Multifunctional Carbon-Based Nanocomposite Hydrogels for Wound Healing and Health Management
Abstract
1. Introduction
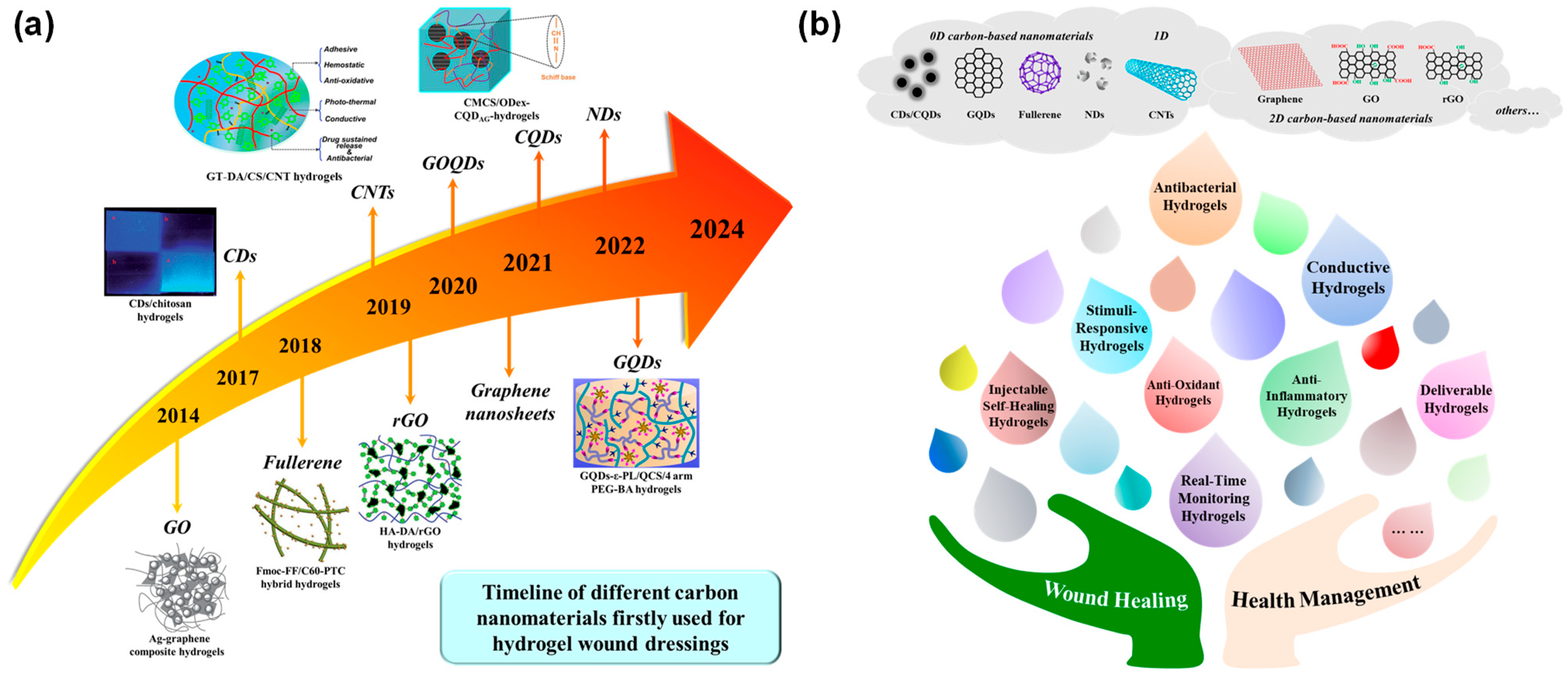
2. Low-Dimensional Carbon-Based Nanomaterials for Multifunctional Hydrogels
2.1. 0D-CBNs
2.1.1. Carbon Dots and Carbon Quantum Dots
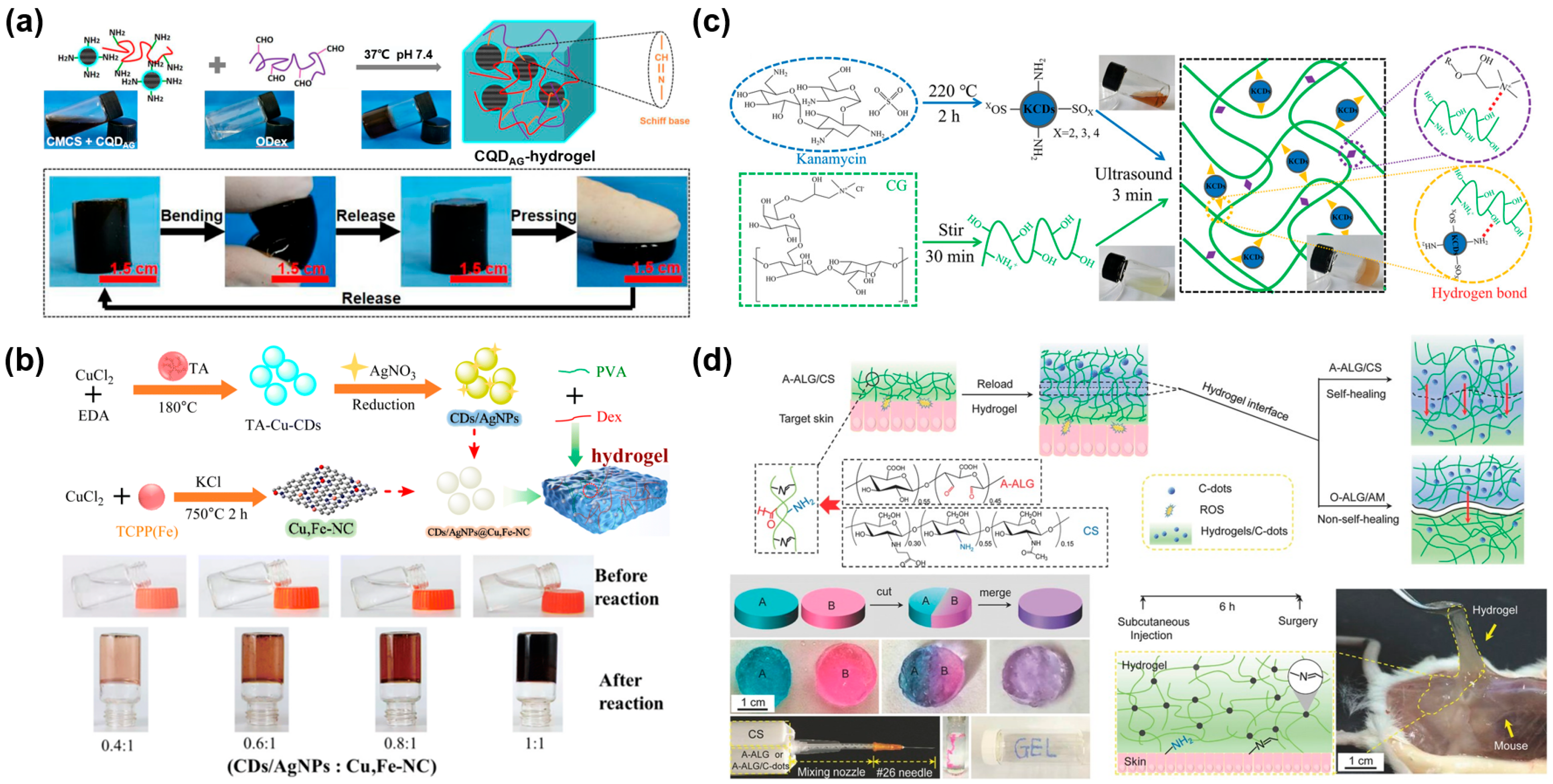
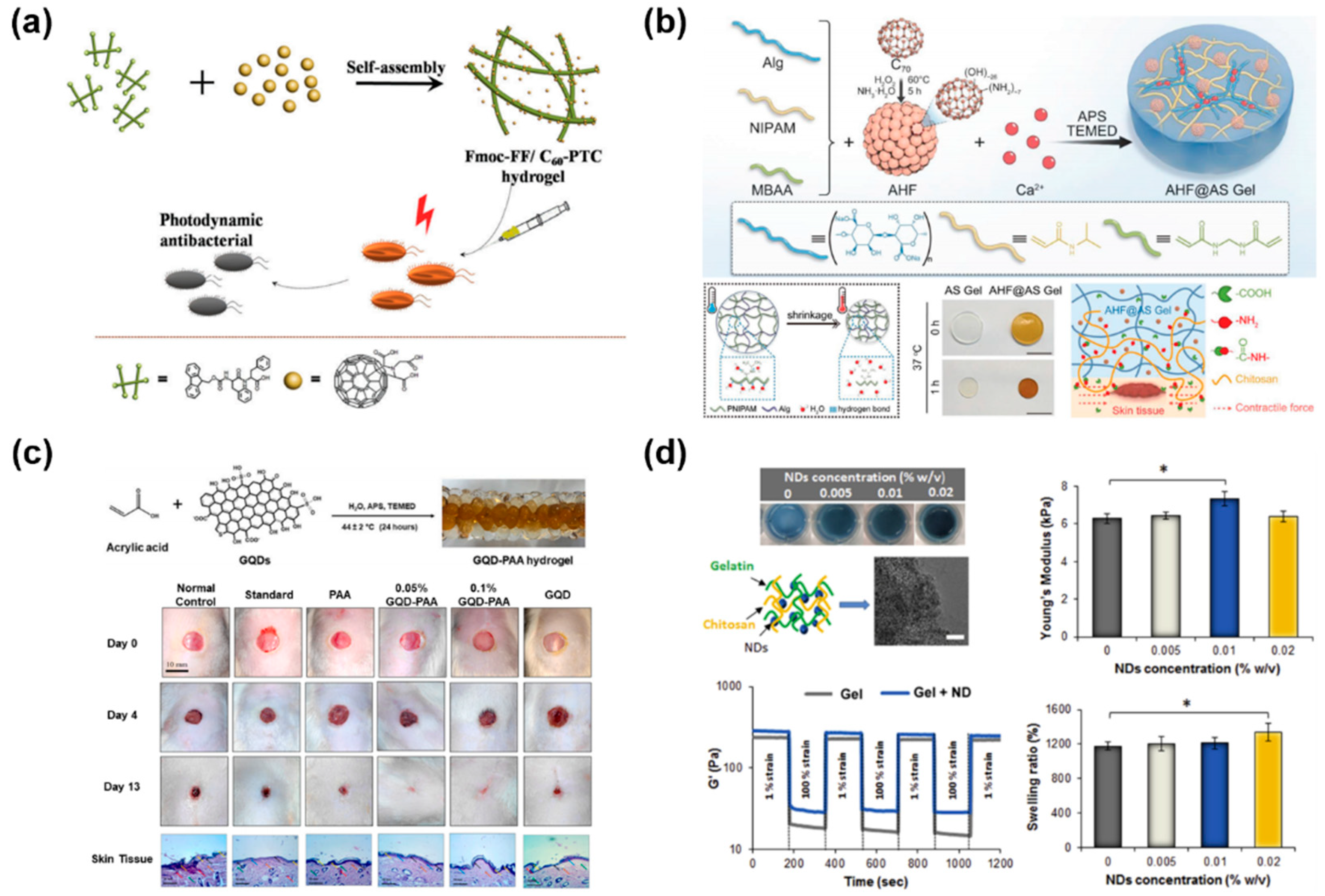
2.1.2. Fullerene
2.1.3. Graphene Quantum Dots
2.1.4. Nanodiamonds
2.2. 1D-CBNs

2.3. 2D-CBNs

3. Multifunctional Carbon-Based Nanocomposite Hydrogels for Chronic Wound Healing
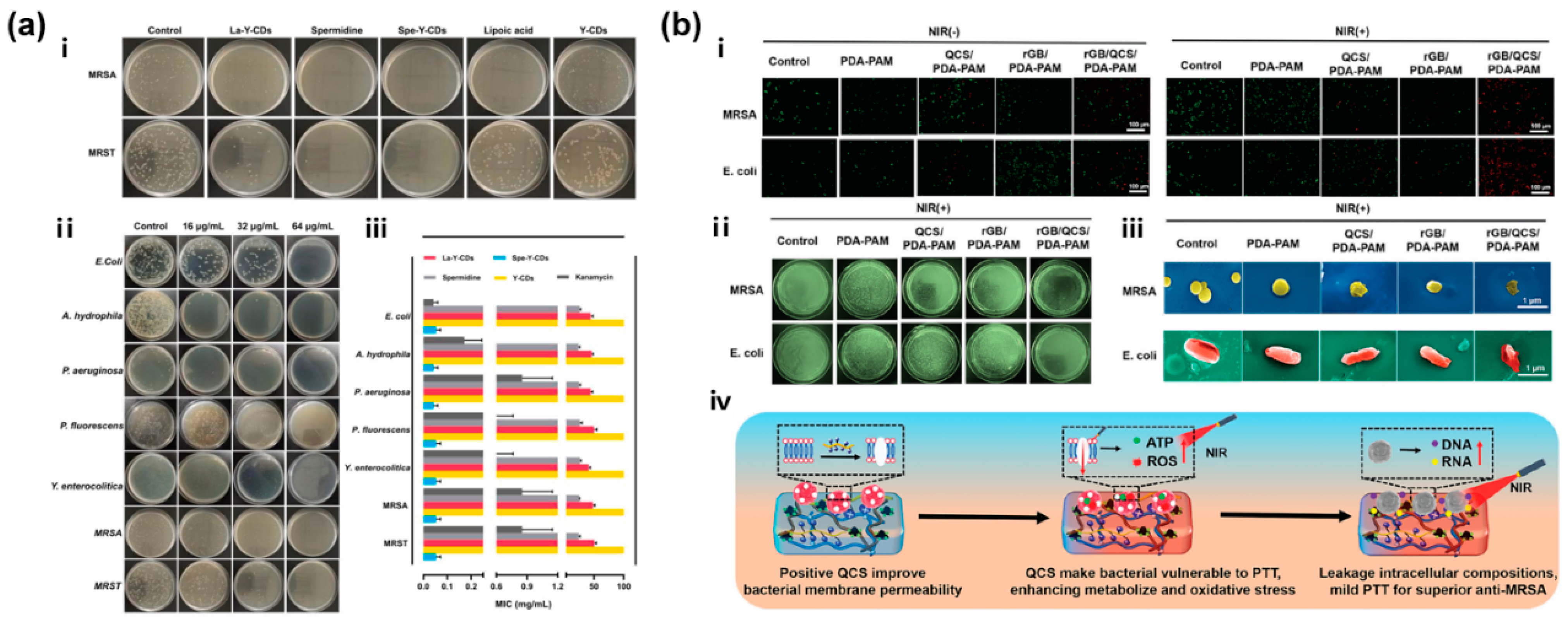
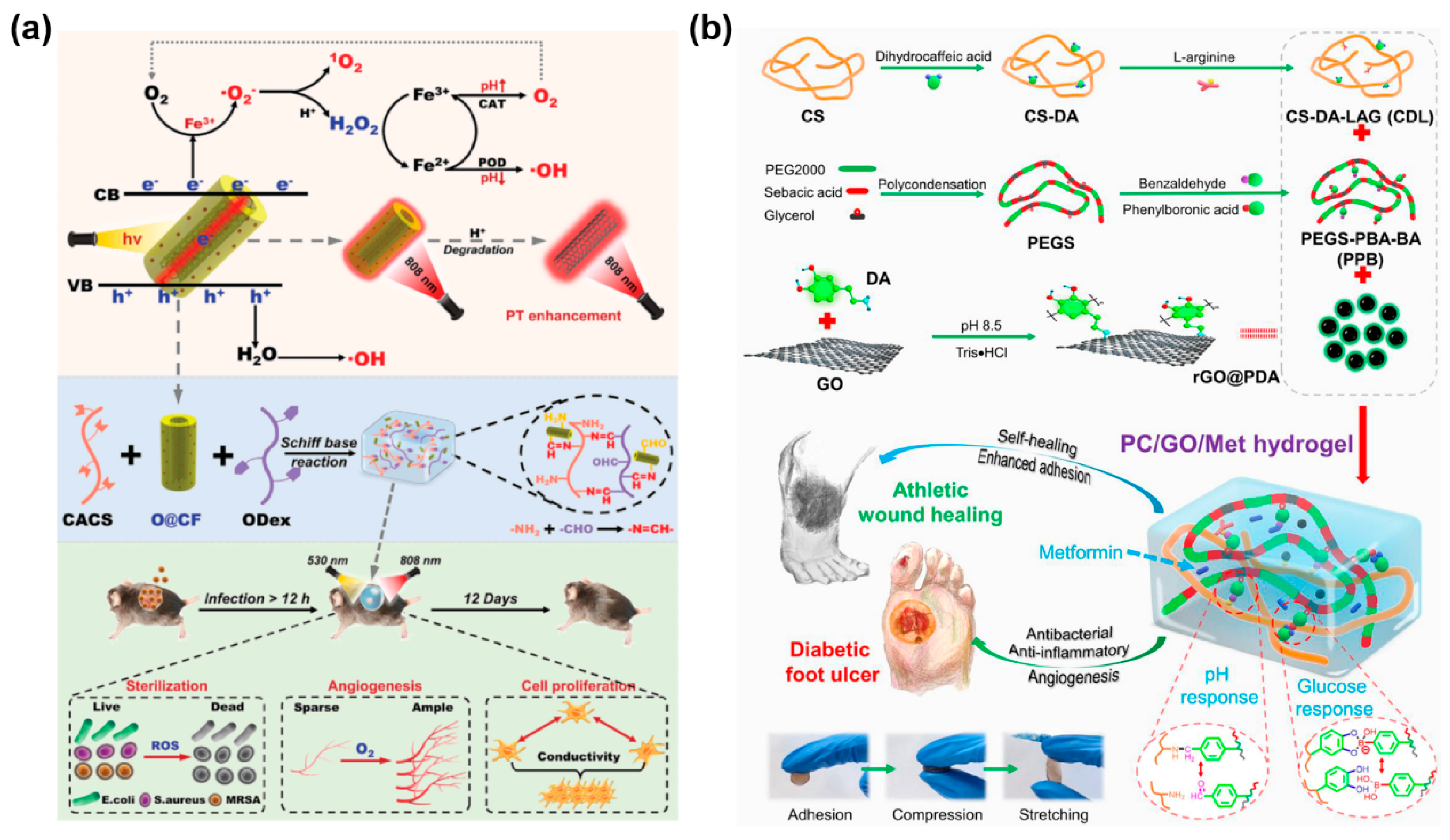
3.1. CBNHs for Infected Wound Healing
3.2. CBNHs for Diabetic Wound Healing
3.3. CBNHs for Burn Wound Healing
4. Carbon-Based Nanocomposite Hydrogels for Real-Time Wound Monitoring and Health Management
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chang, T.-H.; Li, K.; Yang, H.; Chen, P.-Y. Multifunctionality and Mechanical Actuation of 2D Materials for Skin-Mimicking Capabilities. Adv. Mater. 2018, 30, 1802418. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, S.J.M.; Baqersad, J. Mechanical modeling and characterization of human skin: A review. J. Biomech. 2022, 130, 110864. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Skin Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef]
- Zhu, P.; Du, H.; Hou, X.; Lu, P.; Wang, L.; Huang, J.; Bai, N.; Wu, Z.; Fang, N.X.; Guo, C.F. Skin-electrode iontronic interface for mechanosensing. Nat. Commun. 2021, 12, 4731. [Google Scholar] [CrossRef]
- Zhang, A.; Pang, D.; Wang, B.; Wang, J. Dynamic responses of wearable thermoelectric generators used for skin waste heat harvesting. Energy 2023, 262, 125621. [Google Scholar] [CrossRef]
- Webb, P. Temperatures of skin; subcutaneous tissue, muscle and core in resting men in cold, comfortable and hot conditions. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 471–476. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Cheng, N.-C.; Cheng, C.-M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Del. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Del. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Matoori, S.; Veves, A.; Mooney, D.J. Advanced bandages for diabetic wound healing. Sci. Transl. Med. 2021, 13, eabe4839. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Lim, J.Z.; Ng, N.S.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2013, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Keogh, S.J.; Liu, Z.; Stubbs, N.; Walker, R.M. Fortnam, Alginate dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 5, CD011277. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 2020. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, I.K.; Kim, Y.S.; Kim, H.J.; Moon, H.; Mueller, S.; Jeong, Y.-I.L. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater. Res. 2016, 20, 15. [Google Scholar] [CrossRef]
- Dutra, R.A.A.; Salomé, G.M.; Alves, J.R.; Pereira, V.O.S.; Miranda, F.D.; Vallim, V.B.; de Brito, M.J.A.; Ferreira, L.M. Using transparent polyurethane film and hydrocolloid dressings to prevent pressure ulcers. J. Wound Care 2015, 24, 268–275. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; de Santiago, G.T. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Del. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite hydrogels for biomedical applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef] [PubMed]
- Colletta, A.D.; Pelin, M.; Sosa, S.; Fusco, L.; Prato, M.; Tubaro, A. CARBON-BASED nanomaterials and SKIN: An overview. Carbon 2022, 196, 683–698. [Google Scholar] [CrossRef]
- Sadat, Z.; Farrokhi-Hajiabad, F.; Lalebeigi, F.; Naderi, N.; Gorab, M.G.; Cohan, R.A.; Eivazzadeh-Keihan, R.; Maleki, A. A comprehensive review on the applications of carbon-based nanostructures in wound healing: From antibacterial aspects to cell growth stimulation. Biomater. Sci. 2022, 10, 6911–6938. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021, 14, 1. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Lagos, K.J.; García, D.; Cuadrado, C.F.; de Souza, L.M.; Mezzacappo, N.F.; da Silva, A.P.; Inada, N.; Bagnato, V.; Romero, M.P. Carbon dots: Types; preparation, and their boosted antibacterial activity by photoactivation. Current status and future perspectives. WIREs Nanomed. Nanobi. 2023, 15, e1887. [Google Scholar] [CrossRef]
- Khan, Z.G.; Patil, P.O. A comprehensive review on carbon dots and graphene quantum dots based fluorescent sensor for biothiols. Microchem. J. 2020, 157, 105011. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Omidi, M.; Yadegari, A.; Tayebi, L. Wound dressing application of pH-sensitive carbon dots/chitosan hydrogel. RSC Adv. 2017, 7, 10638–10649. [Google Scholar] [CrossRef]
- Pandey, P.K.; Preeti; Rawat, K.; Prasad, T.; Bohidar, H.B. Multifunctional, fluorescent DNA-derived carbon dots for biomedical applications: Bioimaging, luminescent DNA hydrogels, and dopamine detection. J. Mater. Chem. B 2020, 8, 1277–1289. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Hu, X.; Lu, Y.; Zhang, J.; Jin, W.; Liu, W.; Shu, Y.; Cheng, Y.Y.; Li, W.; et al. Caffeic acid-grafted chitosan/sodium alginate/nanoclay-based multifunctional 3D-printed hybrid scaffolds for local drug release therapy after breast cancer surgery. Carbohydr. Polym. 2024, 324, 121441. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, P.; Tang, W.; Du, S.; Yu, M.; Lu, H.; Tan, H.; Xing, X. A facile injectable carbon dot/oxidative polysaccharide hydrogel with potent self-healing and high antibacterial activity. Carbohydr. Polym. 2021, 251, 117040. [Google Scholar] [CrossRef] [PubMed]
- Chekini, M.; Krivoshapkina, E.; Shkodenko, L.; Koshel, E.; Shestovskaya, M.; Dukhinova, M.; Kheiri, S.; Khuu, N.; Kumacheva, E. Nanocolloidal Hydrogel with Sensing and Antibacterial Activities Governed by Iron Ion Sequestration. Chem. Mater. 2020, 32, 10066–10075. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T.; Kim, J. Polyphenol derived bioactive carbon quantum dot-incorporated multifunctional hydrogels as an oxidative stress attenuator for antiaging and in vivo wound-healing applications. Biomater. Sci. 2022, 10, 3527–3539. [Google Scholar] [CrossRef]
