Newly Designed Organic-Inorganic Nanocomposite Membrane for Simultaneous Cr and Mn Speciation in Waters
Abstract
1. Introduction
2. Results and Discussion
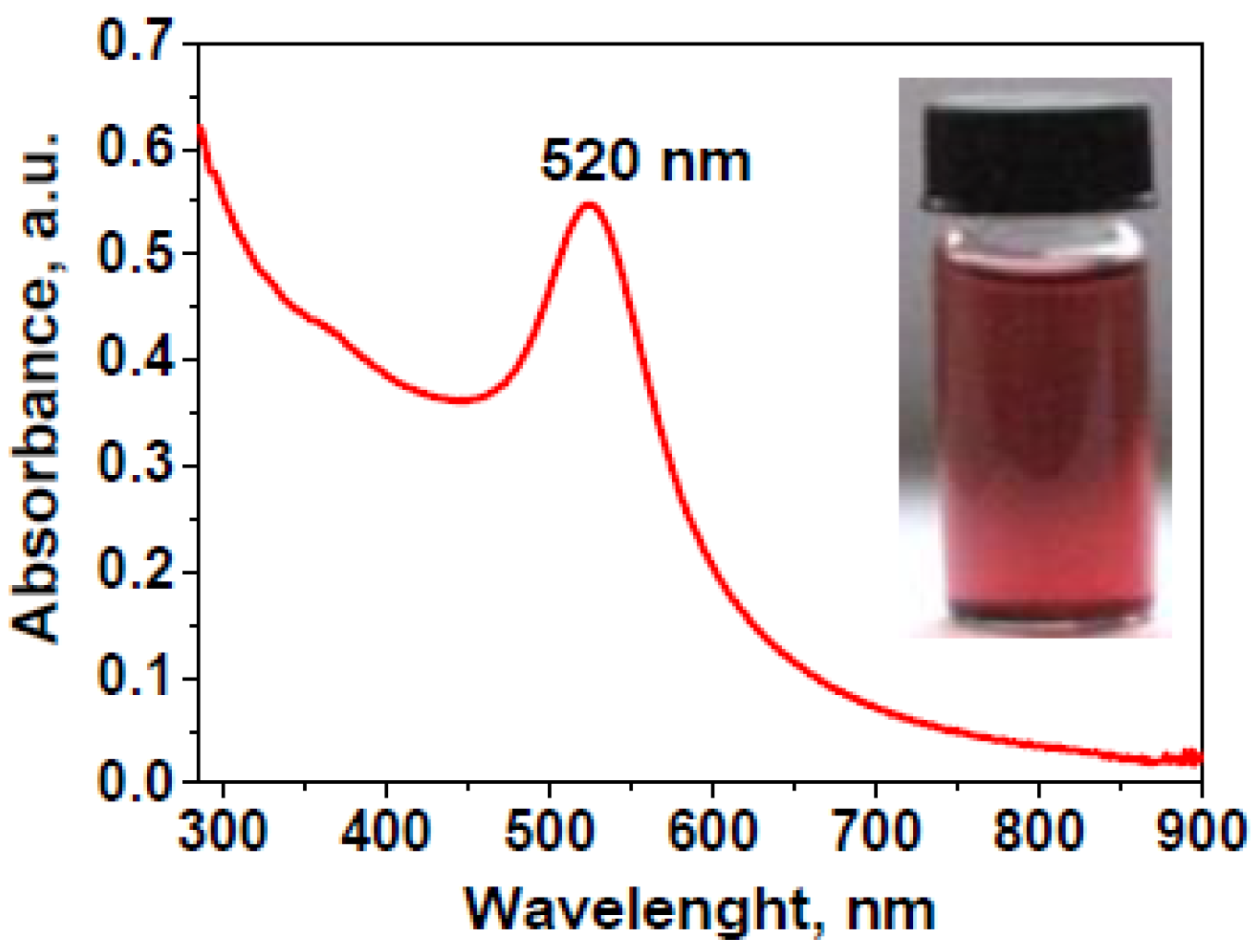
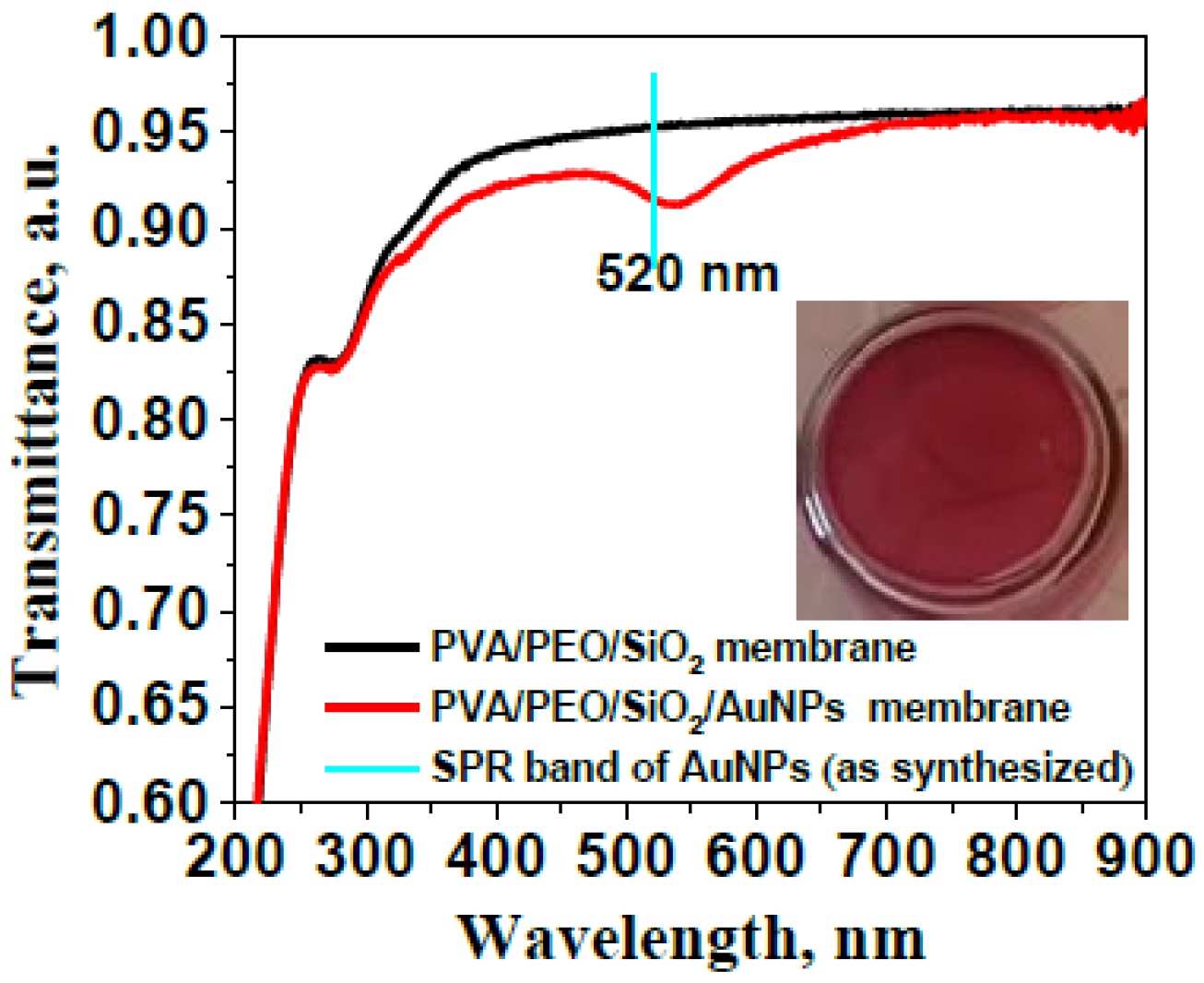
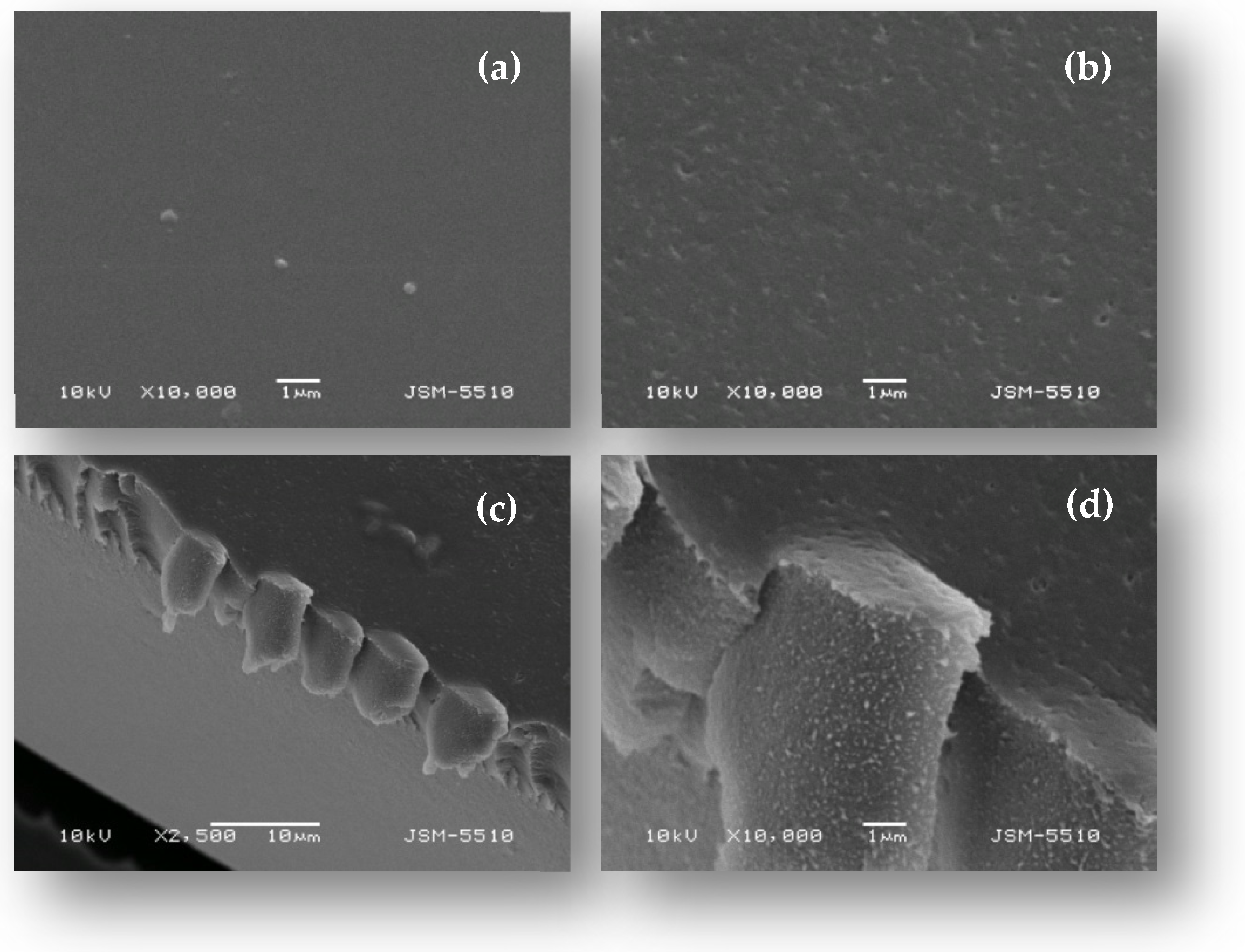
2.1. Characterization of Gold Nanoparticles and the PVA/PEO/SiO2/AuNP Nanocomposite Membrane
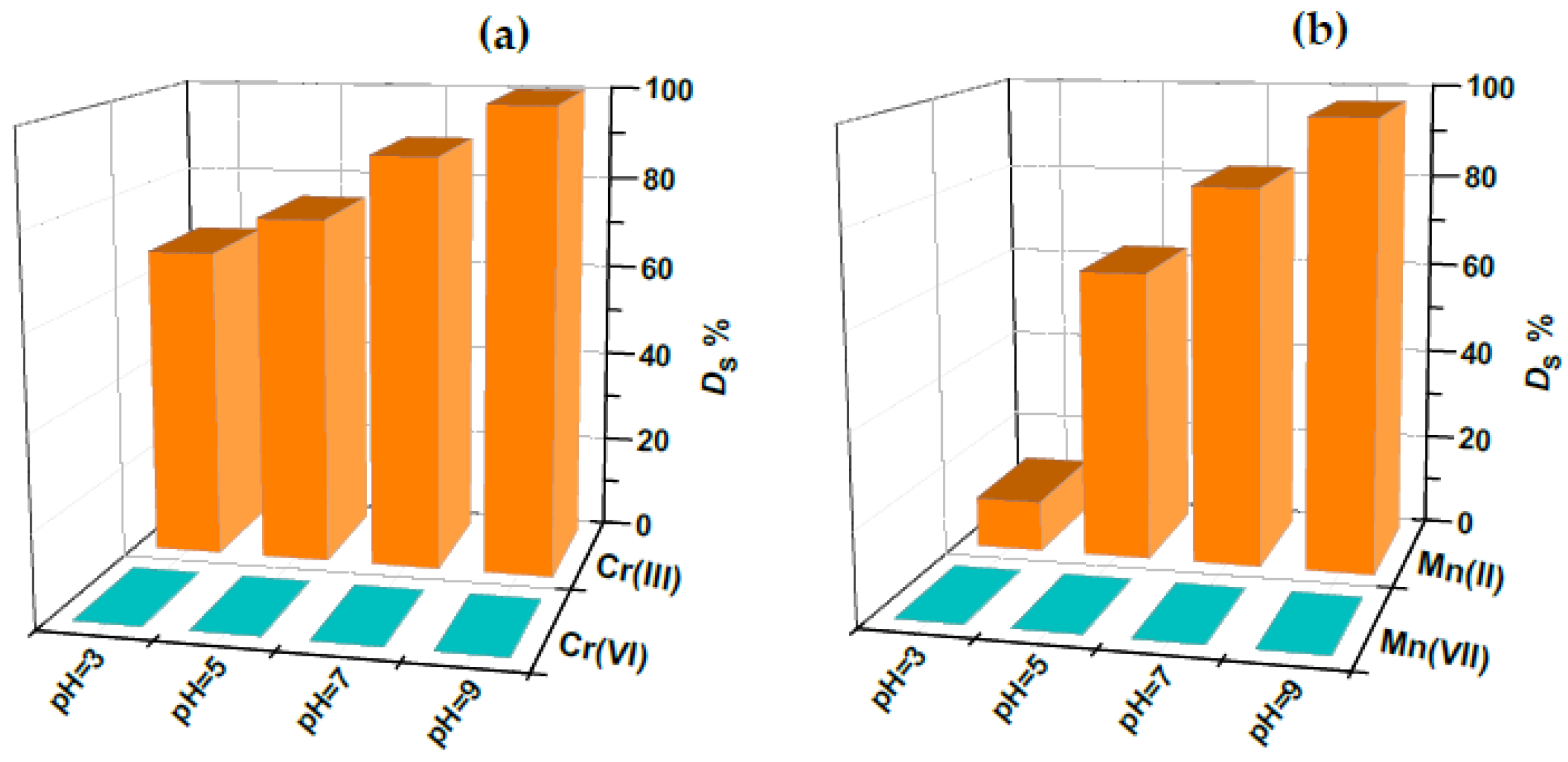
2.2. Adsorption Behavior of the PVA/PEO/SiO2/AuNP Nanocomposite Membrane Toward Cr(III)/Cr(VI) and Mn(II)/Mn(VII)—Optimization Studies
2.3. Desorption Studies
2.4. Investigations on the Mechanism of Cr(III) and Mn(II) Adsorption onto the PVA/PEO/SiO2/AuNP Nanocomposite Membrane
2.4.1. Adsorption Isotherm Models
2.4.2. Modeling of Cr(III) Sorption Kinetics
2.5. Analytical Applications
2.6. Analytical Figures of Merit
3. Conclusions
4. Materials and Methods
4.1. Materials, Reagents, and Instruments
4.2. Synthesis of Starch-Coated AuNPs
4.3. Preparation of the PVA/PEO/SiO2/AuNP Nanocomposite Membrane
4.4. Static Adsorption/Desorption Experiments
4.5. Isotherm and Kinetic Studies
4.6. Analytical Procedure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metze, D.; Jakubowski, N.; Klockow, D. Species in the environment, food, medicine & occupational health. In Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine and Occupational Health; Cornelis, R., Crews, H., Caruso, J., Heumann, K.G., Eds.; John Wiley&Sons, Ltd.: Chichester, UK, 2005; pp. 120–135. [Google Scholar]
- Kot, A.; Namiesńik, J. The role of speciation in analytical chemistry. TrAC Trends Anal. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Llaver, M.; Fiorentini, E.F.; Oviedo, M.N.; Quintas, P.Y.; Wuilloud, R.G. Elemental Speciation Analysis in Environmental Studies: Latest Trends and Ecological Impact. Int. J. Environ. Res. Public Health 2021, 19, 12135. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Eary, L.E.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Chebeir, M.; Chen, G.; Liu, H. Emerging investigators series: Frontier review: Occurrence and speciation of chromium in drinking water distribution systems. Environ. Sci.:Water Res. Technol. 2016, 2, 906–914. [Google Scholar]
- Katz, S.A.; Salem, H. The toxicology of chromium with respect to its chemical speciation—A review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Jablońska-Czapla, M. Manganese and its speciation in environmental samples using hyphenated techniques: A review. J. Elem. 2015, 20, 1061–1075. [Google Scholar] [CrossRef]
