Development of Jellyfish (Stomolophus sp. 2) Gelatine–Chitosan Films: Structural, Physical, and Antioxidant Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Properties of Jellyfish Gelatine (JG)
2.2. Chemical Characterisation of Films
2.2.1. FT-IR
2.2.2. Proton Nuclear Magnetic Resonance (1H-NMR)
2.3. Viscosity Values
2.4. Water Content and Stability of Films
2.5. Thickness, Appearance, and Optical Properties of Films
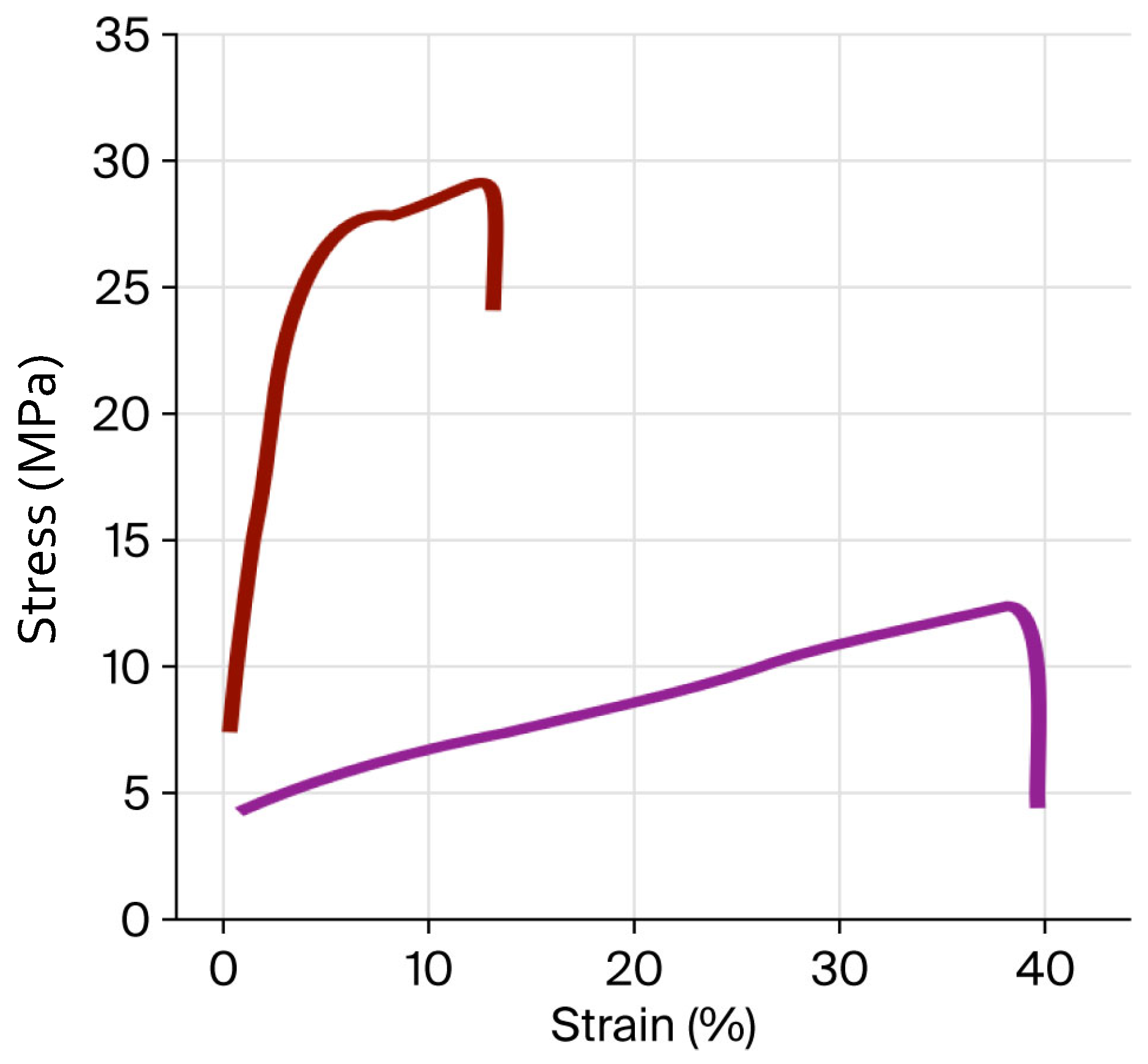
2.6. Stress–Strain Mechanical Properties (SSMPs) of Films
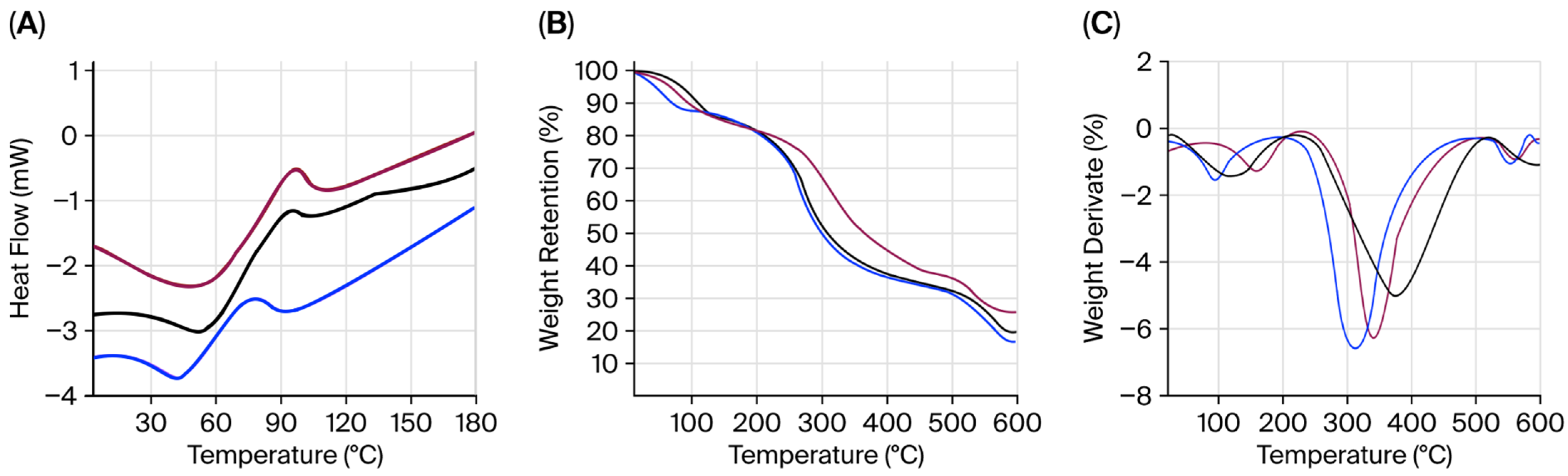
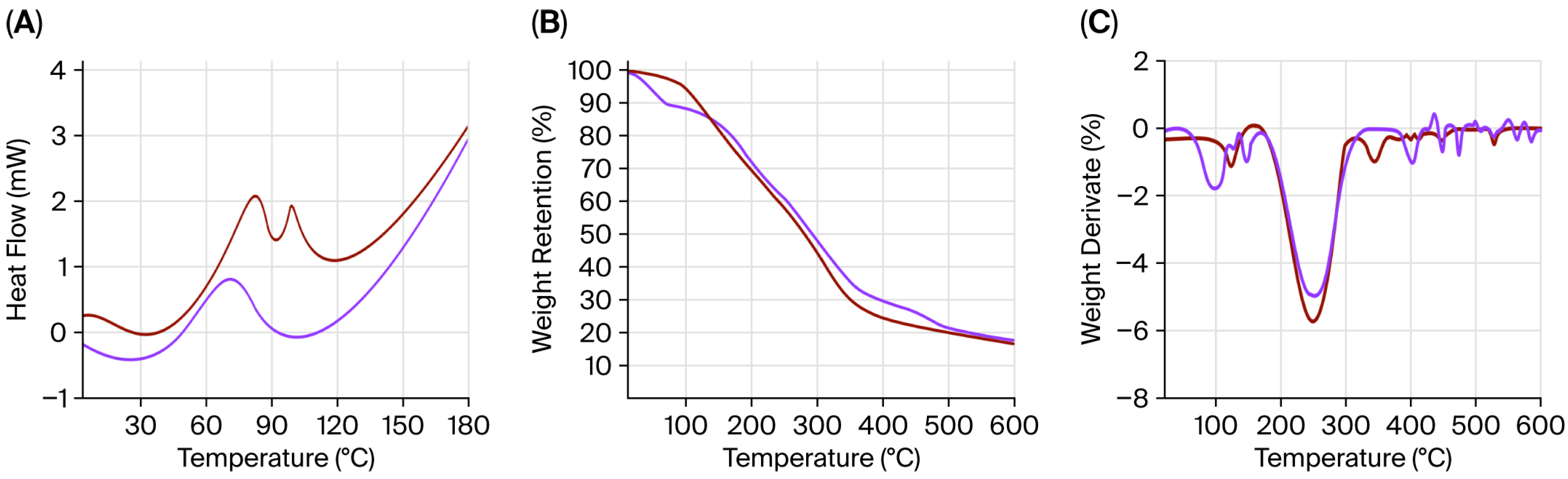
2.7. Thermogravimetric Analysis
2.8. Antioxidant Activity of Films
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Gelatine Extraction and Analysis
4.3. Gelatine–Chitosan Films
4.4. Analysis
4.4.1. Chemical Characterisation of Jellyfish Gelatine–Chitosan (JG-CH) Film
4.4.2. Viscosity
4.4.3. Water Content and Stability
4.4.4. Thickness, Appearance, and Optical Properties
4.4.5. Stress–Strain Mechanical Properties
4.4.6. Thermal Properties
4.4.7. In Vitro Antioxidant Activity of Films
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JG | Jellyfish gelatine |
| CH | Chitosan |
| CG | Commercial gelatine |
| GLY | Glycerol |
| JG-CH | Jellyfish gelatine–chitosan–glycerol film |
| CG-CH | Commercial gelatine–chitosan–glycerol film |
| OH | Hydroxyl group |
| NH2 | Amino group |
| COOH | Carboxyl group |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| IC50 | Half-maximal inhibitory concentration |
| μ | Viscosity |
| W | Stability in aqueous solution |
| ΔEab | Differences in colour |
| TS | Tensile stress |
| εb | Elongation at break |
| E | Elastic modulus |
| SSMP | Stress–strain mechanical properties |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| Tg | Glass transition temperature |
| Tm | Melting point |
| FT-IR | Fourier-transform infrared spectroscopy |
| 1H-NMR | Proton nuclear magnetic resonance |
| C=O | Carbonyl group |
| C-H | Carbon hydrogen bond |
| C-O | Chitosan ether linkage and gelatine carboxylic group |
| NaOH | Sodium hydroxide |
| Hyp | Hydroxyproline |
| Mpa | Megapascal |
References
- Jung, Y.L.; Yoo, H.S. Environmental, social, and governance activities and firm performance: Global evidence and the moderating effect of market competition. Corp. Soc. Responsib. Environ. Manag. 2023, 30, 2830–2839. [Google Scholar] [CrossRef]
