Nutritional and Techno-Functional Properties of Ultrasound-Assisted Moringa oleifera Leaf Protein Concentrate with Potential Applications in Food Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Protein Extraction from M. oleifera Leaves
2.2. Nutritional Profile of M. oleifera Flour and Protein Concentrate
2.3. Amino Acid Profile
2.4. Physicochemical and Functional Properties of MOPC: Potential for Gel Formation
3. Conclusions
4. Materials and Methods
4.1. Biological Material
4.2. Production of MOF
4.3. Optimization of Protein Extraction from M. oleifera Leaves
4.4. Nutritional Composition of MOF and MOPC
4.5. Physicochemical and Functional Properties of MOPC
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF; WHO; World Bank. Joint Child Malnutrition Estimates (JME) Standard Methodology. UNICEF/WHO/World Bank. 2024. Available online: https://data.unicef.org/resources/jme-standard-methodology/?utm_source=chatgpt.com (accessed on 21 August 2025).
- Khan, L.H.; Varshney, V.K. Chemical utilization of Albizia lebbeck leaves for developing protein concentrates as a dietary supplement. J. Diet. Suppl. 2018, 15, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and plant-based protein sources: A scoping review of human health outcomes and environmental impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef] [PubMed]
- Yen, N.T.H.; Quoc, L.P.T. Optimization of ultrasound-assisted extraction of phenolic compounds from fresh Moringa oleifera leaves with a response surface methodology and comparison with the Soxhlet extraction method. Bull. Chem. Soc. Ethiop. 2022, 36, 261–275. [Google Scholar] [CrossRef]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef]
- Sultana, S. Nutritional and functional properties of Moringa oleifera. Metab. Open 2020, 8, 100061. [Google Scholar] [CrossRef]
- Mhlomi, Y.N.; Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. Assessment of rats fed protein-deficient diets supplemented with Moringa oleifera leaf meal. Curr. Res. Nutr. Food Sci. J. 2022, 10, 45–55. [Google Scholar] [CrossRef]
- Akyüz, A.; Ersus, S. Optimization of enzyme-assisted extraction of protein from the sugar beet (Beta vulgaris L.) leaves for alternative plant protein concentrate production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.H.; Gamlath, C.J.O.; Martin, G.J.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Khan, M.K.; Mazhar, H.; Shehzadi, U.; Ahmad, M.H.; Nadeem, M.T.; Ahmad, R.S.; Imran, M.; Khalid, W.; Khalid, M.Z.; Alharbi, S.A.; et al. The effect of ultrasound-assisted extraction process on techno-functional properties of protein from Moringa oleifera leaves. Cogent Food Agric. 2024, 10, 2436645. [Google Scholar] [CrossRef]