- Witholt, R.; Gwiazda, R.H.; Smith, D.R. The neurobehavioral effects of sub-chronic manganese exposure in the presence and absence of pre-parkinsonism. Neurotoxicol. Teratol. 2000, 22, 851–861. [Google Scholar] [CrossRef]
- Yokel, R.A.; Crossgrove, J.S.; Bukaveckas, B.L. Manganese distribution across the blood-brain barrier. II. Manganese efflux from the brain does not appear to be carrier mediated. NeuroToxicology 2003, 24, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.; Callao, M.P. Chromium determination and speciation since 2000. TrAC Trends Anal. Chem. 2006, 25, 1006–1015. [Google Scholar] [CrossRef]
- Namieśnik, J.; Rabajczyk, A. Speciation Analysis of Chromium in Environmental Samples. Crit. Rev. Environ. Sci. Technol. 2011, 42, 327–377. [Google Scholar] [CrossRef]
- Rakhunde, R.; Deshpande, L.; Juneja, H.D. Chemical Speciation of Chromium in Water: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Dawra, N.; Dabas, N. Advances in spectrophotometric determination of Chromium (III) and Chromium(VI) in water: A review. J. Environ. Anal. Chem. 2022, 104, 2994–3015. [Google Scholar] [CrossRef]
- Markiewicz, B.; Komorowicz, I.; Sajnóg, A.; Belter, M.; Barałkiewicz, D. Chromium and its speciation in water samples by HPLC/ICP-MS—Technique establishing metrological traceability: A review since 2000. Talanta 2015, 132, 814–828. [Google Scholar] [CrossRef]
- Rumsby, P.; Rockett, L.; Clegg, H.; Jonsson, J.; Benson, V.; Harman, M.; Doyle, T.; Rushton, L.; Wilkinson, D.; Warwick, P. Speciation of manganese in drinking water. Toxicol. Lett. 2014, 229, S120. [Google Scholar] [CrossRef]
- Pearson, G.F.; Greenway, G.M. Recent developments in manganese speciation. TrAC Trends Analyt Chem. 2005, 24, 803–809. [Google Scholar] [CrossRef]
- Grygo-Szymanko, E.; Tobiasz, A.; Walas, S. Speciation analysis and fractionation of manganese—A review. TrAC Trends Anal. Chem. 2016, 80, 112–124. [Google Scholar] [CrossRef]
- Zhang, M.; Zhan, G.; Chen, Z. Iodometric Amplification Method for the Determinations of Microgram Amounts of Manganese(II), Manganese(VII), Chromium(III) and Chromium(VI) in Aqueous Solution. Anal. Sci. 2005, 14, 1077–1083. