Mechanically Robust and Conductive Gelatin/Glucose Hydrogels Enabled by the Hofmeister Effect for Flexible Strain Sensors
Abstract
1. Introduction
2. Results and Discussion
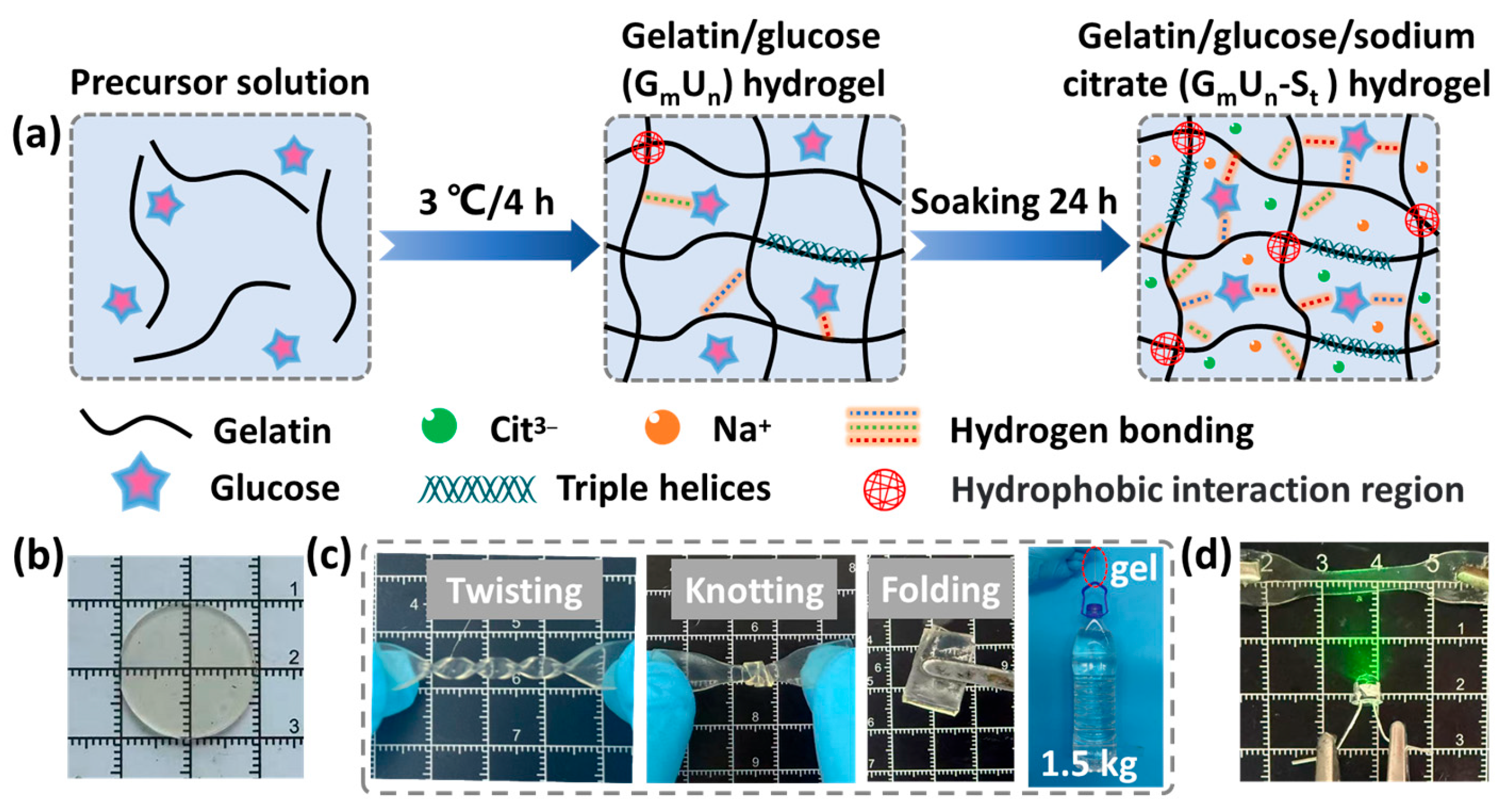
2.1. Synthesis of Tough Hydrogels
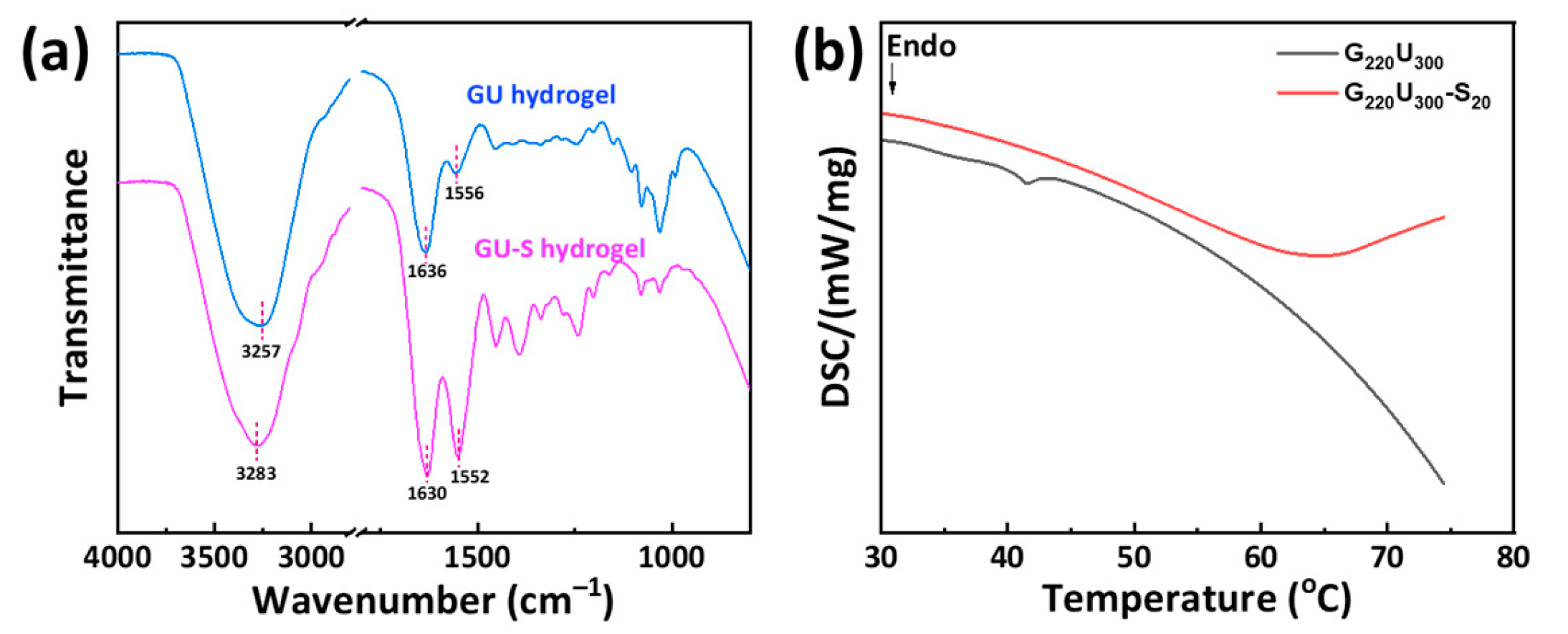
2.2. Structural Characterizations
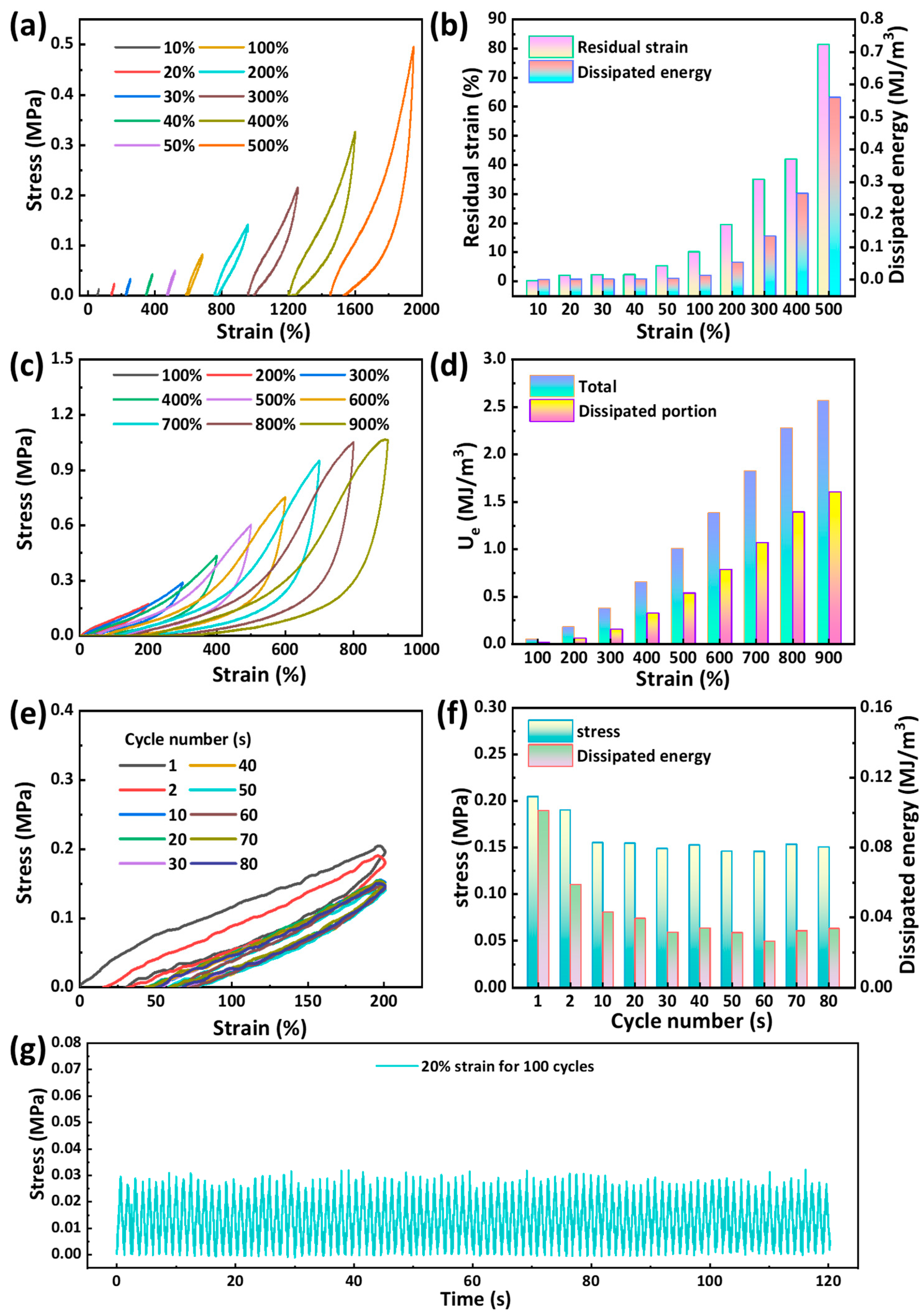
2.3. Mechanical Properties
2.4. Conductivity of GmUn-St Hydrogels
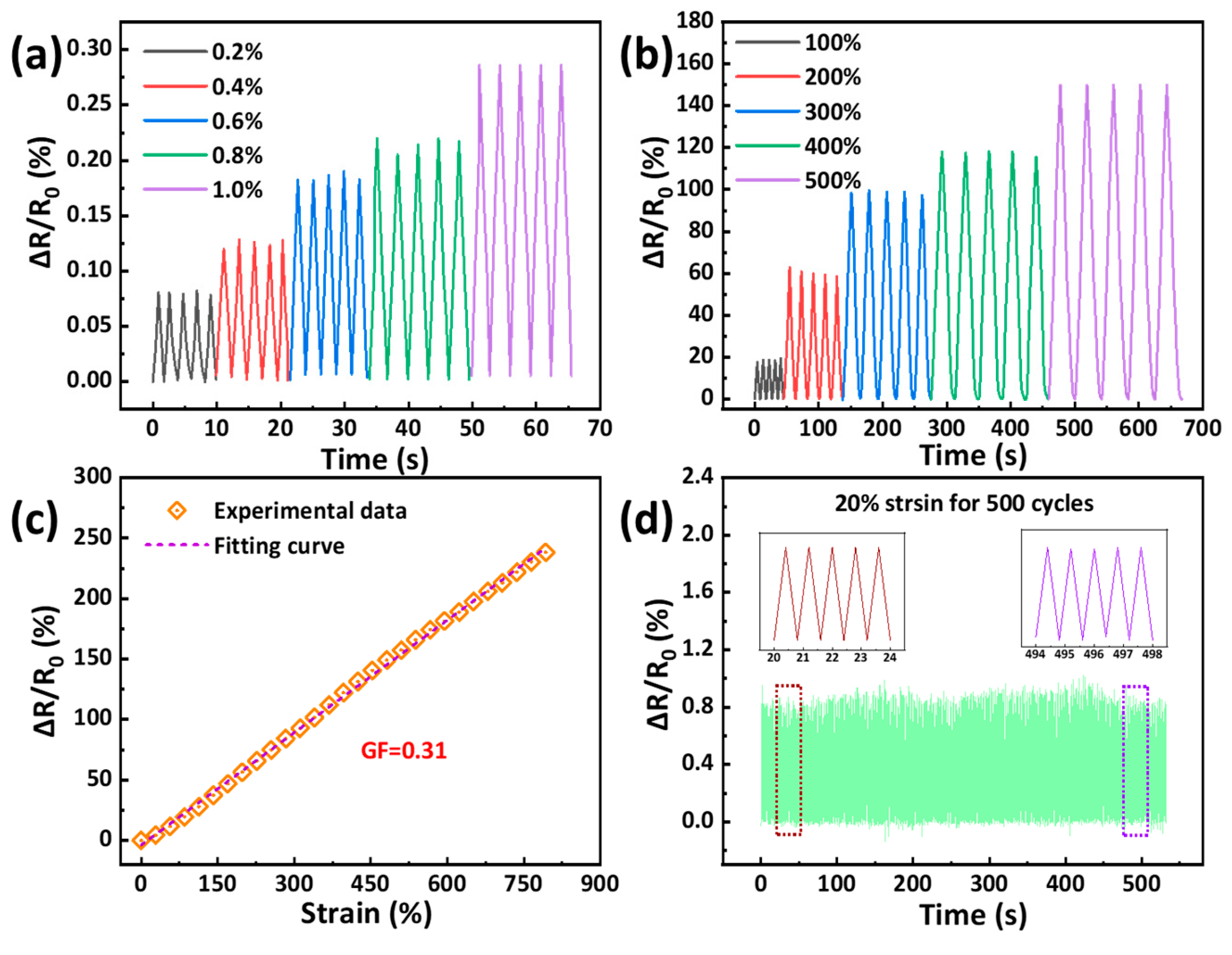
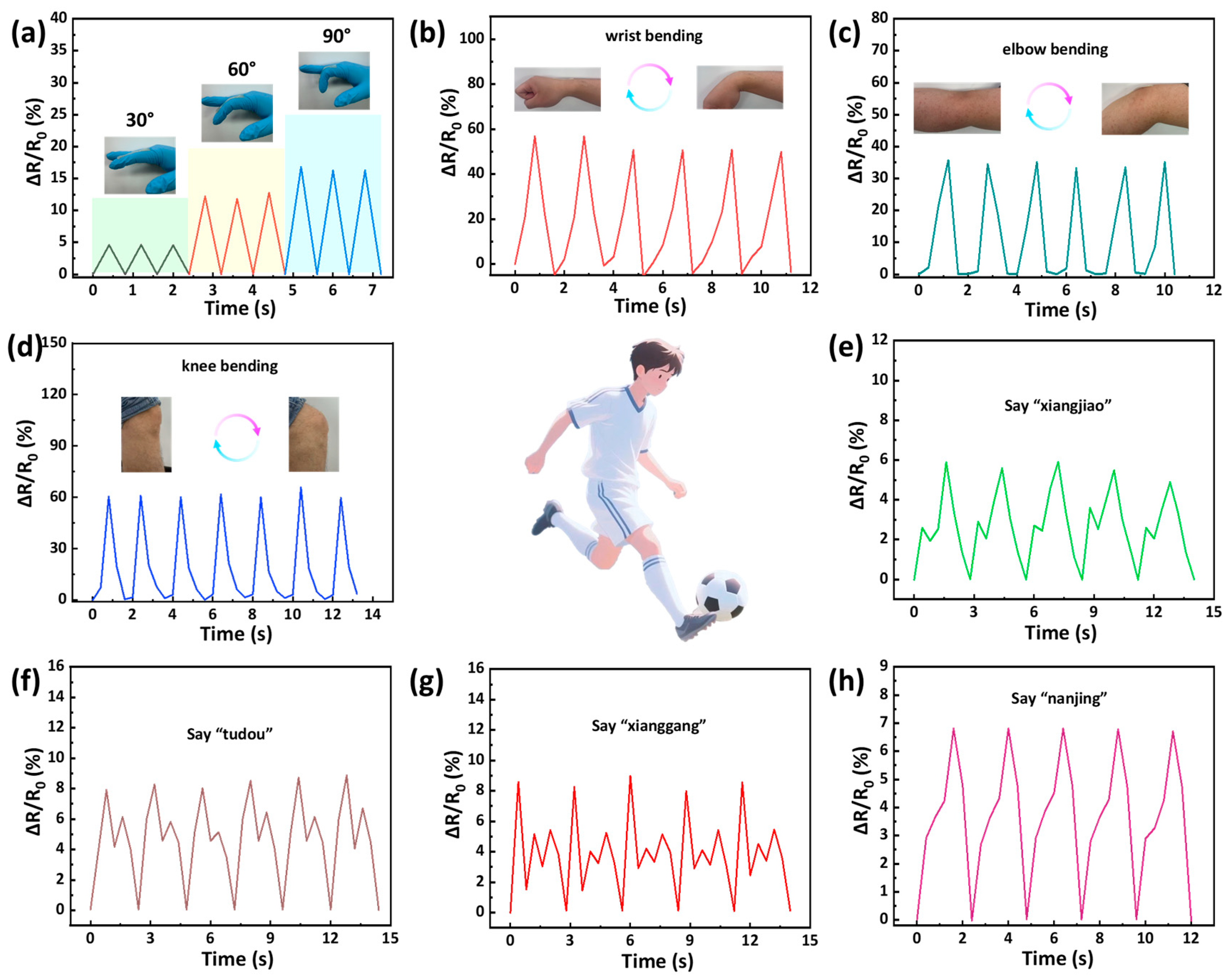
2.5. Sensing Performance and Application for Monitoring Human Motions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.3. Structural and Performance Characterizations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, A.; Afshari, R.; Jain, S.; Zheng, Y.; Lin, M.-H.; Zenkar, S.; Yin, J.; Chen, J.; Peppas, N.A.; Annabi, N. Advances in conducting nanocomposite hydrogels for wearable biomonitoring. Chem. Soc. Rev. 2025, 54, 2595–2652. [Google Scholar] [CrossRef]
- Arwani, R.T.; Tan, S.C.L.; Sundarapandi, A.; Goh, W.P.; Liu, Y.; Leong, F.Y.; Yang, W.; Zheng, X.T.; Yu, Y.; Jiang, C. Stretchable ionic–electronic bilayer hydrogel electronics enable in situ detection of solid-state epidermal biomarkers. Nat. Mater. 2024, 23, 1115–1122. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Yang, X.; Wang, L.; Li, G.; Wong, T.-W.; Li, T.; Yang, W.; Luo, Z. Hydro-locking in hydrogel for extreme temperature tolerance. Science 2025, 387, 967–973. [Google Scholar] [CrossRef]
- Liang, C.; Dudko, V.; Khoruzhenko, O.; Hong, X.; Lv, Z.-P.; Tunn, I.; Umer, M.; Timonen, J.V.; Linder, M.B.; Breu, J. Stiff and self-healing hydrogels by polymer entanglements in co-planar nanoconfinement. Nat. Mater. 2025, 24, 599–606. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Mogadam, E.; Yu, C.H.; Kimball, W.; Annabi, N. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Shon, A.; Joseph, R.; Pavlov, A.; Stefanov, A.; Namkoong, M.; Guo, H.; Bui, D.; Master, R.; Sharma, A. Soft, stretchable conductive hydrogels for high-performance electronic implants. Sci. Adv. 2025, 11, eads4415. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors–A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tropp, J.; Collins, C.P.; Xie, X.; Daso, R.E.; Mehta, A.S.; Patel, S.P.; Reddy, M.M.; Levin, S.E.; Sun, C.; Rivnay, J. Conducting polymer nanoparticles with intrinsic aqueous dispersibility for conductive hydrogels. Adv. Mater. 2024, 36, 2306691. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Mogli, G.; Reina, M.; Chiappone, A.; Lamberti, A.; Pirri, C.F.; Roppolo, I.; Stassi, S. Self-powered integrated tactile sensing system based on ultrastretchable, self-healing and 3D printable ionic conductive hydrogel. Adv. Funct. Mater. 2024, 34, 2307133. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, Y.; Zhu, R.; Mu, Q.; Wang, X.; Wang, Z.; Liu, S.; Li, M.; Ran, R. Strong tough conductive hydrogels via the synergy of ion-induced cross-linking and salting-out. Adv. Funct. Mater. 2022, 32, 2204823. [Google Scholar] [CrossRef]
- Dong, X.; Guo, X.; Liu, Q.; Zhao, Y.; Qi, H.; Zhai, W. Strong and tough conductive organo-hydrogels via freeze-casting assisted solution substitution. Adv. Funct. Mater. 2022, 32, 2203610. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose nanofibrils enhanced, strong, stretchable, freezing-tolerant ionic conductive organohydrogel for multi-functional sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Wang, W.; Guo, P.; Liu, X.; Chen, M.; Li, J.; Hu, Z.; Li, G.; Chang, Q.; Shi, K.; Wang, X. Fully polymeric conductive hydrogels with low hysteresis and high toughness as multi-responsive and self-powered wearable sensors. Adv. Funct. Mater. 