- Bernardi, S.; Lupatini-Menegotto, A.L.; Kalschne, D.L.; Moraes Flores, É.L.; Bittencourt, P.R.S.; Colla, E.; Canan, C. Ultrasound: A suitable technology to improve the extraction and techno-functional properties of vegetable food proteins. Plant Foods Hum. Nutr. 2021, 76, 1–11. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, X.; Zhou, H. Optimization of ultrasound-assisted extraction of defatted wheat germ proteins by reverse micelles. J. Cereal Sci. 2009, 50, 266–271. [Google Scholar] [CrossRef]
- Bernardi, S.; Kalschne, D.L.; Menegotto, A.L.L.; Flores, E.L.M.; Barin, J.S.; Fuchs, R.H.B.; Colla, E.; Canan, C. Feasibility of ultrasound-assisted optimized process of high purity rice bran protein extraction. Cienc. Rural 2020, 50, e20200012. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound-assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Illingworth, K.A.; Lee, Y.Y.; Siow, L.F. Functional properties of Moringa oleifera protein isolates as influenced by different isolation techniques, pH, and ionic strength. Food Bioprocess Technol. 2024, 17, 3060–3073. [Google Scholar] [CrossRef]
- Alavi, F.; Chen, L.; Emam-Djomeh, Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021, 354, 129494. [Google Scholar] [CrossRef]
- Tang, S.Q.; Du, Q.H.; Fu, Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason. Sonochem. 2021, 71, 105357. [Google Scholar] [CrossRef]
- Bou, R.; Navarro-Vozmediano, P.; Domínguez, R.; López-Gómez, M.; Pinent, M.; Ribas-Agustí, A.; Benedito, J.J.; Lorenzo, J.M.; Terra, X.; García-Pérez, J.V.; et al. Application of emerging technologies to obtain legume protein isolates with improved techno-functional properties and health effects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2200–2232. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products: Mechanisms, techniques, combinations, protocols and applications—A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Bao, T.; Zheng, X.; Chen, W.; Wang, J. A recyclable protein resource derived from cauliflower by-products: Potential biological activities of protein hydrolysates. Food Chem. 2017, 221, 114–122. [Google Scholar] [CrossRef] [PubMed]
- del Mar Contreras, M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Garrido, J.; Endara, A.; Landázuri, A.C.; Ramírez-Cárdenas, L. Optimization of the extraction and precipitation process of a leaf protein concentrate from Moringa oleifera Lam. Rev. Fac. Nal. Agron. Medellín 2022, 75, 9813–9821. [Google Scholar]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Fatima, K.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Khalid, W.; Al-Farga, A.; Alansari, W.S.; Shamlan, G.; Eskandrani, A.A. Ultrasound-assisted extraction of protein from Moringa oleifera seeds and its impact on techno-functional properties. Molecules 2023, 28, 2554. [Google Scholar] [CrossRef]
