Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics
Abstract
1. Introduction
2. Methodology
- Studies published in the English language.
- Research articles, reviews, and clinical studies that provided significant insights into the preparation, characterization, and application of nanovesicular systems for drug delivery, especially in the context of dermatological disorders.
- Studies that were published within the time frame of 2010–2024.
- Studies that did not specifically address the use of ethanol and/or glycerin-based nanovesicular systems.
- Articles not related to dermatological drug delivery or those focused on non-relevant therapeutic areas
3. Phospholipid-Based Nanovesicles
3.1. Classical Phospholipid-Based Nanovesicles
3.2. Modified Phospholipid-Based Nanovesicles Containing Glycerol and/or Ethanol for Transdermal Drug Administration
3.3. Phospholipid Vesicular Gel Systems
3.3.1. Formulation of the Modified Types of Phospholipid Vesicular System Containing Ethanol and/or Glycerin
3.3.2. Formulation of Phospholipid Nanovesicular Gel
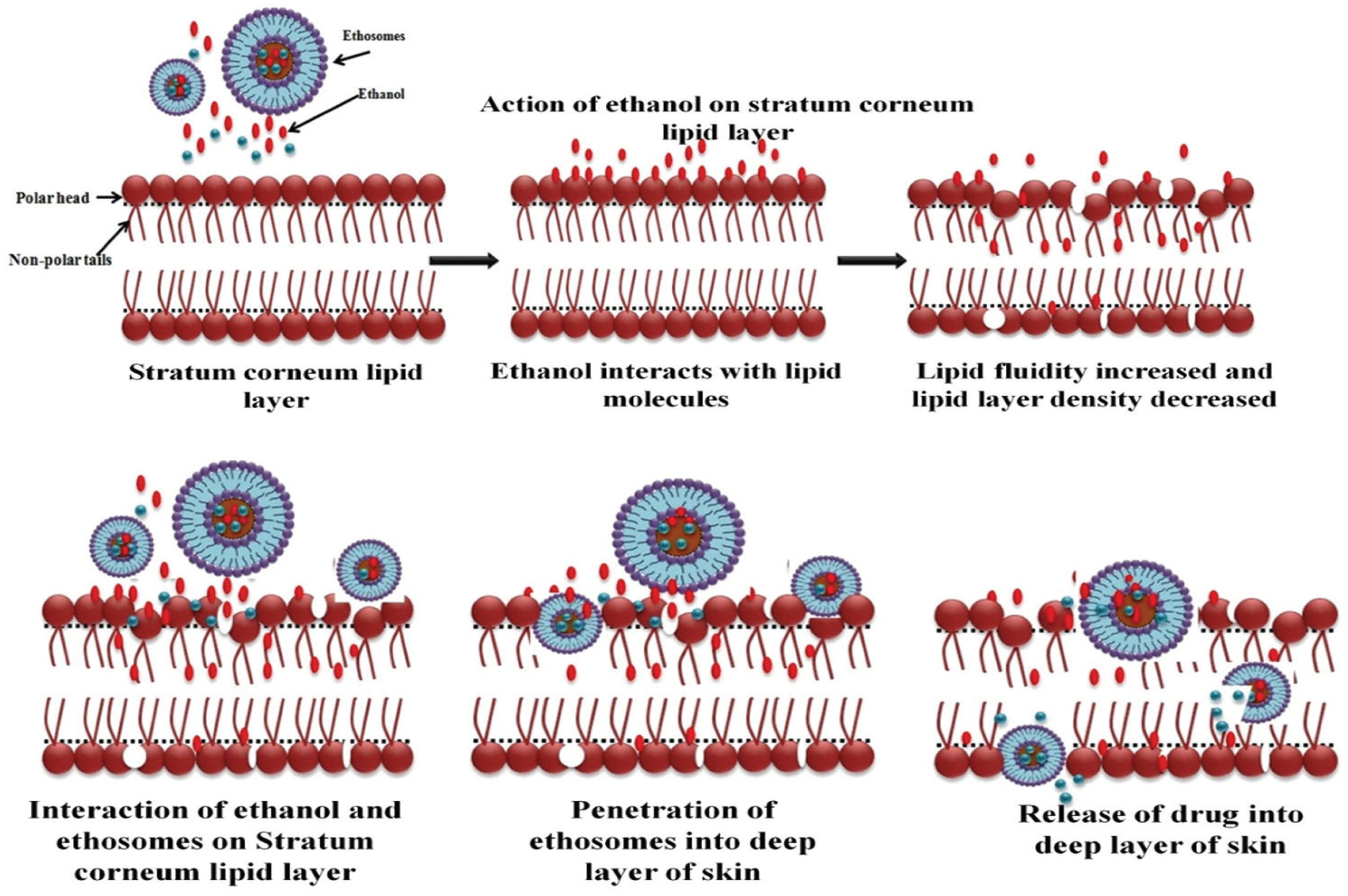
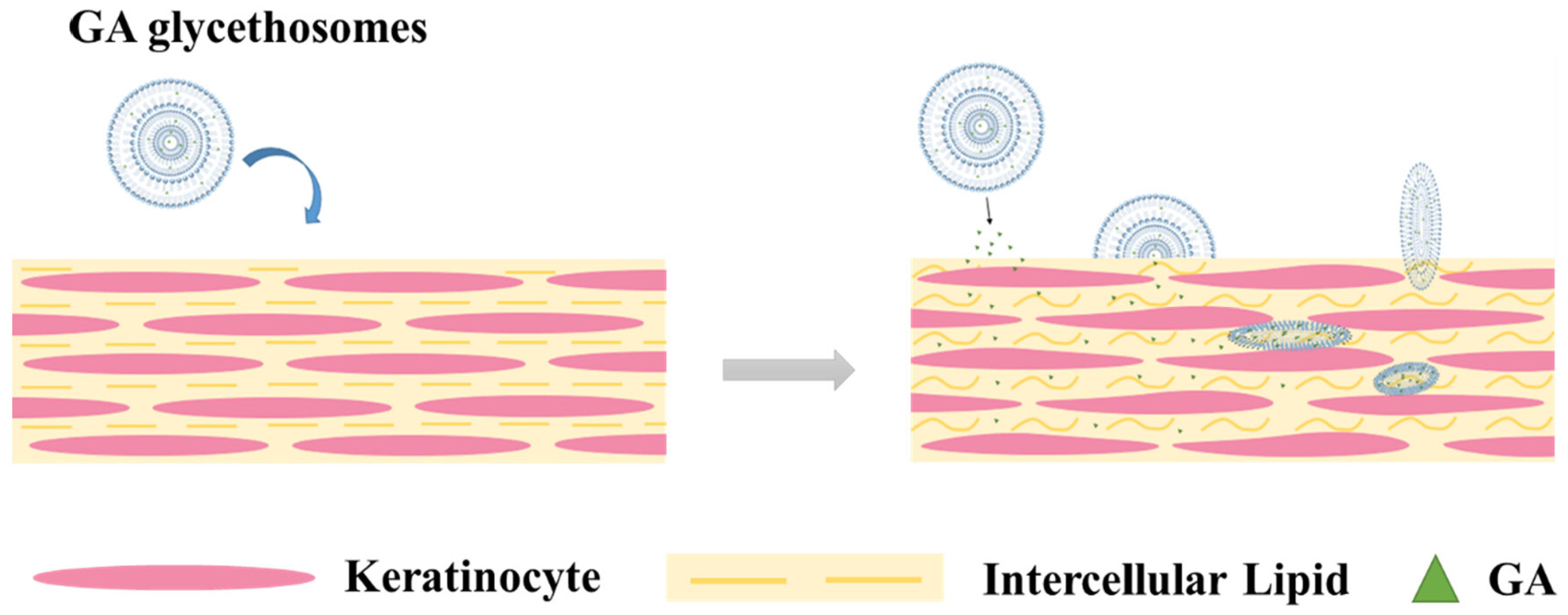
3.4. Mechanism of Actions of Phospholipid Nanovesicular Systems for Enhanced Transdermal Delivery of Drugs
3.5. Application of Phospholipid Nanovesicular Gel Systems Containing Glycerol and/or Ethanol for Transdermal Drug Administration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Zhao, W.; Ma, Q.; Gao, Y.; Wang, W.; Zhang, X.; Dong, Y.; Zhang, T.; Liang, Y.; Han, S.; et al. Functional nano-systems for transdermal drug delivery and skin therapy. Nanoscale Adv. 2023, 5, 1527–1558. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.M.; Riddell, R.P.; Taddio, A.; Racine, N.; Asmundson, G.J.; Noel, M.; Chambers, C.T.; Shah, V.; HELPinKids&Adults Team. Far from “just a poke”: Common painful needle procedures and the development of needle fear. Clin. J. Pain 2015, 31, S3–S11. [Google Scholar] [CrossRef]
- Atia, H.A.; Shahien, M.M.; Ibrahim, S.; Ahmed, E.H.; Elariny, H.A.; Abdallah, M.H. Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency. Gels 2024, 10, 525. [Google Scholar] [CrossRef]
- Gaur, P.K.; Mishra, S.; Purohit, S.; Kumar, Y.; Bhandari, A. Development of a new nanovesicle formulation as transdermal carrier: Formulation, physicochemical characterization, permeation studies and anti-inflammatory activity. Artif. Cells Nanomed. Biotechnol. 2014, 42, 323–330. [Google Scholar] [CrossRef]
- Garg, U.; Jain, K. Dermal and Transdermal Drug Delivery through Vesicles and Particles: Preparation and Applications. Adv. Pharm. Bull. 2022, 12, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef]
- Subramaniyan, G.; Rubina, S.; Ramana, B.V.; Stanley, A.M.; Srinivasan, D. Nano-Revolution in Transdermal Drug Delivery: A Bibliographic Compilation of Vesicular System. Int. J. Pharm. Investig. 2024, 14, 317–326. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Sabry, S.A.; Hasan, A.A. Enhancing transdermal delivery of glimepiride via entrapment in proniosomal gel. J. Young Pharm. 2016, 8, 335. [Google Scholar] [CrossRef]
- Ahad, H.A.; Ishaq, B.M.; Shaik, M.; Bandagisa, F. Designing and characterizing of tramadol hydrochloride transdermal patches prepared with Ficus carica fruit mucilage and povidone. Pak. J. Pharm. Sci. 2016, 29, 945–951. [Google Scholar]
- Malvey, S.; Rao, J.V.; Arumugam, K.M. Transdermal drug delivery system: A mini review. Pharma Innov. 2019, 8, 181–197. [Google Scholar]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.A.; Khafagy, E.-S.; Lila, A.S.A.; El-Horany, H.E.-S.; El-Housiny, S. Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity. Gels 2022, 8, 737. [Google Scholar] [CrossRef]