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Sadeghi, G.H. A nano-structured material for reliable speciation of chromium and manganese in drinking waters, surface waters and industrial wastewater effluents. Talanta 2012, 94, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, A.G.; Nille, O.S.; Gunjal, D.B.; Naik, V.M.; Ngoc, Q.N.; Sohn, D.; Kolekar, G.B.; Gokavi, G.S.; More, V.R. Prompt in situ synthesis of sulphur doped carbon dots from jaggery for parallel determination of iron, chromium and manganese in environmental samples. J. Photochem. Photobiol. A Chem. 2024, 454, 115672. [Google Scholar] [CrossRef]
- Wakshe, S.B.; Dongare, P.R.; Gore, A.H.; Mote, G.V.; Salunkhe, S.Y.; Mahanwar, S.T.; Anbhule, P.V.; Kolekar, G.B. A highly sensitive and selective phthalazine derivative based fluorescent organic nanosheets for simultaneous detection of Cr6+ and Mn7+ in aqueous media. Inorg. Chim. Acta 2021, 526, 120534. [Google Scholar] [CrossRef]
- Hagarová, I.; Nemček, L. Application of Metallic Nanoparticles and Their Hybrids as Innovative Sorbents for Separation and Pre-concentration of Trace Elements by Dispersive Micro-Solid Phase Extraction: A Mini review. Front. Chem. 2021, 9, 672755. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Li, Y.K.; Wang, X.Y.; Liu, X.; Yang, T.; Chen, M.L.; Wang, J.H. Ensuring high selectivity for preconcentration and detection of ultra-trace cadmium using a phage-functionalized metal–organic framework. Analyst 2020, 145, 5280–5288. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3, 22–39. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Pena-Crecente, R.M. Graphene and carbon nanotubes as solid phase extraction sorbents for the speciation of chromium: A review. Anal. Chim. Acta 2018, 1002, 1–17. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C. Layered double hydroxides: Emerging sorbent materials for analytical extractions. TrAC Trends Anal. Chem. 2016, 75, 174–182. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Recent advances in nanomaterials for analysis of trace heavy metals. Crit. Rev. Anal. Chem. 2021, 51, 353–372. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, L.; Zhao, B.; Chen, B.; Hu, B. Advanced functional materials in solid phase extraction for ICP-MS determination of trace elements and their species-A review. Anal. Chim. Acta 2017, 973, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic nanoparticle based solid-phase extraction of heavy metal ions: A review on recent advances. Microchim. Acta 2018, 185, 160. [Google Scholar] [CrossRef] [PubMed]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef]
