Lignin-Mediated Dual Conductive Hydrogels with High Conductivity, Antibacterial Activity and Biocompatibility for Chronic Wound Repair
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Design and Characterizations
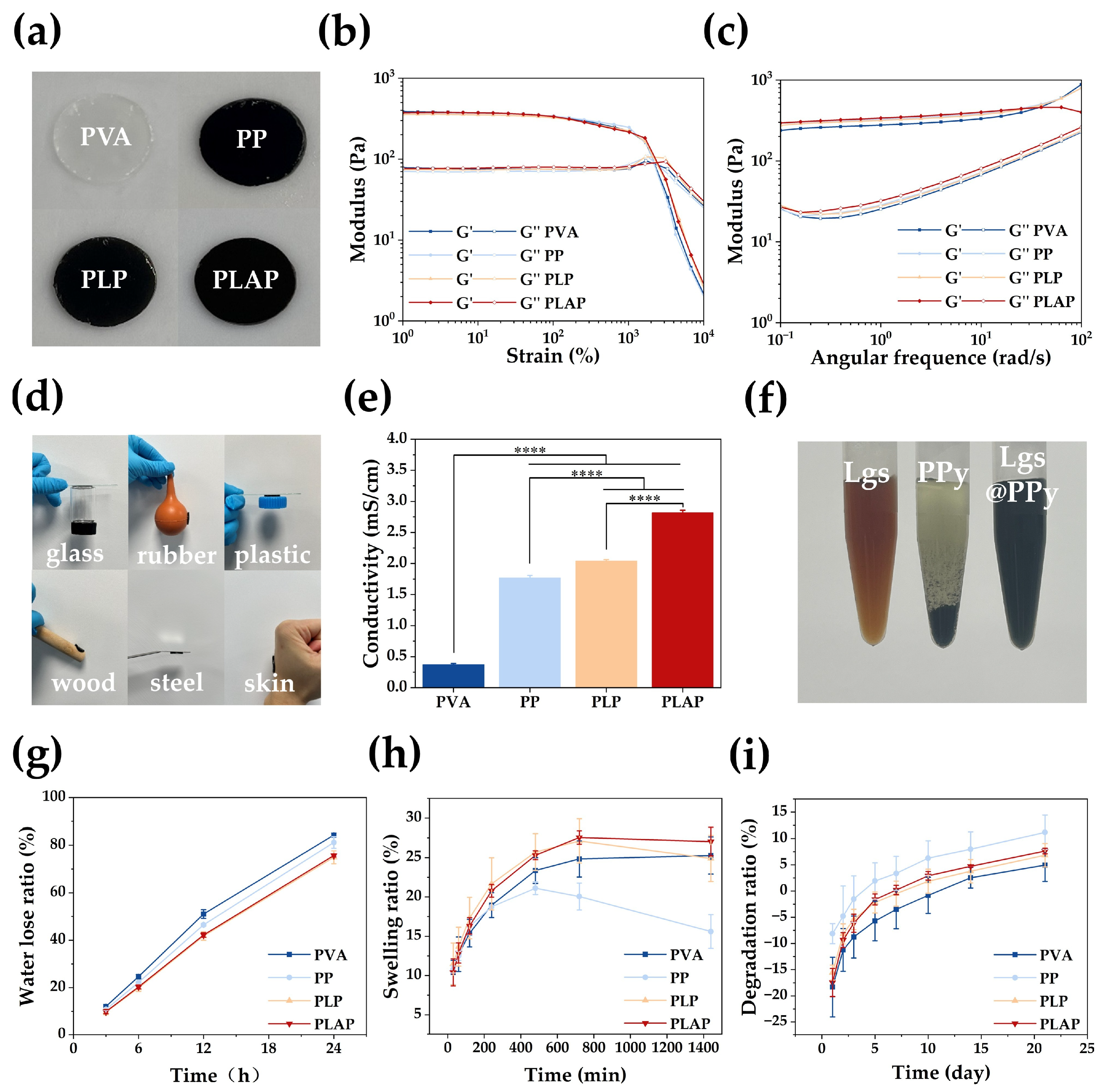
2.2. Properties of the Hydrogels
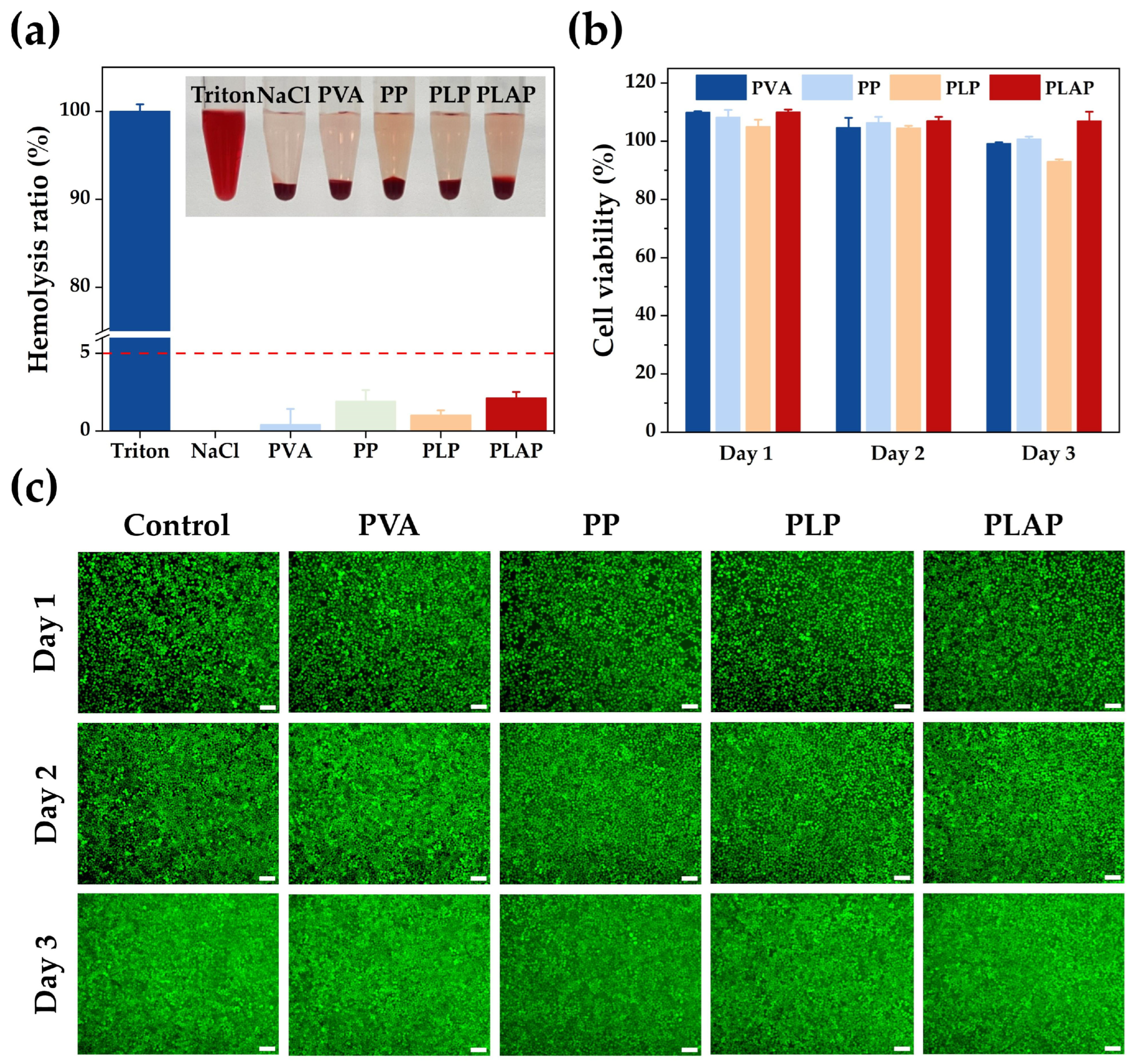
2.3. Biocompatibility of Hydrogels
2.4. Hemostatic Properties of Hydrogels
2.5. In Vitro Antibacterial Activity of Hydrogels
2.6. In Vivo Wound Healing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Lgs-Ag NPs
4.3. Preparation of the Hydrogels
4.4. Rheological Properties of Hydrogels
4.5. Electrical Property of Hydrogels
4.6. Morphology of the Hydrogels
4.7. Porosity of Hydrogels
4.8. Adhesion Properties of Hydrogels
4.9. Moisturizing, Swelling and Degradation Properties of Hydrogels
4.10. Cell Viability
4.11. In Vitro Hemolysis Assay
4.12. Hemostatic Ability Evaluation
4.13. Antibacterial Activity of Hydrogels
4.14. In Vivo Wound Healing Assay
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarbrink, K.; Ni, G.; Sonnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; Garcia-Gonzalez, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Song, R.; Xie, H.; Liu, G. Advances of MXene-based hydrogels for chronic wound healing. Chin. Chem. Lett. 2024, 110442. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Wu, C.; Chen, J.; Ning, N.; Yang, Z.; Guo, Y.; Zhang, J.; Hu, X.; Wang, Y. Conductive dual hydrogen bonding hydrogels for the electrical stimulation of infected chronic wounds. J. Mater. Chem. B 2021, 9, 8138–8146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, D.; Feng, G.; Xu, F.-j. A hydrogel wound dressing ideally designed for chronic wound care. Matter 2023, 6, 1060–1062. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, C.; Guo, W.; Zhang, B.; Ren, S.; Wu, S.; Guo, J.; Qu, W. Conductive hydrogels as an “innovative healer” for the treatment of diabetic wounds. Mater. Chem. Front. 2024, 8, 2944–2977. [Google Scholar] [CrossRef]
- Du, S.; Zhou, N.; Gao, Y.; Xie, G.; Du, H.; Jiang, H.; Zhang, L.; Tao, J.; Zhu, J. Bioinspired hybrid patches with self-adhesive hydrogel and piezoelectric nanogenerator for promoting skin wound healing. Nano Res. 2020, 13, 2525–2533. [Google Scholar] [CrossRef]