- Omura, M.H.; de Oliveira, A.P.H.; de Souza Soares, L.; dos Reis Coimbra, J.S.; de Barros, F.A.R.; Vidigal, M.C.T.R.; Baracat-Pereira, M.C.; de Oliveira, E.B. Effects of protein concentration during ultrasonic processing on physicochemical properties and techno-functionality of plant food proteins. Food Hydrocoll. 2021, 113, 106457. [Google Scholar] [CrossRef]
- Etzbach, L.; Gola, S.; Küllmer, F.; Acir, I.-H.; Wohlt, D.; Ignatzy, L.M.; Bader-Mittermaier, S.; Schweiggert-Weisz, U. Opportunities and challenges of plant proteins as functional ingredients for food production. Proc. Natl. Acad. Sci. USA 2024, 121, e2319019121. [Google Scholar] [CrossRef]
- Anyiam, P.N.; Tangjaidee, P.; Zhang, W.; Rawdkuen, S. A comparative study on novel-assisted extraction techniques for retrieving protein from Moringa oleifera seeds. Foods 2025, 14, 3046. [Google Scholar] [CrossRef]
- Peñalver, R.; Martínez-Zamora, L.; Lorenzo, J.M.; Ros, G.; Nieto, G. Nutritional and antioxidant properties of Moringa oleifera leaves in functional foods. Foods 2022, 11, 1107. [Google Scholar] [CrossRef]
- Kambuno, N.T.; Louisa, M.; Wuyung, P.E.; Supali, T. Impact of ultrasonic-assisted extraction on the protein yield from Moringa oleifera Lam leaves and its functional characterization. SSRN 2023. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Tshabalala, T. Diversity in the nutritional values of some Moringa oleifera Lam. cultivars. Diversity 2023, 15, 834. [Google Scholar] [CrossRef]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guiné, R.P.F.; Correia, P.M.R.; Mehra, R.; Kumar, H. Recent advances in drumstick (Moringa oleifera) leaves bioactive compounds: Composition, health benefits, bioaccessibility, and dietary applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef]
- Sahay, S.; Yadav, U.; Srinivasamurthy, S. Potential of Moringa oleifera as a functional food ingredient: A review. Int. J. Food Sci. Nutr. 2017, 2, 31–37. [Google Scholar]
- Arasaretnam, S.; Kiruthika, A.; Mahendran, T. Nutritional and mineral composition of selected green leafy vegetables. Ceylon J. Sci. 2018, 47, 35–41. [Google Scholar] [CrossRef]
- Abdelazim, A.M.; Afifi, M.; Abu-Alghayth, M.H.; Alkadri, D.H. Moringa oleifera: Recent insights for its biochemical and medicinal applications. J. Food Biochem. 2024, 48, e1270903. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam.: An overview. S. Afr. J. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Castillo-Lopez, R.I.; Leon-Felix, J.; Angulo-Escalante, M.A.; Gutierrez-Dorado, R.; Muy-Rangel, M.D.; Heredia, J.B. Nutritional and phenolic characterization of Moringa oleifera leaves grown in Sinaloa, Mexico. Pak. J. Bot. 2017, 49, 161–168. [Google Scholar]