- Rivas, B.L.; Urbano, B.F.; Sánchez, J. Water-soluble and insoluble polymers, nanoparticles, nanocomposites and hybrids with ability to remove hazardous inorganic pollutants in water. Front. Chem. 2018, 6, 320. [Google Scholar] [CrossRef]
- Shamsipur, M.; Fasihi, J.; Ashtari, K. Grafting of ion-imprinted polymers on the surface of silica gel particles through covalently surface-bound initiators: A selective sorbent for uranyl ion. Anal. Chem. 2007, 79, 7116–7123. [Google Scholar] [CrossRef]
- Buhani, B.; Narsito, N.; Nuryono, N.; Kunarti, E.S. Production of metal ion imprinted polymer from mercapto-silica through sol–gel process as selective adsorbent of cadmium. Desalination 2010, 251, 83–89. [Google Scholar] [CrossRef]
- Li, F.; Jiang, H.; Zhang, S. An ion-imprinted silica-supported organic–inorganic hybrid sorbent prepared by a surface imprinting technique combined with a polysaccharide incorporated sol–gel process for selective separation of cadmium(II) from aqueous solution. Talanta 2007, 71, 1487–1493. [Google Scholar] [CrossRef]
- Djerahov, L.; Vasileva, P.; Karadjova, I. Self-standing chitosan film loaded with silver nanoparticles as a tool for selective determination of Cr (VI) by ICP-MS. Microchem. J. 2016, 129, 23–28. [Google Scholar] [CrossRef]
- Vimala, K.; Murali Mohana, Y.; Samba Sivudu, K.; Varaprasad, K.; Ravindra, S.; Narayana Reddy, N.; Padma, Y.; Sreedhar, B.; Mohana Raju, K. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B Biointerfaces 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Ščančar, J.; Milačič, R. A critical overview of Cr speciation analysis based on high performance liquid chromatography and spectrometric techniques. J. Anal. At. Spectrom. 2014, 29, 427–443. [Google Scholar] [CrossRef]
- Mladenova, E.K.; Dakova, I.G.; Karadjova, I.B. Chitosan membranes as sorbents for trace elements determination in surface waters. Environ Sci. Pollut. Res. 2011, 18, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Priastomo, Y.; Setiawan, H.R.; Kurniawan, Y.S.; Ohto, K. Simultaneous removal of lead (II), chromium (III), and copper (II) heavy metal ions through an adsorption process using C-phenylcalix [4] pyrogallolarene material. J. Environ. Chem. Eng. 2020, 8, 103971. [Google Scholar]
- Abdelwahab, O.; Fouad, Y.O.; Amin, N.K.; Mandor, H. Kinetic and thermodynamic aspects of cadmium adsorption onto raw and activated guava (Psidium guajava) leaves. Environ. Prog. Sustain. 2015, 34, 351–358. [Google Scholar] [CrossRef]