- Zhou, S.; Meenu, M.; Xu, B. Gelling, textural, and sensory properties of grass jelly formulated with different starches. Food Prod. Process Nutr. 2025, 7, 5. [Google Scholar] [CrossRef]
- Venugopal, V. (Ed.) Marine Products for Healthcare; CRC Press: Boca Raton, FL, USA, 2008; p. 528. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Senanayake, S.; Wijesekara, W.; Perera, P.; Pathmalal, M.; Marapana, R. Characterization of biodegradable films prepared from gelatin extracted from jellyfish Acromitus flagellates using hot water extraction and microwave-assisted extraction. Food Pack. Shelf 2024, 44, 101315. [Google Scholar] [CrossRef]
- Thumthanaruk, B.; Rodsuwan, U.; Chancharern, P.; Kerdchoechuen, O.; Laohakunjit, N.; Chism, G.W. Physico-Chemical Properties of Extruded Copolymer Film. J. Food Process. Preserv. 2017, 41, e12808. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.; Yang, H.J.; Won, M.; Song, K.B. Characterisation of jellyfish protein films with added transglutaminase and wasabi extract. Int. J. Food Sci. Technol. 2015, 50, 1683–1689. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S. Modified fish gelatin as an alternative to mammalian gelatin in modern food technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef]
- Rigueto, T.; Rosseto, C.V.; Alessandretti, M.; De Oliveira, I.; Wohlmuth, D.A.R.; Menezes, J.F.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin films from wastes: A review of production, characterization, and application trends in food preservation and agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef] [PubMed]
- Staroszczyk, H.; Kusznierewicz, B.; Malinowska-Pańczyk, E.; Sinkiewicz, I.; Gottfried, K.; Kołodziejska, I. Fish gelatin films containing aqueous extracts from phenolic-rich fruit pomace. LWT 2020, 117, 108613. [Google Scholar] [CrossRef]
- Chiarelli, P.G.; Fair, C.G.; Pegg, R.B.; Mis Solval, K. Modifying and improving the Bloom strength and rheological properties of jellyfish gelatin. Food Hydrocoll. 2025, 159, 110692. [Google Scholar] [CrossRef]
- Xu, X.; Dai, M.; Yan, J.; Du, Y.; Wang, C.; Lai, B.; Wu, H. Comparison of the properties of composite films constructed from chitosan and gelatin from two jellyfish species. Food Hydrocoll. 2025, 167, 111412. [Google Scholar] [CrossRef]
