Hyaluronic Acid/Chitosan/Glycerophosphate-Based In Situ-Forming Hydrogel for Accelerated Wound Healing
Abstract
1. Introduction
2. Results and Discussion
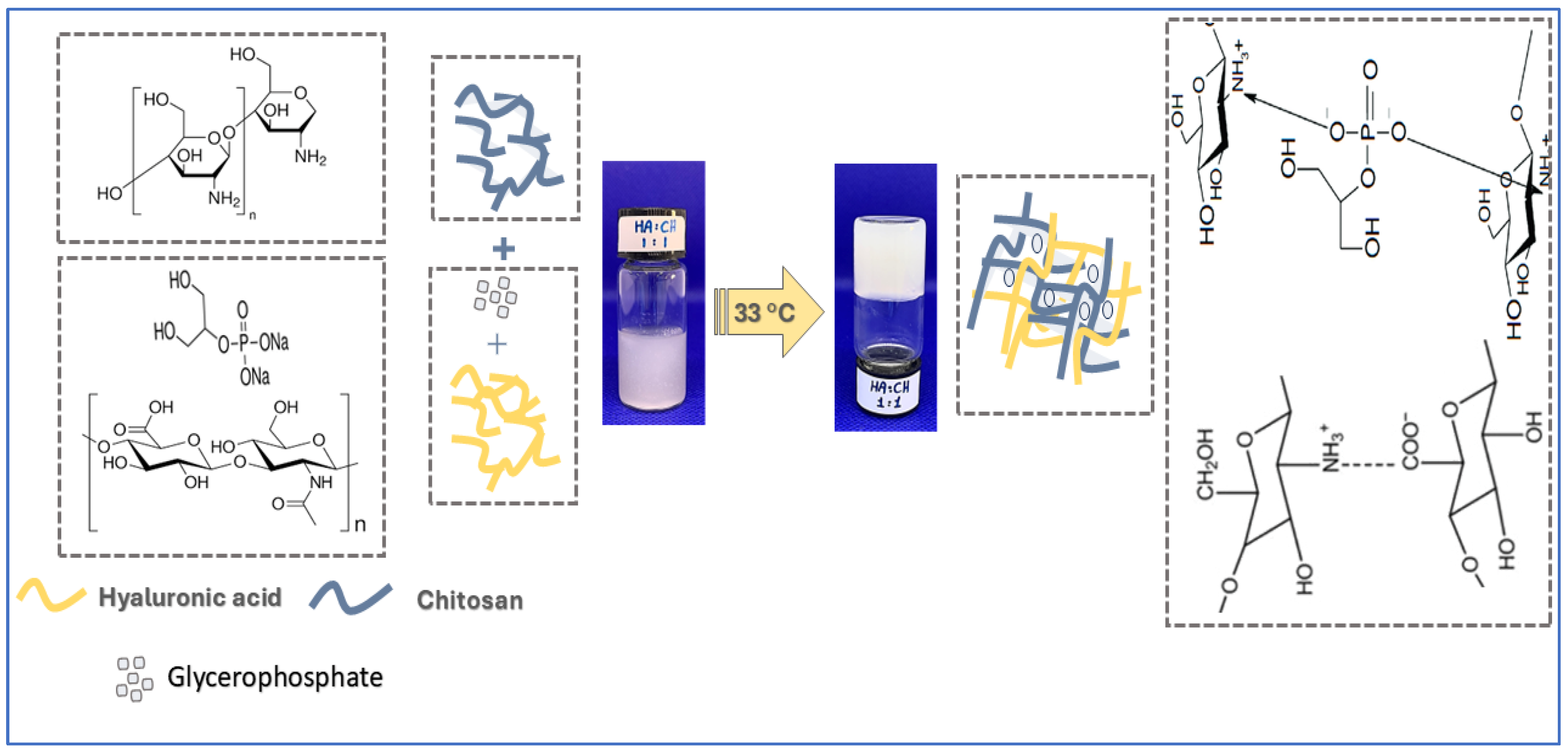
2.1. Preparation of Chitosan-Hyaluronic Acid-Glycerophosphate In Situ-Forming Hydrogels
2.2. Physicochemical Characterization of Hydrogels
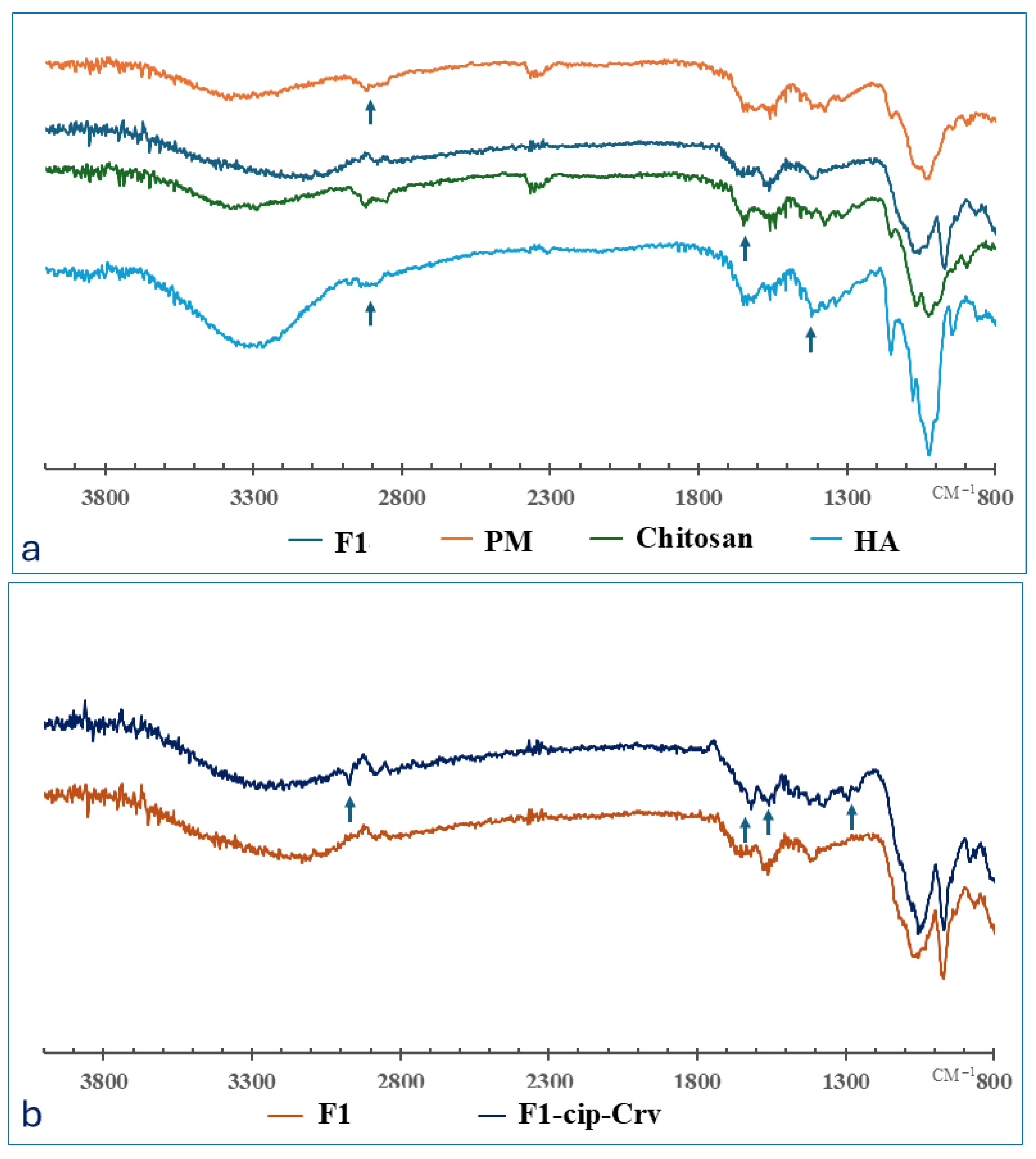
