Alginate Microbeads for Trapping Phenolic Antioxidants in Rosemary (Rosmarinus officinalis L.): Multivariate Optimization Based on Bioactive Properties and Morphological Measurements
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Encapsulation Process Based on BBD–RSM
2.1.1. Impacts of Input Variables on the Encapsulation Efficiency
2.1.2. Impacts of Input Variables on Antioxidant Activity
2.1.3. Impacts of Input Variables on Sphericity
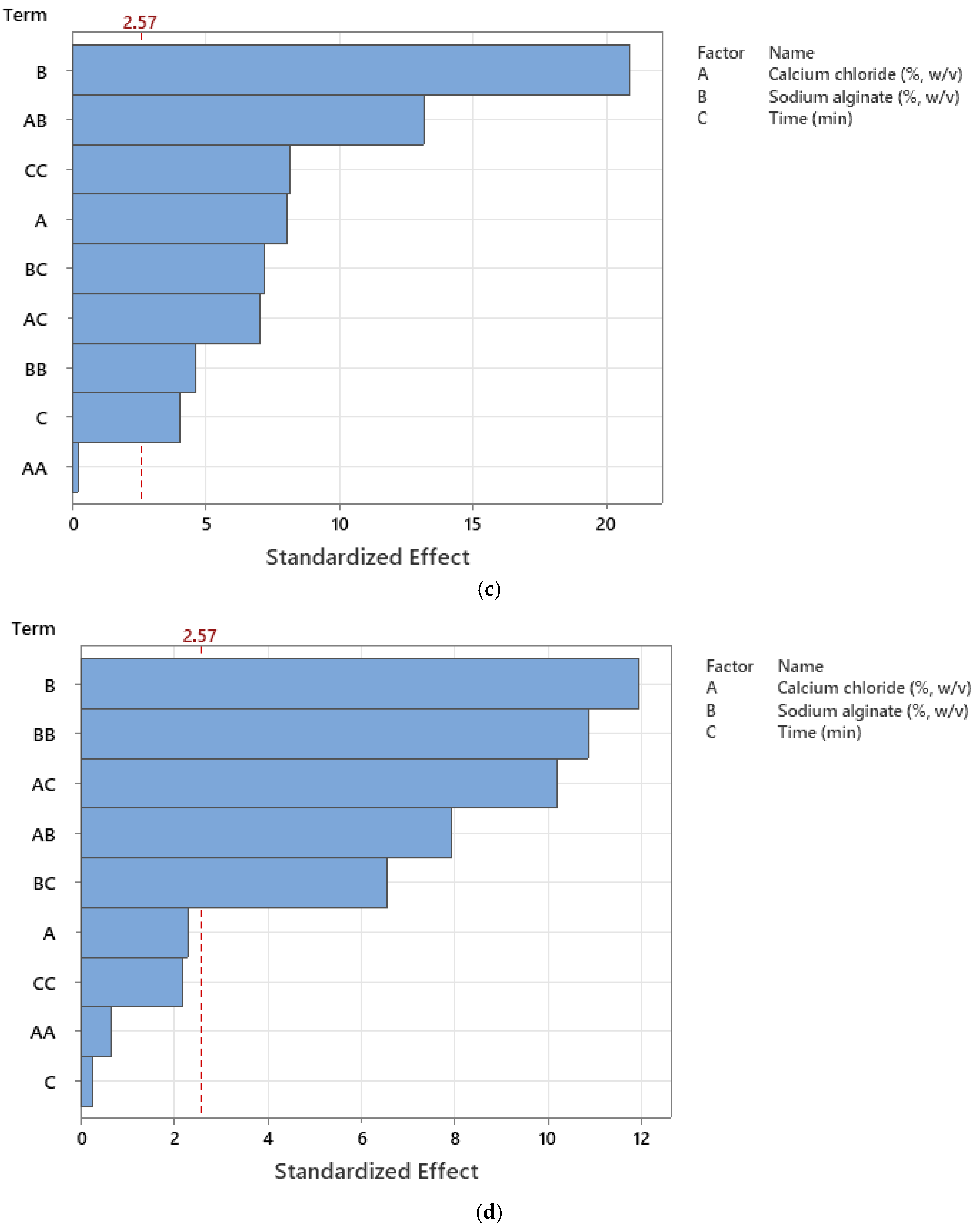
2.1.4. Modeling the Encapsulation Process
2.1.5. Verification of the Estimated Values
2.2. Characterization Analysis of the Microbeads
2.2.1. Shape and Size
2.2.2. Moisture Content, Water Activity, and Bulk Density
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of Phenolic Antioxidants from Rosemary
4.3. Encapsulation of Rosemary Phenolic Antioxidants in Alginate Microbeads
4.4. Physicochemical Measurements
4.5. Measurement of Sphericity and Roundness
4.6. Encapsulation Efficiency
4.7. DPPH Scavenging Activity
4.8. BBD–RSM
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of Bioactives for Food Applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yuan, Y.; Baojun, X. A Critical Review on the Microencapsulation of Bioactive Compounds and Their Application. Food Rev. Int. 2023, 39, 2594–2634. [Google Scholar] [CrossRef]
- Onwulata, C.I. Mıcroencapsulatıon And Functıonal Bıoactıve Foods. J. Food Process. Preserv. 2013, 37, 510–532. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; de la Rosa, L.A.; Wall-Medrano, A. Structural Stability and Viability of Microencapsulated Probiotic Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymer 2022, 14, 1730. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic Food Compounds Encapsulation by Ionic Gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Berling, C.L.; Germer, S.P.M.; Alvim, I.D.; Hubinger, M.D. Encapsulating Anthocyanins from Hibiscus Sabdariffa L. Calyces by Ionic Gelation: Pigment Stability during Storage of Microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef] [PubMed]