- Dehghani, N.; Haghiralsadat, F.; Yazdian, F.; Sadeghian-Nodoushan, F.; Ghasemi, N.; Mazaheri, F.; Pourmadadi, M.; Naghib, S.M. Chitosan/silk fibroin/nitrogen-doped carbon quantum dot/α-tricalcium phosphate nanocomposite electrospinned as a scaffold for wound healing application: In vitro and in vivo studies. Int. J. Biol. Macromol. 2023, 238, 124078. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Karagianni, A.; Tsierkezos, N.G.; Prato, M.; Terrones, M.; Kordatos, K.V. Application of carbon-based quantum dots in photodynamic therapy. Carbon 2023, 203, 273–310. [Google Scholar] [CrossRef]
- Xu, N.; Du, J.; Yao, Q.; Ge, H.; Li, H.; Xu, F.; Gao, F.; Xian, L.; Fan, J.; Peng, X. Precise photodynamic therapy: Penetrating the nuclear envelope with photosensitive carbon dots. Carbon 2020, 159, 74–82. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Tang, Z.; Qu, S. Tuning the photothermal properties of carbon dots in the deep-red to near-infrared wavelength regions for tumor therapy. Mater. Chem. Front. 2023, 7, 2359–2372. [Google Scholar] [CrossRef]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Holá, K.; et al. In vivo theranostics with near-infrared-emitting carbon dots—Highly efficient photothermal therapy based on passive targeting after intravenous administration. Light-Sci. Appl. 2018, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Zhang, Z.; Yan, L.; Guo, L.; Niu, G.; Zhang, J.; Zhao, J.; Zhang, H.; Wang, P.; et al. Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy. Nano Res. 2017, 10, 3113–3123. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Yang, X.; Du, S.; Tang, W.; Cao, W.; Zhou, J.; Gong, X.; Xing, X. Low-drug resistance carbon quantum dots decorated injectable self-healing hydrogel with potent antibiofilm property and cutaneous wound healing. Chem. Eng. J. 2021, 403, 126387. [Google Scholar] [CrossRef]
- Cui, F.; Sun, J.; Ji, J.; Yang, X.; Wei, K.; Xu, H.; Gu, Q.; Zhang, Y.; Sun, X. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J. Hazard. Mater. 2021, 406, 124330. [Google Scholar] [CrossRef]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid-Conjugated PDA Hydrogel through Dual-Light Triggered ROS and Membrane Permeability. Small 2019, 15, 1900322. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; LeCroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L.; et al. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef]
- Huang, H.-H.; Anand, A.; Lin, C.-J.; Lin, H.-J.; Lin, Y.-W.; Harroun, S.G.; Huang, C.-C. LED irradiation of halogen/nitrogen-doped polymeric graphene quantum dots triggers the photodynamic inactivation of bacteria in infected wounds. Carbon 2021, 174, 710–722. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, X.; Ma, T.; Huang, Y.; Yan, C.; Du, Y. Multifunctional cerium doped carbon dots nanoplatform and its applications for wound healing. Chem. Eng. J. 2021, 423, 130301. [Google Scholar] [CrossRef]
- Sun, B.; Wu, F.; Zhang, Q.; Chu, X.; Wang, Z.; Huang, X.; Li, J.; Yao, C.; Zhou, N.; Shen, J. Insight into the effect of particle size distribution differences on the antibacterial activity of carbon dots. J. Colloid Interface Sci. 2021, 584, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Wang, X.; Teng, J.; Xie, Z.; Zheng, M. Injectable self-healing hydrogel fabricated from antibacterial carbon dots and ɛ-polylysine for promoting bacteria-infected wound healing. J. Nanobiotechnol. 2022, 20, 368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Panwar, V.; Salaria, N.; Ghosh, D. Protease-responsive hydrogel, cross-linked with bioactive curcumin-derived carbon dots, encourage faster wound closure. Biomater. Adv. 2022, 139, 212978. [Google Scholar] [CrossRef]
- Zhu, J.; Han, Q.; Li, Q.; Wang, F.; Dong, M.; Liu, N.; Li, X.; Chen, D.; Yang, D.; Song, Y.; et al. A multi-enzyme-like activity exhibiting mussel-inspired nanozyme hydrogel for bacteria-infected wound healing. Biomater. Sci. 2023, 11, 2711–2725. [Google Scholar] [CrossRef]
- Li, T.; Lin, L.; Wang, D.; Fang, H.; Zhang, Z.; Wang, Y.; Chen, Y.; Feng, L. Injectable Hydrogel Incorporated with Iron-Doped Carbon Dots Exhibiting Peroxidase-Like Activity for Antibacterial Therapy and Wound Healing. Adv. Ther. 2024, 7, 2300368. [Google Scholar] [CrossRef]
- Wang, M.; Xia, A.; Wu, S.; Shen, J. Facile Synthesis of the Cu, N-CDs@GO-CS Hydrogel with Enhanced Antibacterial Activity for Effective Treatment of Wound Infection. Langmuir 2021, 37, 7928–7935. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, P.; Yin, C.; Li, Y.; Liao, Z.; Yang, C.; Liu, H.; Wang, W.; Fan, C.; Sun, D.; et al. Antibiotic-Derived Carbon-Nanodot-Decorated Hydrogel for Reactive Oxygen Species-Enhanced Anti-Infection Through Biofilm Damage. Adv. Funct. Mater. 2023, 33, 2300341. [Google Scholar] [CrossRef]
- Nayak, S.; Prasad, S.R.; Mandal, D.; Das, P. Hybrid DNA–Carbon Dot–Poly(vinylpyrrolidone) Hydrogel with Self-Healing and Shape Memory Properties for Simultaneous Trackable Drug Delivery and Visible-Light-Induced Antimicrobial Photodynamic Inactivation. ACS Appl. Bio Mater. 2020, 3, 7865–7875. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Liu, H.; Wu, Y.; Wang, Y.; Cao, Z.; Li, W.; Peng, Y.; Xiong, H.; Jin, B.; et al. A refractory wound healing hydrogel with integrated functions of photothermal anti-infection, superoxide dismutase mimicking activity, and intelligent infection management. Mater. Des. 2022, 224, 111280. [Google Scholar] [CrossRef]
- Xiang, T.; Guo, Q.; Jia, L.; Yin, T.; Huang, W.; Zhang, X.; Zhou, S. Multifunctional Hydrogels for the Healing of Diabetic Wounds. Adv. Healthc. Mater. 2024, 13, 2301885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Zhang, Y.; Wang, W.; Zhang, X.; Zhao, Y.; Wang, Y.; Bi, H. A Reloadable Self-Healing Hydrogel Enabling Diffusive Transport of C-Dots Across Gel–Gel Interface for Scavenging Reactive Oxygen Species. Adv. Healthc. Mater. 2017, 6, 1700746. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Roberts, A.P.; Mount, A.S.; Klaine, S.J.; Ke, P.C. Translocation of C60 and Its Derivatives Across a Lipid Bilayer. Nano Lett. 2007, 7, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef]