- Pan American Health Organization. Recommended Nutrient Intakes and Population Nutrient Intake Goals for the Caribbean; Pan American Health Organization: Washington, DC, USA, 2020. [Google Scholar]
- Valdez-Solana, M.A.; Mejía-García, V.Y.; Téllez-Valencia, A.; García-Arenas, G.; Salas-Pacheco, J.; Alba-Romero, J.J.; Sierra-Campos, E. Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. J. Chem. 2015, 2015, 860381. [Google Scholar] [CrossRef]
- Masitlha, E.P.; Seifu, E.; Teketay, D. Nutritional composition and mineral profile of leaves of Moringa oleifera provenances grown in Gaborone, Botswana. Food Prod. Process Nutr. 2024, 6, 3. [Google Scholar] [CrossRef]
- FAO; IAEA. Development of a Protein Database and the Way Forward for Reviewing Protein Requirements—Report of a Joint FAO/IAEA Technical Meeting in Vienna, 10–13 October 2022; FAO: Rome, Italy; IAEA: Vienna, Austria, 2024; pp. 1–68. [Google Scholar] [CrossRef]
- Javed, M.S.; Alvi, S.Q.; Amjad, A.; Sardar, H.; Anwar, M.J.; Javid, A.; Fayssal, S.A.; Kumar, P.; Fayssal, T.A.; Wabaidur, S.M.; et al. Protein extracted from Moringa oleifera Lam. leaves: Bio-evaluation and characterization as suitable plant-based meat-protein alternative. Regul. Toxicol. Pharmacol. 2024, 146, 105536. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, K.A.; Lee, Y.Y.; Siow, L.F. The effect of isolation techniques on the physicochemical properties of Moringa oleifera protein isolates. Food Chem. Adv. 2022, 1, 100029. [Google Scholar] [CrossRef]
- Xiao, C.W.; Hendry, A.; Kenney, L.; Bertinato, J. L-Lysine supplementation affects dietary protein quality and growth and serum amino acid concentrations in rats. Sci. Rep. 2023, 13, 19943. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Ali, R.; Zhang, H.; Zafar, M.H.; Wang, M. Research progress in the role and mechanism of Leucine in regulating animal growth and development. Front. Physiol. 2023, 14, 1252089. [Google Scholar] [CrossRef]
- Bon, L.I.; Maksimovich, N.Y.; Burak, I.N. Amino acids that play an important role in the functioning of the nervous system: A review. Clin. Trials Clin. Res. 2023, 2, 1–5. [Google Scholar]
- Benhammouche, T.; Melo, A.; Martins, Z.; Faria, M.A.; Pinho, S.C.M.; Ferreira, I.M.; Zaidi, F. Nutritional quality of protein concentrates from Moringa oleifera leaves and in vitro digestibility. Food Chem. 2021, 348, 128858. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Aruna, T.E.; Raji, A.O. Nutritive value and acceptability of bread fortified with moringa seed powder. J. Saudi Soc. Agric. Sci. 2019, 18, 195–200. [Google Scholar] [CrossRef]
- Yadav, H.; Gaur, A.; Bansal, S.C. Effect of Moringa oleifera leaf powder supplementation in children with severe acute malnutrition in Gwalior District of Central India: A randomised controlled trial. J. Clin. Diagn. Res. 2022, 16, 9–14. [Google Scholar] [CrossRef]