- Samadi, N.; Hasanzadeh, R.; Rasad, M. Adsorption isotherms, kinetic, and desorption studies on removal of toxic metal ions from aqueous solutions by polymeric adsorbent. J. Appl. Polym. Sci. 2015, 132, 41642. [Google Scholar] [CrossRef]
- Embaby, M.A.; Moniem, S.M.; Fathy, N.A.; El-Kady, A.A. Nanocarbon hybrid for simultaneous removal of arsenic, iron and manganese ions from aqueous solutions. Heliyon 2021, 7, e08218. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent—A review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Kaptso, K.G.; Njintang, Y.N.; Komnek, A.E.; Hounhouigan, J.; Scher, J.; Mbofung, C.M. Physical properties and rehydration kinetics of two varieties of cowpea (Vigna unguiculata) and bambara groundnuts (Voandzeia subterranea) seeds. J. Food Eng. 2008, 86, 91–99. [Google Scholar] [CrossRef]
- Cai, Q.; Turner, B.D.; Sheng, D.; Sloan, S. The kinetics of fluoride sorption by zeolite: Effects of cadmium, barium and manganese. J. Contam. Hydrol. 2015, 177, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Madaeni, S.S.; Vatanpour, V. Thermodynamic investigation and mathematical modeling of ion-imprinted membrane adsorption. J. Membr. Sci. 2012, 389, 334–342. [Google Scholar] [CrossRef]
- Martell, J.D.; Milner, P.J.; Siegelman, R.L.; Long, J.R. Kinetics of cooperative CO2 adsorption in diamine-appended variants of the metal–organic framework Mg2(dobpdc). Chem. Sci. 2020, 11, 6457–6471. [Google Scholar] [CrossRef]
- Quaranta, M.; Gehring, T.; Odell, B.; Brown, J.M.; Blackmond, D.G. Unusual inverse temperature dependence on reaction rate in the asymmetric autocatalytic alkylation of pyrimidyl aldehydes. J. Am. Chem. Soc. 2010, 132, 15104–15107. [Google Scholar] [CrossRef]
- Mathew, S.P.; Klussmann, M.; Iwamura, H.; Wells, D.H., Jr.; Armstrong, A.; Blackmond, D.G. A mechanistic rationalization of unusual kinetic behavior in proline-mediated C–O and C–N bond-forming reactions. Chem. Commun. 2006, 41, 4291–4293. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis and pharmacology. In Pharmacology: Principles and Practice, 1st ed.; Hacker, M., Messer, W.S., Bachmann, K.A., Eds.; Academic Press/Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 75–102. [Google Scholar]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Newberry, N.R.; Gilbert, M.J. Biphasic dose-response curve to muscarine on the rat superior cervical ganglion. Eur. J. Pharmacol. 1989, 163, 237–244. [Google Scholar] [CrossRef]