- Eranda, D.H.U.; Chaijan, M.; Panpipat, W.; Karnjanapratum, S.; Cerqueira, M.A.; Castro-Muñoz, R. Gelatin-chitosan interactions in edible films and coatings doped with plant extracts for biopreservation of fresh tuna fish products: A review. Int. J. Biol. Macromol. 2024, 280, 135661. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Lima, C.; De Oliveira, R.; Figueiró, S.; Wehmann, C.; Góes, J.; Sombra, A. DC conductivity and dielectric permittivity of collagen–chitosan films. Mater. Chem. Phys. 2006, 99, 284–288. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; De Guzzi Plepis, A.M. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Copinet, A.; Tighzert, L.; Coma, V. Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J. Polym. Environ. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Cisneros-Mata, M.A.; Mangin, T.; Bone, J.; Rodriguez, L.; Smith, S.L.; Gaines, S.D. Fisheries governance in the face of climate change: Assessment of policy reform implications for Mexican fisheries. PLoS ONE 2019, 14, e0222317. [Google Scholar] [CrossRef]
- Brotz, L.; Cisneros-Montemayor, A.M.; Cisneros-Mata, M.Á. The race for jellyfish: Winners and losers in Mexico’s Gulf of California. Mar. Policy. 2021, 134, 104775. [Google Scholar] [CrossRef]
- Gómez-Salinas, L.C.; López-Martínez, J.; Morandini, A.C. The young stages of the cannonball jellyfish (Stomolophus sp. 2) from the central Gulf of California (Mexico). Divers 2021, 13, 229. [Google Scholar] [CrossRef]
- Jarms, G.; Morandini, A.C. World Atlas of Jellyfish; Dölling und Galitz Verlag: Hamburg, Germany, 2019; p. 816. [Google Scholar]
- Banha, T.N.S.; Morandini, A.C.; Rosário, R.P.; Martinelli Filho, J.E. Scyphozoan jellyfish (Cnidaria, Medusozoa) from Amazon coast: Distribution, temporal variation and length–weight relationship. J. Plankton Res. 2020, 42, 767–778. [Google Scholar] [CrossRef]
- Sastré-Velásquez, C.D.; Rodríguez-Armenta, C.; Minjarez-Osorio, C.; De la Re-Vega, E. Current status of the knowledge of the cannonball Jellyfish (Stomolophus meleagris). Epistemus 2022, 16, 75–83. [Google Scholar] [CrossRef]
- Cruz, D. La Pesquería de la Medusa Bola de Cañón en México (The Cannonball Jellyfish Fishery in Mexico). Available online: https://sirbaa.com/category/recursos-pesqueros/ (accessed on 19 June 2025).
