New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research
Abstract
1. Introduction
2. Pathophysiology of RA
2.1. Genetic Factors
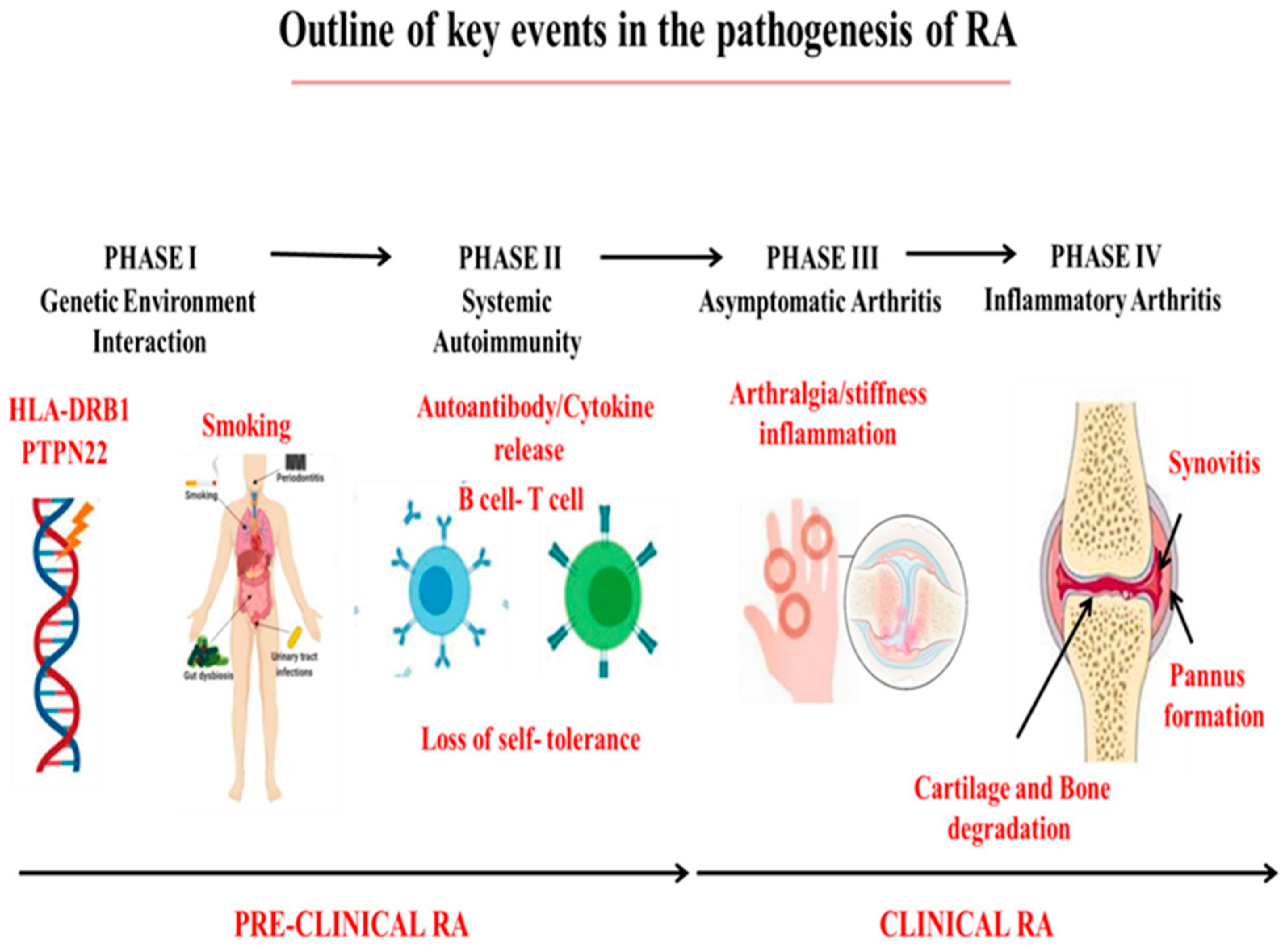
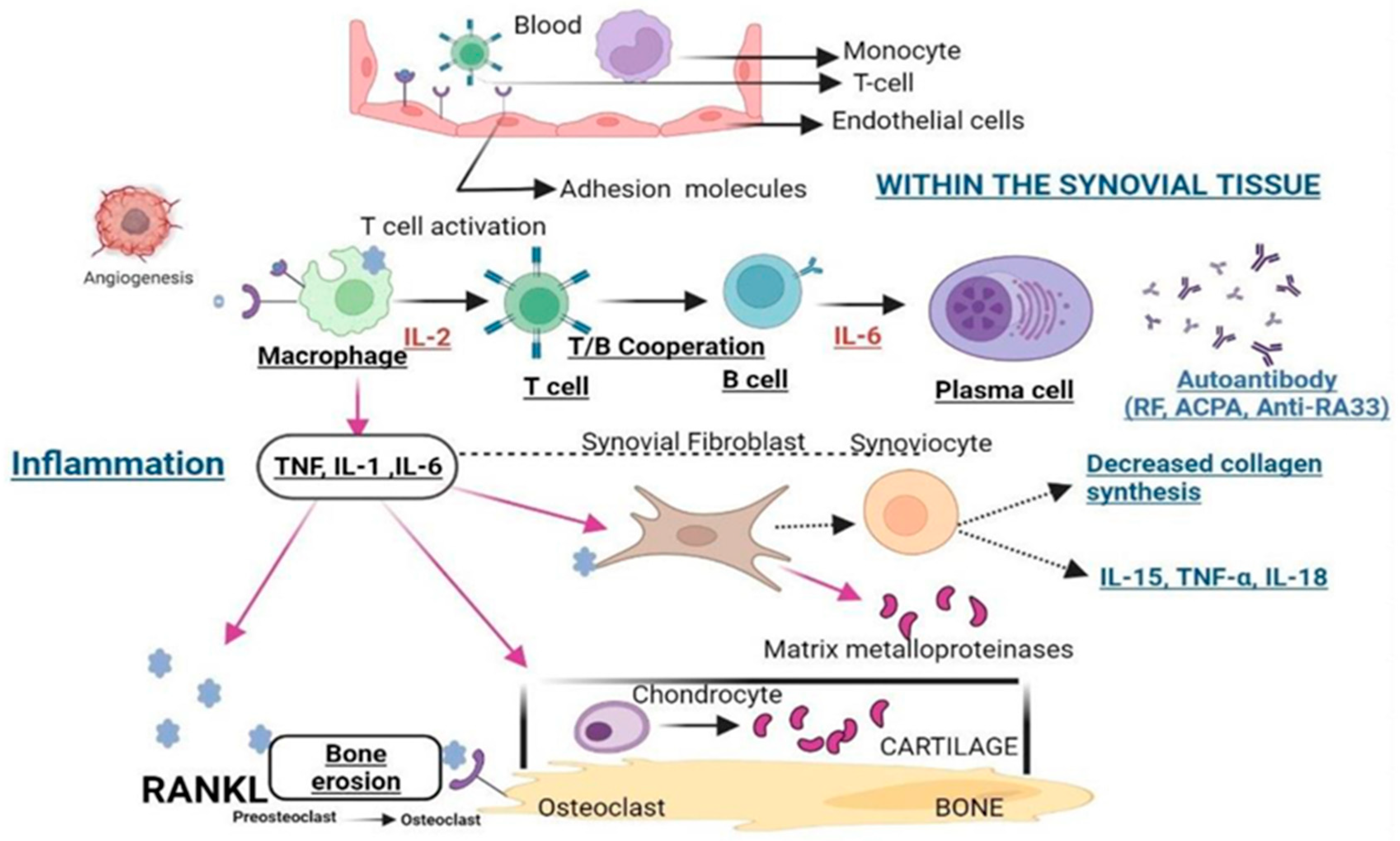
2.2. Abnormal Activation of Immune Cells
2.3. Environmental Factors
3. Overview of Methodologies for RA Treatment
3.1. Physical Therapy
3.2. Drug Therapy
3.3. Surgical Treatment
4. Advantages of Hydrogels in the Treatment of RA
4.1. Biocompatibility and Antibacterial Properties
4.2. Adjustable Physical Properties
4.3. Lubricating Properties
4.4. Structural Superiority
5. Functional Hydrogels for the Treatment of RA
5.1. Tissue Engineering Scaffold Hydrogels
5.2. Lubricating Hydrogels
5.3. Immunomodulatory Hydrogels
5.4. Drug Delivery Hydrogels
6. Hydrogels as Drug Carriers for the Treatment of RA via Different Administration Routes
6.1. Oral Administration
6.2. Parenteral Administration
6.3. Transdermal Administration
6.4. Intra-Articular Administration
6.5. Application Prospects and Challenges
6.5.1. Scalability Challenge
6.5.2. Manufacturing Cost Challenge
6.5.3. Long Term Internal Stability Challenge
6.5.4. Potential Immunogenicity Challenge
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | rheumatoid arthritis |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| DMARDs | disease-modifying antirheumatic drugs |
| SNP | single nucleotide polymorphisms |
| HLP | Human leukocyte antigen |
| CTL | cytotoxic T lymphocytes |
| APC | Antigen-presenting cells |
| RF | rheumatoid factor |
| ACPA | anti-citrullinated protein antibodies |
| TNF-α | tumor necrosis factor-α |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| MMPs | matrix metalloproteinases |
| MTX | methotrexate |
| PVA | polyvinyl alcohol |
| PEG | polyethylene glycol |
| H2O2 | hydrogen peroxide |
| PVA/PEG/GO | polyvinyl alcohol/polyethylene glycol/graphene oxide |
| ROS | reactive oxygen species |
| CM | metformin-loaded hollow copper sulfide |
| NPs | nanoparticles |
| siBiMPNH | pH-responsive injectable peptide hydrogel |
| IL-1β | interleukin-1β |
| MPDANP | mesoporous polydopamine nanoparticles |
| SPT@TPL | double-dynamically crosslinked sodium alginate hydrogel |
| TPL | antirheumatic drug triptolide |
| MAA | methacrylic acid |
| EG | ethylene glycol |
| NVP | N-vinylpyrrolidone |
| NiH | nanomedicine-in-hydrogel composite |
| cNPs | cationic nanoparticles |
| cfDNA | cell-free DNA |
| cGAS | cyclic guanosine monophosphate-adenosine monophosphate synthase |
| CIA | collagen-induced arthritis |
| mPEG–P(LP-co-LC) | methoxy poly(ethylene glycol)–poly(L-phenylalanine-co-L-cystine) |
| MSN | silica nanoparticle matrix |
| DES | deep eutectic solvents |
| Leon | Leonurine |
| FA-PDA | folate-functionalized polydopamine |
| OCF/siHMGB1 | oxidized chondroitin sulfate-chitosan-sodium glycerol β-phosphate-fibronectin/small interfering high mobility group protein |
References
- Kessler, J.; Totoson, P.; Devaux, S.; Moretto, J.; Wendling, D.; Demougeot, C. Animal models to study pathogenesis and treatments of cardiac disorders in rheumatoid arthritis: Advances and challenges for clinical translation. Pharmacol. Res. 2021, 170, 105494. [Google Scholar] [CrossRef]
- Agnihotri, P.; Malik, S.; Saquib, M.; Chakraborty, D.; Kumar, V.; Biswas, S. Exploring the impact of 2-hydroxyestradiol on heme oxygenase-1 to combat oxidative stress in rheumatoid arthritis. Int. J. Biol. Macromol. 2024, 283, 137935. [Google Scholar] [CrossRef]
- Han, J.; Wu, B.; Wang, D. The potential efficacy of sesquiterpenes and their derivatives in treating rheumatoid arthritis: A systematic review. Int. Immunopharmacol. 2024, 141, 112946. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Y.; Xu, X.; Ye, Q. Targeted delivery of methotrexate by modified yeast β-glucan nanoparticles for rheumatoid arthritis therapy. Carbohydr. Polym. 2022, 284, 119183. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lv, G.; Wu, Y.; Guo, W.; Wang, Y.; Hu, J.; Li, N.; Zheng, F.; Dai, Y.; Pi, Z.; et al. Licorice-regulated gut–joint axis for alleviating collagen-induced rheumatoid arthritis. Phytomedicine 2024, 135, 156203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, Q.; Yao, G.; Wang, Z.; Zhu, L.; Zeng, Z.; Jia, L.; Du, Y.; Xue, J.; Gao, C. A cationic hydrogel with anti-IL-17A-specific nanobodies for rheumatoid arthritis treatment via inhibition of inflammatory activities of neutrophils. Nano Today 2024, 59, 102507. [Google Scholar] [CrossRef]
