Oral Delivery of a GI-Stable Apigenin–Cyclodextrin Complex via Pectin-Coated Nanoliposomes In Situ Gel: A DoE-Optimized Targeted Colon Cancer Therapy by Modulating Gut Drug Sensitivity
Abstract
1. Introduction
2. Results and Discussion
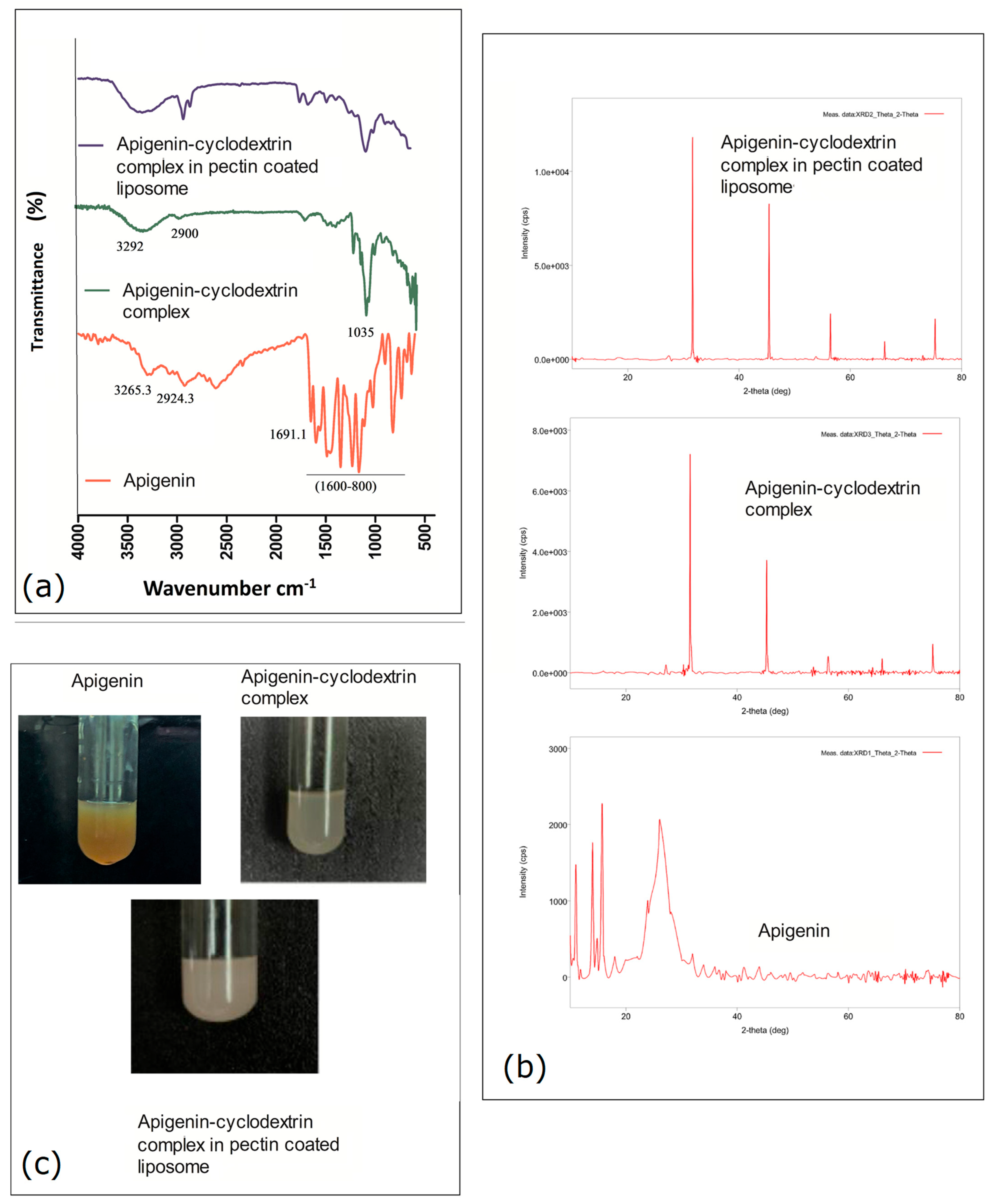
2.1. Pre-Formulation Study Using FTIR
2.2. XRD Observation Analysis
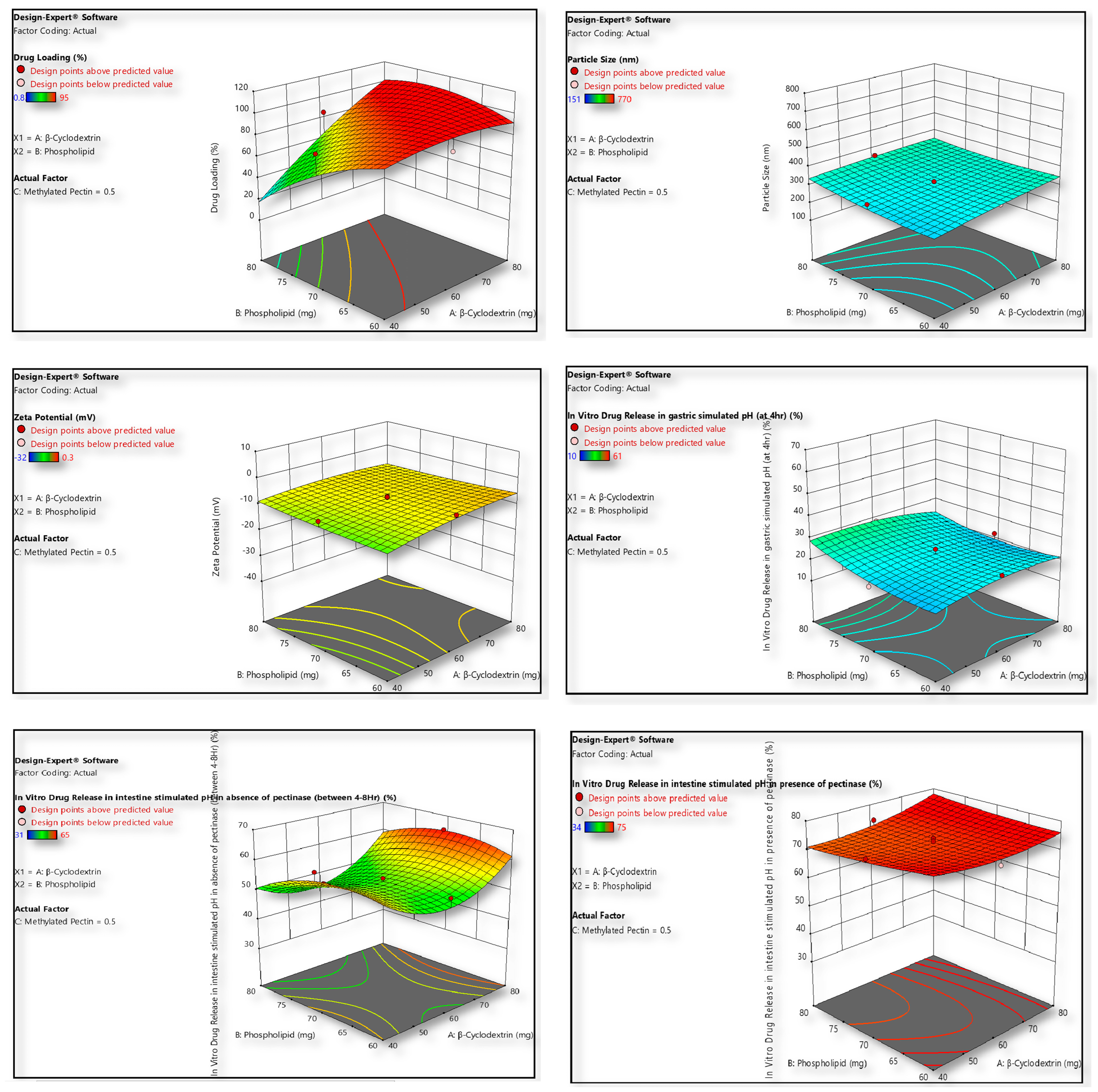
2.3. Formulation Optimization Strategy by Design Expert
2.4. Physical Evaluation of Optimized Formula
2.4.1. Drug Loading
2.4.2. Particle Morphology Characteristics
2.4.3. Swelling Index (SI)
2.5. In Vitro Drug Release Pattern Applying GI Simulated Media
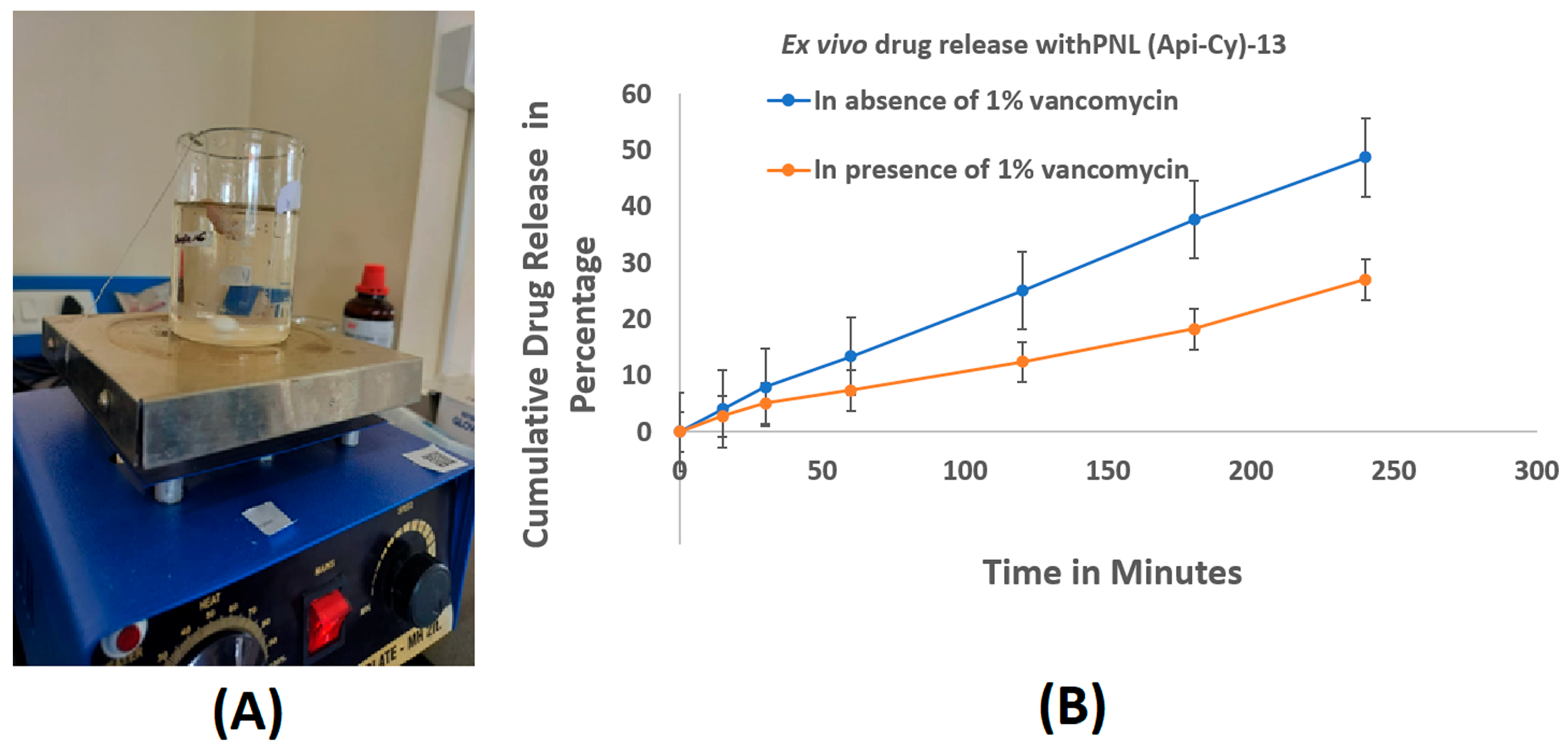
2.6. Ex Vivo Intestinal Drug Release in Presence and Absence of Vancomycin
2.7. Cytotoxicity Profile of Experimental Coated Nanoliposomes
2.8. Cellular Uptake Study Using FITC-Labelled PNL (Api-Cy)-13
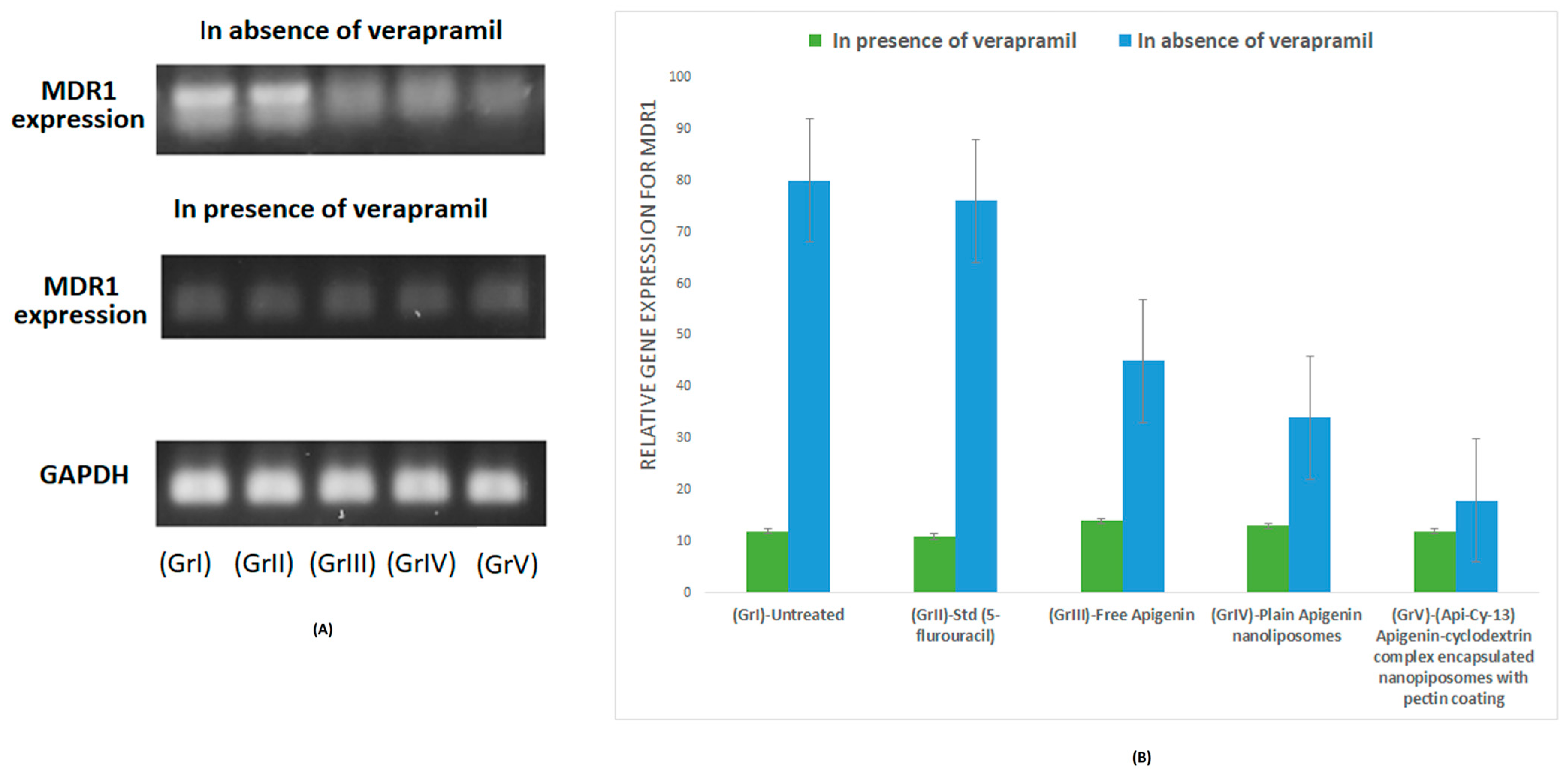
2.9. MDR1 mRNA Expression Analysis via RT-PCR
2.10. In Vivo Studies
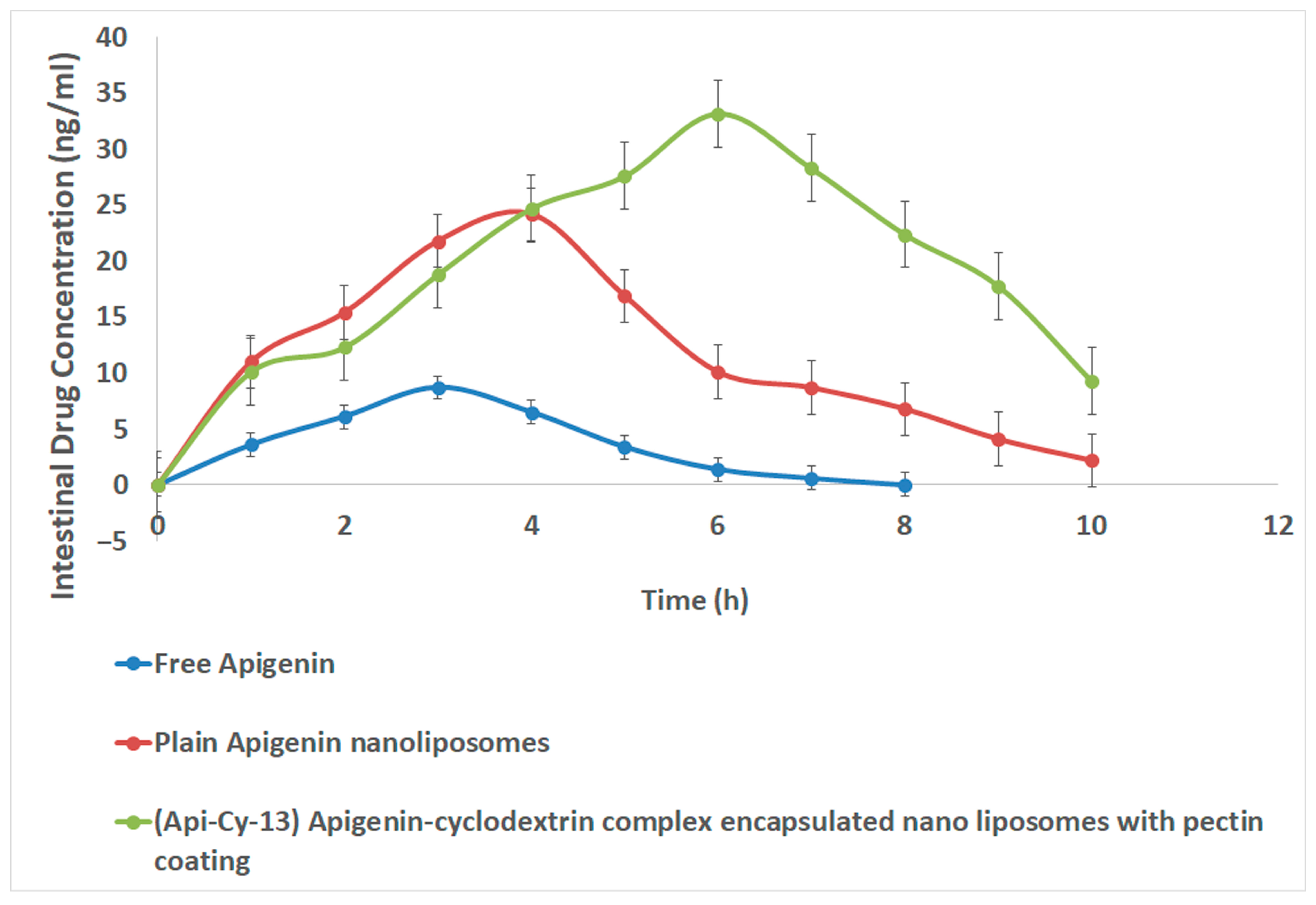
2.10.1. Intestinal Pharmacokinetics
2.10.2. Systemic Safety Studies
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments
4.1.1. Pre-Formulation Studies
FTIR Study
XRD Analysis Study
4.1.2. Design of Experiments (DOE) Strategy for Formulation Optimization
4.1.3. Preparation of Cyclodextrin-Embedded Coated Nanoliposomes
Preparation of Apigenin and β-Cyclodextrin Inclusion Complexes Using Ani-Solvent Solution
Preparation of Nanoliposomes
Coating of Pectin on Liposomes and Its Purification
4.1.4. Physicochemical Characterization (In Vitro)
Drug Entrapment
- ➢
- % Drug loading = (amount of drug in liposome/amount of liposome used) × 100
- ➢
- % Yield = (weight of dry powdered liposome/total weight of all the components used in the formulation) × 100
Particle Size and ζ- Potential Extents
SEM Study for Particle Morphology Analysis
Determination of Swelling Index (SI)
In Vitro Drug Digestion and Release Study
4.1.5. Evaluating Intestinal Drug Release Profile of Optimized Nanoformulation in Presence of 1% Vancomycin Using Chick Ileum Ex Vivo Method
4.1.6. In Vitro Cytotoxicity Assay and Cell Uptake Assay Using Colon Cancer Cell Lines
4.1.7. Cellular Uptake Study Using Flow Cytometry in HCT116 Cells
4.1.8. Real-Time PCR Study
4.1.9. In Vivo Studies