2.2.1. FTIR Analysis
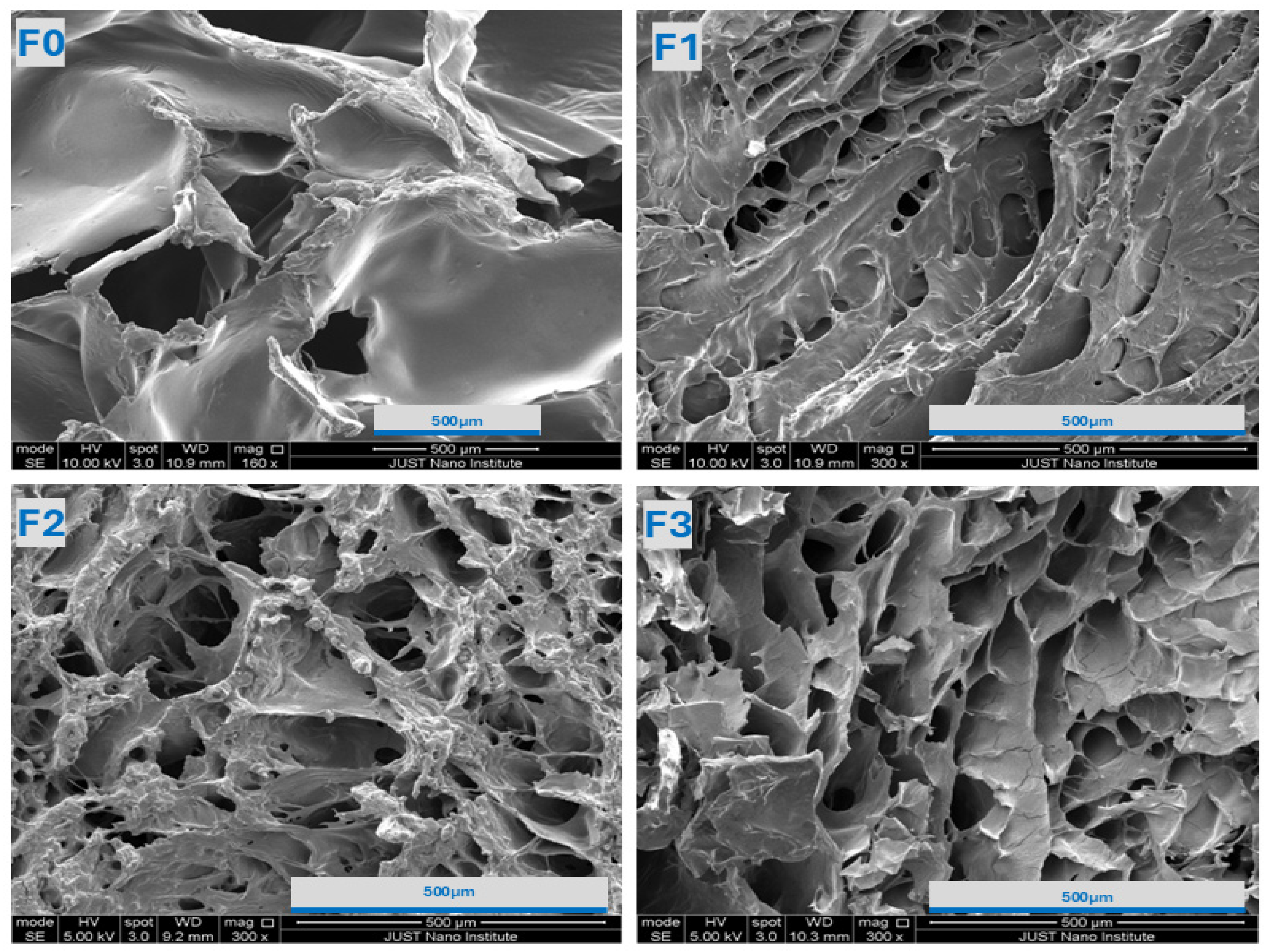
2.2.2. SEM Analysis
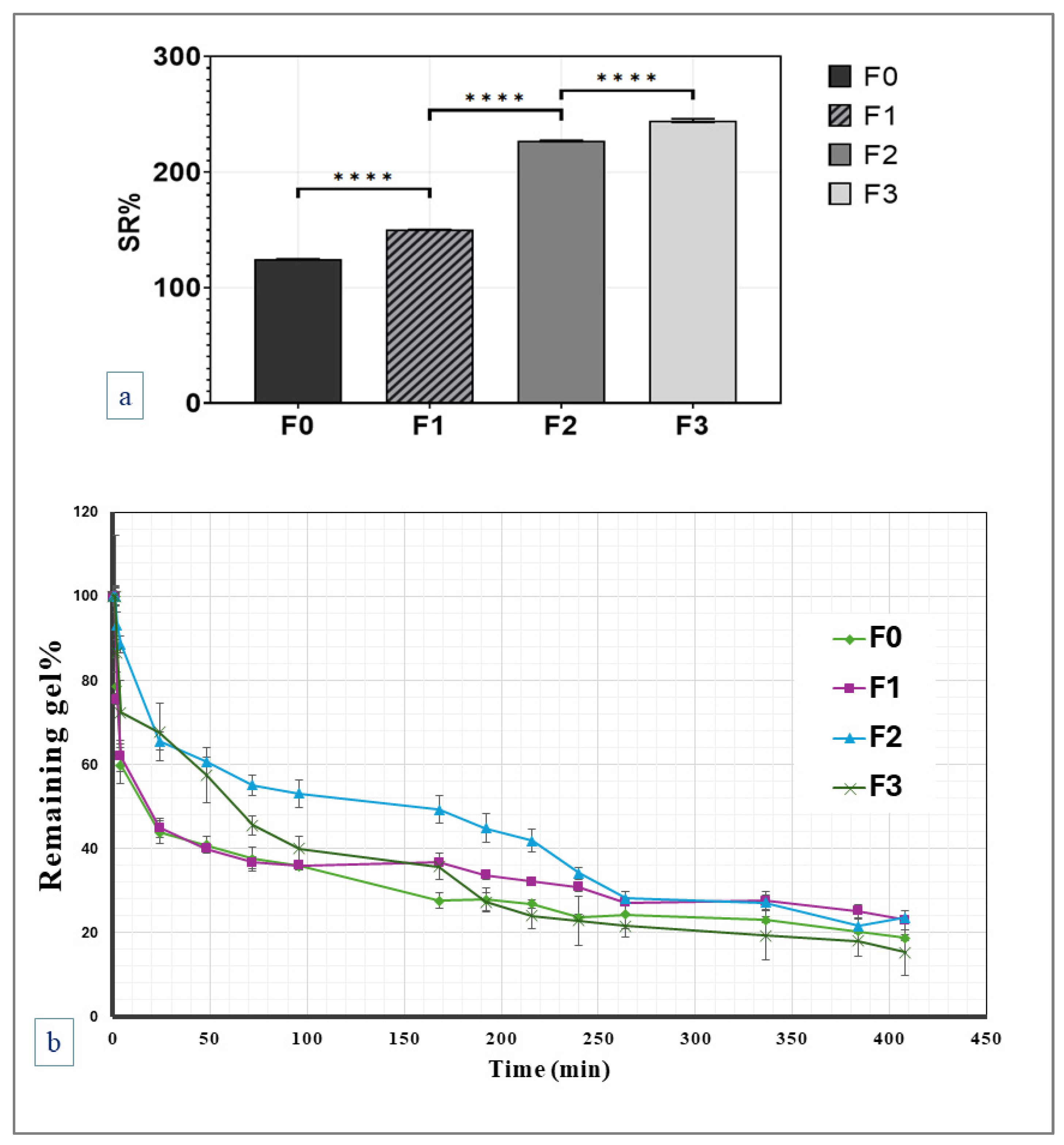
2.2.3. Swelling and In Vitro Degradation Studies
2.2.4. Rheological Studies
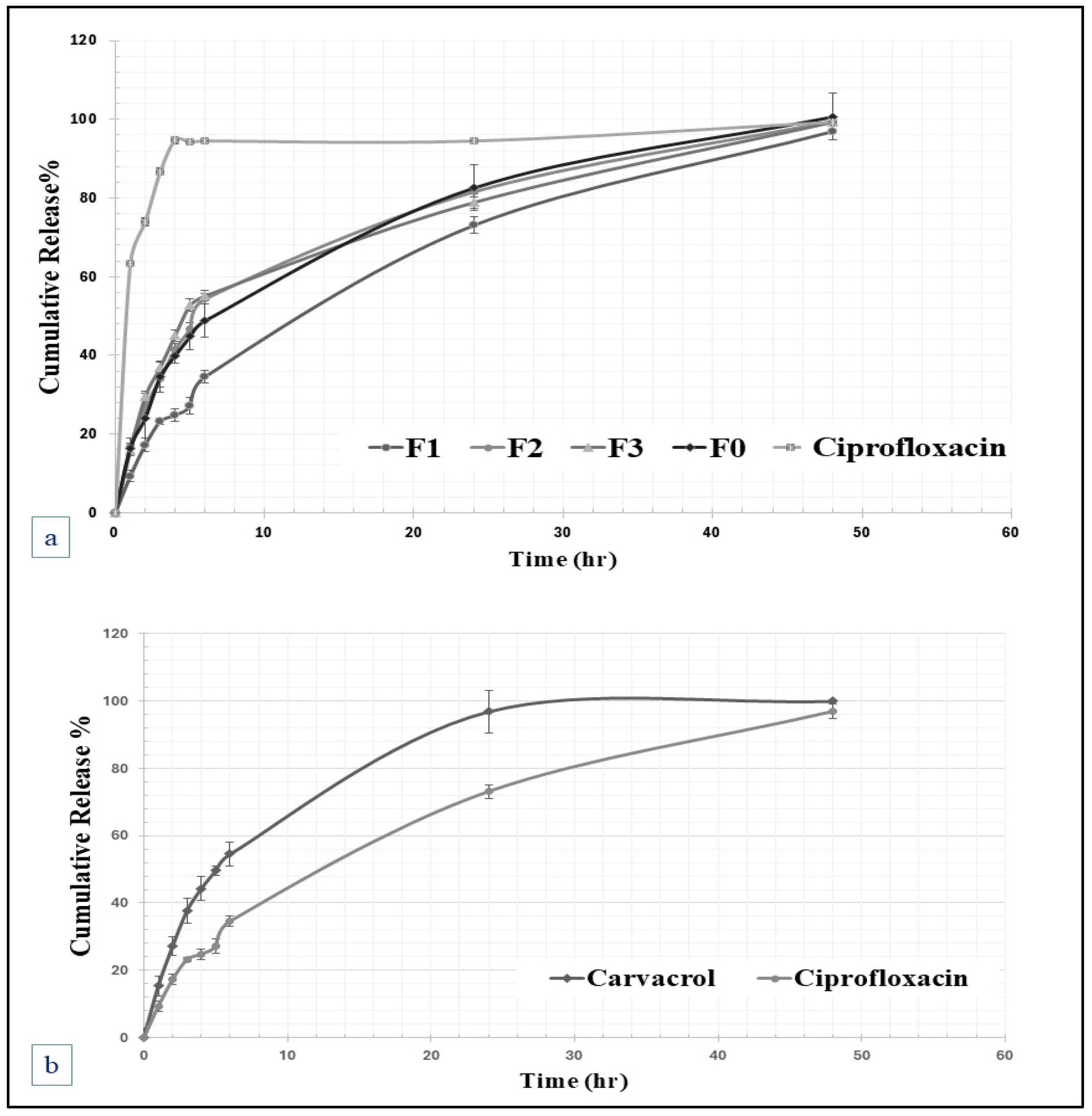
2.2.5. In Vitro Release and Kinetics of Drug Release
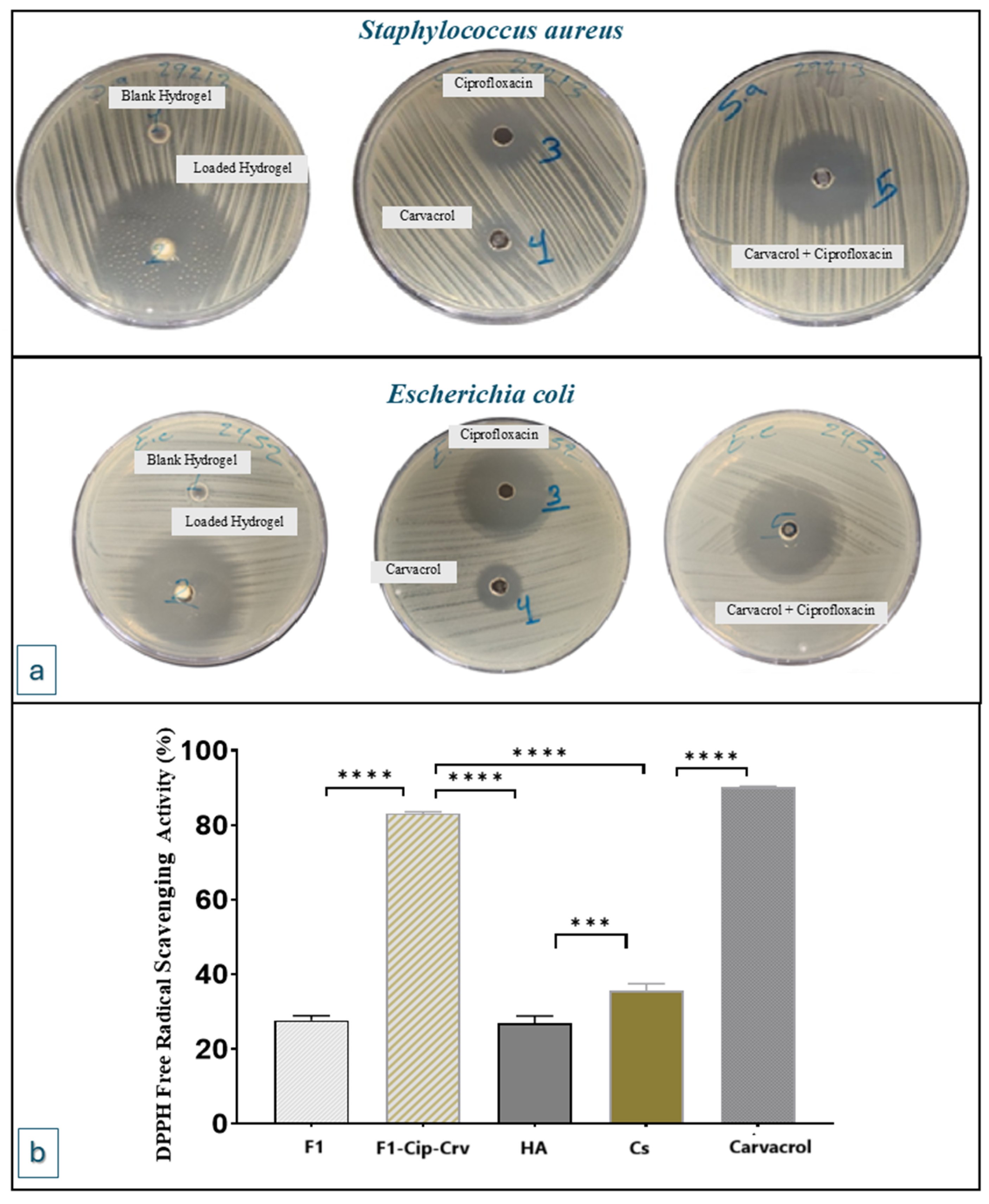
2.3. Antibacterial Activity
2.4. Antioxidant DPPH Solution Scavenging Activity