- Villalba-Urquidy, B.S.; Velázquez-Valdez, L.P.; Bracamontes-Picos, J.S.; Del Toros-Sánchez, C.L.; Chan-Higuera, J.E.; Ezquerra-Brauer, J.M. Conversion of dry-salted cannonball jellyfish (Stomolophus meleagris) umbrella and oral arms to cornmeal snacks and gelatin with antioxidant properties. Fishes 2022, 7, 277. [Google Scholar] [CrossRef]
- Esparza-Espinoza, D.M.; Santacruz-Ortega, H.C.; Plascencia-Jatomea, M.; Aubourg, S.P.; Salazar-Leyva, J.A.; Rodríguez-Felix, F.; Ezquerra-Brauer, J.M. Chemical-structure identification of crude gelatin from jellyfish (Stomolophus meleagris) and evaluation of its potential biological activity. Fishes 2023, 8, 246. [Google Scholar] [CrossRef]
- Yan, J.; Dai, M.; Zhang, Z.; Wang, C.; Lai, B.; Wu, H. Comparison of gel and functional properties of gelatin derived from two jellyfish, Stomolophus meleagris and Rhopilema esculentum kishinouye. Food Hydrocoll. 2025, 159, 110658. [Google Scholar] [CrossRef]
- Villalba-Urquidy, B.d.S.; Torres-Arreola, W.; Del Toro-Sánchez, C.L.; Medina, I.; Burgos-Hernández, A.; Santacruz-Ortega, H.d.C.; Ezquerra Brauer, J.M. Collagen extracts from blue cannonball jellyfish (Stomolophus meleagris): Antioxidant properties, chemical structure, and proteomic identification. Ital. J. Food Sci. 2025, 37, 180–193. [Google Scholar] [CrossRef]
- Rodsuwan, U.; Thumthanaruk, B.; Kerdchoechuen, O.; Laohakunjit, N. Functional properties of type A gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 507–514. Available online: http://www.ifrj.upm.edu.my/23%20(02)%202016/(8).pdf (accessed on 17 September 2022).
- Karim, A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, J.; Zhu, M.; Yang, H.; Liu, J.; Liu, Y. Study of physicochemical and gelation properties of fish gelatin from different sources. Appl. Sci. 2023, 13, 5337. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 5, 3273–3327. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Suriyati, S.; Meriatna, M.; Sulhatun, S.; Lestari, D.W. Preparation and characterization of chitosan-gelatin-glycerol biocomposite for primary wound dressing. Int. J. Eng. Sci. Inf. Technol. 2022, 2, 64–69. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles, W.; Desbrières, J. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Guerrero, P.; Retegi, A.; Gabilondo, N.; De la Caba, K. Mechanical and thermal properties of soy protein films processed by casting and compression. J. Food Eng. 2010, 100, 145–151. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Razmjooee, K.; Saber-Samandari, S.; Toghraie, D. Fabrication of chitosan-gelatin films incorporated with thymol-loaded alginate microparticles for controlled drug delivery, antibacterial activity and wound healing: In vitro and in vivo studies. Int. J. Biol. Macromol. 2022, 223, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, G.D.; Nes, E.; Amurao, M.; Rahal, A.; Krasnosselskaia, L.; Cameron, I. An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biol. Int. 2006, 30, 66–73. [Google Scholar] [CrossRef]
- Damonte, G.; Vallin, A.; Fina, A.; Monticelli, O. On the Development of an Effective Method to Produce Conductive PCL Film. Nanomaterials 2021, 11, 1385. [Google Scholar] [CrossRef]
- Sreeja, S.J.; Tamilarutselvi, K.; Tamilselvi, A.; Sarojini, K.P.; Jayala Jasmin, K.; Malini, M.M. Production of chitin and conversion into chitosan from crab (Scylla tranquebarica) shells and evaluation of its antioxidant activities. Biomass Conv. Bioref. 2023, 14, 17193–17199. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Kennedy, C.; Wess, T. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Salomé Machado, A.A.; Martins, V.C.A.; Plepis, A.M.G. Thermal and rheological behavior of collagen. Chitosan Blends. J. Therm. Anal. Cal. 2002, 67, 491–498. [Google Scholar] [CrossRef]
- Moraes, I.C.; Carvalho, R.A.; Bittante, A.M.Q.; Solorza-Feria, J.; Sobral, P.J. Film forming solutions based on gelatin and poly(vinyl alcohol) blends: Thermal and rheological characterizations. J. Food Eng. 2009, 95, 588–596. [Google Scholar] [CrossRef]
- Zheng, T.; Tang, P.; Lirui, S.; Bu, H.; Li, G. Rheological behaviour of collagen/chitosan blended solutions. J. Appl. Polym. Sci. 2021, 138, 50840. [Google Scholar] [CrossRef]
- Duan, Q.; Chen, Y.; Yu, L.; Xie, F. Chitosan–gelatin films: Plasticizers/nanofillers affect chain interactions and material properties in different ways. Polymers 2022, 14, 3797. [Google Scholar] [CrossRef]
- Voron’ko, N.G.; Derkach, S.R.; Kuchina, Y.A.; Sokolan, N.I. The chitosan–gelatin (bio)polyelectrolyte complexes formation in an acidic medium. Carbohydr. Polym. 2016, 138, 265–272. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Farhat, N.; Javier, L.; Van Loosdrecht, M.; Kruithof, J.; Vrouwenvelder, J. Role of feed water biodegradable substrate concentration on biofouling: Biofilm characteristics, membrane performance and cleanability. Water Res. 2019, 150, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Y.; Gao, Q.; Li, Y.; Bao, X.; Zheng, J.; Zhou, Z. Identification and Verification of the Blue Pigment in the Edible Jellyfish (Rhopilema esculentum). Aquac. Res. 2023, 2023, 2994722. [Google Scholar] [CrossRef]
