Cochlospermum regium Leaf Extract Gel: A Natural Strategy Against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
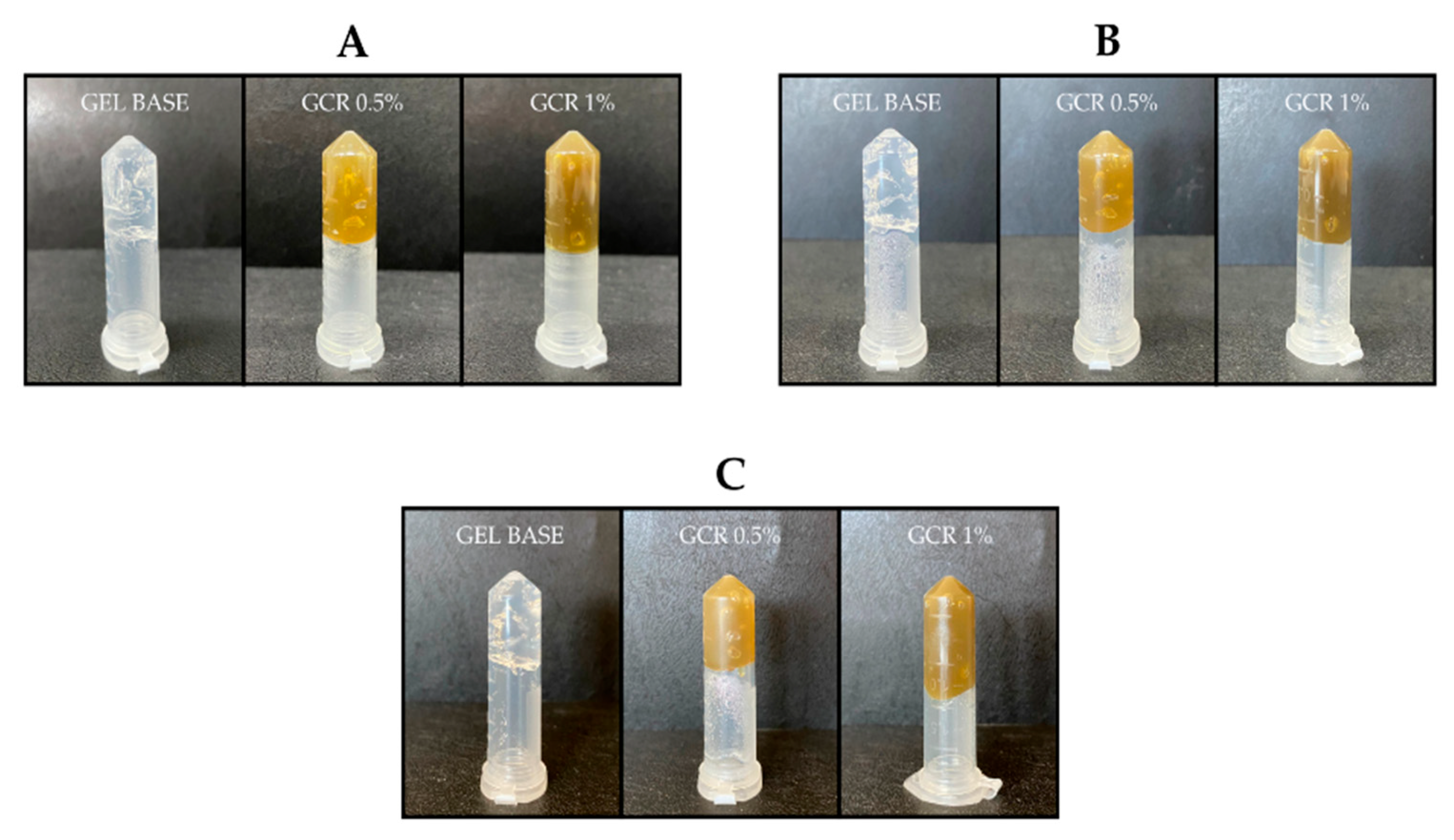
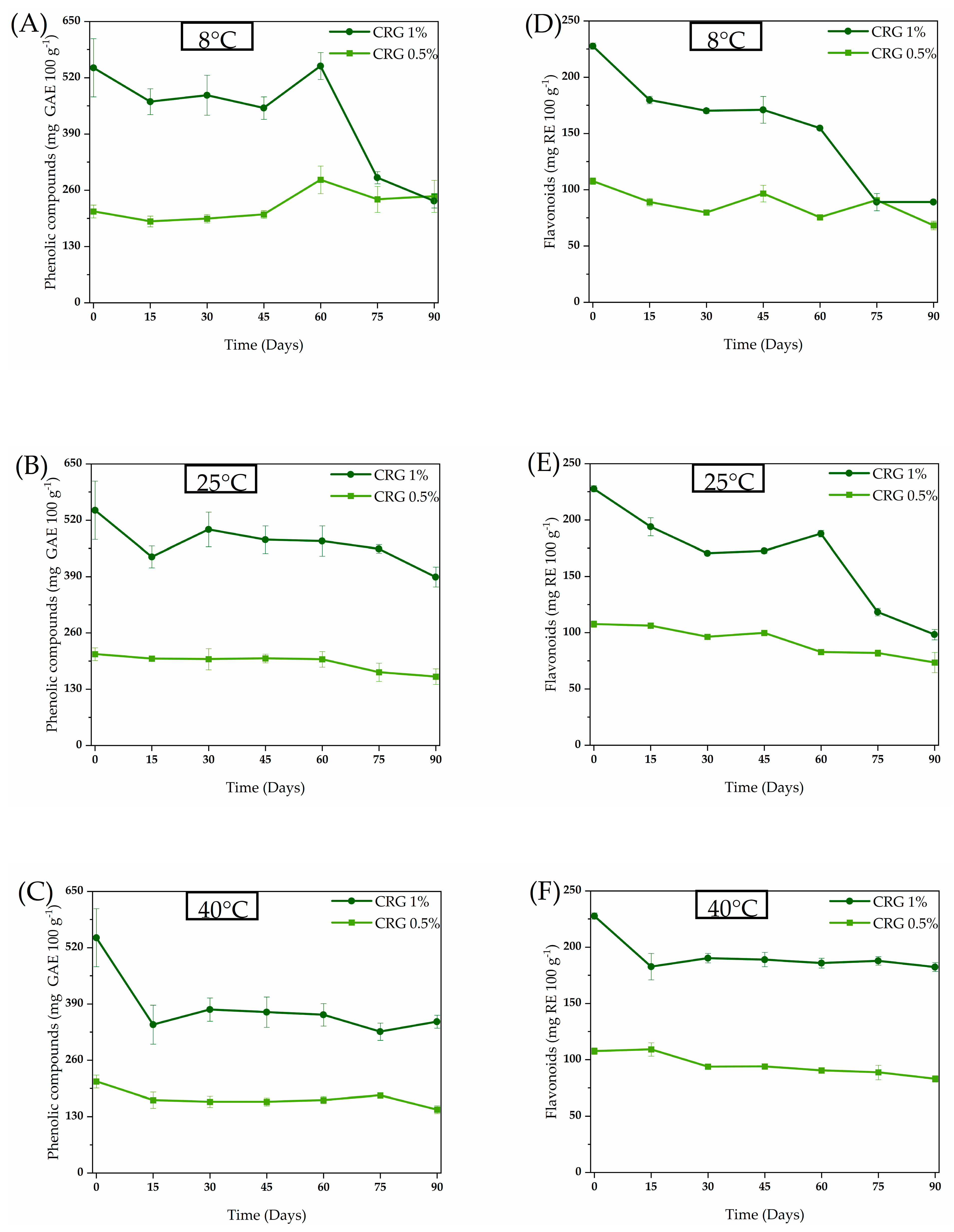
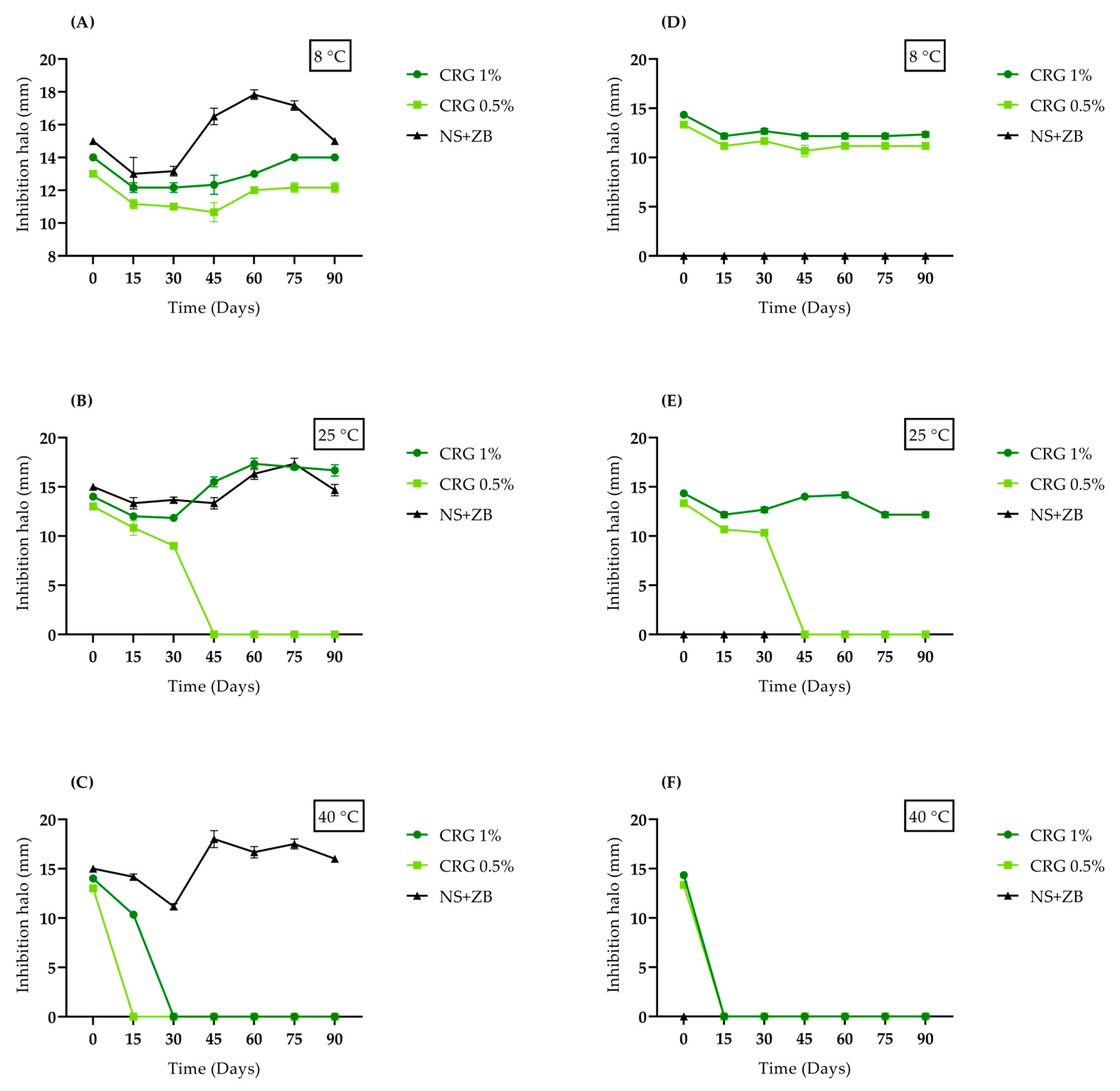
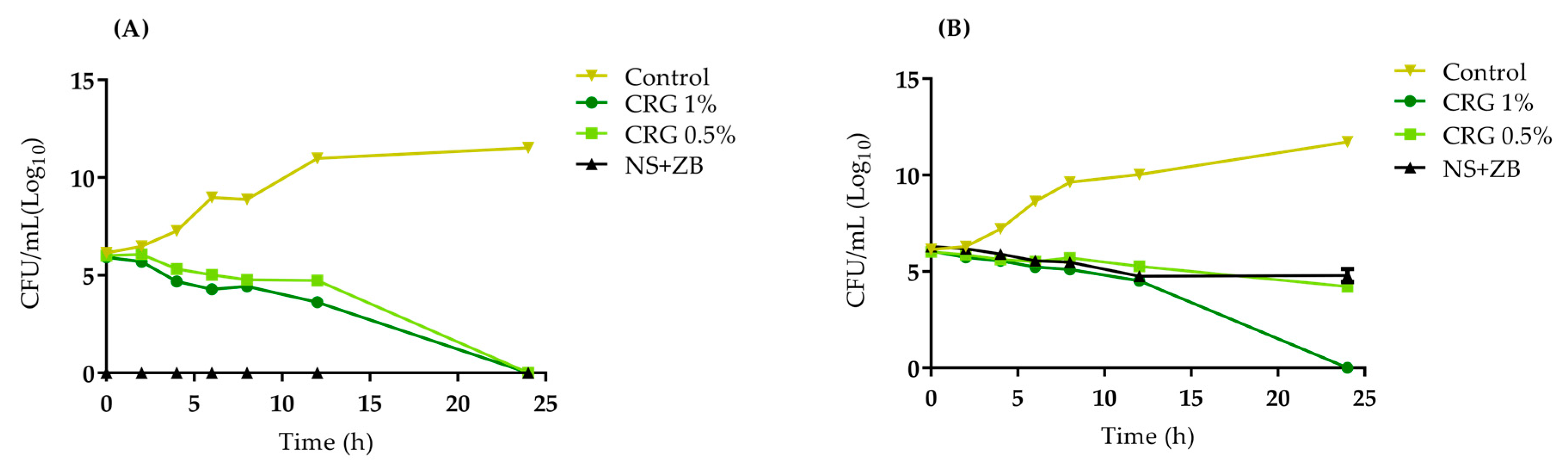
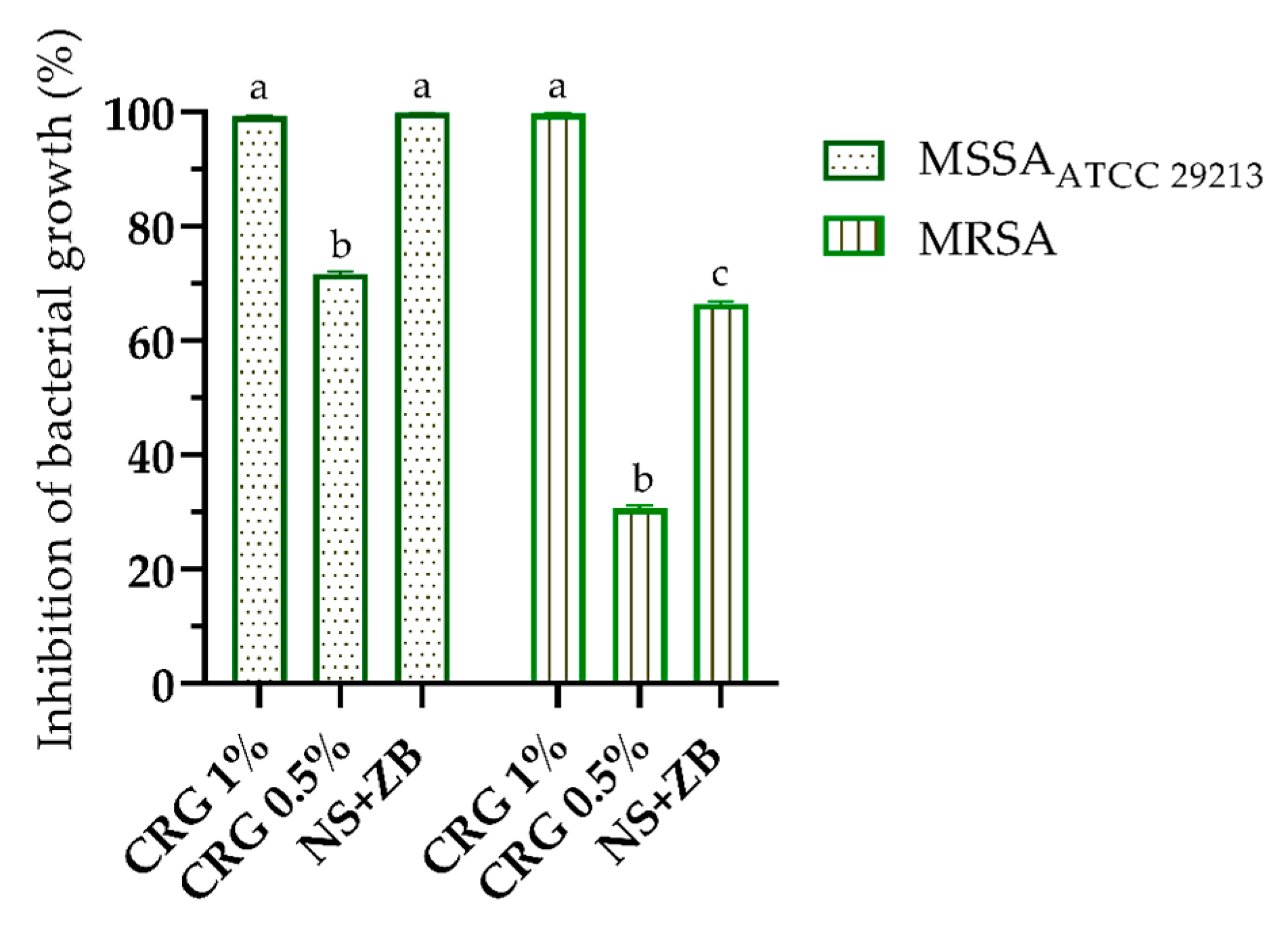
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Plant Collection and Preparation of the Ethanolic Extract from Cochlospermum regium Leaves
4.2. Preparation of Carbopol Gel Containing Cochlospermum regium Leaf Extract
4.2.1. Evaluation of Physical Characteristics, Injectability, and Inversion Test
4.2.2. Quantification of Phenolic and Flavonoid Compounds
4.3. Antibacterial Activity
4.3.1. Microorganisms
4.3.2. Agar Diffusion Test
4.3.3. Time Kill
4.3.4. Ex Vivo Antibacterial Activity Assay
4.4. Antioxidant Activity
4.5. Biocompatibility Assays
4.5.1. Ames Test
4.5.2. Hemolytic Potential
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRG 0.5% | Cochlospermum regium gel containing 0.5% extract |
| CRG 1% | Cochlospermum regium gel containing 1% extract |
| S. aureus | Staphylococcus aureus |
| EECR | Ethanolic extract of Cochlospermum regium leaves |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| NS + ZB | Neomycin sulfate + Zinc bacitracin |
| IC50 | Lowest concentration capable of causing 50% inhibition |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| GAE | Gallic acid equivalents |
| RE | Rutin equivalents |
| ATCC | American Type Culture Collection |