- de Moura, S.C.S.R.; Berling, C.L.; Garcia, A.O.; Queiroz, M.B.; Alvim, I.D.; Hubinger, M.D. Release of Anthocyanins from the Hibiscus Extract Encapsulated by Ionic Gelation and Application of Microparticles in Jelly Candy. Food Res. Int. 2019, 121, 542–552. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic Gelation Method in the Synthesis of Nanoparticles/Microparticles for Biomedical Purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of Alginate Alkaline Extraction Technology from Sargassum Latifolium and Its Potential Antioxidant and Emulsifying Properties. Carbohydr. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation Optimization of Lemon Balm Antioxidants in Calcium Alginate Hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef]
- Niizawa, I.; Espinaco, B.Y.; Zorrilla, S.E.; Sihufe, G.A. Natural Astaxanthin Encapsulation: Use of Response Surface Methodology for the Design of Alginate Beads. Int. J. Biol. Macromol. 2019, 121, 601–608. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Jarzębski, M.; Pratap-Singh, A.; Guo, Y.; Abd-Manap, Y. Encapsulation of Betacyanins from the Peel of Red Dragon Fruit (Hylocereus Polyrhizus L.) in Alginate Microbeads. Food Hydrocoll. 2021, 113, 106535. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimized Encapsulation of Anthocyanin-Rich Extract from Haskap Berries (Lonicera Caerulea L.) in Calcium-Alginate Microparticles. J. Berry Res. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; He, Y.-M.; Ma, X.-Y.; Liu, L.-N.; Ke, Y.-J. Optimization of Preparation and Properties of Gardenia Yellow Pigment-Loaded Alginate Beads. Korean J. Chem. Eng 2021, 38, 1669–1675. [Google Scholar] [CrossRef]
- Celli, G.B.; Teixeira, A.G.; Duke, T.G.; Brooks, M.S.L. Encapsulationof Lycopene from Watermelon in Calcium-Alginate Microparticles Using an Optimised Inverse-Gelation Method by Response Surface Methodology. Int. J. Food Sci. Technol. 2016, 51, 1523–1529. [Google Scholar] [CrossRef]
- Wani, K.M.; Uppaluri, R.V.S. Efficacy of Ionic Gelation Based Encapsulation of Bioactives from Papaya Leaf Extract: Characterization and Storage Stability. Biomass Convers. Biorefinery 2023, 14, 19911–19928. [Google Scholar] [CrossRef]