Intestinal Pharmacokinetics
Safety Studies
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.-L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Patel, P.; Vyas, N.; Raval, M. Safety and Toxicity issues of Polymeric Nanoparticles: A Serious Concern. In Nanotechnology in Medicine, 1st ed.; Rai, M., Patel, M., Patel, R., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 156–173. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Mukherjee, B.; Dhara, M.; Dutta, D.; Chakraborty, A.; Chakraborty, S.; Sengupta, S.; Mondal, L.; Dutta, L.; Pal, K. Biopolymer-based materials in nanomedicine: Synthesis and characterization. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–28. [Google Scholar] [CrossRef]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Pectin co-functionalized dual layered solid lipid nanoparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr. Polym. 2021, 252, 117180. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef]
- Gunawan, M.; Ramadon, D.; Setio Putri, K.S.; Iswandana, R. Considerations in excipient selection for colon-targeted dosage forms. J. Appl. Pharm. Sci. 2024, 15, 063–085. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Dhara, M.; Al Hoque, A.; Sen, R.; Dutta, D.; Mukherjee, B.; Paul, B.; Laha, S. Phosphorothioated amino-AS1411 aptamer functionalized stealth nanoliposome accelerates bio-therapeutic threshold of apigenin in neoplastic rat liver: A mechanistic approach. J. Nanobiotechnol. 2023, 21, 28. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, C.; Cui, B.; Zhao, M.; Yu, B.; Guo, L.; Liu, P.; Fang, Y. Encapsulation of apigenin into β-cyclodextrin metal-organic frameworks with high embedment efficiency and stability. Food Chem. 2024, 443, 138543. [Google Scholar] [CrossRef]
- Hong, S.; Dia, V.P.; Baek, S.J.; Zhong, Q. Nanoencapsulation of apigenin with whey protein isolate: Physicochemical properties, in vitro activity against colorectal cancer cells, and bioavailability. LWT 2022, 154, 112751. [Google Scholar] [CrossRef]
- KM, A.S.; Angolkar, M.; Rahamathulla, M.; Thajudeen, K.Y.; Ahmed, M.M.; Farhana, S.A.; Shivanandappa, T.B.; Paramshetti, S.; Osmani, R.A.M.; Natarajan, J. Box-Behnken Design-Based Optimization and Evaluation of Lipid-Based Nano Drug Delivery System for Brain Targeting of Bromocriptine. Pharmaceuticals 2024, 17, 720. [Google Scholar] [CrossRef]