| CFU | Colony-forming unit |
| MH | Mueller–Hinton (agar/broth) |
| TA98 | Salmonella enterica serovar Typhimurium strain TA98 |
| TA100 | Salmonella enterica serovar Typhimurium strain TA100 |
| S9 | fraction exogenous metabolic activation system |
| PBS | Phosphate-buffered saline |
| MI | Mutagenicity index |
| ANOVA | Analysis of variance |
| SisGen | Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado |
References
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.d.O. Ethnobotanical Study of Medicinal Plants Used by Ribeirinhos in the North Araguaia Microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Johnson-Fulton, S.B.; Watson, L.E. Comparing Medicinal Uses of Cochlospermaceae throughout Its Geographic Range with Insights from Molecular Phylogenetics. Diversity 2018, 10, 123. [Google Scholar] [CrossRef]
- Galvão, F.d.O.; Dantas, F.G.d.S.; Santos, C.R.d.L.; Marchioro, S.B.; Cardoso, C.A.L.; Wender, H.; Sangalli, A.; de Almeida-Apolonio, A.A.; de Oliveira, K.M.P. Cochlospermum regium (Schrank) Pilger Leaf Extract Inhibit Methicillin-Resistant Staphylococcus aureus Biofilm Formation. J. Ethnopharmacol. 2020, 261, 113167. [Google Scholar] [CrossRef] [PubMed]
- Galvão, F.; Dos Santos, E.; Gomes da Silva Dantas, F.; Irlan da Silva Santos, J.; da Paz Costa Sauda, T.; Carvalho Dos Santos, A.; Carvalho Souza, R.I.; da Silva Pinto, L.; Ferreira Moraes, C.A.; Sangalli, A.; et al. Chemical Composition and Effects of Ethanolic Extract and Gel of Cochlospermum regium (Schrank) Pilg. Leaves on Inflammation, Pain, and Wounds. J. Ethnopharmacol. 2023, 302, 115881. [Google Scholar] [CrossRef]
