Impact of Alkali-Activated Tannery Sludge-Derived Geopolymer Gel on Cement Properties: Workability, Hydration Process, and Compressive Strength
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Sludge Dosage and Alkali Equivalent on Workability
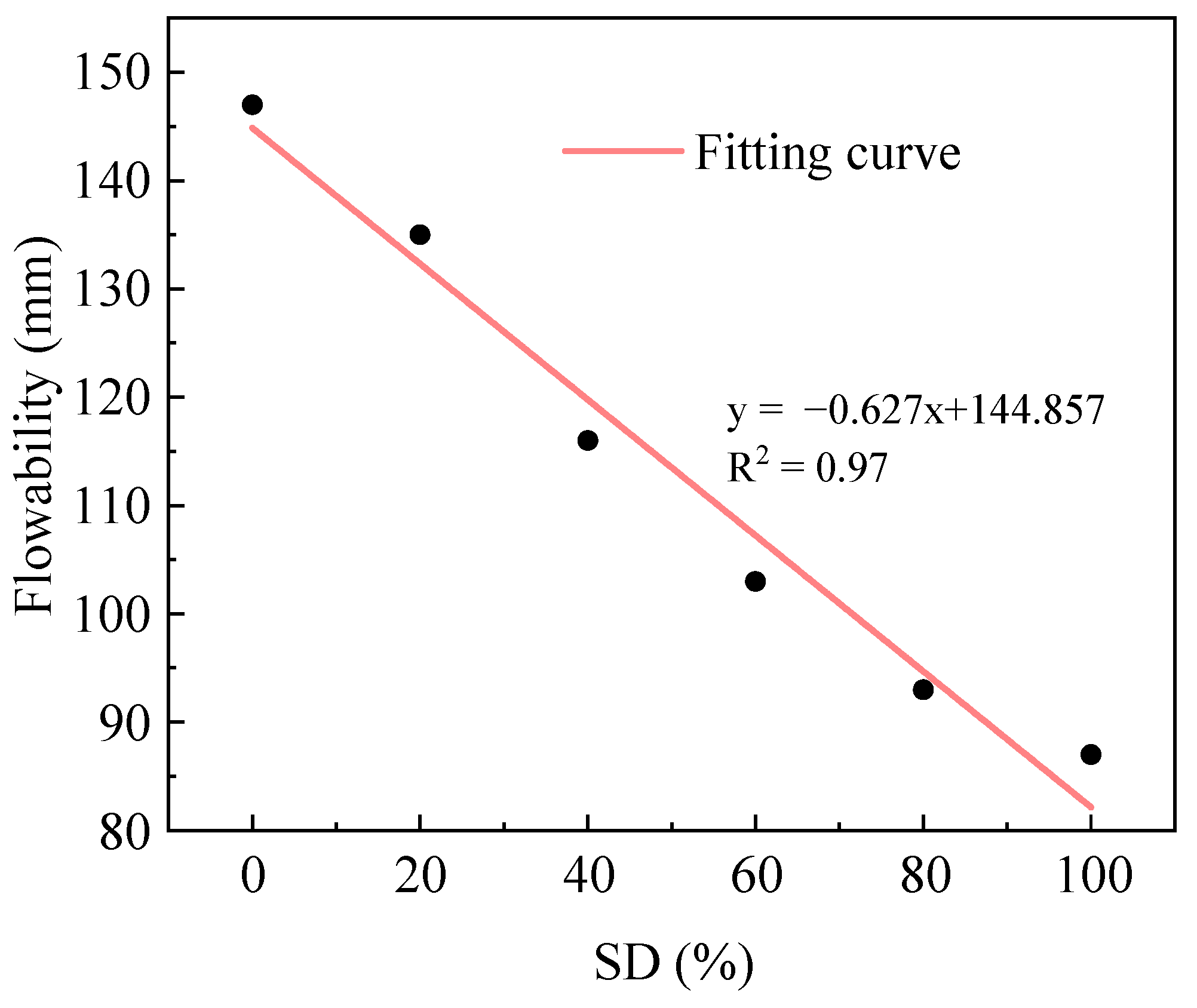
