Optimizing Gelatin Methacryloyl for Craniofacial Muscle Regeneration: Material Design and Application
Abstract
1. Introduction
2. Results and Discussion
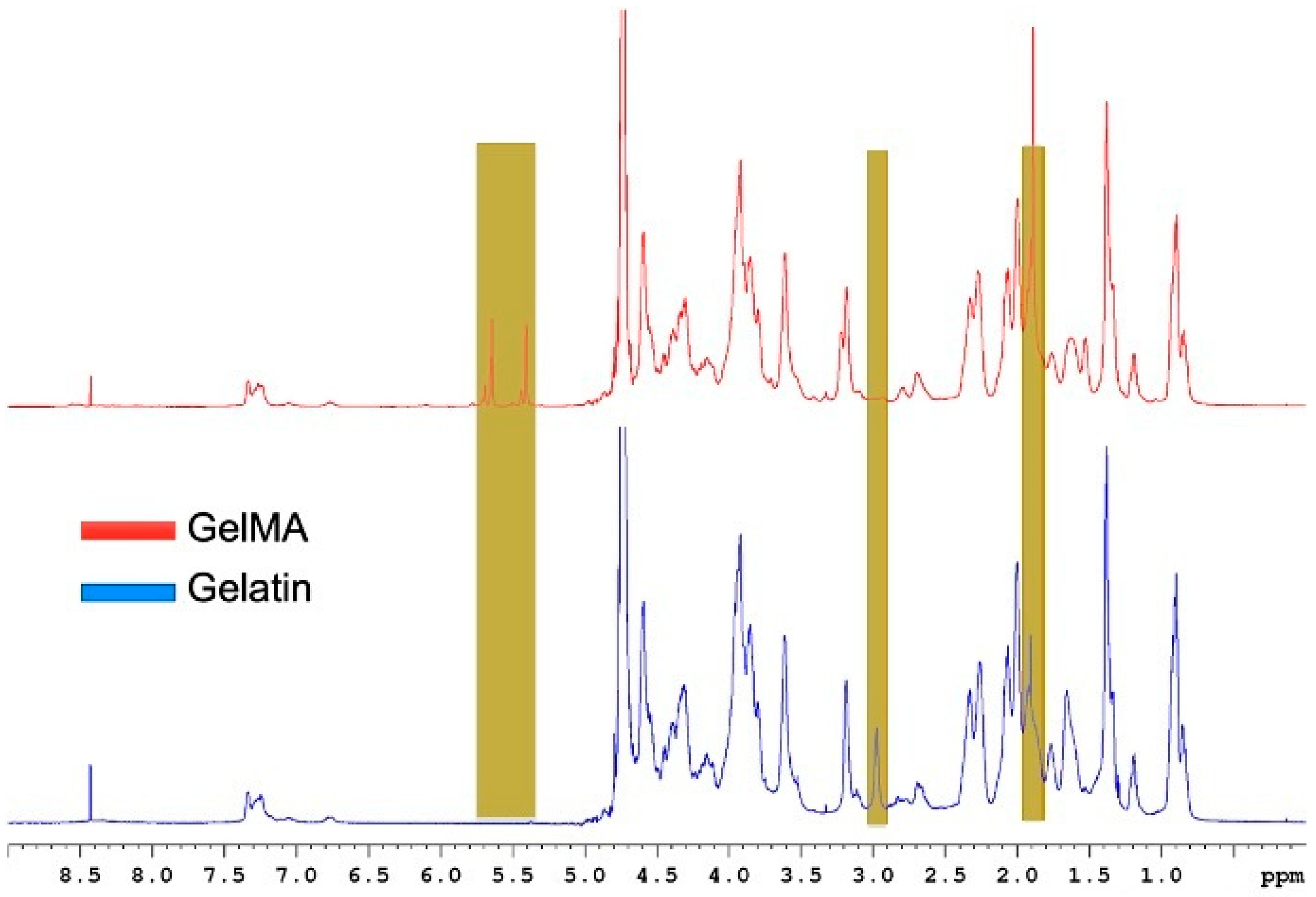
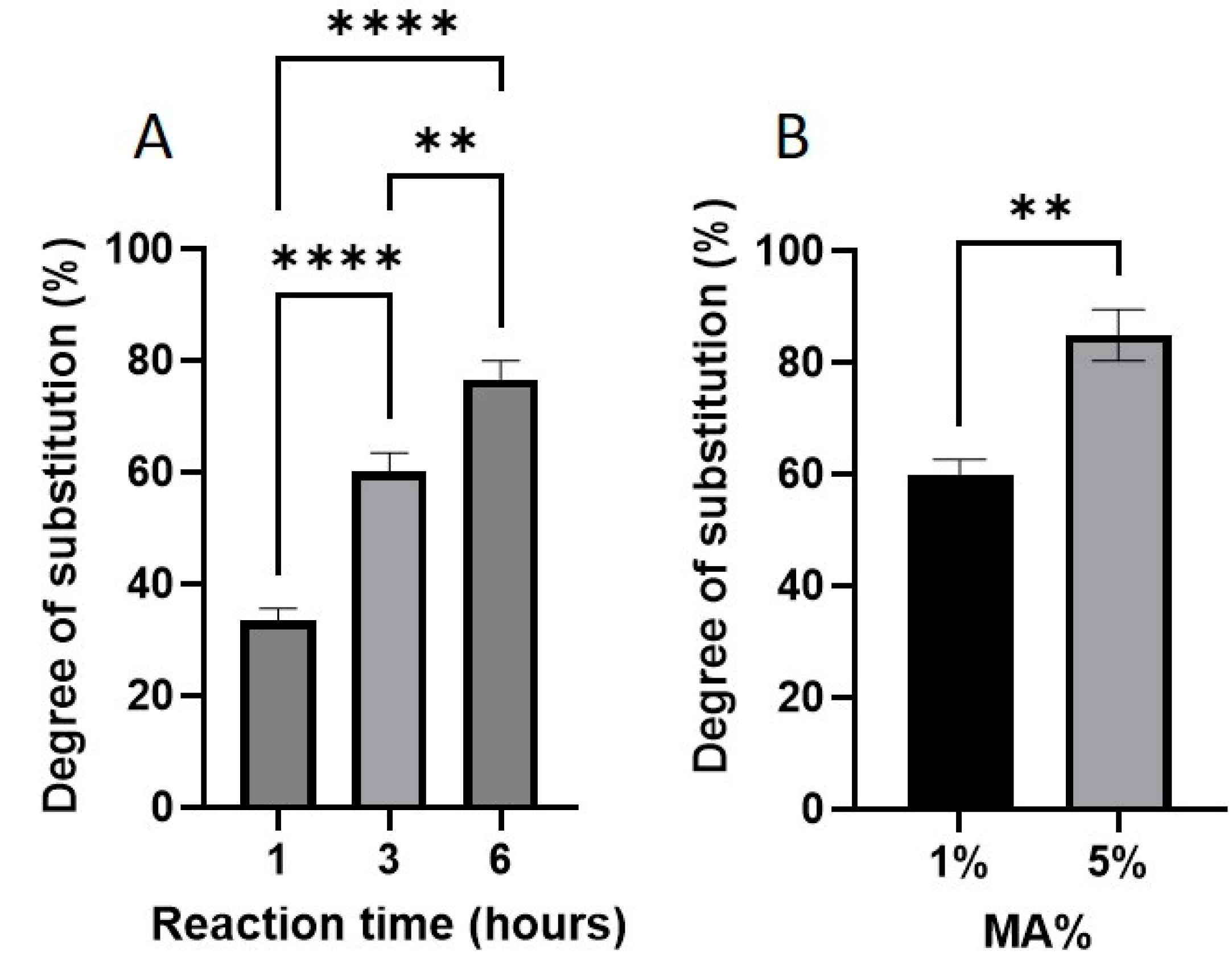
2.1. Degree of Substitution by 1H-NMR
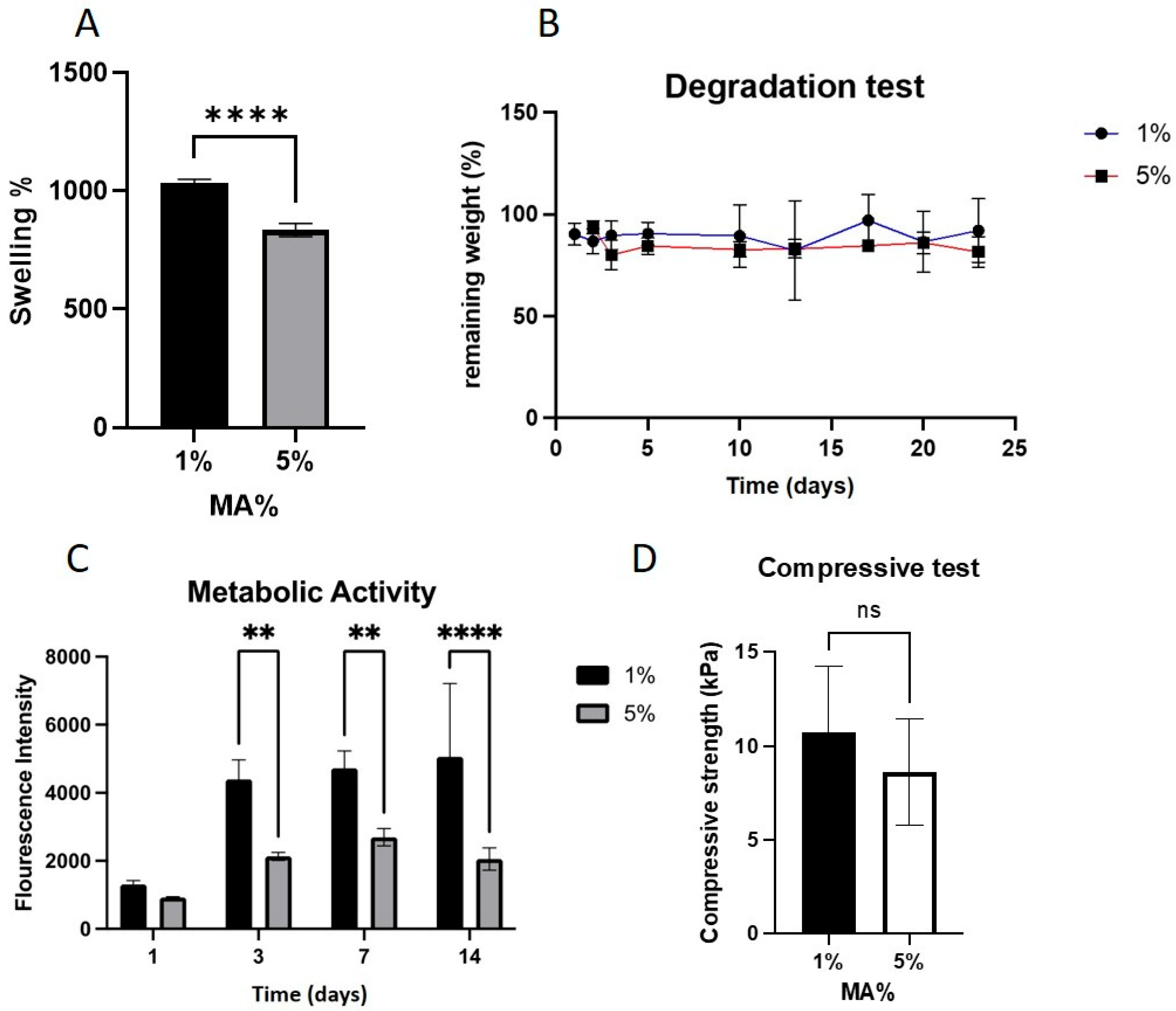
2.2. MA Concentration
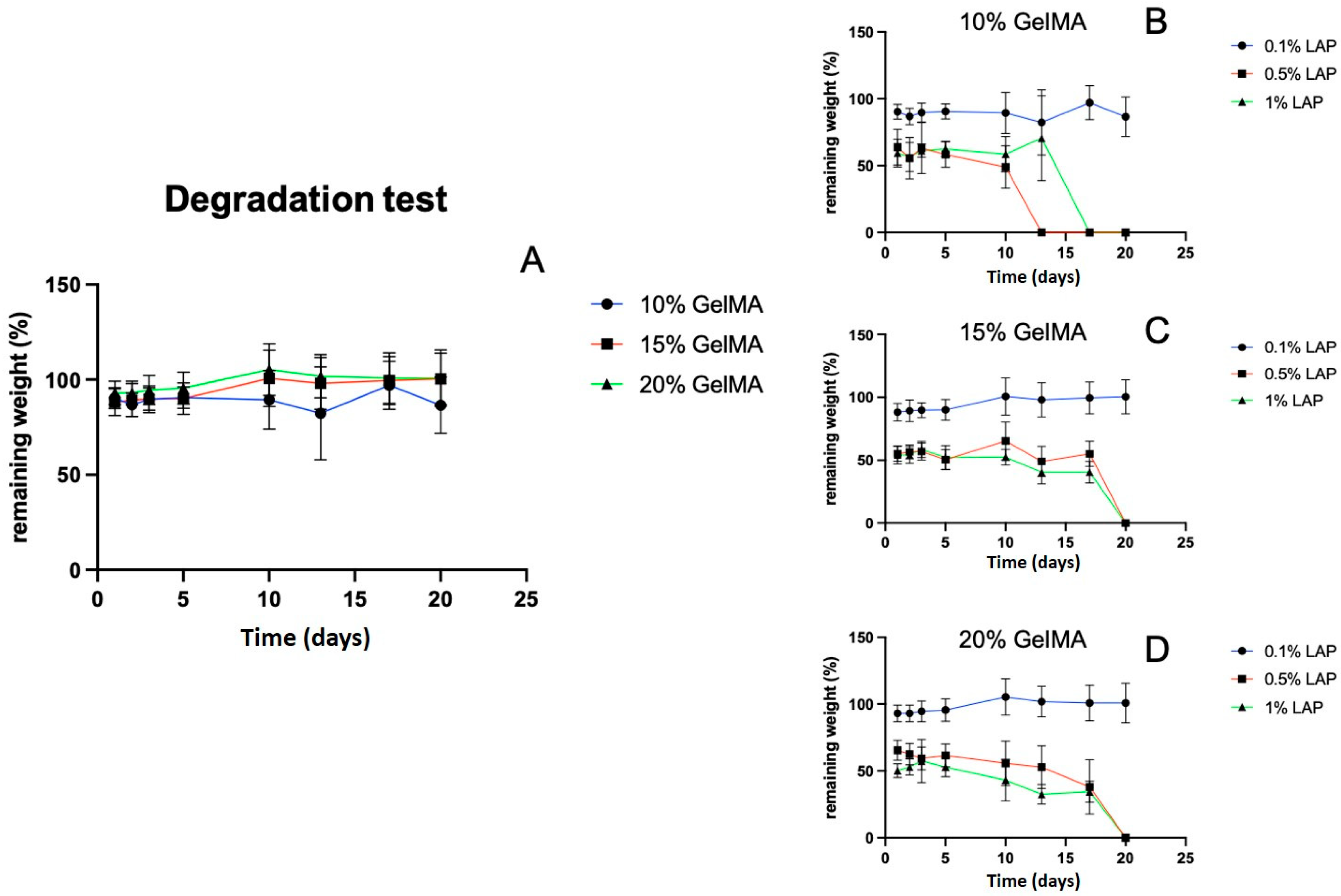
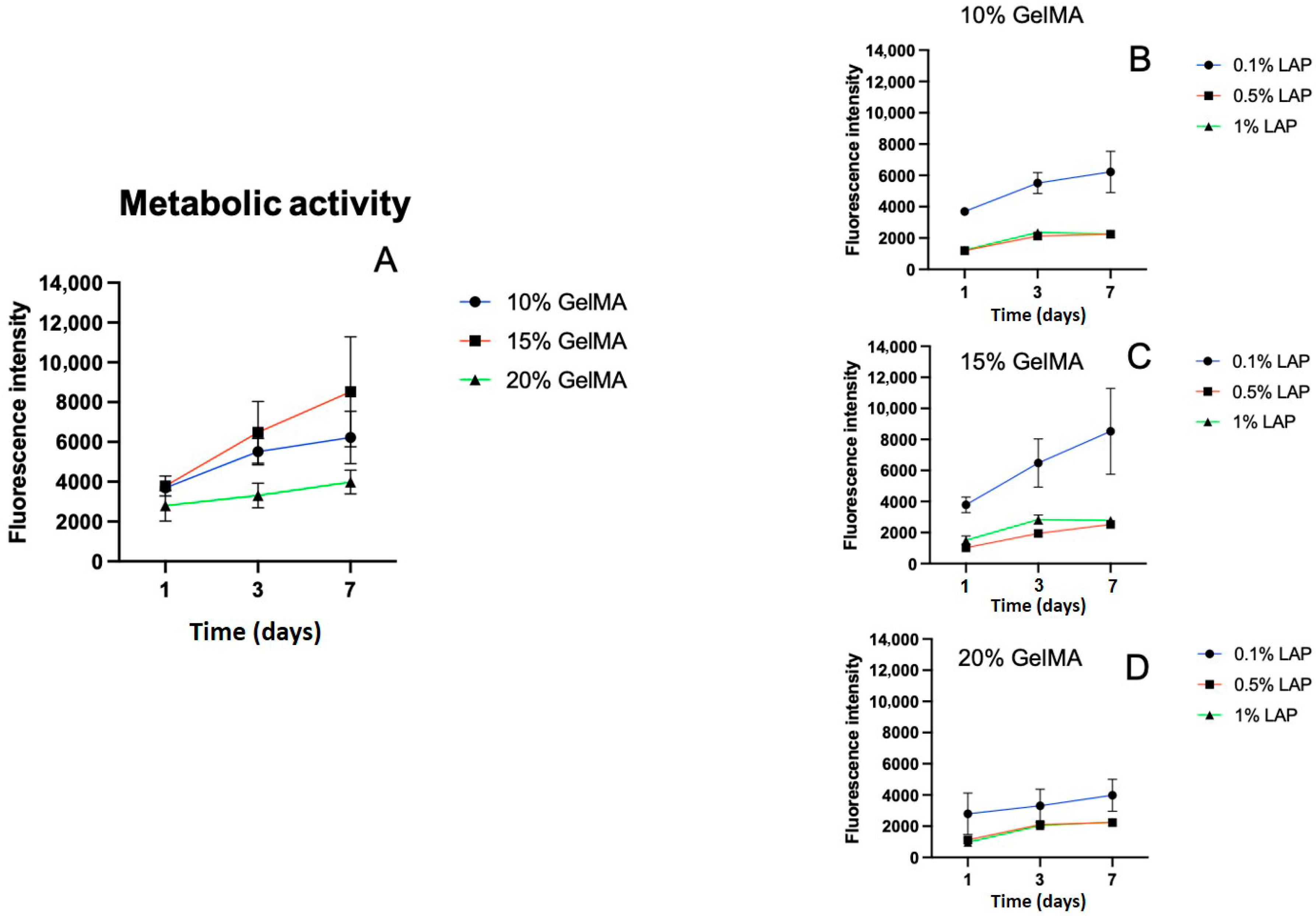
2.3. GelMA and LAP Concentrations
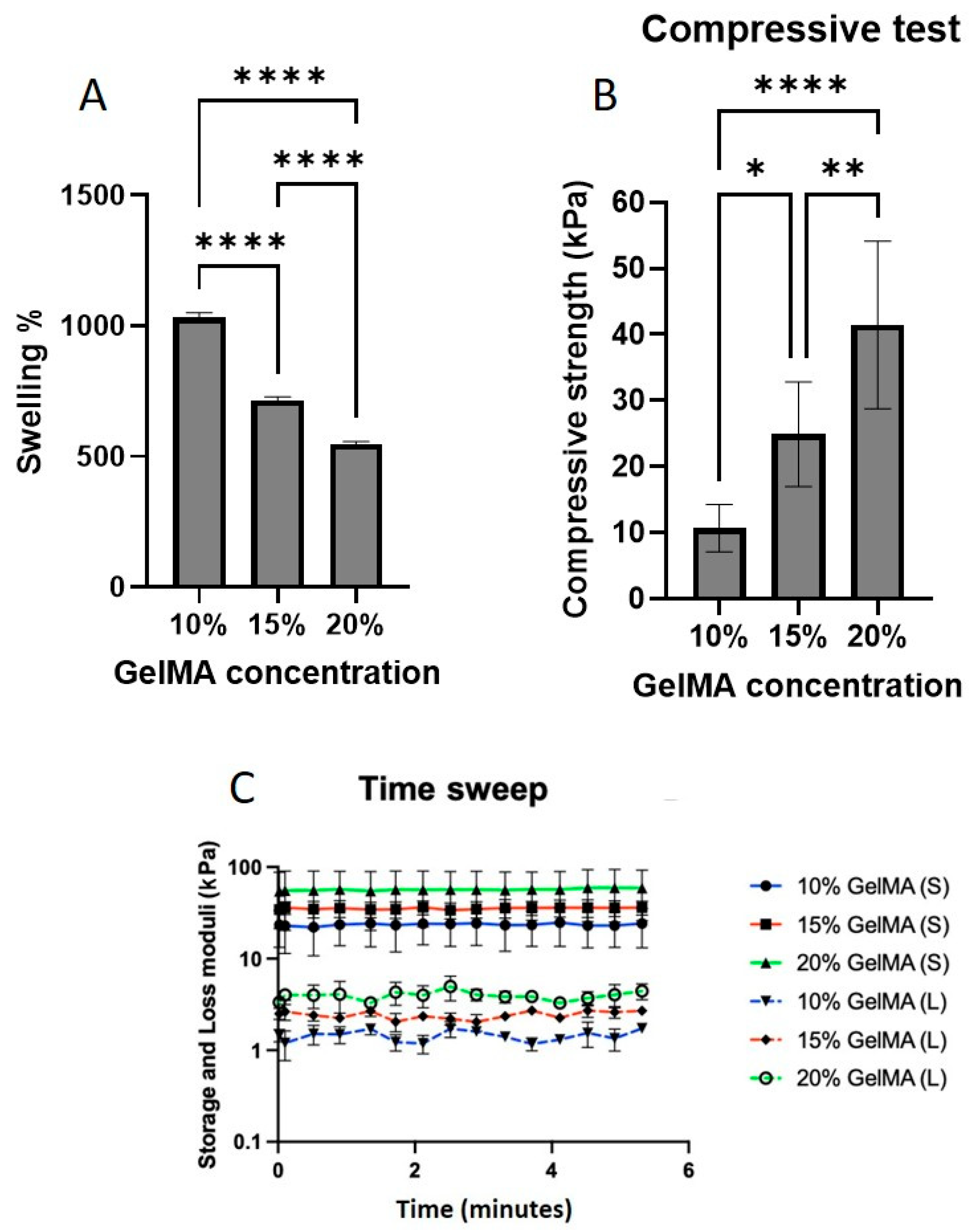
2.4. UV Exposure Time
3. Conclusions
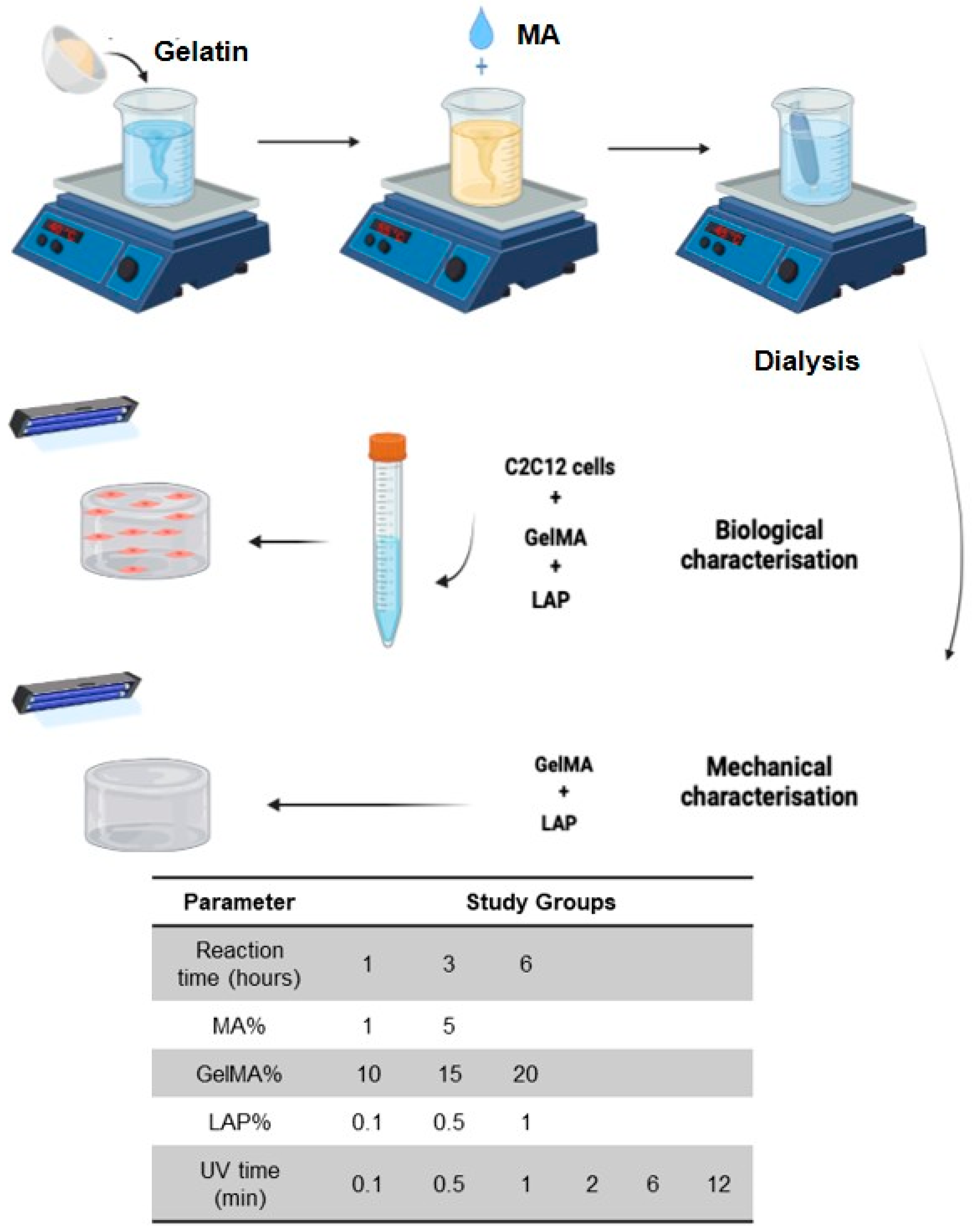
4. Materials and Methods
4.1. GelMA Synthesis
4.2. Proton-Nuclear Magnetic Resonance Characterization
4.3. Hydrogel Preparation and UV Crosslinking
4.4. Swelling Test
4.5. Mechanical Testing
4.6. Degradation in PBS
4.7. Gel Sterilization and 3D Cell Culture
4.8. Cell Metabolic Activity