- Long, Y.; Wei, H.; Li, J.; Yao, G.; Yu, B.; Ni, D.; Gibson, A.L.; Lan, X.; Jiang, Y.; Cai, W.; et al. Effective Wound Healing Enabled by Discrete Alternative Electric Fields from Wearable Nanogenerators. ACS Nano 2018, 12, 12533–12540. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Li, W.; Li, D.; Song, P.; Kang, Y.; Han, X.; Wang, X.; Tian, H.; Rauf, A.; et al. Construction of piezoelectric, conductive and injectable hydrogels to promote wound healing through electrical stimulation. Acta Biomater. 2025, 191, 205–215. [Google Scholar] [CrossRef]
- Houghton, P.E. Electrical stimulation therapy to promote healing of chronic wounds: A review of reviews. Chronic Wound Care Manag. Res. 2017, 4, 25–44. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Lee, Y.; Lee, M.-G.; Song, W.J.; Park, J.-U.; Sun, J.-Y. Accelerated wound healing with an ionic patch assisted by a triboelectric nanogenerator. Nano Energy 2021, 79, 105463. [Google Scholar] [CrossRef]
- Kao, F.C.; Ho, H.H.; Chiu, P.Y.; Hsieh, M.K.; Liao, J.C.; Lai, P.L.; Huang, Y.F.; Dong, M.Y.; Tsai, T.T.; Lin, Z.H. Self-assisted wound healing using piezoelectric and triboelectric nanogenerators. Sci. Technol. Adv. Mater. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Kim, H.J.; Zhang, S.; Zhou, X.; Chen, Y.; Ling, H.; Xue, Y.; Chen, Z.; Qu, M.; et al. Flexible patch with printable and antibacterial conductive hydrogel electrodes for accelerated wound healing. Biomaterials 2022, 285, 121479. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, F.; Ye, H.; Jiang, J.; Li, S.; Dai, B.; Li, J.; Yang, J.; Song, X.; Zhang, J.; et al. MXene-based flexible electronic materials for wound infection detection and treatment. Npj Flexible Electron. 2024, 8, 30. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Zeng, Y.; Cao, X.; Zhao, H.; Yang, Y.Y.; Yuan, P.; Lu, X.; Ding, X. Co3O4 Nanowires Capable of Discharging Low Voltage Electricity Showing Potent Antibacterial Activity for Treatment of Bacterial Skin Infection. Adv. Healthc. Mater. 2022, 11, e2102044. [Google Scholar] [CrossRef]
- Xu, G.; Lu, Y.; Cheng, C.; Li, X.; Xu, J.; Liu, Z.; Liu, J.; Liu, G.; Shi, Z.; Chen, Z.; et al. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv. Funct. Mater. 2021, 31, 2100852. [Google Scholar] [CrossRef]
- Tang, Q.; Ke, Q.; Chen, Q.; Zhang, X.; Su, J.; Ning, C.; Fang, L. Flexible, Breathable, and Self-Powered Patch Assembled of Electrospun Polymer Triboelectric Layers and Polypyrrole-Coated Electrode for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17641–17652. [Google Scholar] [CrossRef]
- Liang, Y.; Qiao, L.; Qiao, B.; Guo, B. Conductive hydrogels for tissue repair. Chem. Sci. 2023, 14, 3091–3116. [Google Scholar] [CrossRef]
