Novel Gels for Post-Piercing Care: Evaluating the Efficacy of Pranoprofen Formulations in Reducing Inflammation
Abstract
1. Introduction
2. Results and Discussion
2.1. Gels Formulation
2.2. Physicochemical Characterization of the Gels
2.2.1. PH and Morphological Analysis
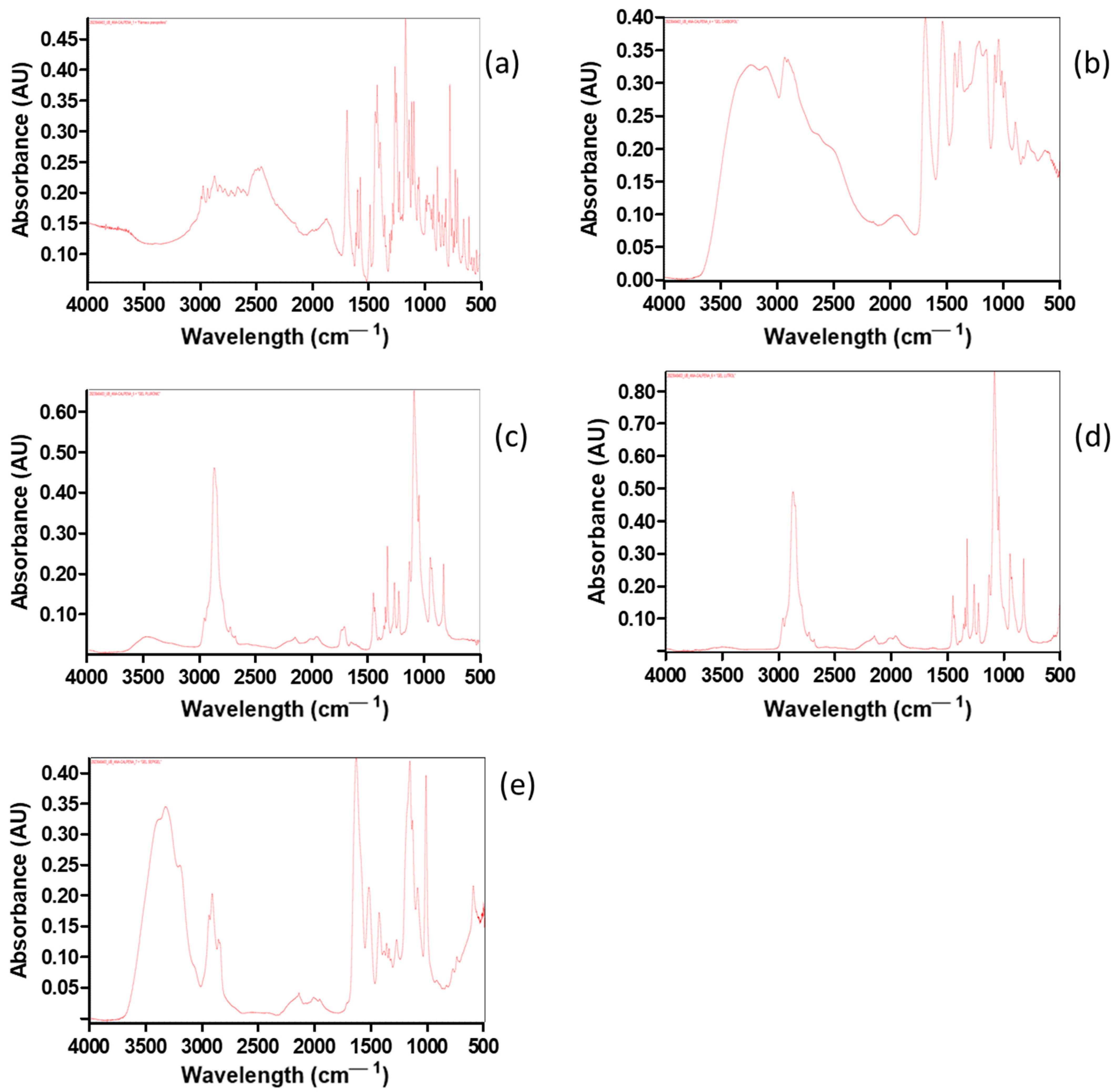
2.2.2. Fourier Transform Infrared (FT-IR) Spectroscopy
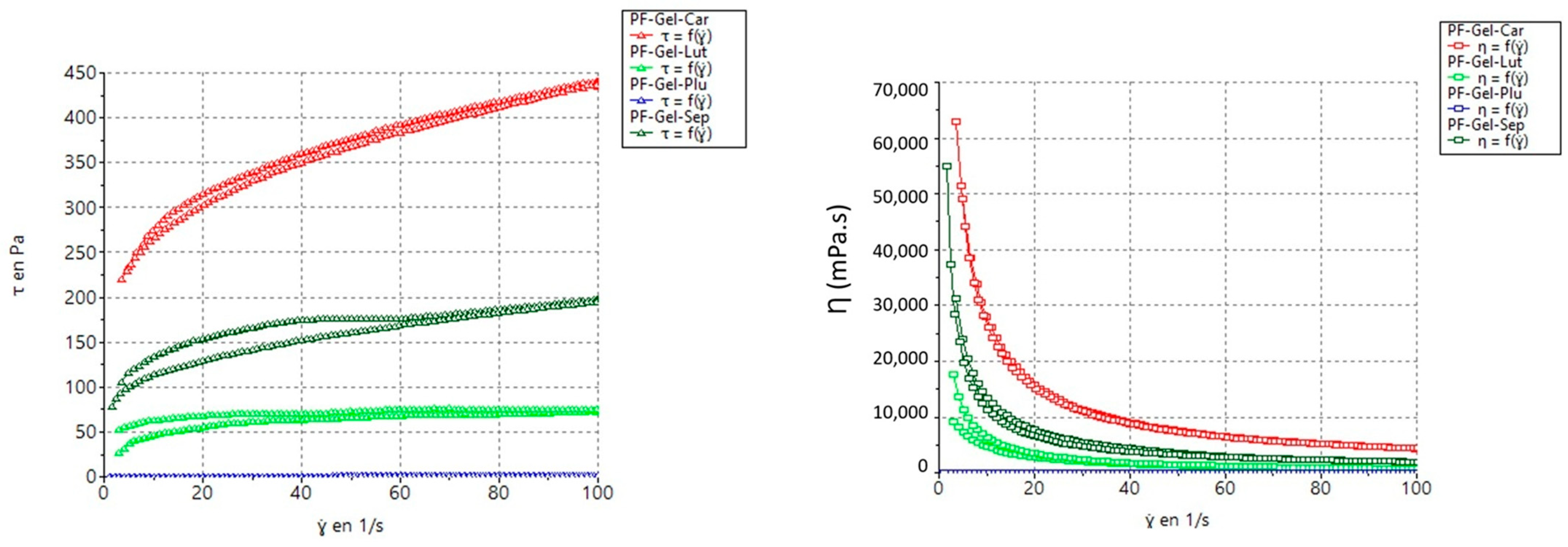
2.2.3. Rheological Measurements of the Gels
| Sample | Model | Consistency (K) K (Pa·s) | Flow Index (n) | Yield Stress (τ0) (Pa) |
|---|---|---|---|---|
| PF-GEL-LUT | Cross | 30.57 | 0.1949 | — |
| PF-GEL-CAR | Herschel-Bulkley | 95.29 | 0.2805 | 92.97 |
| PF-GEL-PLU | Cross | 0.02242 | 0.961 | 0.00418 |
| PF-GEL-SEP | Herschel-Bulkley | 21.89 | 0.3976 | 58.09 |
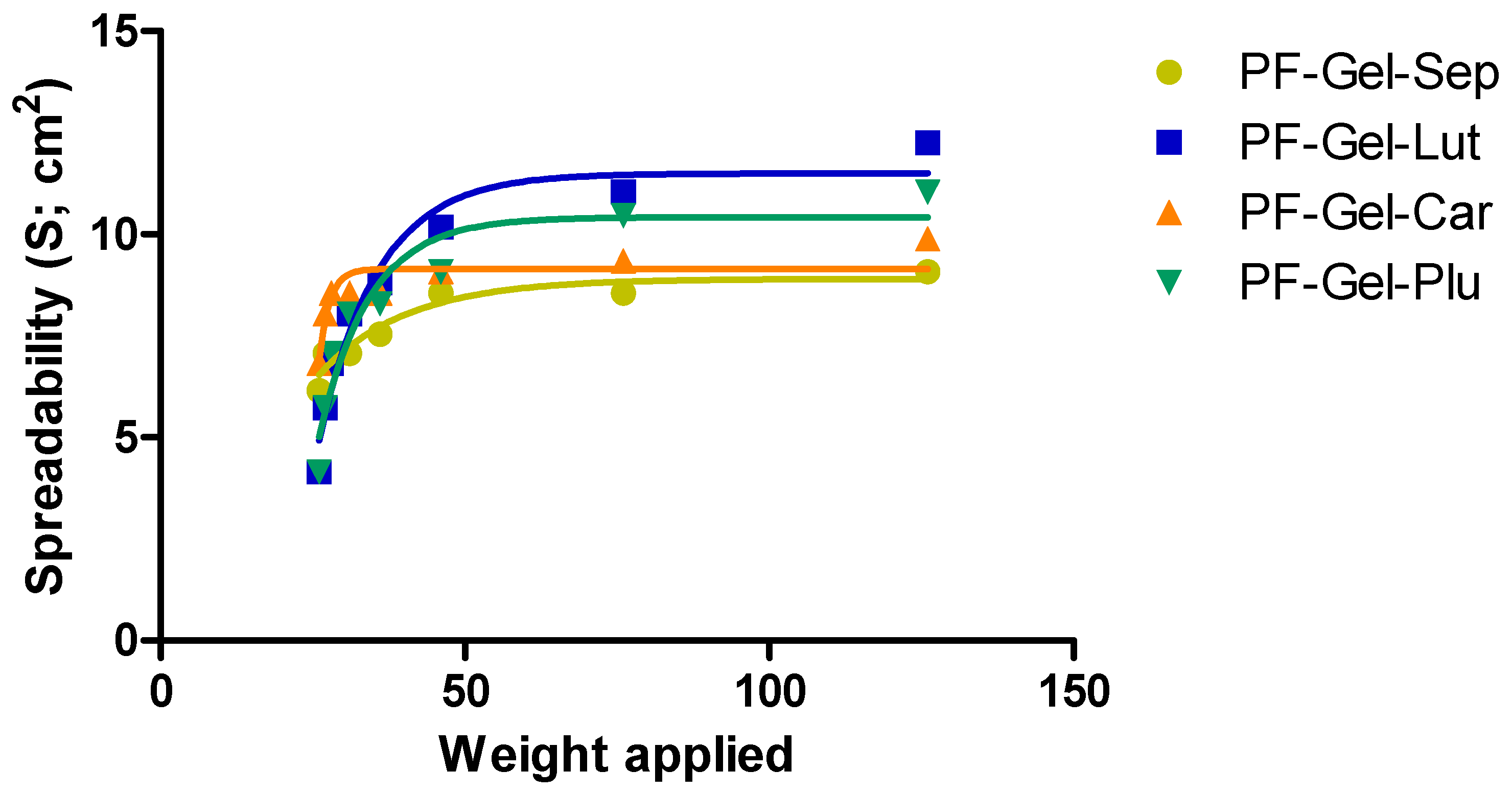
2.2.4. Spreadability Test
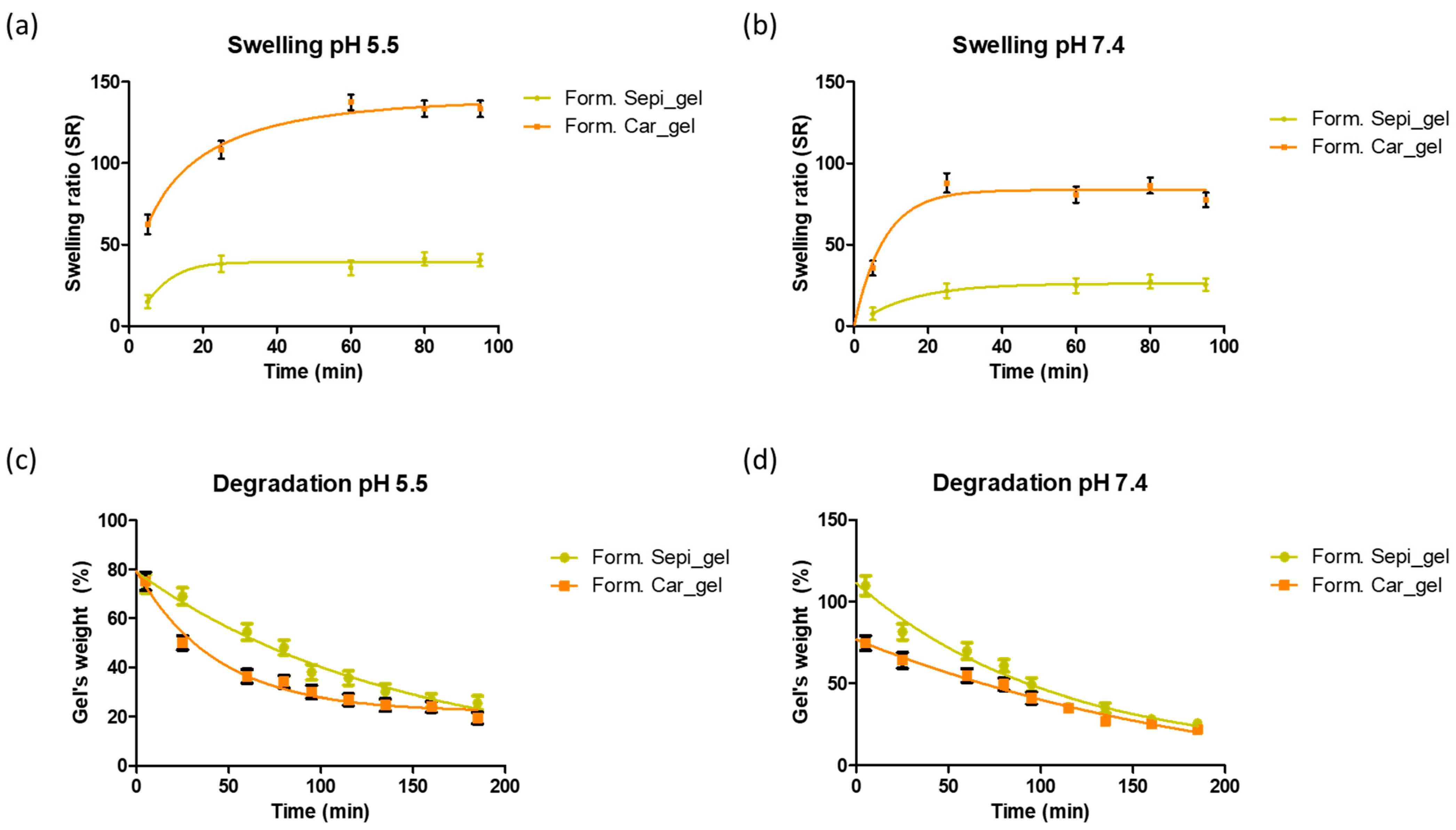