- Al Kindi, A.; Alkahtani, A.M.; Nalubega, M.; El-Chami, C.; O’Neill, C.; Arkwright, P.D.; Pennock, J.L. Staphylococcus aureus Internalized by Skin Keratinocytes Evade Antibiotic Killing. Front. Microbiol. 2019, 10, 2242. [Google Scholar] [CrossRef]
- Ray, G.T.; Suaya, J.A.; Baxter, R. Microbiology of Skin and Soft Tissue Infections in the Age of Community-Acquired Methicillin-Resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2013, 76, 24–30. [Google Scholar] [CrossRef] [PubMed]
- DEL GIUDICE, P. Skin Infections Caused by Staphylococcus aureus. Acta Derm. Venereol. 2020, 100, 5725. [Google Scholar] [CrossRef]
- Bessa, G.R.; Quinto, V.P.; Machado, D.C.; Lipnharski, C.; Weber, M.B.; Bonamigo, R.R.; D’Azevedo, P.A. Staphylococcus aureus Resistance to Topical Antimicrobials in Atopic Dermatitis. Bras. Dermatol. 2016, 91, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Chellathurai, B.J.; Anburose, R.; Alyami, M.H.; Sellappan, M.; Bayan, M.F.; Chandrasekaran, B.; Chidambaram, K.; Rahamathulla, M. Development of a Polyherbal Topical Gel for the Treatment of Acne. Gels 2023, 9, 163. [Google Scholar] [CrossRef]
- Inácio, M.C.; Paz, T.A.; Bertoni, B.W.; Vieira, M.A.R.; Marques, M.O.M.; Pereira, A.M.S. Histochemical Investigation of Cochlospermum regium (Schrank) Pilg. Leaves and Chemical Composition of Its Essential Oil. Nat. Prod. Res. 2014, 28, 727–731. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and Application as Delivery Systems of Phenolic and Aroma Compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Batista, P.A.; Dario, M.F.; Gonçalves, F.S.M.; Ji, S.; Batista, P.A.; Dario, M.F.; Gonçalves, F.S.M. Avaliação Da Estabilidade e Atividade Antioxidante de Formulações Com o Extrato de Mangifera indica L. Evaluation of the stability and antioxidant activity of formulations with Mangifera indica L. extract. O Mundo Da Saúde 2024, 48, e15832024. [Google Scholar] [CrossRef]
- de Souza, C.C.; Pinto, G.I.; Kerppers, I.I.; de Paula, D. Desenvolvimento e Caracterização de Formulação Tópica de Extrato de Uvarana para Tratamento de Feridas. Rev. Eletrônica Farmácia 2016, 13, 191–200. [Google Scholar] [CrossRef]
- Alvarenga, G.F.; Carlos, L.A.; Arruda, A.C.; Martins, L.M.; Oliveira, K.G.; Silva, E.C. Blend de Maracujá e Capuchinha: Efeito do Processamento Térmico Sobre Compostos Bioativos e Características Sensoriais. Braz. J. Food Res. 2017, 8, 112–125. [Google Scholar] [CrossRef]
- de Oliveira, K.G.; Queiroz, V.A.V.; Carlos, L.d.A.; Cardoso, L.d.M.; Pinheiro-Sant’Ana, H.M.; Anunciação, P.C.; de Menezes, C.B.; da Silva, E.C.; Barros, F. Effect of the Storage Time and Temperature on Phenolic Compounds of Sorghum Grain and Flour. Food Chem. 2017, 216, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Mrázková, M.; Sumczynski, D.; Orsavová, J. Influence of Storage Conditions on Stability of Phenolic Compounds and Antioxidant Activity Values in Nutraceutical Mixtures with Edible Flowers as New Dietary Supplements. Antioxidants 2023, 12, 962. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Hamed, A.A.; Hassan, H.M.; Belbahri, L.; Rateb, M.E.; Sayed, A.M. Flavonoids as Potential Anti-MRSA Agents through Modulation of PBP2a: A Computational and Experimental Study. Antibiotics 2020, 9, 562. [Google Scholar] [CrossRef]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, Characterization and Stability Evaluation of Topical Gel Loaded With Ethosomes Containing Achillea millefolium L. Extract. Front. Pharmacol. 2021, 12, 603227. [Google Scholar] [CrossRef]
- Das, S.; Wong, A.B.H. Stabilization of Ferulic Acid in Topical Gel Formulation via Nanoencapsulation and pH Optimization. Sci. Rep. 2020, 10, 12288. [Google Scholar] [CrossRef]
- Deuschle, V.C.K.N.; Deuschle, R.A.N.; Bortoluzzi, M.R.; Athayde, M.L. Physical Chemistry Evaluation of Stability, Spreadability, in Vitro Antioxidant, and Photo-Protective Capacities of Topical Formulations Containing Calendula officinalis L. Leaf Extract. Braz. J. Pharm. Sci. 2015, 51, 63–75. [Google Scholar] [CrossRef]
- Nair, S.V.; Baranwal, G.; Chatterjee, M.; Sachu, A.; Vasudevan, A.K.; Bose, C.; Banerji, A.; Biswas, R. Antimicrobial Activity of Plumbagin, a Naturally Occurring Naphthoquinone from Plumbago Rosea, against Staphylococcus aureus and Candida albicans. Int. J. Med. Microbiol. 2016, 306, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Sousa, J.C.; Silva, A.C.; Melo, L.D.R.; Sillankorva, S. Chestnut Honey and Bacteriophage Application to Control Pseudomonas aeruginosa and Escherichia coli Biofilms: Evaluation in an Ex Vivo Wound Model. Front. Microbiol. 2018, 9, 1725. [Google Scholar] [CrossRef]