- Ho, M.; Ramirez, A.B.; Akbarnia, N.; Croiset, E.; Prince, E.; Fuller, G.G.; Kamkar, M. Direct Ink Writing of Conductive Hydrogels. Adv. Funct. Mater. 2025, 2415507. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. In-situ doping of a conductive hydrogel with low protein absorption and bacterial adhesion for electrical stimulation of chronic wounds. Acta Biomater. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Shan, M.; Chen, X.; Zhang, X.; Zhang, S.; Zhang, L.; Chen, J.; Wang, X.; Liu, X. Injectable Conductive Hydrogel with Self-Healing, Motion Monitoring, and Bacteria Theranostics for Bioelectronic Wound Dressing. Adv. Healthc. Mater. 2024, 13, e2303876. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2017, 28, 1704195. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid Fabrication of Self-Healing, Conductive, and Injectable Gel as Dressings for Healing Wounds in Stretchable Parts of the Body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, C.; Chen, X.; Huang, J.; Guo, Z. Advances in Electrically Conductive Hydrogels: Performance and Applications. Small Methods 2024, e2401156. [Google Scholar] [CrossRef]
- Gajendiran, M.; Choi, J.; Kim, S.-J.; Kim, K.; Shin, H.; Koo, H.-J.; Kim, K. Conductive biomaterials for tissue engineering applications. J. Ind. Eng. Chem. 2017, 51, 12–26. [Google Scholar] [CrossRef]
- Lin, X.; Yang, X.; Li, P.; Xu, Z.; Zhao, L.; Mu, C.; Li, D.; Ge, L. Antibacterial Conductive Collagen-Based Hydrogels for Accelerated Full-Thickness Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 22817–22829. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Xu, Y.; Chen, J.; Ning, N.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Highly Stretchable and Conductive Self-Healing Hydrogels for Temperature and Strain Sensing and Chronic Wound Treatment. ACS Appl. Mater. Interfaces 2020, 12, 40990–40999. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically Conductive and 3D-Printable Oxidized Alginate-Gelatin Polypyrrole:PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, e2001876. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, R.; He, P.; Li, X.; Shi, Z.; Yang, G. A capacitive polypyrrole-wrapped carbon cloth/bacterial cellulose antibacterial dressing with electrical stimulation for infected wound healing. Adv. Compos. Hybrid Mater. 2024, 7, 10. [Google Scholar] [CrossRef]