2.2.5. Swelling and Degradation Tests
2.2.6. Evaluation of the Porosity of the Gels
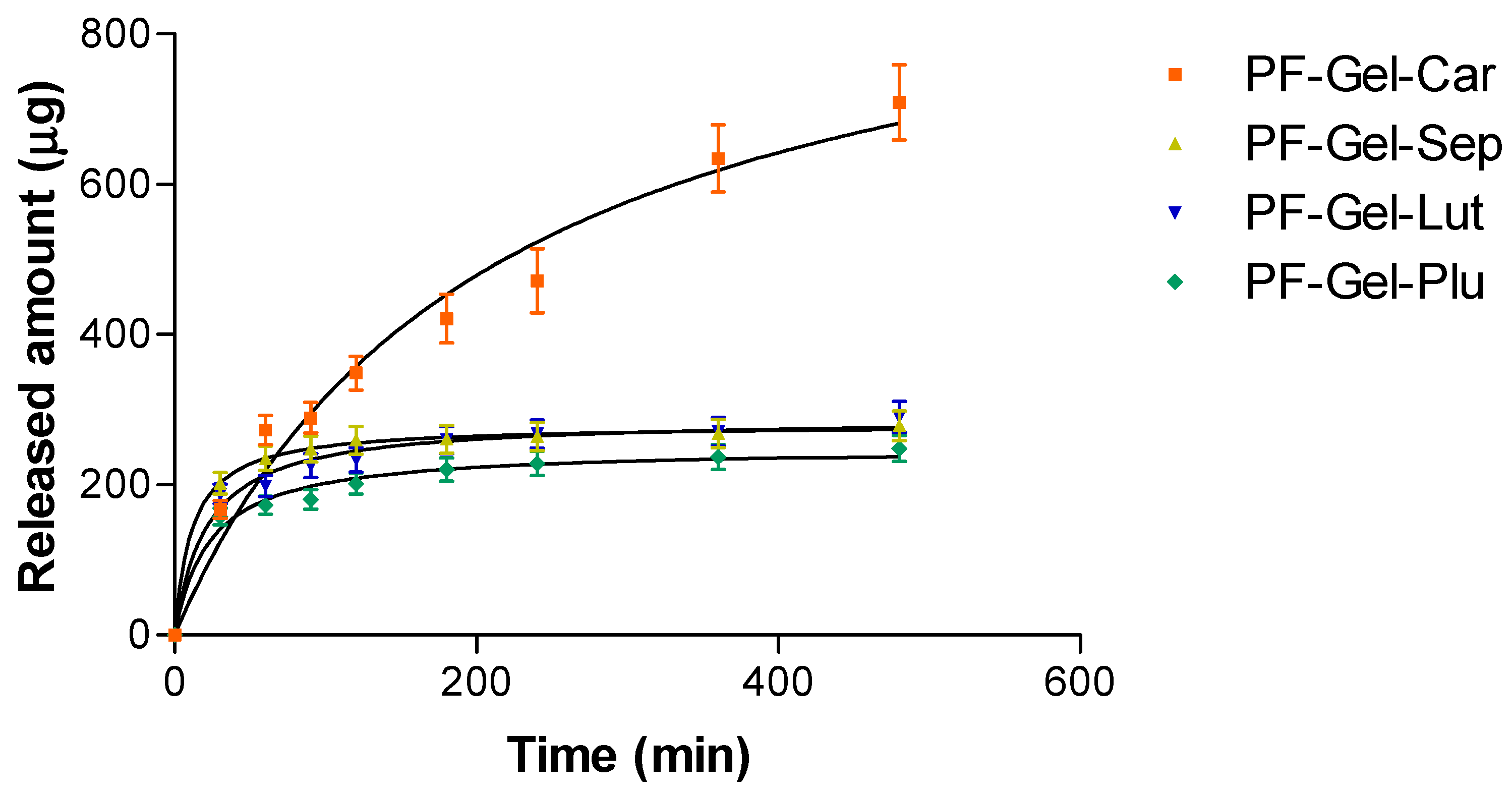
2.3. In Vitro Release Kinetics of Pranoprofen from Topical Gel Formulations
2.4. Ex Vivo Permeation Studies
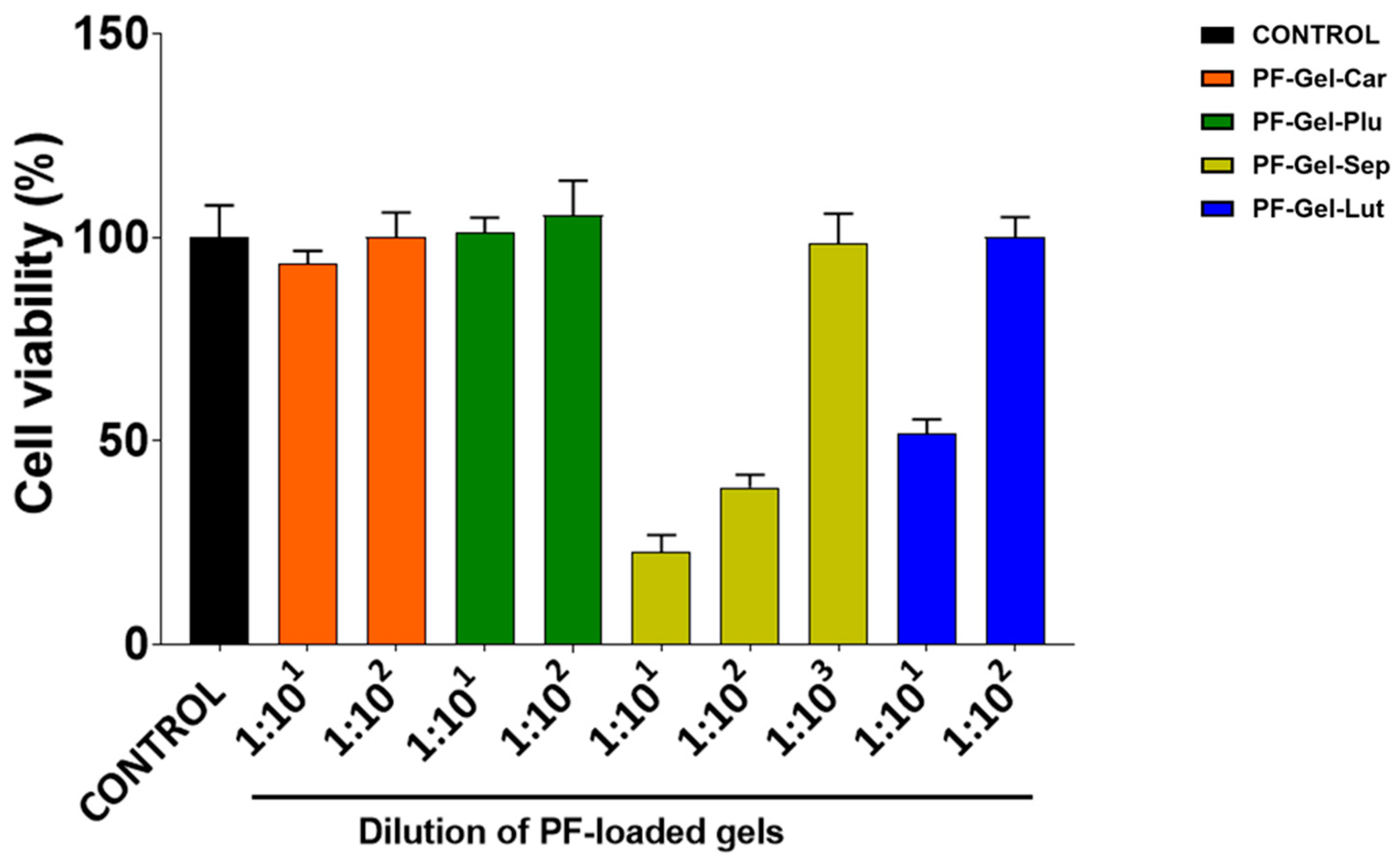
2.5. Cell Viability Studies
2.6. Anti-Inflammatory Efficacy
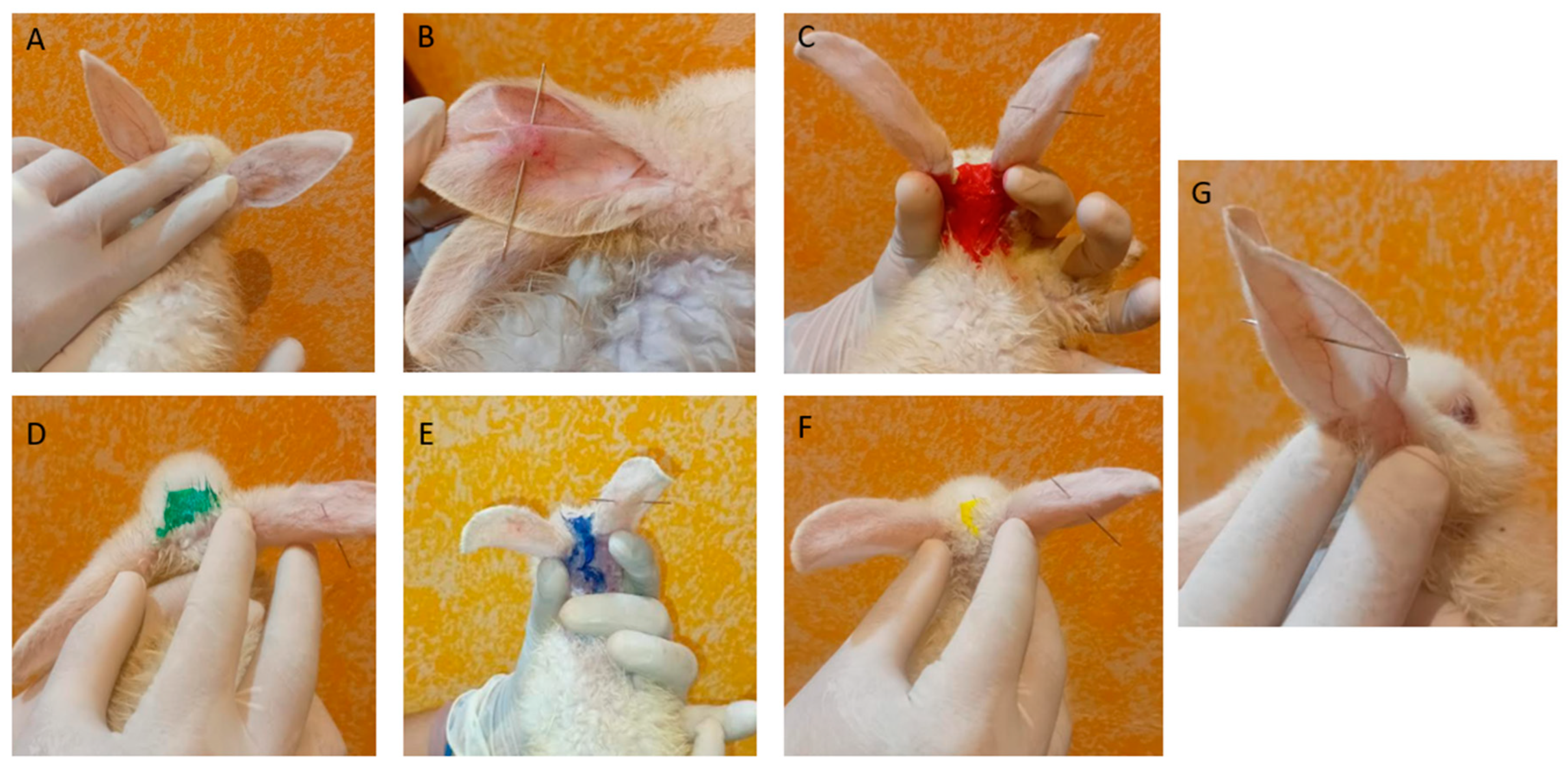
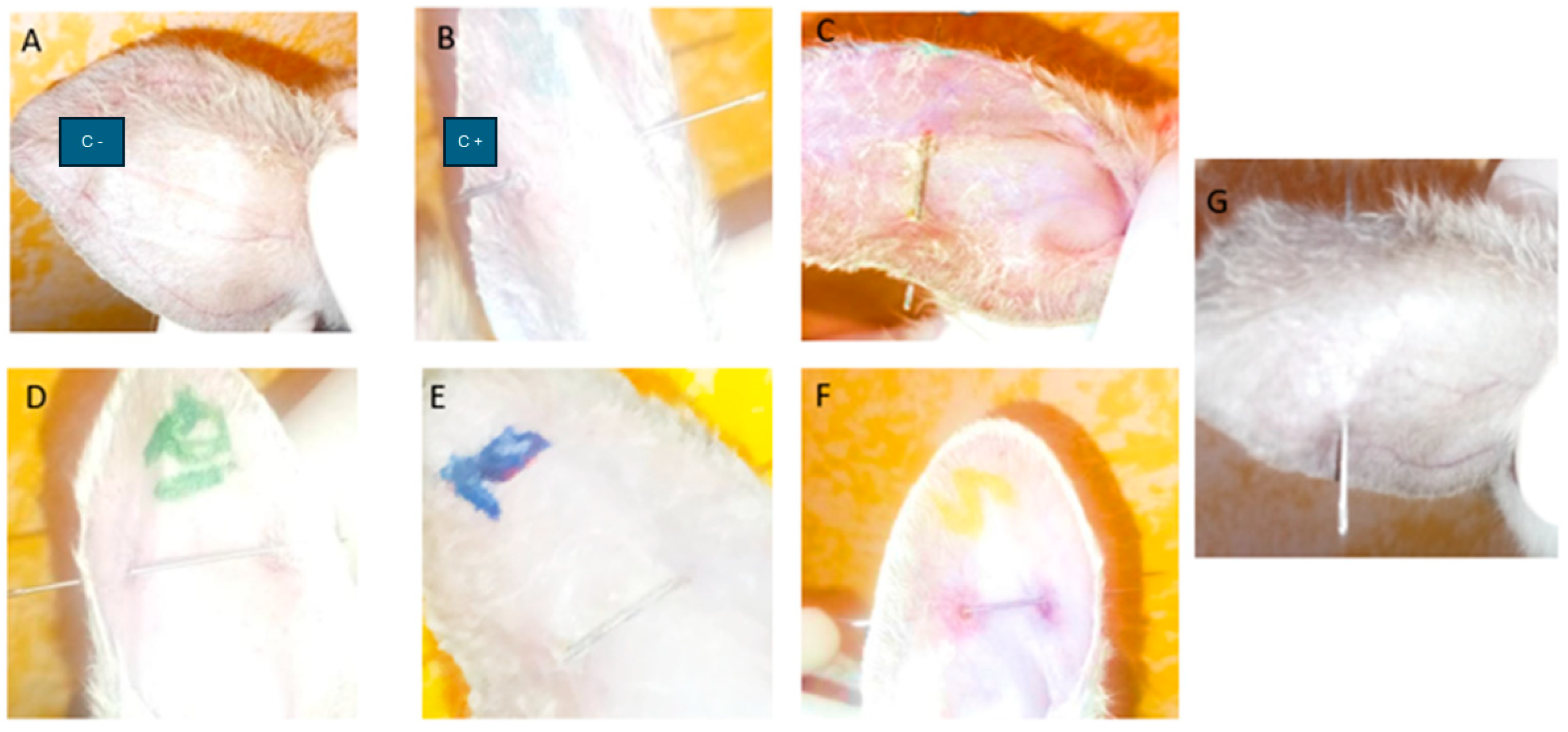
2.6.1. Anti-Inflammatory Efficacy of PF Gels on Mice
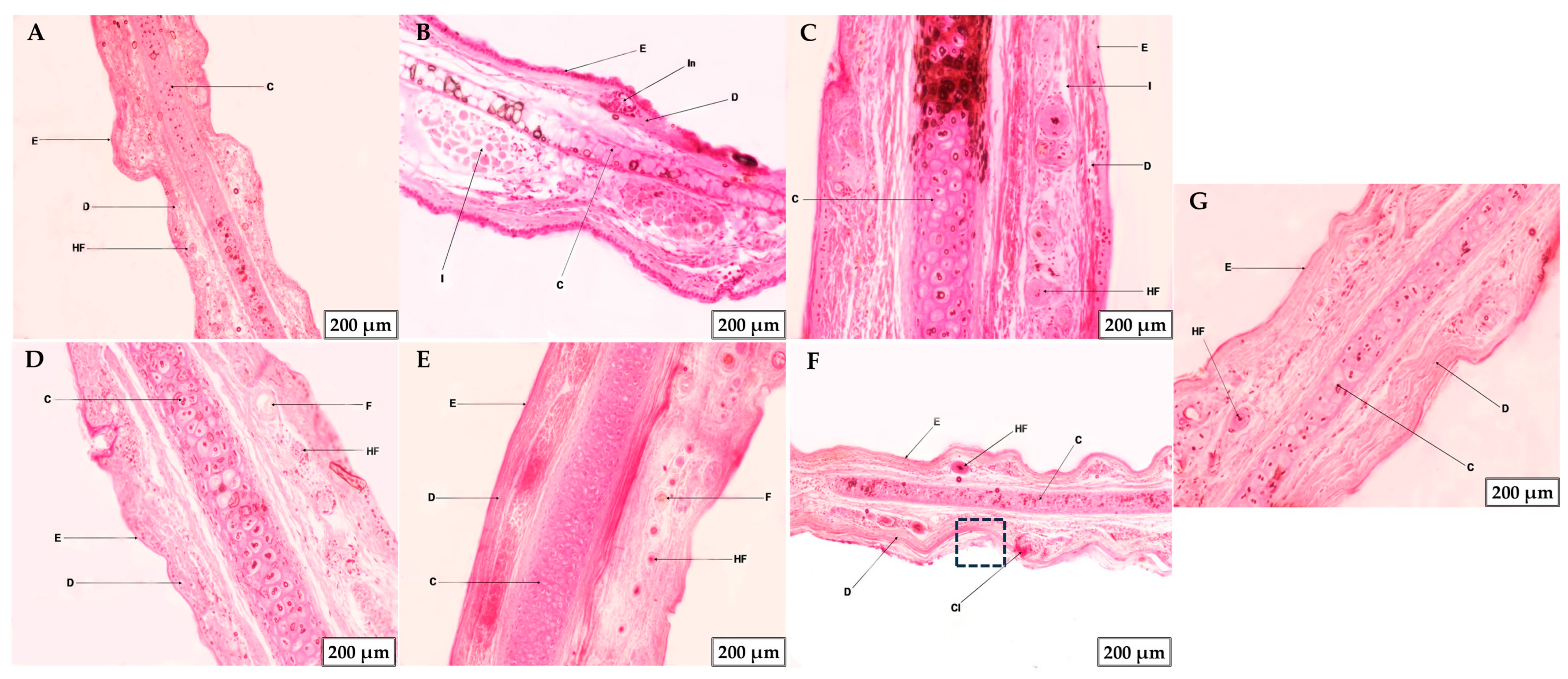
2.6.2. Histological Studies or Microscopic Analysis of Tissue Morphology
2.7. The Safety Profile of the Formulations
2.7.1. Tolerance Study by HET-CAM Assay
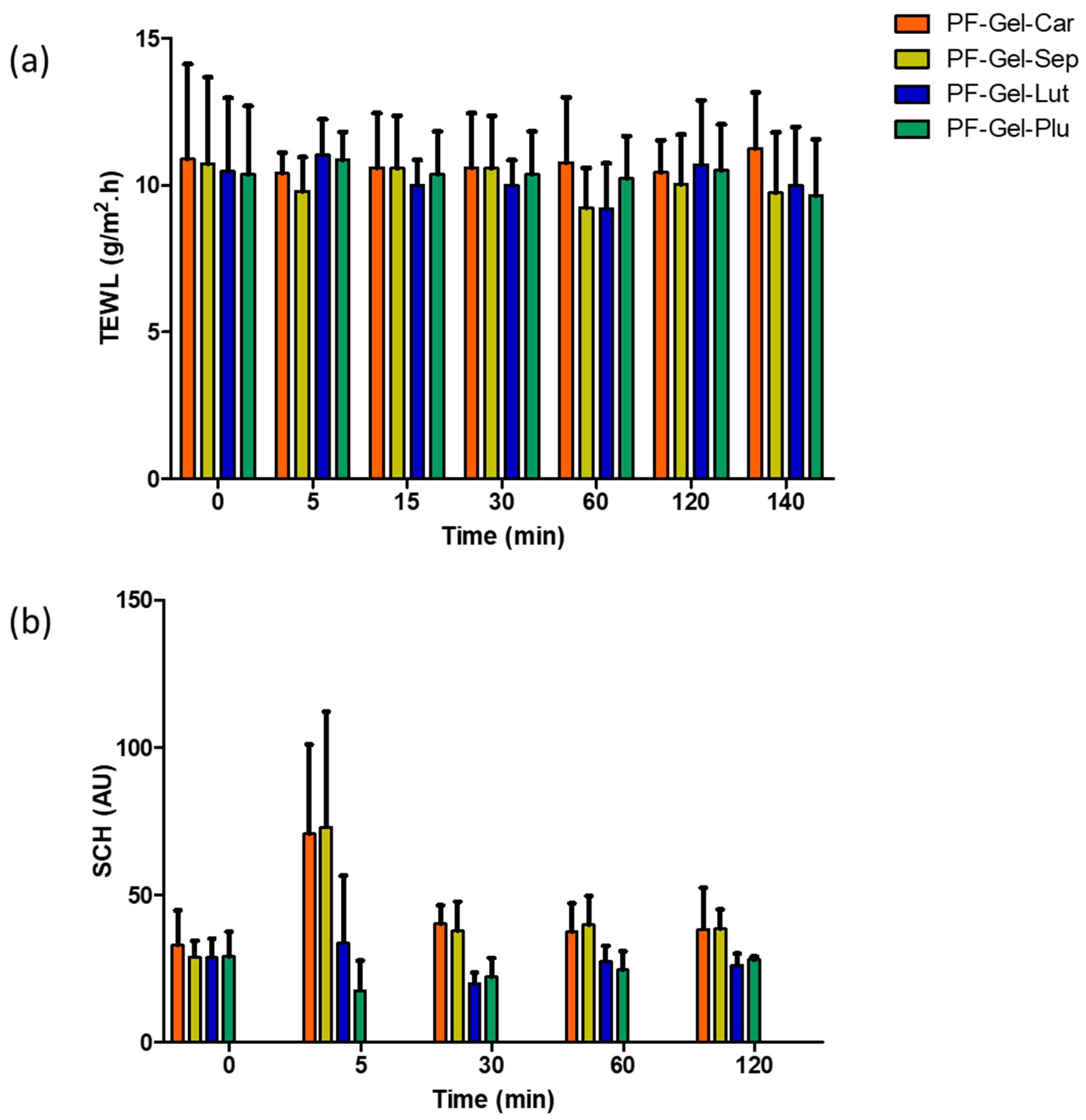
2.7.2. Tolerance of the Blank Gels on Human Skin Assessment by Means of the Biochemical Properties of the Skin
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Gels Formulation