2.1.1. Influence of Sludge Dosage on Flowability
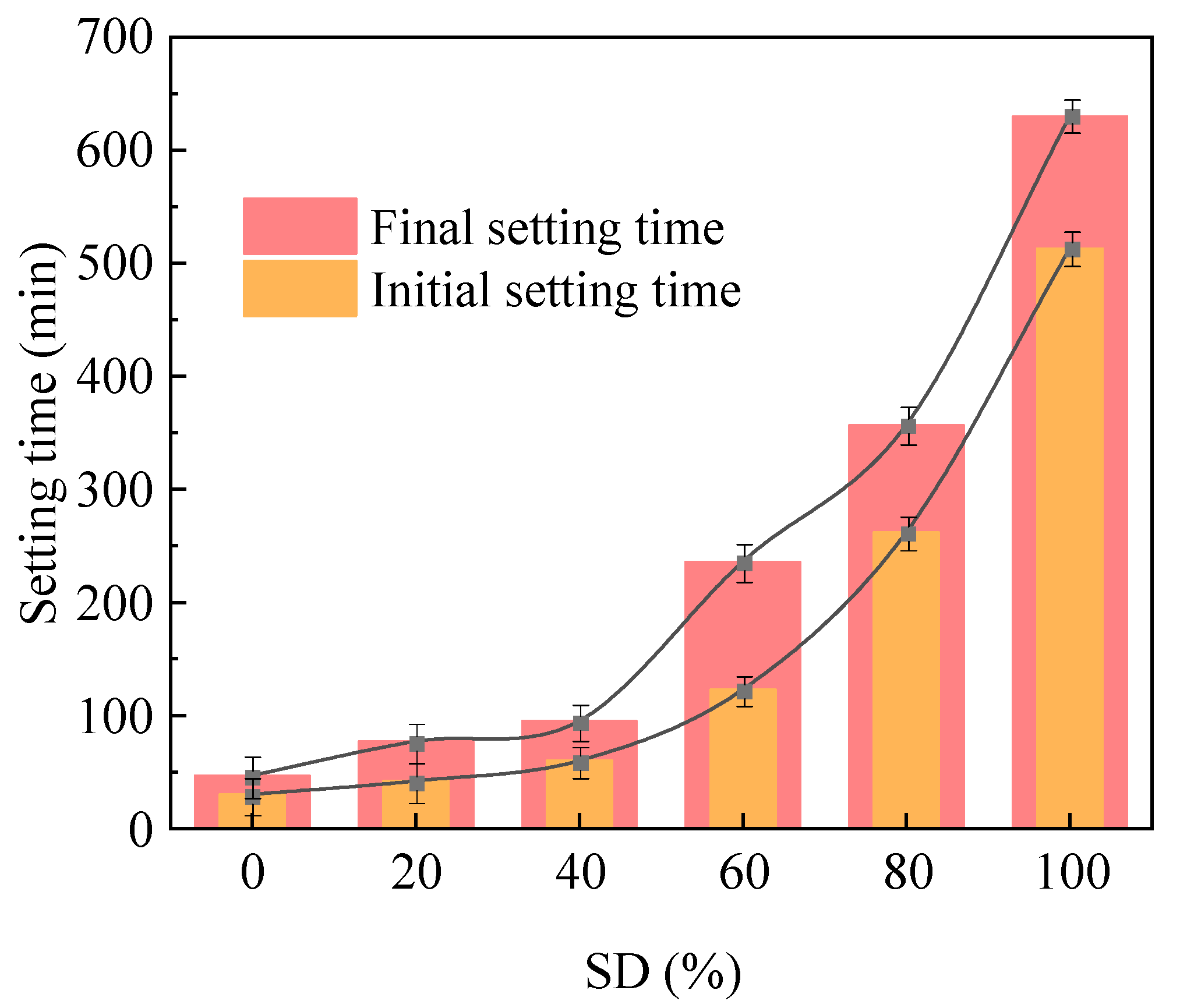
2.1.2. Impact of Sludge Dosage on Setting Behavior
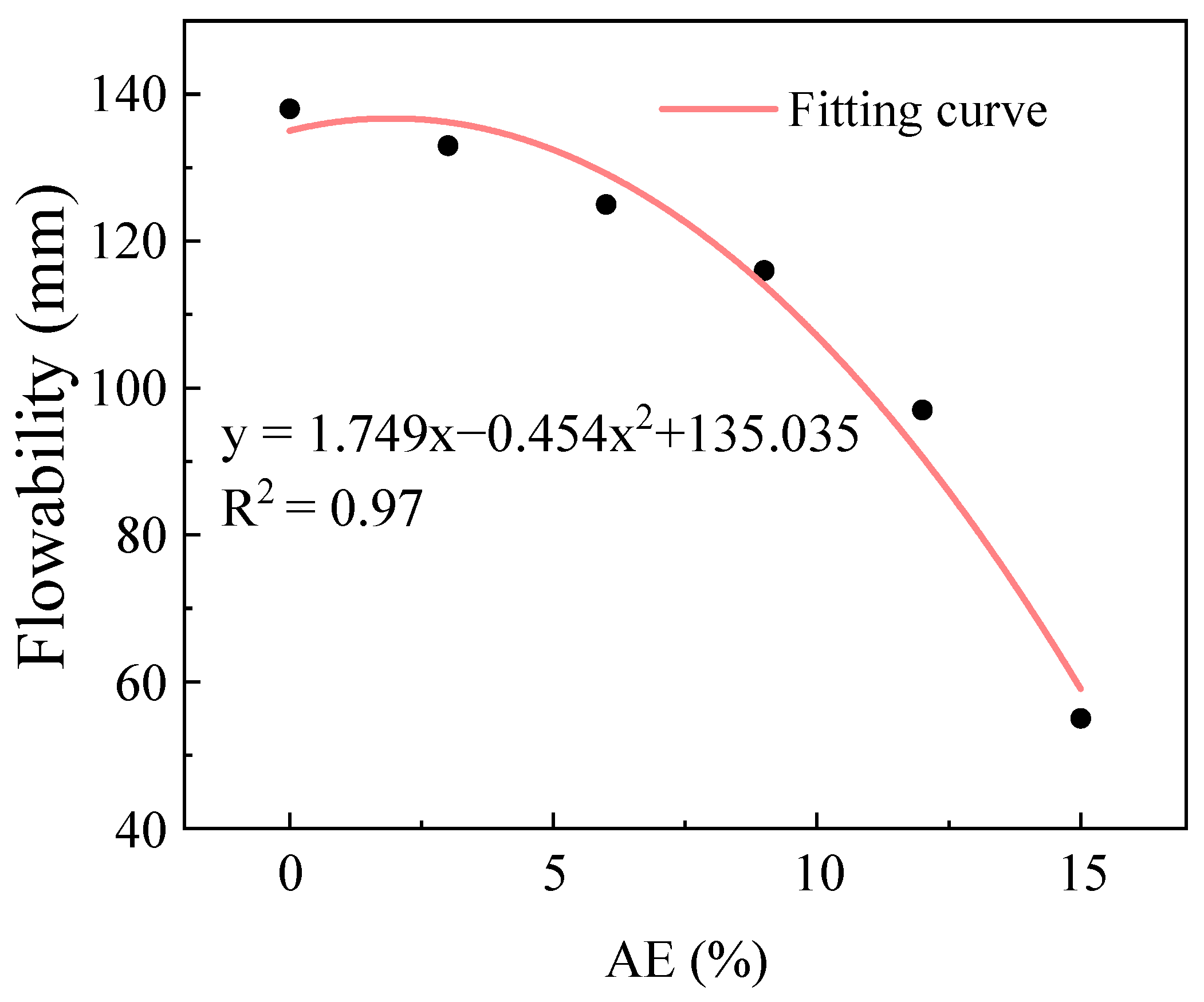
2.1.3. Influence of Alkali Equivalent on Flowability
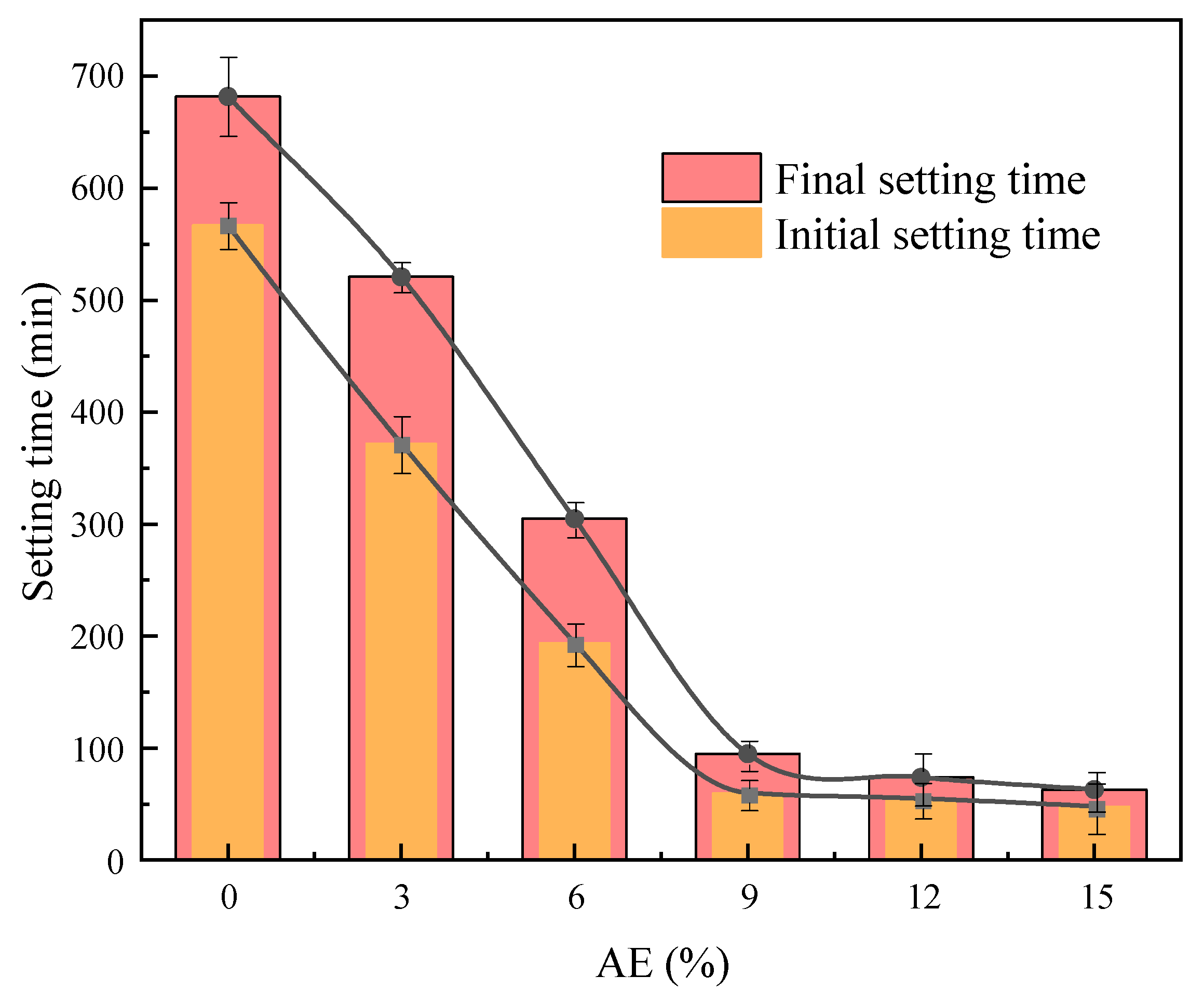
2.1.4. Impact of Alkali Equivalent on Setting Behavior