- van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, T.N. Impact of Ceramides and Penetration Enhancers on the Nanostructure of Stratum Corneum Lipid Model Membranes; Universitäts-und Landesbibliothek Sachsen-Anhalt: Halle, Germany, 2012. [Google Scholar]
- Chen, Q.; Yi, S.; Yang, L.; Zhu, L. Penetration pathways, influencing factors and predictive models for dermal absorption of exobiotic molecules: A critical review. Sci. Total Environ. 2024, 927, 172390. [Google Scholar] [CrossRef] [PubMed]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Nishchaya, K.; Rai, V.K. Nanoemulsion-based dosage forms for the transdermal drug delivery applications: A review of recent advances. Expert Opin. Drug Deliv. 2022, 19, 303–319. [Google Scholar] [CrossRef]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef]
- Burki, F.A.; Shah, K.U.; Razaque, G.; Shah, S.U.; Nawaz, A.; Saeed, M.D.; Rehman, M.U.; Bibi, H.; Alfatama, M.; Elsayed, T.M. Optimization of Chitosan-Decorated Solid Lipid Nanoparticles for Improved Flurbiprofen Transdermal Delivery. ACS Omega 2023, 8, 19302–19310. [Google Scholar] [CrossRef]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef]
- Touitou, E.; Natsheh, H. Topical administration of drugs incorporated in carriers containing phospholipid soft vesicles for the treatment of skin medical conditions. Pharmaceutics 2021, 13, 2129. [Google Scholar] [CrossRef]
- Myneni, G.S.; Radha, G.; Soujanya, G. Novel vesicular drug delivery systems: A review. J. Pharm. Res. 2021, 11, 1650–1664. [Google Scholar]
- Mosallam, S.; Albash, R.; Abdelbari, M.A. Advanced vesicular systems for antifungal drug delivery. AAPS PharmSciTech 2022, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Ahad, A.; Gupta, D.K.; Aqil, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Nanovesicles for the treatment of skin disorders. In Applications of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 285–302. [Google Scholar]
- Bseiso, E.A.; Nasr, M.; Sammour, O.; Abd El Gawad, N.A. Recent advances in topical formulation carriers of antifungal agents. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 457. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, R.; Liu, C.; Yang, C. Improved stability and skin penetration through glycethosomes loaded with glycyrrhetinic acid. Int. J. Cosmet. Sci. 2022, 44, 249–261. [Google Scholar] [CrossRef]
- Touitou, E. Compositions for Applying Active Substances to or Through the Skin. U.S. Patent 5,540,934, 30 July 1996. [Google Scholar]
- Guillot, A.J.; Martínez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin drug delivery using lipid vesicles: A starting guideline for their development. J. Control. Release 2023, 355, 624–654. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release Off. J. Control. Release Soc. 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Ainbinder, D.; Touitou, E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. 2005, 12, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Vasava, P.; Khan, S.L.; Siddiqui, F.A.; Islam, F.; Chopra, H.; Emran, T.B. Ethosomes: A novel drug carrier. Ann. Med. Surg. 2022, 82, 104595. [Google Scholar] [CrossRef]
- Shinde, P.; Page, A.; Bhattacharya, S. Ethosomes and their monotonous effects on skin cancer disruption. Front. Nanotechnol. 2023, 5, 1087413. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Wu, Z.-H.; Zhao, J.-H.; Feng, N.-P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int. J. Pharm. 2014, 471, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ç.; Şeker Karatoprak, G.; Değim, İ.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef]