4.3. Physicochemical Characterization of the Gels
4.3.1. PH and Morphological Analysis
4.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
4.3.3. Rheological Measurements of the Gels
4.3.4. Spreadability Test
4.3.5. Swelling and Degradation Tests
4.3.6. Evaluation of the Porosity of the Gels
4.3.7. In Vitro Release Kinetics of Pranoprofen from Topical Gel Formulations
| Parameter | Condition |
|---|---|
| Receptor fluid | Transcutol 5% + Milli Q water |
| Cell volume | 5 mL |
| Membrane | Nylon membrane |
| Diffusion area | 0.64 cm2 |
| Temperature | 32 ± 0.5 °C |
| Stirring | 600 r.p.m. |
| Dose | 0.1 g |
| Sample volume | 0.5 mL |
| Sampling times | 0, 30 (min), 1, 1.5, 2, 3, 4, 6, and 8 (h) |
| Replicates | n = 3 |
4.4. Ex Vivo Permeation Studies
4.4.1. Biological Tissues
4.4.2. Ex Vivo Evaluation of Skin Permeability
4.4.3. Analytical Method by HPLC
4.5. Cell Viability Studies
4.6. Anti-Inflammatory Efficacy Study of PRA on Rabbit Ears
4.6.1. Anti-Inflammatory Efficacy
4.6.2. Histological Studies or Microscopic Analysis of Tissue Morphology
4.7. The Safety Profile of the Formulations
4.7.1. Tolerance Study by HET-CAM (Irritancy Assessment)
4.7.2. Tolerance of the Blank Gels on Human Skin Assessment by Means of the Biochemical Properties of the Skin
- A common method for evaluating and quantifying skin-insensible water loss is transepidermal water loss (TEWL), which is frequently used to evaluate the integrity of the skin’s barrier function. The TEWL was measured by a basic device called a Tewameter® (TM 300 Courage-Khazaka Electronics GmbH, Cologne, Germany). The majority of measuring techniques make use of skin contact equipment. The room temperature was 25 °C, and participants were given 15 min to acclimatize to the room’s conditions and allow their skin to reach equilibrium and adapt to environmental conditions before taking measurements. Additionally, participants were requested to refrain from applying topical lotions or oils to the areas of the skin that would be assessed before the measurement process [89]. After that, the skin was covered evenly with around 0.5 g of PF-Gel-Plu, PF-Gel-Lut, PF-Gel-Car, and PF-Gel-Sep. Measurements were made at time intervals (0, 5, 15, 30 min; and 1, 2, 2:20 h). For sixty seconds, the electrode was placed on the skin’s surface without applying any pressure. Low TEWL values often indicate a functioning barrier that is intact on the skin, whereas an elevated TEWL indicates a skin barrier that is damaged or disrupted. The results were presented as mean ± standard deviation (n = 4) and represented as g/m2/h for transepidermal water loss (TEWL).