2.2. Hydration Process Evolution Under Composite Admixture Variations
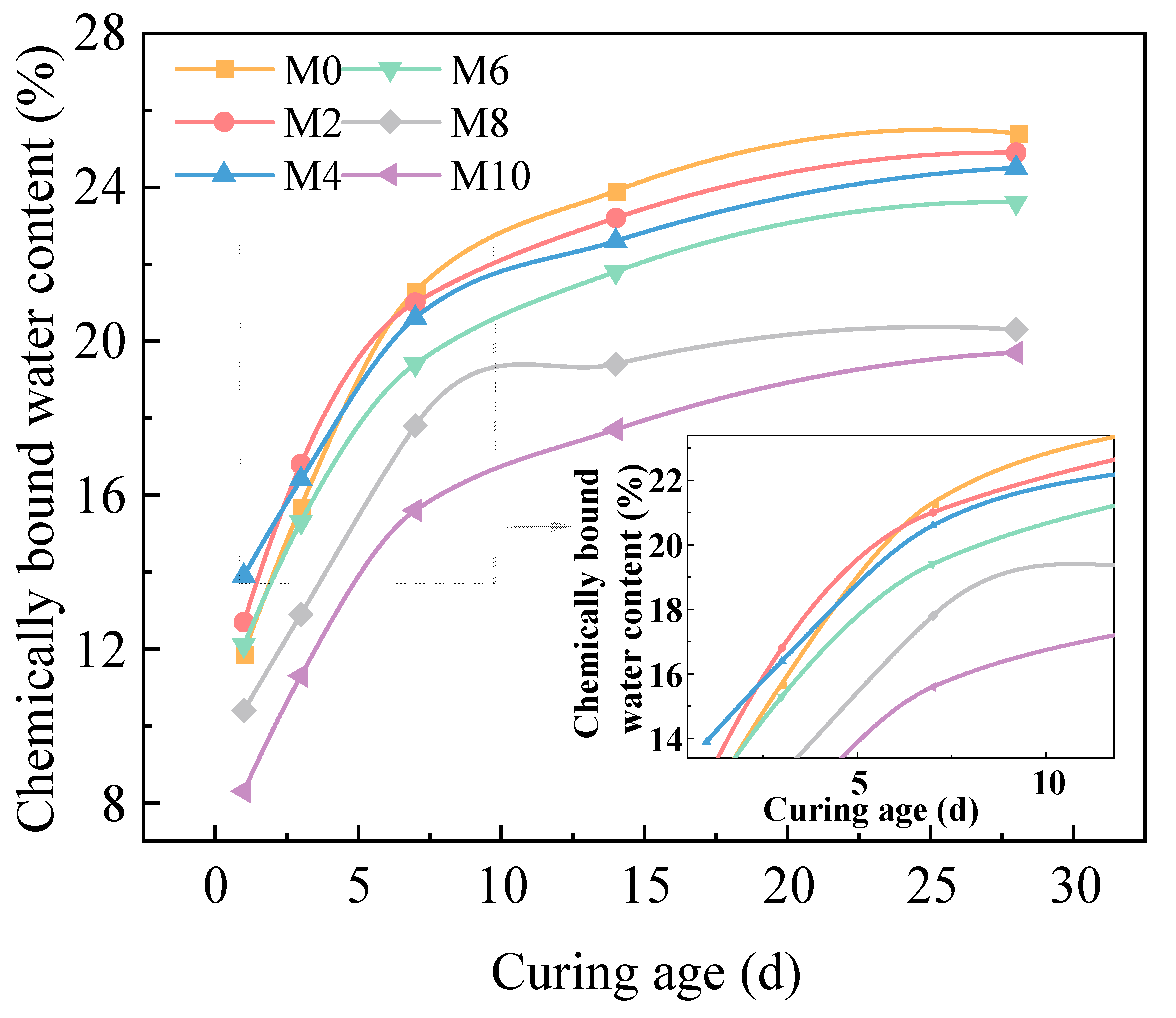
2.2.1. Effect of Sludge Dosage on Chemically Bound Water
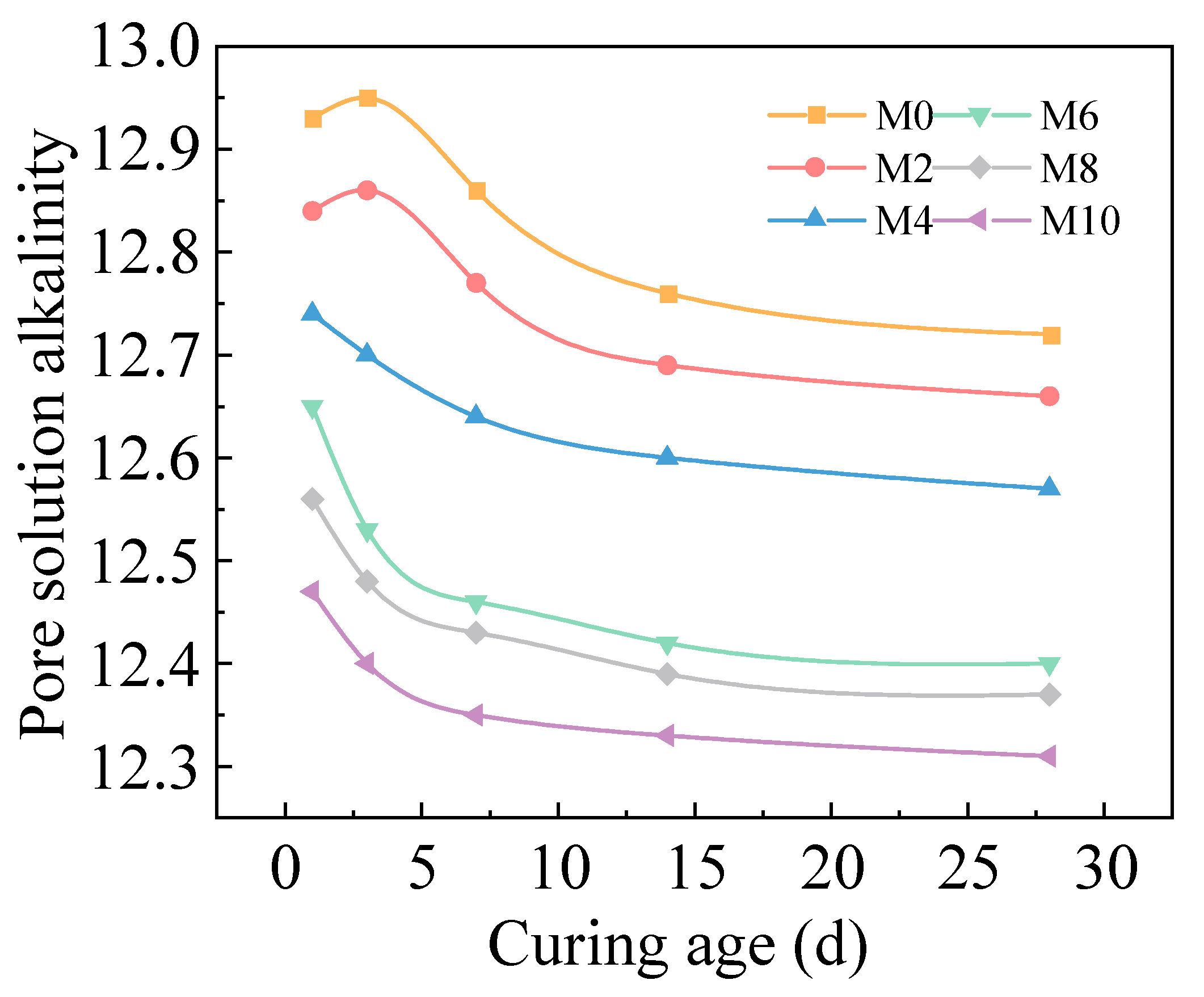
2.2.2. pH Dynamics in Pore Solution with Sludge Dosage