- Zhan, B.; Wang, J.; Li, H.; Xiao, K.; Fang, X.; Shi, Y.; Jia, Y. Ethosomes: A Promising Drug Delivery Platform for Transdermal Application. Chemistry 2024, 6, 993–1019. [Google Scholar] [CrossRef]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N.-P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef]
- Aljohani, A.A.; Alanazi, M.A.; Munahhi, L.A.; Hamroon, J.D.; Mortagi, Y.; Qushawy, M.; Soliman, G.M. Binary ethosomes for the enhanced topical delivery and antifungal efficacy of ketoconazole. OpenNano 2023, 11, 100145. [Google Scholar] [CrossRef]
- Saadallah, M.N.; Almajidi, Y.Q.; Ali, A. Binary Ethosomal Gel for Enhanced Transdermal Delivery of Tazarotene: Development, Refinement, in vitro Evaluation, and Skin Penetration Investigations. Al-Rafidain J. Med. Sci. 2023, 5, S42–S50. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Wei, Y.-H.; Zhou, Y.; Li, Y.-Q.; Wu, X.-A. Ethosomes, binary ethosomes and transfersomes of terbinafine hydrochloride: A comparative study. Arch. Pharmacal Res. 2012, 35, 109–117. [Google Scholar] [CrossRef]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.; Simões, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef]
- Esposito, E.; Pecorelli, A.; Ferrara, F.; Lila, M.A.; Valacchi, G. Feeding the Body Through the Skin: Ethosomes and Transethosomes as a New Topical Delivery System for Bioactive Compounds. Annu. Rev. Food Sci. Technol. 2024, 15, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Afzal, O.; Altamimi, A.S.A.; Alamri, M.A.; Haider, T.; Faheem Haider, M. Development and optimization of transethosomal gel of apigenin for topical delivery: In-vitro, ex-vivo and cell line assessment. Int. J. Pharm. 2023, 631, 122506. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.; Khafagy, E.-S.; Shawky, S.; El-Horany, H.E.-S.; El-Housiny, S. Development and optimization of erythromycin loaded transethosomes cinnamon oil based emulgel for antimicrobial efficiency. Gels 2023, 9, 137. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and optimization of fisetin loaded glycerol based soft nanovesicles by Box-Behnken design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Gupta, P.; Mazumder, R.; Padhi, S. Glycerosomes: Advanced Liposomal Drug Delivery System. Indian J. Pharm. Sci. 2020, 82, 385–397. [Google Scholar] [CrossRef]
- Sharma, D.; Rani, A.; Singh, V.D.; Shah, P.; Sharma, S.; Kumar, S. Glycerosomes: Novel Nano-Vesicles for Efficient Delivery of Therapeutics. Recent Adv. Drug Deliv. Formul. Former. Recent Pat. Drug Deliv. Formul. 2023, 17, 173–182. [Google Scholar] [CrossRef]
- Younes, N.F.; Habib, B.A. Augmented local skin accumulation efficiency of sertaconazole nitrate via glycerosomal hydrogel: Formulation, statistical optimization, ex vivo performance and in vivo penetration. J. Drug Deliv. Sci. Technol. 2022, 72, 103364. [Google Scholar] [CrossRef]
- Shahien, M.M.; Alshammari, A.; Ibrahim, S.; Ahmed, E.H.; Atia, H.A.; Elariny, H.A.; Abdallah, M.H. Development of Glycerosomal pH Triggered In Situ Gelling System to Ameliorate the Nasal Delivery of Sulpiride for Pediatric Psychosis. Gels 2024, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Melis, V.; Manca, M.L.; Bullita, E.; Tamburini, E.; Castangia, I.; Cardia, M.C.; Valenti, D.; Fadda, A.M.; Peris, J.E.; Manconi, M. Inhalable polymer-glycerosomes as safe and effective carriers for rifampicin delivery to the lungs. Colloids Surf. B Biointerfaces 2016, 143, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Sultana, N.; Rashid, M.A.; Alhamhoom, Y.; Ali, A.; Waheed, A.; Ansari, M.S.; Aqil, M.; Mujeeb, M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels 2023, 9, 752. [Google Scholar] [CrossRef]