- Moreno-Ricardo, M.A.; Gómez-Contreras, P.; González-Delgado, Á.D.; Hernández-Fernández, J.; Ortega-Toro, R. Development of films based on chitosan, gelatin and collagen extracted from bocachico scales (Prochilodus magdalenae). Heliyon 2024, 10, e25194. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.A.; Hosseini, S.M.; Yousefi, M. Application of electrospinning technique in development of intelligent food packaging: A short review of recent trends. Food Sci. Nut. 2020, 8, 4656–4665. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2025, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Meng, F.; Wang, S.; Li, P.; Geng, C.; Zhang, X.; Zhou, Z.; Kong, F. Tough, antibacterial fish scale gelatin/chitosan film with excellent water vapor and UV-blocking performance comprising liquefied chitin and silica sol. Int. J. Biol. Macromol. 2022, 222, 3250–3260. [Google Scholar] [CrossRef]
- Jang, B.Z. Advanced Polymer Composites: Principles and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 1994; p. 297. ISBN 0871704919. [Google Scholar]
- Lueyot, A.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wonganu, B.; Wongsa-Ngasri, P.; Charoenlappanit, S.; Roytrakul, S.; Thumthanaruk, B. Influence of collagen and some proteins on gel properties of jellyfish gelatin. PLoS ONE 2021, 16, e0253254. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J. Antioxidant and antimicrobial poly(vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Li, X.; Ren, Z.; Wang, R.; Liu, L.; Zhang, J.; Ma, F.; Khan, M.Z.H.; Zhao, D.; Liu, X. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 2021, 349, 129208. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysates. J. Agri. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Vazquez-Ortiz, F.; Moron-Fuenmayor, O.; Gonzalez-Mendez, N. Hydroxyproline measurement by HPLC: Improved method of total collagen determination in meat samples. J. Liq. Chromatogr. Rel. Technol. 2004, 27, 2771–2780. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Protoggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, Y.; Liu, G. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 2010, 46, 205–208. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Rodríguez-Feliz, F.; Castillo-Ortega, M.M.; Yepiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan chomposite films: Thermal, structural, mechanical and antifungal properties. Cabohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; García-Amezquita, L.E.; Ramos-Martínez, A.; Tirado Gallegos, J.M.; Bello-Pérez, L.A.; Salgado-Delgado, R. Soluciones formadoras de películas a base de almidón oxidado de avena mezclada con quitosano: Caracterización reológica y propiedades mecánicas de sus películas. Rev. Iberoam. Pol. 2013, 14, 293–304. [Google Scholar]
- Vieira, C.B.; Sousa, J.R.; Do Vale, D.A.; Guimarães, C.P.; De Sousa, K.C.; Mattos, A.L.A.; Silva, A.L.C.; De Sá Moreira Souza Filho, M.; Souza, B.W.S. Edible films based on sulfated polysaccharides from the seaweed Gracilaria birdiae: Physicochemical, optical and mechanical properties. Carbohydr. Res. 2025, 552, 109473. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.L. Edible wheat gluten films influence of the main process variables on film properties using response surface methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- Kurek, M.; Descours, E.; Poldan, P.; Julou, A.; Pitois, A.; Klepac, D.; Vallet, N.; Galić, K. Possibility of storing olive oil in antioxidant biobased pouches made of chitosan and gelatin. Food Hydrocoll. 2024, 151, 109835. [Google Scholar] [CrossRef]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food. Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- ASTM D882-10; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. ASTM International: Conshohocken, PA, USA, 2010. [CrossRef]
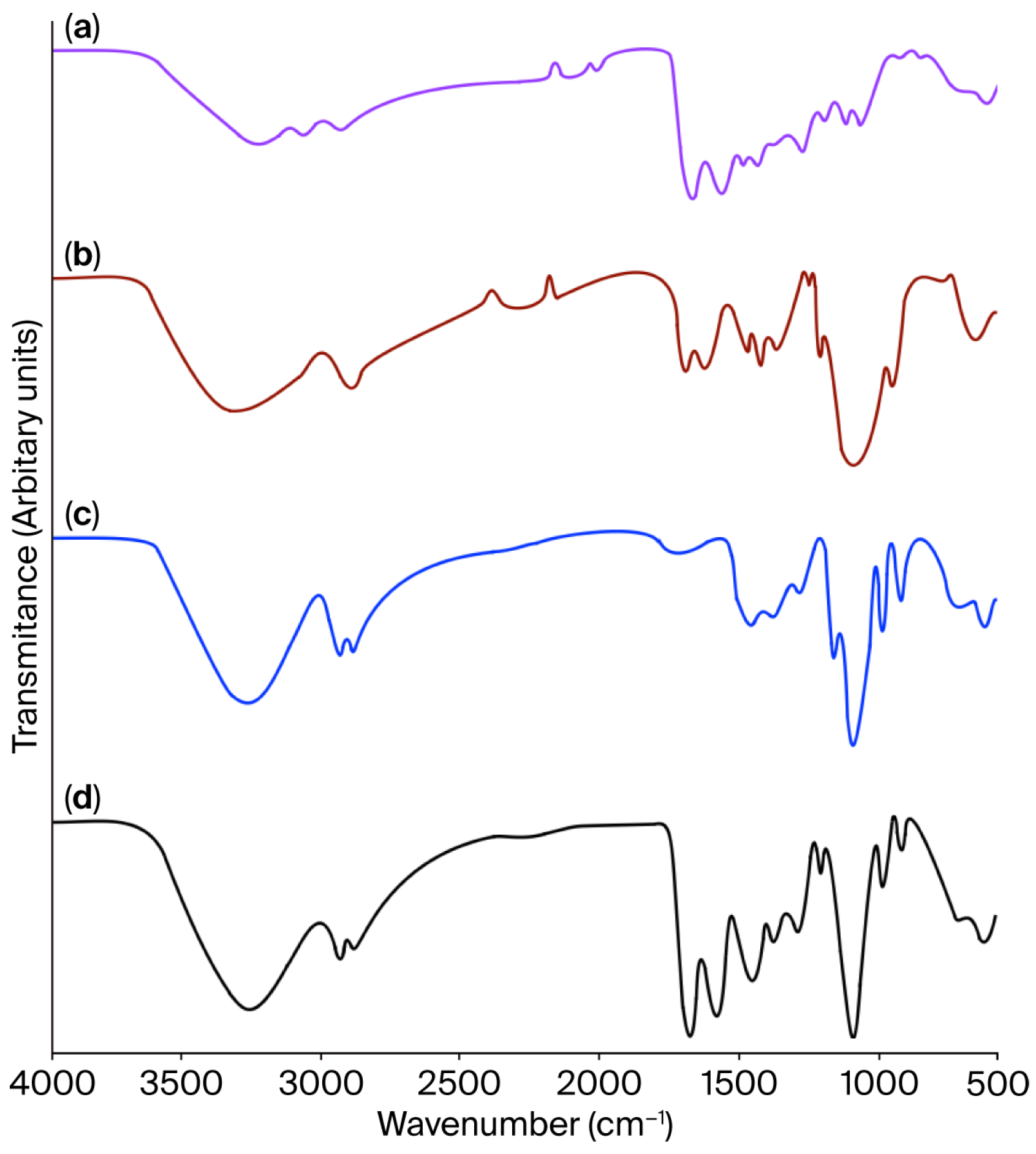
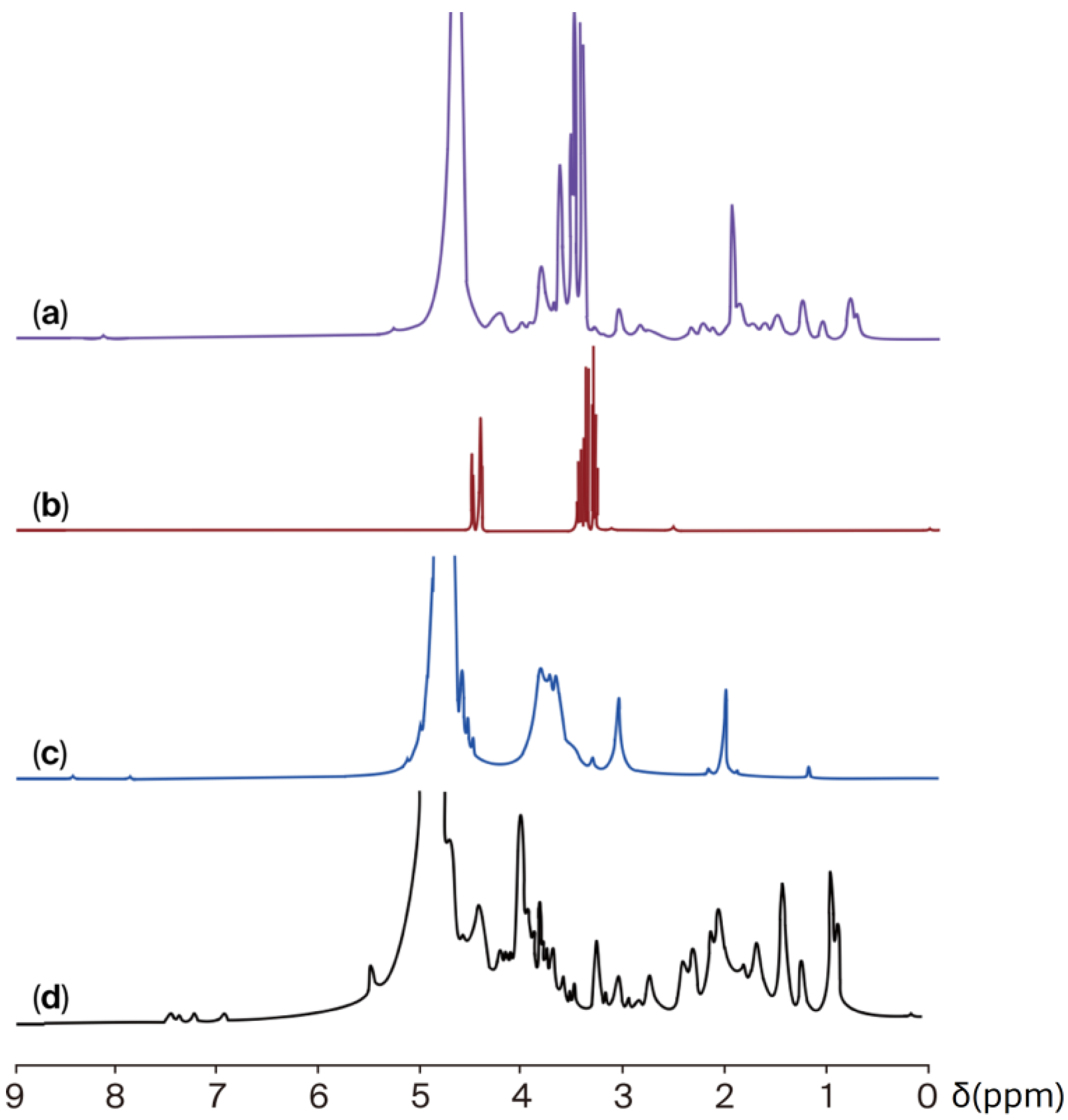
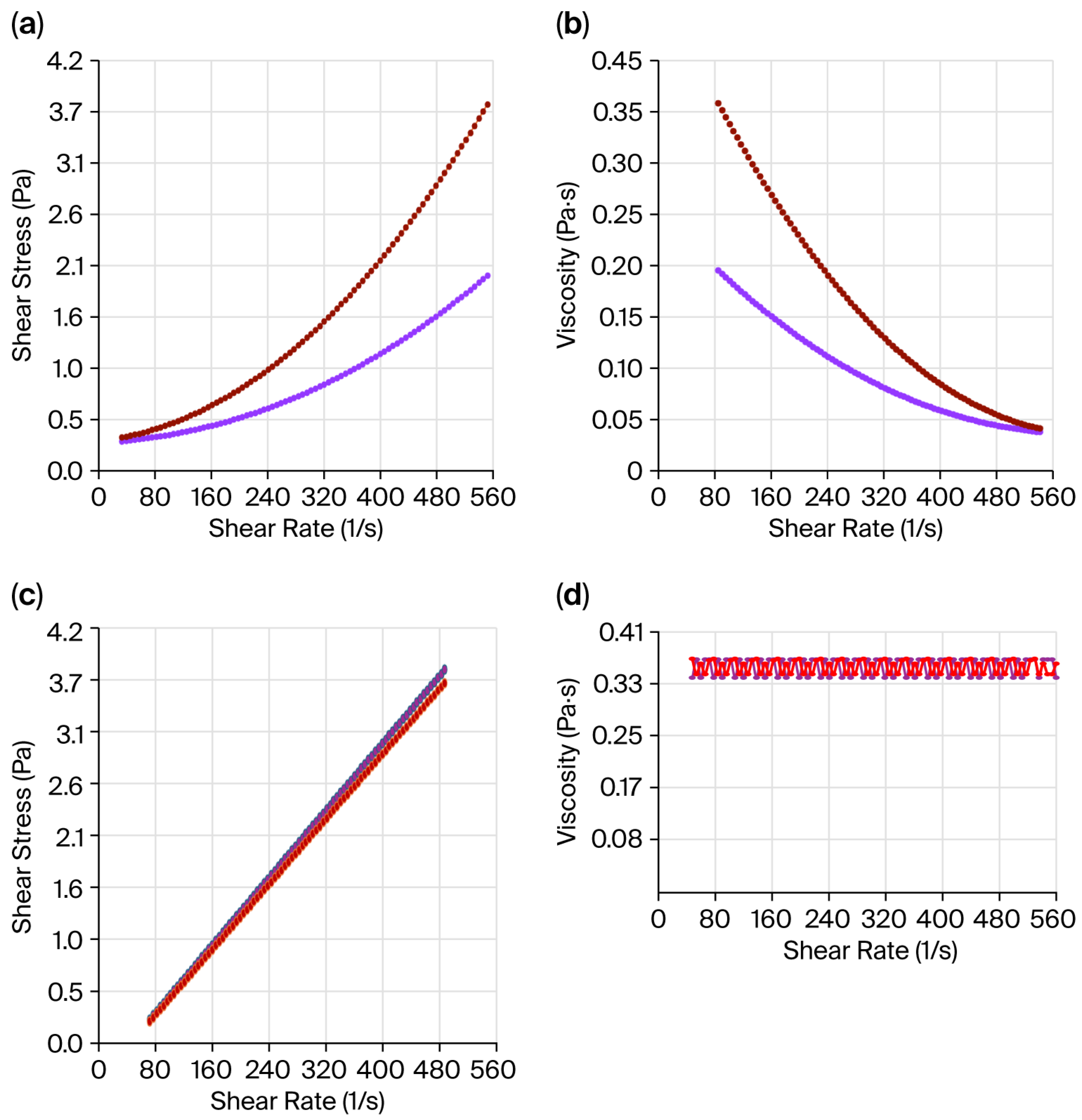
| Assignments | JG | CH | JG-CH Film |
|---|---|---|---|
| N-H stretching, Amide A | 3247 | 3260 | 3249 |
| CH2 and NH3+ asymmetric stretching, Amide B | 2950 | – | – |
| C-H stretching vibration of methylene, -CH2 groups | 2835 | 2835 | 2835 |
| C=O stretching, Amide I | 1635 | 1645 | 1630 |
| N–H and C–N torsional vibration, Amide II | 1585 | 1591 | 1545 |
| CH residual groups, Amide III | 1283 | – | 1285 |
| Primary alcohol OH group | 1480 | 1480 | 1480 |
| -CH2 torsion and C-N tension vibration | – | 1406–1249 | – |
| Pyranosic and C-O-C groups | – | 1080 | – |
| N-H and C-OH out-of-plane bending | 682–562 | 685–564 | 675–550 |