- Das, R.S.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing on alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar] [CrossRef]
- Lafarga, T.; Álvarez, C.; Bobo, G.; Aguiló-Aguayo, I. Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT Food Sci. Technol. 2018, 98, 106–112. [Google Scholar] [CrossRef]
- Roger, R.A.; Rawdkuen, S. Properties of Moringa oleifera leaf protein from alkaline–acid extraction. Food Appl. Biosci. J. 2020, 8, 43–67. [Google Scholar]
- Zhang, M.; Fan, L.; Liu, Y.; Li, J. Relationship between protein native conformation and ultrasound efficiency for improving the physicochemical stability of water-in-oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129737. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Armel, A.A.J.; Edith, F.N.; Moses, M.C. Influence of compounds contents and particle size on some functional properties of Moringa oleifera leaves (Lam.) powders. Asian Food Sci. J. 2021, 20, 60–71. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Xia, S.; Cheong, L.; Tu, M. Recent progress in plant-based proteins: From extraction and modification methods to applications in the food industry. Food Chem. X 2024, 23, 101540. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. (Eds.) Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ogunsina, B.S.; Radha, C.; Govardhan Singh, R.S. Physicochemical and functional properties of full-fat and defatted Moringa oleifera kernel flour. Int. J. Food Sci. Technol. 2010, 45, 2433–2439. [Google Scholar] [CrossRef]
- Bocarando-Guzmán, M.D.; Luna-Suárez, S.; Hernández-Cázares, A.S.; Herrera-Corredor, J.A.; Hidalgo-Contreras, J.V.; Ríos-Corripio, M.A. Comparison of the physicochemical and functional properties of flour and protein isolate from moringa (Moringa oleifera Lam.) leaves. Int. J. Food Prop. 2022, 25, 733–747. [Google Scholar] [CrossRef]
- Illingworth, D.; Zhang, T.; Chen, X. Ultrasound-assisted extraction enhances yield and gel-forming ability of plant protein concentrates for functional food applications. Food Hydrocoll. 2024, 152, 108019. [Google Scholar]
- Wu, Y.-H.; Lu, L.-Q.; Li, J.-M.; Liu, X.-L.; Fu, Z.; Ren, M.-H. Incorporation of amylose improves rheological and textural properties of Moringa oleifera seed salt-soluble protein. Food Chem. X 2024, 23, 101757. [Google Scholar] [CrossRef]
- Huang, J.-W.; Liu, X.-L.; He, L.-L.; Wei, Q.-Y.; Wu, Y.-H.; Fu, Z. Effects of Moringa oleifera seed protein on the gel behavior and in vitro digestive properties of japonica, indica, and glutinous rice starch. J. Food Sci. 2025, 90, e70503. [Google Scholar] [CrossRef]
- Hu, H.; Fan, T.; Zhao, X.; Zhang, X.; Sun, Y.; Liu, H. Influence of pH and salt concentration on functional properties of walnut protein from different extraction methods. J. Food Sci. Technol. 2017, 54, 2833–2841. [Google Scholar] [CrossRef]