- Wu, P.; Chen, H.; Cheng, G.; Hou, X. Exploring surface chemistry of nano-TiO2 for automated speciation analysis of Cr(III) and Cr(VI) in drinking water using flow injection and ET-AAS detection. J. Anal. At. Spectrom. 2009, 24, 1098. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Li, Y.; Jiang, Y.; Yan, X.-P. Magnetic immobilization of amine-functionalized magnetite microspheres in a knotted reactor for on-line solid-phase extraction coupled with ICP-MS for speciation analysis of trace chromium. J. Anal. At. Spectrom. 2010, 25, 1467. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, T.; Wang, Y.; Lian, H.; Hu, X. Magnetic solid-phase extraction combined with graphite furnace atomic absorption spectrometry for speciation of Cr(III) and Cr(VI) in environmental waters. Talanta 2013, 116, 361–367. [Google Scholar] [CrossRef]
- Diniz, K.M.; Tarley, C.R.T. Speciation analysis of chromium in water samples through sequential combination of dispersive magnetic solid phase extraction using mesoporous amino-functionalized Fe3O4/SiO2 nanoparticles and cloud point extraction. Microchem. J. 2015, 123, 185–195. [Google Scholar] [CrossRef]
- Islam, A.; Ahmad, H.; Zaidi, N.; Kumar, S. A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim. Acta. 2015, 183, 289–296. [Google Scholar] [CrossRef]
- Heena, G.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Speciation of Cr (III) and Cr (VI) Ions via Fabric Phase Sorptive Extraction for Their Quantification via HPLC with UV Detection. J. Chromatogr. Sep. Tech. 2016, 7, 1–6. [Google Scholar]
- Dakova, I.; Vasileva, P.; Karadjova, I. Cr(III) Ion-Imprinted Hydrogel Membrane for Chromium Speciation Analysis in Water Samples. Gels 2022, 8, 757. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, L.; Lu, D.; Cheng, X.; Zhou, X. Separation and chromium speciation by single-wall carbon nanotubes microcolumn and inductively coupled plasma mass spectrometry. Microchim. Acta 2010, 169, 123–128. [Google Scholar]
- Şahan, S.; Saçmacı, Ş.; Kartal, Ş.; Saçmacı, M.; Şahin, U.; Ülgen, A. Development of a new on-line system for the sequential speciation and determination of chromium species in various samples using a combination of chelating and ion exchange resins. Talanta 2014, 120, 391–397. [Google Scholar] [CrossRef]