- Vitonyte, J.; Manca, M.L.; Caddeo, C.; Valenti, D.; Peris, J.E.; Usach, I.; Nacher, A.; Matos, M.; Gutiérrez, G.; Orrù, G. Bifunctional viscous nanovesicles co-loaded with resveratrol and gallic acid for skin protection against microbial and oxidative injuries. Eur. J. Pharm. Biopharm. 2017, 114, 278–287. [Google Scholar] [CrossRef]
- Manca, M.L.; Peris, J.E.; Melis, V.; Valenti, D.; Cardia, M.C.; Lattuada, D.; Escribano-Ferrer, E.; Fadda, A.M.; Manconi, M. Nanoincorporation of curcumin in polymer-glycerosomes and evaluation of their in vitro–in vivo suitability as pulmonary delivery systems. RSC Adv. 2015, 5, 105149–105159. [Google Scholar] [CrossRef]
- Abdallah, M.H.; El-Horany, H.E.-S.; El-Nahas, H.M.; Ibrahim, T.M. Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route. Pharmaceutics 2023, 15, 2521. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, Z.; Wang, L.; Lei, F.; Wang, Z.; Ma, X.; Gong, Z.; Wang, J.; He, J.; Wang, D. Preparation of Paeonol Ethosomes by Microfluidic Technology Combined with Gaussians and Evaluation of Biological Activity by Zebrafish. ACS Omega 2024, 9, 44425–44435. [Google Scholar] [CrossRef]
- Cheng, Y.; Hay, C.D.; Mahuttanatan, S.M.; Hindley, J.W.; Ces, O.; Elani, Y. Microfluidic technologies for lipid vesicle generation. Lab. Chip 2024, 24, 4679–4716. [Google Scholar] [CrossRef]
- Manca, M.L.; Cencetti, C.; Matricardi, P.; Castangia, I.; Zaru, M.; Sales, O.D.; Nacher, A.; Valenti, D.; Maccioni, A.M.; Fadda, A.M.; et al. Glycerosomes: Use of hydrogenated soy phosphatidylcholine mixture and its effect on vesicle features and diclofenac skin penetration. Int. J. Pharm. 2016, 511, 198–204. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Diez-Sales, O.; Manca, M.L.; Manconi, M.; Sauri, A.R.; Escribano-Ferrer, E.; Nácher, A. Mangiferin glycethosomes as a new potential adjuvant for the treatment of psoriasis. Int. J. Pharm. 2020, 573, 118844. [Google Scholar] [CrossRef]
- Breitsamer, M.; Winter, G. Vesicular phospholipid gels as drug delivery systems for small molecular weight drugs, peptides and proteins: State of the art review. Int. J. Pharm. 2019, 557, 1–8. [Google Scholar] [CrossRef]
- Brandl, M. Vesicular phospholipid gels: A technology platform. J. Liposome Res. 2007, 17, 15–26. [Google Scholar] [CrossRef]
- Massing, U.; Cicko, S.; Ziroli, V. Dual asymmetric centrifugation (DAC)—A new technique for liposome preparation. J. Control. Release 2008, 125, 16–24. [Google Scholar] [CrossRef]
- Ali, S.; Koehler, J.K.; Silva, L.; Gedda, L.; Massing, U.; Edwards, K. Dual centrifugation as a novel and efficient method for the preparation of lipodisks. Int. J. Pharm. 2024, 653, 123894. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, L.; Zhang, Y.; Li, W.; Sun, X.; Gong, T.; Zhang, Z. Vesicular phospholipid gels using low concentrations of phospholipids for the sustained release of thymopentin: Pharmacokinetics and pharmacodynamics. Die Pharm. 2013, 68, 811–815. [Google Scholar]
- Abdallah, M.H.; Shahien, M.M.; Alshammari, A.; Ibrahim, S.; Ahmed, E.H.; Atia, H.A.; Elariny, H.A. The Exploitation of Sodium Deoxycholate-Stabilized Nano-Vesicular Gel for Ameliorating the Antipsychotic Efficiency of Sulpiride. Gels 2024, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Shawky, S.; Shahien, M.M.; El-Horany, H.E.; Ahmed, E.H.; El-Housiny, S. Development and Evaluation of Nano-Vesicular Emulsion-Based Gel as a Promising Approach for Dermal Atorvastatin Delivery Against Inflammation. Int. J. Nanomed. 2024, 19, 11415–11432. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Lila, A.S.A.; Anwer, M.K.; Khafagy, E.-S.; Mohammad, M.; Soliman, M.S. Formulation, Development and Evaluation of Ibuprofen Loaded Nano-transferosomal Gel for the Treatment of Psoriasis. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Elsewedy, H.S.; AbuLila, A.S.; Almansour, K.; Unissa, R.; Elghamry, H.A.; Soliman, M.S. Quality by design for optimizing a novel liposomal jojoba oil-based emulgel to ameliorate the anti-inflammatory effect of brucine. Gels 2021, 7, 219. [Google Scholar] [CrossRef]