2024, 34, 2316346. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough hydrogels with rapid self-reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, S.; Shi, S.; Jiang, Y.; Du, Z.; Wang, B.; Li, X. Hybrid assembly of conducting nanofiber network for ultra-stretchable and highly sensitive conductive hydrogels. J. Mater. Sci. Technol. 2024, 169, 1–10. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Liu, C.; Ye, Y.; Sun, Z.; Wang, B.; Luo, Z. Conductive polymer hydrogels crosslinked by electrostatic interaction with PEDOT: PSS dopant for bioelectronics application. Chem. Eng. J. 2022, 429, 132430. [Google Scholar] [CrossRef]
- Deng, Y.; Hussain, I.; Kang, M.; Li, K.; Yao, F.; Liu, S.; Fu, G. Self-recoverable and mechanical-reinforced hydrogel based on hydrophobic interaction with self-healable and conductive properties. Chem. Eng. J. 2018, 353, 900–910. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Guo, J.; Liu, L.; Fu, H.; Yao, J.; Krucinska, I.; Draczynski, Z. Highly strong, tough, and stretchable conductive hydrogels based on silk sericin-mediated multiple physical interactions for flexible sensors. ACS Appl. Polym. Mater. 2021, 4, 618–626. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Yang, J.; Niu, S.; Pan, X.; Lu, W.; Li, L.; Xu, Y.; Cui, J.; Ho, G.W. Scalable Layered Heterogeneous Hydrogel Fibers with Strain-Induced Crystallization for Tough, Resilient, and Highly Conductive Soft Bioelectronics. Adv. Mater. 2024, 36, 2409632. [Google Scholar] [CrossRef]
