Enhancing Solid-State Li-Ion Batteries with MOF–Polymer Composite Electrolytes—Effect Mechanisms and Interface Engineering
Abstract
1. Introduction
2. MOF-Polymer Composite Electrolytes (MPCEs)
2.1. Design and Synthesis Strategies of MPCEs
2.2. Effect Mechanisms of MOF in MPCEs
2.2.1. Ion Transport Regulation
| MPCEs | Ionic Conductivity (σ) (S cm−1) | Lithium Ion Transference Number (tLi+) | Ref. |
|---|---|---|---|
| PVDF-HFP@ZIF-8@PVPG | 0.55 × 10−3 (22 °C) | 0.87 | [35] |
| PVDF-HFP-Cu-MOF-74 | 7.9 × 10−4 (25 °C) | 0.69 | [36] |
| PVDF-HFP-Ni,Go-MOF | 0.68 × 10−3 (25 °C) | 0.49 | [37] |
| ZIF-8/polyether (F127) | 0.74 × 10−4 (30 °C) | 0.58 | [38] |
| PVDF-HFP-UIO-66 | 6.9 × 10−4 (25 °C) | 0.59 | [39] |
| PVDF-HFP-UIO-66 | 3.37 × 10−4 (25 °C) | 0.90 | [40] |
| PVDF-HFP-ZIF-8 | 0.46× 10−3 (25 °C) | 0.74 | [41] |
| PAN-HKUST-1 | 2.40 × 10−3 (25 °C) | 0.698 | [42] |
| PDMA-MOF-808 | 10–4 (25 °C) | 0.77 | [33] |
| PVDF-HFP-UiO-66 | 5.55 × 10–4 (25 °C) | 0.52 | [43] |
| PolyDOL (PDOL)-fluorinated-UIO66 | 3.96 × 10–4 (25 °C) | 0.65 | [44] |
| PEO-UIO-66@67 | 9.2 × 10−4 (25 °C) | 0.74 | [45] |
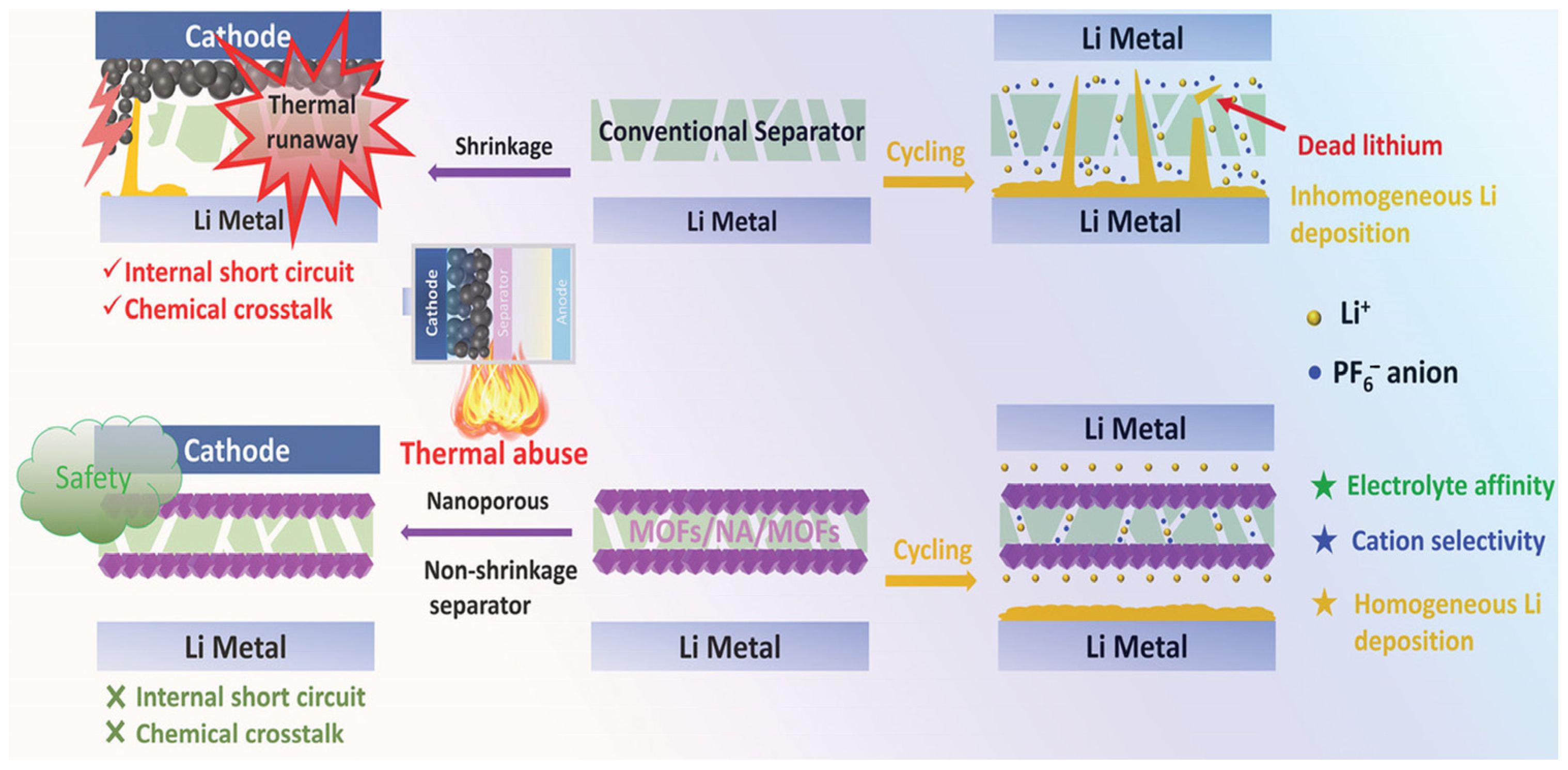
2.2.2. Dendrite Suppression
2.2.3. Electrochemical Stability Window
2.2.4. Optimize Solid Electrolyte Interphase (SEI) Layer
3. Interface Engineering Using MPCEs
3.1. Interfacial Challenges in SSBs
3.1.1. Fundamental Challenges for the Cathode–CPE Interface
3.1.2. Basic Challenges for the Li Metal Anode–CPE Interface
3.2. Interfacial Engineering Strategies for MPCEs
3.2.1. In Situ Polymerization
3.2.2. In Situ Growth of MOFs in Polymer Matrices
3.2.3. Gel-like Ionic Conductor
3.2.4. Composite Cathodes (CC) Design
4. Current Challenges and Limitations
5. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.-K.; Zhao, C.-Z.; Du, J.; Zhang, X.-Q.; Chen, A.-B.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, 2205315. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced Liquid Electrolytes for Rechargeable Li Metal Batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.-C. Designing Nonflammable Liquid Electrolytes for Safe Li-Ion Batteries. Adv. Mater. 2025, 37, 2312451. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Zeng, X.-X.; Zhang, X.-D.; Zuo, T.-T.; Yan, M.; Yin, Y.-X.; Shi, J.-L.; Wu, X.-W.; Guo, Y.-G.; Wan, L.-J. Engineering Janus Interfaces of Ceramic Electrolyte via Distinct Functional Polymers for Stable High-Voltage Li-Metal Batteries. J. Am. Chem. Soc. 2019, 141, 9165–9169. [Google Scholar] [CrossRef]
- Shen, H.; Yi, E.; Heywood, S.; Parkinson, D.Y.; Chen, G.; Tamura, N.; Sofie, S.; Chen, K.; Doeff, M.M. Scalable Freeze-Tape-Casting Fabrication and Pore Structure Analysis of 3D LLZO Solid-State Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Cretu, S.; Bradley, D.G.; Feng, L.P.W.; Kudu, O.U.; Nguyen, L.L.; Nguyen, T.T.; Jamali, A.; Chotard, J.-N.; Seznec, V.; Hanna, J.V.; et al. The Impact of Intergrain Phases on the Ionic Conductivity of the LAGP Solid Electrolyte Material Prepared by Spark Plasma Sintering. ACS Appl. Mater. Interfaces 2023, 15, 39186–39197. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Mallaiah, Y.; Jeedi, V.R.; Swarnalatha, R.; Raju, A.; Narender Reddy, S.; Sadananda Chary, A. Impact of polymer blending on ionic conduction mechanism and dielectric properties of sodium based PEO-PVdF solid polymer electrolyte systems. J. Phys. Chem. Solids 2021, 155, 110096. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.-Q.; Shen, L.; Liu, Q.; Ma, J.-B.; Lv, W.; He, Y.-B.; Yang, Q.-H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Chen, L.; Xue, P.; Liang, Q.; Liu, X.; Tang, J.; Li, J.; Liu, J.; Tang, M.; Wang, Z. A Single-Ion Polymer Composite Electrolyte Via In Situ Polymerization of Electrolyte Monomers into a Porous MOF-Based Fibrous Membrane for Lithium Metal Batteries. ACS Appl. Energy Mater. 2022, 5, 3800–3809. [Google Scholar] [CrossRef]