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GelMA | Gelatin Methacryloyl |
| MA | Methacrylic Anhydride |
| DS | Degree of Substitution |
| PBS | Phosphate-Buffered Saline |
| LAP | lithium phenyl-2 4 6-trimethyl-benzoyl phosphinate |
| UV | Ultraviolet |
| 1H NMR | Proton Nuclear Magnetic Resonance |
References
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the mechanical properties of gelatin methacryloyl hydrogels through manipulation of the photocrosslinking conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef]
- Zhou, M.; Lee, B.H.; Tan, L.P. A dual crosslinking strategy to tailor rheological properties of gelatin methacryloyl. Int. J. Bioprint. 2017, 3, 130–137. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, K.; Lee, H.J. Effect of GelMA hydrogel properties on long-term encapsulation and myogenic differentiation of C2C12 spheroids. Gels 2023, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 42186. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.; et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci. Rep. 2014, 4, 4271. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.P.; Lwin, N.C.; Adnan, K.; Mehta, J.S. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Basara, G.; Yue, X.; Zorlutuna, P. Dual crosslinked gelatin methacryloyl hydrogels for photolithography and 3D printing. Gels 2019, 5, 34. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 14887. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ahadian, S.; Ramón-Azcón, J.; Hosseini, V.; Fujie, T.; Parthiban, S.P.; Shiku, H.; Matsue, T.; Kaji, H.; Ramalingam, M.; et al. Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 2017, 11, 582–595. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C 2020, 109, 110578. [Google Scholar] [CrossRef]