- Yang, B.; Wang, C.; Yu, Q.; Ma, P.; Zhao, Q.; Wu, Y.; Ma, K.; Tan, S. Strong acid enabled comprehensive training of poly (sodium acrylate) hydrogel networks. Angew. Chem. Int. Ed. 2024, 63, e202406407. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Chen, W.; Yang, J.; Yu, H.; Lv, R.; Fu, M. Free-standing and flexible polyvinyl alcohol-sodium alginate-polypyrrole electrodes based on interpenetrating network hydrogels. J. Colloid Interface Sci. 2024, 664, 299–308. [Google Scholar] [CrossRef]
- Ghazizadeh, E.; Sadeghi, M.; Deigner, H.-P.; Neshastehriz, A. Engineered Sustainable Mxene-PVA Hydrogel as an Inspiring Co-Delivery Carrier for Targeting Solid Tumors. Pharmaceutics 2025, 17, 823. [Google Scholar] [CrossRef]
- Dang, X.; Guo, B.; Fu, Y.; Fei, Y.; Wang, X. All-natural biomass-based multifunctional conductive hydrogel for an all-in-one wearable strain sensor. Cell Biomater. 2025, 1, 100076. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and tough hydrogel through physical cross-linking and molecular alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef]
- Guo, G.; Chen, Y.; Liu, X.; Zhu, D.Y.; Zhang, B.; Lin, N.; Gao, L. Tough and durable hydrogels with robust skin layers formed via soaking treatment. J. Mater. Chem. B 2018, 6, 8043–8054. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, C.; Jiang, S.; Liu, L.; Yao, J. Strengthening gelatin hydrogels using the Hofmeister effect. Soft Matter 2021, 17, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Y.; Li, D.; Mo, X.; Pan, J. Hofmeister effect-enhanced gelatin/oxidized dextran hydrogels with improved mechanical properties and biocompatibility for wound healing. Acta Biomater. 2022, 151, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Bao, Y.; Lin, B.; Cheng, G.; Yuan, N.; Ding, J. Multifunctional PVA/gelatin DN hydrogels with strong mechanical properties enhanced by Hofmeister effect. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133833. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, C.; Jiang, S.; Liu, L.; Yao, J. Hofmeister effect in gelatin-based hydrogels with shape memory properties. Colloids Surf. B Biointerfaces 2022, 217, 112674. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Tian, Y.; Wang, S.; Du, M. Strong fish gelatin hydrogels enhanced by carrageenan and potassium sulfate. Food Hydrocoll. 2021, 119, 106841. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Wang, X.; He, S.; Yao, F.; Li, J. A transparent, ultrastretchable and fully recyclable gelatin organohydrogel based electronic sensor with broad operating temperature. J. Mater. Chem. A 2020, 8, 4447–4456. [Google Scholar] [CrossRef]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Ma, W. In vivo noninvasive monitoring of glucose concentration in human epidermis by mid-infrared pulsed photoacoustic spectroscopy. Anal. Chem. 2013, 85, 1013–1020. [Google Scholar] [CrossRef]
- Wei, S.; Xu, J.; Zhao, W.; Li, X.; Zhao, W.; Yan, S. Mechanically robust gelatin gel for sensitive touch sensor based on electrode potential. Adv. Funct. Mater. 2024, 34, 2408648. [Google Scholar] [CrossRef]
- He, Q.; Huang, Y.; Wang, S. Hofmeister effect-assisted one step fabrication of ductile and strong gelatin hydrogels. Adv. Funct. Mater. 2018, 28, 1705069. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, X.; Li, J.; Tian, Z.; Xiao, B.; Yi, S.; Duan, L. Coordination with zirconium: A facile approach to improve the mechanical properties and thermostability of gelatin hydrogel. Int. J. Biol. Macromol. 2022, 205, 595–603. [Google Scholar] [CrossRef]