- Xu, M.; Liang, S.; Shi, H.; Miao, J.; Tian, F.; Cui, W.; Shao, R.; Xu, Z. High-Strength MOF-Based Polymer Electrolytes with Uniform Ionic Flow for Lithium Dendrite Suppression. Small 2024, 20, 2406007. [Google Scholar] [CrossRef]
- Duan, S.; Qian, L.; Zheng, Y.; Zhu, Y.; Liu, X.; Dong, L.; Yan, W.; Zhang, J. Mechanisms of the Accelerated Li+ Conduction in MOF-Based Solid-State Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Adv. Mater. 2024, 36, 2314120. [Google Scholar] [CrossRef]
- Gerbaldi, C.; Nair, J.R.; Kulandainathan, M.A.; Kumar, R.S.; Ferrara, C.; Mustarelli, P.; Stephan, A.M. Innovative high performing metal organic framework (MOF)-laden nanocomposite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 2014, 2, 9948–9954. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, P.; Wang, N.; Huang, F.; Dou, S. Research progress in stable interfacial constructions between composite polymer electrolytes and electrodes. Energy Environ. Sci. 2022, 15, 2753–2775. [Google Scholar] [CrossRef]
- Tuo, K.; Sun, C.; Liu, S. Recent Progress in and Perspectives on Emerging Halide Superionic Conductors for All-Solid-State Batteries. Electrochem. Energy Rev. 2023, 6, 17. [Google Scholar] [CrossRef]
- Bai, M.; Tang, X.; Zhang, M.; Wang, H.; Wang, Z.; Shao, A.; Ma, Y. An in-situ polymerization strategy for gel polymer electrolyte Si||Ni-rich lithium-ion batteries. Nat. Commun. 2024, 15, 5375. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Judez, X.; Chakir, M.; Armand, M.; Buannic, L. High Voltage Solid State Batteries: Targeting High Energy Density with Polymer Composite Electrolytes. J. Electrochem. Soc. 2020, 167, 020548. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, M.; Sun, F.; Arlt, T.; Frerichs, J.E.; Dong, K.; Wang, J.; Hilger, A.; Wilde, F.; Kolek, M.; et al. Performance and behavior of LLZO-based composite polymer electrolyte for lithium metal electrode with high capacity utilization. Nano Energy 2020, 77, 105196. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, Y.; Liang, Z.; Gao, L.; Xia, W.; Zhao, Y.; Zou, R. Metal–organic frameworks for solid-state electrolytes. Energy Environ. Sci. 2020, 13, 2386–2403. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Su-Yeon, L.; Kil, G.-S.; Park, D.-W.; Koo, B.H.; Lee, C.G. Preparation and characterizations of polyaniline (PANI)/ZnO nanocomposites film using solution casting method. Thin Solid Film. 2011, 519, 8375–8378. [Google Scholar] [CrossRef]
- Krstić, M.; Radojević, M.; Stojanović, D.; Radojević, V.; Uskoković, P.; Ibrić, S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur. J. Pharm. Sci. 2017, 101, 160–166. [Google Scholar] [CrossRef]
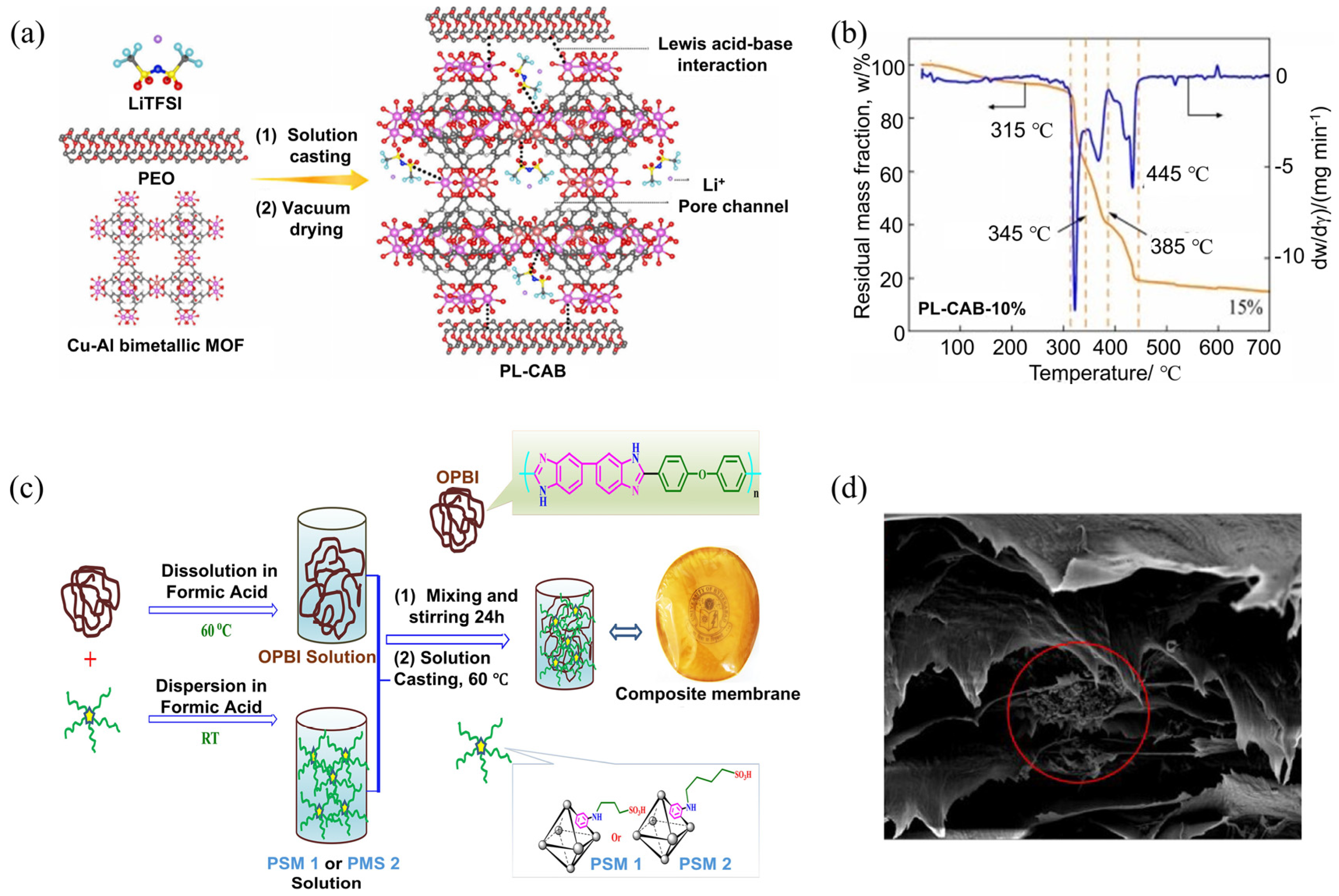
- Song, L.-B.; Long, T.-Y.; Xiao, M.-Z.; Liu, M.; Zhao, T.-T.; Kuang, Y.-J.; Jiang, L.; Xiao, Z.-L. Improvement of ionic conductivity of solid polymer electrolyte based on Cu−Al bimetallic metal-organic framework fabricated through molecular grafting. Trans. Nonferrous Met. Soc. China 2024, 34, 2943–2958. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Das, A.; Jana, T.; Das, S.K. Fabricating a MOF Material with Polybenzimidazole into an Efficient Proton Exchange Membrane. ACS Appl. Energy Mater. 2020, 3, 7964–7977. [Google Scholar] [CrossRef]
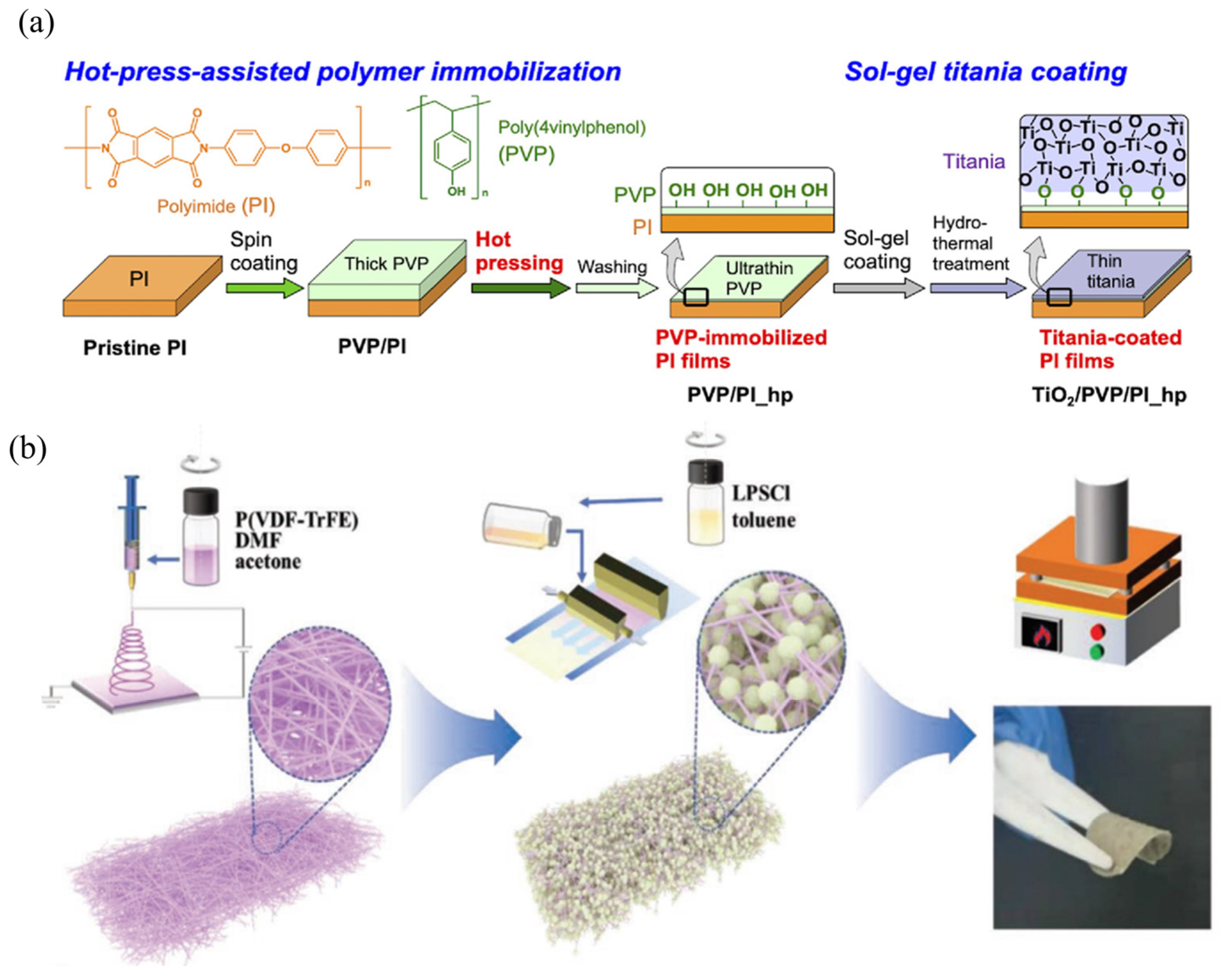
- Chen, Y.; Li, S.; Pei, X.; Zhou, J.; Feng, X.; Zhang, S.; Cheng, Y.; Li, H.; Han, R.; Wang, B. A Solvent-Free Hot-Pressing Method for Preparing Metal–Organic-Framework Coatings. Angew. Chem. Int. Ed. 2016, 55, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Hirashima, M. Preparation of Polymer-Immobilized Polyimide Films Using Hot Pressing and Titania Coatings. Langmuir 2021, 37, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, L.; Han, J.; Wen, K.; Guan, S.; Xue, C.; Zhang, Z.; Xu, B.; Lin, Y.; Shen, Y.; et al. Super Long-Cycling All-Solid-State Battery with Thin Li6PS5Cl-Based Electrolyte. Adv. Energy Mater. 2022, 12, 2200660. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Ji, C. Advances in Preparation and Biomedical Applications of Sodium Alginate-Based Electrospun Nanofibers. Gels 2025, 11, 704. [Google Scholar] [CrossRef]