- Stratesteffen, H.; Köpf, M.; Kreimendahl, F.; Blaeser, A.; Jockenhövel, S.; Fischer, H. GelMA-collagen blends enable drop-on-demand 3D printability and promote angiogenesis. Biofabrication 2017, 9, 045004. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, M.; Ferracci, G.; Cho, N.J.; Lee, B.H. Hydrolytic stability of methacrylamide and methacrylate in gelatin methacryloyl and decoupling of gelatin methacrylamide from gelatin methacryloyl through hydrolysis. Macromol. Chem. Phys. 2018, 219, 1800266. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Çelebi-Saltik, B.; Barros, N.; Nasiri, R.; Banton, E.; Shamloo, A.; Ashammakhi, N.; Dokmeci, M.; Ahadian, S. Three-dimensional bioprinting of functional skeletal muscle tissue using gelatin methacryloyl-alginate bioinks. Micromachines 2019, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Aldana, A.A.; Malatto, L.; Ur Rehman, M.A.; Boccaccini, A.R.; Abraham, G.A. Fabrication of gelatin methacrylate (Gel-MA) scaffolds with nano- and micro-topographical and morphological features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Hasirci, V. Cell-loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Keskin-Erdogan, Z.; Patel, K.D.; Chau, D.Y.S.; Day, R.M.; Kim, H.W.; Knowles, J.C. Utilization of GelMA with phosphate glass fibers for glial cell alignment. J. Biomed. Mater. Res. A 2021, 109, 2212–2224. [Google Scholar] [CrossRef]
- Costantini, M.; Testa, S.; Fornetti, E.; Barbetta, A.; Trombetta, M.; Cannata, S.M.; Gargioli, C.; Rainer, A. Engineering muscle networks in 3D gelatin methacryloyl hydrogels: Influence of mechanical stiffness and geometrical confinement. Front. Bioeng. Biotechnol. 2017, 5, 22. [Google Scholar] [CrossRef]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.O.; Afewerki, S.; de Paula, M.M.M.; Ghannadian, P.; Marciano, F.R.; Zhang, Y.S.; Webster, T.J.; Khademhosseini, A. Electrospun nanofiber blend with improved mechanical and biological performance. Int. J. Nanomed. 