cost and sustainable cross-linked polyvinyl alcohol–tartaric acid composite binder for high-performance lithium–sulfur batteries. ACS Appl. Energy Mater. 2023, 6, 6327–6337. [Google Scholar] [CrossRef]
- Babu, M.P.; Moodakare, S.B.; Vedarajan, R.; Ramanujam, K. Quasi-gel polymer electrolyte interfaced with electrodes through solvent-swollen poly(ethylene oxide) for high-performance lithium/lithium-ion batteries. ACS Appl. Mater. Interfaces 2024, 16, 45399–45410. [Google Scholar] [CrossRef]
- Hu, R.; Liu, P.; Zhu, W.; Guo, X.; Liu, Z.; Jin, Z.; Yang, R.; Han, J.; Tian, H.; Ma, Y.; et al. Design, fabrication, and wearable medical application of a high-resolution flexible capacitive temperature sensor based on the thermotropic phase transition composites of PEO/PVDF-HFP/H3PO4. Adv. Funct. Mater. 2025, 35, 2417205. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Yuan, Q.; Li, B.; Wu, Y.; Dou, Y.; Zhang, X.; Han, J. Low volume-expansion, insertion-type layered silicate hierarchical structure for superior storage of Li, Na, K. Adv. Funct. Mater. 2023, 33, 301914. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, X.; Liu, M.Z.; Wang, Y.P.; Feng, S.Y.; Wu, T.H. A self-floating carbon fiber-based evaporator with a novel sandwich-Janus structure for highly efficient solar-driven interfacial evaporation. Desalination 2025, 597, 118363. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.P.; Jing, D.; Yang, G.L.; Ji, J.Y.; Li, X.K. Integrated Janus evaporator with an enhanced donnan effect and thermal localization for salt-tolerant solar desalination and thermal-to-electricity generation. ACS Appl. Mater. Interfaces 2023, 15, 49892–49901. [Google Scholar] [CrossRef]
- Guo, Y.H.; Zhao, X.; Zhao, F.; Jiao, Z.H.; Zhou, X.Y.; Yu, G.H. Tailoring surface wetting states for ultrafast solar-driven water evaporation. Energy Environ. Sci. 2020, 13, 2087–2095. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.G.; Li, X.G.; Song, W.; Li, Y.F. Porous polyvinyl alcohol/biochar hydrogel induced high yield solar steam generation and sustainable desalination. J. Environ. Chem. Eng. 2022, 10, 107690. [Google Scholar] [CrossRef]
- Wei, D.; Wang, C.B.; Zhang, J.; Zhao, H.; Asakura, Y.; Eguchi, M.; Xu, X.T.; Yamauchi, Y.K. Water activation in solar-powered vapor generation. Adv. Mater. 2023, 35, 2212100. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wu, D.P.; Zhao, J.Y.; Lu, Y.; Yang, X.F.; Xu, H.L. Dual-zone photothermal evaporator for antisalt accumulation and highly efficient solar steam generation. Adv. Funct. Mater. 2021, 31, 2102618. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Shi, Y.; Qian, X.; Alexander, M.; Zhao, X.; Mendez, S.; Yang, R.; Qu, L.; Yu, G. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 2018, 13, 489–495. [Google Scholar] [CrossRef]
- Liu, H.; Chen, B.; Chen, Y.; Zhou, M.; Tian, F.; Li, Y.; Jiang, J.; Zhai, W. Bioinspired self-standing, self-floating 3D solar evaporators breaking the trade-off between salt cycle and heat localization for continuous seawater desalination. Adv. Mater. 2023, 35, 2301596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Y.; Bai, X.; Wang, S.; Liang, B.; Fu, G.; Wu, Z. Synthesis of mesoporous Fe3Si aerogel as a photo-thermal material for highly efficient and stable corrosive-water evaporation. J. Mater. Chem. A 2018, 6, 23263–23269. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Z.; Liu, P.; Fu, X.; Wang, J.; Cao, Y.; Tang, R.; Du, X.; Chen, W.; Li, S.; et al. Flatband λ-Ti3O5 towards extraordinary solar steam generation. Nature 2023, 622, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pan, G.; Yan, M.; Wang, F.; Wu, Y.; Guo, C. Janus membrane with enhanced interfacial activation for solar evaporation. J. Energy Chem. 2023, 87, 1–11. [Google Scholar] [CrossRef]