- Sasso, C.; Fenoll, M.; Stephan, O.; Beneventia, D. Use of wood derivatives as doping /dispersing agents in the preraration of polypyrrole aqueous dispersios. BioResources 2008, 3, 1187–1195. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Chen, H.; Wang, N.; Liu, X.; Sun, G.; Qiao, W. Effective wound dressing based on Poly (vinyl alcohol)/Dextran-aldehyde composite hydrogel. Int. J. Biol. Macromol. 2019, 132, 1098–1105. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Ji, X.; Wang, Q.; Tian, Z.; Liu, S. Recent advances in lignosulfonate filled hydrogel for flexible wearable electronics: A mini review. Int. J. Biol. Macromol. 2022, 212, 393–401. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, J.; Zhao, H.; Zhang, W.; Shi, G.; He, Y.; Zhou, S.; Qiao, X.; Pang, X. Preparation of Lignosulfonate@AgNPs Colloidal Nanocrystal Clusters through In Situ Reduction, Confined Growth, and Self-Assembly. ACS Sustain. Chem. Eng. 2023, 11, 11130–11139. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; An, X.; Seta, F.T.; Li, C.; Zhang, H.; Luo, B.; Hu, Q.; Zhang, R.; Nie, S.; et al. Facile preparation of lignosulfonate induced silver nanoparticles for high efficient removal of organic contaminants in wastewater. Ind. Crops Prod. 2021, 169, 113644. [Google Scholar] [CrossRef]
- Xiao, S.; Tan, Y.; Xu, J.; Xiong, C.; Wang, X.; Su, S. Lignosulfonate as dispersant for layered double hydroxide in nitrile–butadiene rubber composites. Appl. Clay Sci. 2014, 97–98, 91–95. [Google Scholar] [CrossRef]
- Ren, K.; Shi, Y.; Wen, C.; Kang, X.; Tian, Y.; Guan, Y.; Ning, C.; Yang, X.; Zhou, L.; Fu, R.; et al. Lignin-Based Conductive Hydrogels with Plasticity, Recyclability, and Self-Adhesion as Flexible Strain Sensors for Human Motion Monitoring. ACS Appl. Polym. Mater. 2024, 6, 5297–5307. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Chu, J.; Ma, J.; Fan, Y.; Wang, Z.; Ni, Y. Lignin-Directed Control of Silver Nanoparticles with Tunable Size in Porous Lignocellulose Hydrogels and Their Application in Catalytic Reduction. ACS Sustain. Chem. Eng. 2020, 8, 12655–12663. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, X.; Wu, Y.; Qian, Y.; Zhou, M.; Deng, Y.; Li, Y. Aggregation-induced emission: The origin of lignin fluorescence. Polym. Chem. 2016, 7, 3502–3508. [Google Scholar] [CrossRef]
- Ahmad, A.; Roy, P.G.; Hassan, A.; Zhou, S.; Azam, M.; Sial, M.A.Z.G.; Irfan, A.; Kanwal, F.; Begum, R.; Farooqi, Z.H. Catalytic degradation of various dyes using silver nanoparticles fabricated within chitosan based microgels. Int. J. Biol. Macromol. 2024, 283, 137965. [Google Scholar] [CrossRef]