- Ding, B.; Chen, H.; Wang, C.; Zhai, Y.; Zhai, G. Preparation and In Vitro Evaluation of Apigenin Loaded Lipid Nanocapsules. J. Nanosci. Nanotechnol. 2013, 13, 6546–6552. [Google Scholar] [CrossRef]
- Subudhi, M.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S. Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- Nguyen, S.; Alund, S.J.; Hiorth, M.; Kjøniksen, A.-L.; Smistad, G. Studies on pectin coating of liposomes for drug delivery. Colloids Surf. B Biointerfaces 2011, 88, 664–673. [Google Scholar] [CrossRef]
- Das, S. Pectin based multi-particulate carriers for colon-specific delivery of therapeutic agents. Int. J. Pharm. 2021, 605, 120814. [Google Scholar] [CrossRef]
- Gutiérrez-Alvarado, K.; Chacón-Cerdas, R.; Starbird-Pérez, R. Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry 2022, 4, 121–136. [Google Scholar] [CrossRef]
- Kim, A.H.; Lee, Y.; Kim, E.; Ji, S.C.; Chung, J.-Y.; Cho, J.-Y. Assessment of Oral Vancomycin-Induced Alterations in Gut Bacterial Microbiota and Metabolome of Healthy Men. Front. Cell. Infect. Microbiol. 2021, 11, 629438. [Google Scholar] [CrossRef]
- Jafar, M.; Khalid, M.S.; Alghamdi, H.; Amir, M.; Al Makki, S.A.; Alotaibi, O.S.; Al Rmais, A.A.; Imam, S.S.; Alshehri, S.; Gilani, S.J. Formulation of Apigenin-Cyclodextrin-Chitosan Ternary Complex: Physicochemical Characterization, In Vitro and In Vivo Studies. AAPS PharmSciTech 2022, 23, 71. [Google Scholar] [CrossRef]
- Goleij, P.; Ferdousmakan, S.; Tabari, M.A.K.; Amini, A.; Larsen, D.S.; Daglia, M.; Javan, A.; Li, T.; Khan, H.; Xu, Y. Targeting drug resistant colorectal cancer with apigenin nanoarchitectures. Transl. Oncol. 2025, 59, 102455. [Google Scholar] [CrossRef]
- Haque, S.; Nawaz, M.; Rasool, N.; Abbas, G.; Ahmed, M.; Irfan, M.; Iqbal, M.; Nasar, A.; Choudhary, M.I. Expression and Functional Relevance of P-Glycoprotein (MDR1) in Drug Resistance: A qPCR-Based Study in Cancer Cell Lines. Molecules 2020, 25, 3405. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H. Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and In Vitro/In Vivo Evaluation. Int. J. Mol. Sci. 2015, 16, 2693–2710. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Platon, I.