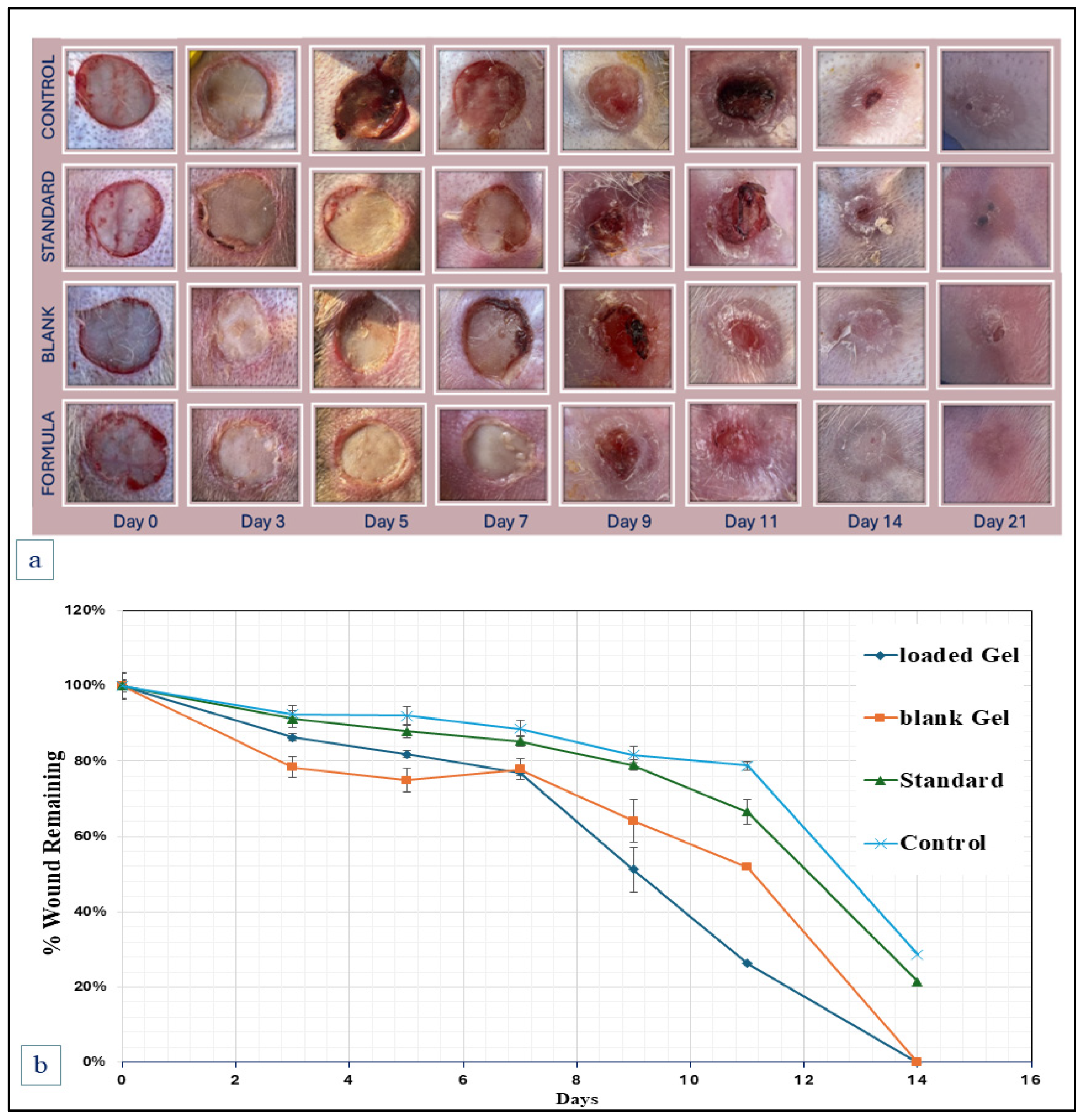
2.5. Animal Wound Healing Studies
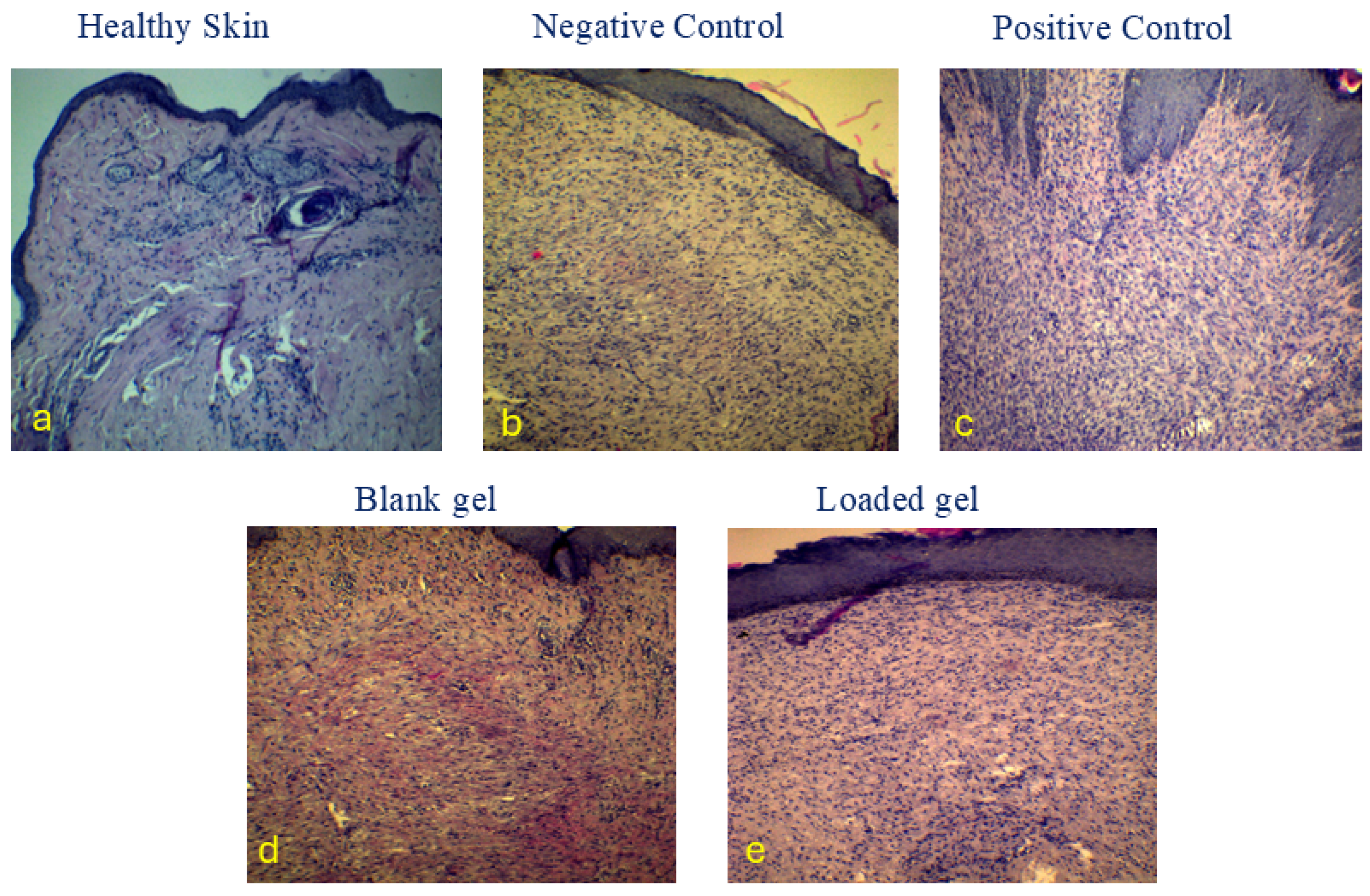
2.6. Histopathologic Examination (H&E Stain)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
Gel Preparation
4.3. Physicochemical Characterization:
4.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.3.2. Scanning Electron Microscopy SEM Analysis
4.3.3. Swelling and In Vitro Degradation Studies
4.4. Rheological Study
4.5. Drug Content
4.6. In Vitro Release Test
4.7. Antibacterial Activity Test
4.8. Antioxidant DPPH Solution Scavenging Test
4.9. In Vivo Wound Healing Study
4.10. Histopathologic Examination (H&E Stain)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Başhan, V.; Yucesan, M.; Demirel, H.; Gul, M. Health, safety, and environmental failure evaluation by hybridizing fuzzy multi-attribute decision-making methods for maritime scrubber systems. Environ. Monit. Assess. 2022, 194, 641. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Hyaluronic Acid Sodium Bethesda, MD: National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hyaluronic-acid-sodium (accessed on 30 September 2025).