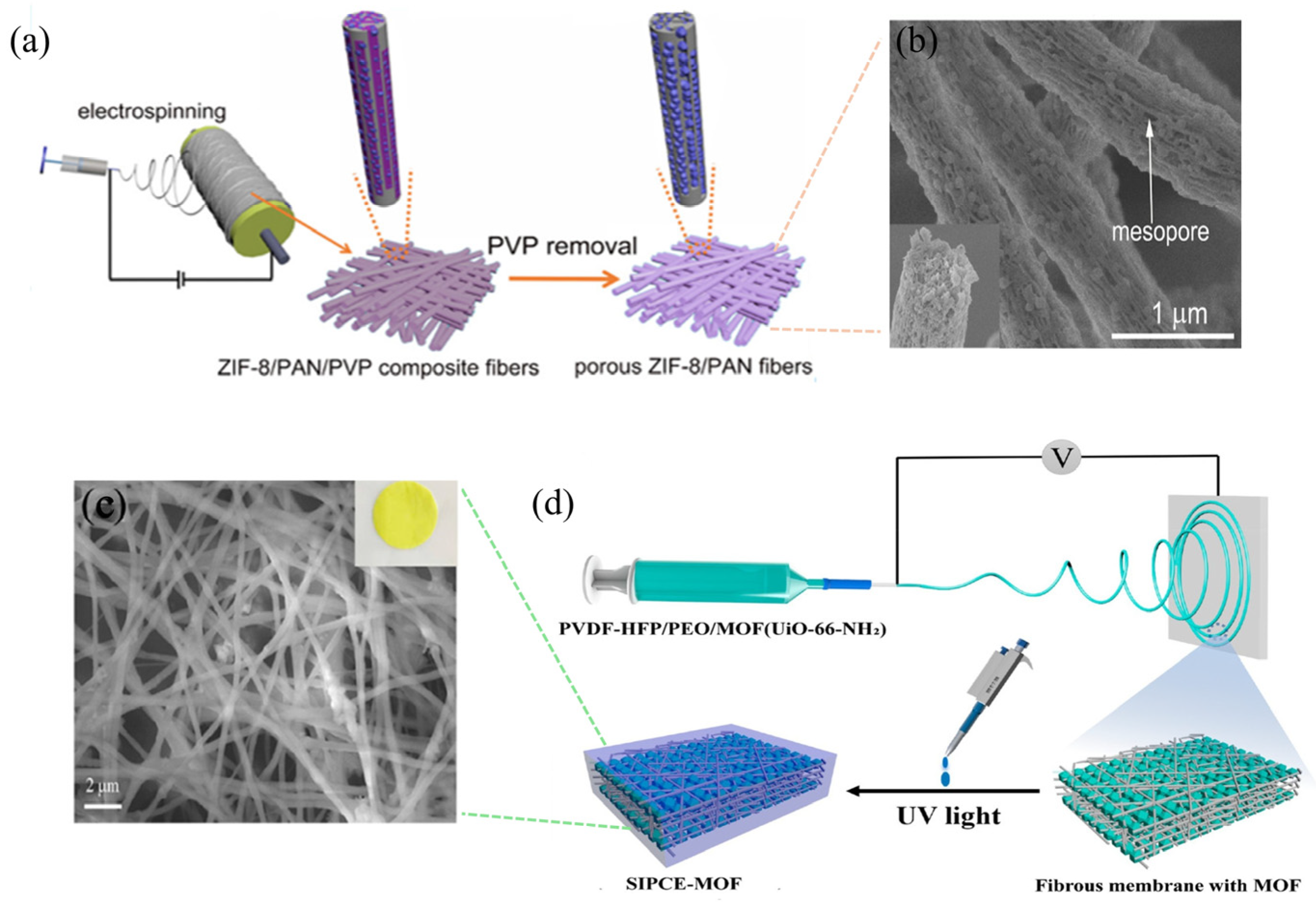
- Zhao, R.; Shi, X.; Ma, T.; Rong, H.; Wang, Z.; Cui, F.; Zhu, G.; Wang, C. Constructing Mesoporous Adsorption Channels and MOF-Polymer Interfaces in Electrospun Composite Fibers for Effective Removal of Emerging Organic Contaminants. ACS Appl. Mater. Interfaces 2021, 13, 755–764. [Google Scholar] [CrossRef]
- Li, B.; Wang, C.; Yu, R.; Han, J.; Jiang, S.; Zhang, C.; He, S. Recent progress on metal–organic framework/polymer composite electrolytes for solid-state lithium metal batteries: Ion transport regulation and interface engineering. Energy Environ. Sci. 2024, 17, 1854–1884. [Google Scholar] [CrossRef]
- Yao, X.; Lan, L.; Hun, Q.; Lu, X.; Wei, J.; Liang, X.; Shen, P.; Long, Y.; Guo, Y. Preparation and Performance of PVDF-HFP/PAN-Based Gel Polymer Electrolytes. Gels 2025, 11, 317. [Google Scholar] [CrossRef]
- Luo, H.-B.; Chen, W.-Z.; Mo, Y.-H.; Gao, J.-L.; Zhang, G.-Q.; Tong, Y.-B.; Cao, D.-Q.; Ren, X.-M. In Situ Polymer-Integrated Metal–Organic Framework for Solid-State Electrolyte Membrane. Inorg. Chem. 2025, 64, 21255–21262. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Jian, Z.; Liao, X.; Liao, W.; Huang, Y.; Chen, D.; Li, R.K.Y.; Liu, C. Tailored architecture of composite electrolyte for all-solid-state sodium batteries with superior rate performance and cycle life. Nano Res. 2024, 17, 4171–4180. [Google Scholar] [CrossRef]
- Ren, H.; Li, S.; Wang, B.; Gong, Y.; Zhang, H.; Wang, J.; Lv, Q.; Wang, D.; Liu, H.; Dou, S. Mapping the design of electrolyte additive for stabilizing zinc anode in aqueous zinc ion batteries. Energy Storage Mater. 2024, 68, 103364. [Google Scholar] [CrossRef]
- Zhao, J.; Ouyang, S.; Yang, H.; Guo, S.; Bai, Y.; Wu, D.; Duan, X.; Zhang, X.-M. Design of quasi-solid-state electrolyte based on MOF/polymer composites with high conductivity and lithium transfer number for lithium metal batteries. Chem. Eng. J. 2025, 521, 166854. [Google Scholar] [CrossRef]
- Zeng, Q.; Shi, L.; Wang, J.; Zha, X.; Yang, W.; Yang, Y. High-performance MOF-derived polymer electrolytes with modified ionic transport for solid-state lithium metal batteries. J. Energy Storage 2025, 110, 115314. [Google Scholar] [CrossRef]
- Tang, J.; Wang, D.; Qin, W.; Fang, S.; Xu, T.; Huang, J.; Tang, M.; Wang, Z. In-situ solvent-free preparation of MOF/polymer composite electrolytes with highly dispersed and defected MOF nanoparticles for lithium-metal batteries. Chem. Eng. J. 2025, 513, 163082. [Google Scholar] [CrossRef]
- Li, X.; Pan, H.; Yin, G.; Xiang, Y.; Lin, X.; Liu, Z.; Jiang, Y.; Hui, Q.; Zhang, X.; Xu, M. Achieving multifunctional MOF/polymer-based quasi-solid electrolytes via functional molecule encapsulation in MOFs. Inorg. Chem. Front. 2025, 12, 4438–4448. [Google Scholar] [CrossRef]
- Cheng, P.; Jia, X.; Liu, S.; Pan, H.; Jiang, Y.; Zhang, X. Metal–organic framework-driven composite polymer electrolytes with high lithium mobility for high-safety and high-energy-density lithium batteries. J. Colloid Interface Sci. 2026, 703, 139066. [Google Scholar] [CrossRef]
- Wang, M.; Tong, L.; Lv, S.; Li, M.; Zhao, J.; Li, X.; Li, C.; Chen, X.; Wu, J.; Li, X.; et al. In Situ-Engineered MOF/Polymer Hybrid Electrolyte With 3D Continuous Ion Channels for High-Voltage and Thermal-Resistant Lithium Metal Batteries. Interdiscip. Mater. 2025, 4, 763–774. [Google Scholar] [CrossRef]
- Ren, Z.-P.; Cong, B.; Xu, F.; Ouyang, S.; Zhao, J.-H.; Yang, H.-J.; Guo, S.; Wu, D.; Duan, X.; Zhang, X.-M. Construction of excellent solid-state electrolyte by incorporating Li-IL into open-pore MOF/polymer-based materials. Mater. Chem. Front. 2024, 8, 3166–3174. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, S.; Jia, X.; Jiang, Y.; Zhang, X. Robust MOF-Based Composite Solid-State Electrolyte Membrane for High-Performance Lithium–Metal Batteries. Nano Lett. 2025, 25, 6152–6159. [Google Scholar] [CrossRef]