- Li, J.; Guan, M.; Wang, T.; Zhen, M.; Zhao, F.; Shu, C.; Wang, C. Gd@C82-(ethylenediamine)8 Nanoparticle: A New High-Efficiency Water-Soluble ROS Scavenger. ACS Appl. Mater. Interfaces 2016, 8, 25770–25776. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An injectable dipeptide–fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Yu, W.; Zhang, W.; Tang, H.; Yuan, W.-E. In situ forming ROS-scavenging hybrid hydrogel loaded with polydopamine-modified fullerene nanocomposites for promoting skin wound healing. J. Nanobiotechnol. 2023, 21, 129. [Google Scholar] [CrossRef]
- Kong, X.; Chen, H.; Li, F.; Zhang, F.; Jiang, Y.; Song, J.; Sun, Y.; Zhao, B.; Shi, J. Three-dimension chitosan hydrogel loading melanin composite nanoparticles for wound healing by anti-bacteria, immune activation and macrophage autophagy promotion. Int. J. Biol. Macromol. 2023, 237, 124176. [Google Scholar] [CrossRef]
- Sun, J.; Jia, W.; Qi, H.; Huo, J.; Liao, X.; Xu, Y.; Wang, J.; Sun, Z.; Liu, Y.; Liu, J.; et al. An Antioxidative and Active Shrinkage Hydrogel Integratedly Promotes Re-Epithelization and Skin Constriction for Enhancing Wound Closure. Adv. Mater. 2024, 36, 2312440. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Gao, X.; Shi, Y.; Cheng, C.; Shi, Z.; Jiao, M.; Cao, F.; Xu, Z.; Li, X.; Zhang, J. Augmented Graphene Quantum Dot-Light Irradiation Therapy for Bacteria-Infected Wounds. ACS Appl. Mater. Interfaces 2020, 12, 40153–40162. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Li, Y.; Hu, J.; Chen, Y.; Huang, J.; Shen, L.; Pan, D.; Li, P. Graphitic-N-doped graphene quantum dots for photothermal eradication of multidrug-resistant bacteria in the second near-infrared window. J. Mater. Chem. B 2022, 10, 3357–3365. [Google Scholar] [CrossRef]
- Cheng, C.; Zhong, H.; Zhang, Y.; Gao, X.; Wang, J.; Liu, J.; Han, X. Bacterial responsive hydrogels based on quaternized chitosan and GQDs-ε-PL for chemo-photothermal synergistic anti-infection in diabetic wounds. Int. J. Biol. Macromol. 2022, 210, 377–393. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.-K. In Vivo Biodistribution and Toxicology of Carboxylated Graphene Quantum Dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef]
- Yu, M.; Li, P.; Huang, R.; Xu, C.; Zhang, S.; Wang, Y.; Gong, X.; Xing, X. Antibacterial and antibiofilm mechanisms of carbon dots: A review. J. Mater. Chem. B 2023, 11, 734–754. [Google Scholar] [CrossRef]
- Shivam, K.; Selvam, A.; Sangam, S.; Majood, M.; Pahari, S.; Patra, R.; Sharma, A.K.; Mukherjee, M. Graphene quantum dots-hybrid hydrogel as an avant-garde biomimetic scaffold for diabetic wound healing. Biomater. Adv. 2023, 149, 213395. [Google Scholar] [CrossRef]
- Purohit, S.S.; Biswal, A.; Mohapatra, P.; Mishra, L.; Mishra, M.; Biswal, S.B.; Swain, S.K. In Vivo Wound Healing in Drosophila melanogaster and Mouse Models: Synergistic Effect of Bovine Serum Albumin and Graphene Quantum Dots. ACS Appl. Bio Mater. 2023, 6, 5531–5540. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Hsiao, W.W.-W.; Hui, Y.Y.; Tsai, P.-C.; Chang, H.-C. Fluorescent Nanodiamond: A Versatile Tool for Long-Term Cell Tracking, Super-Resolution Imaging, and Nanoscale Temperature Sensing. Acc. Chem. Res. 2016, 49, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Iakoubovskii, K.; Baidakova, M.V.; Wouters, B.H.; Stesmans, A.; Adriaenssens, G.J.; Vul, A.Y.; Grobet, P.J. Structure and defects of detonation synthesis nanodiamond. Diamond Relat. Mater. 2000, 9, 861–865. [Google Scholar] [CrossRef]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Niwase, K.; Tanaka, T.; Kakimoto, Y.; Ishihara, K.N.; Shingu, P.H. Raman Spectra of Graphite and Diamond Mechanically Milled with Agate or Stainless Steel Ball-Mill. Mater. Trans. JIM 1995, 36, 282–288. [Google Scholar] [CrossRef][Green Version]
- Boudou, J.-P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, N.; Sundaravel, B.; Panda, K.; David, W.; Kamarrudin, M.; Dash, S.; Panigrahi, B.K.; Tyagi, A.K.; Lin, I.N.; et al. Effect of CH4/H2 plasma ratio on ultra-low friction of nano-crystalline diamond coating deposited by MPECVD technique. Tribol. Int. 2011, 44, 980–986. [Google Scholar] [CrossRef]
- Amaral, M.; Abreu, C.S.; Oliveira, F.J.; Gomes, J.R.; Silva, R.F. Tribological characterization of NCD in physiological fluids. Diamond Relat. Mater. 2008, 17, 848–852. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Pacelli, S.; Acosta, F.; Chakravarti, A.R.; Samanta, S.G.; Whitlow, J.; Modaresi, S.; Ahmed, R.P.H.; Rajasingh, J.; Paul, A. Nanodiamond-based injectable hydrogel for sustained growth factor release: Preparation, characterization and in vitro analysis. Acta Biomater. 2017, 58, 479–491. [Google Scholar] [CrossRef]
- Zeng, Y.; Yi, T.; Ma, J.; Han, M.; Xu, X.; Dan, C.; Chen, X.; Wang, R.; Zhan, Y. Precisely controlled polydopamine-mediated antibacterial system: Mathematical model of polymerization, prediction of antibacterial capacity, and promotion of wound healing. Nanotechnology 2022, 33, 455102. [Google Scholar] [CrossRef]
- Yu, D.; Liu, F. Synthesis of Carbon Nanotubes by Rolling up Patterned Graphene Nanoribbons Using Selective Atomic Adsorption. Nano Lett. 2007, 7, 3046–3050. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.E.; Tune, D.D.; Flavel, B.S. Double-Walled Carbon Nanotube Processing. Adv. Mater. 2015, 27, 3105–3137. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host–Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Wang, L.; Hu, S.; Ullah, M.W.; Li, X.; Shi, Z.; Yang, G. Enhanced cell proliferation by electrical stimulation based on electroactive regenerated bacterial cellulose hydrogels. Carbohydr. Polym. 2020, 249, 116829. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z.; et al. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef]
- Chao, Y.; Yu, S.; Zhang, H.; Gong, D.; Li, J.; Wang, F.; Chen, J.; Zhu, J.; Chen, J. Architecting Lignin/Poly(vinyl alcohol) Hydrogel with Carbon Nanotubes for Photothermal Antibacterial Therapy. ACS Appl. Bio Mater. 2023, 6, 1525–1535. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Schulte, K. Influence of different carbon nanotubes on the mechanical properties of epoxy matrix composites—A comparative study. Compos. Sci. Technol. 2005, 65, 2300–2313. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Song, W.; Ma, Y.; Wu, J.; Fan, L. Poly(vinyl pyrrolidone) wrapped multi-walled carbon nanotube/poly(vinyl alcohol) composite hydrogels. Compos. Part A-Appl. Sci. Manuf. 2011, 42, 1398–1405. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Electrochemically functionalized carbon nanotubes for device applications. J. Mater. Chem. 2008, 18, 3071–3083. [Google Scholar] [CrossRef]