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Rezigue, M.; Omari, D.; Shakhatreh, G. Ferric ions crosslinked hyaluronic acid beads: Potentials for drug delivery use. Drug Dev. Ind. Pharm. 2024, 50, 865–877. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Hamed, R.; Obaidat, R.; Hmedat, A.; Aburayya, R.; Hijazi, S.; Akkam, Y. Hyaluronic acid and K-carrageenan metal ionic cross-linked polymers: A promising injectable hydrogels for prolonged chemotherapeutic drug delivery. J. Biomater. Sci. Polym. Ed. 2025, 1–30. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.; Leroux, J.; Atkinson, B.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Combes, C.; Selmani, A.; Jalal, F. Temperature-Controlled pH-Dependent Formation of Ionic Polysaccharide Gels. Google Patents No. 6344488, 5 February 2002. [Google Scholar]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.-R.; Wu, C.-W. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Weng, H.; Jia, W.; Li, M.; Chen, Z. New injectable chitosan-hyaluronic acid based hydrogels for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119767. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, N.; Chi, Y.; Peng, Q.; Dai, G.; Qian, L.; Xu, K.; Zhong, W.; Yue, W. Injectable and self-healing hydrogel based on chitosan-tannic acid and oxidized hyaluronic acid for wound healing. ACS Biomater. Sci. Eng. 2022, 8, 3754–3764. [Google Scholar] [CrossRef]
- Li, J.; Su, J.; Liang, J.; Zhang, K.; Xie, M.; Cai, B.; Li, J. A hyaluronic acid/chitosan composite functionalized hydrogel based on enzyme-catalyzed and Schiff base reaction for promoting wound healing. Int. J. Biol. Macromol. 2024, 255, 128284. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Baradaran Rahimi, V.; Askari, V.R. Thermosensitive chitosan--β--glycerophosphate hydrogels as targeted drug delivery systems: An overview on preparation and their applications. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6640893. [Google Scholar] [CrossRef]
- El-Aassar, M.; El Fawal, G.; Kamoun, E.A.; Fouda, M.M. Controlled drug release from cross-linked κ-carrageenan/hyaluronic acid membranes. Int. J. Biol. Macromol. 2015, 77, 322–329. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.R.; Devi, K.P. Swelling controlled-release systems: Recent developments and applications. Int. J. Pharm. 1988, 48, 1–13. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Abu Alata, W.; Abu-Sini, M.; Abulebdah, D.H.; Hammad, A.M.; Aburayya, R. Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels. Gels 2023, 9, 592. [Google Scholar] [CrossRef]
- Gaia, Z.; Barbara, V.; Caterina, V.; Marco, R.; Giuseppina, S.; Silvia, R. Exploring Sangelose-cyclodextrin in situ-forming hydrogels for vitreous substitution: Design, development, and characterization. Mater. Des. 2025, 257, 114480. [Google Scholar]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar]
- Okur, M.E.; Ayla, Ş.; Batur, Ş.; Yoltaş, A.; Genç, E.; Pertek, S.; Okur, N.Ü. Evaluation of In Situ Gel Containing Pycnogenol for Cutaneous Wound Healing. Medeni. Med. J. 2019, 34, 67–75. [Google Scholar]
- Lee, C.M.; Jin, S.-P.; Doh, E.J.; Lee, D.H.; Chung, J.H. Regional variation of human skin surface temperature. Ann. Dermatol. 2019, 31, 349–352. [Google Scholar] [CrossRef]
- Fairuz, A.; Sapuan, S.; Zainudin, E.; Jaafar, C. The effect of gelation and curing temperatures on mechanical properties of pultruded kenaf fibre reinforced vinyl ester composites. Fibers Polym. 2015, 16, 2645–2651. [Google Scholar] [CrossRef]
- Paul, D. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Shemchuk, O.; d’Agostino, S.; Fiore, C.; Sambri, V.; Zannoli, S.; Grepioni, F.; Braga, D. Natural antimicrobials meet a synthetic antibiotic: Carvacrol/thymol and ciprofloxacin cocrystals as a promising solid-state route to activity enhancement. Cryst. Growth Des. 2020, 20, 6796–6803. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Yang, Y.; Li, P.; Wang, X.; Zhang, K.; Zeng, K.; Ming, J.; Lei, X. Chitosan-based emulsion gel beads developed on the multiple-unit floating delivery system for gastric sustained release of proanthocyanidins. Food Hydrocoll. 2025, 159, 110704. [Google Scholar] [CrossRef]