- Essifi, K.; Lakrat, M.; Berraaouan, D.; Fauconnier, M.L.; El Bachiri, A.; Tahani, A. Optimization of Gallic Acid Encapsulation in Calcium Alginate Microbeads Using Box-Behnken Experimental Design. Polym. Bull. 2021, 78, 5789–5814. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Katakam, P.; Khan, A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Mar. Drugs 2021, 19, 467. [Google Scholar] [CrossRef]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Lucini, L.; Rocchetti, G.; Dalmina, M.; Pearson, J.P.; de Mello Castanho Amboni, R.D. Encapsulation of Stevia Rebaudiana Bertoni Aqueous Crude Extracts by Ionic Gelation—Effects of Alginate Blends and Gelling Solutions on the Polyphenolic Profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef]
- Baudequin, T.; Wee, H.; Cui, Z.; Ye, H. Towards Ready-to-Use Iron-Crosslinked Alginate Beads as Mesenchymal Stem Cell Carriers. Bioengineering 2023, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.k.; Pande, V. Development and Evaluation of Dual Cross-Linked Pulsatile Beads for Chronotherapy of Rheumatoid Arthritis. J. Pharm. 2013, 2013, 906178. [Google Scholar] [CrossRef][Green Version]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus Officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrition 2016, 8, 731. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus Officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrition 2017, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.d.P.; Herrero, M. Rosemary (Rosmarinus Officinalis) as a Functional Ingredient: Recent Scientific Evidence. Curr. Opin. Food Sci. 2017, 14, 13–19. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Reglero, G.; Ramírez De Molina, A. Rosemary (Rosmarinus Officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Visentin, A.; Rodríguez-Rojo, S.; Navarrete, A.; Maestri, D.; Cocero, M.J. Precipitation and Encapsulation of Rosemary Antioxidants by Supercritical Antisolvent Process. J. Food Eng. 2012, 109, 9–15. [Google Scholar] [CrossRef]
- Rashidaie Abandansarie, S.S.; Ariaii, P.; Charmchian Langerodi, M. Effects of Encapsulated Rosemary Extract on Oxidative and Microbiological Stability of Beef Meat during Refrigerated Storage. Food Sci. Nutr. 2019, 7, 3969–3978. [Google Scholar] [CrossRef]