- Fujigaya, T.; Nakashima, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 2015, 16, 024802. [Google Scholar] [CrossRef] [PubMed]
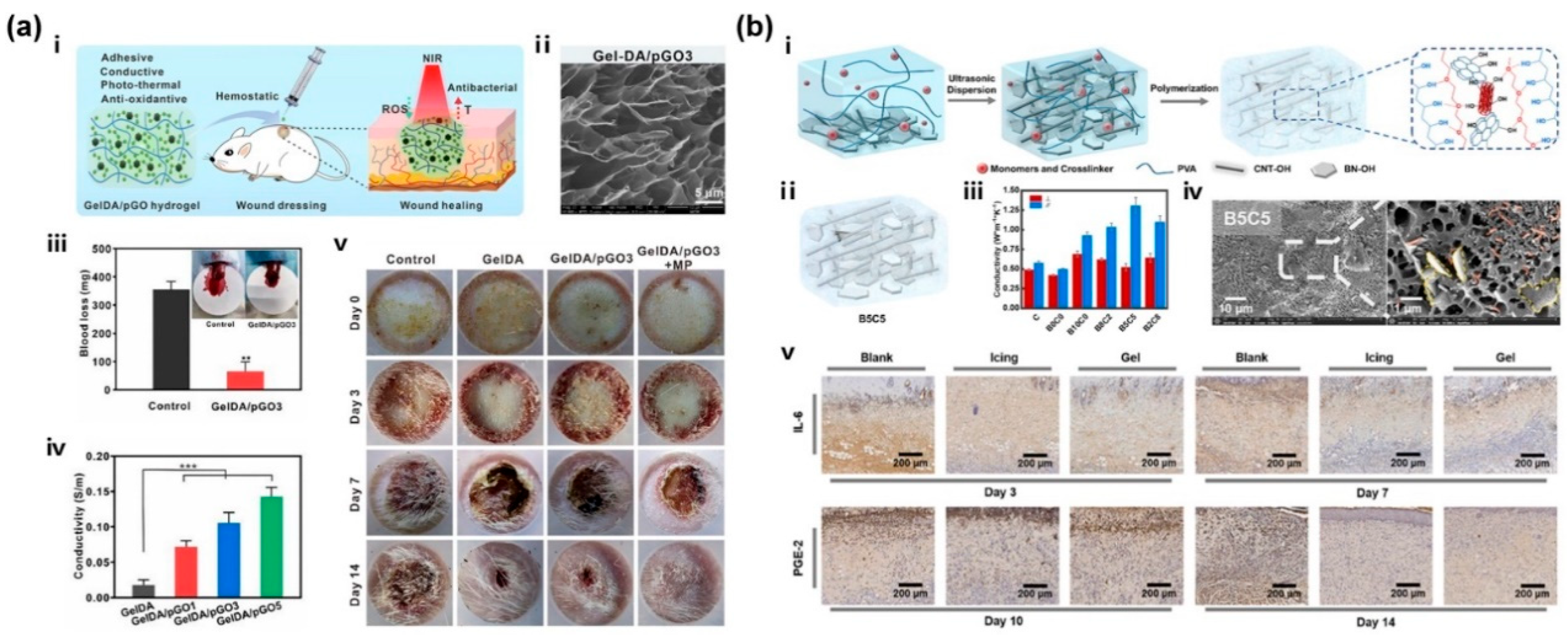
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Mutlu, H.; Malik, S.; Theato, P. Conductive hydrogel composites with autonomous self-healing properties. Soft Matter 2020, 16, 10969–10976. [Google Scholar] [CrossRef]
- Shen, K.; Liu, Z.; Xie, R.; Zhang, Y.; Yang, Y.; Zhao, X.; Zhang, Y.; Yang, A.; Cheng, Y. Nanocomposite conductive hydrogels with Robust elasticity and multifunctional responsiveness for flexible sensing and wound monitoring. Mater. Horiz. 2023, 10, 2096–2108. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Zhang, H.; Zhu, Z.; Fu, S. Cellulose nanocrystal mediated fast self-healing and shape memory conductive hydrogel for wearable strain sensors. Int. J. Biol. Macromol. 2021, 170, 272–283. [Google Scholar] [CrossRef]
- Xu, M.; Li, Q.; Fang, Z.; Jin, M.; Zeng, Q.; Huang, G.; Jia, Y.-G.; Wang, L.; Chen, Y. Conductive and antimicrobial macroporous nanocomposite hydrogels generated from air-in-water Pickering emulsions for neural stem cell differentiation and skin wound healing. Biomater. Sci. 2020, 8, 6957–6968. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzmán, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Durán-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 2020, 242, 116383. [Google Scholar] [CrossRef]
- Bottini, M.; Bruckner, S.; Nika, K.; Bottini, N.; Bellucci, S.; Magrini, A.; Bergamaschi, A.; Mustelin, T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006, 160, 121–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, Y.; Han, F.; Shen, K.; Luo, L.; Li, Y.; Jia, Y.; Zhang, J.; Cai, W.; et al. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact. Mater. 2023, 26, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Song, N.; Huang, Y.; He, C.; Zhang, M.; Zhao, W.; Zhao, C. Improved Cooling Performance of Hydrogel Wound Dressings via Integrating Thermal Conductivity and Heat Storage Capacity for Burn Therapy. Biomacromolecules 2022, 23, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Eitan, A.; Jiang, K.; Dukes, D.; Andrews, R.; Schadler, L.S. Surface Modification of Multiwalled Carbon Nanotubes: Toward the Tailoring of the Interface in Polymer Composites. Chem. Mater. 2003, 15, 3198–3201. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired; antibacterial; conductive; antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Kittana, N.; Assali, M.; Abu-Rass, H.; Lutz, S.; Hindawi, R.; Ghannam, L.; Zakarneh, M.; Mosa, A. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. Int. J. Nanomed. 2018, 13, 7195–7206. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of Curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, M.; Lv, W.; Li, J.; Huang, R.; Wang, Y. Engineering Carbon Nanotube-Based Photoactive COF to Synergistically Arm a Multifunctional Antibacterial Hydrogel. Adv. Funct. Mater. 2024, 34, 2310845. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Adaptive Hydrogels Based on Nanozyme with Dual-Enhanced Triple Enzyme-Like Activities for Wound Disinfection and Mimicking Antioxidant Defense System. Adv. Healthc. Mater. 2022, 11, 2101849. [Google Scholar] [CrossRef]
- Guerrero-Bermea, C.; Rajukumar, L.P.; Dasgupta, A.; Lei, Y.; Hashimoto, Y.; Sepulveda-Guzman, S.; Cruz-Silva, R.; Endo, M.; Terrones, M. Two-dimensional and three-dimensional hybrid assemblies based on graphene oxide and other layered structures: A carbon science perspective. Carbon 2017, 125, 437–453. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-R.; Qin, H.; Cong, H.-P.; Yu, S.-H. A Highly Stretchable and Real-Time Healable Supercapacitor. Adv. Mater. 2019, 31, 1900573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, X.; Xin, X.; Tang, Z.-R.; Xu, Y.-J. Ti3C2Tx-Based Three-Dimensional Hydrogel by a Graphene Oxide-Assisted Self-Convergence Process for Enhanced Photoredox Catalysis. ACS Nano 2019, 13, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Gao, C.; Guo, R.; Lin, C.; Li, J.; Guo, W. Human mesenchymal stem cells encapsulated-coacervated photoluminescent nanodots layered bioactive chitosan/collagen hydrogel matrices to indorse cardiac healing after acute myocardial infarction. J. Photochem. Photobiol. B Biol. 2020, 206, 111789. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Qin, R.; Ao, F.; Wang, X.; Zhang, H.; Yang, M.; Liu, X. Tissue-nanoengineered hyperbranched polymer based multifunctional hydrogels as flexible “wounped treatment-health monitoring” bioelectronic implant. Appl. Mater. Today 2022, 29, 101576. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Madanchi, H.; Aliabadi, H.A.M.; Maleki, A. Graphene oxide/alginate/silk fibroin composite as a novel bionanostructure with improved blood compatibility, less toxicity and enhanced mechanical properties. Carbohydr. Polym. 2020, 248, 116802. [Google Scholar] [CrossRef]
- Schwamb, T.; Burg, B.R.; Schirmer, N.C.; Poulikakos, D. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 2009, 20, 405704. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, L.; Zhang, Z.; Zhou, M.; Gao, Y.; Wang, Y.; Liu, Y.; Qin, J. Biodegradable pectin-based thermo-responsive composite GO/hydrogel with mussel inspired tissue adhesion for NIR enhanced burn wound healing. Chem. Eng. J. 2024, 480, 148067. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. A synergistic antibacterial effect between terbium ions and reduced graphene oxide in a poly(vinyl alcohol)–alginate hydrogel for treating infected chronic wounds. J. Mater. Chem. B 2019, 7, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bai, Q.; Wu, W.; Sun, N.; Cui, N.; Lu, T. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef]