- Priya, S.; Daryani, J.; Desai, V.M.; Singhvi, G. Bridging the gap in rheumatoid arthritis treatment with hyaluronic acid-based drug delivery approaches. Int. J. Biol. Macromol. 2024, 271, 132586. [Google Scholar] [CrossRef]
- Karnam, S.; Jindal, A.B.; Paul, A.T. Quality by design-based optimization of teriflunomide and quercetin combinational topical transferosomes for the treatment of rheumatoid arthritis. Int. J. Pharm. 2024, 666, 124829. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zheng, H.; Li, B.; Huang, F.; Qiu, Y.; Yang, Y.; Sheng, W.; Peng, C.; Tian, X.; Wang, W.; et al. Nanomedicines targeting activated immune cells and effector cells for rheumatoid arthritis treatment. J. Control. Release 2024, 371, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Aryaeian, N.; Akhlaghi, M.; Abolghasemi, J.; Fallah, S.; Heydarian, A.; Rostami, R.; Bayat, M.H.; Mahmoudi, M. The effect of purslane supplementation on clinical outcomes, inflammatory and antioxidant markers in patients with rheumatoid arthritis: A parallel double-blinded randomized controlled clinical trial. Phytomedicine 2024, 135, 156006. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Shen, C.-P.; Wang, T.-T.; Huang, Y.; Jin, Y.; Zhou, M.-Y.; Zhang, M.-Y.; Gu, S.-L.; Wang, M.-Q.; Liu, Z.-C.; et al. Shikonin suppresses rheumatoid arthritis by inducing apoptosis and autophagy via modulation of the AMPK/mTOR/ULK-1 signaling pathway. Phytomedicine 2024, 128, 155512. [Google Scholar] [CrossRef] [PubMed]
- Jahanbekam, S.; Mozafari, N.; Bagheri-Alamooti, A.; Mohammadi-Samani, S.; Daneshamouz, S.; Heidari, R.; Azarpira, N.; Ashrafi, H.; Azadi, A. Ultrasound-responsive hyaluronic acid hydrogel of hydrocortisone to treat osteoarthritis. Int. J. Biol. Macromol. 2023, 240, 124449. [Google Scholar] [CrossRef]
- He, Y.; Chen, H.; Li, M.; Tang, Z.; Yu, H.; Huang, C.; Zhang, X.; Ling, X.; Xie, X.; Wei, G.; et al. Analysis of TLR10 gene polymorphisms in patients with rheumatoid arthritis. Int. Immunopharmacol. 2024, 138, 112565. [Google Scholar] [CrossRef] [PubMed]
- Pathade, V.; Nene, S.; Ratnam, S.; Khatri, D.K.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Emerging insights of peptide-based nanotherapeutics for effective management of rheumatoid arthritis. Life Sci. 2023, 312, 121257. [Google Scholar] [CrossRef]
- Srivastava, S.; Rasool, M. Genetics, epigenetics and autoimmunity constitute a Bermuda triangle for the pathogenesis of rheumatoid arthritis. Life Sci. 2024, 357, 123075. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dan, A.; Kumawat, M.K.; Pawar, V.; Chauhan, D.S.; Kaushik, A.; Bhatia, D.; Srivastava, R.; Dhanka, M. Pathophysiology to advanced intra-articular drug delivery strategies: Unravelling rheumatoid arthritis. Biomaterials 2023, 303, 122390. [Google Scholar] [CrossRef]
- Ting, Y.T.; Petersen, J.; Ramarathinam, S.H.; Scally, S.W.; Loh, K.L.; Thomas, R.; Suri, A.; Baker, D.G.; Purcell, A.W.; Reid, H.H.; et al. The interplay between citrullination and HLA-DRB1 polymorphism in shaping peptide binding hierarchies in rheumatoid arthritis. J. Biol. Chem. 2018, 293, 3236–3251. [Google Scholar] [CrossRef] [PubMed]
- Nagafuchi, Y.; Ota, M.; Hatano, H.; Inoue, M.; Kobayashi, S.; Okubo, M.; Sugimori, Y.; Nakano, M.; Yamada, S.; Yoshida, R.; et al. Control of naive and effector CD4 T cell receptor repertoires by rheumatoid-arthritis-risk HLA alleles. J. Autoimmun. 2022, 133, 102907. [Google Scholar] [CrossRef]
- Chang, H.-H.; Ho, C.-H.; Tomita, B.; Silva, A.A.; Sparks, J.A.; Karlson, E.W.; Rao, D.A.; Lee, Y.C.; Ho, I.C. Utilizing a PTPN22 gene signature to predict response to targeted therapies in rheumatoid arthritis. J. Autoimmun. 2019, 101, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Sardana, Y.; Bhatti, G.K.; Singh, C.; Sharma, P.K.; Reddy, P.H.; Bhatti, J.S. Progression of pre-rheumatoid arthritis to clinical disease of joints: Potential role of mesenchymal stem cells. Life Sci. 2023, 321, 121641. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Cheng, J.; Qi, Y.; Jiang, X. Genetic link between gut microbiota, immune cells, and rheumatoid arthritis: Mechanism of action of CD28 proteins. Int. J. Biol. Macromol. 2024, 282, 137212. [Google Scholar] [CrossRef]