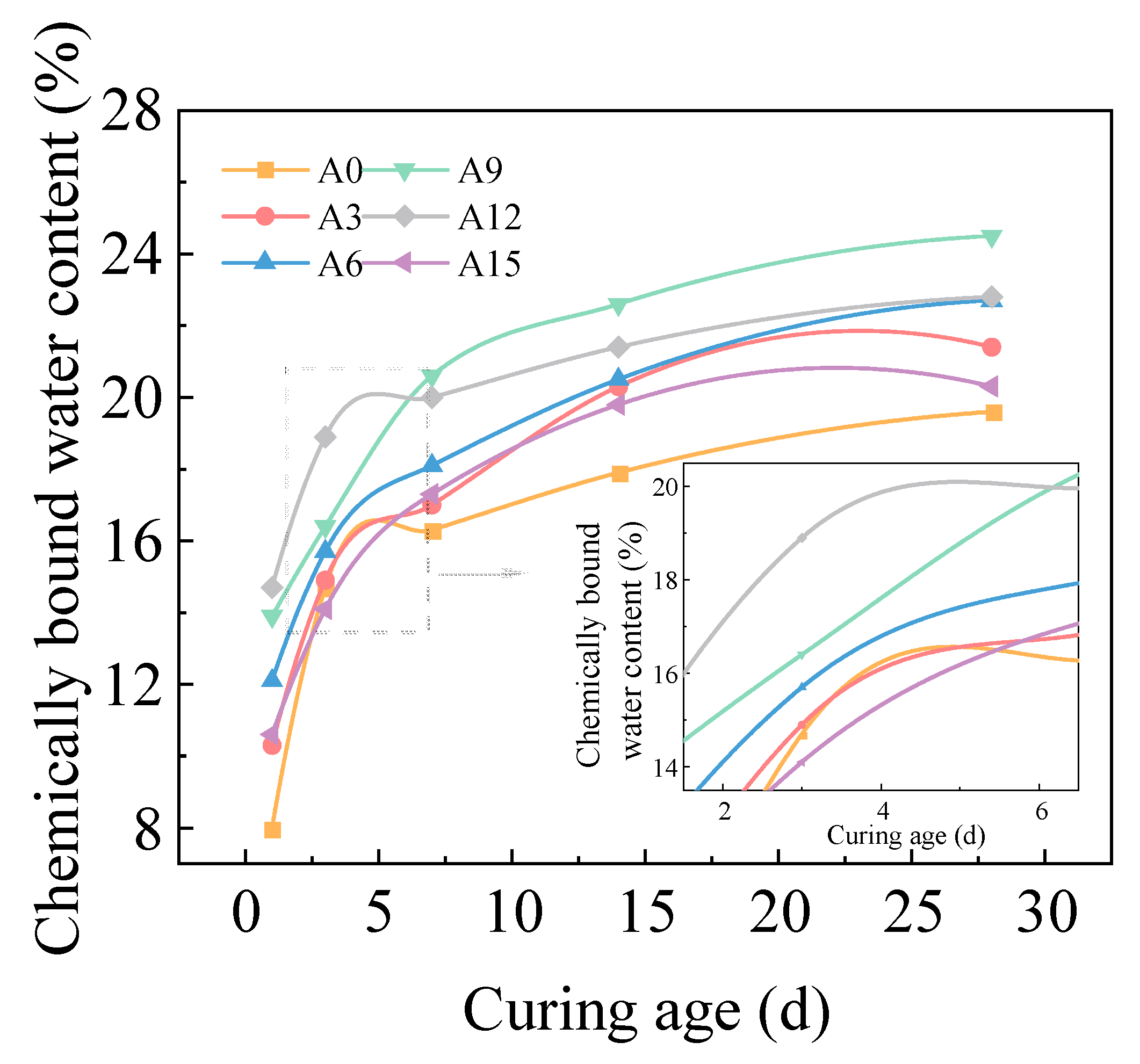
2.2.3. Effect of Alkali Equivalent on Chemically Bound Water
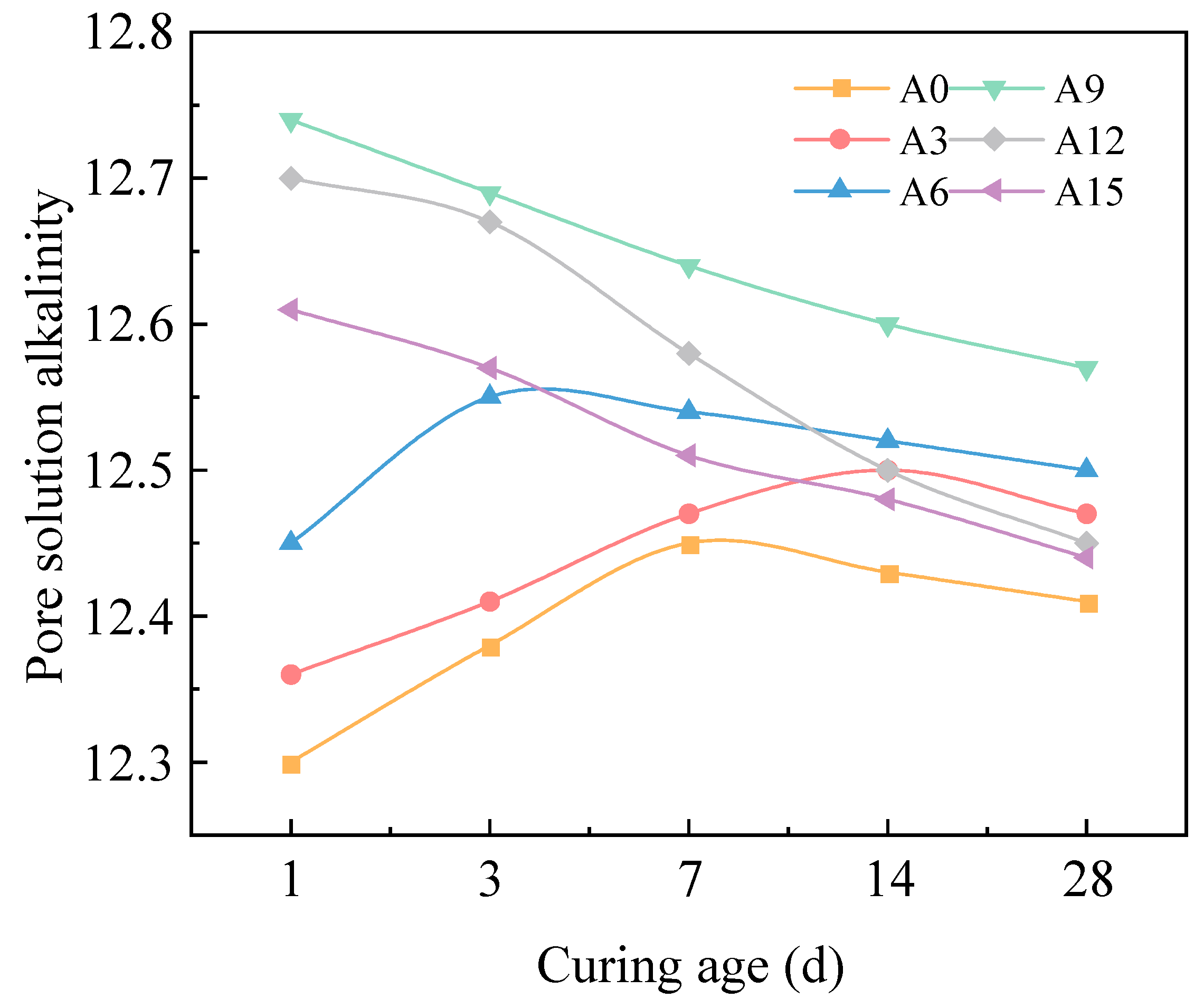
2.2.4. pH Dynamics in Pore Solution with Alkali Equivalent
2.3. Variation Rules of Compressive Strength
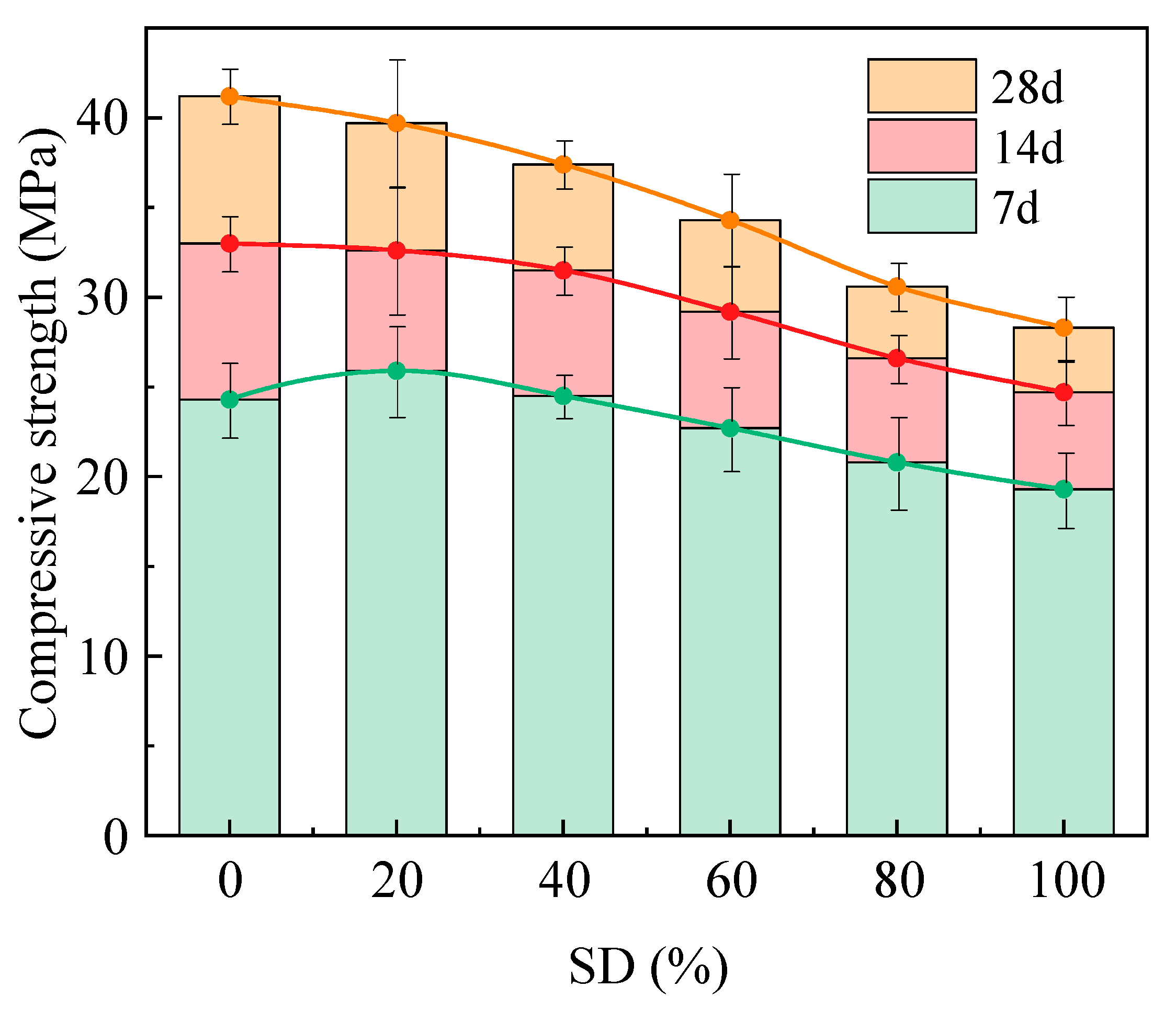