- Shan, M.; Chen, X.; Zhang, X.; Zhang, S.; Zhang, L.; Chen, J.; Wang, X.; Liu, X. Injectable Conductive Hydrogel with Self-Healing, Motion Monitoring, and Bacteria Theranostics for Bioelectronic Wound Dressing. Adv. Healthcare Mater. 2024, 13, 2303876. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119824. [Google Scholar] [CrossRef]
- Ou, X.; Guan, L.; Guo, W.; Zhang, X.; Wu, S.; Guo, D.; Li, R.; Zvyagin, A.V.; Lin, Q.; Qu, W. Graphene oxide-based injectable conductive hydrogel dressing with immunomodulatory for chronic infected diabetic wounds. Mater. Des. 2022, 224, 111284. [Google Scholar] [CrossRef]
- Hao, P.-C.; Burnouf, T.; Chiang, C.-W.; Jheng, P.-R.; Szunerits, S.; Yang, J.-C.; Chuang, E.-Y. Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J. Nanobiotechnology 2023, 21, 318. [Google Scholar] [CrossRef]
- Qureshi, M.A.U.R.; Arshad, N.; Rasool, A.; Rizwan, M.; Rasheed, T. Guar gum-based stimuli responsive hydrogels for sustained release of diclofenac sodium. Int. J. Biol. Macromol. 2023, 250, 126275. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Razak, S.I.A.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef]
- Darban, Z.; Singh, H.; Singh, U.; Bhatia, D.; Gaur, R.; Kuddushi, M.; Dhanka, M.; Shahabuddin, S. β-Carotene laden antibacterial and antioxidant gelatin/polyglyceryl stearate nano-hydrogel system for burn wound healing application. Int. J. Biol. Macromol. 2024, 255, 128019. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, S.; Zhang, D.; Guo, S.; Chang, R.; He, Y.; Yao, M.; Guan, F. Functionalized injectable hyaluronic acid hydrogel with antioxidative and photothermal antibacterial activity for infected wound healing. Int. J. Biol. Macromol. 2022, 210, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Liu, X.; Cao, Y.; Feng, H.; Wang, R.; Xu, Z.; Xiao, L.; Zhu, W. Preparation of quaternized N-halamine modified graphene oxide based antibacterial hydrogel and wound healing of bacterial infection. Colloids Surf. B. Biointerfaces 2023, 229, 113451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Du, H.; Xie, Z.; Li, M.; Zhu, J.; Xu, J.; Zhang, L.; Tao, J.; Zhu, J. Self-adhesive photothermal hydrogel films for solar-light assisted wound healing. J. Mater. Chem. B 2019, 7, 3644–3651. [Google Scholar] [CrossRef]
- Xie, C.; Luo, J.; Luo, Y.; Zhou, J.; Guo, X.; Lu, X. Electroactive Hydrogels with Photothermal/Photodynamic Effects for Effective Wound Healing Assisted by Polydopamine-Modified Graphene Oxide. ACS Appl. Mater. Interfaces 2023, 15, 42329–42340. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yu, W. Functionalized Graphene Oxide-Reinforced Chitosan Hydrogel as Biomimetic Dressing for Wound Healing. Macromol. Biosci. 2021, 21, 2000432. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Chi, R.; Shi, J.; Yang, Y.; Zhang, X. Reduced graphene oxide loaded with MoS2 and Ag3PO4 nanoparticles/PVA interpenetrating hydrogels for improved mechanical and antibacterial properties. Mater. Des. 2019, 183, 108166. [Google Scholar] [CrossRef]
- Huang, H.; He, D.; Liao, X.; Zeng, H.; Fan, Z. An excellent antibacterial and high self-adhesive hydrogel can promote wound fully healing driven by its shrinkage under NIR. Mater. Sci. Eng. C 2021, 129, 112395. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Chen, X.; Xu, C.; Guo, J.; Deng, M.; Qu, X.; Huang, P.; Feng, Z.; Zhang, J. Versatile Hydrogel Dressing with Skin Adaptiveness and Mild Photothermal Antibacterial Activity for Methicillin-Resistant Staphylococcus Aureus-Infected Dynamic Wound Healing. Adv. Sci. 2023, 10, 2206585. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, T.; Zhu, W.; Li, H.-B.; Shen, A.-G. A cooling-driven self-adaptive and removable hydrogel coupled with combined chemo-photothermal sterilization for promoting infected wound healing. Nanoscale 2023, 15, 11163–11178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Xie, X.; Liao, J. An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 2022, 293, 119722. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, Y.; Zhang, S.; Ma, S.; Zhang, H. Multi-functional conductive hydrogels based on heparin–polydopamine complex reduced graphene oxide for epidermal sensing and chronic wound healing. J. Nanobiotechnol. 2023, 21, 343. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C 2021, 118, 111447. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem. Eng. J. 2019, 378, 122043. [Google Scholar] [CrossRef]
- Babaluei, M.; Mojarab, Y.; Mottaghitalab, F.; Farokhi, M. Injectable hydrogel based on silk fibroin/carboxymethyl cellulose/agarose containing polydopamine functionalized graphene oxide with conductivity, hemostasis, antibacterial, and anti-oxidant properties for full-thickness burn healing. Int. J. Biol. Macromol. 2023, 249, 126051. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Khan, A.; Rehman, W.; Alanazi, M.M.; Khan, Y.; Rasheed, L.; Saboor, A.; Iqbal, S. Development of Novel Multifunctional Electroactive, Self-Healing, and Tissue Adhesive Scaffold to Accelerate Cutaneous Wound Healing and Hemostatic Materials. ACS Omega 2023, 8, 39110–39134. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Han, K.; Bai, Q.; Zeng, Q.; Sun, N.; Zheng, C.; Wu, W.; Zhang, Y.; Lu, T. A multifunctional mussel-inspired hydrogel with antioxidant, electrical conductivity and photothermal activity loaded with mupirocin for burn healing. Mater. Des. 2022, 217, 110598. [Google Scholar] [CrossRef]
- He, J.; Li, Z.; Wang, J.; Li, T.; Chen, J.; Duan, X.; Guo, B. Photothermal antibacterial antioxidant conductive self-healing hydrogel with nitric oxide release accelerates diabetic wound healing. Compos. Part B-Eng. 2023, 266, 110985. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, L.; Zhang, X.; He, J.; Huang, Y.; Li, H. Generation of a photothermally responsive antimicrobial, bioadhesive gelatin methacryloyl (GelMA) based hydrogel through 3D printing for infectious wound healing. Int. J. Biol. Macromol. 2024, 260, 129372. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Guo, Y.; Yin, Y.; Qu, X.; Zhang, X.; Li, S.; Xu, X.; Zhou, Z. Composite Hydrogel for the Targeted Capture and Photothermal Killing of Bacteria toward Facilitating Wound Healing. Langmuir 2023, 39, 6413–6424. [Google Scholar] [CrossRef]