- Ali, A.; Jori, C.; Jori, C.; Kumar, A.; Khan, R. Recent trends in stimuli-responsive hydrogels for the management of rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2023, 89, 104985. [Google Scholar] [CrossRef]
- Su, Q.-Y.; Li, H.-C.; Jiang, X.-J.; Jiang, Z.-Q.; Zhang, Y.; Zhang, H.-Y.; Zhang, S.-X. Exploring the therapeutic potential of regulatory T cell in rheumatoid arthritis: Insights into subsets, markers, and signaling pathways. Biomed. Pharmacother. 2024, 174, 116440. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yoshimura, M.; Higashioka, K.; Miyawaki, K.; Ota, Y.; Ayano, M.; Kimoto, Y.; Mitoma, H.; Ono, N.; Arinobu, Y.; et al. Type 1 helper T cells generate CXCL9/10-producing T-bet+ effector B cells potentially involved in the pathogenesis of rheumatoid arthritis. Cell. Immunol. 2021, 360, 104263. [Google Scholar] [CrossRef] [PubMed]
- Dehnavi, S.; Sadeghi, M.; Afshari, J.T.; Mohammadi, M. Interactions of mesenchymal stromal/stem cells and immune cells following MSC-based therapeutic approaches in rheumatoid arthritis. Cell. Immunol. 2023, 393–394, 104771. [Google Scholar] [CrossRef] [PubMed]
- Filali, S.; Noack, M.; Géloën, A.; Pirot, F.; Miossec, P. Effects of pro-inflammatory cytokines and cell interactions on cell area and cytoskeleton of rheumatoid arthritis synoviocytes and immune cells. Eur. J. Cell Biol. 2023, 102, 151303. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Li, X.; Wang, J.; Liu, Y.; Wang, Z.; Yang, X.; Gao, J.; Wu, J.; Sun, T.; et al. Critical role of G protein-coupled receptor 40 in B cell response and the pathogenesis of rheumatoid arthritis in mice and patients. Cell Rep. 2024, 43, 114858. [Google Scholar]
- Dong, X.; Liu, H.; Sun, R.; Tan, S.; Li, G.; Jin, X.; Zhang, X.; Xiao, Z.; Li, D.; Cao, J.-L.; et al. Inflammatory microenvironment activation-targeted self-delivery nanomedicine for effective rheumatoid arthritis treatment. Chem. Eng. J. 2024, 500, 157120. [Google Scholar] [CrossRef]
- Nooreen, R.; Nene, S.; Jain, H.; Prasannanjaneyulu, V.; Chitlangya, P.; Otavi, S.; Khatri, D.K.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Polymer nanotherapeutics: A versatile platform for effective rheumatoid arthritis therapy. J. Control. Release 2022, 348, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Derksen, V.F.A.M.; Huizinga, T.W.J.; van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; He, L.; Wang, J.; Tang, D.; Wu, C.; Peng, W. Targeting IL-17 and its receptors: A feasible way for natural herbal medicines to modulate fibroblast-like synoviocytes in rheumatoid arthritis. Biochem. Pharmacol. 2024, 230, 116598. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Xu, C.; Zhang, G.; Che, X.; Yang, J. Potential mechanisms of rheumatoid arthritis therapy: Focus on macrophage polarization. Int. Immunopharmacol. 2024, 142, 113058. [Google Scholar] [CrossRef]
- Xing, J.; Jia, J.; Zhang, H.; Han, H.; Li, Q. Fluorinated dendrimer-mediated miR-30a delivery regulates the inflammation of macrophages and mitigates the symptoms of rheumatoid arthritis. J. Control. Release 2024, 376, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-W.; Tang, C.-H. The role of macrophage polarization in rheumatoid arthritis and osteoarthritis: Pathogenesis and therapeutic strategies. Int. Immunopharmacol. 2024, 142, 113056. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Chen, S.; Ngai, T.; Miao, S.; Pang, J.; Zhang, L.; Hu, W.; Wang, X.; Liu, B.; Li, X.; et al. The nanozymes of protein nanotubes-constructed microspheres with dual peroxidase- and catalase-like properties for M1-to-M2 macrophages repolarization and the synergistic anti-rheumatoid arthritis effect with loaded capsaicin. Nano Today 2024, 56, 102290. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, H.; Zhang, Z.; Liang, X.; Liu, Y.; Wang, C.; Yang, X.; Tang, J.; Mao, J.; Nie, Y.; et al. pH-responsive size-adjustable liposomes induce apoptosis of fibroblasts and macrophages for rheumatoid arthritis treatment. Acta Biomater. 2024, 179, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lee, K.-T.; Lin, Y.-Y.; Tsai, C.-H.; Ko, C.-Y.; Fong, Y.-C.; Hou, S.-M.; Chen, W.-L.; Huang, C.-C.; Tang, C.-H. NGF facilitates ICAM-1-dependent monocyte adhesion and M1 macrophage polarization in rheumatoid arthritis. Int. Immunopharmacol. 2024, 130, 111733. [Google Scholar] [CrossRef]