-V.; Lazar, M.M.; Dinu, M.V.; Aprotosoaie, A.C. Trends in polysaccharide-based hydrogels and their role in enhancing the bioavailability and bioactivity of phytocompounds. Carbohydr. Polym. 2024, 334, 122033. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Ma, Y.; Cui, X.; Chen, C.; Zhu, G.; Sun, Y.; Tong, L. Development of A Nanostructured Lipid Carrier-Based Drug Delivery Strategy for Apigenin: Experimental Design Based on CCD-RSM and Evaluation against NSCLC In Vitro. Molecules 2023, 28, 6668. [Google Scholar] [CrossRef]
- Sirisha Mulukuri, N.V.L.; Kumar, S.; Dhara, M.; Dheeraj Rajesh, G.; Kumar, P. Statistical modeling, optimization and characterization of andrographolide loaded emulgel for its therapeutic application on skin cancer through enhancing its skin permeability. Saudi Pharm. J. 2024, 32, 102068. [Google Scholar] [CrossRef]
- Huang, Y.; Zu, Y.; Zhao, X.; Wu, M.; Feng, Z.; Deng, Y.; Zu, C.; Wang, L. Preparation of inclusion complex of apigenin-hydroxypropyl-β-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int. J. Pharm. 2016, 511, 921–930. [Google Scholar] [CrossRef]
- Wu, W.; Zu, Y.; Zhao, X.; Zhang, X.; Wang, L.; Li, Y.; Wang, L.; Zhang, Y.; Lian, B. Solubility and dissolution rate improvement of the inclusion complex of apigenin with 2-hydroxypropyl-β-cyclodextrin prepared using the liquid antisolvent precipitation and solvent removal combination methods. Drug Dev. Ind. Pharm. 2017, 43, 1366–1377. [Google Scholar] [CrossRef]
- Al Hoque, A.; Dutta, D.; Paul, B.; Kumari, L.; Ehsan, I.; Dhara, M.; Mukherjee, B.; Quadir, M.; Kaipparettu, B.A.; Laha, S.; et al. ΔPSap4#5 surface-functionalized abiraterone-loaded nanoparticle successfully inhibits carcinogen-induced prostate cancer in mice: A mechanistic investigation. Cancer Nanotechnol. 2023, 14, 73. [Google Scholar] [CrossRef]
- Mulukuri, N.V.L.S.; Dhara, M.; Gupta, D.; Devi, K.; Kumar, P. Development and Optimization of Novel Emulgel Loaded with Andrographolide-Rich Extract and Sesame Oil Using Quality by Design Approach: In Silico and In Vitro Cytotoxic Evaluation against A431 Cells. Gels 2023, 9, 507. [Google Scholar] [CrossRef]