- Suneetha, M.; Zo, S.; Choi, S.M.; Han, S.S. Antibacterial; biocompatible; hemostatic, and tissue adhesive hydrogels based on fungal-derived carboxymethyl chitosan-reduced graphene oxide-polydopamine for wound healing applications. Int. J. Biol. Macromol. 2023, 241, 124641. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Hassan, A.; Qureshi, S.; Stojanović, G.M. Ihsan-Ul-Haq, Multifunctional Arabinoxylan-functionalized-Graphene Oxide Based Composite Hydrogel for Skin Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865059. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Yan, J.; Meng, G.; Cui, L.; Li, W.; Liu, Z.; Guo, X. Green Reduction of Graphene Oxide by Macromolecular CMCS to Prepare Self-Healing Conductive Hydrogel Wound Dressing with Drug/Photothermal Antibacterial Activity. Macromol. Mater. Eng. 2022, 307, 2100878. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, M.; Wang, M.; Shao, Z.; Jiang, X.; Wang, K.; Yao, Z.; Yang, S.; Zhang, X.; Gao, W.; et al. Antioxidant, and Antibacterial Scaffolds for Rapid Angiogenesis and Diabetic Wound Repair. Adv. Funct. Mater. 2021, 31, 2100924. [Google Scholar] [CrossRef]
- Hao, J.; Sun, M.; Li, D.; Zhang, T.; Li, J.; Zhou, D. An IFI6-based hydrogel promotes the healing of radiation-induced skin injury through regulation of the HSF1 activity. J. Nanobiotechnol. 2022, 20, 288. [Google Scholar] [CrossRef]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Rational design of antimicrobial peptide conjugated graphene-silver nanoparticle loaded chitosan wound dressing. Int. J. Biol. Macromol. 2023, 246, 125347. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Rehman, R.A.; Moradi, A.-R.; Rizwan, M.; Ashammakhi, N.; Hasan, A. Graphene Oxide-Functionalized Bacterial Cellulose–Gelatin Hydrogel with Curcumin Release and Kinetics: In Vitro Biological Evaluation. ACS Omega 2023, 8, 40024–40035. [Google Scholar] [CrossRef]
- Li, X.; Tan, Z.; Guo, B.; Yu, C.; Yao, M.; Liang, L.; Wu, X.; Zhao, Z.; Yao, F.; Zhang, H.; et al. Magnet-oriented hydrogels with mechanical–electrical anisotropy and photothermal antibacterial properties for wound repair and monitoring. Chem. Eng. J. 2023, 463, 142387. [Google Scholar] [CrossRef]
- Dey, A.; Khan, F.; Mukhopadhyay, M.; Mukhopadhyay, J.; Mukherjee, M.; Dutta, K.; Das, S. Amphiphilically engineered sodium deoxycholate based nanocomposite hydrogels with strong bactericidal and water absorption characteristics. Mater. Today Commun. 2023, 34, 105353. [Google Scholar] [CrossRef]
- Qiao, Z.; Ding, J.; Wu, C.; Zhou, T.; Wu, K.; Zhang, Y.; Xiao, Z.; Wei, D.; Sun, J.; Fan, H. One-Pot Synthesis of Bi2S3/TiO2/rGO Heterostructure with Red Light-Driven Photovoltaic Effect for Remote Electrotherapy-Assisted Wound Repair. Small 2023, 19, 2206231. [Google Scholar] [CrossRef]
- Zhou, L.; Min, T.; Bian, X.; Dong, Y.; Zhang, P.; Wen, Y. Rational Design of Intelligent and Multifunctional Dressing to Promote Acute/Chronic Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4055–4085. [Google Scholar] [CrossRef]
- Chin, J.S.; Madden, L.; Chew, S.Y.; Becker, D.L. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv. Drug Del. Rev. 2019, 149–150, 2–18. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, B.; Liou, Y.-C.; Huang, C. The pathogenesis and diagnosis of sepsis post burn injury. Burn. Trauma 2021, 9, tkaa047. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model. Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhou, D.; Gao, Y.; Zeng, M.; Wang, W. miRNA delivery for skin wound healing. Adv. Drug Del. Rev. 2018, 129, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Del. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Controlled Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Gong, C.; Guan, W.; Liu, X.; Zheng, Y.; Li, Z.; Zhang, Y.; Zhu, S.; Jiang, H.; Cui, Z.; Wu, S. Biomimetic Bacteriophage-Like Particles Formed from Probiotic Extracts and NO Donors for Eradicating Multidrug-Resistant Staphylococcus aureus. Adv. Mater. 2022, 34, 2206134. [Google Scholar] [CrossRef]
- Zhou, J.; Mei, J.; Liu, Q.; Xu, D.; Wang, X.; Zhang, X.; Zhu, W.; Zhu, C.; Wang, J. Spatiotemporal On–Off Immunomodulatory Hydrogel Targeting NLRP3 Inflammasome for the Treatment of Biofilm-Infected Diabetic Wounds. Adv. Funct. Mater. 2023, 33, 2211811. [Google Scholar] [CrossRef]
- Huang, B.; Guan, W.; Wang, C.; Wu, S.; Cui, Z.; Zheng, Y.; Li, Z.; Zhu, S.; Jiang, H.; Chu, P.K.; et al. S–Cu–FC/CuS modified GO carboxymethyl cellulose hydrogel for enhanced photocatalytic sterilization through homo-heterojunction interface accelerated charge transfer. Biomater. Sci. 2023, 11, 3589–3602. [Google Scholar] [CrossRef]
- Lu, T.; Sun, M.; Zhou, Y.; Tu, W.; Ni, Z.; Li, X.; Hu, T. Self-powered chitosan/graphene oxide hydrogel band-aids with bioadhesion for promoting infected wounds healing. Int. J. Biol. Macromol. 2024, 283, 137374. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Kolipaka, T.; Pandey, G.; Abraham, N.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Rajinikanth, P.S.; Tickoo, V.; Srivastava, S. Stimuli-responsive polysaccharide-based smart hydrogels for diabetic wound healing: Design aspects, preparation methods and regulatory perspectives. Carbohydr. Polym. 2024, 324, 121537. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Li, H.; Chang, L.; Du, W.W.; Gupta, S.; Khorshidi, A.; Sefton, M.; Yang, B.B. Anti-microRNA-378a Enhances Wound Healing Process by Upregulating Integrin Beta-3 and Vimentin. Mol. Ther. 2014, 22, 1839–1850. [Google Scholar] [CrossRef]
- Kasiewicz, L.N.; Whitehead, K.A. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng. Transl. Med. 2019, 4, 75–82. [Google Scholar] [CrossRef]
- Lee, R.S.B.; Hamlet, S.M.; Moon, H.-J.; Ivanovski, S. Re-establishment of macrophage homeostasis by titanium surface modification in type II diabetes promotes osseous healing. Biomaterials 2021, 267, 120464. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Lin, W.-C.; Huang, C.-C.; Lin, S.-J.; Li, M.-J.; Chang, Y.; Lin, Y.-J.; Wan, W.-L.; Shih, P.-C.; Sung, H.-W. In situ depot comprising phase-change materials that can sustainably release a gasotransmitter H2S to treat diabetic wounds. Biomaterials 2017, 145, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Chen, B.; Zhao, T.; Gu, Z. Superabsorbent poly(acrylic acid) and antioxidant poly(ester amide) hybrid hydrogel for enhanced wound healing. Regener. Biomater. 2021, 8, rbaa059. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Wu, S.; Dong, H.; Deng, S.; Liu, Y.; Zhang, W.; Feng, G.; Lei, L.; Xie, H. Accelerated Healing of Infected Diabetic Wounds by a Dual-Layered Adhesive Film Cored with Microsphere-Loaded Hydrogel Composite Dressing. ACS Appl. Mater. Interfaces 2023, 15, 33207–33222. [Google Scholar] [CrossRef]