- Gan, C.; Langa, E.; Wang, G.; Van Bambeke, F.; Ballestero, D.; Pino-Otín, M.R. Mechanisms of action and resistance prevention of synergistic thymol and carvacrol combinations with antibiotics in Staphylococcus aureus and Acinetobacter baumannii. Nat. Prod. Bioprospecting 2025, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Synergistic antibacterial activity of carvacrol loaded chitosan nanoparticles with Topoisomerase inhibitors and genotoxicity evaluation. Saudi J. Biol. Sci. 2023, 30, 103765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, X.; Yang, K.; Guo, L.; Yang, J.; Lan, Z.; Guo, Y.; Xiao, L.; Wang, X. Fabrication and characterization of carvacrol encapsulated gelatin/chitosan composite nanofiber membrane as active packaging material. Int. J. Biol. Macromol. 2024, 282, 137114. [Google Scholar] [CrossRef]
- Aristatile, B.; Al-Numair, K.S.; Al-Assaf, A.H.; Veeramani, C.; Pugalendi, K.V. Protective effect of carvacrol on oxidative stress and cellular DNA damage induced by UVB irradiation in human peripheral lymphocytes. J. Biochem. Mol. Toxicol. 2015, 29, 497–507. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem. Res. Int. 2016, 2016, 2645237. [Google Scholar] [CrossRef]
- Potra Cicalău, G.I.; Ciavoi, G.; Scrobotă, I.; Marcu, A.O.; Romanul, I.; Marian, E.; Vicaș, L.G.; Ganea, M. Assessing the Antioxidant Benefits of Topical Carvacrol and Magnolol Periodontal Hydrogel Therapy in Periodontitis Associated with Diabetes in Wistar Rats. Dent. J. 2023, 11, 284. [Google Scholar] [CrossRef]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A. Wound healing models: A systematic review of animal and non-animal models. Wound Med. 2019, 24, 8–17. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Breen, A.; Mc Redmond, G.; Dockery, P.; O’Brien, T.; Pandit, A. Assessment of wound healing in the alloxan-induced diabetic rabbit ear model. J. Investig. Surg. 2008, 21, 261–269. [Google Scholar] [CrossRef]
- O’Loughlin, A.; Kulkarni, M.; Vaughan, E.E.; Creane, M.; Liew, A.; Dockery, P.; Pandit, A.; O’bRien, T. Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model. Stem Cell Res. Ther. 2013, 4, 158. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the cosmetic ingredient review expert panel safety assessment of polymethyl methacrylate (PMMA), methyl methacrylate crosspolymer, and methyl methacrylate/glycol dimethacrylate crosspolymer. Int. J. Toxicol. 2011, 30 (Suppl. 3), 54S–65S. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Chenite, A.; Sun, J.; Hurtig, M.; Serreqi, A.; Lu, Z.; Rossomacha, E.; Buschmann, M.D. Cytocompatible gel formation of chitosan-glycerol phosphate solutions supplemented with hydroxyl ethyl cellulose is due to the presence of glyoxal. J. Biomed. Mater. Res. Part A 2007, 83, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, J.; Di, Y. A study of human skin and surface temperatures in stable and unstable thermal environments. J. Therm. Biol. 2013, 38, 440–448. [Google Scholar] [CrossRef]
- Caldwell, J.N.; Matsuda-Nakamura, M.; Taylor, N.A. Three-dimensional interactions of mean body and local skin temperatures in the control of hand and foot blood flows. Eur. J. Appl. Physiol. 2014, 114, 1679–1689. [Google Scholar] [CrossRef]
- Tarawneh, O.; Abu Mahfouz, H.; Hamadneh, L.; Deeb, A.A.; Al-Sheikh, I.; Alwahsh, W.; Abed, A.F. Assessment of persistent antimicrobial and anti-biofilm activity of p-HEMA hydrogel loaded with rifampicin and cefixime. Sci. Rep. 2022, 12, 3900. [Google Scholar] [CrossRef]
- Wang, W.; Shi, D.; Zhang, Y.; Li, W.; Li, F.; Feng, H.; Ma, L.; Yang, C.; Peng, Z.; Song, G.; et al. An injectable hydrogel based on hyaluronic acid prepared by Schiff base for long-term controlled drug release. Int. J. Biol. Macromol. 2023, 245, 125341. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Alsmadi, M.M.; Bardaweel, S.; Hailat, N. Characterization and optimization of colon specific nanosponges immobilized polymeric microbeads formulation for the combined delivery of 5-fluorouracil and curcumin. J. Drug Deliv. Sci. Technol. 2024, 99, 105968. [Google Scholar] [CrossRef]