- Fang, F.; Hua, M.; Yu, G. Study of the mechanism of fibroblast-like synoviocytes-derived exosomes inducing macrophages M1 polarization and CD8+T cells immune regulation ferroptosis and autophagy in rheumatoid arthritis. Immunol. Lett. 2024, 270, 106936. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Balandraud, N.; Roudier, J. Epstein-Barr virus and rheumatoid arthritis. Jt. Bone Spine 2018, 85, 165–170. [Google Scholar] [CrossRef]
- Batra, P.; Shende, P. β-cyclodextrin-based supramolecular micelles of diosgenin conjugated with anti-CD64 antibody for rheumatoid arthritis. Colloids Surf. A Physicochem. Eng. Aspects 2024, 688, 133521. [Google Scholar] [CrossRef]
- Gumtorntip, W.; Kasitanon, N.; Louthrenoo, W.; Chattipakorn, N.; Chattipakorn, S.C. Potential roles of air pollutants on the induction and aggravation of rheumatoid arthritis: From cell to bedside studies. Environ. Pollut. 2023, 334, 122181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, F.; Cheng, H.; Zhang, D.; Zhang, Y.; Wang, L.; Tang, F.; Wang, J.; Wan, Y.; Wu, J.; et al. Chronic cold environment regulates rheumatoid arthritis through modulation of gut microbiota-derived bile acids. Sci. Total Environ. 2023, 903, 166837. [Google Scholar] [CrossRef]
- Sahraei, F.; Rahemi, Z.; Sadat, Z.; Zamani, B.; Ajorpaz, N.M.; Afshar, M.; Mianehsaz, E. The effect of Swedish massage on pain in rheumatoid arthritis patients: A randomized controlled trial. Complement. Ther. Clin. Pract. 2022, 46, 101524. [Google Scholar] [CrossRef]
- An, X.; Yang, J.; Cui, X.; Zhao, J.; Jiang, C.; Tang, M.; Dong, Y.; Lin, L.; Li, H.; Wang, F. Advances in local drug delivery technologies for improved rheumatoid arthritis therapy. Adv. Drug Deliv. Rev. 2024, 209, 115325. [Google Scholar] [CrossRef] [PubMed]
- Karnam, S.; Donthi, M.R.; Jindal, A.B.; Paul, A.T. Recent innovations in topical delivery for management of rheumatoid arthritis: A focus on combination drug delivery. Drug Discov. Today 2024, 29, 104071. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ma, T.; Wang, W.; Sun, J.; Zhang, X.; Hu, J.; Zhu, C. Carbon monoxide (CO)-releasing micelles enable efficient treatment of MRSA-induced septic arthritis and rheumatoid arthritis. Nano Today 2024, 57, 102389. [Google Scholar] [CrossRef]
- Yang, L.; He, X.; Xue, Y.; Zhi, D.; Meng, Q.; Zhao, W.; Gong, X.; Yue, D.; Dong, K.; Tian, Y. Amelioration of melittin on adjuvant-induced rheumatoid arthritis: Integrated transcriptome and metabolome. Int. J. Biol. Macromol. 2024, 270, 132293. [Google Scholar] [CrossRef]
- Ali, A.; Jori, C.; Kumar, J.; Kumar, A.; Ansari, M.M.; Ahmad, A.; Ali, N.; Yadav, P.; Parvez, S.; Navik, U.; et al. Sinapic acid-pullulan based inflammation responsive nanomicelles for the local treatment of experimental inflammatory arthritis. Int. J. Biol. Macromol. 2024, 278, 134903. [Google Scholar]
- Zhang, Y.; Gao, Y.; Li, N.; Xu, L.; Wang, Y.; Liu, H. Polypropylene sulfide methotrexate nanoparticles target the synovial lymphatic system to restore immune tolerance in rheumatoid arthritis. Int. J. Pharm. 2024, 665, 124713. [Google Scholar] [CrossRef] [PubMed]
- Crowson, L.P.; Davis, J.M.; Hanson, A.C.; Myasoedova, E.; Kronzer, V.L.; Makol, A.; Peterson, L.S.; Bekele, D.I.; Crowson, C.S. Time Trends in Glucocorticoid Use in Rheumatoid Arthritis During the Biologics Era: 1999–2018. Semin. Arthritis Rheum. 2023, 61, 152219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-R.; Cheng, Q.-H.; Yang, Y.-Z.; Yang, X.; Zhang, Z.-Z.; Guo, H.-Z. Meta-analysis of outcomes after total knee arthroplasty in patients with rheumatoid arthritis and osteoarthritis. Asian J. Surg. 2024, 47, 43–54. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Borrelli, C.; Buckley, C.T. Injectable Disc-Derived ECM Hydrogel Functionalised with Chondroitin Sulfate for Intervertebral Disc Regeneration. Acta Biomater. 2020, 117, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, H.; Jing, X.; Wang, X.; Shi, Y.; He, C.; Ma, B.; Nie, J.; Zhang, J.; Ma, G. Injectable In Situ Photocrosslinked Hydrogel Dressing for Infected Wound Healing. ACS Appl. Bio Mater. 2023, 6, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Li, C.; Cong, H.; Yu, B. Research progress of injectable hydrogels in the treatment of bone tissue diseases. Chem. Eng. J. 2024, 498, 155139. [Google Scholar] [CrossRef]