- He, X.; Kim, H.; Dong, T.G.; Gates, I.; Lu, Q. Green synthesis of Ag/lignin nanoparticle-loaded cellulose aerogel for catalytic degradation and antimicrobial applications. Cellulose 2022, 29, 9341–9360. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Magotra, V.K.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Palem, R.R.; et al. Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities. Polymers 2022, 14, 648. [Google Scholar] [CrossRef]
- Fan, L.; Xiao, C.; Guan, P.; Zou, Y.; Wen, H.; Liu, C.; Luo, Y.; Tan, G.; Wang, Q.; Li, Y.; et al. Extracellular Matrix-Based Conductive Interpenetrating Network Hydrogels with Enhanced Neurovascular Regeneration Properties for Diabetic Wounds Repair. Adv. Healthc. Mater. 2022, 11, e2101556. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993: 2018; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- Sethi, S.; Medha; Thakur, S.; Kaith, B.S. Preliminary in vitro hemocompatibility assessment of biopolymeric hydrogels for versatile biomedical applications. Polym. Bull. 2023, 81, 4499–4522. [Google Scholar] [CrossRef]
- Garg, S.S.; Dubey, R.; Sharma, S.; Vyas, A.; Gupta, J. Biological macromolecules-based nanoformulation in improving wound healing and bacterial biofilm-associated infection: A review. Int. J. Biol. Macromol. 2023, 247, 125636. [Google Scholar] [CrossRef]
- Lei, Z.; Liang, H.; Sun, W.; Chen, Y.; Huang, Z.; Yu, B. A biodegradable PVA coating constructed on the surface of the implant for preventing bacterial colonization and biofilm formation. J. Orthop. Surg. Res. 2024, 19, 175. [Google Scholar] [CrossRef]
- Xu, T.; Gao, H.; Zhou, J.; He, M.; Ji, X.; Dai, H.; Rojas, O.J. Design of AgNPs doped chitosan/sodium lignin sulfonate/polypyrrole films with antibacterial and endotoxin adsorption functions. Int. J. Biol. Macromol. 2023, 229, 321–328. [Google Scholar] [CrossRef]
- Noreen, H.; Iqbal, J.; Arshad, A.; Faryal, R.; Ataur, R.; Khattak, R. Sunlight induced catalytic degradation of bromophenol blue and antibacterial performance of graphene nanoplatelets/polypyrrole nanocomposites. J. Solid State Chem. 2019, 275, 141–148. [Google Scholar] [CrossRef]
- Piggot, T.J.; Holdbrook, D.A.; Khalid, S. Electroporation of the E. coli and S. aureus membranes: Molecular dynamics simulations of complex bacterial membranes. J. Phys. Chem. B 2011, 115, 13381–13388. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar free healing of full thickness diabetic wounds: A unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix. Int. J. Biol. Macromol. 2021, 178, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Maklakova, M.; Villarreal-Gómez, L.J.; Nefedova, E.; Shkil, N.; Pestryakov, A.; Bogdanchikova, N. Role of Biofilm Formation in the Drop of Bacterial Resistance to Antibiotics after Animal Therapy with Silver Nanoparticles. ACS Appl. Nano Mater. 2024, 7, 16553–16563. [Google Scholar] [CrossRef]