2.3.1. Effect of Sludge Dosage on Compressive Strength
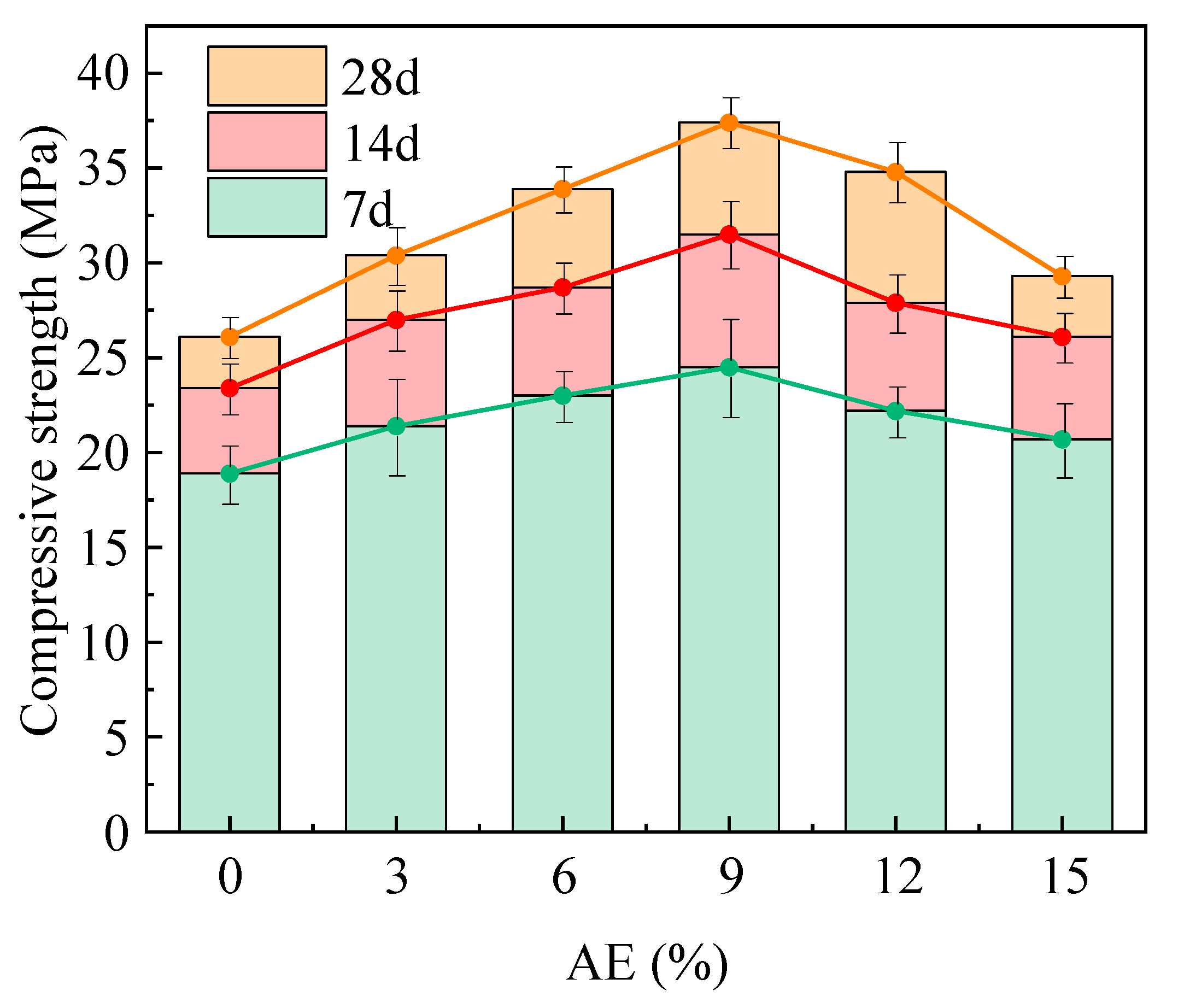
2.3.2. Effect of Alkali Equivalent on Compressive Strength
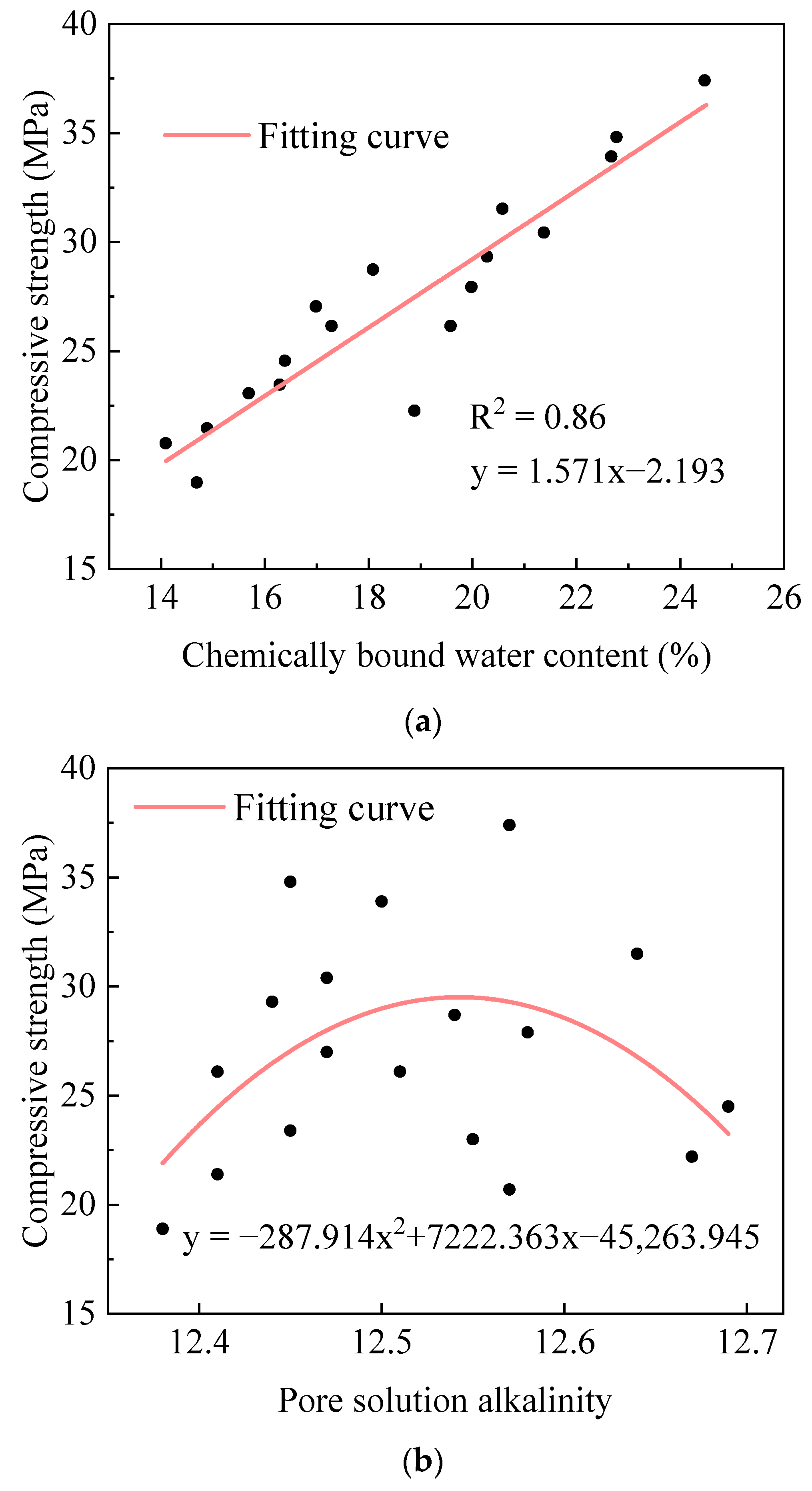
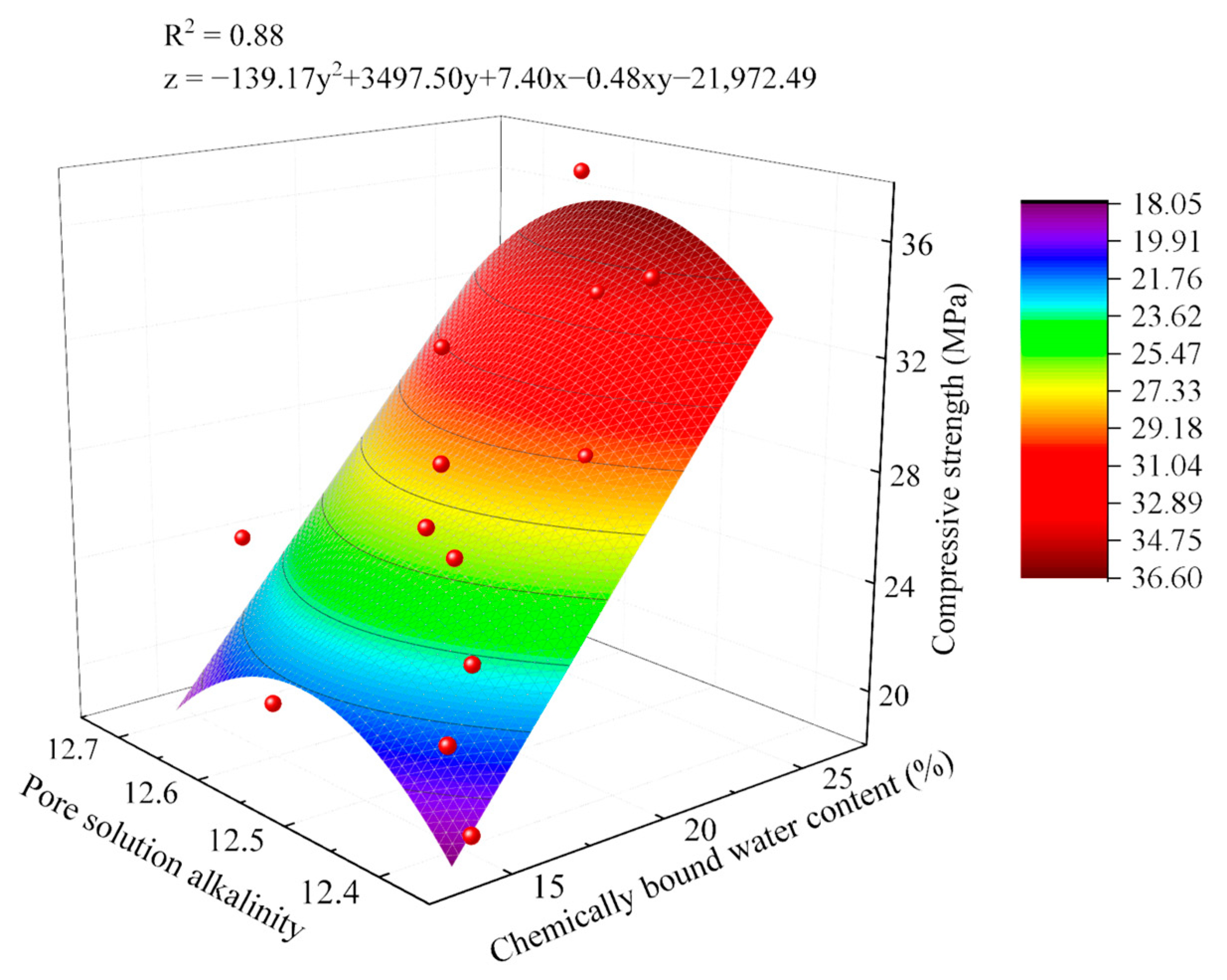
2.4. Hydration–Strength Relationships of AACC
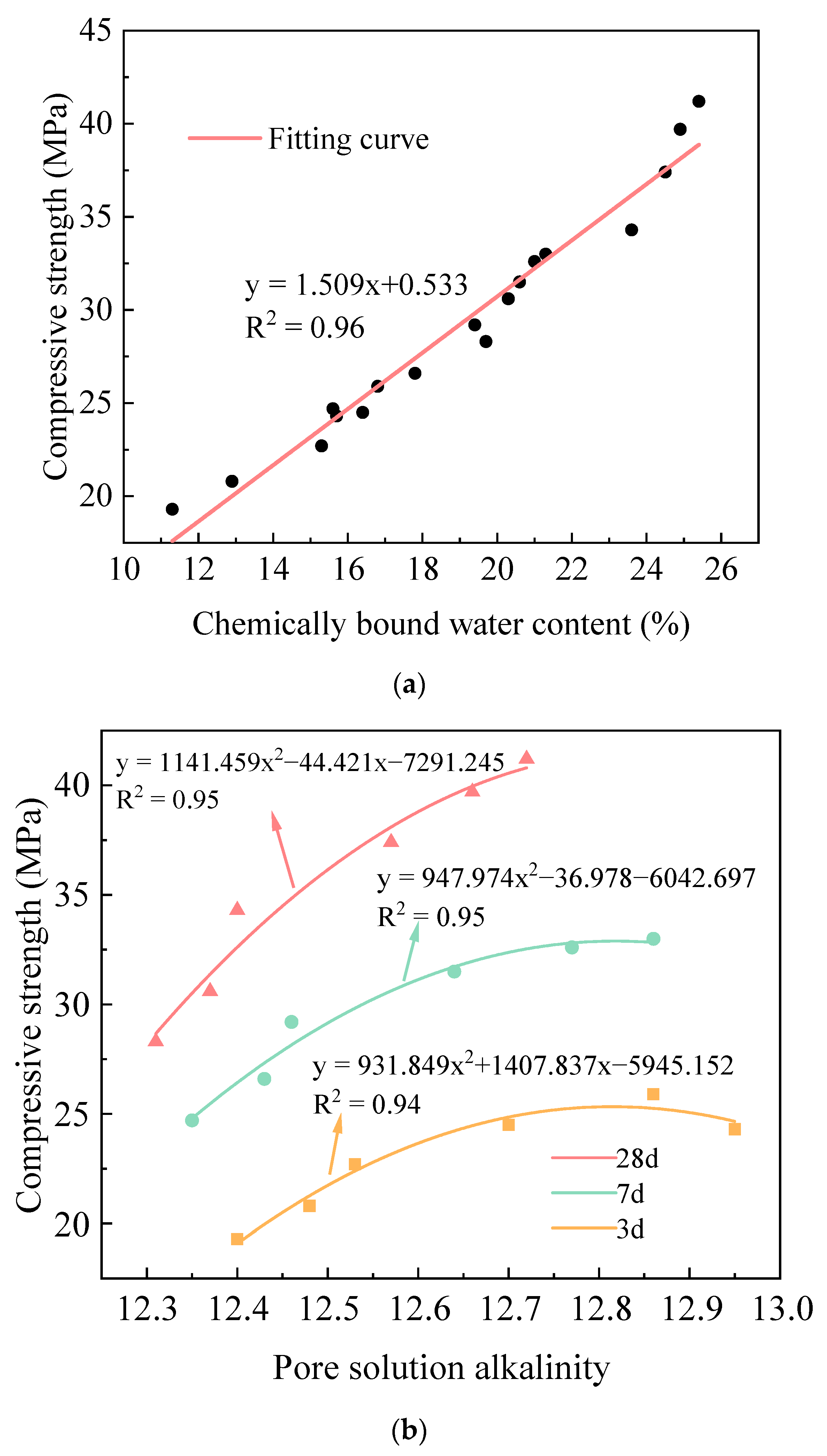