- Kanakidi, L.-D.; Tsimogiannis, D.; Kiokias, S.; Oreopoulou, V. Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification. Nutraceuticals 2022, 2, 1–21. [Google Scholar] [CrossRef]
- Nutrizio, M.; Jurić, S.; Kucljak, D.; Švaljek, S.L.; Vlahoviček-Kahlina, K.; Režek Jambrak, A.; Vinceković, M. Encapsulation of Rosemary Extracts Using High Voltage Electrical Discharge in Calcium Alginate/Zein/Hydroxypropyl Methylcellulose Microparticles. Foods 2023, 12, 1570. [Google Scholar] [CrossRef]
- Tomé, A.C.; da Silva, F.A. Alginate Based Encapsulation as a Tool for the Protection of Bioactive Compounds from Aromatic Herbs. Food Hydrocoll. Health 2022, 2, 100051. [Google Scholar] [CrossRef]
- Popescu, L.; Cojocari, D.; Ghendov-Mosanu, A.; Lung, I.; Soran, M.L.; Opriş, O.; Kacso, I.; Ciorîţă, A.; Balan, G.; Pintea, A.; et al. The Effect of Aromatic Plant Extracts Encapsulated in Alginate on the Bioactivity, Textural Characteristics and Shelf Life of Yogurt. Antioxidants 2023, 12, 893. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhattacharjee, P. Encapsulation of Colour from Peels of Eggplant in Calcium Alginate Matrix. Nutrafoods 2015, 14, 87–96. [Google Scholar] [CrossRef]
- Alkhatib, H.; Doolaanea, A.A.; Assadpour, E.; Mohmad Sabere, A.S.; Mohamed, F.; Jafari, S.M. Optimizing the Encapsulation of Black Seed Oil into Alginate Beads by Ionic Gelation. J. Food Eng. 2022, 328, 111065. [Google Scholar] [CrossRef]
- Kaltsa, O.; Alibade, A.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Encapsulation of Moringa Oleifera Extract in Ca-Alginate Chocolate Beads: Physical and Antioxidant Properties. J. Food Qual. 2021, 2021, 5549873. [Google Scholar] [CrossRef]
- Gholamian, S.; Nourani, M.; Bakhshi, N. Formation and Characterization of Calcium Alginate Hydrogel Beads Filled with Cumin Seeds Essential Oil. Food Chem. 2021, 338, 128143. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.; Kim, S.; Lee, C.; Cho, S.; Kim, S.B. Changes in the Physical Properties of Calcium Alginate Gel Beads under a Wide Range of Gelation Temperature Conditions. Foods 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.N.; Kalal, A.Y.; Suryavanshi, S.K.; Patil, P.G.; Sharma, D.; Sharma, J. Process Optimization by Response Surface Methodology for Microencapsulation of Pomegranate Seed Oil. J. Food Process. Preserv. 