- Yue, Y.; Yokota, Y.; Matsuba, G. Polyelectrolyte-layered hydrogels with electrically tunable toughness, viscoelasticity, hysteresis, and crack resistance. Macromolecules 2022, 55, 1230–1238. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, M.; Cao, X.; Wang, T.; Wang, Y.; Xu, Y.; Liu, W.; Chen, L.; Huang, Y.; Liu, X. Highly flexible interconnected Li+ ion-sieve porous hydrogels with self-regulating nanonetwork structure for marine lithium recovery. Chem. Eng. J. 2022, 445, 136780. [Google Scholar] [CrossRef]
- Chhetry, A.; Sharma, S.; Barman, S.C.; Yoon, H.; Ko, S.; Park, C.; Yoon, S.; Kim, H.; Park, J.Y. Black phosphorus@ laser-engraved graphene heterostructure-based temperature–strain hybridized sensor for electronic-skin applications. Adv. Funct. Mater. 2021, 31, 2007661. [Google Scholar] [CrossRef]
- Shin, S.-H.; Lee, W.; Kim, S.-M.; Lee, M.; Koo, J.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Ion-conductive self-healing hydrogels based on an interpenetrating polymer network for a multimodal sensor. Chem. Eng. J. 2019, 371, 452–460. [Google Scholar] [CrossRef]
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R.; Sani, E.S.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering biodegradable and biocompatible bio-ionic liquid conjugated hydrogels with tunable conductivity and mechanical properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef]
- Ara, L.; Sher, M.; Khan, M.; Rehman, T.U.; Shah, L.A.; Yoo, H.-M. Dually-crosslinked ionic conductive hydrogels reinforced through biopolymer gellan gum for flexible sensors to monitor human activities. Int. J. Biol. Macromol. 2024, 276, 133789. [Google Scholar] [CrossRef]