- Moreno Ruiz, Y.P.; de Almeida Campos, L.A.; Alves Agreles, M.A.; Galembeck, A.; Macário Ferro Cavalcanti, I. Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics 2023, 12, 104. [Google Scholar] [CrossRef]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl Cellulose-Based Hydrogel: Dielectric Study, Antimicrobial Activity and Biocompatibility. Arab. J. Sci. Eng. 2020, 46, 17–30. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Li, J.; Mishra, D.; Ren, Y.; Gates, I.; Hu, J.; Lu, Q. Ultrastretchable, Adhesive, and Antibacterial Hydrogel with Robust Spinnability for Manufacturing Strong Hydrogel Micro/Nanofibers. Small 2021, 17, e2103521. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Lu, F.; Jiang, H.; Wei, F. Si-induced insertion of Li into SiC to form Li-rich SiC twin crystal. Particuology 2023, 74, 56–63. [Google Scholar] [CrossRef]
- GB/T 35892-2018; Laboratory Animal—Guideline for Ethical Review of Animal Welfare. Standards Press of China: Beijing, China, 2018.





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Chen, M.; Zhao, W.; Zhang, S.; Liu, J.; Zhou, Y.; Jiang, L.; Zhang, J. Lignin-Mediated Dual Conductive Hydrogels with High Conductivity, Antibacterial Activity and Biocompatibility for Chronic Wound Repair. Gels 2025, 11, 283. https://doi.org/10.3390/gels11040283
Lin J, Chen M, Zhao W, Zhang S, Liu J, Zhou Y, Jiang L, Zhang J. Lignin-Mediated Dual Conductive Hydrogels with High Conductivity, Antibacterial Activity and Biocompatibility for Chronic Wound Repair. Gels. 2025; 11(4):283. https://doi.org/10.3390/gels11040283
Chicago/Turabian StyleLin, Jianhong, Mengyao Chen, Wei Zhao, Shengyu Zhang, Jialin Liu, Yang Zhou, Lei Jiang, and Jiantao Zhang. 2025. "Lignin-Mediated Dual Conductive Hydrogels with High Conductivity, Antibacterial Activity and Biocompatibility for Chronic Wound Repair" Gels 11, no. 4: 283. https://doi.org/10.3390/gels11040283
APA StyleLin, J., Chen, M., Zhao, W., Zhang, S., Liu, J., Zhou, Y., Jiang, L., & Zhang, J. (2025). Lignin-Mediated Dual Conductive Hydrogels with High Conductivity, Antibacterial Activity and Biocompatibility for Chronic Wound Repair. Gels, 11(4), 283. https://doi.org/10.3390/gels11040283