- Hernández, R.J.A.; Ulloa, J.A.; Ulloa, R.B.E.; Rosas, U.P. Valorization of noni (Morinda citrifolia) seeds as source of a protein concentrate and its physicochemical, functional, and structural characterization. Waste Biomass Valor. 2024, 15, 2033–2043. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; Volume 1. [Google Scholar]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids: Amino Acid Analysis Using Zorbax Eclipse-AAA Columns and the Agilent 1100 HPLC; Agilent Technologies: Palo Alto, CA, USA, 2000; pp. 1–10. [Google Scholar]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016, 212, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- Coffmann, C.W.; Garciaj, V.V. Functional properties and amino acid content of a protein isolate from mung bean flour. Int. J. Food Sci. Technol. 1977, 12, 473–484. [Google Scholar] [CrossRef]
- Yuceer, M. Structural and rheological characterization of liquid egg white modified with phospholipase A2 enzyme. J. Food Process Preserv. 2020, 44, e14450. [Google Scholar] [CrossRef]
- Lawal, O.S.; Adebowale, K.O.; Adebowale, Y.A. Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res. Int. 2007, 40, 1003–1011. [Google Scholar] [CrossRef]
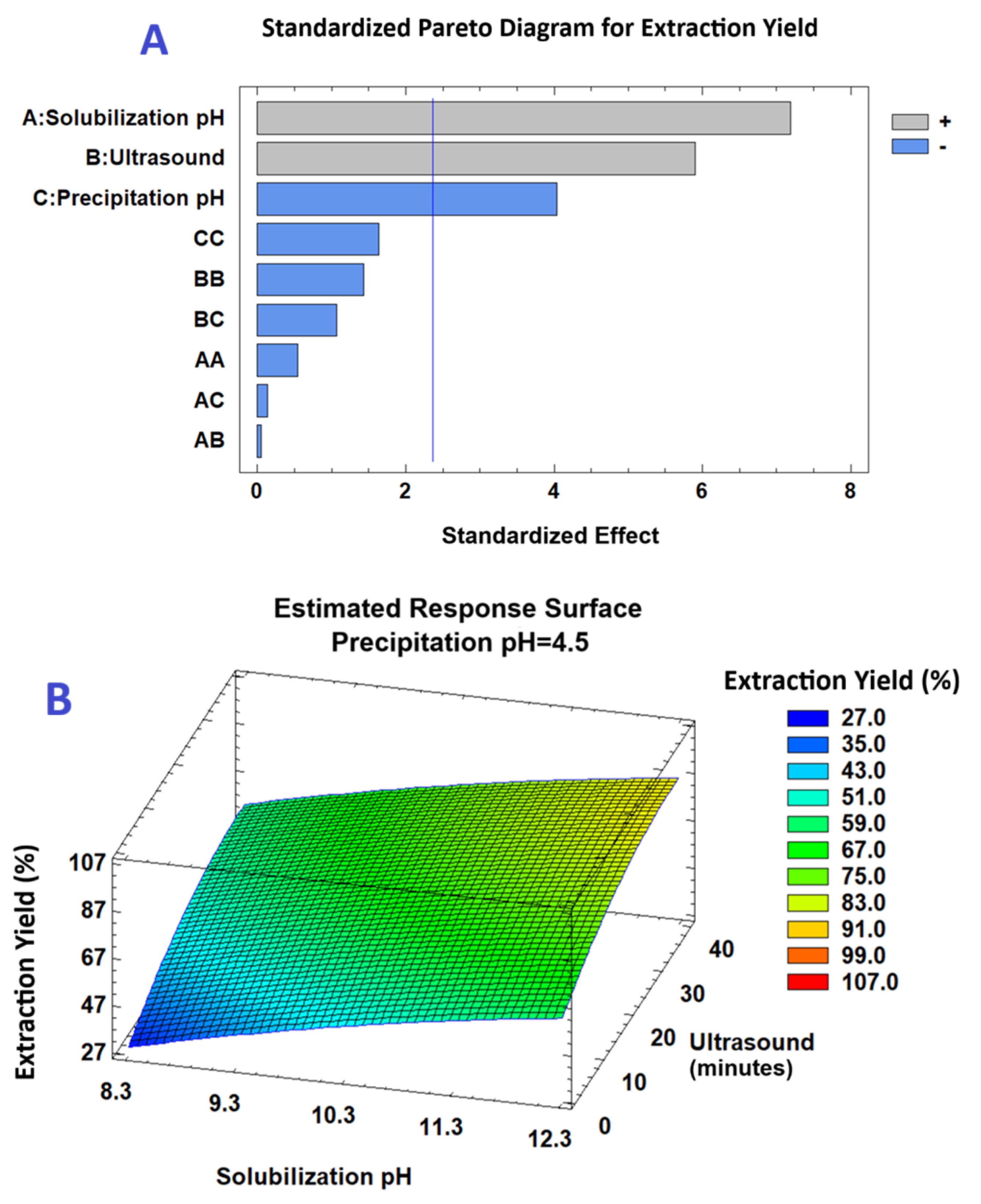

| Experimental Run | Solubilization pH | Extraction Time (min) | Precipitation pH | Extraction Yield (%) | Protein Content (%) |
|---|---|---|---|---|---|
| 1 | 8.31 | 20 | 4.5 | 45.07 | 42.47 |
| 2 | 9 | 10 | 3.5 | 51.4 | 50.75 |
| 3 | 11 | 10 | 3.5 | 65.18 | 50.84 |
| 4 | 10 | 20 | 4.5 | 64.48 | 52.84 |
| 5 | 11.68 | 20 | 4.5 | 82.44 | 50.39 |
| 6 | 10 | 36.81 | 4.5 | 75.75 | 46.45 |
| 7 | 10 | 20 | 4.5 | 65.43 | 52.17 |
| 8 | 11 | 30 | 5.5 | 65.44 | 51.69 |
| 9 | 9 | 30 | 5.5 | 52.80 | 50.92 |
| 10 | 9 | 10 | 5.5 | 45.95 | 53.52 |
| 11 | 10 | 20 | 6.18 | 52.40 | 50.40 |
| 12 | 11 | 30 | 3.5 | 78.21 | 47.71 |
| 13 | 10 | 3.18 | 4.5 | 45.33 | 55.45 |
| 14 | 11 | 10 | 5.5 | 57.76 | 51.53 |
| 15 | 10 | 20 | 4.5 | 65.65 | 51.53 |
| 16 | 10 | 20 | 2.81 | 67.25 | 49.28 |
| 17 | 9 | 30 | 3.5 | 65.86 | 45.38 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| A | 942.096 | 1 | 942.096 | 51.63 | 0.0002 |
| B | 635.764 | 1 | 635.764 | 34.84 | 0.0006 |
| C | 296.881 | 1 | 296.881 | 16.27 | 0.0050 |
| A2 | 5.32921 | 1 | 5.32921 | 0.29 | 0.6057 |
| A × B | 0.045 | 1 | 0.045 | 0.00 | 0.9618 |
| A × C | 0.3528 | 1 | 0.3528 | 0.02 | 0.8933 |
| B2 | 37.5154 | 1 | 37.5154 | 2.06 | 0.1947 |
| B × C | 20.9952 | 1 | 20.9952 | 1.15 | 0.3190 |
| C2 | 48.6333 | 1 | 48.6333 | 2.67 | 0.1466 |
| Residual error | 127.721 | 7 | 18.2459 | ||
| Corrected total | 2091.12 | 16 | |||
| R2 = 93.89% | |||||