- Wei, Z.; Zuo, Y.; Wu, E.; Huang, L.; Qian, Y.; Wang, J.; Chen, Z. Highly biocompatible, antioxidant and antibacterial gelatin methacrylate/alginate—Tannin hydrogels for wound healing. Int. J. Biol. Macromol. 2024, 279, 135417. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, J.; Zhou, Y.; Guo, Y.; Liao, X.; He, L.; Li, D.; Li, X.; Liu, Y. From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 231, 123308. [Google Scholar] [CrossRef]
- Shaygani, H.; Mofrad, Y.M.; Demneh, S.M.R.; Hafezi, S.; Almasi-Jaf, A.; Shamloo, A. Cartilage and bone injectable hydrogels: A review of injectability methods and treatment strategies for repair in tissue engineering. Int. J. Biol. Macromol. 2024, 282, 136689. [Google Scholar] [CrossRef]
- Aliakbarzadeh, S.; Abdouss, M.; Khonakdar, H.A.; Rahdar, A.; Fathi-karkan, S. Gelatin methacrylate/poly(2-ethyl-2-oxazoline) porous hydrogel loaded with kartogenin drug as a biocompatible scaffold for cartilage tissue regeneration. J. Mol. Liq. 2024, 404, 124982. [Google Scholar] [CrossRef]
- Li, S.; Ge, X.; Zhong, Z.; Dong, X.; Xing, Z.; Wei, Y.; Zhang, Q. Robust and low swelling polyacrylamide/sodium alginate dual-network hydrogels via multiple freezing strategy toward amphibious motion sensors. Polymer 2024, 315, 127850. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Hang, R.; Yao, X.; Bai, L.; Wang, H.; Huang, D.; Hang, R. Tailoring surface stiffness to modulate senescent macrophage immunomodulation: Implications for osteo-/angio-genesis in aged bone regeneration. Biomater. Adv. 2024, 165, 214010. [Google Scholar] [CrossRef] [PubMed]
- Kolour, A.K.; Shahrousvand, M.; Mohammadi-Rovshandeh, J.; Puppi, D.; Farzaneh, D. Absorbable and biodegradable enzyme-crosslinked gelatin/alginate semi-IPN hydrogel wound dressings containing curcumin. Int. J. Biol. Macromol. 2024, 279, 134938. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, F.; Li, X.; Liang, Q.; Zhuo, Z.; Huang, J.; Duan, L.; Xiong, J.; Wang, D. Repair of osteochondral defects using injectable chitosan-based hydrogel encapsulated synovial fluid-derived mesenchymal stem cells in a rabbit model. Mater. Sci. Eng. C 2019, 99, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Gong, X.; Wang, C.; Liu, P.; Shi, W.; Cheng, J.; Cao, C.; Hu, X.; Wang, J.; Ao, Y. Injectable decellularized extracellular matrix hydrogel with cell-adaptable supramolecular network enhances cartilage regeneration by regulating inflammation and facilitating chondrogenesis. Chem. Eng. J. 2024, 498, 155138. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.; Brouwer, L.A.; Buist-Homan, M.; Wolters, J.C.; Moshage, H.; Harmsen, M.C. Collagen-rich liver-derived extracellular matrix hydrogels augment survival and function of primary rat liver sinusoidal endothelial cells and hepatocytes. Int. J. Biol. Macromol. 2024, 278, 134717. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, Z.; Nasrollahzadeh, M.; Heidari, F.; Nasrabadi, D. A drug-loaded nano chitosan/hyaluronic acid hydrogel system as a cartilage tissue engineering scaffold for drug delivery. Int. J. Biol. Macromol. 2024, 283, 137314. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.M.F.A.; Wong, L.K.; Zia, A.W.; Wu, H. Development of biomedical hydrogels for rheumatoid arthritis treatment. Asian J. Pharm. Sci. 2024, 19, 100887. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Wong, C.-C.; Weng, P.-W.; Chiang, C.-W.; Lin, P.-Y.; Lee, P.-W.; Jheng, P.-R.; Hao, P.-C.; Chen, Y.-T.; Cho, E.-C.; et al. Bioinspired and self-restorable alginate-tyramine hydrogels with plasma reinforcement for arthritis treatment. Int. J. Biol. Macromol. 2023, 250, 126105. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, T.; Alotaibi, H.F.; Prokopovich, P. Polymer colloids as drug delivery systems for the treatment of arthritis. Adv. Colloid Interface Sci. 2020, 285, 102273. [Google Scholar] [CrossRef]
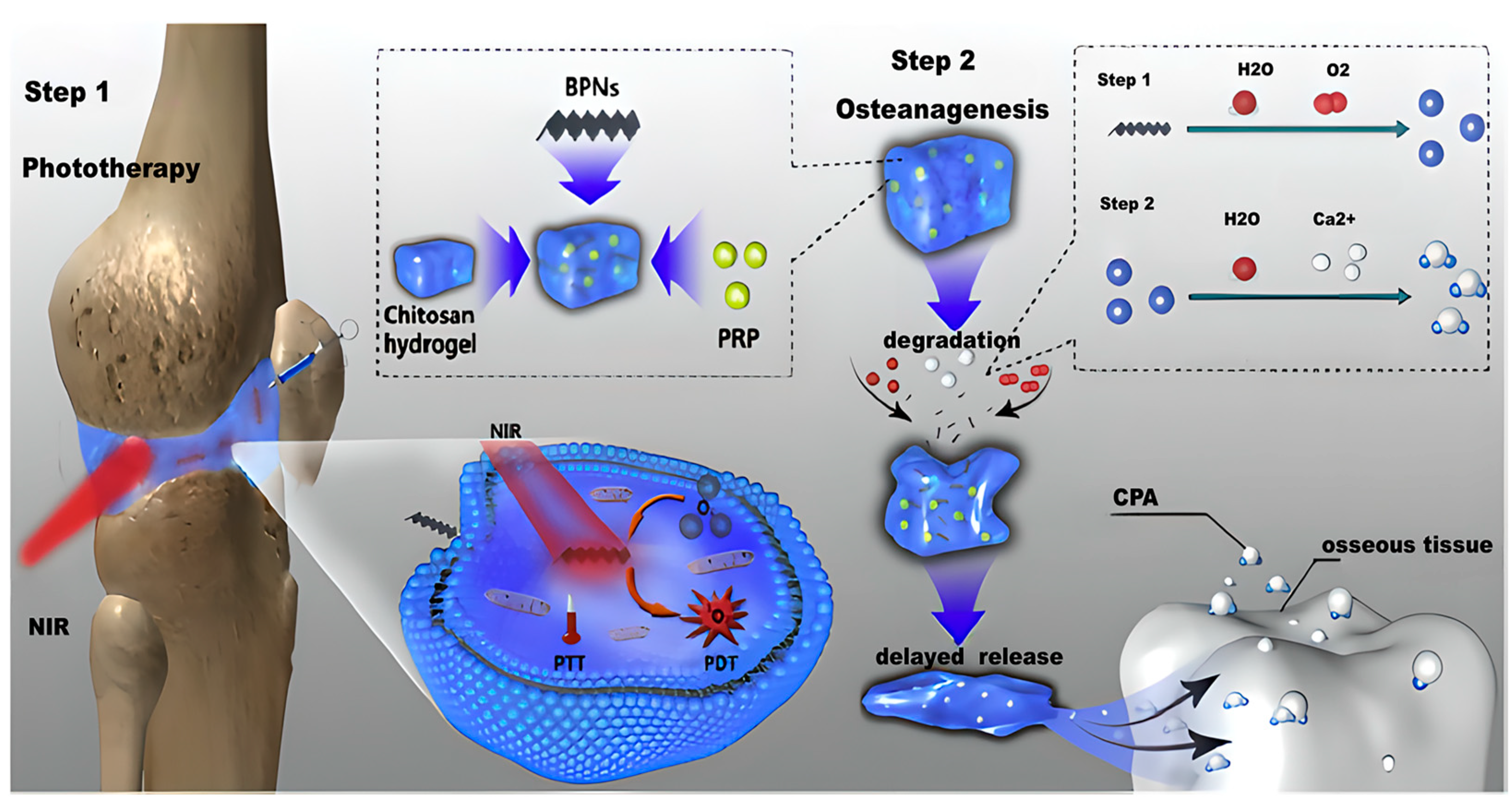