- Zhou, C.; Mei, Q.; Huang, L.; Mao, T.; Li, S.; Wang, Z.; Wan, H.; Gu, H.; Han, K. Flexible Janus black silicon photothermal conversion membranes for highly efficient solar-driven interfacial water purification. ACS Appl. Mater. Interfaces 2024, 16, 26153–26166. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, J.; Liu, Y.; Yang, L.; Wang, L.; Yu, J.; Qin, X. Reconfiguration and self-healing integrated Janus electrospinning nanofiber membranes for durable seawater desalination. Nano Res. 2022, 16, 489–495. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, H.; Xu, Y.; Chen, M.; Ai, L. Janus Co@C/NCNT photothermal membrane with multiple optical absorption for highly efficient solar water evaporation and wastewater purification. Colloids Surf. A Physicochem. Eng. Aspects 2022, 647, 128960. [Google Scholar] [CrossRef]
- Du, Y.; Wen, J.; Deng, K.; Zou, L.; Liu, X.; Liu, P.; Liu, B.; Lv, X.; Tian, W.; Ji, J. Janus film evaporator with improved light-trapping and gradient interfacial hydrophilicity toward sustainable solar-driven desalination and purification. Sep. Purif. Technol. 2023, 322, 124312. [Google Scholar] [CrossRef]
- Sui, Z.; Xue, X.; Wang, Q.; Li, M.; Zou, Y.; Zhang, W.; Lu, C. Facile fabrication of 3D Janus foams of electrospun cellulose nanofibers/rGO for high efficiency solar interface evaporation. Carbohydr. Polym. 2024, 331, 121859. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xin, Y.; Li, Z.; Sun, B.; Fan, X. Rapid preparation of Janus biomass evaporator by dielectric barrier discharge plasma for high-efficiency desalination and wastewater purification. Chem. Eng. J. 2024, 484, 149669. [Google Scholar] [CrossRef]
- Hou, L.; Wang, N.; Yu, L.; Liu, J.; Zhang, S.; Cui, Z.; Li, S.; Li, H.; Liu, X.; Jiang, L. High-Performance Janus solar evaporator for water purification with broad spectrum absorption and ultralow heat loss. ACS Energy Lett. 2023, 8, 553–564. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Xiong, L.; Wang, B.; Wang, G.; Ma, S.; Han, X. SiO2/MXene/poly(tetrafluoroethylene)-based Janus membranes as solar absorbers for solar steam generation. ACS Appl. Nano Mater. 2021, 4, 14274–14284. [Google Scholar] [CrossRef]
- Tan, X.; Cheng, Y.; Wang, S. Design of interface-stable Janus solar-energy evaporator. Int. J. Therm. Sci. 2022, 179, 107712. [Google Scholar] [CrossRef]
- Song, R.; Zhang, N.; Wang, P.; Ding, H.; Wang, J.; Li, S. A self-floating Janus PPy@Ni sponge salt-resisting solar evaporator for efficient interfacial evaporation. Appl. Surf. Sci. 2023, 616, 156448. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Hu, L.; Wang, H.; Yang, Z.; Wu, X.; Zhang, Y. Preparation of Janus-Structured Evaporators for Enhanced Solar-Driven Interfacial Evaporation and Seawater Desalination. Gels 2025, 11, 368. https://doi.org/10.3390/gels11050368
Liao J, Hu L, Wang H, Yang Z, Wu X, Zhang Y. Preparation of Janus-Structured Evaporators for Enhanced Solar-Driven Interfacial Evaporation and Seawater Desalination. Gels. 2025; 11(5):368. https://doi.org/10.3390/gels11050368
Chicago/Turabian StyleLiao, Junjie, Luyang Hu, Haoran Wang, Zhe Yang, Xiaonan Wu, and Yumin Zhang. 2025. "Preparation of Janus-Structured Evaporators for Enhanced Solar-Driven Interfacial Evaporation and Seawater Desalination" Gels 11, no. 5: 368. https://doi.org/10.3390/gels11050368
APA StyleLiao, J., Hu, L., Wang, H., Yang, Z., Wu, X., & Zhang, Y. (2025). Preparation of Janus-Structured Evaporators for Enhanced Solar-Driven Interfacial Evaporation and Seawater Desalination. Gels, 11(5), 368. https://doi.org/10.3390/gels11050368