- Akl, M.A.; Ryad, S.; Ibrahim, M.F.; Kassem, A.A. Formulation, and optimization of transdermal Atorvastatin Calcium-Loaded Ultra-flexible vesicles; ameliorates poloxamer 407-caused dyslipidemia. Int. J. Pharm. 2023, 638, 122917. [Google Scholar] [CrossRef]
- Hemati, H.; Haghiralsadat, F.; Hemati, M.; Sargazi, G.; Razi, N. Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing. Gels 2023, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Gilani, S.J.; Zafar, A.; Jumah, M.N.B.; Alshehri, S. Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics 2023, 15, 581. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Shitole, M.; Nangare, S.; Patil, U.; Jadhav, N.R. Review on drug delivery applications of ethosomes: Current developments and prospects. Thai J. Pharm. Sci. 2022, 46, 251–265. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Zaru, M.; Valenti, D.; Peris, J.E.; Matricardi, P.; Maccioni, A.M.; Fadda, A.M. Glycerosomes: Investigation of role of 1,2-dimyristoyl-sn-glycero-3-phosphatidycholine (DMPC) on the assembling and skin delivery performances. Int. J. Pharm. 2017, 532, 401–407. [Google Scholar] [CrossRef]
- Pham, Q.D.; Björklund, S.; Engblom, J.; Topgaard, D.; Sparr, E. Chemical penetration enhancers in stratum corneum—Relation between molecular effects and barrier function. J. Control. Release 2016, 232, 175–187. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Li, Z.; Li, N.; Feng, N. Essential oil-mediated glycerosomes increase transdermal paeoniflorin delivery: Optimization, characterization, and evaluation in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3521–3532. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Nahar, M.; Jain, V.; Jain, N.K. Enhanced transdermal delivery of an anti-HIV agent via ethanolic liposomes. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 590–596. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Wu, Z.-H.; Zhao, J.-H.; Feng, N.-P. Evaluation of skin viability effect on ethosome and liposome-mediated psoralen delivery via cell uptake. J. Pharm. Sci. 2014, 103, 3120–3126. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Li, X.; Chen, S.-Y.; Huang, L.-Y.; Bian, Y.-Y.; Wang, J.; Shu, Y.-T.; Yan, G.-J.; Dong, J.; et al. Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int. J. Pharm. 2021, 592, 119936. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Barry, B.W.; Williams, A. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Qadir, A.; Warsi, M.H.; Mujeeb, M.; Aqil, M.; Mir, S.R.; Sharma, S. Ethosomes-based gel formulation of karanjin for treatment of acne vulgaris: In vitro investigations and preclinical assessment. 3 Biotech 2021, 11, 456. [Google Scholar] [CrossRef]
- Ajikumar, A.; Dharan, S.S.; Chandra, S.M.; Soman, M.; Mathew, L.T. Formulation and evaluation of Ethosomal gel containing clarithromycin for the treatment of acne vulgaris. J. Pharm. Sci. Res. 2022, 14, 825–833. [Google Scholar]
- Shetty, S.; Jose, J.; Kumar, L.; Charyulu, R.N. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J. Cosmet. Dermatol. 2019, 18, 862–869. [Google Scholar] [CrossRef]