2018, 13, 7891–7903. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yao, Y.; Wong, S.; Bian, L.; Mak, A.F.T. Change in viability of C2C12 myoblasts under compression, shear and oxidative challenges. J. Biomech. 2016, 49, 1305–1310. [Google Scholar] [CrossRef]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological properties of coordinated physical gelation and chemical cross-linking in gelatin methacryloyl (GelMA) hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef]
- Claaßen, C.; Claaßen, M.H.; Truffault, V.; Sewald, L.; Tovar, G.E.M.; Borchers, K. Quantification of substitution of gelatin methacryloyl: Best practice and current pitfalls. Biomacromolecules 2018, 19, 42–52. [Google Scholar] [CrossRef]
- Quint, J.P.; Samandari, M.; Abbasi, L.; Mollocana, E.; Rinoldi, C.; Mostafavi, A.; Tamayol, A. Nanoengineered myogenic scaffolds for skeletal muscle tissue engineering. Nanoscale 2014, 3, 797–814. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.; Alvarez, M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. A 2018, 106, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Aljaber, M.B.; Verisqa, F.; Keskin-Erdogan, Z.; Patel, K.D.; Chau, D.Y.S.; Knowles, J.C. Influence of gelatin source and Bloom number on gelatin methacryloyl hydrogels mechanical and biological properties for muscle regeneration. Biomolecules 2023, 13, 811. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Aljaber, M.B.S. The Utilisation of GelMA-Based Surface-Patterned Scaffolds for Craniofacial Muscle Regeneration Applications; University College London: London, UK, 2024. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaber, M.B.; Alageel, O.; Chau, D.Y.S.; Knowles, J.C. Optimizing Gelatin Methacryloyl for Craniofacial Muscle Regeneration: Material Design and Application. Gels 2025, 11, 945. https://doi.org/10.3390/gels11120945
Aljaber MB, Alageel O, Chau DYS, Knowles JC. Optimizing Gelatin Methacryloyl for Craniofacial Muscle Regeneration: Material Design and Application. Gels. 2025; 11(12):945. https://doi.org/10.3390/gels11120945
Chicago/Turabian StyleAljaber, Mohammad B., Omar Alageel, David Y. S. Chau, and Jonathan C. Knowles. 2025. "Optimizing Gelatin Methacryloyl for Craniofacial Muscle Regeneration: Material Design and Application" Gels 11, no. 12: 945. https://doi.org/10.3390/gels11120945
APA StyleAljaber, M. B., Alageel, O., Chau, D. Y. S., & Knowles, J. C. (2025). Optimizing Gelatin Methacryloyl for Craniofacial Muscle Regeneration: Material Design and Application. Gels, 11(12), 945. https://doi.org/10.3390/gels11120945