- Hakim, M.L.; Wiranata, A.; Darmanto, S.; Widagdo, D.; Santos, G.N.; Muflikhun, M.A. Durability against folded test of SR/CNT/SR flexible strain sensors for human therapy motion monitoring. Sens. Actuators A Phys. 2025, 387, 116397. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, Z.; Wang, X.; Huang, X.; Li, J.; Wei, Y. Tough, antifreezing, and conductive hydrogel based on gelatin and oxidized dextran. Adv. Mater. Technol. 2022, 7, 2101382. [Google Scholar] [CrossRef]
- Qie, H.; Wang, Z.; Ren, J.; Lü, S.; Liu, M. A tough shape memory hydrogel strain sensor based on gelatin grafted polypyrrole. Polymer 2022, 263, 125524. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, C.; Chen, Y.; Wang, Y.; Feng, Q.; Liu, Y.; Yu, M.; Yuan, Y.; Xu, C.-Y.; Liu, F. Transient, printable and recyclable gelatin hydrogels with enhanced mechanical sensing and electromagnetic shielding performance by incorporation of reduced graphene oxide. Chem. Eng. J. 2023, 475, 145794. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Y.; Maimaitiyiming, X. Tough polyvinyl alcohol-gelatin biological macromolecules ionic hydrogel temperature, humidity, stress and strain, sensors. Int. J. Biol. Macromol. 2023, 249, 125978. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, H.; Hu, Q.; Yang, Y.; Ni, S.; Peng, F.; Jin, X. High stretchable and tough xylan-g-gelatin hydrogel via the synergy of chemical cross-linking and salting out for strain sensors. Int. J. Biol. Macromol. 2024, 261, 129759. [Google Scholar] [CrossRef]
- Li, W.; Ming, Y.; Yang, L.; Ni, Y.; Chen, Y.; Xu, W.; Li, L.; Zheng, C.; Lin, W. Conductive Hydrogel Motion Sensor with Low-Temperature Stability for Winter Sports and Sensing Rescue. Polymers 2025, 17, 1365. [Google Scholar] [CrossRef]
- Song, W.; Chen, H.; Lu, P.; Miao, Z.; Zhao, Y.; He, Z.; Ren, Z.; Nica, V.; Qian, L. Polymeric ionic liquid modifier as ion-induced crosslinker and functional enhancer: Facile fabrication of multifunctional gelatin hydrogels for flexible electronics. Int. J. Biol. Macromol. 2025, 318, 145296. [Google Scholar] [CrossRef]