- Chiani, M.; Norouzian, D.; Shokrgozar, M.A.; Azadmanesh, K.; Najmafshar, A.; Mehrabi, M.R.; Akbarzadeh, A. Folic acid conjugated nanoliposomes as promising carriers for targeted delivery of bleomycin. Artif. Cells Nanomed. Biotechnol. 2018, 46, 757–763. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Meng, X.; Fryganas, C.; Fogliano, V.; Hoppenbrouwers, T. Double-coated nanoliposomes improve the bioavailability of flavanone hesperetin. Food Hydrocoll. 2024, 151, 109872. [Google Scholar] [CrossRef]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Bains, A.; Ali, N.; Shang, Z.; Patil, A.; Patil, S. Exploring Anticancer Potential of Lactobacillus Strains: Insights into Cytotoxicity and Apoptotic Mechanisms on HCT 115 Cancer Cells. Biol. Targets Ther. 2024, 18, 285–295. [Google Scholar] [CrossRef]
- Shin, H.J.; Kwak, M.; Joo, S.; Lee, J.Y. Quantifying fluorescent nanoparticle uptake in mammalian cells using a plate reader. Sci. Rep. 2022, 12, 20146. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Silva, A.M. Natural Products as a Tool to Modulate the Activity and Expression of Multidrug Resistance Proteins of Intestinal Barrier. J. Xenobiot. 2023, 13, 172–192. [Google Scholar] [CrossRef]
- Silic-Benussi, M.; Sharova, E.; Corradin, A.; Urso, L.; Raimondi, V.; Cavallari, I.; Buldini, B.; Francescato, S.; Minuzzo, S.A.; D’Agostino, D.M.; et al. Repurposing Verapamil to Enhance Killing of T-ALL Cells by the mTOR Inhibitor Everolimus. Antioxidants 2023, 12, 625. [Google Scholar] [CrossRef]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.-W.; Park, B.J.; Han, H.-K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef]



| Variable (Independent) | Level Followed | ||
|---|---|---|---|
| (−1) | 0 | (+1) | |
| β-Cyclodextrin (mg) | 40 | 60 | 80 |
| Phospholipid (mg) | 60 | 70 | 80 |
| Methylated Pectin (%) | 0.5 | 0 | 1 |
| Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|
| Run | A: β-Cyclodextrin (mg) | B: Phospholipid (mg) | C: Methylated Pectin (%) |
| 1 | 60 | 60 | 0.5 |
| 2 | 60 | 80 | 0.5 |
| 3 | 60 | 70 | 0.5 |
| 4 | 40 | 80 | 1 |
| 5 | 40 | 60 | 1 |
| 6 | 80 | 60 | 0 |
| 7 | 40 | 70 | 0.5 |
| 8 | 60 | 70 | 1 |
| 9 | 80 | 80 | 1 |
| 10 | 80 | 60 | 1 |
| 11 | 40 | 60 | 0 |
| 12 | 80 | 80 | 0 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 | Response 6 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Std | Run | A: β-Cyclodextrin | B: Phospholipid | C: Methylated Pectin | Drug Loading | Particle Size | Zeta Potential | In Vitro Drug Release in Gastric Simulated pH (at 4 h) | In Vitro Drug Release in Intestine Simulated pH in Absence of Pectinase (Between 4 and 8 h) | In Vitro Drug Release in Intestine Simulated pH in Presence of Pectinase |