- Chirayath, R.B.; Viswanathan A, A.; Jayakumar, R.; Biswas, R.; Vijayachandran, L.S. Development of Mangifera Indica Leaf Extract Incorporated Carbopol Hydrogel and Its Antibacterial Efficacy against Staphylococcus aureus. Colloids Surf. B Biointerfaces 2019, 178, 377–384. [Google Scholar] [CrossRef]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel Wound Dressings Accelerating Healing Process of Wounds in Movable Parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef]
- Nunes, W.B.; de Carvalho, S. Evaluation of the Mutagenic Potential of Cochlospermum regium in Drosophila melanogaster Male Germ Cells. Genet. Mol. Biol. 2003, 26, 545–549. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and Hemocompatible pH-Responsive Hydrogel for Skin Wound Healing Application: In Vitro Drug Release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Kakade, P.M.; Kadam, P.M. Formulation Development and Evaluation of Antimicrobial Polyherbal Gel. Ann. Pharm. Fr. 2017, 75, 349–358. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Boubekeur, N.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Nithya, S.; Nimal, T.R.; Baranwal, G.; Suresh, M.K.; C.P., A.; Anil Kumar, V.; Gopi Mohan, C.; Jayakumar, R.; Biswas, R. Preparation, Characterization and Efficacy of Lysostaphin-Chitosan Gel against Staphylococcus aureus. Int. J. Biol. Macromol. 