- Gang, F.; Zhang, Q.; Jiang, L.; Xiao, Y.; Xu, N.; Wang, Y.; Xiao, Y.; Li, A.; Liu, Z.; Liu, B.; et al. Thermochemotherapy Meets Tissue Engineering for Rheumatoid Arthritis Treatment. Adv. Funct. Mater. 2021, 31, 2104131. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Wang, D.; Liu, H.; Zhang, J.; Li, Z.; Wang, J.; Ren, X.; Zhao, Y. Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat. Commun. 2022, 13, 6758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, J.; Wang, Z.; Wang, H.; An, M.; Yakufu, M.; Wang, W.; Liu, Y.; Liu, W.; Li, C. An injectable self-lubricating supramolecular polymer hydrogel loaded with platelet lysate to boost osteoarthritis treatment. J. Control. Release 2024, 376, 20–36. [Google Scholar] [CrossRef]
- Hu, F.; Lu, H.; Ye, Z.; Zhang, S.; Wang, W.; Gao, L. Slow-release lubrication of artificial joints using self-healing polyvinyl alcohol/polyethylene glycol/ graphene oxide hydrogel. J. Mech. Behav. Biomed. Mater. 2021, 124, 104807. [Google Scholar] [CrossRef]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.A.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef]
- Du, X.; Lin, Y.; Shuai, Z.; Duan, J.; Wang, C.; Liu, J.; Jiang, J.; Wu, J.; Zhou, M.; Zhang, Z.; et al. Nanocomposite hydrogel to deliver the immunomodulator lenalidomide and anti-inflammatory hesperidin locally to joints affected by rheumatoid arthritis. Chem. Eng. J. 2023, 476, 146270. [Google Scholar] [CrossRef]
- Liao, H.; Qi, W.; Xue, Z.; Wu, K.; Jiang, L.; Wu, C.; Huang, Z.; Li, Q.; Lu, Y. A multifunctional supramolecular hydrogel that rapidly binds TNF-α for efficient reduction of synovial inflammation and cartilage destruction in rheumatoid arthritis. Chem. Eng. J. 2023, 477, 147125. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Ge, Y.; Zhu, Y.; Tian, T.; Wei, J.; Jin, Y.; Zhao, Y.; Jia, Q.; Wu, J.; et al. Microenvironment Responsive Hydrogel Exerting Inhibition of Cascade Immune Activation and Elimination of Synovial Fibroblasts for Rheumatoid Arthritis Therapy. J. Control. Release 2024, 370, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, C.; Liu, H.; Liu, H.; Feng, Y.; Li, Z.; Liu, H.; Wang, J.; Yang, B.; Lin, Q. Infliximab-based self-healing hydrogel composite scaffold enhances stem cell survival, engraftment, and function in rheumatoid arthritis treatment. Acta Biomater. 2021, 121, 653–664. [Google Scholar] [CrossRef]
- Yue, Y.; Shi, F.; Wang, J.; Ning, Q.; Zhang, Z.; Lv, H. Sulfated hyaluronic acid gel for the treatment of rheumatoid arthritis in rats. Int. J. Biol. Macromol. 2024, 256, 128537. [Google Scholar] [CrossRef]
- Xu, M.; Fu, T.; Zhang, C.; An, Z.; Yan, J.; Lu, Z.; Wu, H.; Liu, J.; Qiu, L.; Shi, L.; et al. Prolonged, staged, and self-regulated methotrexate release coupled with ROS scavenging in an injectable hydrogel for rheumatoid arthritis therapy. J. Control. Release 2024, 375, 60–73. [Google Scholar] [CrossRef] [PubMed]
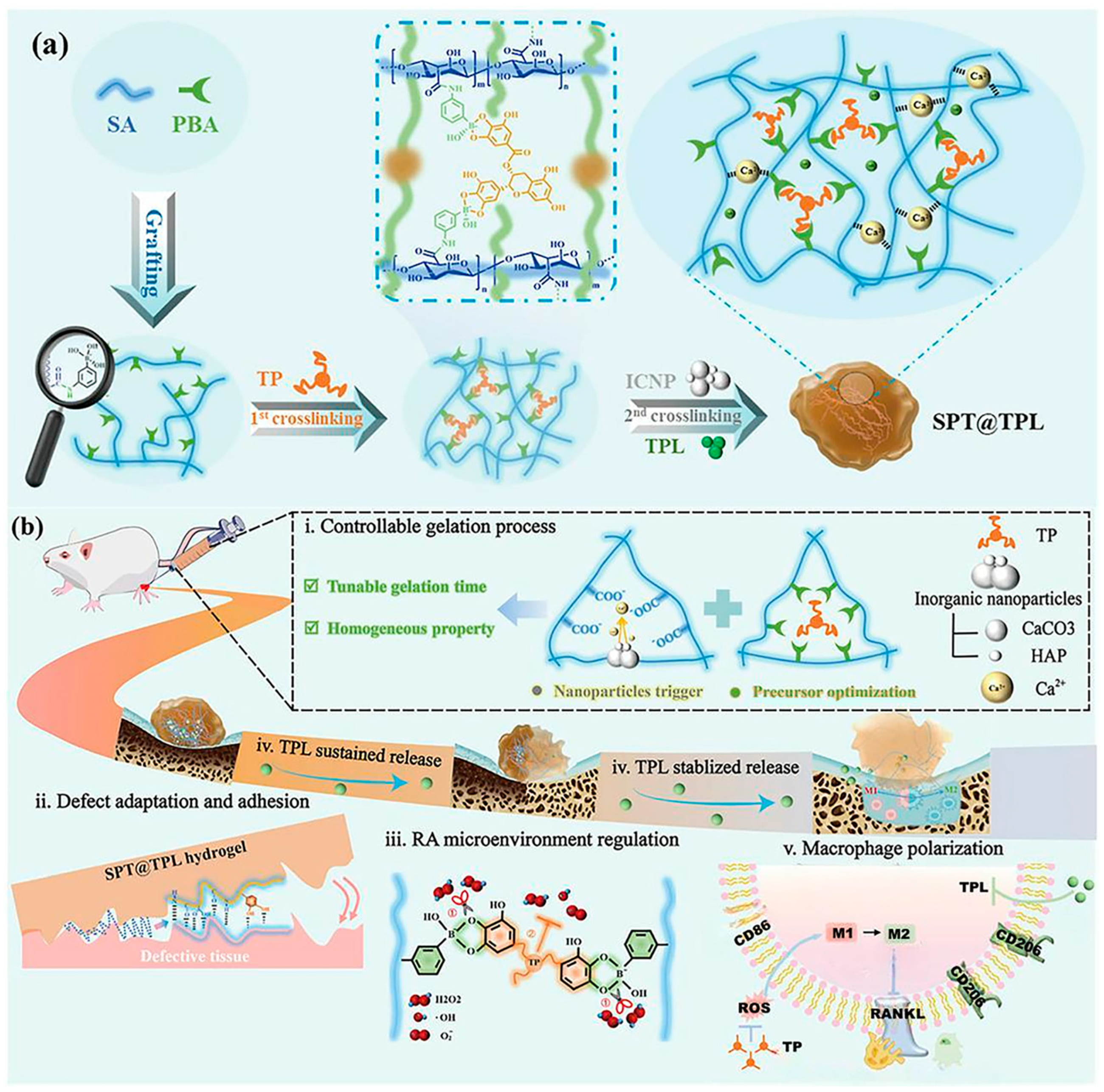
- Wang, T.; Huang, C.; Fang, Z.; Bahatibieke, A.; Fan, D.; Wang, X.; Zhao, H.; Xie, Y.; Qiao, K.; Xiao, C.; et al. A dual dynamically cross-linked hydrogel promotes rheumatoid arthritis repair through ROS initiative regulation and microenvironment modulation-independent triptolide release. Mater. Today Bio 2024, 26, 101042. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, S.; Liu, Z.; Wang, X. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ran, B.; Xie, X.; Gu, W.; Ye, X.; Liao, J. Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy. Gels 2022, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef]
- Khan, D.; Ahmed, N.; Muhammad, A.; Shah, K.U.; Mir, M.; Rehman, A.U. A macromolecule infliximab loaded reverse nanomicelles-based transdermal hydrogel: An innovative approach against rheumatoid arthritis. Biomater. Adv. 2025, 167, 214093. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Guido, J.F.; Gupta, M.; Zhang, A.; Peppas, N.A. pH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: Effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. J. Control. Release 2016, 221, 18–25. [Google Scholar] [CrossRef]