- Obaidat, R.; Kwiak, A.D.A.; Hamed, R. Development of combined therapy of metronidazole and ibuprofen using in situ microgels for the treatment of periodontitis. J. Drug Deliv. Sci. Technol. 2022, 71, 103314. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan/β-glycerophosphate in situ gelling mucoadhesive systems for intravesical delivery of mitomycin-C. Int. J. Pharm. X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Sadeghi, D.; Solouk, A.; Samadikuchaksaraei, A.; Seifalian, A.M. Preparation of internally-crosslinked alginate microspheres: Optimization of process parameters and study of pH-responsive behaviors. Carbohydr. Polym. 2021, 255, 117336. [Google Scholar] [CrossRef]
- Essifi, K.; Lakrat, M.; Berraaouan, D.; Fauconnier, M.-L.; El Bachiri, A.; Tahani, A. Optimization of gallic acid encapsulation in calcium alginate microbeads using Box-Behnken Experimental Design. Polym. Bull. 2021, 78, 5789–5814. [Google Scholar] [CrossRef]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-bacterial, anti-biofilm and anti-quorum sensing activities of honey: A review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef]
- Zeng, Q.; Han, Y.; Li, H.; Chang, J. Design of a thermosensitive bioglass/agarose–alginate composite hydrogel for chronic wound healing. J. Mater. Chem. B 2015, 3, 8856–8864. [Google Scholar] [CrossRef] [PubMed]
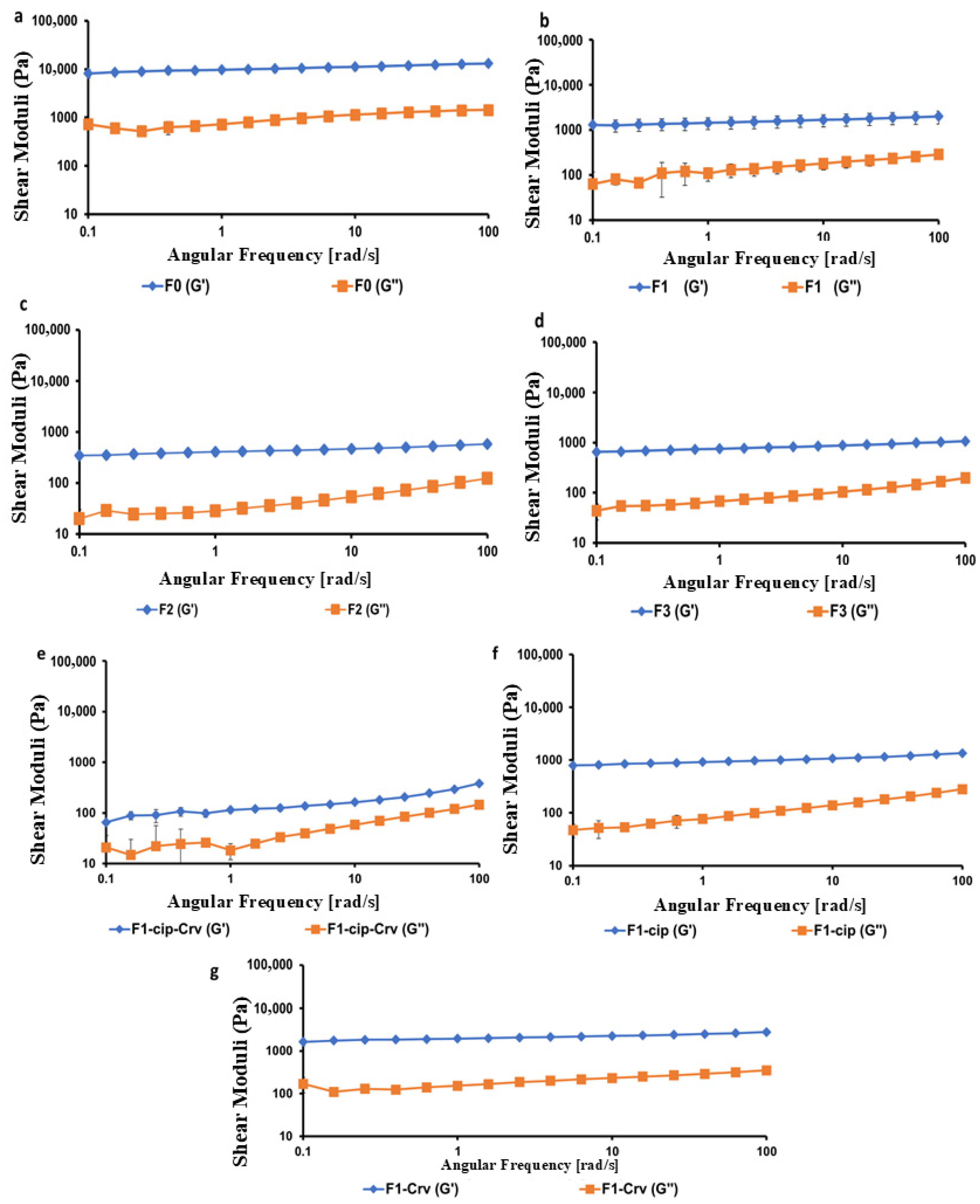
| Sample | LVR (%) | Gel Strength (G′/G″) at 6.31 Rad/s | Gelation Point (Tsol→gel, °C) | Curing Temperature (T-curing, °C) |
|---|---|---|---|---|
| F0 | 0.01–15.8 | 10.24 ± 0.40 | 25.11 ± 0.76 | 30.55 ± 1.80 |
| F1 | 0.01–6.31 | 9.73 ± 0.50 | 26.00 ± 0.49 | 34.14 ± 3.48 |
| F2 | 0.01–25.1 | 9.79 ± 0.52 | 31.49 ± 0.63 | 38.30 ± 0.42 |
| F3 | 0.01–2.51 | 9.02 ± 0.51 | 31.97 ± 0.82 | 38.19 ± 1.40 |
| F1-cip-Crv | 0.01–10.0 | 3.03 ± 0.16 | 28.94 ± 0.36 | 35.91 ± 1.50 |
| F1-cip | 0.01–15.8 | 8.34 ± 0.39 | 28.91 ± 0.66 | 37.35 ± 0.82 |
| F1-Crv | 0.01–25.1 | 10.00 ± 0.72 | 32.14 ± 1.20 | 38.52 ± 5.30 |
| Sample | Zone of Inhibition (mm) | |
|---|---|---|
| Staphylococcus aureus ATCC 29213 | Escherichia coli ATCC 2452 | |
| F1-Cip-Crv | 40 ± 0.01 | 40 ± 0.01 |
| Ciprofloxacin | 18 ± 1.15 | 28.7 ± 0.57 |
| Carvacrol | 13 ± 0.01 | 15.7 ± 0.57 |
| Ciprofloxacin-Carvacrol | 30 ± 0.01 | 34.7 ± 0.57 |
| Hydrogel | Polymeric Ratio (HA:CS) | GP (%w/v) | Ciprofloxacin (mg/mL) | Carvacrol (µL) | pH | Gelation Time at 33 °C (min) |
|---|---|---|---|---|---|---|
| F0 | (0:1) | 60 | 14 | 600 | 8.01 | 3.2 ± 0.3 |
| F1 | (1:1) | 60 | - | - | 7.83 | 3.2 ± 0.3 |
| F2 | (1:2) | 60 | 14 | 600 | 7.7 | 3.2 ± 0.3 |
| F3 | (1:3) | 60 | 14 | 600 | 7.46 | 3.7 ± 0.8 |
| F1-Cip-Crv | (1:1) | 60 | 14 | 600 | 7.63 | 3.8 ± 0.3 |
| F1-Cip | (1:1) | 60 | 14 | - | 7.77 | 3.2 ± 0.3 |
| F1-Crv | (1:1) | 60 | - | 600 | 7.83 | 3.7 ± 0.3 |
| PM | (1:1) | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashaqbeh, H.; Hamed, R.; Alzoubi, H.; Obaidat, R.; Alnaeif, M.; Rezigue, M.; Abukassab, H.T.; Al-Farhan, W.; Obeid, M. Hyaluronic Acid/Chitosan/Glycerophosphate-Based In Situ-Forming Hydrogel for Accelerated Wound Healing. Gels 2025, 11, 835. https://doi.org/10.3390/gels11100835
Mashaqbeh H, Hamed R, Alzoubi H, Obaidat R, Alnaeif M, Rezigue M, Abukassab HT, Al-Farhan W, Obeid M. Hyaluronic Acid/Chitosan/Glycerophosphate-Based In Situ-Forming Hydrogel for Accelerated Wound Healing. Gels. 2025; 11(10):835. https://doi.org/10.3390/gels11100835
Chicago/Turabian StyleMashaqbeh, Hadeia, Rania Hamed, Hiba Alzoubi, Rana Obaidat, Mohammad Alnaeif, Meriem Rezigue, Hala T. Abukassab, Wasan Al-Farhan, and Mohammad Obeid. 2025. "Hyaluronic Acid/Chitosan/Glycerophosphate-Based In Situ-Forming Hydrogel for Accelerated Wound Healing" Gels 11, no. 10: 835. https://doi.org/10.3390/gels11100835
APA StyleMashaqbeh, H., Hamed, R., Alzoubi, H., Obaidat, R., Alnaeif, M., Rezigue, M., Abukassab, H. T., Al-Farhan, W., & Obeid, M. (2025). Hyaluronic Acid/Chitosan/Glycerophosphate-Based In Situ-Forming Hydrogel for Accelerated Wound Healing. Gels, 11(10), 835. https://doi.org/10.3390/gels11100835