- Liu, S.; Yan, Y.; Zhang, S.; Yan, D.; Cai, Y.; Yin, S.; Lu, Q.; Ren, W.; Zhang, Q.; Xing, Y. A 3D interconnected functionalized metal organic framework-reinforced solid electrolyte by in-situ polymerization for stable lithium metal battery. Energy Storage Mater. 2025, 80, 104396. [Google Scholar] [CrossRef]
- Abdelmaoula, A.E.; Du, L.; Xu, L.; Cheng, Y.; Mahdy, A.A.; Tahir, M.; Liu, Z.; Mai, L. Biomimetic brain-like nanostructures for solid polymer electrolytes with fast ion transport. Sci. China Mater. 2022, 65, 1476–1484. [Google Scholar] [CrossRef]
- He, Y.; Chang, Z.; Wu, S.; Qiao, Y.; Bai, S.; Jiang, K.; He, P.; Zhou, H. Simultaneously Inhibiting Lithium Dendrites Growth and Polysulfides Shuttle by a Flexible MOF-Based Membrane in Li–S Batteries. Adv. Energy Mater. 2018, 8, 1802130. [Google Scholar] [CrossRef]
- Aruchamy, K.; Ramasundaram, S.; Divya, S.; Chandran, M.; Yun, K.; Oh, T.H. Gel Polymer Electrolytes: Advancing Solid-State Batteries for High-Performance Applications. Gels 2023, 9, 585. [Google Scholar] [CrossRef]
- Lin, G.; Jia, K.; Bai, Z.; Liu, C.; Liu, S.; Huang, Y.; Liu, X. Metal-Organic Framework Sandwiching Porous Super-Engineering Polymeric Membranes as Anionphilic Separators for Dendrite-free Lithium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2207969. [Google Scholar] [CrossRef]
- Huo, S.; Sheng, L.; Xue, W.; Wang, L.; Xu, H.; Zhang, H.; He, X. Challenges of polymer electrolyte with wide electrochemical window for high energy solid-state lithium batteries. InfoMat 2023, 5, e12394. [Google Scholar] [CrossRef]
- Xue, C.; Guan, S.; Hu, B.; Wang, X.; Xin, C.; Liu, S.; Yu, J.; Wen, K.; Li, L.; Nan, C.-W. Significantly improved interface between PVDF-based polymer electrolyte and lithium metal via thermal-electrochemical treatment. Energy Storage Mater. 2022, 46, 452–460. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Yang, X.; You, J.; Xu, Y.; Wang, H.; Ma, Y.; Gao, G. Influence of additives in a PVDF-based solid polymer electrolyte on conductivity and Li-ion battery performance. Sustain. Energy Fuels 2018, 2, 492–498. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.; Feng, Y.; Yuan, Y.; Wu, Y.; Zhao, H.; Kumar, R.V.; Feng, G.; Xi, K. Microporous Materials in Polymer Electrolytes: The Merit of Order. Adv. Mater. 2024, 36, e2405079. [Google Scholar] [CrossRef]
- Jiang, H.; Du, Y.; Zhao, L.; Liu, X.; Kong, J.; Liu, P.; Zhou, T. Mof-derived ionic conductor enhancing the performance of polymer electrolyte for solid-state lithium batteries. Chem. Eng. J. 2024, 487, 150455. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Han, P.; Lai, Y.; Zhang, Z.; Liu, J. Enhanced electrochemical performance of poly(ethylene oxide) based composite polymer electrolyte by incorporation of nano-sized metal-organic framework. J. Power Sources 2013, 240, 653–658. [Google Scholar] [CrossRef]
- Han, Q.; Wang, S.; Jiang, Z.; Hu, X.; Wang, H. Composite Polymer Electrolyte Incorporating Metal-Organic Framework Nanosheets with Improved Electrochemical Stability for All-Solid-State Li Metal Batteries. ACS Appl. Mater. Interfaces 2020, 12, 20514–20521. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Croce, F.; Persi, L. Impedance Spectroscopy Study of PEO-Based Nanocomposite Polymer Electrolytes. J. Electrochem. Soc. 2000, 147, 1718. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Yang, J.; Zhang, J. Neuron-Like Silicone Nanofilaments@Montmorillonite Nanofillers of PEO-Based Solid-State Electrolytes for Lithium Metal Batteries with Wide Operation Temperature. Angew. Chem. Int. Ed. 2024, 63, e202400091. [Google Scholar] [CrossRef]
- Tian, W.; Lin, G.; Yuan, S.; Jin, T.; Wang, Q.; Jiao, L. Competitive Coordination and Dual Interphase Regulation of MOF-Modified Solid-State Polymer Electrolytes for High-Performance Sodium Metal Batteries. Angew. Chem. Int. Ed. Engl. 2025, 64, e202423075. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, P.; Fan, L.-Z. Asymmetric Polymer Electrolyte Constructed by Metal–Organic Framework for Solid-State, Dendrite-Free Lithium Metal Battery. Adv. Funct. Mater. 2021, 31, 2007198. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Sun, X.; Yang, Y. Mitigating Interfacial Instability in Polymer Electrolyte-Based Solid-State Lithium Metal Batteries with 4 V Cathodes. ACS Energy Lett. 2020, 5, 3244–3253. [Google Scholar] [CrossRef]