- Water content in the stratum corneum is measured by SCH. Using an 825 Corneometer® (Courage & Khazaka Electronics GmbH, Cologne, Germany), the stratum corneum’s (SCH) level of hydration was assessed. At intervals of 0 min, 5 min, 15 min, 30 min, 1 h, and 2 h, measurements were taken, and the number of selected candidates was equal to the TEWEL test. Additionally, the capacitance method was used to conduct the tests.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harmon, M.L.; Downey, B.C.; Drwencke, A.M.; Tucker, C.B. Development and Application of a Novel Approach to Scoring Ear Tag Wounds in Dairy Calves. J. Dairy Sci. 2023, 106, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Covello, F.; Salerno, C.; Giovannini, V.; Corridore, D.; Ottolenghi, L.; Vozza, I. Piercing and Oral Health: A Study on the Knowledge of Risks and Complications. Int. J. Environ. Res. Public Health 2020, 17, 613. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Chávez, L.M.; Alva-Alayo, C.A.; Aguilar-Villanueva, G.A.; Zavala-Alvarado, K.A.; Alverca-Meza, C.A.; Aguirre-Sánchez, M.M.; Amaya-Castro, A.A. Bacterial Infections in Patients with Nipple Piercings: A Qualitative Systematic Review of Case Reports and Case Series. GMS Infect. Dis. 2022, 10, 03. [Google Scholar]
- Malcangi, G.; Patano, A.; Palmieri, G.; Riccaldo, L.; Pezzolla, C.; Mancini, A.; Inchingolo, A.D.; Di Venere, D.; Piras, F.; Inchingolo, F.; et al. Oral Piercing: A Pretty Risk—A Scoping Review of Local and Systemic Complications of This Current Widespread Fashion. Int. J. Environ. Res. Public Health 2023, 20, 5744. [Google Scholar] [CrossRef]
- Gobbo Oliveira Erünlü, N.; Rieder, S.; Kuntzer, T.; Bérard, J.; Wellnitz, O. Effects of Sensor Ear Tags with Twin Pin Fixing System on Health and Well-Being of Cattle. Schweiz. Arch. Tierheilkd. 2023, 165, 512–523. [Google Scholar] [CrossRef]
- Junqueira’s Basic Histology: Text and Atlas, 17th ed; Mescher, A.L., Ed.; McGraw Hill: New York, NY, USA, 2024. [Google Scholar]
- Newton, K.; Dixit, V.; Kayagaki, N. Dying Cells Fan the Flames of Inflammation. Science 2021, 374, 1076–1080. [Google Scholar] [CrossRef]
- Kiss, A.L. Inflammation in Focus: The Beginning and the End. Pathol. Oncol. Res. 2022, 27, 1610136. [Google Scholar] [CrossRef]
- Tate, A.R.; Rao, G.H.R. Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks? Biomolecules 2024, 14, 948. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Nathan, C. Points of Control in Inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Ziadlou, R.; Pandian, G.N.; Hafner, J.; Akdis, C.A.; Stingl, G.; Maverakis, E.; Brüggen, M. Subcutaneous Adipose Tissue: Implications in Dermatological Diseases and Beyond. Allergy 2024, 79, 3310–3325. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.M.; Edwards, D.S. Welfare Implications of Identification of Cattle by Ear Tags. Vet. Rec. 1996, 138, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Hayer, J.J.; Nysar, D.; Schmitz, A.; Leubner, C.D.; Heinemann, C.; Steinhoff-Wagner, J. Wound Lesions Caused by Ear Tagging in Unweaned Calves: Assessing the Prevalence of Wound Lesions and Identifying Risk Factors. Animal 2022, 16, 100454. [Google Scholar] [CrossRef]
- Woods, J.M.; Adcock, S.J.J. Healing Progression of Tail Docking and Ear Tag Wounds in Lambs. Sci. Rep. 2025, 15, 3061. [Google Scholar] [CrossRef]
- Wong, S.S.-Y.; Wong, S.C.-Y.; Yuen, K.-Y. Infections Associated with Body Modification. J. Formos. Med. Assoc. 2012, 111, 667–681. [Google Scholar] [CrossRef]
- Pereira, G.M.; Heins, B.J.; O’Brien, B.; McDonagh, A.; Lidauer, L.; Kickinger, F. Validation of an Ear Tag–Based Accelerometer System for Detecting Grazing Behavior of Dairy Cows. J. Dairy Sci. 2020, 103, 3529–3544. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D.; Han, J. Effect of a Topical Combination of Latanoprost and Pranoprofen on Intraocular Pressure and the Ocular Surface in Open-Angle Glaucoma Patients. J. Ophthalmol. 2018, 2018, 7474086. [Google Scholar] [CrossRef]
- Hilaliyati, N.; Nasution, I.A.R.; Najmi, R.L.; Meirita, E.; Wahyuni, F.; Yuhana, S.I. Optimizing NSAID Therapy in Elderly with Osteoarthritis: A Case Study from a Primary Care Setting in Indonesia. Int. J. Pharm. Sci. Med. 2025, 10, 35–41. [Google Scholar] [CrossRef]
- Rincón, M.; Espinoza, L.C.; Silva-Abreu, M.; Sosa, L.; Pesantez-Narvaez, J.; Abrego, G.; Calpena, A.C.; Mallandrich, M. Quality by Design of Pranoprofen Loaded Nanostructured Lipid Carriers and Their Ex Vivo Evaluation in Different Mucosae and Ocular Tissues. Pharmaceuticals 2022, 15, 1185. [Google Scholar] [CrossRef]
- Shtroblia, V.; Petakh, P.; Kamyshna, I.; Halabitska, I.; Kamyshnyi, O. Recent Advances in the Management of Knee Osteoarthritis: A Narrative Review. Front. Med. 2025, 12, 1523027. [Google Scholar] [CrossRef]
- Rincón, M.; Calpena, A.C.; Clares, B.; Espina, M.; Garduño-Ramírez, M.L.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Skin-Controlled Release Lipid Nanosystems of Pranoprofen for the Treatment of Local Inflammation and Pain. Nanomedicine 2018, 13, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Rincón, M.; Calpena, A.C.; Fabrega, M.-J.; Garduño-Ramírez, M.L.; Espina, M.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials 2018, 8, 1022. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, L.; Feng, P.; Qiu, H.; Wu, X.; Lu, S.; Zhou, M.; Xu, L.; Zhu, Y. Pranoprofen Nanoparticles With Poly(L-Lactide)-b-Poly(Ethylene Glycol)-b-Poly(L-Lactide) as the Matrix Toward Improving Ocular Anti-Inflammation. Front. Bioeng. Biotechnol. 2020, 8, 581621. [Google Scholar] [CrossRef]
- El Bejjaji, S.; Ramos-Yacasi, G.; Suñer-Carbó, J.; Mallandrich, M.; Goršek, L.; Quilchez, C.; Calpena, A.C. Nanocomposite Gels Loaded with Flurbiprofen: Characterization and Skin Permeability Assessment in Different Skin Species. Gels 2024, 10, 362. [Google Scholar] [CrossRef]
- Berenguer, D.; Sosa, L.; Alcover, M.; Sessa, M.; Halbaut, L.; Guillén, C.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C. Development and Characterization of a Semi-Solid Dosage Form of Meglumine Antimoniate for Topical Treatment of Cutaneous Leishmaniasis. Pharmaceutics 2019, 11, 613. [Google Scholar] [CrossRef]
- Singh, M.P.; Nagori, B.P.; Shaw, N.R.; Tiwari, M.; Jhanwar, B. Formulation Development & Evaluation of Topical Gel Formulations Using Different Gelling Agents and Its Comparison with Marketed Gel Formulation. Int. J. Pharm. Erud. 2013, 3, 5268–5278. [Google Scholar]
- Zakzak, K.; Semenescu, A.-D.; Moacă, E.-A.; Predescu, I.; Drăghici, G.; Vlaia, L.; Vlaia, V.; Borcan, F.; Dehelean, C.-A. Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives. Gels 2024, 10, 477. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of Thermo-Reversible Pluronic F-127 Gels In Pharmaceutical Formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Nigam, P. Equilibrium Penetration of Pluronic F-68 in Lipid Monolayers. Chem. Phys. Lipids 2020, 228, 104888. [Google Scholar] [CrossRef]
- Arafa, W.M.; Elkomy, M.H.; Aboud, H.M.; Ali, M.I.; Abdel Gawad, S.S.; Aboelhadid, S.M.; Mahdi, E.A.; Alsalahat, I.; Abdel-Tawab, H. Tunable Polymeric Mixed Micellar Nanoassemblies of Lutrol F127/Gelucire 44/14 for Oral Delivery of Praziquantel: A Promising Nanovector against Hymenolepis Nana in Experimentally-Infected Rats. Pharmaceutics 2022, 14, 2023. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef] [PubMed]