| mg | Mg | % | % | nm | mV | % | % | % | ||
| 11 | 1 | 60 | 60 | 0.5 | 84 | 319 | −6.11 | 24 | 54 | 72 |
| 12 | 2 | 60 | 80 | 0.5 | 85 | 353 | −8.5 | 28 | 46 | 74 |
| 17 | 3 | 60 | 70 | 0.5 | 86 | 315 | −7.11 | 25 | 47 | 72 |
| 7 | 4 | 40 | 80 | 1 | 82 | 632 | −26 | 13 | 49 | 62 |
| 5 | 5 | 40 | 60 | 1 | 79 | 611 | −32 | 11 | 54 | 65 |
| 2 | 6 | 80 | 60 | 0 | 91 | 155 | 0.2 | 45 | 44 | 41 |
| 9 | 7 | 40 | 70 | 0.5 | 82 | 320 | −8.4 | 19 | 62 | 74 |
| 14 | 8 | 60 | 70 | 1 | 88 | 697 | −24.1 | 10 | 51 | 61 |
| 8 | 9 | 80 | 80 | 1 | 93 | 721 | −22 | 12 | 58 | 70 |
| 6 | 10 | 80 | 60 | 1 | 90 | 770 | −19 | 11 | 57 | 68 |
| 1 | 11 | 40 | 60 | 0 | 81 | 151 | 0.12 | 44 | 40 | 43 |
| 4 | 12 | 80 | 80 | 0 | 92 | 211 | 0.3 | 49 | 40 | 41 |
| 10 | 13 | 80 | 70 | 0.5 | 95 | 313 | −9.5 | 22 | 65 | 75 |
| 15 | 14 | 60 | 70 | 0.5 | 87 | 320 | −6.9 | 22 | 54 | 74 |
| 16 | 15 | 60 | 70 | 0.5 | 83 | 321 | −7.23 | 21 | 52 | 73 |
| 3 | 16 | 40 | 80 | 0 | 82 | 244 | −0.012 | 61 | 31 | 34 |
| 13 | 17 | 60 | 70 | 0 | 84 | 158 | 0.231 | 51 | 37 | 39 |
| (a) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Drug Loading (Y1) | Particle Size (Y2) | Zeta Potential (Y3) | |||||||||||||||
| Sum of Squares | df | Mean Square | F-Value | p-Value | Sum of Squares | df | Mean Square | F-Value | p-Value | Sum of Squares | df | Mean Square | F-Value | p-Value | ||||
| Model | 11,925.26 | 9 | 1325.03 | 6.66 | 0.0103 | significant | 7.051 × 105 | 9 | 78,345.51 | 216.24 | <0.0001 | significant | 1696.84 | 9 | 188.54 | 47.73 | <0.0001 | significant |
| A-β-Cyclodextrin | 4724.54 | 1 | 4724.54 | 23.74 | 0.0018 | 4494.40 | 1 | 4494.40 | 12.40 | 0.0097 | 26.54 | 1 | 26.54 | 6.72 | 0.0358 | |||
| B-Phospholipid | 2351.93 | 1 | 2351.93 | 11.82 | 0.0109 | 2402.50 | 1 | 2402.50 | 6.63 | 0.0367 | 0.0334 | 1 | 0.0334 | 0.0085 | 0.9293 | |||
| C-Methylated Pectin | 0.4000 | 1 | 0.4000 | 0.0020 | 0.9655 | 6.310 × 105 | 1 | 6.3 × 105 | 1741.62 | <0.0001 | 1536.09 | 1 | 1536.09 | 388.91 | <0.0001 | |||
| AB | 3295.10 | 1 | 3295.10 | 16.55 | 0.0048 | 1431.13 | 1 | 1431.13 | 3.95 | 0.0872 | 9.61 | 1 | 9.61 | 2.43 | 0.1628 | |||