- Pramanik, S.; Dey, S.; Chattopadhyay, P. A new chelating resin containing azophenolcarboxylate functionality: Synthesis, characterization and application to chromium speciation in wastewater. Anal. Chim. Acta 2007, 584, 469–476. [Google Scholar] [CrossRef]
- Chen, S.; Qin, X.; Gu, W.; Zhu, X. Speciation analysis of Mn (II)/Mn (VII) using Fe3O4@ionic liquids-β-cyclodextrin polymer magnetic solid phase extraction coupled with ICP-OES. Talanta 2016, 161, 325–332. [Google Scholar] [CrossRef]
- Shirkhanloo, H.; Khaligh, A.; Zavvar Mousavi, H.; Rashidi, AM. Ultrasound assisted-dispersive-micro-solid phase extraction based on bulky amino bimodal mesoporous silica nanoparticles for speciation of trace manganese (II)/(VII) ions in water samples. Microchem. J. 2016, 124, 637–645. [Google Scholar] [CrossRef]
- Qian, A.X.S.; He, G.H.F.; Han, X. Separation and preconcentration of MnVII/MnII speciation on crosslinked chitosan and determination by flame atomic absorption spectrometry. Analyst 2001, 126, 239–241. [Google Scholar] [CrossRef]
- Rakhtshah, J.; Shirkhanloo, H.; Mobarake, M.D. Simultaneously speciation and determination of manganese(II) and (VII) ions in water, food, and vegetable samples based on immobilization of N-acetylcysteine on multi-walled carbon nanotubes. Food Chem. 2022, 389, 133124. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.K.; Kattumuri, V.; Bhaskaran, S.; Katti, K.V.; Kannan, R. Facile and general method for synthesis of sugar-coated gold nanoparticles. Int. J. Green Nanotechnol. Biomed. 2009, 1, B53–B59. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, P.; Donkova, B.; Karadjova, I.; Dushkin, C. Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 203–210. [Google Scholar] [CrossRef]
- Yordanova, T.; Vasileva, P.; Karadjova, I. Noble metal nanocomposites as tools for fast and reliable speciation analysis of mercury in water samples. J. Environ. Anal. Chem. 2020, 105, 1152–1170. [Google Scholar] [CrossRef]



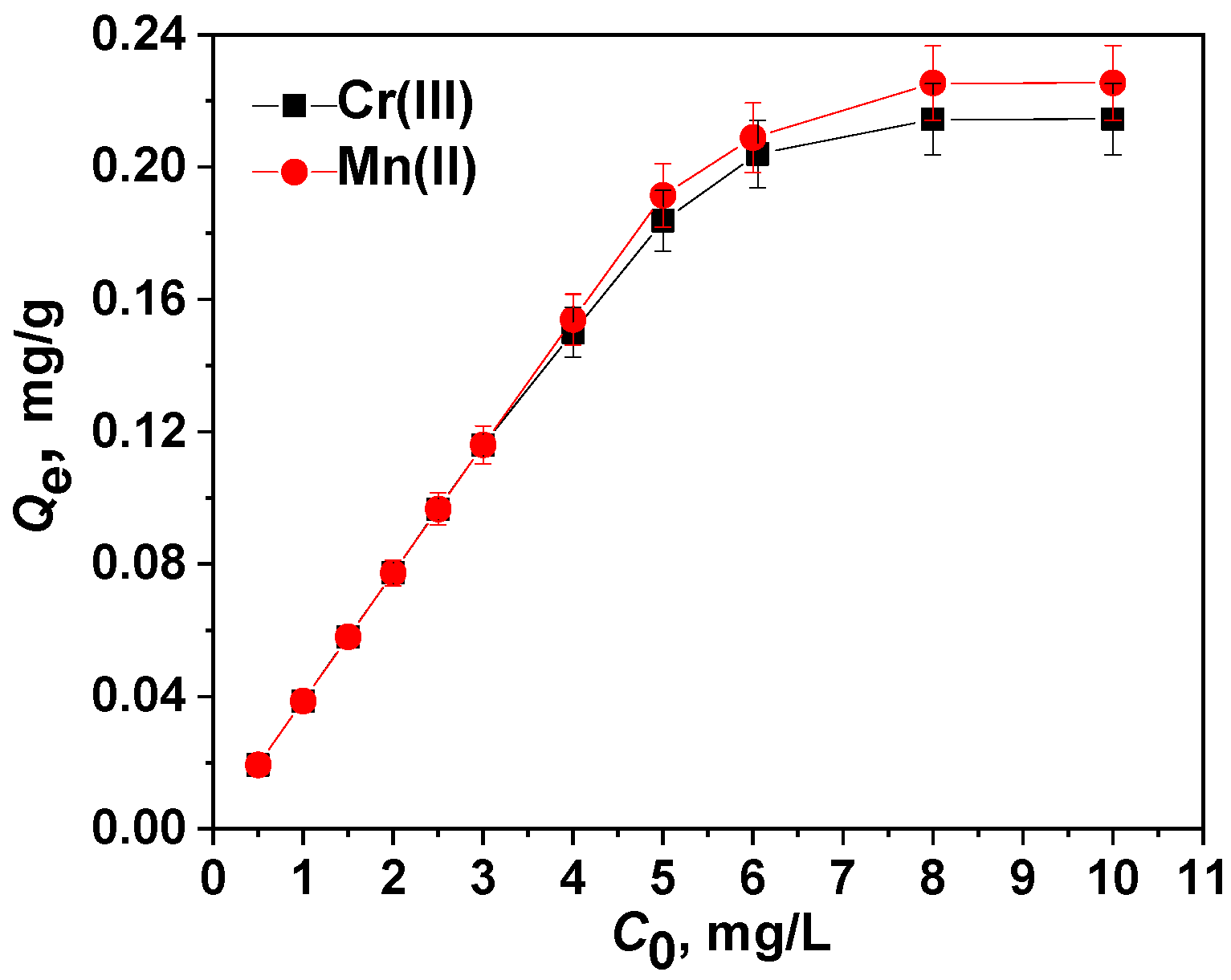
| Isotherm Model | Parameters | Cr(III) | Mn(II) |
|---|---|---|---|
| Langmuir | Qm, mg/g | 0.2149 | 0.2256 |
| KL, L/mg | 71.66 | 154.8 | |
| RL (at C0 = 1 mg/L) | 0 < 0.0138 < 1 | 0 < 0.0064 < 1 | |
| R2 | 0.9998 | 0.9999 | |
| Freundlich | KF, mg/g | 0.1964 | 0.2126 |
| 1/n | 0.0908 << 1 | 0.0632 << 1 | |
| R2 | 0.7748 | 0.8127 | |
| DKR | Xm, mg/g | 0.2159 | 0.2161 |
| β, mol2/J2 | 1.156 × 10−8 | 2.192 × 10−11 | |
| E, J/mol | 6.578 | 151.0 | |
| R2 | 0.9900 | 0.8858 |