2021, 45, e15561. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Aguirre-Calvo, D.; Perullini, M.; Santagapita, P.R. A Detailed Microstructural and Multiple Responses Analysis through Blocking Design to Produce Ca(II)-Alginate Beads Loaded with Bioactive Compounds Extracted from by-Products. Food Hydrocoll. Health 2021, 1, 100030. [Google Scholar] [CrossRef]
- Zazzali, I.; Jaramillo, G.; Gabilondo, J.; Peixoto Mallmann, L.; Rodrigues, E.; Perullini, M.; Santagapita, P.R. Fine-Tuning of Functional and Structural Properties of Ca(II)-Alginate Beads Containing Artichoke Waste Extracts. Food Hydrocoll. Health 2022, 2, 100097. [Google Scholar] [CrossRef]
- Lee, B.B.; Ravindra, P.; Chan, E.S. Size and Shape of Calcium Alginate Beads Produced by Extrusion Dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of Alginate Microspheres Containing Thyme Essential Oil Using Ionic Gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.L.; Marian, E. Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, A.; Sharma, S.; Khuller, G.K. Inhalable Alginate Nanoparticles as Antitubercular Drug Carriers against Experimental Tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC—Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
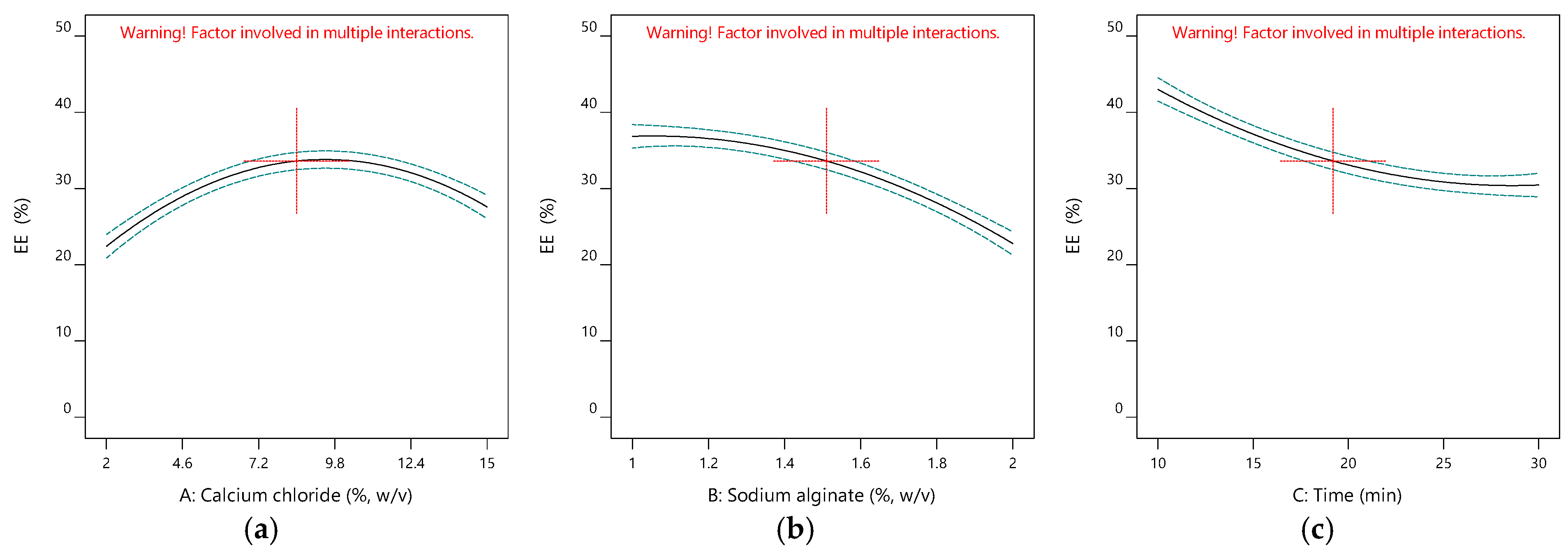

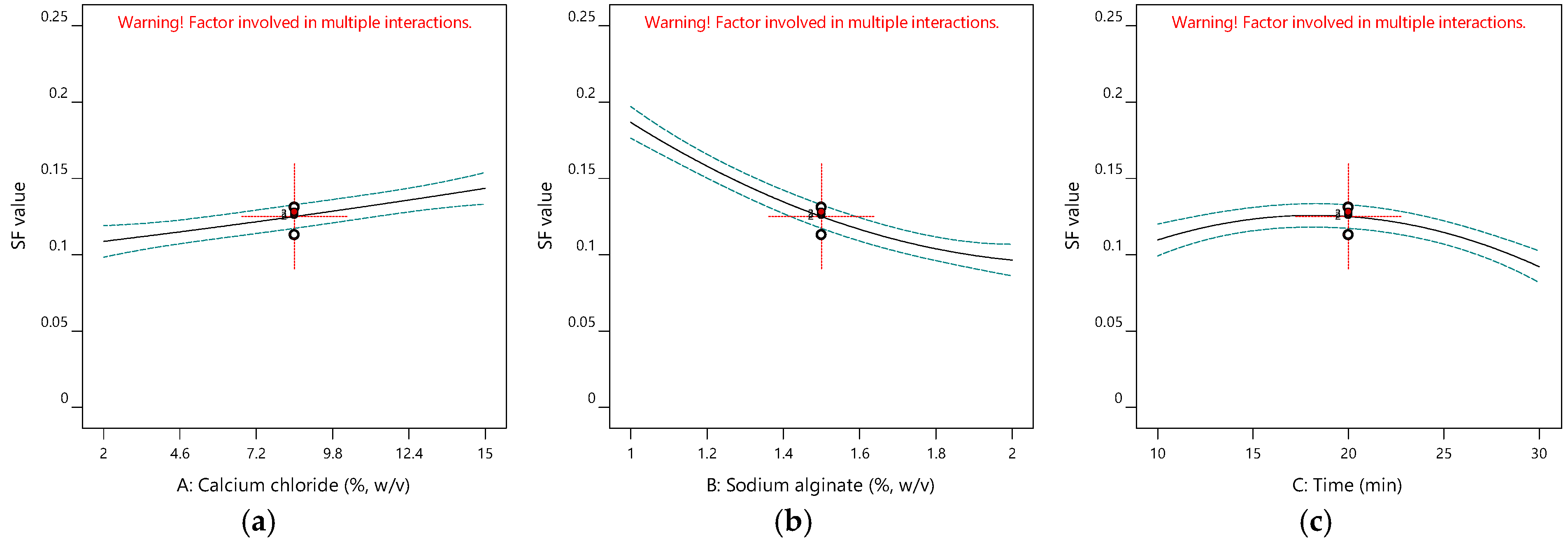
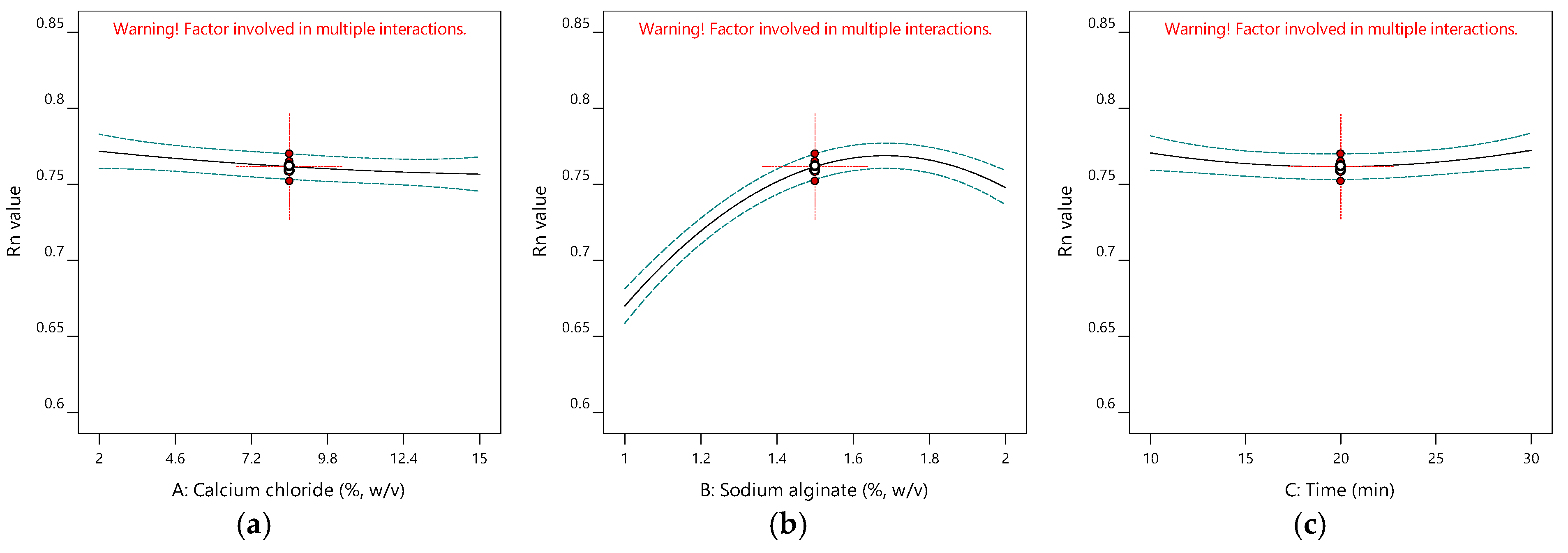

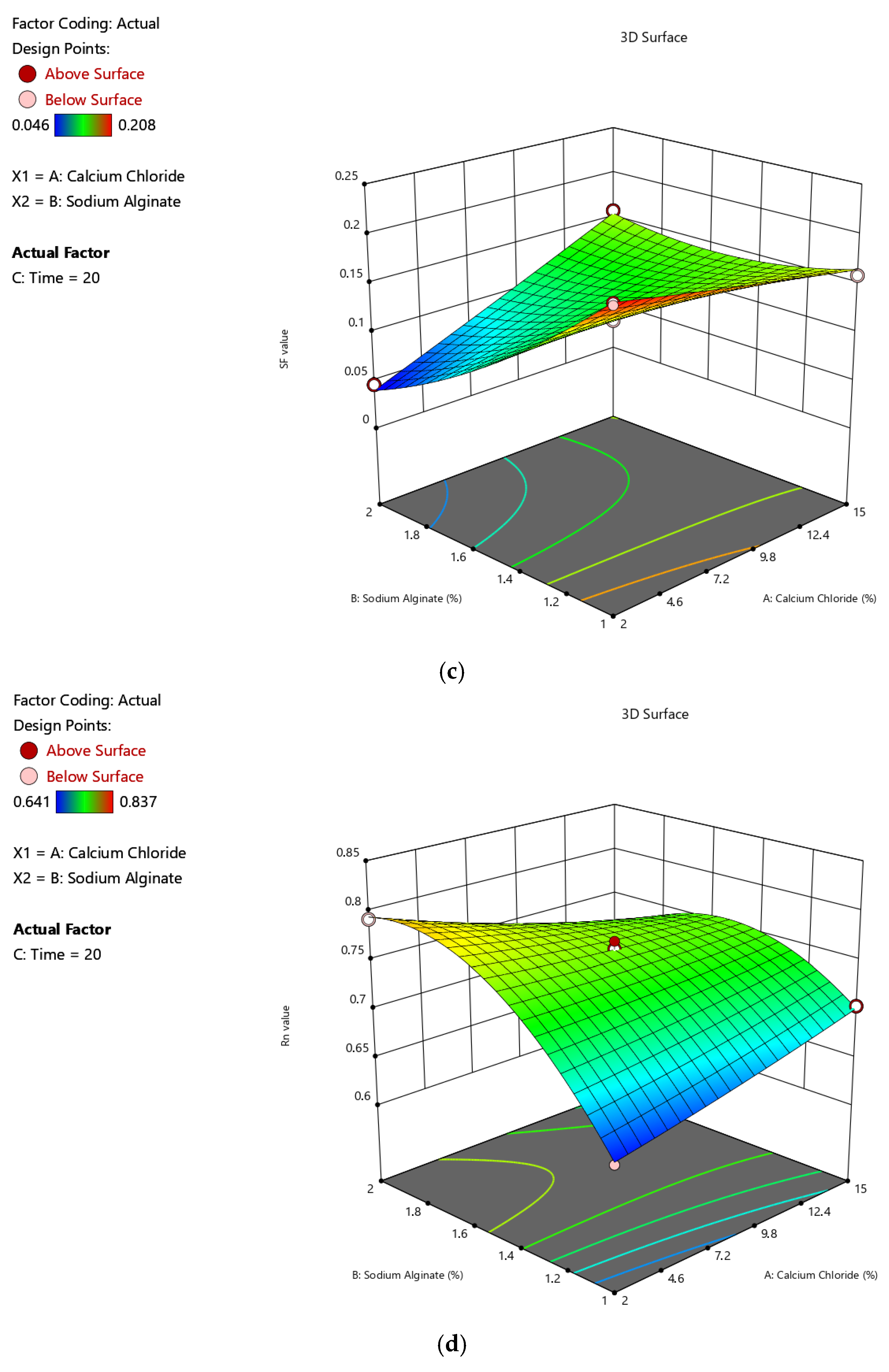


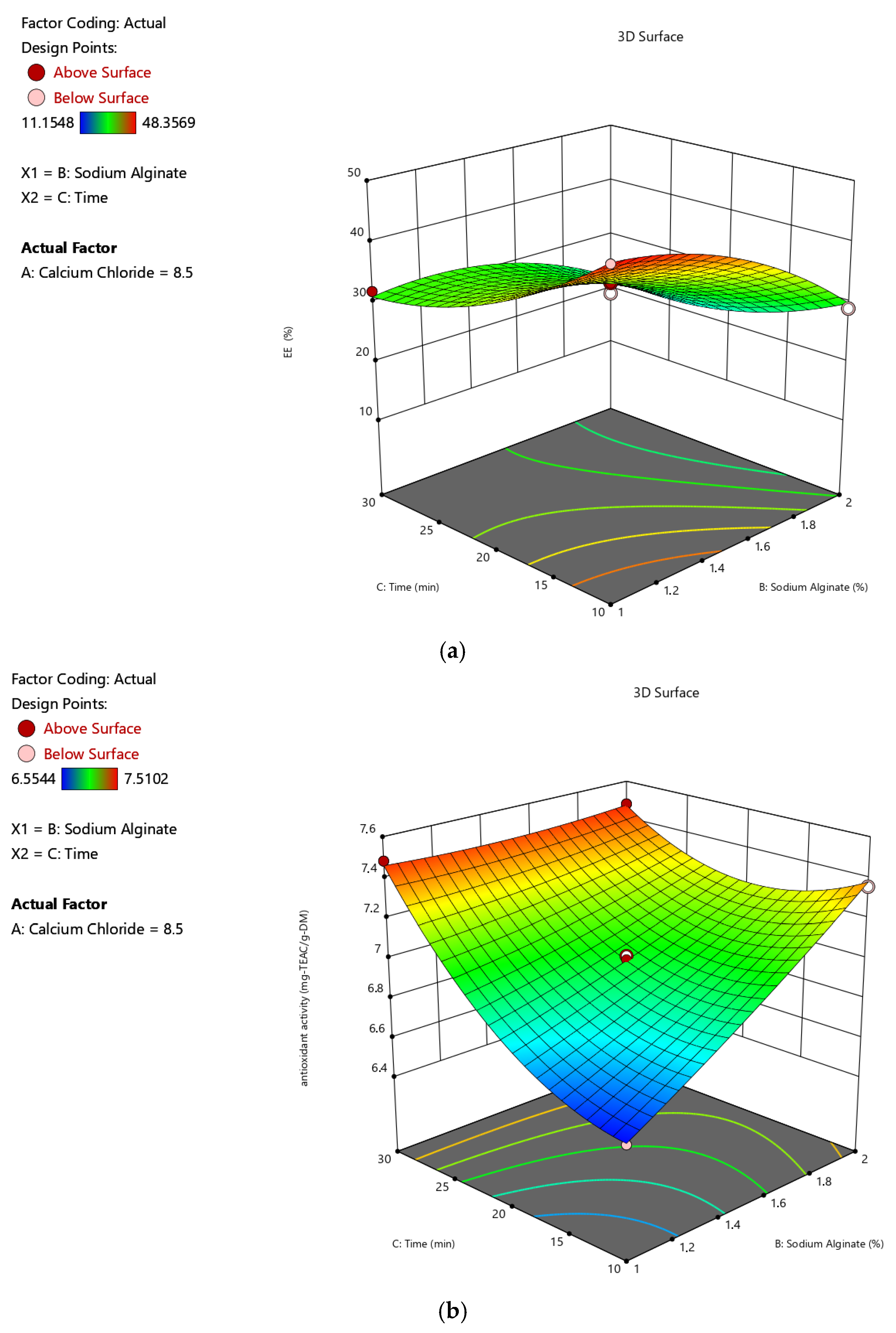
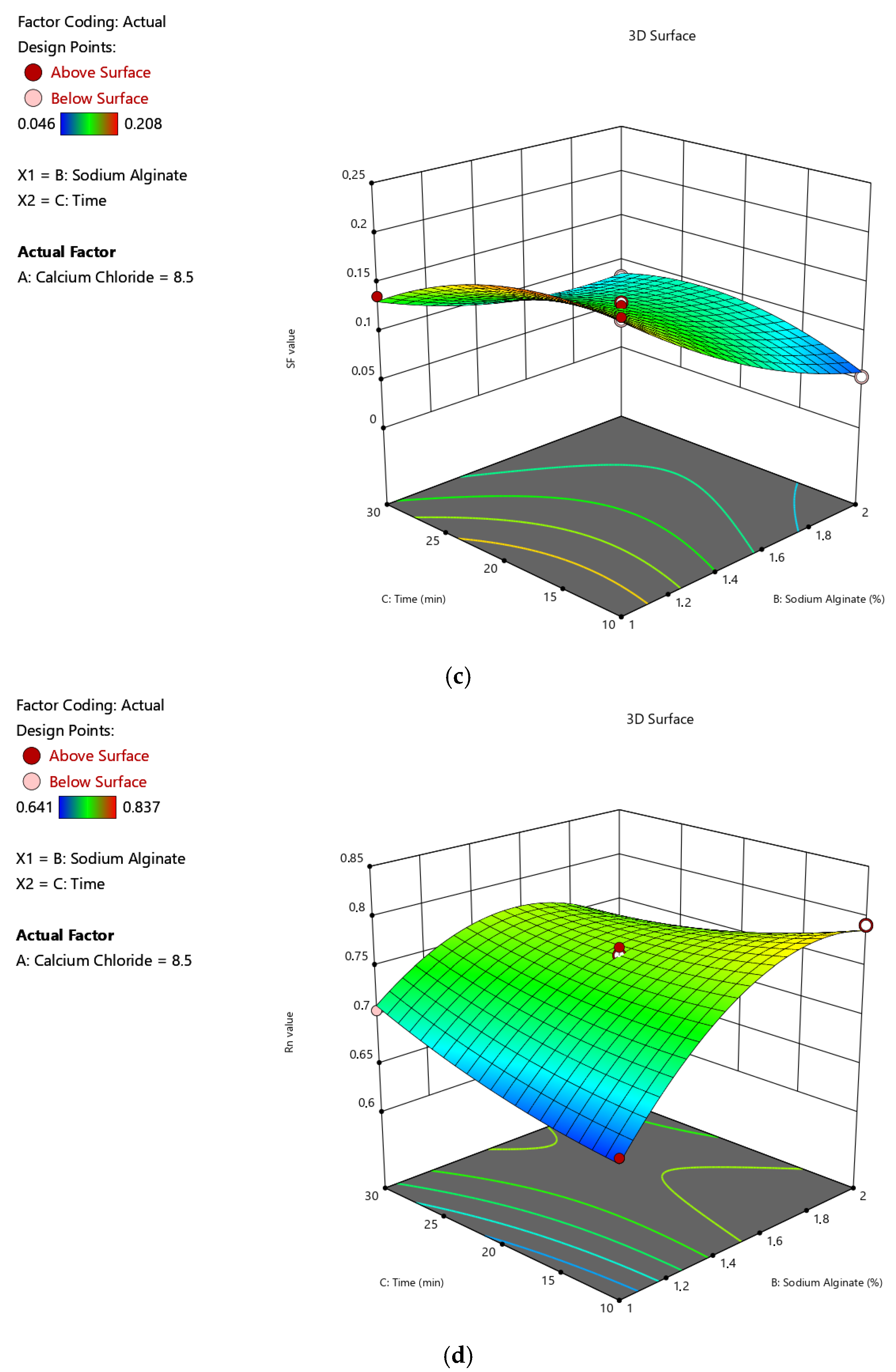



| Run | A (%, w/v) | B (%, w/v) | C (min) | Y1 (%) | Y2 (mg-TEAC/g-DM) | Y3 | Y4 |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1.5 | 30 | 22.388 | 7.316 ± 0.003 | 0.052 ± 0.02 | 0.837 ± 0.01 |
| 2 | 8.5 | 1.5 | 20 | 33.344 | 7.018 ± 0.000 | 0.113 ± 0.02 | 0.762 ± 0.01 |
| 3 | 8.5 | 2 | 10 | 29.085 | 7.359 ± 0.002 | 0.054 ± 0.02 | 0.792 ± 0.01 |
| 4 | 8.5 | 1.5 | 20 | 31.616 | 6.983 ± 0.001 | 0.131 ± 0.04 | 0.759 ± 0.03 |
| 5 | 8.5 | 1.5 | 20 | 34.183 | 7.000 ± 0.001 | 0.127 ± 0.04 | 0.770 ± 0.02 |
| 6 | 2 | 1.5 | 10 | 30.172 | 7.212 ± 0.000 | 0.114 ± 0.03 | 0.731± 0.02 |