- Teng, L.; Song, Y.; Hu, L.; Bai, Q.; Zhang, X.; Dong, C.-M. Nitric Oxide-Releasing Poly(L-glutamic acid) Hybrid Hydrogels for Accelerating Diabetic Wound Healing. Chin. J. Chem. 2023, 41, 2103–2112. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.; Xue, H.; Liu, G.; Wang, N.; Shao, Z. MiR-21 regulating PVT1/PTEN/IL-17 axis towards the treatment of infectious diabetic wound healing by modified GO-derived biomaterial in mouse models. J. Nanobiotechnol. 2022, 20, 309. [Google Scholar] [CrossRef]
- Wu, S.; Qin, B.; Tang, X.; Cui, T.; Yin, S.; Dong, H.; Liu, Y.; Deng, S.; Zhang, H.; Feng, G.; et al. Enzyme-responsive microneedle patch for bacterial infection and accelerated healing of diabetic wounds. Chem. Eng. J. 2023, 466, 143126. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Roy, M.; Goswami, P.; Roy, S.; Das, A.K.; Ghosh, S.K.; Dhara, S. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J. Mater. Chem. B 2020, 8, 9277–9294. [Google Scholar] [CrossRef]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef]
- Holzer, J.C.J.; Tiffner, K.; Kainz, S.; Reisenegger, P.; de Mattos, I.B.; Funk, M.; Lemarchand, T.; Laaff, H.; Bal, A.; Birngruber, T.; et al. A novel human ex-vivo burn model and the local cooling effect of a bacterial nanocellulose-based wound dressing. Burns 2020, 46, 1924–1932. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns & Trauma 2014, 2, 2321–3868.143616. [Google Scholar] [CrossRef]
- Li, Q.; Shen, X.; Liu, C.; Xing, D. Facile wound dressing replacement: Carbon dots for dissolving alginate hydrogels via competitive complexation. Int. J. Biol. Macromol. 2023, 240, 124455. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Song, N.; Huang, Y.; Wang, W.; He, C.; Zhao, W.; Zhao, C. Enhanced thermal conductive and moisturizing hydrogels by constructing 3D networks of BN-OH and CNT-OH in alignment for burn therapy. Mater. Des. 2023, 233, 112239. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Song, J.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X.; et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2020, 30, 1905493. [Google Scholar] [CrossRef]
- Mokrý, M.; Gál, P.; Vidinský, B.; Kušnír, J.; Dubayová, K.; Mozeš, Š.; Sabo, J. In Vivo Monitoring the Changes of Interstitial pH and FAD/NADH Ratio by Fluorescence Spectroscopy in Healing Skin Wounds. Photochem. Photobiol. 2006, 82, 793–797. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the Neutrophil Phagosome: Oxidants, Myeloperoxidase, and Bacterial Killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef]
- McLister, A.; McHugh, J.; Cundell, J.; Davis, J. New Developments in Smart Bandage Technologies for Wound Diagnostics. Adv. Mater. 2016, 28, 5732–5737. [Google Scholar] [CrossRef]
- Hajnsek, M.; Schiffer, D.; Harrich, D.; Koller, D.; Verient, V.; Palen, J.V.D.; Heinzle, A.; Binder, B.; Sigl, E.; Sinner, F.; et al. An electrochemical sensor for fast detection of wound infection based on myeloperoxidase activity. Sens. Actuators B Chem. 2015, 209, 265–274. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef]
- Zheng, X.T.; Zhong, Y.; Chu, H.E.; Yu, Y.; Zhang, Y.; Chin, J.S.; Becker, D.L.; Su, X.; Loh, X.J. Carbon Dot-Doped Hydrogel Sensor Array for Multiplexed Colorimetric Detection of Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17675–17687. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Wei, W.; Dai, H. A Multifunctional Hydrogel with Photothermal Antibacterial and AntiOxidant Activity for Smart Monitoring and Promotion of Diabetic Wound Healing. Adv. Funct. Mater. 2024, 34, 2402531. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Gong, M.; Zhan, Y.; Yu, Z.; Shen, C.; Zhang, Y.; Yu, L.; Chen, Z. Integrated photo-inspired antibacterial polyvinyl alcohol/carboxymethyl cellulose hydrogel dressings for pH real-time monitoring and accelerated wound healing. Int. J. Biol. Macromol. 2023, 238, 124123. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Tang, J.; Ning, H.; Hu, N.; Zhu, Y.; Gong, Y.; Xu, C.; Zhao, Q.; Jiang, X.; Hu, X.; et al. Multifunctional Ionic Skin with Sensing, UV-Filtering, Water-Retaining, and Anti-Freezing Capabilities. Adv. Funct. Mater. 2021, 31, 2011176. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Wang, Q.; Dan, N.; Chen, Y.; Li, Z.; Dan, W.; Wang, Y. Wearable and Flexible Hydrogels for Strain Sensing and Wound Electrical Stimulation. Ind. Eng. Chem. Res. 2023, 62, 5468–5481. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Wang, M.; Liu, D.; Wang, Z. In situ reduction strategy towards high conductivity, anti-freezing and super-stretchable rGO based hydrogel for diverse flexible electronics. Nano Res. 2024, 17, 4016–4022. [Google Scholar] [CrossRef]

| Nanocomposite Hydrogels | Functionalization of GO | Interaction Mechanism | Functional Requirements | References |
|---|---|---|---|---|
| QNGH | quaternized N-halamine (QN) | Hydrogen bonds | Conductive, antibacterial, and real-time monitoring properties | [153] |
| PEI-rGO-PDA | polyethyleneimine (PEI) | Covalent bonds, hydrogen bonds | Self-adhesive, photothermal, and antibacterial properties, certain anti-inflammatory effect | [154] |
| ICG-PGO-CaP-PVA | PDA, indocyanine green (ICG) | Reversible hydrogen bonds | Photothermal and photodynamic antibacterial effects, self-healing, Ca2+ release, electroactivity, ROS-scavenging activity | [155] |
| CS-CGO | CS | Hydrogen bonds | High strength, excellent biocompatibility | [156] |
| rGO/MoS2/Ag3PO4 | MoS2, Ag3PO4 | Strong interfacial interactions | Photothermal and photodynamic antibacterial properties, certain anti-inflammatory effects, radical scavenging activity | [157] |
| PDA@Ag5GO1 | PDA, Ag NPs | Covalent bonds | Self-adhesive and antibacterial properties, NIR-driven shrinkage, certain anti-inflammatory effects | [158] |