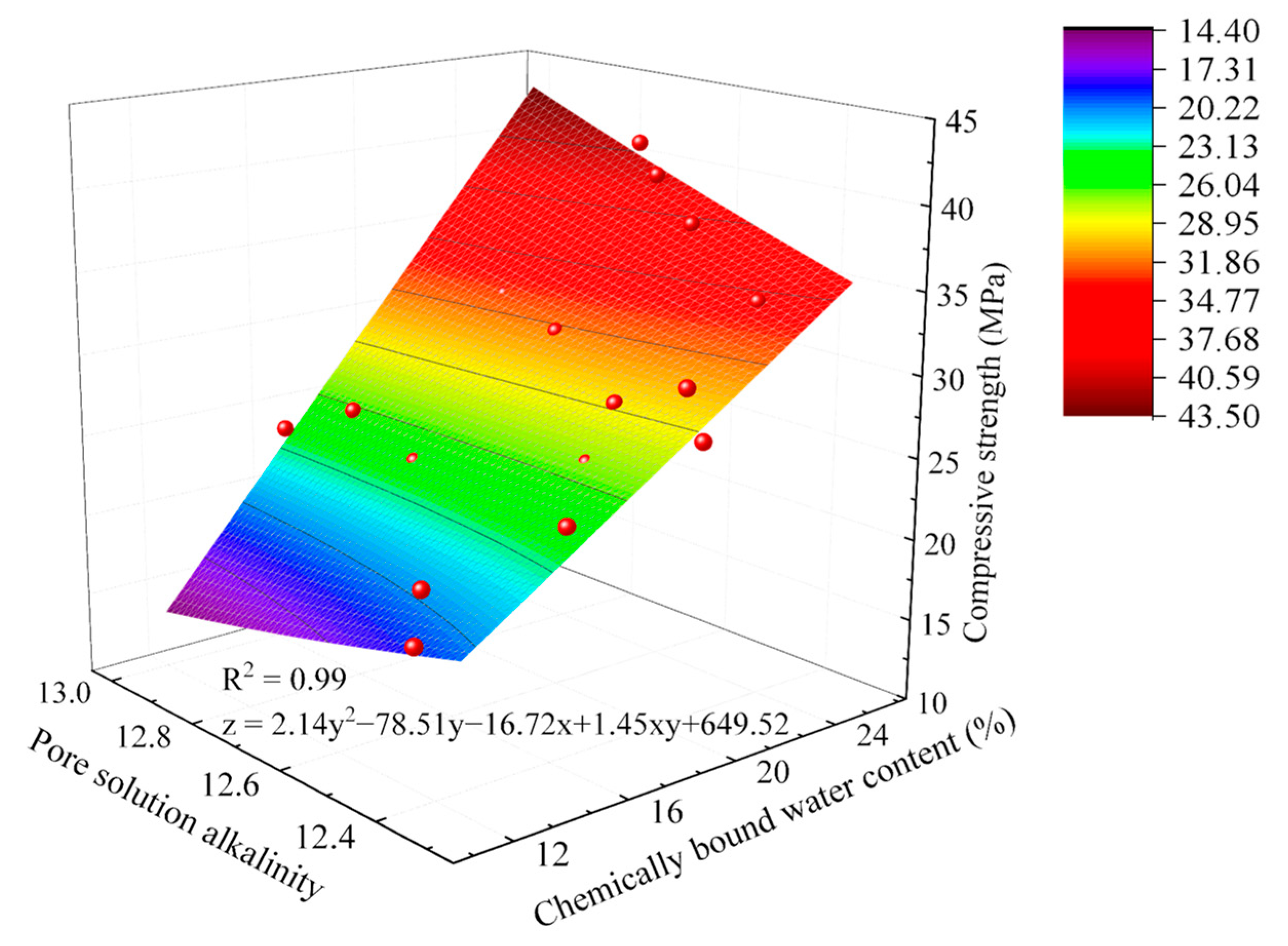
2.4.1. Hydration–Strength Relationships Under Sludge Dosage Gradients
2.4.2. Hydration–Strength Relationships Under Alkali Equivalent Gradients
3. Conclusions
4. Materials and Methods
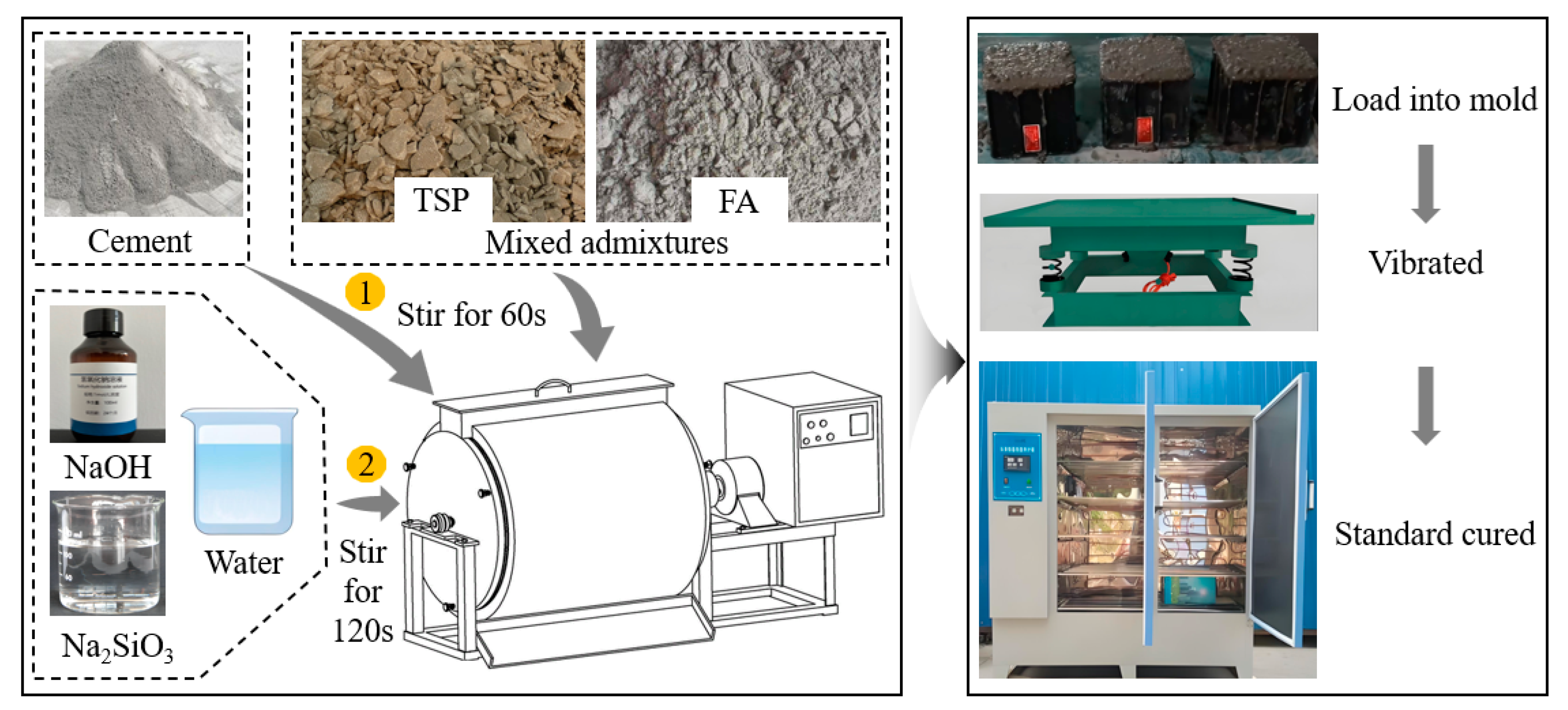
4.1. Raw Materials
- 1.