| Kinetic Sigmoidal Model | Parameters | Cr(III) | Mn(II) |
|---|---|---|---|
| Slogistic1 | qe, calc, mg/g | 0.04446 | 0.04102 |
| τ, h | 8.895 | 8.301 | |
| k, dimensionless | 0.5115 | 0.6566 | |
| R2 | 0.9905 | 0.9811 | |
| Dose response | qe, calc, mg/g | 0.04313 | 0.04056 |
| qmin, calc, mg/g | 0.0025 | 0.00144 | |
| logEC50, h | 9.087 | 8.443 | |
| p, dimensionless | 0.2840 | 0.3310 | |
| R2 | 0.9945 | 0.9803 |
| Sample | Cr(III) | Cr(VI) | Mn (II) | Mn(VII) |
|---|---|---|---|---|
| River Iskar, µg/L | 0.15 ± 0.06 | <DL | 4.54 ± 0.5 | n.a. |
| Added, µg/L | 0.010 | |||
| Found, µg/L | 0.14 ± 0.08 | 0.009 ± 0.008 | 4.32 ± 0.06 | |
| Tap water, Bistritsa, µg/L | 0.092 ± 0.008 | <DL | 15.4 ± 0.5 | <DL |
| Added, µg/L | 0.05 | 2.00 | ||
| Found, µg/L | 0.093 ± 0.009 | 0.052 ± 0.007 | 14.9 ± 0.5 | 1.95 ± 0.45 |
| Wastewater | 1.23 ± 0.09 | 0.034 ± 0.004 | 256 ± 25 | 21 ± 2 |
| Added, µg/L | 0.10 | 10 | ||
| Found, µg/L | 1.34 ± 0.07 | 0.14 ± 0.006 | 249 ± 23 | 32 ± 3 |
| Cr (Cr(III) + Cr(VI)) | Mn (Mn(II) + Mn(VII)) | |
|---|---|---|
| Certified value, µg/L | 0.252 ± 0.012 | 2.12 ± 0.10 |
| Proposed procedure, µg/L | 0.245 ± 0.016 | 2.04 ± 0.09 |
| Recovery, % | 97.2 ± 0.3 | 96.2 ± 0.2 |
| Parameters | Cr(III) | Cr(VI) | Mn(II) | Mn(VII) |
|---|---|---|---|---|
| Detection limit, µg/L | 0.09 | 0.1 | 0.04 | 0.05 |
| Determination limit, µg/L | 0.26 | 0.3 | 0.12 | 0.15 |
| RSD, % in tap water for the species content (LOD-100 µg/L) | 3–7 | 4–7 | 3–7 | 4–7 |
| RSD, % in wastewater for the species content (LOD-500 µg/L) | 4–10 | 3–9 | 3–10 | 3–8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, P.; Karadjova, I. Newly Designed Organic-Inorganic Nanocomposite Membrane for Simultaneous Cr and Mn Speciation in Waters. Gels 2025, 11, 205. https://doi.org/10.3390/gels11030205
Vasileva P, Karadjova I. Newly Designed Organic-Inorganic Nanocomposite Membrane for Simultaneous Cr and Mn Speciation in Waters. Gels. 2025; 11(3):205. https://doi.org/10.3390/gels11030205
Chicago/Turabian StyleVasileva, Penka, and Irina Karadjova. 2025. "Newly Designed Organic-Inorganic Nanocomposite Membrane for Simultaneous Cr and Mn Speciation in Waters" Gels 11, no. 3: 205. https://doi.org/10.3390/gels11030205
APA StyleVasileva, P., & Karadjova, I. (2025). Newly Designed Organic-Inorganic Nanocomposite Membrane for Simultaneous Cr and Mn Speciation in Waters. Gels, 11(3), 205. https://doi.org/10.3390/gels11030205