- Ma, X.; Xu, W.; Chen, J.; Wang, Y.; Xiong, W.; Li, J.; You, L.; Wang, S. Breathable Gelatin Conductive Hydrogels Using a Template Method and Reverse Use of Hofmeister Effect for Wearable Sensors. ACS Appl. Polym. Mater. 2024, 6, 6290–6301. [Google Scholar]
- Xu, L.; Li, X.; Gao, J.; Yan, M.; Wang, Q. Environment-tolerant gelatin based ionic conductive organohydrogel for flexible sensor. Mater. Today Commun. 2024, 40, 109542. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Fan, X.; Song, B.; Gu, H. Tara tannin-cross-linked, underwater-adhesive, super self-healing, and recyclable gelatin-based conductive hydrogel as a strain sensor. Ind. Eng. Chem. Res. 2022, 61, 17915–17929. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, W.; Yang, X.; Li, H.; Liang, X.; Ding, H. Mechanically Robust and Conductive Gelatin/Glucose Hydrogels Enabled by the Hofmeister Effect for Flexible Strain Sensors. Gels 2025, 11, 694. https://doi.org/10.3390/gels11090694
Sang W, Yang X, Li H, Liang X, Ding H. Mechanically Robust and Conductive Gelatin/Glucose Hydrogels Enabled by the Hofmeister Effect for Flexible Strain Sensors. Gels. 2025; 11(9):694. https://doi.org/10.3390/gels11090694
Chicago/Turabian StyleSang, Wei, Xu Yang, Hui Li, Xiaoxu Liang, and Hongyao Ding. 2025. "Mechanically Robust and Conductive Gelatin/Glucose Hydrogels Enabled by the Hofmeister Effect for Flexible Strain Sensors" Gels 11, no. 9: 694. https://doi.org/10.3390/gels11090694
APA StyleSang, W., Yang, X., Li, H., Liang, X., & Ding, H. (2025). Mechanically Robust and Conductive Gelatin/Glucose Hydrogels Enabled by the Hofmeister Effect for Flexible Strain Sensors. Gels, 11(9), 694. https://doi.org/10.3390/gels11090694