| 7 | 8.5 | 2 | 30 | 22.368 | 7.479 ± 0.006 | 0.082 ± 0.01 | 0.723 ± 0.02 |
| 8 | 2 | 2 | 20 | 11.155 | 7.510 ± 0.000 | 0.046 ± 0.02 | 0.792 ± 0.02 |
| 9 | 15 | 2 | 20 | 17.255 | 6.926 ± 0.000 | 0.158 ± 0.03 | 0.709 ± 0.02 |
| 10 | 8.5 | 1 | 10 | 48.357 | 6.554 ± 0.002 | 0.197 ± 0.04 | 0.654 ± 0.03 |
| 11 | 15 | 1.5 | 30 | 20.438 | 7.502 ± 0.001 | 0.133 ± 0.02 | 0.723 ± 0.02 |
| 12 | 8.5 | 1.5 | 20 | 33.288 | 6.989 ± 0.001 | 0.128 ± 0.02 | 0.767 ± 0.02 |
| 13 | 15 | 1 | 20 | 29.536 | 6.979 ± 0.001 | 0.159 ± 0.03 | 0.704 ± 0.05 |
| 14 | 8.5 | 1.5 | 20 | 33.730 | 6.983 ± 0.001 | 0.126 ± 0.03 | 0.765 ± 0.01 |
| 15 | 8.5 | 1 | 30 | 31.929 | 7.483 ± 0.004 | 0.137 ± 0.05 | 0.706 ± 0.03 |
| 16 | 2 | 1 | 20 | 24.775 | 6.593 ± 0.000 | 0.208 ± 0.06 | 0.641 ± 0.05 |
| 17 | 15 | 1.5 | 10 | 40.035 | 6.672 ± 0.003 | 0.109 ± 0.03 | 0.805 ± 0.03 |
| Y1 | Source | SS * | DF ** | MS *** | F-Value | p-Value | |
| Model | 1227.10 | 9 | 136.34 | 116.65 | <0.0001 | Significant **** | |
| A | 44.06 | 1 | 44.06 | 37.70 | 0.0005 | ||
| B | 374.46 | 1 | 374.46 | 320.36 | <0.0001 | ||
| C | 319.12 | 1 | 319.12 | 273.02 | <0.0001 | ||
| AB | 0.4483 | 1 | 0.4483 | 0.3836 | 0.5553 | ||
| AC | 34.88 | 1 | 34.88 | 29.84 | 0.0009 | ||
| BC | 23.58 | 1 | 23.58 | 20.17 | 0.0028 | ||
| A2 | 312.44 | 1 | 312.44 | 267.30 | <0.0001 | ||
| B2 | 65.29 | 1 | 65.29 | 55.86 | 0.0001 | ||
| C2 | 55.80 | 1 | 55.80 | 47.74 | 0.0002 | ||
| Residual | 8.18 | 7 | 1.17 | ||||
| Lack of Fit | 4.40 | 3 | 1.47 | 1.55 | 0.3317 | not significant | |
| Pure Error | 3.78 | 4 | 0.9446 | ||||
| Cor Total | 1235.28 | 16 | |||||
| C.V.: 3.72% R2 = 0.9934 Adjusted R2 = 0.9849 Predicted R2 = 0.9382 | |||||||
| Source | SS | DF | MS | F-Value | p-Value | ||
| Y2 | Model | 1.58 | 9 | 0.1753 | 203.27 | <0.0001 | significant |
| A | 0.0382 | 1 | 0.0382 | 44.30 | 0.0003 | ||
| B | 0.3461 | 1 | 0.3461 | 401.37 | <0.0001 | ||
| C | 0.4915 | 1 | 0.4915 | 570.02 | <0.0001 | ||
| AB | 0.2351 | 1 | 0.2351 | 272.59 | <0.0001 | ||
| AC | 0.1316 | 1 | 0.1316 | 152.64 | <0.0001 | ||
| BC | 0.1635 | 1 | 0.1635 | 189.57 | <0.0001 | ||
| A2 | 0.0014 | 1 | 0.0014 | 1.58 | 0.2490 | ||
| B2 | 0.0027 | 1 | 0.0027 | 3.12 | 0.1204 | ||