- Cement.The experimental study employed P.O 42.5 ordinary Portland cement produced by Henan Tianrui Group Co., Ltd (Pingdingshan, China) with a specific surface area of 348.7 m2/kg (measured via Blaine method) and a density of 3120 g/m3.
- 2.
- Composite admixture.The composite admixture consisted of TS and FA. The FA, classified as Grade F low calcium FA, was produced by Borun Refractory Materials Co., Ltd (Gongyi, China) exhibiting a specific surface area of 330 m2/kg and a density of 2550 kg/m3. The raw TS was obtained from Henan Yongsheng Leather Industry Co., Ltd (Zhumadian, China) after concentration and filter pressing. Through laboratory processing including drying, crushing, mechanical grinding, and sieving, TS powder with particle sizes below 0.15 mm was prepared, demonstrating a specific surface area of 310 m2/kg and density of 1850 kg/m3. The chemical composition of the cementitious materials is presented in Table 1.
- 3.
- Alkali activator.The alkali activator used in the experiment was formulated through composite blending of sodium hydroxide (NaOH) and sodium silicate solution (Na2SiO3). The sodium hydroxide, with a purity of 95%, primarily served to adjust the modulus and alkalinity of the sodium silicate. The industrial grade sodium silicate solution (water glass) was supplied by Yourui Refractory Materials Co., Ltd (Jiaxing, China) exhibiting a transparent viscous state.
4.2. Mix Proportion Design and Preparation Process
4.3. Test Method
- The compressive strength test of AACC refers to Test Method for Cement Mortar Strength (ISO Method) (GB/T17671-2021) [48]. Cubic specimens with dimensions of 40 × 40 × 40 mm are prepared, with three samples per group. An electro-hydraulic servo pressure testing machine is used to measure the compressive strength of specimens cured for 7 d and 28 d.
- The test selected flowability as the indicator for flowability evaluation, following the cement paste flowability test method specified in Methods for testing uniformity of concrete admixtures (GB/T8077-2023) [49]. Additionally, the setting time of fresh alkali-activated composite cementitious paste was determined according to Test Methods for Water Requirement of Normal Consistency, Setting Time, and Soundness of Cement (GB/T1346-2011) [50].
- The chemically bound water content is measured using the loss on ignition method as follows: hardened paste specimens cured to the specified age are immersed in anhydrous ethanol to terminate hydration. The specimens are then manually crushed, ground into powder, and sieved through a 0.08 mm square mesh sieve. The sieved powder is dried in an oven at 75 °C until a constant mass is achieved. The dried powder mass (m1) is weighed using an analytical balance. The dried powder is heated in a muffle furnace to 1050 °C for 3 h. After cooling to ambient temperature, the mass is reweighed (m2). The chemically bound water content Wn of the sample is calculated as follows:In the formula, LC, LTS, and LFA represent the loss on ignition of cement, TS, and FA, respectively; β denotes the mass fraction of cement and supplementary cementitious materials in the composite cementitious system.
- The alkalinity of pore solution is tested using the extraction leaching method with the following specific steps: after terminating the hydration of hardened paste specimens with different mix proportions at various ages, grind them into powder and sieve through a 0.08 mm square mesh sieve; weigh 3 g of the sieved powder and place it in a beaker; add 30 g of distilled water and stir with a magnetic stirrer for 0.5 h (at 200 rpm); let it stand for 12 h, then test the pH value of the supernatant using a Leici PHS-25 pH meter.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Yuan, D.; Li, W.; Wang, L.; Li, Y.; Che, L.; Tian, W.; Salama, E.; Ossman, M.; Lin, F. Efficient removal of toxic organics and reduction of Cr (VI) to Cr (III) from tannery sludge: A comparative study of microwave pyrolysis and conventional pyrolysis. Sep. Purif. Technol. 2025, 354, 128736. [Google Scholar] [CrossRef]
- Tuci, G.A.; Valentino, F.; Parmar, A.C.; Pavan, P.; Gottardo, M. Minimizing tannery sludge in landfilling through a mixed microbial culture approach: Effect of oxidizing pretreatment, temperature and hydraulic retention time on process performances and chromium fate. Biochem. Eng. J. 2023, 200, 109073. [Google Scholar] [CrossRef]
- Priyanka, K.M.; Saravanakumar, M.P. Ameliorating tannery sludge dewaterability by disintegration of extracellular biopolymers with FeCl3-MnSO4/oxalic acid/sodium percarbonate: Spectroscopic profiling of dissolved organic matter in sludge filtrate. J. Water Process Eng. 2024, 58, 104741. [Google Scholar] [CrossRef]
- Moktadir, M.A.; Shi, T.; Ayub, Y.; Ren, J.; He, C. Upcycling potential of hazardous tannery sludge to value-added products: Process modelling, simulation, and 3E analysis. J. Environ. Chem. Eng. 2024, 12, 113710. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Wang, X.; Liu, X.; Xu, Z.; Wang, Y. A novel strategy of tannery sludge disposal—Converting into biochar and reusing for Cr (VI) removal from tannery wastewater. J. Environ. Sci. 2024, 138, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, P.; Bie, Y.; Wang, L.; Guo, L. Mechanical properties and micro mechanism of alkali-activated tannery sludge / fly ash composite cement-based recycled concrete. Constr. Build. Mater. 2023, 391, 131813. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, S.; Zhu, C.; Hao, Y.; Yan, L.; Li, J.; Chen, X.; Chen, B.; Ma, X. Immobilization of chromium in real tannery sludge via heat treatment with coal fly ash. Chemosphere 2023, 335, 139180. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Gong, H.; Yang, Z. Chromium speciation in tannery sludge residues after different thermal decomposition processes. J. Clean. Prod. 2021, 314, 128071. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal and recovery of heavy metals from tannery sludge subjected to plasma pyro-gasification process. J. Clean. Prod. 2020, 273, 123166. [Google Scholar] [CrossRef]
- Venkatesh, G.; Krishnaiah, S.; Ramesh, G. Assessment of tannery sludge additions on physico-mechanical properties of clay bricks. Can. J. Civ. Eng. 2023, 51, 545–560. [Google Scholar] [CrossRef]
- Jothilingam, M.; Preethi, V.; Chandana, P.S.; Janardhanan, G. Fabrication of sustainable green bricks by the effective utilization of tannery sludge as main additive. Structures 2023, 48, 182–194. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Bie, Y.; Duan, P.; Wang, L. Multi-scale performance study of concrete with recycled aggregate from tannery sludge. Case Stud. Constr. Mater. 2022, 17, e01698. [Google Scholar] [CrossRef]
- Sunmathi, N.; Padmapriya, R.; Sudarsan, J.S.; Nithiyanantham, S. Optimum utilization and resource recovery of tannery sludge: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10405–10414. [Google Scholar] [CrossRef]
- Hasan, M.A.; Hashem, M.A.; Payel, S.; Nithiyanantham, S. Stabilization of liming sludge in brick production: A way to reduce pollution in tannery. Constr. Build. Mater. 2022, 314, 125702. [Google Scholar] [CrossRef]
- Bie, Y.; Yin, X.; Chen, S. The durability of slag and tannery sludge based geopolymer corroded by acid and base solutions. Constr. Build. Mater. 2024, 433, 136746. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, Y.; Li, X.; Yang, X.; Rao, F.; Zhong, L. Durability of alkali-activated materials with different C-S-H and N-A-S-H gels in acid and alkaline environment. J. Mater. Res. Technol. 2022, 16, 619–630. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhang, Y. Research on hydration characteristics of OSR-GGBFS-FA alkali-activated materials. Constr. Build. Mater. 2024, 411, 134321. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, X.; Liu, M.; Fang, B.; Wang, C.; Mi, H. Compatibility of sodium hydroxide, sodium silicate and calcium-enriched additives in alkali-activated materials: From the perspectives of flowability, strength and microstructure. Constr. Build. Mater. 2023, 403, 133102. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, T.; Yang, Z.; Wang, C.; Wu, H.-C. Influence of different activators on microstructure and strength of alkali-activated nickel slag cementitious materials. Constr. Build. Mater. 2020, 235, 117449. [Google Scholar] [CrossRef]