| Adjusted R2 = 86.03% | |||||
| Standard error of estimate = 4.27 | |||||
| Mean absolute error = 2.10 | |||||
| Durbin–Watson statistic = 2.59 (p = 0.857) | |||||
| Lag-1 residual autocorrelation = −0.36 | |||||
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| A | 15.4375 | 1 | 15.4375 | 3.13 | 0.1200 |
| B | 49.7893 | 1 | 49.7893 | 10.10 | 0.0155 |
| C | 16.177 | 1 | 16.177 | 3.28 | 0.1129 |
| A2 | 33.3679 | 1 | 33.3679 | 6.77 | 0.0353 |
| A × B | 3.125 | 1 | 3.125 | 0.63 | 0.4520 |
| A × C | 1.6562 | 1 | 1.6562 | 0.34 | 0.5803 |
| B2 | 0.168812 | 1 | 0.168812 | 0.03 | 0.8584 |
| B × C | 4.59045 | 1 | 4.59045 | 0.93 | 0.3666 |
| C2 | 2.98783 | 1 | 2.98783 | 0.61 | 0.4617 |
| Residual error | 34.4952 | 7 | 4.92789 | ||
| Corrected total | 160.594 | 16 | |||
| R2 = 78.52% | |||||
| Adjusted R2 = 50.90% | |||||
| Standard error of estimate = 2.21 | |||||
| Mean absolute error = 1.16 | |||||
| Durbin–Watson statistic = 2.22 (p = 0.475) | |||||
| Lag-1 residual autocorrelation = −0.32 | |||||
| Component (g/100 g) | MOF | MOPC |
|---|---|---|
| Water | 7.71 ± 0.30 a | 5.09 ± 0.26 b |
| Protein | 29.38 ± 1.00 b | 53.97 ± 1.43 a |
| Lipids | 5.46 ± 0.38 b | 15.35 ± 0.15 a |
| Carbohydrates | 10.44 ± 0.62 a | 5.43 ± 0.56 b |
| Dietary fiber | 37.98 ± 1.16 a | 18.09 ± 0.64 b |
| Insoluble fiber | 32.06 ± 1.88 a | 17.38 ± 0.99 b |
| Soluble fiber | 5.92 ± 0.89 a | 0.71 ± 0.07 b |
| Ash | 9.03 ± 0.05 a | 2.07 ± 0.041 b |
| Mineral | MOF (mg/100 g) | RDDI (1–3 Years) (mg/Day) | RDDI (19–30 Years) (mg/Day) |
|---|---|---|---|
| Calcium | 1751.85 ± 3.60 | 500 | 1000 |
| Magnesium | 512.55 ± 1.80 | 80 | 100–310 |
| Potassium | 1144.58 ± 1.06 | 3000 | 4700 |
| Iron | 8.73 ± 0.05 | 5.8 | 13.7–29.4 |
| Sodium | 85.81 ± 1.06 | 1000 | 1500 |
| Lead | ND | - | - |
| Amino Acids | FAO and IAEA (2024) [42] (g/kg/d of Protein) | MOF (g/kg of Protein) | MOPC (g/kg of Protein) |
|---|---|---|---|
| Histidine | 15 | 18.18 | 16.08 |
| Isoleucine | 30 | 50.29 | 48.64 |
| Leucine | 59 | 10.44 | 110.98 |
| Lysine | 45 | 102.13 | 94.90 |
| Methionine + cystine | 22 | 26.31 | 13.30 |
| Phenylalanine + tyrosine | 38 | 78.53 | 86.76 |
| Threonine | 23 | 54.16 | 42.29 |
| Tryptophan | 6 | 11.61 | 38.71 |
| Valine | 30 | 63.83 | 57.18 |
| Arginine | - | 59.19 | 63.93 |
| Aspartic acid | - | 70.79 | 63.33 |
| Glycine | - | 112.57 | 100.66 |
| Glutamic acid | - | 50.68 | 43.88 |
| Serine | - | 229.01 | 136.39 |
| Proline | - | 41.01 | 33.35 |
| Parameters | |||||
|---|---|---|---|---|---|
| pH | 4.63 ± 0.01 | ||||
| Water activity (aw) | 0.44 ± 0.007 | ||||
| Color | |||||
| L* (lightness) | 23.90 ± 1.57 | ||||
| C* (chroma) | 16.36 ± 0.58 | ||||
| h° (hue angle) | 88.11 ± 1.09 | ||||
| Water absorption (g/g) | 1.66 ± 0.07 | ||||
| Oil absorption (g/g) | 2.52 ± 0.18 | ||||