- Carrillo-Conde, B.R.; Brewer, E.; Lowman, A.; Peppas, N.A. Complexation Hydrogels as Oral Delivery Vehicles of Therapeutic Antibodies: An In Vitro and Ex Vivo Evaluation of Antibody Stability and Bioactivity. Ind. Eng. Chem. Res. 2015, 54, 10197–10205. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Pferdehirt, L.; Saleh, L.S.; Savadipour, A.; Springer, L.E.; Lenz, K.L.; Thompson, D.M.; Oswald, S.J.; Pham, C.T.N.; Guilak, F. Hydrogel Encapsulation of Genome-Engineered Stem Cells for Long-Term Self-Regulating Anti-Cytokine Therapy. Gels 2023, 9, 169. [Google Scholar] [CrossRef] [PubMed]
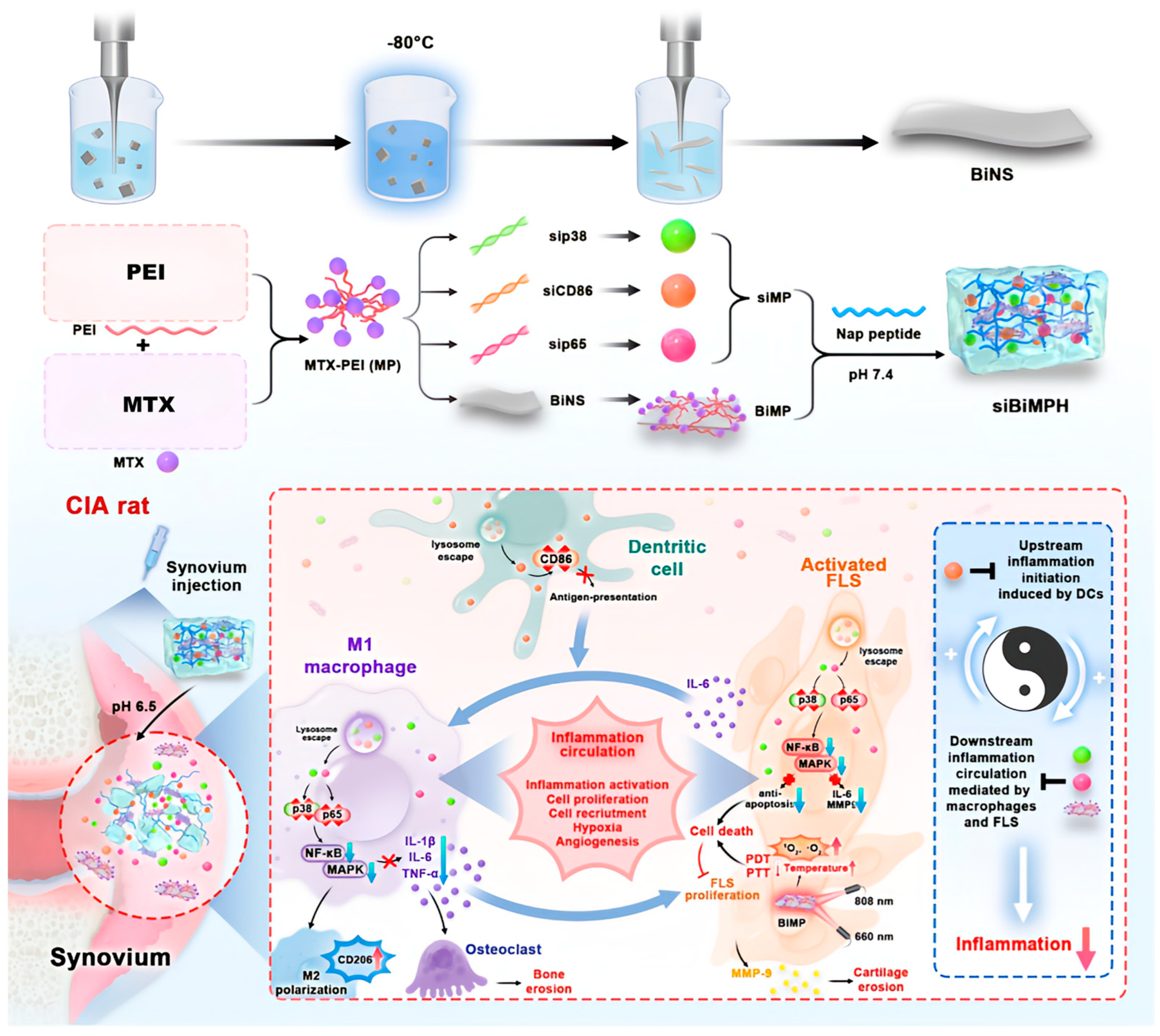
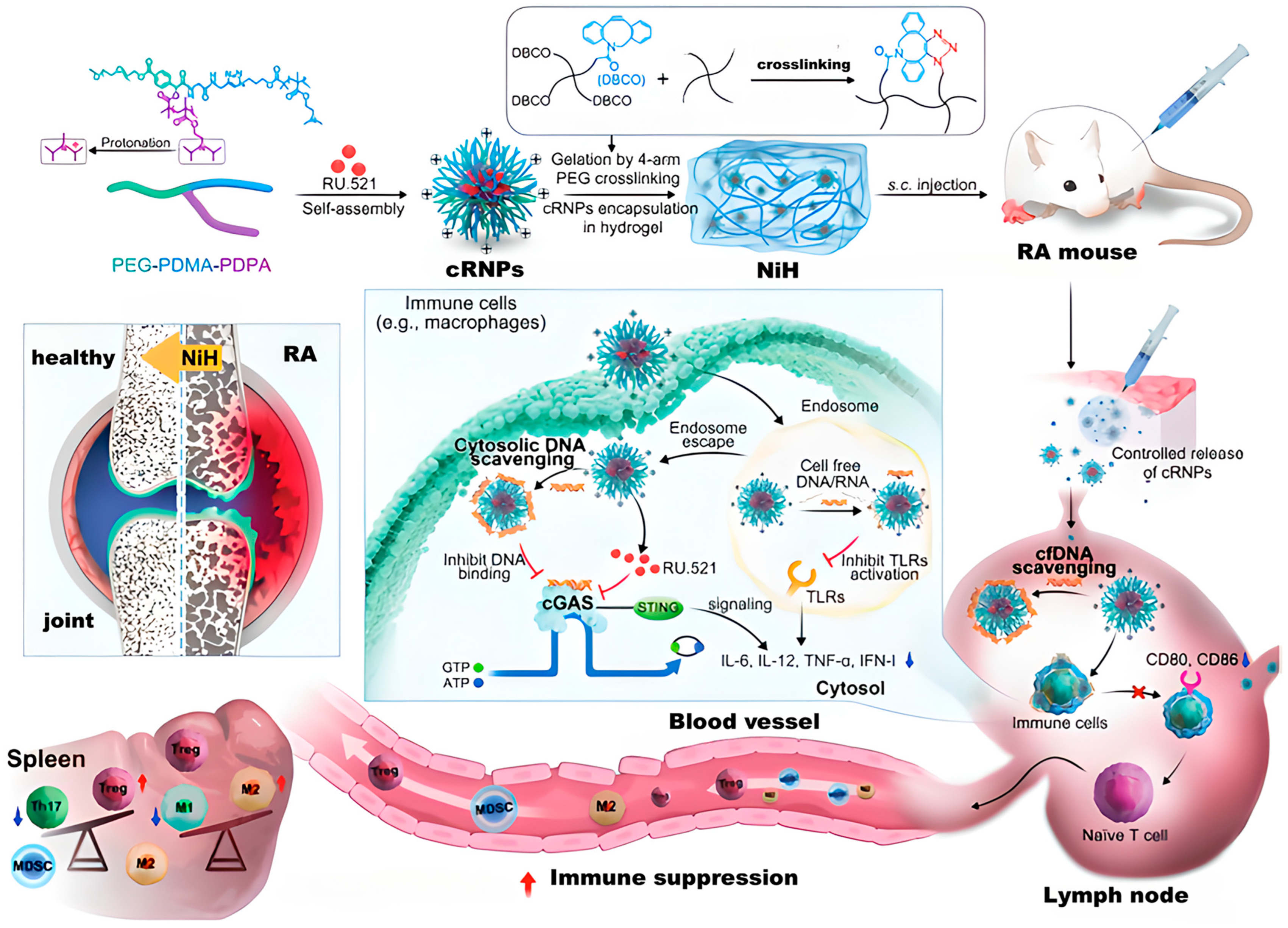
- Cheng, F.; Su, T.; Liu, Y.; Zhou, S.; Qi, J.; Guo, W.; Zhu, G. Targeting Lymph Nodes for Systemic Immunosuppression Using Cell-Free-DNA-Scavenging And cGAS-Inhibiting Nanomedicine-In-Hydrogel for Rheumatoid Arthritis Immunotherapy. Adv. Sci. 2023, 10, 2302575. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, M.; Cao, J.; Fang, T.; Zhang, J.; Zhen, Y.; Wu, F.; Yu, X.; Liu, Y.; Li, J.; et al. Applications and recent advances in transdermal drug delivery systems for the treatment of rheumatoid arthritis. Acta Pharm. Sin. B 2023, 13, 4417–4441. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, S.; Kim, M.S. Intra-articular hydrogel formulation prolongs the in vivo stability of Toll-like receptor antagonistic peptides for rheumatoid arthritis treatment. J. Control. Release 2024, 372, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Villarreal, F.A.; Stephens, M.; Pavlicin, R.; Beussman, M.; Peppas, N.A. Therapeutic delivery systems for rheumatoid arthritis based on hydrogel carriers. Adv. Drug Deliv. Rev. 2024, 208, 115300. [Google Scholar] [CrossRef]
- Hu, N.; Zhu, L.; Zhang, L.; Wang, J.; Wang, Y.; Luo, J.; He, L.; Hao, Z.; Zhang, L. Immunomodulatory effect and safety of TNF-α RNAi mediated by oral yeast microcapsules in rheumatoid arthritis therapy. Mater. Today Bio 2022, 16, 100384. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Yang, M.; Feng, X.; Wang, Y.; Chang, F.; Ding, J. Reduction-Responsive Polypeptide Nanogel for Intracellular Drug Delivery in Relieving Collagen-Induced Arthritis. ACS Biomater. Sci. Eng. 2018, 4, 4154–4162. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Gupta, N.V.; Sastri, K.T.; Sharadha, M.; Chand, P.; Kumar, H.; Osmani, R.A.M.; Gowda, D.V.; Jaind, V. Current progressions in transdermal drug delivery systems for management of rheumatoid and osteoarthritis: A comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 73, 103476. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2021, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yin, S.; Jiao, R.; Chen, W.; Wang, W.; Zhang, H.; Liu, Z.; Chen, Z.; Tian, X. The combination of hydrogels and rutin-loaded black phosphorus nanosheets treats rheumatoid arthritis. Mater. Today Bio 2024, 29, 101264. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, H.; Cao, Y.; Lin, Y.; Yang, Y.; Gao, M.; Zhang, W.; Wang, C. Deep eutectic solvents—Hydrogels for the topical management of rheumatoid arthritis. J. Control. Release 2023, 354, 664–679. [Google Scholar] [CrossRef]
- Makled, S.; Abbas, H.; Ali, M.E.; Zewail, M. Melatonin hyalurosomes in collagen thermosensitive gel as a potential repurposing approach for rheumatoid arthritis management via the intra-articular route. Int. J. Pharm. 2024, 661, 124449. [Google Scholar] [CrossRef] [PubMed]
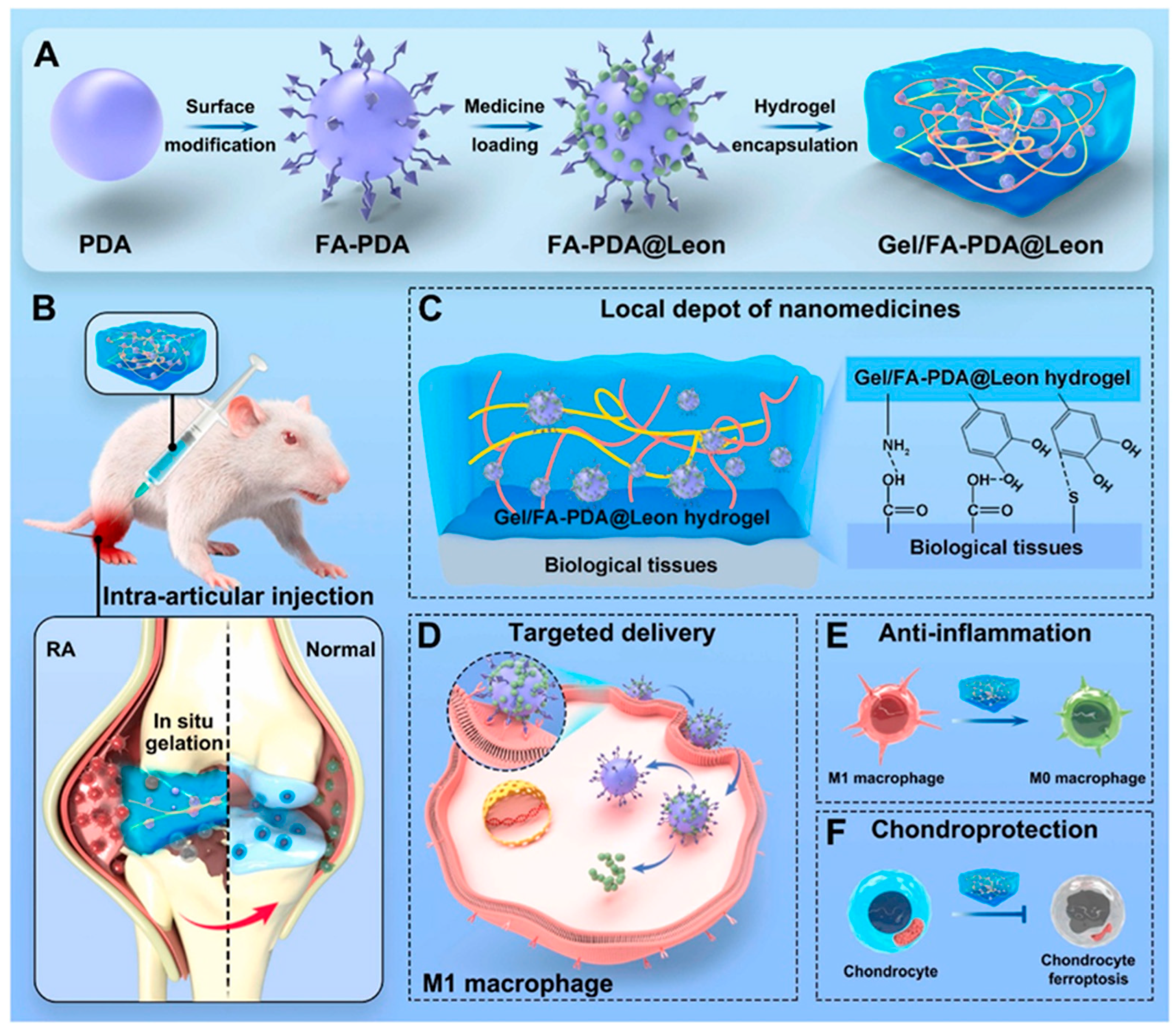
- Yang, R.; Yan, L.; Xu, T.; Zhang, K.; Lu, X.; Xie, C.; Fu, W. Injectable bioadhesive hydrogel as a local nanomedicine depot for targeted regulation of inflammation and ferroptosis in rheumatoid arthritis. Biomaterials 2024, 311, 122706. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qin, J.; Ma, X.; Wang, Q.; Wu, W.; Huang, H.; Cai, L. Chitosan-based self-healing thermosensitive hydrogel loaded with siHMGB1 for treatment of rheumatoid arthritis via macrophage repolarization. Int. J. Biol. Macromol. 2024, 282, 137102. [Google Scholar] [CrossRef] [PubMed]


| Type | Function | Matrix Material | Ref. |
|---|---|---|---|
| Tissue engineering scaffold hydrogels | It is used to provide support for the repair of tissues such as articular cartilage and synovium. The three-dimensional structure of hydrogels can mimic the environment of the extracellular matrix, which is beneficial to cell adhesion, proliferation, and differentiation. | Natural materials such as polymers such as chitosan, alginate, and hyaluronic acid, proteins (gelatin, collagen, silk fibroin), as well as polymers such as PVA and PEG. | [68] |