- Gupta, P.; Kushwaha, P.; Hafeez, A. Development and characterization of topical ethosomal gel for improved antifungal therapeutics. J. Mol. Liq. 2024, 405, 125111. [Google Scholar] [CrossRef]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal drug delivery systems: An update review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, W.; Ba, W.; Li, H.; Fan, J. Preparation and partial pharmacodynamic studies of luliconazole ethosomes. Clin. Exp. Pharmacol. Physiol. 2022, 49, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Y.; Shahid, H.; Ahmed, S.; Khursheed, A.; Jamshaid, T.; Jamshaid, M.; Mengistie, A.A.; Dawoud, T.M.; Siddique, F. Synthesis of vitamin D3 loaded ethosomes gel to cure chronic immune-mediated inflammatory skin disease: Physical characterization, in vitro and ex vivo studies. Sci. Rep. 2024, 14, 23866. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameri, A.A.F.; Al-Gawhari, F.J. Formulation Development of Meloxicam Binary Ethosomal Hydrogel for Topical Delivery: In Vitro and In Vivo Assessment. Pharmaceutics 2024, 16, 898. [Google Scholar] [CrossRef]
- Halagali, P.; Wannur, V.I.; Patil, A.K.A.; Torgal, V.D.; Naik, S.M.; Marennavar, S.A.; Shahapurmath, S.; Patil, P. Formulation and Evaluation of Quercetin Ethosomal Hydrogel for Topical Delivery System. Int. J. Pharm. Investig. 2024, 14, 749–758. [Google Scholar] [CrossRef]
- Valsalan Soba, S.; Babu, M.; Panonnummal, R. Ethosomal Gel Formulation of Alpha Phellandrene for the Transdermal Delivery in Gout. Adv. Pharm. Bull. 2021, 11, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.B.; Alqahtani, A.; Lakshmi, S.; Gnanaprakash, K.; Kumarappan, C. Studies on formulation development and evaluation of tolnaftate-loaded glycerosomes. Mater. Res. Innov. 2021, 25, 233–242. [Google Scholar] [CrossRef]
- Johl, S.; Bhattacharyya, S. Novel Deformable Vesicle for the Transdermal Delivery of Terbinafine Hydrochloride-Formulation and Cytotoxic Evaluation. Ind. J. Pharm. Edu. Res. 2024, 58, 460–469. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Abdel Hakim, L.F. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box–Behnken statistical design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wu, T.; He, Z.; Guo, T.; Feng, N. Optimizing glycerosome formulations via an orthogonal experimental design to enhance transdermal triptolide delivery. Acta Pharm. 2022, 72, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Shadab; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Khan, N.; Alfaleh, M.A.; Asfour, H.Z.; Riadi, Y.; Bilgrami, A.L.; Akhter, M.H. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers 2021, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Huanbutta, K.; Rattanachitthawat, N.; Luangpraditkun, K.; Sriamornsak, P.; Puri, V.; Singh, I.; Sangnim, T. Development and Evaluation of Ethosomes Loaded with Zingiber zerumbet Linn Rhizome Extract for Antifungal Skin Infection in Deep Layer Skin. Pharmaceutics 2022, 14, 2765. [Google Scholar] [CrossRef] [PubMed]
- Rattanachitthawat, N.; Sriamornsak, P.; Puri, V.; Singh, I.; Huanbutta, K.; Sangnim, T. Bioactivity assessment of Zingiber zerumbet Linn rhizome extract for topical treatment of skin diseases. J. Appl. Pharm. Sci. 2023, 13, 168–174. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Khalid, M. Development of Piperine-Loaded Soft Binary Ethosomal Gel to Improve Transdermal Delivery: Box-Bhekhen Design Optimization, Ex-Vivo Permeation, and Antimicrobial Evaluation. J. Clust. Sci. 2024, 35, 311–325. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.; Srivastava, D.; Fatima, Z.; Prasad, R. Crisaborole-Enthused Glycerosomal Gel for an Augmented Skin Permeation. Recent Adv. Drug Deliv. Formul. Former. Recent Pat. Drug Deliv. Formul. 2024, 18, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Das, S.; Do, C.; Park, Y.C. Effect of Cholesterol on Nano-Structural Alteration of Light-Activatable Liposomes via Laser Irradiation: Small Angle Neutron Scattering Study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128548. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Wonglertnirant, N.; Songprakhon, P.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Visualization of ultradeformable liposomes penetration pathways and their skin interaction by confocal laser scanning microscopy. Int. J. Pharm. 2013, 441, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]