- Pang, Y.; Pan, J.; Yang, J.; Zheng, S.; Wang, C. Electrolyte/Electrode Interfaces in All-Solid-State Lithium Batteries: A Review. Electrochem. Energy Rev. 2021, 4, 169–193. [Google Scholar] [CrossRef]
- He, H.; Deng, N.; Wang, X.; Gao, L.; Tang, C.; Wu, E.; Ren, J.; Yang, X.; Feng, N.; Gao, D.; et al. Design Strategies, Characterization Mechanisms, and Applications of MOFs in Polymer Composite Electrolytes for Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2025, 35, 2421670. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Z.; Pan, X.; Zhang, M.; Lian, T.; Shi, K.; Qian, J.; Li, L.; Wu, F.; Chen, R. In-Built Compatible Electrode-Electrolyte Interphases for Quasi-Solid-State Li-SPAN Batteries. Angew. Chem. Int. Ed. 2025, 64, e202510624. [Google Scholar] [CrossRef]
- Yang, Q.; Liao, M.; Ren, P.; Li, D.; Deng, N.; Kang, W.; Cai, G. Dual functional composite solid electrolyte constructed by double-layer sulfonated polyethersulfone (SPES)/poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) nanofiber membrane in high-performance all-solid-state lithium metal batteries. Chem. Eng. J. 2024, 495, 153448. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, L.; Lee, S.; Bae, W.; Park, S.; Jeon, M.; Kim, W.; Jang, H. In situ induced crosslinking highly conductive solid polymer electrolyte with intimated electrodes interfacial compatibility for safe Li-ion batteries. J. Power Sources 2023, 557, 232568. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; de Zea Bermudez, V.; Fidalgo-Marijuan, A.; Zhang, Q.; Lanceros-Méndez, S. Metal–organic frameworks and zeolite materials as active fillers for lithium-ion battery solid polymer electrolytes. Mater. Adv. 2021, 2, 3790–3805. [Google Scholar] [CrossRef]
- Huo, H.; Wu, B.; Zhang, T.; Zheng, X.; Ge, L.; Xu, T.; Guo, X.; Sun, X. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Storage Mater. 2019, 18, 59–67. [Google Scholar] [CrossRef]
- Li, M.; Song, S.; Li, Y.; Chu, M.; Chen, T.; Seung Lee, C.; Bae, J. Novel DNA-based polysulfide sieves incorporated with MOF providing excellent 3D Li+ pathway for high-performance lithium-sulfur batteries. Appl. Surf. Sci. 2023, 614, 156163. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Song, S.; Li, Y.; Bae, J. HKUST-1@IL-Li Solid-state Electrolyte with 3D Ionic Channels and Enhanced Fast Li(+) Transport for Lithium Metal Batteries at High Temperature. Nanomaterials 2021, 11, 736. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, S.; Li, Y.; Li, X.; Chu, F.; Wang, X.; Yu, M.; Jiang, X.; Ruan, X.; Tu, J.; et al. In situ coordinated ultrathin MOF-polymer electrolyte membrane with vertically aligned transfer channels for solid lithium metal batteries. J. Membr. Sci. 2024, 707, 122955. [Google Scholar] [CrossRef]
- Yu, X.; Grundish, N.S.; Goodenough, J.B.; Manthiram, A. Ionic Liquid (IL) Laden Metal–Organic Framework (IL-MOF) Electrolyte for Quasi-Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 24662–24669. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cha, H.; Kostecki, R.; Chen, G. Composite Cathode Design for High-Energy All-Solid-State Lithium Batteries with Long Cycle Life. ACS Energy Lett. 2023, 8, 521–528. [Google Scholar] [CrossRef]