- Atli, E. The Effects of Ethylparaben and Propylparaben on the Development and Fecundity of Drosophila Melanogaster. Environ. Toxicol. Pharmacol. 2022, 92, 103856. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Mlynarczyk, D.T.; Kolasinski, E.; Güzel, E.; Dlugaszewska, J.; Popenda, Ł.; Jurga, S.; Goslinski, T.; Sobotta, L. Zinc(II), Palladium(II), and Metal-Free Phthalocyanines Bearing Nipagin-Functionalized Substituents against Candida Auris and Selected Multidrug-Resistant Microbes. Pharmaceutics 2022, 14, 1686. [Google Scholar] [CrossRef]
- Hagel, J.M.; Chen, X.; Facchini, P.J. Production of Methylparaben in Escherichia Coli. J. Ind. Microbiol. Biotechnol. 2019, 46, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Yosipovitch, G. Skin PH: From Basic SciencE to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- McEwan Jenkinson, D.; Mabon, R.M. The Effect of Temperature and Humidity on Skin Surface PH and the Ionic Composition of Skin Secretions in Ayrshire Cattle. Br. Vet. J. 1973, 129, 282–295. [Google Scholar] [CrossRef]
- Fox, L.K.; Oura, L.Y.; Ames, C.R. Short Communication: Teat Skin PH. J. Dairy Sci. 2003, 86, 3951–3952. [Google Scholar] [CrossRef]
- Equine Network LLC. Ask Horse Journal: Equine Birthmark? Equine Network LLC: Albuquerque, NM, USA, 2013. [Google Scholar]
- Ajito, T.; Suzuki, K.; Okumura, J.; Hatano, N. Skin PH of Domestic Animals. Jpn. J. Vet. Clin. 2001, 24, 9–12. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal PH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Ahmadi, N.; Rincón, M.; Silva-Abreu, M.; Sosa, L.; Pesantez-Narvaez, J.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Mallandrich, M. Semi-Solid Dosage Forms Containing Pranoprofen-Loaded NLC as Topical Therapy for Local Inflammation: In Vitro, Ex Vivo and In Vivo Evaluation. Gels 2023, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, J.; Wang, Q.; Ju, Y. Charge-Transfer Interaction Mediated Organogels from 18β-Glycyrrhetinic Acid Appended Pyrene. Beilstein J. Org. Chem. 2013, 9, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.D.L.; Cardoso, A.V. Effect of Hydroxyurea (HU) on Gelatinization Mechanism of Type I Collagen Suspensions. Matéria 2018, 23, 12211. [Google Scholar] [CrossRef]
- Can, A.; Erdal, M.; Güngör, S.; Özsoy, Y. Optimization and Characterization of Chitosan Films for Transdermal Delivery of Ondansetron. Molecules 2013, 18, 5455–5471. [Google Scholar] [CrossRef]
- Jamshaid, M.A.; Abbas, M.; Yousaf, M.; Younas, M.; Muzaffar, H.; Hussain, T.; Nawaz, R.A. Formulation and Evaluation of Sustained Releasedomperidone Hydrochloride Transdermal Patches to Treat Motion Sickness. J. Contemp. Pharm. 2022, 6, 41–48. [Google Scholar] [CrossRef]
- Shah, S.; Prabhu, P.; Gundad, S. Formulation Development and Investigation of Domperidone Transdermal Patches. Int. J. Pharm. Investig. 2011, 1, 240. [Google Scholar] [CrossRef]
- Prakash, J.; Dasgupta, K.; Tripathi, B.M.; Bahadur, J.; Ghosh, S.K.; Chakravartty, J.K. A New Approach to Fabricate SiC Nanowire-Embedded Dense SiC Matrix/Carbon Fiber Composite. J. Mater. Sci. 2014, 49, 6784–6792. [Google Scholar] [CrossRef]
- Kuddushi, M.; Patel, N.K.; Rajput, S.; Shah, A.; El Seoud, O.A.; Malek, N.I. Thermo-Switchable de Novo Ionic Liquid-Based Gelators with Dye-Absorbing and Drug-Encapsulating Characteristics. ACS Omega 2018, 3, 12068–12078. [Google Scholar] [CrossRef]
- Keshari, S.; Verma, A.; Pandey, S. Formulation and Characterization of Transdermal Patches: A Review. Int. J. Pharm. Sci. Med. 2024, 9, 83–90. [Google Scholar] [CrossRef]
- Fu, F.; Tian, L.G.; Xu, S.; Xu, X.G.; Hu, X.B. Synthesis of Stable ACC Using Mesoporous Silica Gel as a Support. Nanoscale Res. Lett. 2014, 9, 450. [Google Scholar] [CrossRef]
- USACE Hydrologic Engineering Center. Hershel-Bulkley Parameters; US Army Corps of Engineers Hydrologic Engineering Center: Davis, CA, USA, 1926. Available online: https://www.hec.usace.army.mil/confluence/rasdocs/rasmuddebris/non-newtonian-user-s-manual/user-inputs-and-model-parameters/hershel-bulkley-parameters (accessed on 17 February 2025).
- Buscall, R.; Kusuma, T.E.; Stickland, A.D.; Rubasingha, S.; Scales, P.J.; Teo, H.-E.; Worrall, G.L. The Non-Monotonic Shear-Thinning Flow of Two Strongly Cohesive Concentrated Suspensions. J. Nonnewton. Fluid Mech. 2015, 222, 112–120. [Google Scholar] [CrossRef]
- Stokes, J.R.; Telford, J.H. Measuring the Yield Behaviour of Structured Fluids. J. Nonnewton. Fluid Mech. 2004, 124, 137–146. [Google Scholar] [CrossRef]
- Applied Flow Technology. Herschel-Bulkley Model; AFT: Colorado Springs, CO, USA; Available online: https://docs.aft.com/fathom/Herschel-Bulkley-Model.htm (accessed on 17 February 2025).
- Goh, E.; Amran, A. Dynamic Viscosity as A Function of Shear Rate: The Comparison of Established Rheological Models with the Newly Derived Rheological Model for the Estimation of Zero–And Infinite–Shear Rate Viscosity of Vegetable Oils. J. Teknol. 2011, 54, 111–120. [Google Scholar]
- Elena, O.B.; Maria, N.A.; Michael, S.Z.; Natalia, B.D.; Alexander, I.B.; Ivan, I.K. Dermatologic Gels Spreadability Measuring Methods Comparative Study. Int. J. Appl. Pharm. 2022, 14, 164–168. [Google Scholar] [CrossRef]
- Ivens, U.I.; Steinkjer, B.; Serup, J.; Tetens, V. Ointment Is Evenly Spread on the Skin, in Contrast to Creams and Solutions. Br. J. Dermatol. 2001, 145, 264–267. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Rashidi, M.R.; Mirza Mohammadi, S.H. Adhesive and Spreading Properties of pharmaceutical Gel Composed of Cellulose Polymer. Jundishapur J. Nat. Pharm. Prod. 2007, 2, 45–58. [Google Scholar]
- Pang, J.H.; Wischke, C.; Lendlein, A. In Vitro Degradation Analysis of 3D-Architectured Gelatin-Based Hydrogels. MRS Adv. 2020, 5, 633–642. [Google Scholar] [CrossRef]
- Dinu, M.V.; Přádný, M.; Drăgan, E.S.; Michálek, J. Morphogical and Swelling Properties of Porous Hydrogels Based on Poly(Hydroxyethyl Methacrylate) and Chitosan Modulated by Ice-Templating Process and Porogen Leaching. J. Polym. Res. 2013, 20, 285. [Google Scholar] [CrossRef]
- Erceg, T.; Dapčević-Hadnađev, T.; Hadnađev, M.; Ristić, I. Swelling Kinetics and Rheological Behaviour of Microwave Synthesized Poly(Acrylamide-Co-Acrylic Acid) Hydrogels. Colloid Polym. Sci. 2021, 299, 11–23. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Yacob, N.; Hashim, K. Morphological Effect on Swelling Behaviour of Hydrogel. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; pp. 153–159. [Google Scholar]
- Suhail, M.; Vu, Q.L.; Wu, P.-C. Formulation, Characterization, and In Vitro Drug Release Study of β-Cyclodextrin-Based Smart Hydrogels. Gels 2022, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Bhalodi, K.; Kothari, C.; Butani, S. Next-Generation Cancer Nanotherapeutics: Pluronic® F127 Based Mixed Micelles for Enhanced Drug Delivery. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 3241–3270. [Google Scholar] [CrossRef] [PubMed]
- AL-Rajabi, M.M.; Alzyod, S.; Patel, A.; Teow, Y.H. A Hybrid Machine Learning Framework for Predicting Drug-Release Profiles, Kinetics, and Mechanisms of Temperature-Responsive Hydrogels. Polym. Bull. 2025. [Google Scholar] [CrossRef]
- Di Spirito, N.A.; Di Baia, C.; Grizzuti, N.; Pasquino, R.; de Gennaro, B. Drug Release from Pluronic F68 Hydrogels. Phys. Fluids 2024, 36, 3. [Google Scholar] [CrossRef]
- Delgado, B.; Carrêlo, H.; Loureiro, M.V.; Marques, A.C.; Borges, J.P.; Cidade, M.T. Injectable Hydrogels with Two Different Rates of Drug Release Based on Pluronic/Water System Filled with Poly(ε-Caprolactone) Microcapsules. J. Mater. Sci. 2021, 56, 13416–13428. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Ghosh, T.K. Dermal Drug Delivery; Ghosh, T.K., Ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9781315374215. [Google Scholar]