| C2 | 0.1660 | 1 | 0.1660 | 192.56 | <0.0001 | ||
| Residual | 0.0060 | 7 | 0.0009 | ||||
| Lack of Fit | 0.0052 | 3 | 0.0017 | 7.82 | 0.0378 | significant | |
| Pure Error | 0.0009 | 4 | 0.0002 | ||||
| Cor Total | 1.58 | 16 | |||||
| C.V.: 0.4141% R2 = 0.9962 Adjusted R2 = 0.9913 Predicted R2 = 0.9470 | |||||||
| Source | SS | DF | MS | F-Value | p-Value | ||
| Y3 | Model | 0.0330 | 9 | 0.0037 | 68.92 | <0.0001 | significant |
| A | 0.0024 | 1 | 0.0024 | 45.35 | 0.0003 | ||
| B | 0.0163 | 1 | 0.0163 | 305.92 | <0.0001 | ||
| C | 0.0006 | 1 | 0.0006 | 11.50 | 0.0116 | ||
| AB | 0.0065 | 1 | 0.0065 | 121.69 | <0.0001 | ||
| AC | 0.0018 | 1 | 0.0018 | 34.72 | 0.0006 | ||
| BC | 0.0019 | 1 | 0.0019 | 36.36 | 0.0005 | ||
| A2 | 5.329 × 10−6 | 1 | 5.329 × 10−6 | 0.1001 | 0.7610 | ||
| B2 | 0.0012 | 1 | 0.0012 | 21.85 | 0.0023 | ||
| C2 | 0.0025 | 1 | 0.0025 | 46.02 | 0.0003 | ||
| Residual | 0.0004 | 7 | 0.0001 | ||||
| Lack of Fit | 0.0002 | 3 | 0.0001 | 1.23 | 0.4087 | not significant | |
| Pure Error | 0.0002 | 4 | 0.0000 | ||||
| Cor Total | 0.0334 | 16 | |||||
| C.V.: 5.98% R2 = 0.9888 Adjusted R2 = 0.9745 Predicted R2 = 0.9053 | |||||||
| Source | SS | Df | MS | F-Value | p-Value | ||
| Y4 | Model | 0.0423 | 9 | 0.0047 | 74.46 | <0.0001 | significant |
| A | 0.0004 | 1 | 0.0004 | 7.14 | 0.0319 | ||
| B | 0.0121 | 1 | 0.0121 | 191.71 | <0.0001 | ||
| C | 6.125 × 10−6 | 1 | 6.125 × 10−6 | 0.0971 | 0.7644 | ||
| AB | 0.0053 | 1 | 0.0053 | 84.50 | <0.0001 | ||
| AC | 0.0088 | 1 | 0.0088 | 140.11 | <0.0001 | ||
| BC | 0.0037 | 1 | 0.0037 | 58.04 | 0.0001 | ||
| A2 | 0.0000 | 1 | 0.0000 | 0.4427 | 0.5271 | ||
| B2 | 0.0117 | 1 | 0.0117 | 185.25 | <0.0001 | ||
| C2 | 0.0004 | 1 | 0.0004 | 6.44 | 0.0387 | ||
| Residual | 0.0004 | 7 | 0.0001 | ||||
| Lack of Fit | 0.0003 | 3 | 0.0001 | 1.92 | 0.2687 | not significant | |
| Pure Error | 0.0002 | 4 | 0.0000 | ||||
| Cor Total | 0.0427 | 16 | |||||
| C.V.: 1.07% R2 = 0.9897 Adjusted R2 = 0.9764 Predicted R2 = 0.8959 | |||||||
| Criteria | ||||||
|---|---|---|---|---|---|---|
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
| A | is in range | 2 | 15 | 1 | 1 | 3 |
| B | is in range | 1 | 2 | 1 | 1 | 3 |