- Didwal, P.N.; Singhbabu, Y.N.; Verma, R.; Sung, B.-J.; Lee, G.-H.; Lee, J.-S.; Chang, D.R.; Park, C.-J. An advanced solid polymer electrolyte composed of poly(propylene carbonate) and mesoporous silica nanoparticles for use in all-solid-state lithium-ion batteries. Energy Storage Mater. 2021, 37, 476–490. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Kou, W.; Yang, Z.; Zhai, P.; Liu, Y.; Wu, W.; Wang, J. A flexible, ion-conducting solid electrolyte with vertically bicontinuous transfer channels toward high performance all-solid-state lithium batteries. Chem. Eng. J. 2021, 404, 126517. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, H.; Xing, C.; Li, Q.; Tang, Y.; Zhang, F.; Yang, K.; Zhang, S. Rational design of biomimetic ant-nest solid polymer electrolyte for high-voltage Li-metal battery with robust mechanical and electrochemical performance. Energy Storage Mater. 2021, 41, 51–60. [Google Scholar] [CrossRef]
- Acebedo, B.; Morant-Miñana, M.C.; Gonzalo, E.; Ruiz de Larramendi, I.; Villaverde, A.; Rikarte, J.; Fallarino, L. Current Status and Future Perspective on Lithium Metal Anode Production Methods. Adv. Energy Mater. 2023, 13, 2203744. [Google Scholar] [CrossRef]
- Hu, A.; Chen, W.; Du, X.; Hu, Y.; Lei, T.; Wang, H.; Xue, L.; Li, Y.; Sun, H.; Yan, Y.; et al. An artificial hybrid interphase for an ultrahigh-rate and practical lithium metal anode. Energy Environ. Sci. 2021, 14, 4115–4124. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Hu, B.; Liang, Q.; Xiong, X. Dynamic gel as artificial interphase layer for ultrahigh-rate and large-capacity lithium metal anode. Nat. Commun. 2023, 14, 4018. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Sui, Y.; Zhan, H.; Wang, C.; Xin, H.L.; Cheng, H.-M.; Kang, F.; Yang, C. Polymorph Evolution Mechanisms and Regulation Strategies of Lithium Metal Anode under Multiphysical Fields. Chem. Rev. 2021, 121, 5986–6056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Y.; Bannenberg, L.J.; Liu, M.; Ganapathy, S.; Wagemaker, M. The lasting impact of formation cycling on the Li-ion kinetics between SEI and the Li-metal anode and its correlation with efficiency. Sci. Adv. 2024, 10, eadj8889. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, T.; Zhang, N.; Xue, T.; Chen, Y.; Ye, Y.; Wu, F.; Chen, R. Spatially Confined LiF Nanoparticles in an Aligned Polymer Matrix as the Artificial SEI Layer for Lithium Metal Anodes. Nano Lett. 2023, 23, 276–282. [Google Scholar] [CrossRef]
- Chen, A.-L.; Shang, N.; Ouyang, Y.; Mo, L.; Zhou, C.; Tjiu, W.W.; Lai, F.; Miao, Y.-E.; Liu, T. Electroactive polymeric nanofibrous composite to drive in situ construction of lithiophilic SEI for stable lithium metal anodes. eScience 2022, 2, 192–200. [Google Scholar] [CrossRef]
- Cao, W.; Lu, J.; Zhou, K.; Sun, G.; Zheng, J.; Geng, Z.; Li, H. Organic-inorganic composite SEI for a stable Li metal anode by in-situ polymerization. Nano Energy 2022, 95, 106983. [Google Scholar] [CrossRef]
- Deng, T.; Cao, L.; He, X.; Li, A.-M.; Li, D.; Xu, J.; Liu, S.; Bai, P.; Jin, T.; Ma, L.; et al. In situ formation of polymer-inorganic solid-electrolyte interphase for stable polymeric solid-state lithium-metal batteries. Chem 2021, 7, 3052–3068. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Jiang, H.; Wang, L.; Zhang, F.; Feng, X.; Xiang, H. Confined in-situ polymerization of poly(1,3-dioxolane) and poly(vinylene carbonate)-based quasi-solid polymer electrolyte with improved uniformity for lithium metal batteries. Mater. Today Energy 2023, 32, 101239. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Li, G.; Wang, C.; Li, B.; Li, M.; Wang, Y.; Ming, H.; Wen, Y.; Qiu, J.; et al. A cross-linked gel polymer electrolyte employing cellulose acetate matrix and layered boron nitride filler prepared via in situ thermal polymerization. J. Power Sources 2021, 484, 229235. [Google Scholar] [CrossRef]
- Wu, M.; Liu, D.; Qu, D.; Lei, J.; Zhang, X.; Chen, H.; Tang, H. In-situ polymerized composite polymer electrolyte with cesium-ion additive enables dual-interfacial compatibility in all-solid-state lithium-metal batteries. J. Colloid Interface Sci. 2022, 615, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Liu, Z.; Ma, J.; Wang, J.; Liu, X.; Liu, H.; Zhang, J.; Cui, G.; Chen, L. In Situ Generation of Poly (Vinylene Carbonate) Based Solid Electrolyte with Interfacial Stability for LiCoO2 Lithium Batteries. Adv. Sci. 2017, 4, 1600377. [Google Scholar] [CrossRef]