| AC | 0.5000 | 1 | 0.5000 | 0.0025 | 0.9614 | 9591.13 | 1 | 9591.13 | 26.47 | 0.0013 | 34.48 | 1 | 34.48 | 8.73 | 0.0213 | |||
| BC | 2.00 | 1 | 2.00 | 0.0100 | 0.9230 | 3916.13 | 1 | 3916.13 | 10.81 | 0.0133 | 1.15 | 1 | 1.15 | 0.2909 | 0.6063 | |||
| A2 | 85.79 | 1 | 85.79 | 0.4310 | 0.5325 | 53.07 | 1 | 53.07 | 0.1465 | 0.7133 | 3.78 | 1 | 3.78 | 0.9566 | 0.3606 | |||
| B2 | 249.94 | 1 | 249.94 | 1.26 | 0.2994 | 606.80 | 1 | 606.80 | 1.67 | 0.2367 | 0.5607 | 1 | 0.5607 | 0.1419 | 0.7175 | |||
| C2 | 178.34 | 1 | 178.34 | 0.8960 | 0.3754 | 30,416.81 | 1 | 30,416.81 | 83.95 | <0.0001 | 46.63 | 1 | 46.63 | 11.81 | 0.0109 | |||
| (b) | ||||||||||||||||||
| Factors | In Vitro Drug Release in gastric simulated pH (at 4 h) (Y4) | In Vitro Drug Release in intestine simulated pH in absence of pectinase (between 4 and 8 h) (Y5) | In Vitro Drug Release in intestine simulated pH in presence of pectinase (Y6) | |||||||||||||||
| Sum of Squares | df | Mean Square | F-value | p-value | Sum of Squares | df | Mean Square | F-value | p-value | Sum of Squares | df | Mean Square | F-value | p-value | ||||
| Model | 4163.68 | 9 | 462.63 | 69.57 | <0.0001 | significant | 1258.41 | 9 | 139.82 | 15.34 | 0.0008 | significant | 3548.38 | 9 | 394.26 | 112.37 | <0.0001 | significant |
| A-β-Cyclodextrin | 8.10 | 1 | 8.10 | 1.22 | 0.3062 | 78.40 | 1 | 78.40 | 8.60 | 0.0220 | 28.90 | 1 | 28.90 | 8.24 | 0.0240 | |||
| B-Phospholipid | 78.40 | 1 | 78.40 | 11.79 | 0.0109 | 62.50 | 1 | 62.50 | 6.85 | 0.0345 | 6.40 | 1 | 6.40 | 1.82 | 0.2189 | |||
| C-Methylated Pectin | 3724.90 | 1 | 3724.90 | 560.13 | <0.0001 | 592.90 | 1 | 592.90 | 65.03 | <0.0001 | 1638.40 | 1 | 1638.40 | 466.96 | <0.0001 | |||
| AB | 24.50 | 1 | 24.50 | 3.68 | 0.0964 | 15.13 | 1 | 15.13 | 1.66 | 0.2387 | 24.50 | 1 | 24.50 | 6.98 | 0.0333 | |||
| AC | 12.50 | 1 | 12.50 | 1.88 | 0.2127 | 0.1250 | 1 | 0.1250 | 0.0137 | 0.9101 | 4.50 | 1 | 4.50 | 1.28 | 0.2947 | |||
| BC | 40.50 | 1 | 40.50 | 6.09 | 0.0430 | 10.13 | 1 | 10.13 | 1.11 | 0.3270 | 8.00 | 1 | 8.00 | 2.28 | 0.1748 | |||
| A2 | 17.12 | 1 | 17.12 | 2.58 | 0.1526 | 217.02 | 1 | 217.02 | 23.80 | 0.0018 | 11.72 | 1 | 11.72 | 3.34 | 0.1103 | |||
| B2 | 23.66 | 1 | 23.66 | 3.56 | 0.1012 | 54.25 | 1 | 54.25 | 5.95 | 0.0448 | 0.9375 | 1 | 0.9375 | 0.2672 | 0.6211 | |||