| Active Molecules | Indication | Composition | Method of Preparation | Key Findings | References |
|---|---|---|---|---|---|
| Karanjin | Acne vulgaris | Phospholipids 90 G (30 mg, w/w) and drug karanjin (3% w/v) were dissolved in chloroform–methanol (2:1, v/v)/isotonic phosphate buffer–ethanol solution (25:75) was used as hydrating medium | Film hydration method | The produced ethosomal gel has physicochemical features suitable for dermal medicinal drug delivery; the ethomal gel showed the significant antibacterial and antioxidant properties of karanjin. | [85] |
| Clarithromycin | Acne vulgaris | Phospholipid, ethanol, propylene glycol, drug, and distilled water | Cold method | The study concluded that the ethosomal gel of clarithromycin could effectively improve the bioavailability of the drug by penetration enhancement, reduce the frequency of administration, give better patient compliance and also follow a sustained drug release mechanism. | [86] |
| Clove oil | Cutaneous candidiasis | Soyaphosphotidyl choline 1–4%, 1–50% ethanol, 0.24 mL of the drug, and aqueous phase up to 100% w/w | Cold method | The ethosomal gel increased drug penetration through skin, thereby reducing the dose, minimizing frequent application, and preventing adverse effects. | [87] |
| Eberconazole nitrate | Antifungal | Phosphatidylcholine (PC), ethanol, and propylene glycol (PG) | Cold method | The ethosome-loaded hydrogel demonstrated controlled drug release with higher a skin permeation and skin retention of the drug. | [88] |
| Zingiber zerumbet (L.) Smith. rhizome extract | Antifungal skin infection | 1% (w/v) phosphatidylcholine and 40% (v/v) ethanol | Cold method | The ethosome system significantly enhanced the skin penetration and retention of the active compound. | [89] |
| Luridazole | Antifungal | 5% (w/v) lecithin, 45% (v/v) ethanol | Thin-film hydration | Ethosomal formulation had minimal skin irritation, a better permeation effect, and antifungal activity. | [90] |
| Vitamin D3 | Psoriasis | Soya lecithin 1–8% (w/v), propylene glycol, and ethanol | Cold process was used with a little bit of modification | Ethosomes loaded with vitamin D3 were successfully prepared and then converted into gel for patients’ easy application. The generated formulation containing vitamin D3 could be useful in overcoming psoriasis. | [91] |
| Meloxicam | Arthirities | Soya lecithin, ethanol, and propylene glycol | Hot technique | Meloxicam was successfully formulated as ethosomal hydrogel for topical delivery, introducing a promising approach that increases drug skin permeability and efficacy over the classical type. | [92] |
| Quercetin | Anti-inflammatory and anti-allergy properties | Phospholipid (soya lecithin), cholesterol, and ethanol (20% to 40%) | Hot technique | The developed ethosomal gel could function as an efficient transdermal delivery system for quercetin in the management of inflammation. | [93] |
| Brucine | Anti-inflammatory effect | Lecithin, cholesterol, and ethanol | Thin film hydration method | The developing ethosomes were successfully used, offering a promising approach for the transdermal delivery of brucine. | [29] |