- Michailidou, F.; Klöcker, N.; Cornelissen, N.V.; Singh, R.K.; Peters, A.; Ovcharenko, A.; Kümmel, D.; Rentmeister, A. Cover Picture: Engineered SAM Synthetases for Enzymatic Generation of AdoMet Analogs with Photocaging Groups and Reversible DNA Modification in Cascade Reactions (Angew. Chem. Int. Ed. 1/2021). Angew. Chem. Int. Ed. 2021, 60, 1. [Google Scholar] [CrossRef]
- Yang, C.-H.; Hsiao, Y.-C.; Lin, L.-Y. Novel In Situ Synthesis of Freestanding Carbonized ZIF67/Polymer Nanofiber Electrodes for Supercapacitors via Electrospinning and Pyrolysis Techniques. ACS Appl. Mater. Interfaces 2021, 13, 41637–41648. [Google Scholar] [CrossRef]
- Chai, Y.; Gao, J.; Yang, L.; Wu, W.; Ning, D.; Chen, Z.; Huang, W.; Zhang, G.; Gao, R.; Zhou, D.; et al. In Situ Coordinated MOF-Polymer Composite Electrolyte for Solid-State Lithium Metal Batteries with Exceptional High-Rate Performance. Small 2025, 21, e2412494. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, H.; Kong, D.; Li, X.; Shen, L.; Lu, Y. Facilitating Lithium-Ion Conduction in Gel Polymer Electrolyte by Metal-Organic Frameworks. ACS Mater. Lett. 2020, 2, 1435–1441. [Google Scholar] [CrossRef]
- Ma, S.; Shen, L.; Liu, Q.; Shi, W.; Zhang, C.; Liu, F.; Baucom, J.A.; Zhang, D.; Yue, H.; Wu, H.B.; et al. Class of Solid-like Electrolytes for Rechargeable Batteries Based on Metal–Organic Frameworks Infiltrated with Liquid Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 43824–43832. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, R.; Wang, H.; Yang, L.; Hu, J.; Chen, H.; Pan, F. A Metal-Organic-Framework-Based Electrolyte with Nanowetted Interfaces for High-Energy-Density Solid-State Lithium Battery. Adv. Mater. 2018, 30, 1704436. [Google Scholar] [CrossRef]
- Salado, M.; Fernández de Luis, R.; Smith, T.H.; Hasanpoor, M.; Lanceros-Mendez, S.; Forsyth, M. Dimensionality Control of Li Transport by MOFs Based Quasi-Solid to Solid Electrolyte (Q-SSEs) for Li−Metal Batteries. Batter. Supercaps 2024, 7, e202400134. [Google Scholar] [CrossRef]
- Verma, P.; Bannon, M.S.; Kuenen, M.K.; Raj, S.; Dhakal, A.; Stone, K.; Nichols, A.W.; Machan, C.W.; Colon, Y.J.; Letteri, R.A.; et al. Expanding the Design Space of Polymer-Metal Organic Framework (MOF) Gels by Understanding Polymer-MOF Interactions. Chem. Mater. 2024, 36, 9356–9369. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, N.; Du, L.; Cheng, Y.; Lei, S.; Li, S.; Liao, X.; Shi, W.; Xu, L.; Mai, L. Rational Design of Ion Transport Paths at the Interface of Metal-Organic Framework Modified Solid Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 22930–22938. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; van Eck, E.R.H.; Wang, C.; Ganapathy, S.; Wagemaker, M. Improving Li-ion interfacial transport in hybrid solid electrolytes. Nat. Nanotechnol. 2022, 17, 959–967. [Google Scholar] [CrossRef]
- Huang, J.; Li, C.; Jiang, D.; Gao, J.; Cheng, L.; Li, G.; Luo, H.; Xu, Z.L.; Shin, D.M.; Wang, Y.; et al. Solid-State Electrolytes for Lithium Metal Batteries: State-of-the-Art and Perspectives. Adv. Funct. Mater. 2024, 35, 2411171. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef] [PubMed]
- Al-Salih, H.; Houache, M.S.E.; Baranova, E.A.; Abu-Lebdeh, Y. Composite Cathodes for Solid-State Lithium Batteries: “Catholytes” the Underrated Giants. Adv. Energy Sustain. Res. 2022, 3, 2200032. [Google Scholar] [CrossRef]
- Wan, Z.; Lei, D.; Yang, W.; Liu, C.; Shi, K.; Hao, X.; Shen, L.; Lv, W.; Li, B.; Yang, Q.-H.; et al. Low Resistance–Integrated All-Solid-State Battery Achieved by Li7La3Zr2O12 Nanowire Upgrading Polyethylene Oxide (PEO) Composite Electrolyte and PEO Cathode Binder. Adv. Funct. Mater. 2019, 29, 1805301. [Google Scholar] [CrossRef]