| Assignments | Wavenumber (cm−1) |
|---|---|
| OH stretching | 3290 |
| CH stretching | 2930–2870 |
| Carboxyl group, C-OH bending | 1420 |
| Primary alcohol, C-OH stretch | 1110 |
| Skeletal vibration, C-C | 990–850 |
| Parameter | JG-CH | CG-CH |
|---|---|---|
| L | 86.52 b ± 0.38 | 87.71 a ± 0.70 |
| A | 0.37 a ± 0.02 | 0.41 a ± 0.01 |
| B | −2.15 a ± 0.05 | −2.08 a ± 0.06 |
| ΔE*ab 2 | 1.61 ± 0.32 | |
| Wavelengths (nm) | Transmittance 2 (%) | |
|---|---|---|
| JG-CH | CG-CH | |
| 200 | 0 | 0 |
| 250 | 41 ± 1.3 | 42 ± 1.8 |
| 300 | 60 ± 4.1 | 68 ± 3.9 |
| 400 | 84 ± 3.3 | 90 ± 2.7 |
| 500 | 88 ± 2.8 | 92 ± 2.3 |
| 600 | 89 ± 3.8 | 92 ± 3.1 |
| 700 | 89 ± 2.4 | 92 ± 2.3 |
| 800 | 90 ± 1.8 | 92 ± 1.3 |
| Opacity index (Abs600/thickness) 3 | 1.89 ± 0.16 a | 1.66 ± 0.09 a |
| Property | JG-CH | CG-CH |
|---|---|---|
| TS (MPa) | 12.07 b ± 0.52 | 29.23 a ± 0.31 |
| εb (%) | 40.05 a ± 2.32 | 17.50 b ± 0.51 |
| E (MPa) | 2.39 a ± 0.14 | 1.41 a ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esparza-Espinoza, D.M.; Rodríguez-Felix, F.; Santacruz-Ortega, H.d.C.; Plascencia-Jatomea, M.; Salazar-Leyva, J.A.; Aubourg, S.P.; Ezquerra-Brauer, J.M. Development of Jellyfish (Stomolophus sp. 2) Gelatine–Chitosan Films: Structural, Physical, and Antioxidant Properties. Gels 2025, 11, 836. https://doi.org/10.3390/gels11100836
Esparza-Espinoza DM, Rodríguez-Felix F, Santacruz-Ortega HdC, Plascencia-Jatomea M, Salazar-Leyva JA, Aubourg SP, Ezquerra-Brauer JM. Development of Jellyfish (Stomolophus sp. 2) Gelatine–Chitosan Films: Structural, Physical, and Antioxidant Properties. Gels. 2025; 11(10):836. https://doi.org/10.3390/gels11100836
Chicago/Turabian StyleEsparza-Espinoza, Dania Marisol, Francisco Rodríguez-Felix, Hisila del Carmen Santacruz-Ortega, Maribel Plascencia-Jatomea, Jesús Aarón Salazar-Leyva, Santiago P. Aubourg, and Josafat Marina Ezquerra-Brauer. 2025. "Development of Jellyfish (Stomolophus sp. 2) Gelatine–Chitosan Films: Structural, Physical, and Antioxidant Properties" Gels 11, no. 10: 836. https://doi.org/10.3390/gels11100836
APA StyleEsparza-Espinoza, D. M., Rodríguez-Felix, F., Santacruz-Ortega, H. d. C., Plascencia-Jatomea, M., Salazar-Leyva, J. A., Aubourg, S. P., & Ezquerra-Brauer, J. M. (2025). Development of Jellyfish (Stomolophus sp. 2) Gelatine–Chitosan Films: Structural, Physical, and Antioxidant Properties. Gels, 11(10), 836. https://doi.org/10.3390/gels11100836