2018, 110, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Yang, J.C.; Forrest, A.; Kelchlin, P.A.; Smith, P.F. In Vitro Pharmacodynamics of Novel Rifamycin ABI-0043 against Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 62, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, Y.; Shi, P.; Hu, J.; Li, S.; Huang, L.; Lin, J.; Lin, X. Screening and Quantitative Analysis of Antioxidants in the Fruits of Livistona chinensis R. Br Using HPLC-DAD–ESI/MS Coupled with Pre-Column DPPH Assay. Food Chem. 2012, 135, 2802–2807. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Kado, N.Y.; Langley, D.; Eisenstadt, E. A Simple Modification of the Salmonella Liquid-Incubation Assay. Increased Sensitivity for Detecting Mutagens in Human Urine. Mutat. Res. 1983, 121, 25–32. [Google Scholar] [CrossRef]
- Rangel, M.; Malpezzi, E.L.A.; Susini, S.M.M.; De Freitas, J. Hemolytic Activity in Extracts of the Diatom nitzschia. Toxicon 1997, 35, 305–309. [Google Scholar] [CrossRef] [PubMed]


| Sample | DPPH | ABTS |
|---|---|---|
| AA | 3.14 ± 0.05 | 4.20 ± 0.18 |
| EECR | 5.66 ± 0.07 | 21.81 ± 0.45 |
| µg/placa | TA98 | TA100 | ||
|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | |
| 0 a | 19 ± 1 | 19 ± 3 | 124 ± 11 | 129 ± 4 |
| 50 | 25 ± 1 (1.3) | 16 ± 1 (0.8) | 130 ± 8 (1.0) | 109 ± 4 (0.8) |
| 150 | 19 ± 2 (1.0) | 20 ± 3 (1.0) | 120 ± 4 (0.9) | 108 ± 7 (0.8) |
| 500 | 18 ± 1 (0.9) | 23 ± 1 (1.2) | 127 ± 6 (1.0) | 115 ± 4 (0.8) |