- Zha, W.; Chen, F.; Yang, D.; Shen, Q.; Zhang, L. High-performance Li6.4La3Zr1.4Ta0.6O12/Poly(ethylene oxide)/Succinonitrile composite electrolyte for solid-state lithium batteries. J. Power Sources 2018, 397, 87–94. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Hu, J.; Liu, Y.; Liu, Y.; Feng, Q.; Zhu, G.; Fan, L.-Z. Solid-state lithium metal batteries enabled with high loading composite cathode materials and ceramic-based composite electrolytes. J. Power Sources 2019, 442, 227230. [Google Scholar] [CrossRef]
- Hu, J.; He, P.; Zhang, B.; Wang, B.; Fan, L.-Z. Porous film host-derived 3D composite polymer electrolyte for high-voltage solid state lithium batteries. Energy Storage Mater. 2020, 26, 283–289. [Google Scholar] [CrossRef]
- Li, X.; Hou, Q.; Huang, W.; Xu, H.-S.; Wang, X.; Yu, W.; Li, R.; Zhang, K.; Wang, L.; Chen, Z.; et al. Solution-Processable Covalent Organic Framework Electrolytes for All-Solid-State Li–Organic Batteries. ACS Energy Lett. 2020, 5, 3498–3506. [Google Scholar] [CrossRef]
- Jeong, K.; Park, S.; Jung, G.Y.; Kim, S.H.; Lee, Y.-H.; Kwak, S.K.; Lee, S.-Y. Solvent-Free, Single Lithium-Ion Conducting Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 5880–5885. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, L.; Zhang, Z.; Li, C.; Bang, K.T.; Liem, A.; Luo, H.; Hu, C.; Lee, Y.M.; Lu, Y.; et al. High-Performance All-Solid-State Lithium Metal Batteries Enabled by Ionic Covalent Organic Framework Composites. Adv. Energy Mater. 2024, 14, 2400762. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, W.; Song, W.L.; Wang, M.; Chen, H.; Jiao, S.; Fang, D. Electrocatalysis for Continuous Multi-Step Reactions in Quasi-Solid-State Electrolytes Towards High-Energy and Long-Life Aluminum-Sulfur Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202696. [Google Scholar] [CrossRef]
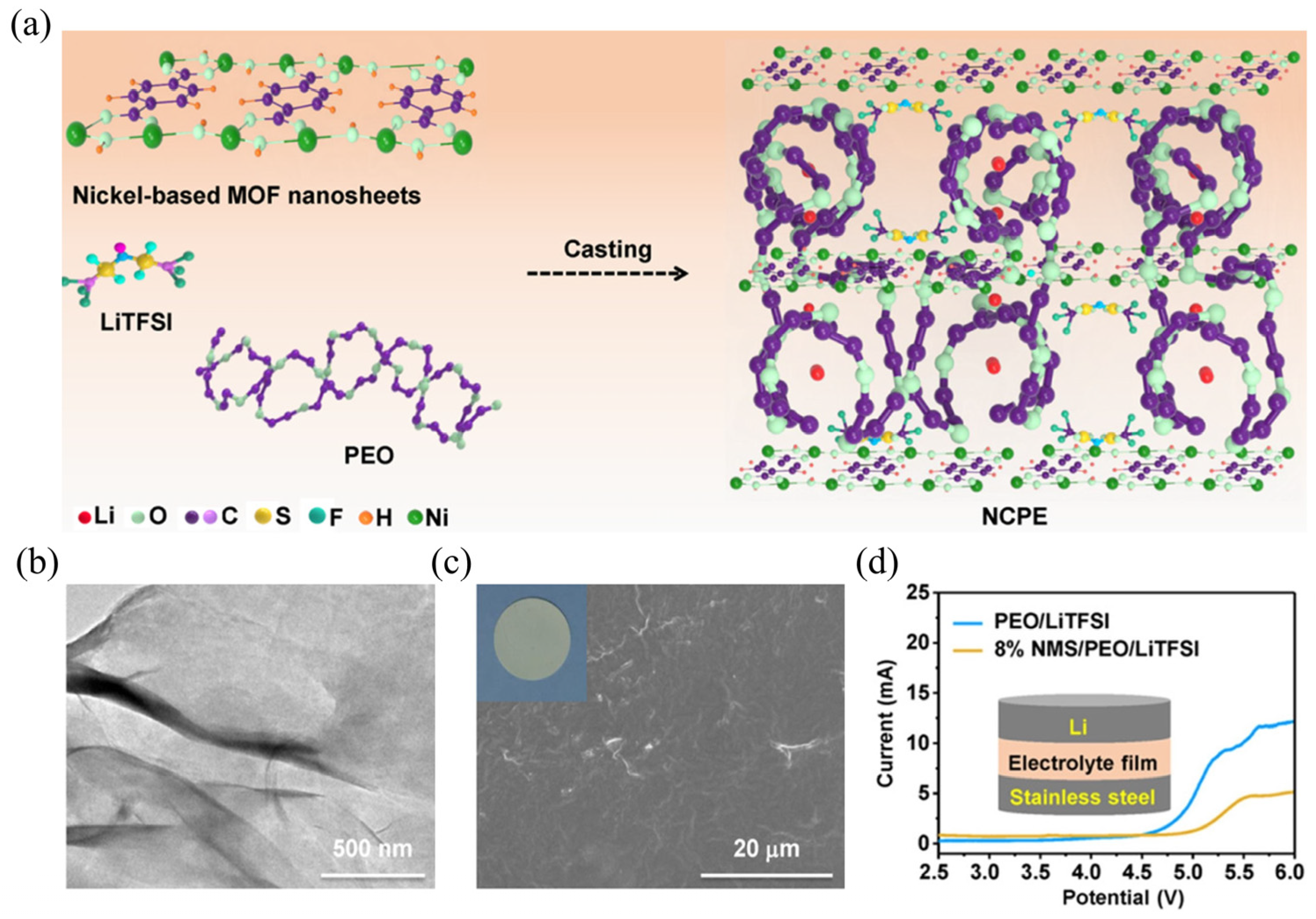
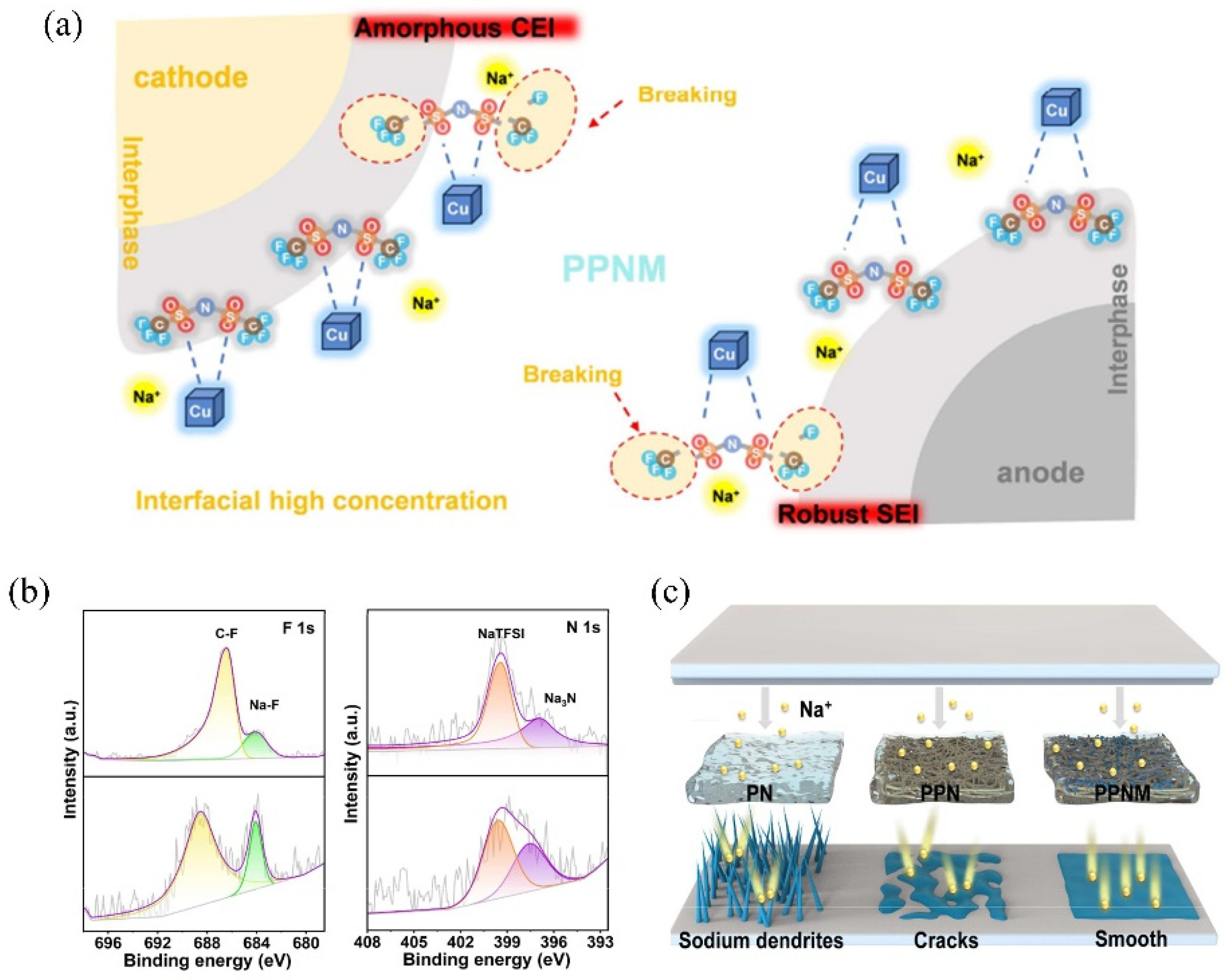
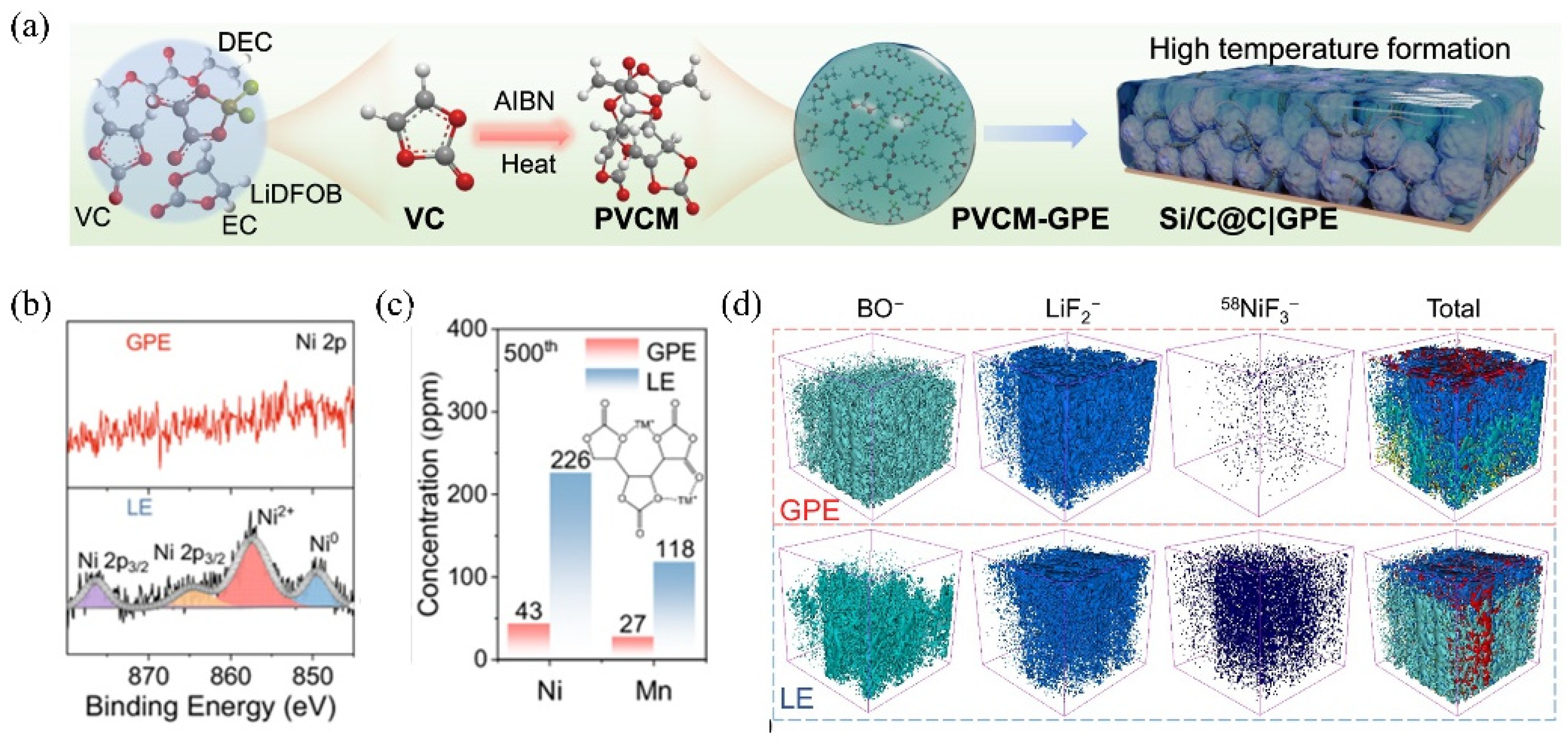
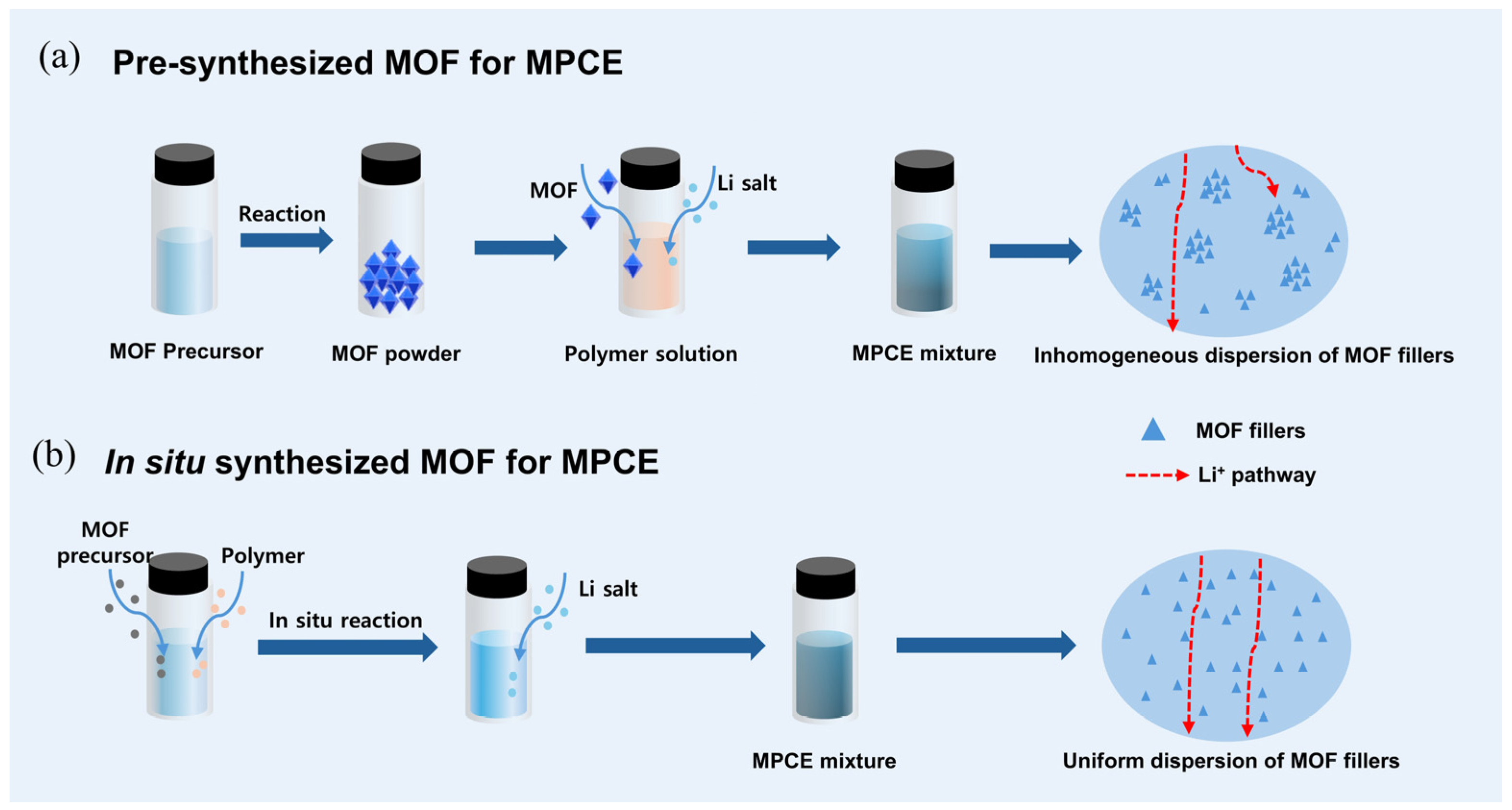
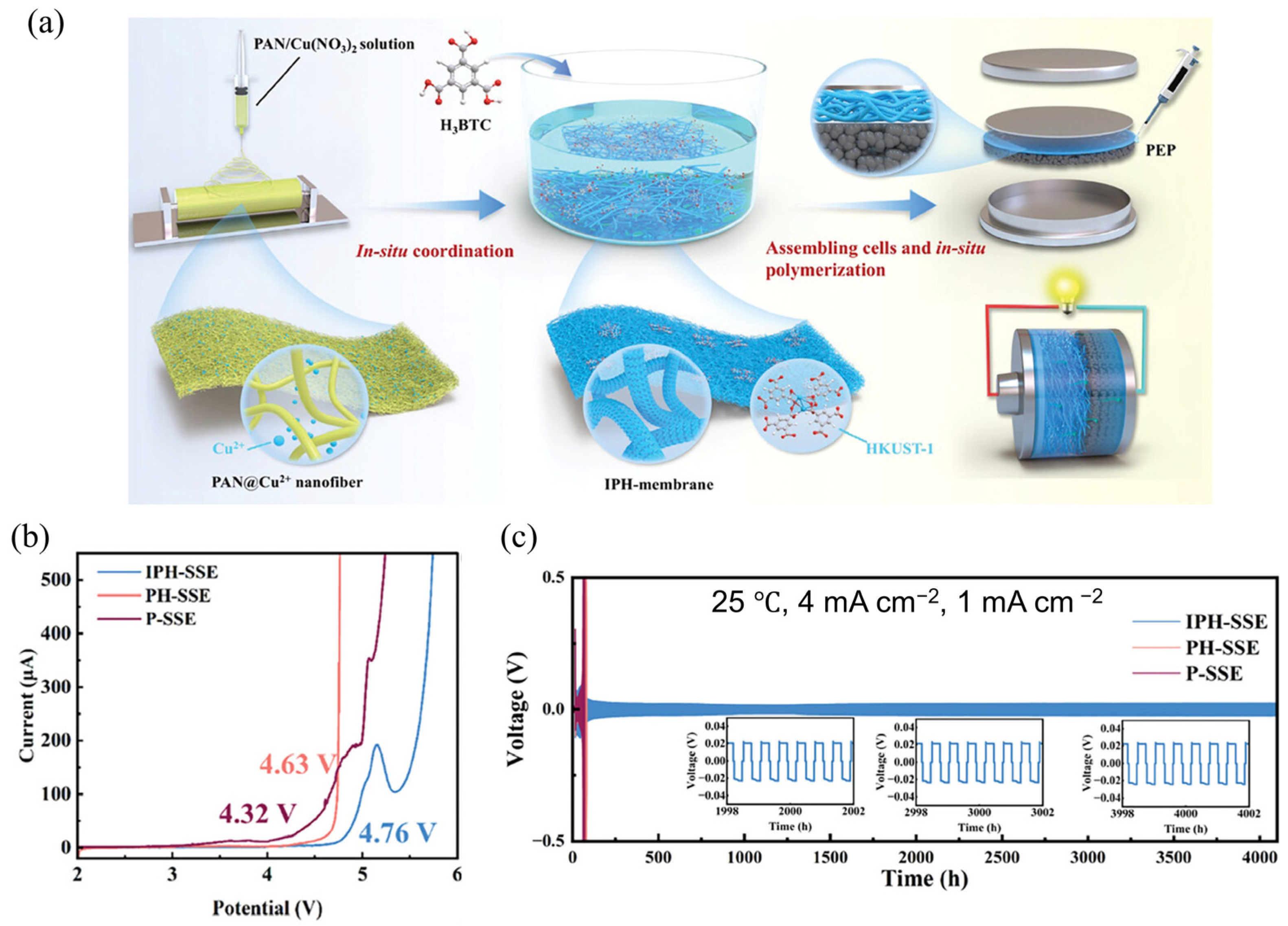
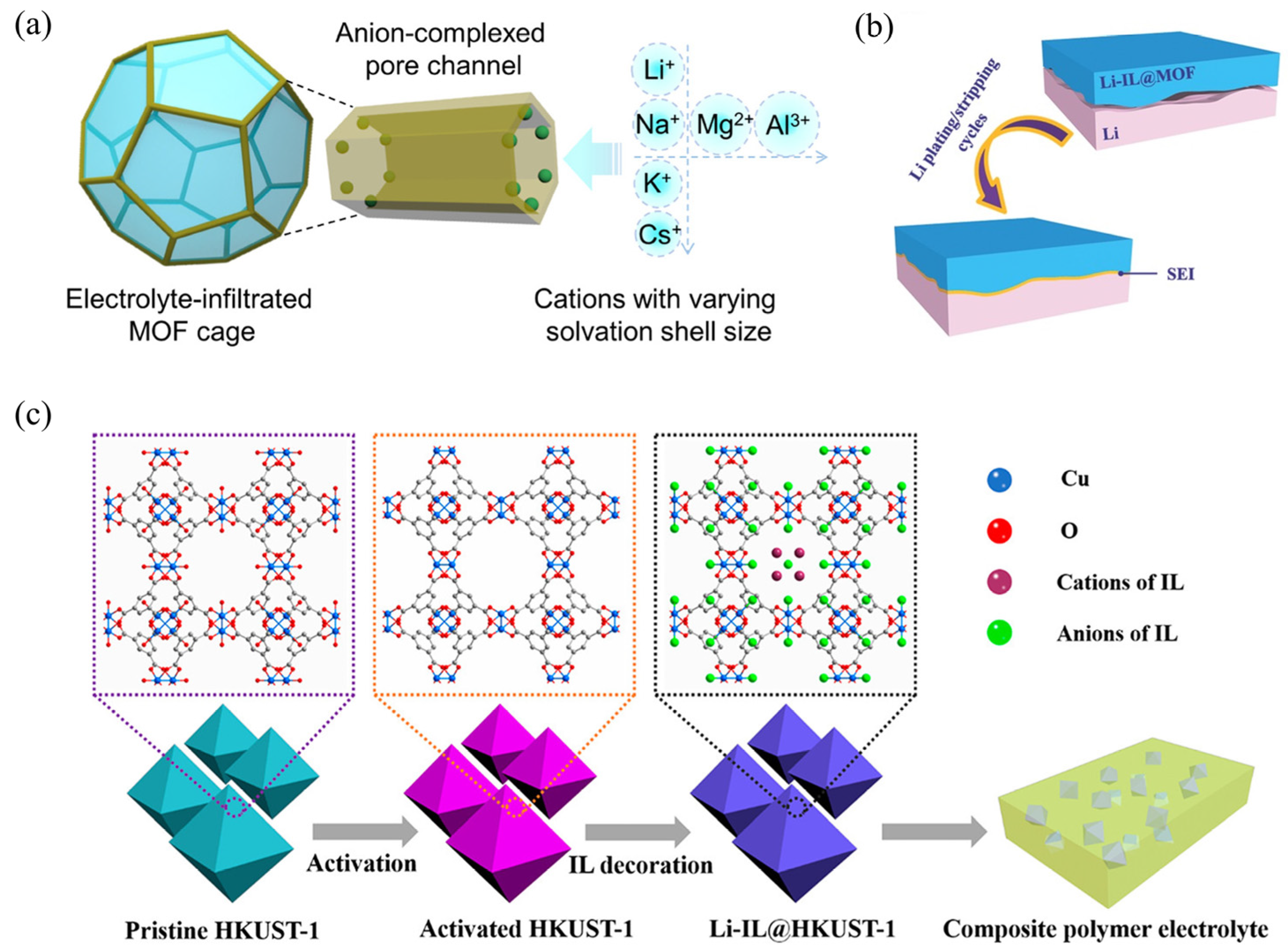
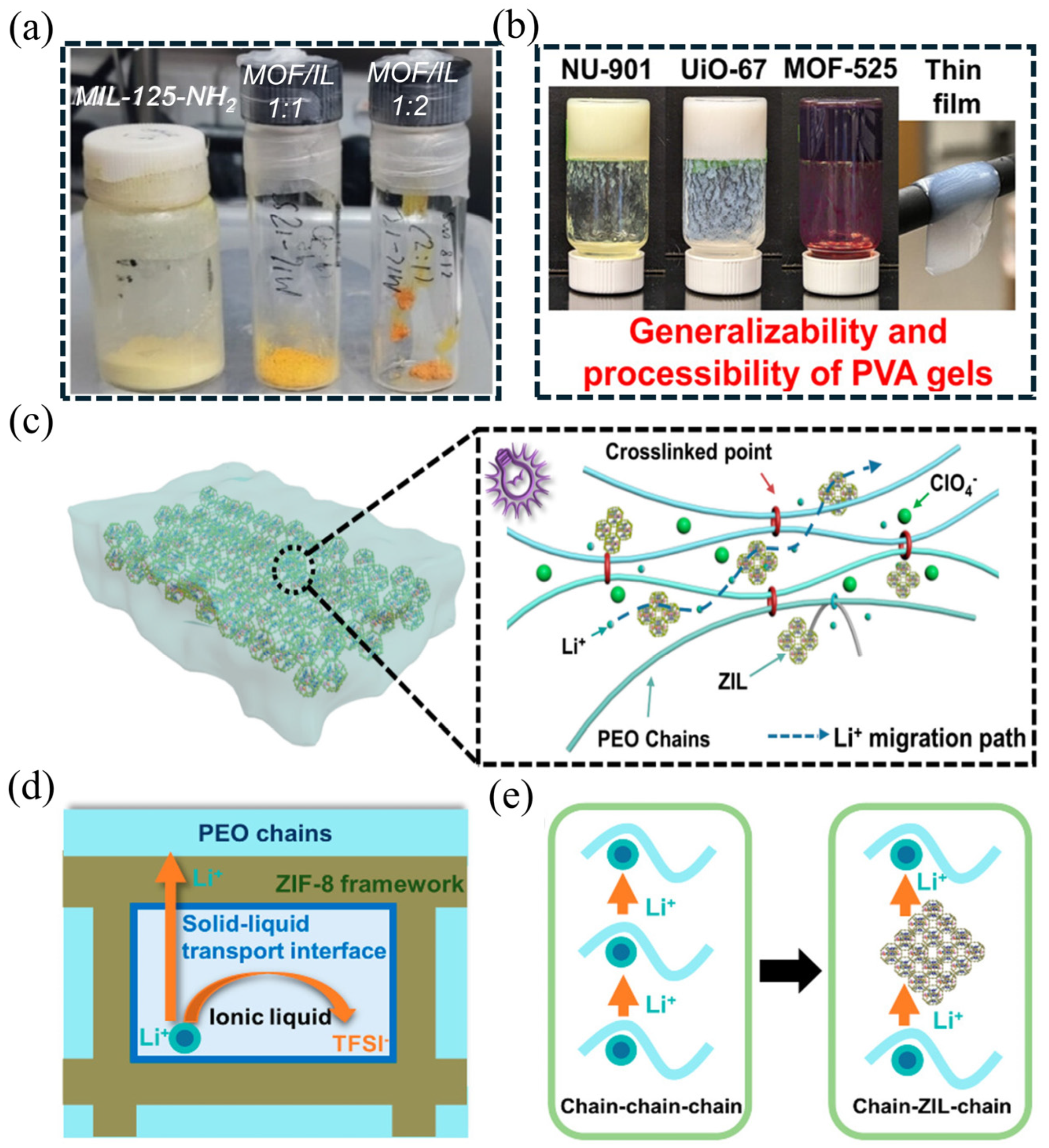
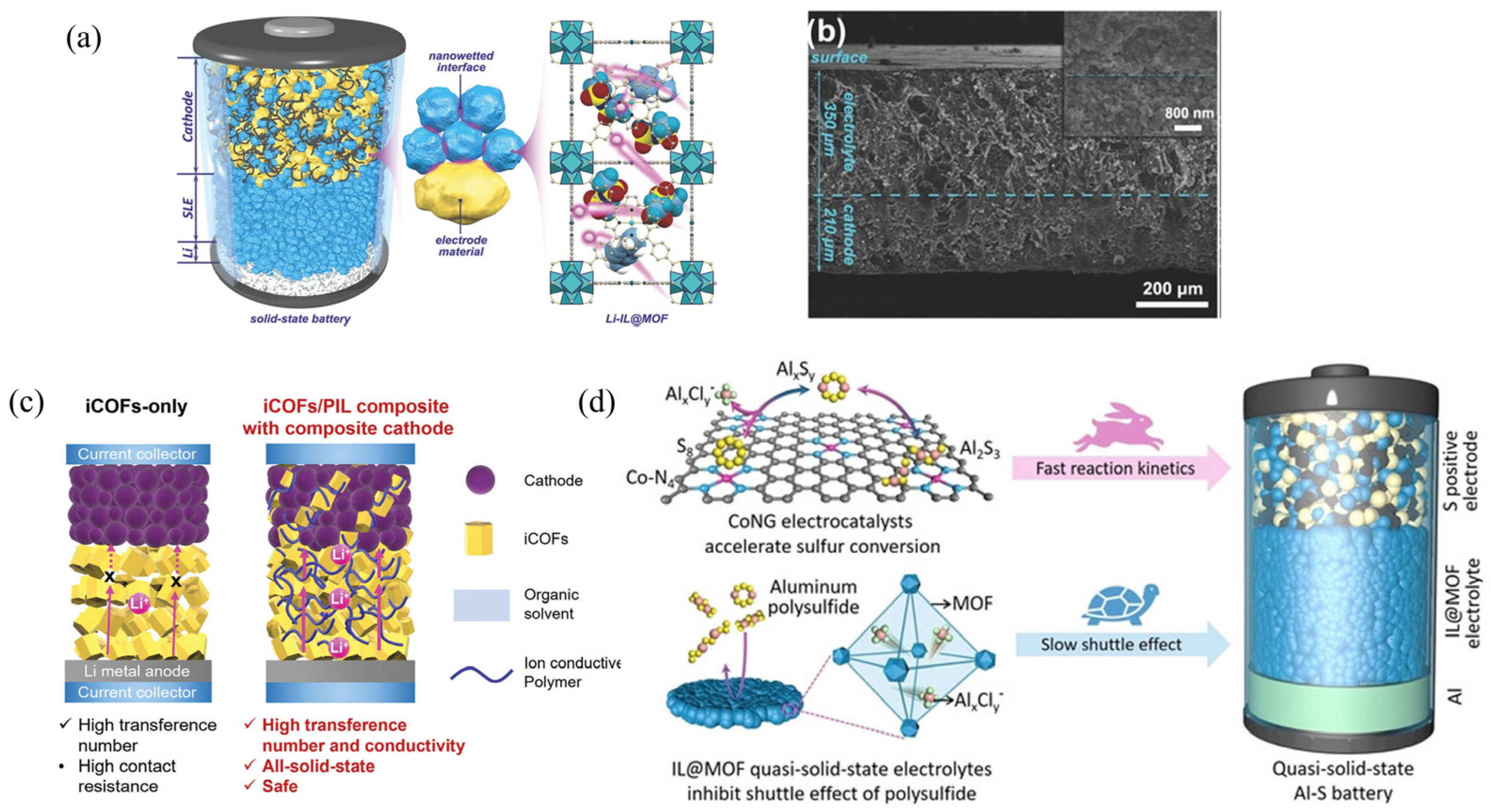
| Strategy | Mechanism | Applicability/Complexity | Strengths | Limitations | Typical σ/tLi⁺ | Ref. |
|---|---|---|---|---|---|---|
| In situ polymerization | Monomer infiltration, then polymerization, forming an intimate interphase | Medium–high; requires monomer compatibility | Low interfacial resistance; conformal contact | Shrinkage; limited to monomer-compatible systems | σ ~10−4–10−3 S/cm; tLi⁺ ~0.4–0.6 | [12] |
| In situ MOF growth | MOF grown inside the polymer matrix for uniform dispersion | High; synthesis-sensitive | High MOF loading; strong MOF–polymer coupling | Polymer damage risk; synthetic control needed | σ ~10−4–10−3 S/cm; tLi⁺ up to ~0.77 | [70] |
| Gel-like ionic conductor | Liquid/plasticizer inside MOF pores improves wettability | Low–medium | High σ; excellent interface wetting | Mechanical weakness; safety concerns | σ up to ~10−3 S/cm | [71] |
| Composite cathode design | Catholyte integrated into the cathode, reducing interfacial resistance | Moderate | Reduced voids; suitable for thick electrodes | Complex formulation; mass tradeoffs | Low R_interface; σ depends on the system | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Reddy, N.P.; Li, M. Enhancing Solid-State Li-Ion Batteries with MOF–Polymer Composite Electrolytes—Effect Mechanisms and Interface Engineering. Gels 2025, 11, 946. https://doi.org/10.3390/gels11120946
Chen T, Reddy NP, Li M. Enhancing Solid-State Li-Ion Batteries with MOF–Polymer Composite Electrolytes—Effect Mechanisms and Interface Engineering. Gels. 2025; 11(12):946. https://doi.org/10.3390/gels11120946
Chicago/Turabian StyleChen, Tao, Nandarapu Purushotham Reddy, and Man Li. 2025. "Enhancing Solid-State Li-Ion Batteries with MOF–Polymer Composite Electrolytes—Effect Mechanisms and Interface Engineering" Gels 11, no. 12: 946. https://doi.org/10.3390/gels11120946
APA StyleChen, T., Reddy, N. P., & Li, M. (2025). Enhancing Solid-State Li-Ion Batteries with MOF–Polymer Composite Electrolytes—Effect Mechanisms and Interface Engineering. Gels, 11(12), 946. https://doi.org/10.3390/gels11120946