| Alpha Phellandrene | Gout | Soya lecithin, cholesterol, ethanol, and propylene | Cold approach | The enhanced skin penetration with deposition indirectly showed the formulation’s topical efficacy. | [94] |
| Tolnaftate | Antifungal | Hydrogenated soy phosphatidyl choline (HSPC), dipalmitoylphosphatidyl choline (DPPC), distearoylphosphatidyl choline (DSPC), cholesterol, glycerol | Thin film hydration method | Glycerosomes confirmed the effective ability to improve the topical delivery of tolnaftate. The diffusibility of tolnaftate through glycerosomes was appreciable when compared to control liposomes. | [95] |
| Terbinafine hydrochloride | Mycoses | Phospholipid, cholesterol, and glycerol–water mixture | Thin film hydration method | Glycerosomes could be a potential formulation approach for treating dermal infection and offer a better alternative to commercially available products. | [96] |
| Rutin | Inflammation associated with sunburn | Phospholipid 90 G, cholesterol, glycerol, and water | Thin film hydration method | Suitable alternation for topical administration of drug to maximize the therapeutic efficacy of the drugs. | [56] |
| Paeoniflorin | Rheumatoid arthritis | Lipoid S 80, cholesterol, glycerol, and water | Reverse-phase evaporation method | Superior transdermal flux; safe and applicable vehicle for the treatment of rheumatoid arthritis. | [80] |
| Celecoxib and Cupferron | Inflammatory illnesses | Soybean phosphatidyl choline, cholesterol, glycerol, Tween 80, and water | Hydration film methods | The study confirmed that the anti-inflammatory effect of topically applied drug-loaded glycerosomal gel formulations can have a profound therapeutic application in topical delivery for the treatment of inflammation. | [97] |
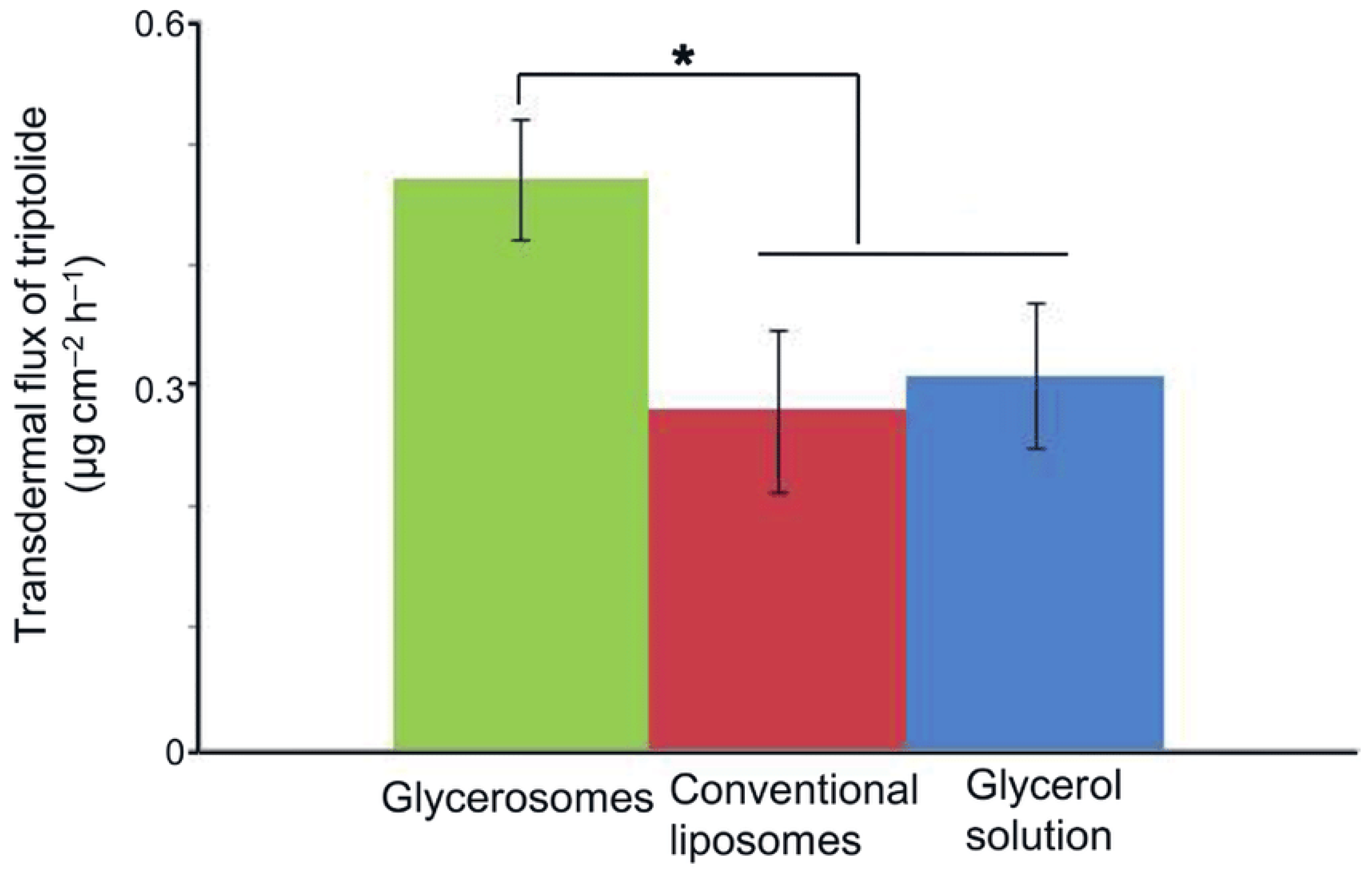
| Triptolide | Various immune and inflammatory diseases and rheumatoid arthritis | Soy lecithin, cholesterol, glycerol | Injection method | The optimized glycerosome formulation would increase triptolide transdermal permeability compared to liposomes. | [98] |
| Plumbagin | Skin cancer | Phospholipid 90 G, cholesterol, glycerol–water mixture (30% w/v glycerol) | Thin film hydration technique | The developed formulation exhibited sustained release and gave excellent flux across various strata of cutaneous layer. | [99] |
| Gycyrrhetinic acid | Anti-inflammatory and antioxidant | Soy phosphatidylcholine (PC60), cholesterol, ethanol, glycerol and water | Ethanol injection and sonication method | Glycethosomes could effectively enhance the fluidity of lipids in the stratum corneum and reduce the skin barrier resistance so as to achieve the effect of promoting transdermal permeability. | [26] |
| Risperidone | Schizophrenia | Epikuron 200 phospholipid, ethanol, glycerin, and propylene glycol, cholesterol | Ethanol injection and sonication method | The optimized glycethosome gel boosted RS. | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.H.; Shahien, M.M.; El-Horany, H.E.-S.; Ahmed, E.H. Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics. Gels 2025, 11, 358. https://doi.org/10.3390/gels11050358
Abdallah MH, Shahien MM, El-Horany HE-S, Ahmed EH. Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics. Gels. 2025; 11(5):358. https://doi.org/10.3390/gels11050358
Chicago/Turabian StyleAbdallah, Marwa H., Mona M. Shahien, Hemat El-Sayed El-Horany, and Enas Haridy Ahmed. 2025. "Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics" Gels 11, no. 5: 358. https://doi.org/10.3390/gels11050358
APA StyleAbdallah, M. H., Shahien, M. M., El-Horany, H. E.-S., & Ahmed, E. H. (2025). Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics. Gels, 11(5), 358. https://doi.org/10.3390/gels11050358