| rGB/QCS/PDA-PAM | 3-Aminophenylboronic acid | Phenol–amine covalent bonds, dynamic borate ester bonds, hydrogen bonds, π–π stacking | Photothermal antibacterial activity, ROS production, adhesion, self-healing, hemostasis, and bacterial capture ability | [159] |
| QCS-CD-AD/GO | β-cyclodextrin | Host–guest interaction, hydrogen bonds | Injectable self-healing properties, conductivity, photothermal antibacterial activity, certain anti-inflammatory effects | [160] |
| rGO@PDA/Ag-PF127 | PDA, Ag NPs | Dynamic borate ester bonds, thermo-reversible gel–sol transition | Photothermal–chemical antimicrobial performance with Ag+ release, adhesive, antioxidant, and hemostatic properties, certain anti-inflammatory effects | [161] |
| GO-BPEI/CMCS/PEG-CHO | Branched polyethyleneimine (BPEI) | Dynamic Schiff base bonds | Injectable, self-healing and photothermal properties | [162] |
| QCS/rGO-PDA/PNIPAm | PDA | Schiff base bonds, hydrogen bonds, cation–π interaction | Thermoresponsive self-contraction, tissue adhesion, temperature-dependent drug release, conductive, self-healing, antibacterial, antioxidant, and anti-inflammatory properties | [163] |
| Hep-PDA-rGO-PAM | Hep, PDA | Hydrophobic bonds, hydrogen bonds, ionic forces | Conductive, antibacterial, antioxidative, and real-time motion monitoring properties | [164] |
| β-GO/RB/PVA | -NH2, β-CD-DA | Hydrogen bonds | Photothermal and photodynamic antibacterial properties, certain anti-inflammatory effects | [165] |
| ZnO QDs@GO-CS | ZnO QDs | Electrostatic interactions | Photothermal and chemodynamic antibacterial activity with Zn2+ release and ROS generation | [166] |
| SF/CMC/AG&GO@PDA | PDA | Covalent bonds, hydrogen bonds | Injectable, conductive, antibacterial, hemostatic, and anti-inflammatory properties | [167] |
| Cu, N-CDs@GO-CS | Cu, N-CDs | Electrostatic interactions | Photothermal, photodynamic and inherent antibacterial effects | [67] |
| Gel/GO-βCD-BNN6 | β-CD, BNN6 | Covalent bonds, hydrogen bonds | Photothermal effect, NO release, antibacterial activity, anti-inflammatory effect, adhesiveness | [168] |
| GATP-PVA | Ag NPs, TGA | Hydrogen bonds, π–π interactions, electrostatic interactions | Electroactive, self-healing, tissue adhesive, antibacterial, and antioxidant properties, autolytic debridement | [169] |
| PC/GO/Met | PDA | Schiff-base bonds, phenylboronate ester dynamic bonds | pH/glucose dual-responsive metformin release, adhesive, self-healing, antibacterial, antioxidant, conductive, hemostatic, and anti-inflammatory properties | [170] |
| GelDA/pGO | PDA | Covalent bonds | Adhesive, hemostatic, conductive, antioxidant, and photothermal antibacterial properties | [171] |
| CMCS/THB/Cu/GB | BNN6 | Dynamic Schiff base bonds, coordination complexation, non-covalent interactions | Conductive, self-healing, antioxidant, and photothermal antibacterial properties, NO release | [172] |
| GelMA/C-CNF/GelMA-DA | PDA | Covalent bonds | Adhesive, hemostatic, conductive, antioxidant, and photothermal antibacterial properties | [173] |
| ABA-GO/CNC/CMCS | 3-Aminobenzene boronic acid | Electrostatic interaction, hydrogen bonds | Photothermal antibacterial, bacterial capture, and anti-inflammatory abilities | [174] |
| FC-rGO-PDA | PDA | Covalent bonds, π–π stacking, hydrogen bonds, electrostatic interactions | Antibacterial, hemostatic, and tissue adhesive properties | [175] |
| GO-arabinoxylan/PVA | Arabinoxylan | Covalent bonds, hydrogen bonds | Antibacterial and anticancer activities | [176] |
| PEP-AG | Ag NPs | Ag–amino coordination interaction | Thermoresponsive, sprayable, and antibacterial properties | [177] |
| OP/CMCS-RGO | CMCS | Schiff base condensation, hydrogen bonds | Self-healing and conductive properties, drug/photothermal antibacterial activity | [178] |
| GDFE | PDA | Hydrophilic-hydrophobic interaction, hydrogen bonds, Schiff base bonds | Injectable, thermosensitive, self-healing, antibacterial, antioxidant, conductive, and anti-inflammatory properties | [179] |
| IFI6-PDA@GO/SA | PDA | Hydrogen and π bonding interactions | Sprayable, antibacterial, and antioxidant characteristics | [180] |
| CGAPL | Ag NPs, ε-poly-L-lysine | Hydrogen bond, electrostatic, Schiff base, and hydrophobic/π–π interactions | Antimicrobial and anti-inflammatory effects | [181] |
| HA-DA/rGO | PDA | Covalent bonds, hydrogen bonds, π–π stacking | Adhesive, antioxidative, hemostatic, conductive, photothermal antibacterial, and drug release properties | [182] |
| PGCNSH | PDA | Covalent bonds, hydrogen bonding | Conductive and implantable properties, physiological signals detection | [183] |
| GO-f-BC/gelatin | bacterial cellulose (BC) | Covalent bonds, hydrogen bonding | Drug release, antibacterial activity | [184] |
| GOH-MPG | PDA, Fe3O4 | Schiff base bonds, hydrogen bonds | Anisotropic, conductive, and photothermal antibacterial properties, rehabilitation training monitoring, certain anti-inflammatory effects | [185] |
| SDS-rGO-NaDC | Sodium dodecylsulfate | Hydrogen bonding, π–π stacking, hydrophobic interactions | Antibacterial activity | [186] |
| BCHA | Bi2S3, TiO2 NPs | Dynamic imine bonds, hydrogen bonds | Adhesive, photovoltaic, conductive, and anti-inflammatory properties, free radical scavenging ability | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Chen, Y.; Sun, M.; Chen, Y.; Tu, W.; Zhou, Y.; Li, X.; Hu, T. Multifunctional Carbon-Based Nanocomposite Hydrogels for Wound Healing and Health Management. Gels 2025, 11, 345. https://doi.org/10.3390/gels11050345
Lu T, Chen Y, Sun M, Chen Y, Tu W, Zhou Y, Li X, Hu T. Multifunctional Carbon-Based Nanocomposite Hydrogels for Wound Healing and Health Management. Gels. 2025; 11(5):345. https://doi.org/10.3390/gels11050345
Chicago/Turabian StyleLu, Tianyi, Yaqian Chen, Meng Sun, Yuxian Chen, Weilong Tu, Yuxuan Zhou, Xiao Li, and Tao Hu. 2025. "Multifunctional Carbon-Based Nanocomposite Hydrogels for Wound Healing and Health Management" Gels 11, no. 5: 345. https://doi.org/10.3390/gels11050345
APA StyleLu, T., Chen, Y., Sun, M., Chen, Y., Tu, W., Zhou, Y., Li, X., & Hu, T. (2025). Multifunctional Carbon-Based Nanocomposite Hydrogels for Wound Healing and Health Management. Gels, 11(5), 345. https://doi.org/10.3390/gels11050345