| 1500 | 18 ± 2 (0.9) | 24 ± 3 (1.2) | 128 ± 5 (1.0) | 148 ± 6 (1.1) |
| 5000 | 16 ± 2 (0.8) | 17 ± 0.5 (0.8) | 117 ± 6 (0.9) | 123 ± 3 (0.9) |
| C+ | 555 ± 25 b | 880 ± 9 c | 1303 ± 7 d | 803 ± 8 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvão, F.; Leite, C.; Andrade, J.; Castilho, P.; Castro, T.; Cardoso, C.; Ferreira, D.; Negri, M.; Dantas, F.; Oliveira, K. Cochlospermum regium Leaf Extract Gel: A Natural Strategy Against Methicillin-Resistant Staphylococcus aureus. Gels 2025, 11, 831. https://doi.org/10.3390/gels11100831
Galvão F, Leite C, Andrade J, Castilho P, Castro T, Cardoso C, Ferreira D, Negri M, Dantas F, Oliveira K. Cochlospermum regium Leaf Extract Gel: A Natural Strategy Against Methicillin-Resistant Staphylococcus aureus. Gels. 2025; 11(10):831. https://doi.org/10.3390/gels11100831
Chicago/Turabian StyleGalvão, Fernanda, Cleison Leite, João Andrade, Pamella Castilho, Thiago Castro, Claudia Cardoso, Deisiany Ferreira, Melyssa Negri, Fabiana Dantas, and Kelly Oliveira. 2025. "Cochlospermum regium Leaf Extract Gel: A Natural Strategy Against Methicillin-Resistant Staphylococcus aureus" Gels 11, no. 10: 831. https://doi.org/10.3390/gels11100831
APA StyleGalvão, F., Leite, C., Andrade, J., Castilho, P., Castro, T., Cardoso, C., Ferreira, D., Negri, M., Dantas, F., & Oliveira, K. (2025). Cochlospermum regium Leaf Extract Gel: A Natural Strategy Against Methicillin-Resistant Staphylococcus aureus. Gels, 11(10), 831. https://doi.org/10.3390/gels11100831