| pH 2 | pH 4 | pH 6 | pH 8 | pH 10 | |
| Solubility (%) | 24.26 ± 0.15 a | 0.23 ± 0.05 e | 6.54 ± 0.56 d | 21.65 ± 0.12 c | 22.42 ± 0.15 b |
| Gelling (%) | 55.55 ± 9.62 b | 28.88 ± 7.69 b,c | 55.55 ± 19.24 b | 100 ± 0.00 a | 6.66 ± 0.00 c |
| Foaming capacity (%) | 21.11 ± 1.92 a | 15.55 ± 3.84 a | 3.33 ± 0.00 b | 15.00 ± 1.66 a | 20.00 ± 3.33 a |
| Foam stability (%) | 94.44 ± 9.62 a | 41.66 ± 14.43 b | 30.10 ± 26.27 b | 41.38 ± 23.57 b | 35.95 ± 9.59 b |
| Emulsifying capacity (%) | 27.60 ± 6.60 b | 4.00 ± 0.00 c | 4.00 ± 0.00 c | 0.00 ± 0.00 c | 58.66 ± 2.30 a |
| Emulsion stability (%) | 14.78 ± 5.52 c | 36.66 ± 11.54 b | 36.66 ± 11.54 b | 0.00 ± 0.00 c | 79.52 ± 0.82 a |
| Experimental Run | Solubilization pH | Extraction Time (minutes) | Precipitation pH |
|---|---|---|---|
| 1 | 8.31 | 20 | 4.5 |
| 2 | 9 | 10 | 3.5 |
| 3 | 11 | 10 | 3.5 |
| 4 | 10 | 20 | 4.5 |
| 5 | 11.68 | 20 | 4.5 |
| 6 | 10 | 36.81 | 4.5 |
| 7 | 10 | 20 | 4.5 |
| 8 | 11 | 30 | 5.5 |
| 9 | 9 | 30 | 5.5 |
| 10 | 9 | 10 | 5.5 |
| 11 | 10 | 20 | 6.18 |
| 12 | 11 | 30 | 3.5 |
| 13 | 10 | 3.18 | 4.5 |
| 14 | 11 | 10 | 5.5 |
| 15 | 10 | 20 | 4.5 |
| 16 | 10 | 20 | 2.81 |
| 17 | 9 | 30 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tranquilino-Rodríguez, E.; Bautista-Durán, E.; Virgen-Ortiz, J.J.; Garnica-Romo, M.G.; Alvarez-Cortés, O.; Ochoa-Manzo, G.M.; Martínez-Flores, H.E. Nutritional and Techno-Functional Properties of Ultrasound-Assisted Moringa oleifera Leaf Protein Concentrate with Potential Applications in Food Gels. Gels 2025, 11, 843. https://doi.org/10.3390/gels11110843
Tranquilino-Rodríguez E, Bautista-Durán E, Virgen-Ortiz JJ, Garnica-Romo MG, Alvarez-Cortés O, Ochoa-Manzo GM, Martínez-Flores HE. Nutritional and Techno-Functional Properties of Ultrasound-Assisted Moringa oleifera Leaf Protein Concentrate with Potential Applications in Food Gels. Gels. 2025; 11(11):843. https://doi.org/10.3390/gels11110843
Chicago/Turabian StyleTranquilino-Rodríguez, Eunice, Estefanía Bautista-Durán, José Juan Virgen-Ortiz, Ma. Guadalupe Garnica-Romo, Osvaldo Alvarez-Cortés, Gabriela Monserrat Ochoa-Manzo, and Héctor Eduardo Martínez-Flores. 2025. "Nutritional and Techno-Functional Properties of Ultrasound-Assisted Moringa oleifera Leaf Protein Concentrate with Potential Applications in Food Gels" Gels 11, no. 11: 843. https://doi.org/10.3390/gels11110843
APA StyleTranquilino-Rodríguez, E., Bautista-Durán, E., Virgen-Ortiz, J. J., Garnica-Romo, M. G., Alvarez-Cortés, O., Ochoa-Manzo, G. M., & Martínez-Flores, H. E. (2025). Nutritional and Techno-Functional Properties of Ultrasound-Assisted Moringa oleifera Leaf Protein Concentrate with Potential Applications in Food Gels. Gels, 11(11), 843. https://doi.org/10.3390/gels11110843