| C2 | 149.58 | 1 | 149.58 | 22.49 | 0.0021 | 295.39 | 1 | 295.39 | 32.40 | 0.0007 | 1345.35 | 1 | 1345.35 | 383.44 | <0.0001 | |||
| Pharmacokinetics Parameter | Free Apigenin | Plain Apigenin Nanoliposomes | (Api-Cy-13) Apigenin-Cyclodextrin Complex Encapsulated Nano Liposomes with Pectin Coating |
|---|---|---|---|
| Cmax (ng/mL) | 8.7 ± 0.54 | 24.2 ± 0.53 | 33.2 ± 0.65 |
| Tmax (h) | 3.0 ± 0.4 | 4.0 ± 0.5 | 6.0 ± 0.25 |
| AUC 0-last (ng·h/mL) | 30.3 ± 2.7 | 120.1 ± 7.9 | 199.85 ± 6.90 |
| T1/2 (h) | 0.86 ± 0.08 | 1.50 ± 0.15 | 1.94 ± 0.07 |
| MRT (h) | 3.22 ± 0.25 | 4.27 ± 0.30 | 5.56 ± 0.29 |
| AUMC (ng·h2/mL) | 97.5 ± 8.4 | 512.3 ± 47.9 | 1111.1 ± 44.0 |
| AUC 0-∞ (ng·h/mL) | 31.05 ± 2.6 | 124.85 ± 9.0 | 225.91 ± 13.4 |
| Clearance (L/h/kg) (dose = 1) | 0.032 ± 0.003 | 0.008 ± 0.001 | 0.0044 ± 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhara, M.; Vemula, K.D.; Karim, Z.; Vadakkepushpakath, A.N.; Shetty, T.; Munchinamane, A.P. Oral Delivery of a GI-Stable Apigenin–Cyclodextrin Complex via Pectin-Coated Nanoliposomes In Situ Gel: A DoE-Optimized Targeted Colon Cancer Therapy by Modulating Gut Drug Sensitivity. Gels 2025, 11, 873. https://doi.org/10.3390/gels11110873
Dhara M, Vemula KD, Karim Z, Vadakkepushpakath AN, Shetty T, Munchinamane AP. Oral Delivery of a GI-Stable Apigenin–Cyclodextrin Complex via Pectin-Coated Nanoliposomes In Situ Gel: A DoE-Optimized Targeted Colon Cancer Therapy by Modulating Gut Drug Sensitivity. Gels. 2025; 11(11):873. https://doi.org/10.3390/gels11110873
Chicago/Turabian StyleDhara, Moumita, Kusum Devi Vemula, Ziaul Karim, Anoop Narayanan Vadakkepushpakath, Tanvi Shetty, and Anushree Prakasha Munchinamane. 2025. "Oral Delivery of a GI-Stable Apigenin–Cyclodextrin Complex via Pectin-Coated Nanoliposomes In Situ Gel: A DoE-Optimized Targeted Colon Cancer Therapy by Modulating Gut Drug Sensitivity" Gels 11, no. 11: 873. https://doi.org/10.3390/gels11110873
APA StyleDhara, M., Vemula, K. D., Karim, Z., Vadakkepushpakath, A. N., Shetty, T., & Munchinamane, A. P. (2025). Oral Delivery of a GI-Stable Apigenin–Cyclodextrin Complex via Pectin-Coated Nanoliposomes In Situ Gel: A DoE-Optimized Targeted Colon Cancer Therapy by Modulating Gut Drug Sensitivity. Gels, 11(11), 873. https://doi.org/10.3390/gels11110873