- Guo, S.; Lu, Y.; Wan, X.; Wu, F.; Zhao, T.; Shen, C. Preparation, characterization of highly dispersed reduced graphene oxide/epoxy resin and its application in alkali-activated slag composites. Cem. Concr. Compos. 2020, 105, 103424. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Guo, H.; Yu, Y.; Guo, Y.; Yue, G.; Li, Q.; Wang, L. Study on preparation and performance of alkali-activated low carbon recycled concrete: Corn cob biomass aggregate. J. Mater. Res. Technol. 2023, 23, 90–105. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, R.; Zhang, J.J.; Shui, Z.H. A low-carbon alkali activated slag based ultra-high performance concrete (UHPC): Reaction kinetics and microstructure development. J. Clean. Prod. 2022, 363, 132416. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhan, Y.; Gao, H.; Zhu, R. Early properties and reaction mechanism of hybrid alkali-activated cements (HAACs). Mater. Today Commun. 2024, 41, 110849. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. Self-cementation solidification of heavy metals in lead-zinc smelting slag through alkali-activated materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Zhang, Y.; Gu, G.; Hu, J. Micro-assessment of heavy metal immobilization within alkali-activated copper tailings-slag geopolymer. Cem. Concr. Compos. 2024, 149, 105510. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, T.; Deng, L.; Li, Y.; Guo, H.; Zhou, J.; Li, L.; Peng, Y. Ion-adsorption type rare earth tailings for preparation of alkali-based geopolymer with capacity for heavy metals immobilization. Cem. Concr. Compos. 2022, 134, 104768. [Google Scholar] [CrossRef]
- Palomo, A.S.; Fermandez-Jimandez, A.; Covalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. OPC-fly ash cementitious system study of gel binders produced during alkline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Zhu, X.; Jamal, A.S.; Zhang, M.; Liu, B.; Shi, J. Energy consumption, carbon emissions and cost analysis of accelerated curing: A case study of hybrid alkali-activated cement. Renew. Sustain. Energy Rev. 2025, 210, 115206. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Park, S.; Pyo, S. Thermodynamic modeling and mechanical properties of hybrid alkaline cement composites. Constr. Build. Mater. 2022, 322, 126381. [Google Scholar] [CrossRef]
- Jin, M.; Yang, G.; Wang, Z.; Zhao, P.; Zheng, X.; Pan, Z.; Wu, J. Early strength and durability of tannery sludge/metakaolin based geopolymer as concrete pavement repair material. Mag. Concr. Res. 2025, 77, 15–30. [Google Scholar] [CrossRef]
- Chen, S.; Duan, P.; Zhao, M.; Shi, H.; Bie, Y. Solidification Mechanism and Strength Characteristics of Alkali-Activated Tannery Sludge–Slag Geopolymer. Buildings 2024, 14, 1060. [Google Scholar] [CrossRef]
- Godiya, C.B.; Ruotolo, L.A.M.; Cai, W. Functional biobased hydrogels for the removal of aqueous hazardous pollutants: Current status, challenges, and future perspectives. J. Mater. Chem. A 2020, 41, 21585–21612. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, M.; Zhang, W.; Qu, G.; Chen, C.; Cheng, E.; Ou, Z. Investigation on crack resistance of full aeolian sand high-performance concrete synergistically modified by supplementary cementitious materials and superabsorbent polymer. Constr. Build. Mater. 2024, 411, 134681. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Wang, J.; Li, S.; Lv, C.; Dong, Y. Effect of fly ash on rheological properties of graphene oxide cement paste. Constr. Build. Mater. 2017, 138, 35–44. [Google Scholar] [CrossRef]
- Wang, S.; Gu, X.; Liu, J.; Zhu, Z.; Wang, H.; Ge, X.; Xu, X.; Nehdi, M. Modulation of the workability and Ca/Si/Al ratio of cement-metakaolin cementitious material system by using fly ash: Synergistic effect and hydration products. Constr. Build. Mater. 2023, 404, 133300. [Google Scholar] [CrossRef]
- Yu, Y.; Gunasekara, C.; Elakneswaran, Y.; Robert, D.; Law, D.; Setunge, S. Correlating strength development with hydration characteristics for multi-blend fly ash concrete. J. Build. Eng. 2025, 103, 112076. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, W.; Ye, J.; Chen, J.; Yan, F.; Ren, X.; Li, J. Mechanism of Na2CO3 on early properties of red mud-based alkali-activated cementitious materials. Constr. Build. Mater. 2024, 449, 138369. [Google Scholar] [CrossRef]
- Ashraf, M.; Nazar, S.; Iqbal, M.; Yang, J.; Ullah, R.; Hasan, M. Exploring the rheological and mechanical properties of alkali activated mortar incorporating waste foundry sand: A comprehensive experimental and machine learning investigation. Results Eng. 2024, 24, 102973. [Google Scholar] [CrossRef]
- Tan, Y.; He, Y.; Cui, X.; Liu, L. Design and performance optimization of alkali-activated waste coal bottom ash/slag porous concrete. Constr. Build. Mater. 2022, 359, 129413. [Google Scholar] [CrossRef]
- Ning, L.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar]
- Hadi, M.N.S.; Zhang, H.; Parkinson, S. Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Wang, L.; Si, C.; Li, C.; Sun, X.; Guo, S. Effect of Potassium Hydroxide-Sodium Water Glass Activator on Properties of Alkali-Activated Slag Cementitious Materials. Bull. Chin. Ceram. Soc. 2022, 41, 2654. [Google Scholar]
- Nie, S.; Skibsted, J. Aluminum distribution in C-(A)-S-H and calcium aluminate hydrate phases of Portland cement—Metakaolin—Limestone blends studied by 27Al and 29Si NMR spectroscopy. Cem. Concr. Res. 2024, 186, 107664. [Google Scholar] [CrossRef]
- Ponomar, V.; Yliniemi, J.; Kilpimaa, K. Influence of calcium oxide and hydroxide on non-ferrous metallurgical slag hydration in alkaline environments. Cem. Concr. Compos. 2024, 152, 105673. [Google Scholar] [CrossRef]
- Xie, L.; Liu, K. Properties and Microstructure of Na2CO3-Activated Binders Modified with Ca(OH)2 and Mg(OH)2. Materials 2022, 15, 1687. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K. The mechanism of limited inhibition by fly ash on expansion due to alkali–silica reaction at the pessimum proportion. Cem. Concr. Res. 2017, 92, 1–15. [Google Scholar] [CrossRef]
- Ge, R.; Tao, E.; Cheng, Y.; Wang, Y.; Yu, J.; Li, Y.; Yang, S. NaH2PO4 synergizes with organic matter to stabilize chromium in tannery sludge. J. Environ. Manag. 2024, 351, 119843. [Google Scholar] [CrossRef]
- GB/T17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standards Press: Beijing, China, 2021.
- GB/T8077-2023; Methods for Testing Uniformity of Concrete Admixtures. Standards Press: Beijing, China, 2023.
- GB/T1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. Standards Press: Beijing, China, 2011.
| Ingredient | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | SO3 | TiO2 | P2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 58.4 | 18.8 | 5.04 | 3.18 | 5.58 | 2.08 | 1.51 | 4.82 | 0.248 | - | - |
| FA | 5.6 | 45.1 | 24.2 | 11.85 | 0.87 | 1.02 | - | 2.1 | 0.82 | 0.85 | - |
| TS | 33.5 | 6.72 | 3.04 | 17.5 | 2.84 | 5.27 | 0.53 | 20.5 | 0.618 | 2.59 | 5.49 |
| Groups | Proportion of TS | Cementitious Materials | Sodium Silicate | NaOH | Water | ||
|---|---|---|---|---|---|---|---|
| Cement | TS | FA | |||||
| M0 | 0% | 1333.6 | 0 | 333.40 | 159.71 | 21.12 | 666.8 |
| M20 | 20% | 1333.6 | 66.68 | 266.72 | 159.71 | 21.12 | 666.8 |
| M40 | 40% | 1333.6 | 133.36 | 200.04 | 159.71 | 21.12 | 666.8 |
| M60 | 60% | 1333.6 | 200.04 | 133.36 | 159.71 | 21.12 | 666.8 |
| M80 | 80% | 1333.6 | 266.72 | 66.68 | 159.71 | 21.12 | 666.8 |
| M100 | 100% | 1333.6 | 333.40 | 0 | 159.71 | 21.12 | 666.8 |
| Groups | AE | Cementitious Materials | Sodium Silicate | NaOH | Water | ||
|---|---|---|---|---|---|---|---|
| Cement | TS | FA | |||||
| A0 | 0% | 1333.6 | 133.36 | 200.04 | 0 | 0 | 666.8 |
| A3 | 3% | 1333.6 | 133.36 | 200.04 | 58.56 | 6.45 | 629.23 |
| A6 | 6% | 1333.6 | 133.36 | 200.04 | 117.12 | 12.91 | 591.66 |
| A9 | 9% | 1333.6 | 133.36 | 200.04 | 175.68 | 19.36 | 554.08 |
| A12 | 12% | 1333.6 | 133.36 | 200.04 | 234.23 | 25.81 | 516.52 |
| A15 | 15% | 1333.6 | 133.36 | 200.04 | 292.80 | 32.26 | 478.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, B.; Nguyen, P.M.V.; Liu, J.; Chen, J.; Zhou, F. Impact of Alkali-Activated Tannery Sludge-Derived Geopolymer Gel on Cement Properties: Workability, Hydration Process, and Compressive Strength. Gels 2025, 11, 339. https://doi.org/10.3390/gels11050339
Chen S, Liu B, Nguyen PMV, Liu J, Chen J, Zhou F. Impact of Alkali-Activated Tannery Sludge-Derived Geopolymer Gel on Cement Properties: Workability, Hydration Process, and Compressive Strength. Gels. 2025; 11(5):339. https://doi.org/10.3390/gels11050339
Chicago/Turabian StyleChen, Shoukai, Beiying Liu, Phu Minh Vuong Nguyen, Jinping Liu, Jialin Chen, and Fei Zhou. 2025. "Impact of Alkali-Activated Tannery Sludge-Derived Geopolymer Gel on Cement Properties: Workability, Hydration Process, and Compressive Strength" Gels 11, no. 5: 339. https://doi.org/10.3390/gels11050339
APA StyleChen, S., Liu, B., Nguyen, P. M. V., Liu, J., Chen, J., & Zhou, F. (2025). Impact of Alkali-Activated Tannery Sludge-Derived Geopolymer Gel on Cement Properties: Workability, Hydration Process, and Compressive Strength. Gels, 11(5), 339. https://doi.org/10.3390/gels11050339