| Lubricating hydrogels | It can mimic the lubricating properties of natural synovial fluid and act as “artificial synovial fluid” in the joint cavity. The water phase and the hydrophilic polymer network inside it endow it with good lubricating ability, which can reduce the friction coefficient during joint activities and minimize wear and tear. | Natural materials such as polymers such as chitosan, alginate, and hyaluronic acid, proteins (gelatin, collagen, silk fibroin), as well as polymers such as PVA and PEG. | [69] |
| Immunomodulatory hydrogels | Regulate the local immune microenvironment of joints by means of adsorbing or neutralizing inflammatory cytokines and interfering with the activation and proliferation of immune cells. | Natural materials such as polymers such as chitosan, alginate, and hyaluronic acid, proteins (gelatin, collagen, silk fibroin), as well as polymers such as PVA and PEG. | [70] |
| Drug delivery hydrogels | Achieve the slow and controllable release of drugs, and utilize its own unique network structure or physicochemical properties to encapsulate the drugs for treating RA. | Natural materials such as polymers such as chitosan, alginate, hyaluronic acid, proteins (gelatin, collagen, silk fibroin), as well as polymers such as PVA and PEG. | [71] |
| Type | Advantage | Disadvantage | Ref. |
|---|---|---|---|
| Oral administration | It is convenient and easy to use. Patients can take it on their own without the need for any invasive procedures. With the drugs encapsulated in hydrogels, their stability is enhanced, and they can exert their effects mainly in the local area of the intestine, thus avoiding the systemic toxicity that may be caused by direct oral administration. | It is rather difficult to control the precision of drug release, and it may also cause certain irritation to the gastrointestinal mucosa. | [88,89] |
| SC injection | It is easy to operate with low invasiveness. The drugs can be slowly released under the skin, and the drug release rate is controllable, which is beneficial for maintaining a stable drug efficacy. | The drugs take effect relatively slowly. Affected by individual differences in subcutaneous tissues, remaining in the subcutaneous tissues for a long time may cause some adverse reactions. Moreover, the drug loading capacity is limited, and repeated injections are required. | [90] |
| IV injection | It takes effect quickly and enables targeted delivery, realizes systemic immune regulation, and improves the condition of RA as a whole. | It has relatively high risks, strict requirements for hydrogels, and relatively high preparation costs. | [91] |
| Transdermal | It is non-invasive and convenient. The drugs can act directly on the local tissues around the joints, precisely target joint inflammation, reduce systemic side effects, and the drug delivery can be controlled at any time. | The skin barrier restricts drug absorption. The drug release rate is affected by the physiological state of the skin. Long term application may cause adverse skin reactions. | [92] |
| Intra-articular injection | Drugs can act precisely within the affected joints, release drugs in a long acting and slow manner, and reduce systemic side effects. | It is difficult to operate, there is a risk of infection, and individual differences affect drug release. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, J.; Ren, F.; Zhang, J.; Song, W.; Ren, L. New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research. Gels 2025, 11, 136. https://doi.org/10.3390/gels11020136
Wang S, Li J, Ren F, Zhang J, Song W, Ren L. New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research. Gels. 2025; 11(2):136. https://doi.org/10.3390/gels11020136
Chicago/Turabian StyleWang, Shuai, Jinyang Li, Fazhan Ren, Jiale Zhang, Wei Song, and Lili Ren. 2025. "New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research" Gels 11, no. 2: 136. https://doi.org/10.3390/gels11020136
APA StyleWang, S., Li, J., Ren, F., Zhang, J., Song, W., & Ren, L. (2025). New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research. Gels, 11(2), 136. https://doi.org/10.3390/gels11020136