- Silva-Abreu, M.; Sosa, L.; Espinoza, L.; Fábrega, M.-J.; Rodríguez-Lagunas, M.; Mallandrich, M.; Calpena, A.; Garduño-Ramírez, M.; Rincón, M. Efficacy of Apremilast Gels in Mouse Model of Imiquimod-Induced Psoriasis Skin Inflammation. Pharmaceutics 2023, 15, 2403. [Google Scholar] [CrossRef]
- Cao, H.; Wang, M.; Ding, J.; Lin, Y. Hydrogels: A Promising Therapeutic Platform for Inflammatory Skin Diseases Treatment. J. Mater. Chem. B 2024, 12, 8007–8032. [Google Scholar] [CrossRef]
- Beirampour, N.; Bustos-Salgado, P.; Garrós, N.; Mohammadi-Meyabadi, R.; Domènech, Ò.; Suñer-Carbó, J.; Rodríguez-Lagunas, M.J.; Kapravelou, G.; Montes, M.J.; Calpena, A.; et al. Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application. Pharmaceutics 2024, 16, 1092. [Google Scholar] [CrossRef]
- Roelandt, T.; Hachem, J.-P. Practical Use and Significance of Transepidermal Water Loss Measurements. In Practical Aspects of Cosmetic Testing; Springer International Publishing: Cham, Switzerland, 2020; pp. 297–304. [Google Scholar]
- Soriano, J.L.; Calpena, A.C.; Rincón, M.; Pérez, N.; Halbaut, L.; Rodríguez-Lagunas, M.J.; Clares, B. Melatonin Nanogel Promotes Skin Healing Response in Burn Wounds of Rats. Nanomedicine 2020, 15, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- Moles-Aranda, C.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Díaz-Tomé, V.; Otero-Espinar, F.; Morales-Molina, J.; Clares-Naveros, B. Novel Polymeric Formulation for Removal of Gastrointestinal Polyps by Digestive Endoscopy. Pharmaceutics 2020, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, N.; Rodríguez-Lagunas, M.J.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Halbaut-Bellowa, L.; Mallandrich, M.; Clares-Naveros, B. Caspofungin-Loaded Formulations for Treating Ocular Infections Caused by Candida spp. Gels 2023, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ruiz, J.L.; Gálvez-Martín, P.; López-Ruiz, E.; Suñer-Carbó, J.; Calpena-Campmany, A.C.; Marchal, J.A.; Clares-Naveros, B. Design and Evaluation of Mesenchymal Stem Cells Seeded Chitosan/Glycosaminoglycans Quaternary Hydrogel Scaffolds for Wound Healing Applications. Int. J. Pharm. 2019, 570, 118632. [Google Scholar] [CrossRef]
- El Moussaoui, S.; Abo-Horan, I.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Clares, B.; Soriano, J.L.; Calpena, A.C.; Fernández-Campos, F.; et al. Polymeric Nanoparticles and Chitosan Gel Loading Ketorolac Tromethamine to Alleviate Pain Associated with Condyloma Acuminata during the Pre- and Post-Ablation. Pharmaceutics 2021, 13, 1784. [Google Scholar] [CrossRef]
- El Moussaoui, S.; Fernández-Campos, F.; Alonso, C.; Limón, D.; Halbaut, L.; Garduño-Ramirez, M.L.; Calpena, A.C.; Mallandrich, M. Topical Mucoadhesive Alginate-Based Hydrogel Loading Ketorolac for Pain Management after Pharmacotherapy, Ablation, or Surgical Removal in Condyloma Acuminata. Gels 2021, 7, 8. [Google Scholar] [CrossRef]
- Márquez Valls, M.; Martínez Labrador, A.; Halbaut Bellowa, L.; Bravo Torres, D.; Granda, P.C.; Miñarro Carmona, M.; Limón, D.; Calpena Campmany, A.C. Biopharmaceutical Study of Triamcinolone Acetonide Semisolid Formulations for Sublingual and Buccal Administration. Pharmaceutics 2021, 13, 1080. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative Statistical Analysis of the Release Kinetics Models for Nanoprecipitated Drug Delivery Systems Based on Poly(Lactic-Co-Glycolic Acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Porbaha, P.; Ansari, R.; Kiafar, M.R.; Bashiry, R.; Khazaei, M.M.; Dadbakhsh, A.; Azadi, A. A Comparative Mathematical Analysis of Drug Release from Lipid-Based Nanoparticles. AAPS PharmSciTech 2024, 25, 208. [Google Scholar] [CrossRef]
- Schoenfelder, H.; Liu, Y.; Lunter, D.J. Systematic Investigation of Factors, such as the Impact of Emulsifiers, Which Influence the Measurement of Skin Barrier Integrity by in-Vitro Trans-Epidermal Water Loss (TEWL). Int. J. Pharm. 2023, 638, 122930. [Google Scholar] [CrossRef]
- Parke, M.A.; Perez-Sanchez, A.; Zamil, D.H.; Katta, R. Diet and Skin Barrier: The Role of Dietary Interventions on Skin Barrier Function. Dermatol. Pract. Concept. 2021, 11, e2021132. [Google Scholar] [CrossRef]
- Schwab, H.; Flora, J.; Mayrovitz, H.N. Impacts of Skin Eccrine Glands on the Measured Values of Transepidermal Water Loss. Cureus 2022, 14, e32266. [Google Scholar] [CrossRef]


| Sample | pH |
|---|---|
| PF-GEL-LUT | 7.67 ± 0.02 |
| PF-GEL-CAR | 5.39 ± 0.01 |
| PF-GEL-PLU | 7.16 ± 0.01 |
| PF-GEL-SEP | 6.23 ± 0.02 |
| Hyperbola (Y = Ymax × X/Kd + X) | PF-Gel-Car | PF-Gel-Sep | PF-Gel-Lut | PF-Gel-Plu |
|---|---|---|---|---|
Best-fit values | ||||
| Ymax (µg) | 975.9 | 280.1 | 288.6 | 249.0 |
| Kd (min) | 207.9 | 11.54 | 21.34 | 23.24 |
Std. Error | ||||
| Ymax | 94.56 | 1.968 | 8.396 | 7.904 |
| Kd | 43.38 | 0.7248 | 3.756 | 4.250 |
| 95% CI (profile likelihood) | ||||
| Ymax | 752.3 to 1200 | 275.5 to 284.8 | 268.7 to 308.5 | 230.3 to 267.7 |
| Kd | 105.3 to 310.4 | 9.827 to 13.26 | 12.45 to 30.22 | 13.19 to 33.29 |
Goodness of Fit | ||||
| Degrees of Freedom | 7 | 7 | 7 | 7 |
| R squared | 0.9918 | 0.9996 | 0.9946 | 0.9939 |
| Sum of Squares | 3229 | 23.4 | 328 | 277.4 |
| Sy.x | 21.48 | 1.828 | 6.846 | 6.295 |
| Formulations | Irritation Score (IS) | Classification |
|---|---|---|
| PF-Gel-Plu | 0.1 | Non-irritating |
| PF-Gel-Car | 0.1 | Non- irritating |
| PF-Gel-Sep | 0.1 | Non-irritating |
| PF-Gel-Lut | 0.1 | Non-irritating |
| Parameter | Condition |
|---|---|
| Receptor fluid | Phosphate buffered saline (PBS pH = 7.4), Transcutol® 5% |
| Cell volume | 5 mL |
| Membrane | Abdominal human skin, Cow breast skin |
| Diffusion area | 0.64 cm2 |
| Thickness | 0.4 mm (Human), 0.7 mm (Cow) |
| Temperature | 32 ± 0.5 °C |
| Stirring | 600 r.p.m. |
| Dose | 0.2 g |
| Sample volume | 0.2 mL |
| Sampling times | 24 h |
| Replicates | n = 5 |
| Parameter | Condition |
|---|---|
| Chromatographic column | Kromasil 100 C18 (15 × 0.46 mm, 5 µm) |
| Mobile phase | Methanol: Acetic 1% (A: B) |
| Flux | 0.8 mL |
| Pump mode | Gradient |
| From 0–10 min: 55% A: 45% B 10–11 min: 20% A: 80% B 11–15 min: 55% A: 45% B | |
| Injection volume | 20.00 µL |
| Run time | 15:00 min |
| Wavelength | 254.0 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, N.; Rincón, M.; Mallandrich, M.; Suñer-Carbó, J.; Sosa, L.; Zelaya, M.; Martinez-Ruiz, S.; Cordero, C.; Calpena, A.C. Novel Gels for Post-Piercing Care: Evaluating the Efficacy of Pranoprofen Formulations in Reducing Inflammation. Gels 2025, 11, 334. https://doi.org/10.3390/gels11050334
Ahmadi N, Rincón M, Mallandrich M, Suñer-Carbó J, Sosa L, Zelaya M, Martinez-Ruiz S, Cordero C, Calpena AC. Novel Gels for Post-Piercing Care: Evaluating the Efficacy of Pranoprofen Formulations in Reducing Inflammation. Gels. 2025; 11(5):334. https://doi.org/10.3390/gels11050334
Chicago/Turabian StyleAhmadi, Negar, Maria Rincón, Mireia Mallandrich, Joaquim Suñer-Carbó, Lilian Sosa, Mireya Zelaya, Sergio Martinez-Ruiz, Cecilia Cordero, and Ana C. Calpena. 2025. "Novel Gels for Post-Piercing Care: Evaluating the Efficacy of Pranoprofen Formulations in Reducing Inflammation" Gels 11, no. 5: 334. https://doi.org/10.3390/gels11050334
APA StyleAhmadi, N., Rincón, M., Mallandrich, M., Suñer-Carbó, J., Sosa, L., Zelaya, M., Martinez-Ruiz, S., Cordero, C., & Calpena, A. C. (2025). Novel Gels for Post-Piercing Care: Evaluating the Efficacy of Pranoprofen Formulations in Reducing Inflammation. Gels, 11(5), 334. https://doi.org/10.3390/gels11050334