| C | is in range | 10 | 30 | 1 | 1 | 3 |
| EE | Maximize | 11.155 | 48.357 | 1 | 1 | 3 |
| Antioxidant activity | Maximize | 6.554 | 7.510 | 1 | 1 | 3 |
| SF | Minimize | 0.046 | 0.208 | 1 | 1 | 3 |
| Rn | Maximize | 0.641 | 0.837 | 1 | 1 | 3 |
| Solutions | ||||||
| A (%, w/v) | B (%, w/v) | C (min) | Response | Predicted | Experimental | Error (%) |
| 6 | 2 | 10 | EE | 29.48 | 30.01 | <2 |
| (%) | ||||||
| Antioxidant activity (mg-TEAC/g-DM) | 7.47 | 7.55 | ||||
| SF | 0.05 | 0.05 | ||||
| Rn | 0.79 | 0.78 | ||||
| Process | Processing Time (min) |
|---|---|
| Immersion | 30 |
| Washing | 45 |
| Recovery | 20 |
| Cooling | 5 |
| Independent Variable | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: Calcium chloride (%, w/v) | 2 | 8.5 | 15 |
| B: Sodium alginate (%, w/v) | 1 | 1.5 | 2 |
| C: Time (min) | 10 | 20 | 30 |
| Dependent variable * | |||
| Y1: EE (%) | |||
| Y2: Antioxidant activity (mg-TEAC/g-DM) | |||
| Y3: SF | |||
| Y4: Rn | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toprakçı, G.; Toprakçı, İ.; Şahin, S. Alginate Microbeads for Trapping Phenolic Antioxidants in Rosemary (Rosmarinus officinalis L.): Multivariate Optimization Based on Bioactive Properties and Morphological Measurements. Gels 2025, 11, 172. https://doi.org/10.3390/gels11030172
Toprakçı G, Toprakçı İ, Şahin S. Alginate Microbeads for Trapping Phenolic Antioxidants in Rosemary (Rosmarinus officinalis L.): Multivariate Optimization Based on Bioactive Properties and Morphological Measurements. Gels. 2025; 11(3):172. https://doi.org/10.3390/gels11030172
Chicago/Turabian StyleToprakçı, Gizem, İrem Toprakçı, and Selin Şahin. 2025. "Alginate Microbeads for Trapping Phenolic Antioxidants in Rosemary (Rosmarinus officinalis L.): Multivariate Optimization Based on Bioactive Properties and Morphological Measurements" Gels 11, no. 3: 172. https://doi.org/10.3390/gels11030172
APA StyleToprakçı, G., Toprakçı, İ., & Şahin, S. (2025). Alginate Microbeads for Trapping Phenolic Antioxidants in Rosemary (Rosmarinus officinalis L.): Multivariate Optimization Based on Bioactive Properties and Morphological Measurements. Gels, 11(3), 172. https://doi.org/10.3390/gels11030172







